Abstract
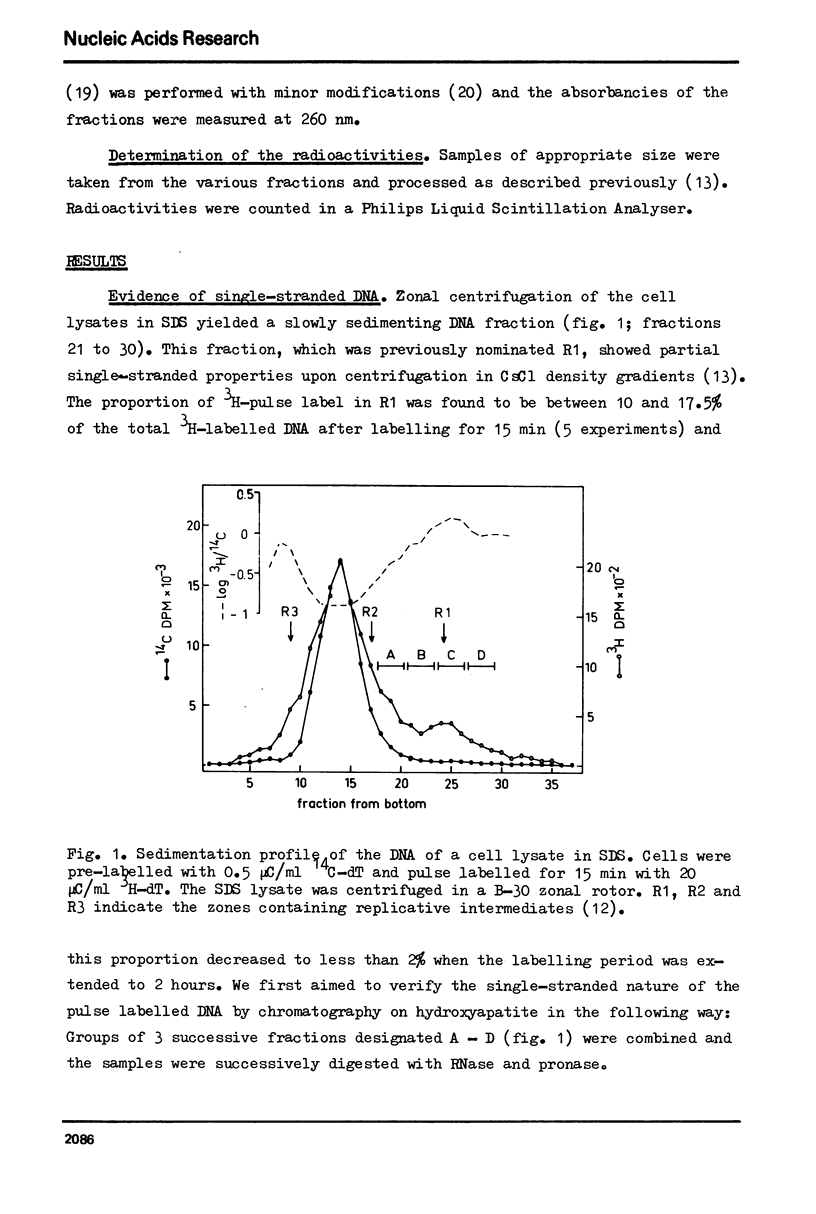
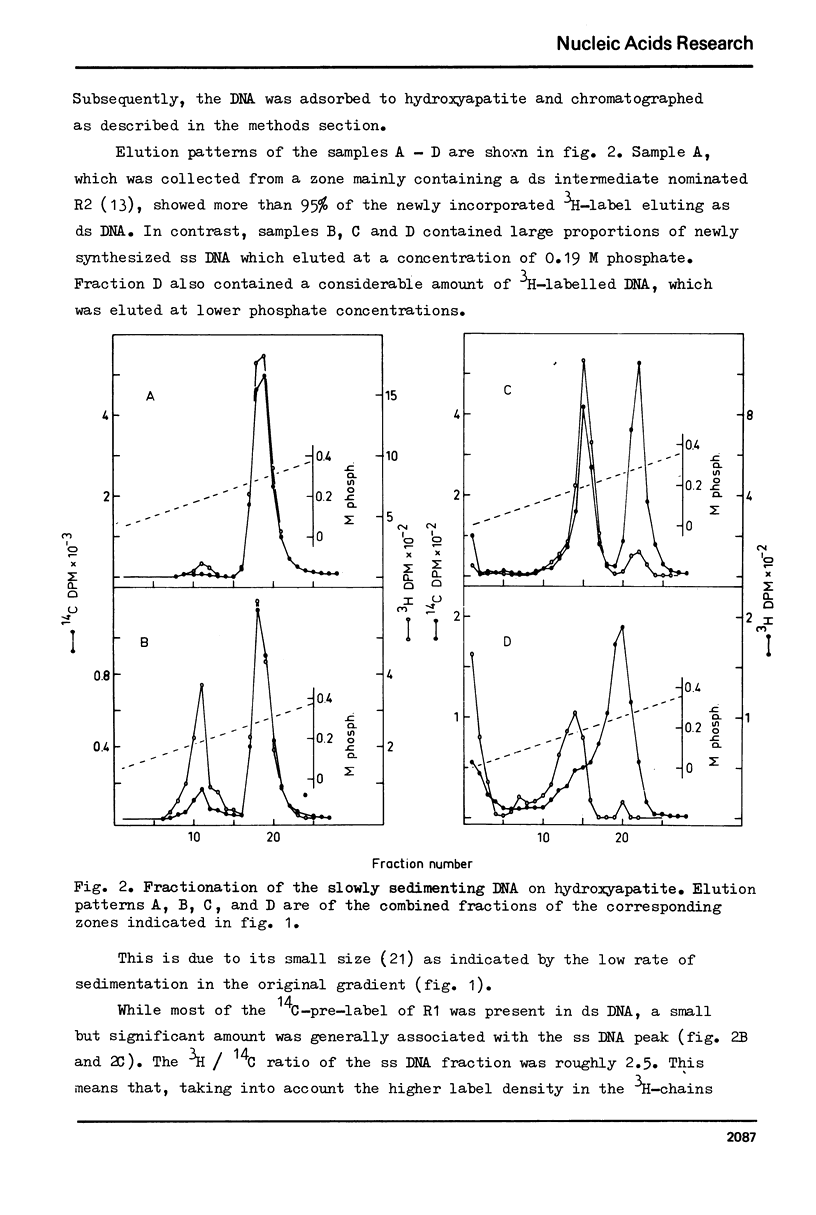
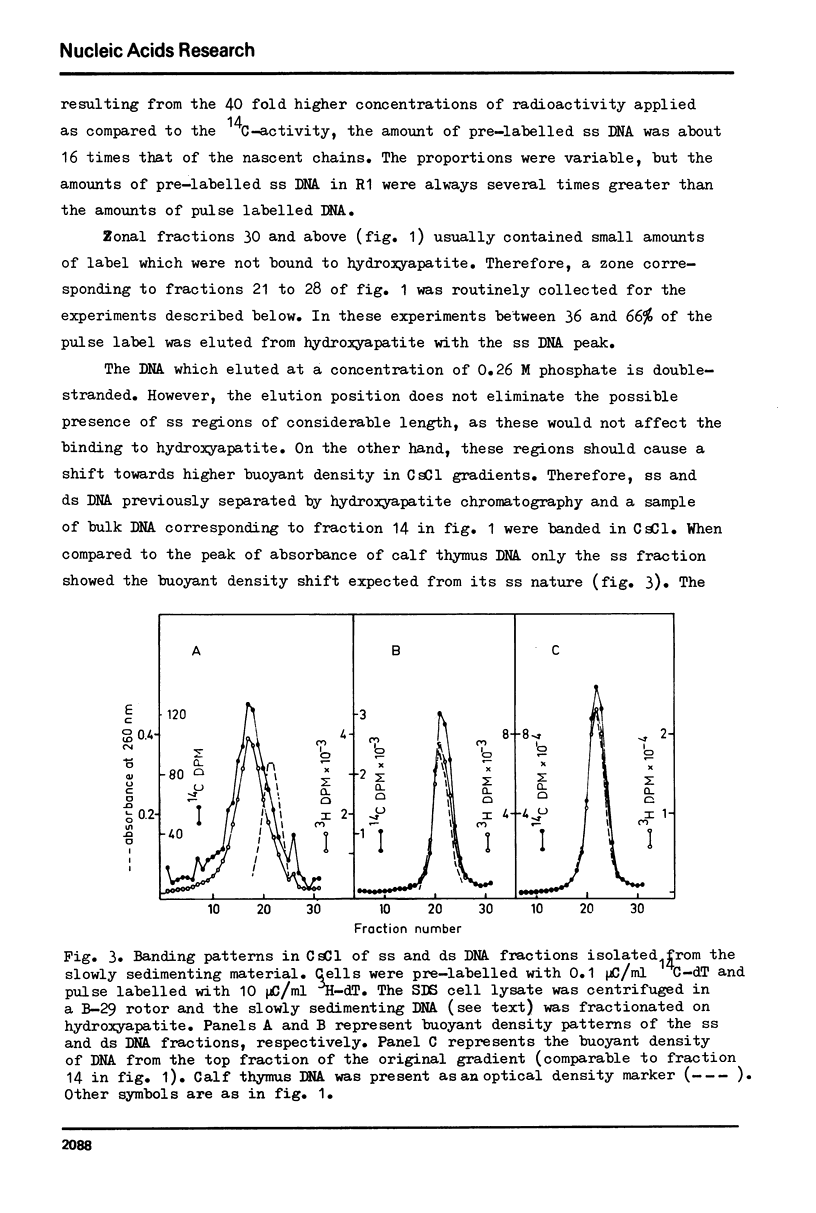
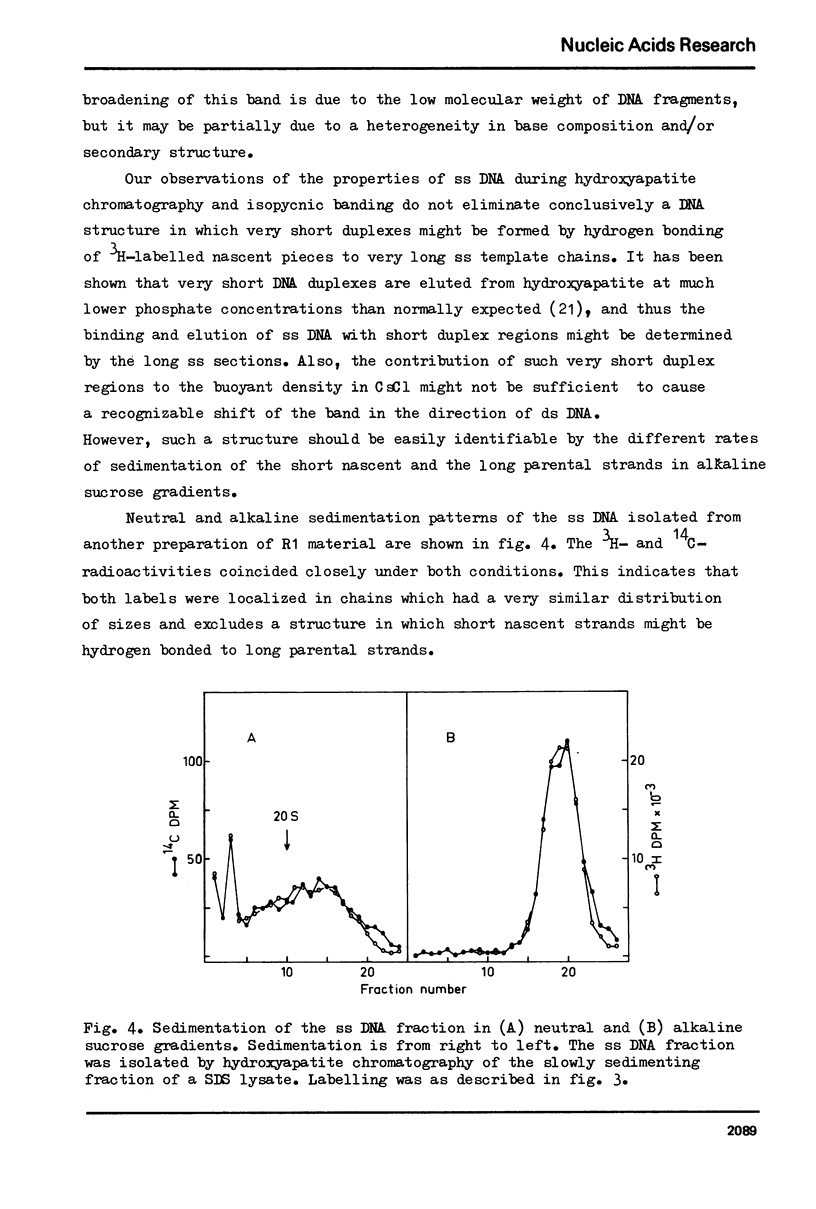
In vitro cultured bovine liver cells were labelled with radioactive thymidine and dissolved in 0.5% sodium dodecyl sulphate. Centrifugation of the lysate through sucrose gradients in a zonal rotor revealed a slowly sedimenting fraction of preferentially pulse labelled DNA. The DNA of this zone was further analysed by chromatography on hydroxy-apatite, banding in CsCl density gradients, and sedimentation in neutral and alkaline sucrose gradients. It contained besides small amounts of fragmented bulk DNA, single-stranded nascent DNA and single-stranded pre-labelled DNA which could be separated from each other by using BrdU as a density label. The density labelling also revealed small amounts of nascent-nascent DNA duplexes. The slowly sedimenting fraction was practically absent from cell lysates which were prepared in 2 M NaCl - 50 microgram/ml pronase. The results suggest that nascent single-strands and nascent-nascent duplexes are released from the forks of replicating DNA by branch migration. Pre-labelled single strands may be released by the same branch migration. Pre-labelled single strands may be released by the same mechanism, but the in vivo structure from which they originate has yet to be elucidated.
Full text
PDF


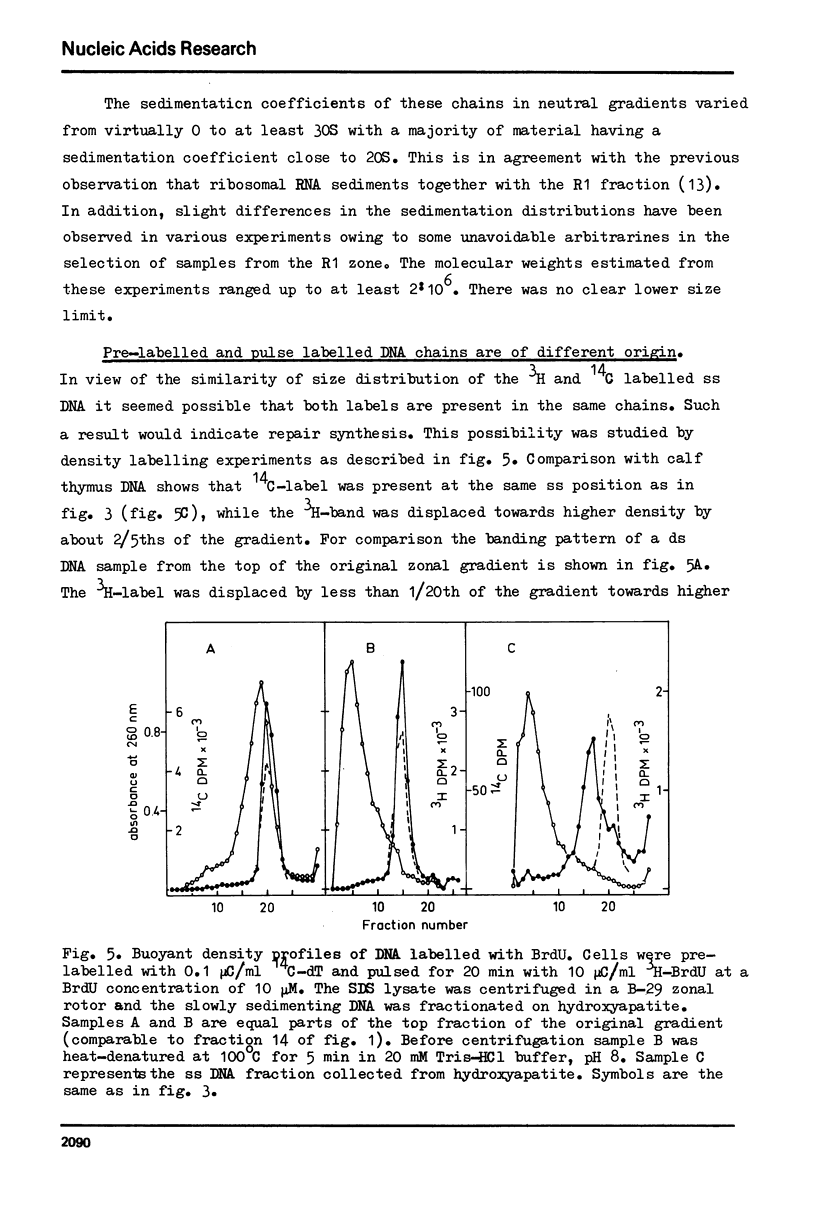
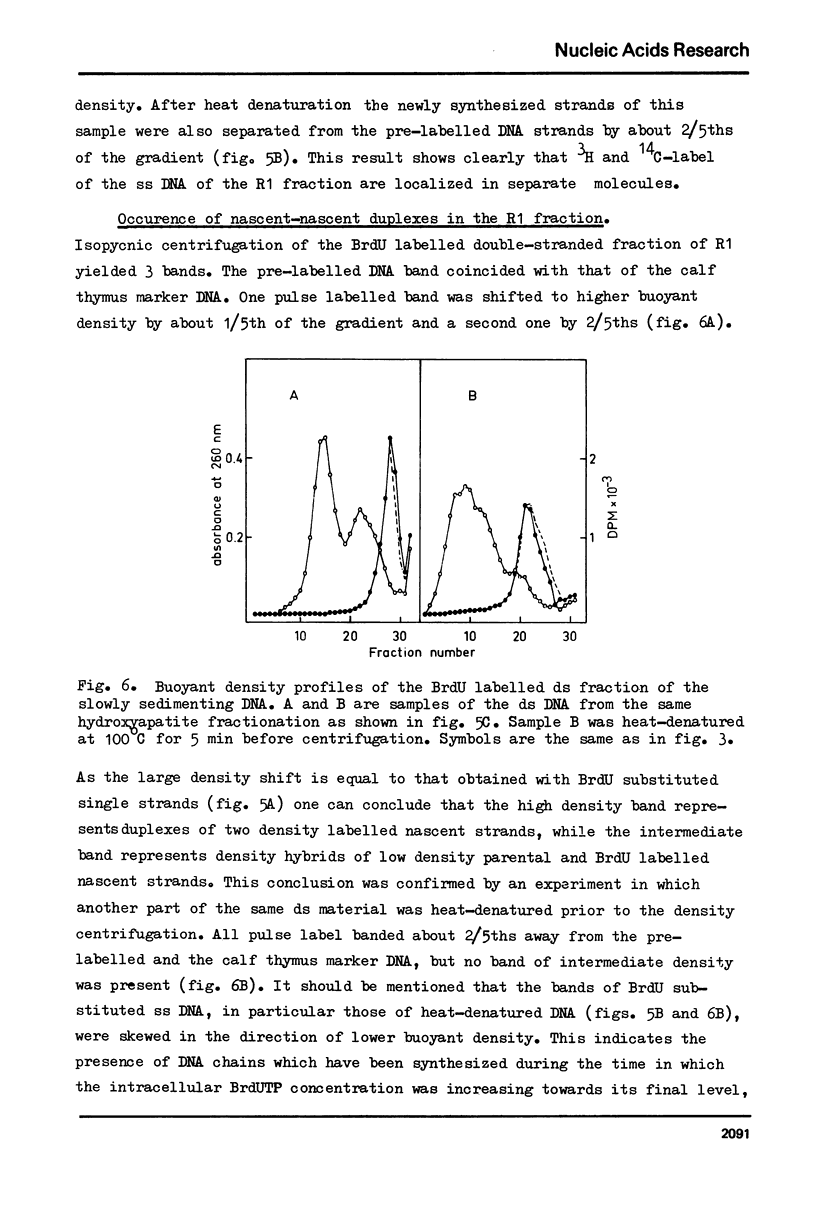
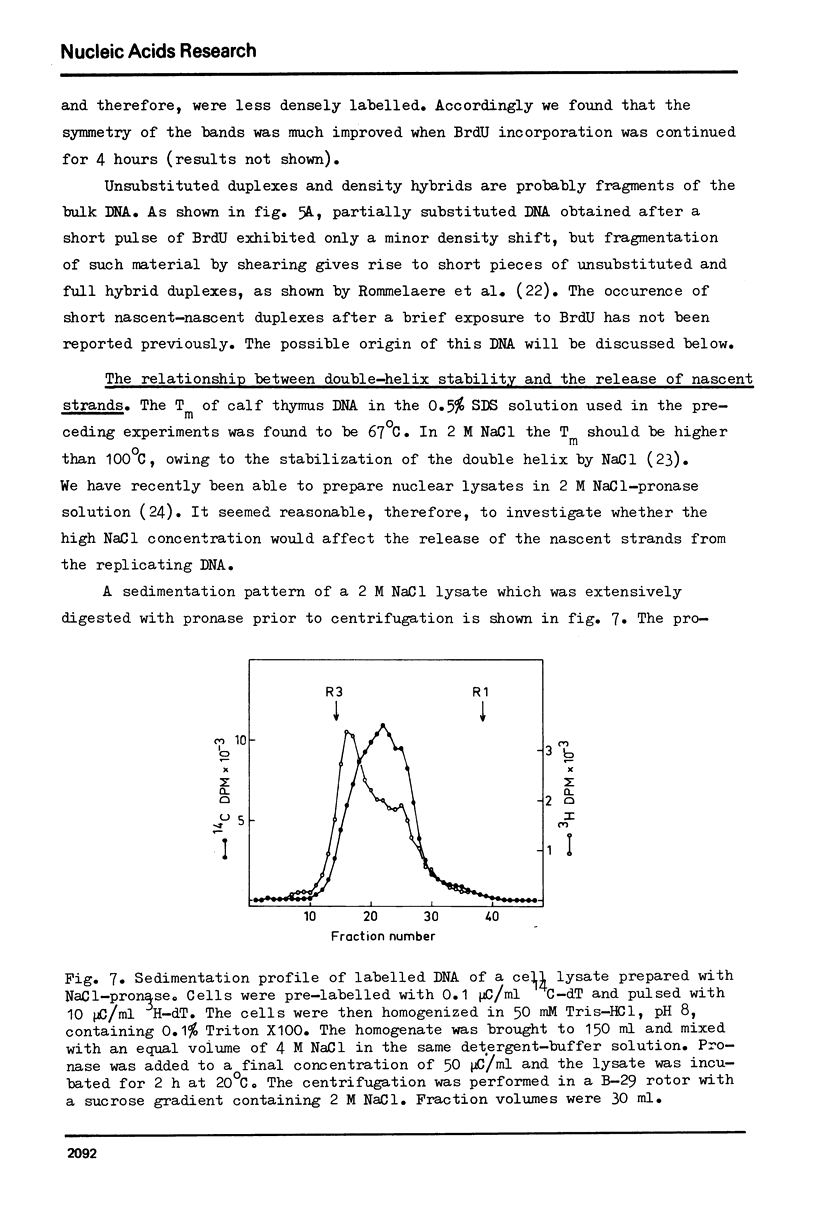





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnberg A., van Bruggen E. F., Borst P. The presence of DNA molecules with a displacement loop in standard mitochondrial DNA preparations. Biochim Biophys Acta. 1971 Aug 26;246(2):353–357. doi: 10.1016/0005-2787(71)90147-x. [DOI] [PubMed] [Google Scholar]
- Bernardi G. Chromatography of nucleic acids on hydroxyapatite. Nature. 1965 May 22;206(4986):779–783. doi: 10.1038/206779a0. [DOI] [PubMed] [Google Scholar]
- Cullen B. R., Bick M. D. Thermal denaturation of DNA from bromodeoxyuridine substituted cells. Nucleic Acids Res. 1976 Jan;3(1):49–62. doi: 10.1093/nar/3.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delius H., Howe C., Kozinski A. W. Structure of the replicating DNA from bacteriophage T4. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3049–3053. doi: 10.1073/pnas.68.12.3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fakan S., Turner G. N., Pagano J. S., Hancock R. Sites of replication of chromosomal DNA in a eukaryotic cell. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2300–2305. doi: 10.1073/pnas.69.8.2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayton G. J., Pearson C. K., Scaife J. R., Keir H. M. Synthesis of deoxyribonucleic acid in BHK-21-C13 cells. Biochem J. 1973 Mar;131(3):499–508. doi: 10.1042/bj1310499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Housman D., Huberman J. A. Changes in the rate of DNA replication fork movement during S phase in mammalian cells. J Mol Biol. 1975 May 15;94(2):173–181. doi: 10.1016/0022-2836(75)90076-5. [DOI] [PubMed] [Google Scholar]
- Huberman J. A., Riggs A. D. On the mechanism of DNA replication in mammalian chromosomes. J Mol Biol. 1968 Mar 14;32(2):327–341. doi: 10.1016/0022-2836(68)90013-2. [DOI] [PubMed] [Google Scholar]
- Kasamatsu H., Robberson D. L., Vinograd J. A novel closed-circular mitochondrial DNA with properties of a replicating intermediate. Proc Natl Acad Sci U S A. 1971 Sep;68(9):2252–2257. doi: 10.1073/pnas.68.9.2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidwell W. R., Mueller G. C. The synthesis and assembly of DNA subunits in isolated HeLa cell nuclei. Biochem Biophys Res Commun. 1969 Aug 22;36(5):756–763. doi: 10.1016/0006-291x(69)90674-3. [DOI] [PubMed] [Google Scholar]
- Kriegstein H. J., Hogness D. S. Mechanism of DNA replication in Drosophila chromosomes: structure of replication forks and evidence for bidirectionality. Proc Natl Acad Sci U S A. 1974 Jan;71(1):135–139. doi: 10.1073/pnas.71.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen C. R. Tables for estimating sedimentation through linear concentration gradients of sucrose solution. Anal Biochem. 1967 Jul;20(1):114–149. doi: 10.1016/0003-2697(67)90271-0. [DOI] [PubMed] [Google Scholar]
- Oishi M. Studies of DNA replication in vivo. I. Isolation of the first intermediate of DNA replication in bacteria as single-stranded DNA. Proc Natl Acad Sci U S A. 1968 May;60(1):329–336. doi: 10.1073/pnas.60.1.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okazaki R., Okazaki T., Sakabe K., Sugimoto K., Sugino A. Mechanism of DNA chain growth. I. Possible discontinuity and unusual secondary structure of newly synthesized chains. Proc Natl Acad Sci U S A. 1968 Feb;59(2):598–605. doi: 10.1073/pnas.59.2.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen R. J., Hill L. R., Lapage S. P. Determination of DNA base compositions from melting profiles in dilute buffers. Biopolymers. 1969;7(4):503–516. doi: 10.1002/bip.1969.360070408. [DOI] [PubMed] [Google Scholar]
- PIECK A. C., KUYPER C. M. Establishment of an epithelial cell strain from calf liver in continuous culture. Experientia. 1961 Mar 15;17:115–116. doi: 10.1007/BF02160817. [DOI] [PubMed] [Google Scholar]
- Paetkau V., Langman L., Miller R. C., Jr The origin of nascent single-stranded fragments in replicating TM DNA. J Mol Biol. 1975 Nov 15;98(4):719–737. doi: 10.1016/s0022-2836(75)80006-4. [DOI] [PubMed] [Google Scholar]
- Painter R. B., Schaefer A. State of newly synthesized HeLa DNA. Nature. 1969 Mar 29;221(5187):1215–1217. doi: 10.1038/2211215a0. [DOI] [PubMed] [Google Scholar]
- Pieck A. C. Extraction, separation and identification of the free nucleotides in liver cells cultivated in vitro. Proc K Ned Akad Wet C. 1971;74(3):303–310. [PubMed] [Google Scholar]
- Probst H., Jenke H. S. Secondary structure of nascent DNA of Ehrlich ascites tumor cells obtained by nitrocellulose column chromatography. Biochem Biophys Res Commun. 1973 Jun 8;52(3):800–806. doi: 10.1016/0006-291x(73)91008-5. [DOI] [PubMed] [Google Scholar]
- Robberson D. L., Kasamatsu H., Vinograd J. Replication of mitochondrial DNA. Circular replicative intermediates in mouse L cells. Proc Natl Acad Sci U S A. 1972 Mar;69(3):737–741. doi: 10.1073/pnas.69.3.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rommelaere J., Faurès-Miller A., Errera M. Isolation of replicating DNA segments from Chinese hamster cells by density equilibrium centrifugation. J Mol Biol. 1974 Dec 15;90(3):491–508. doi: 10.1016/0022-2836(74)90230-7. [DOI] [PubMed] [Google Scholar]
- Sato S., Ariake S., Saito M., Sugimura T. Properties of nascent DNA of Ehrlich ascites tumor cells obtained by nitrocellulose column chromatography. Biochem Biophys Res Commun. 1972 Oct 6;49(1):270–277. doi: 10.1016/0006-291x(72)90040-x. [DOI] [PubMed] [Google Scholar]
- Simpson R. T., Seale R. L. Characterization of chromatin extensively substituted with 5-bromodeoxyuridine. Biochemistry. 1974 Oct 22;13(22):4609–4616. doi: 10.1021/bi00719a022. [DOI] [PubMed] [Google Scholar]
- Von Hippel P. H., Wong K. Y. Dynamic aspects of native DNA structure: kinetics of the formaldehyde reaction with calf thymus DNA. J Mol Biol. 1971 Nov 14;61(3):587–613. doi: 10.1016/0022-2836(71)90066-0. [DOI] [PubMed] [Google Scholar]
- Wanka F., Moors J., Krijzer F. N. Dissociation of nuclear DNA replication from concomitant protein synthesis in synchronous cultures of Chlorella. Biochim Biophys Acta. 1972 Apr 26;269(1):153–161. doi: 10.1016/0005-2787(72)90082-2. [DOI] [PubMed] [Google Scholar]
- Wanka F., Mullenders L. H., Bekers A. G., Pennings L. J., Aelen J. M., Eygensteyn J. Association of nuclear DNA with a rapidly sedimenting structure. Biochem Biophys Res Commun. 1977 Jan 24;74(2):739–747. doi: 10.1016/0006-291x(77)90364-3. [DOI] [PubMed] [Google Scholar]
- Wanka F. Separation of rapidly labeled intermediates of DNA synthesis in mammalian cells. Biochem Biophys Res Commun. 1973 Oct 15;54(4):1410–1417. doi: 10.1016/0006-291x(73)91143-1. [DOI] [PubMed] [Google Scholar]
- Wilson D. A., Thomas C. A., Jr Hydroxyapatite chromatography of short double-helical DNA. Biochim Biophys Acta. 1973 Dec 21;331(3):333–340. doi: 10.1016/0005-2787(73)90019-1. [DOI] [PubMed] [Google Scholar]



