Abstract
Background
Social familiarity, which is based on the ability to recognise familiar conspecific individuals following prior association, may affect all major life activities of group-living animals such as foraging, reproduction and anti-predator behaviours. A scarcely experimentally tested explanation why social familiarity is beneficial for group-living animals is provided by limited attention theory. Limited attention theory postulates that focusing on a given task, such as inspection and assessment of unfamiliar group members, has cognitive and associated physiological and behavioural costs with respect to the attention paid to other tasks, such as anti-predator vigilance and response. Accordingly, we hypothesised that social familiarity enhances the anti-predator success of group-living predatory mites, Phytoseiulus persimilis, confronted with an intraguild predator, the predatory mite Amblyseius andersoni.
Methodology/Principal Findings
We videotaped and analysed the response of two P. persimilis larvae, held in familiar or unfamiliar pairs, to attacks by a gravid A. andersoni female, using the behavioural analyses software EthoVision Pro®. Familiar larvae were more frequently close together, reacted more quickly to predator attacks, survived more predator encounters and survived longer than unfamiliar larvae.
Significance
In line with the predictions of limited attention theory, we suggest that social familiarity improves anti-predator behaviours because it allows prey to shift attention to other tasks rather than group member assessment.
Introduction
Predation is a major selective force shaping the behaviour of prey [1], [2]. To enhance survival under predation risk, animals evolved various behavioural anti-predator mechanisms such as crypsis, defensive or fleeing behaviours, or grouping together to enhance dilution or collective vigilance [3], [4]. Group-living may have partly evolved to reduce predation risk because solitary animals have a relatively limited ability to process multiple information and perform multiple tasks simultaneously, e.g. foraging and anti-predator vigilance [3], [5], [6]–[11]. The more individuals participate in vigilance, the more efficient potential predators can be detected and the less time and energy individual group members have to invest in vigilance [1], [2], [12]. Vigilance may be adjusted to the degree of predation risk, group size and composition, or experience [1], [2], [4], [12]–[16]. For example, group-living animals commonly spend less time being vigilant and invest more time in foraging with increasing group size [1], [2], [12].
Within groups, individuals are expected to position themselves such to maximize their own survival chance under the risk of predation, which depends on the relationships and interactions with other group members [3]. Within-group arrangement is usually non-random and may be influenced by life-stage, age, kinship, size, dominance hierarchy, sex, or social familiarity [3], [16]. We here focused on social familiarity, which requires the ability to discriminate familiar and unfamiliar individuals based on prior association [17]. Many group-living animals of diverse taxa preferentially associate with familiar individuals, which may have positive effects on foraging, life history traits or survival ([18], [19], authors unpublished). Possible cognitive implications of social familiarity are indicated by limited attention theory [7], which postulates that focusing on a given task has cognitive and associated physiological and behavioural costs with respect to the attention paid to other tasks. Familiar individuals usually require less attention than unfamiliar ones [19]–[23] and assorting with familiar individuals should thus lead to increased efficiency in other tasks such as anti-predator behaviour [20], [21]. This has, for example, been shown for groups of familiar trout, which responded more quickly to simulated predator attacks and had higher feeding rates than groups of unfamiliar trout [21]. Studies demonstrating attention shifts induced by social familiarity and its adaptive significance, i.e. its effects on survival or reproduction, under the risk of predation are lacking.
We investigated the influence of social familiarity on anti-predator behaviour of larvae of the group-living predatory mite Phytoseiulus persimilis threatened by the intraguild predator Amblyseius andersoni. Both predatory mite species live on plants and commonly belong to the same predator guild, sharing the herbivorous two-spotted spider mite, Tetranychus urticae, as prey (e.g. [24], [25]). P. persimilis is specialized on spider mite prey and lives in groups inside the spider mite patches, while A. andersoni is a diet-generalist poorly adapted to exploit tetranychid mites [24]. P. persimilis’ development proceeds from the egg to larva, protonymph, deutonymph and the adult [26]. The larvae are six-legged, non-feeding and little mobile whereas later life stages are eight-legged [26]. Due to their limited mobility and small size (∼0.2 mm long), P. persimilis larvae are highly vulnerable to intraguild predation by the large gravid A. andersoni females (∼0.4 mm long) (e.g. [25]). Both A. andersoni and P. persimilis are eyeless and sense the environment predominantly by contact and volatile chemosensory cues [27]. Accordingly, P. persimilis is able to perceive and respond to chemical cues of the intraguild predator A. andersoni with and without physical predator presence [28], [29]. Previous studies revealed that juvenile and adult P. persimilis, including larvae, are able to discriminate familiar from unfamiliar individuals, and that social familiarity modulates within-group association, aggression, reproduction and foraging [30]–[33]. Larvae tend to aggregate and remain rather immobile until moulting into protonymphs. Here, we hypothesized that social familiarity adaptively modulates the anti-predator response of group-living larvae by, for example, reducing their reaction time, which is a common indicator of attention (e.g. [21], [34]), and ultimately increases their survival probabilities under the risk of predation.
Results
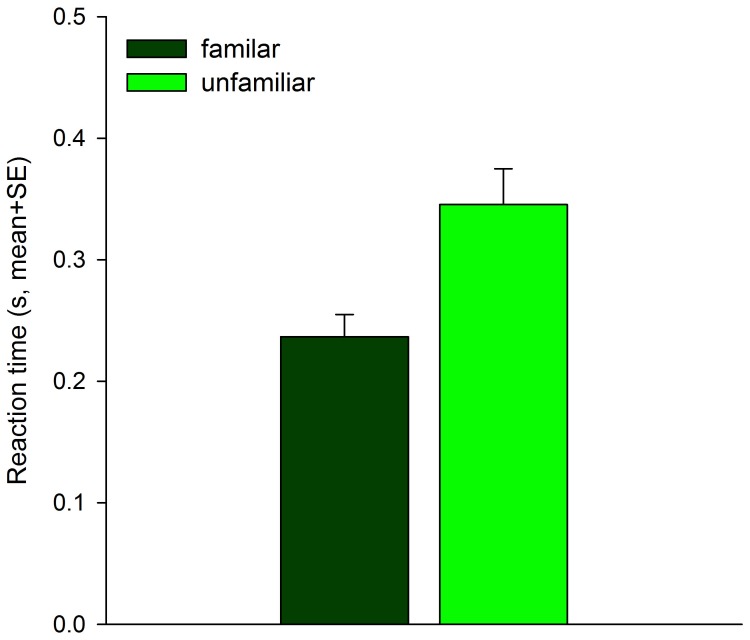
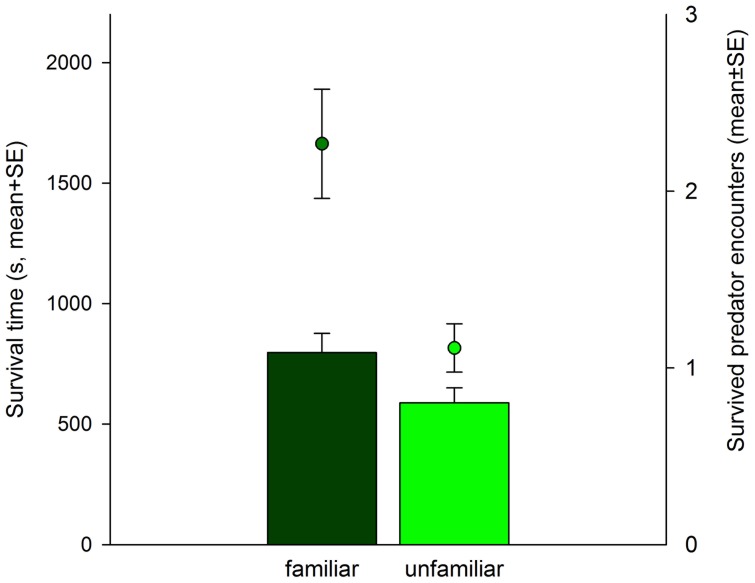
Familiar P. persimilis larvae reacted more quickly to predator attacks (Wald-χ21 = 5.011, p = 0.025), survived more predator encounters (Wald-χ21 = 10.480, p = 0.001) and survived longer (Wald-χ2 1 = 4.298, p = 0.038) than unfamiliar larvae did (Fig. 1, Fig. 2). As a consequence of surviving longer, familiar larvae covered longer distances (mm, mean±SE; familiar: 16.48±2.51, unfamiliar: 9.37±1.33; Wald-χ2 1 = 5.996, p = 0.014) and moved longer than unfamiliar larvae did (s, mean±SE; familiar: 65.62±3.19, unfamiliar: 51.66±3.85; Wald-χ2 1 = 7.484, p = 0.006). Unfamiliar and familiar larvae did not differ in velocity (mm/s, mean±SE; familiar: 0.27±0.08, unfamiliar: 0.23±0.02; Wald-χ2 1 = 2.233, p = 0.135), absolute angular velocity (°/mm, mean±SE; familiar: 49.16±14.33, unfamiliar: 25.78±5.57; Wald-χ2 1 = 1.389, p = 0.239), absolute meandering (°/mm, mean±SE; familiar: 26.88±6.09, unfamiliar: 65.68±27.46; Wald-χ2 1 = 1.939, p = 0.146;) and absolute turning angles (Wald-χ2 1 = 3.004, p = 0.083;°, mean±SE; familiar: 21.05±3.65, unfamiliar: 11.55±1.86). Inter-individual distances of familiar and unfamiliar pairs of larvae were similar (mm, mean±SE; familiar: 6.19±0.46, unfamiliar: 7.21±0.44; t-test: t78 = −1.592, p = 0.115) but relative proximity, i.e. the percentage of time being close together, was higher in familiar (n = 43) than unfamiliar (n = 46) larvae (%, grouped median; familiar: 0.152, unfamiliar: 0.072; Mann-Whitney-U-test: Z = −2.0, p = 0.045).
Figure 1. Reaction time of prey larvae to predator encounters.
Mean reaction time of P. persimilis larvae held in familiar or unfamiliar pairs and threatened by a gravid intraguild predator female of A. andersoni.
Figure 2. Survival time of prey larvae and survived predator encounters.
Mean survival time (left y-axis, bars) and number of predator encounters survived (right y-axis, symbols) of P. persimilis larvae held in familiar or unfamiliar pairs and threatened by a gravid intraguild predator female of A. andersoni.
Discussion
Fleeing is a common response to avoid predation [2], [13], [35]. Whether fleeing is successful is largely determined by reaction distance, encounter direction, reaction time and escape trajectory, but may also be influenced by other factors such as experience and group characteristics [4], [13], [35]. In our experiment, familiar P. persimilis larvae spent more time aggregated (indicated by relative proximity), reacted more quickly to attacks of their predator A. andersoni, and thus survived more predator encounters than unfamiliar larvae. The shorter reaction times suggest that social familiarity allowed the mites to shift attention from attention-demanding neighbour assessment to anti-predator vigilance, in accordance with limited attention theory [7], [8], [21], and ultimately enhanced their survival under predation risk.
Thus far, the impact of social familiarity on anti-predator success has been predominantly demonstrated in fish [15]. One apparent phenomenon in group-living fish is that shoals of familiar individuals are more cohesive, indicating that familiarity stabilises shoal structure. For example, stronger cohesion in minnow shoals reduced the capture success of attacking predators, presumably due to enhanced predator confusion [20]. Moreover, individuals within familiar shoals performed typical anti-predator manoeuvres such as dashing or freezing more efficiently than individuals within unfamiliar shoals [20]. Along the same line, Griffiths et al. [21] demonstrated that individuals within familiar groups of brown trout reacted more quickly to simulated predator attacks than individuals within unfamiliar groups. Social familiarity reduced the aggressive interactions between group members and thus enabled the trout to switch attention from intraspecific aggression to anti-predator vigilance [21]. Our study provides evidence that similar phenomena may occur and similar mechanisms may operate in arthropods such as the predatory mite P. persimilis.
In related studies we observed the ability of different life stages of P. persimilis to preferentially associate with familiar individuals [33]. Here, we show that social familiarity enhances the anti-predator response of larvae. The fragile larvae are often aggregated, possibly to reduce the risk of heterospecific predation and cannibalism [36]. In general, all life stages (egg, larva, protonymph, deutonymph, adult) of P. persimilis live together in the ephemeral patches of its preferred prey, the spider mite T. urticae [37], [38]. All developmentally more advanced life stages are potential cannibals of larvae [36] and the prey patches are often shared with potential intraguild predators [25]. Thus, the larvae may be often exposed to predator cues and repeatedly encounter potential predators within a patch but not every encounter is life-threatening. Deciding if and when to respond to predator cues involves trade-offs between the benefit of avoiding predator attacks and a variety of costs including energy loss [4], [13]. The costs of fleeing may be loss of energy needed for development or leaving a site with favorable abiotic conditions or possibly attracting the attention of predators due to leaving a safe group formation. Thus, mechanisms optimizing the response of the larvae to a predator such as social familiarity should be selected for [4], [13]. Our study shows that social familiarity provides an adaptive advantage, because larvae held in familiar groups reacted faster to approaching predators than unfamiliar larvae did, which ultimately conferred a fitness gain through longer survival times.
Materials and Methods
Origin and Rearing of Experimental Animals
Experimental animals were offspring from females withdrawn from laboratory-reared populations of P. persimilis and A. andersoni, originally founded with specimens field-collected in Trapani, Sicily [25]. Both species were maintained separately on artificial rearing units each consisting of a plastic tile placed on a water-saturated foam cube (13×13×4 cm) in a plastic box (20×20×5 cm) half-filled with tap water. The edges of the tile were covered by water-saturated tissue paper. P. persimilis was fed mixed life-stages of T. urticae, reared on whole bean plants Phaseolus vulgaris, by adding detached spider mite-infested bean leaves onto arenas in 3 to 4 day intervals. A. andersoni was fed mixed life-stages of T. urticae brushed from infested bean leaves onto the rearing arenas. Rearing and experimental arenas were stored at 25±1°C, 60±5% relative humidity and 16∶8 h light:dark.
Generating Familiar and Unfamiliar P. persimilis Larvae
Oviposition arenas used to obtain similarly aged P. persimilis eggs consisted of single bean leaves (∼60 cm2) placed adaxial surface down on a water-saturated, filter paper-covered foam cube (13×13×4 cm) in a plastic box (20×20×5 cm) half-filled with tap water. Strips of moist tissue paper were folded over the edges of the leaves to prevent the mites from escaping. Before placing 40 to 70 P. persimilis females for oviposition onto arenas, mixed life-stages of T. urticae were brushed from infested bean leaves onto arenas to serve as prey for the predators. P. persimilis females were randomly chosen from the laboratory-reared population and eggs were collected after ∼4 h.
P. persimilis larvae were familiarised in artificial cages each consisting of a circular cavity (1.5 cm diameter) drilled in an acrylic plate (0.3 cm thick). The cages were covered by gauze on the lower side and on the upper side by a removable microscope slide held in place by a metal clamp [39]. Six P. persimilis eggs, randomly taken from the oviposition arenas, were placed together in such a familiarisation cage, where the larvae were allowed to familiarise for ∼8 h after hatching [33]. All larvae were derived from the same group of ovipositing females and had thus the same degree of genetic relatedness to each other. The only difference between familiar and unfamiliar larvae was inter-larvae familiarity or not generated in the familiarisation cages.
Experimental Procedure
To start the experiment, either two familiar or two unfamiliar P. persimilis larvae were placed in the centre of a circular experimental arena. Before the experiment the two larvae of a pair were marked with differently sized black water colour dots using a fine brush on their dorsal shields to make them distinguishable for the tracking module of the behavioural analyses software EthoVision Pro®. Familiar pairs were taken out of the same familiarisation cage, whereas larvae of unfamiliar pairs were randomly taken out of two different familiarisation cages using a fine brush. Experimental arenas were flat polypropylene discs (1.4 cm diameter) floating on the surface of a water column in an acrylic cylinder (height 2.0 cm, inner diameter 1.6 cm), leaving a ∼0.1 cm water barrier between the inner margin of the cylinder and the edge of the disc, preventing the mites from escaping. After ∼10 min acclimatisation of the larvae to the novel environment, a single gravid A. andersoni female, starved for 20 h before the experiment, was released in the centre of the arena and videotaping was started. The A. andersoni females were starved to increase the likelihood of attack on P. persimilis larvae. The experiment ended when a larva was killed by A. andersoni or died in the water barrier. 49 familiar and 49 unfamiliar pairs of larvae were tested. Each disc, each P. persimilis larva and each A. andersoni female was used only once. Replicates with familiar and unfamiliar larvae were videotaped at similar daytimes in an air-conditioned room without natural light.
Behavioural Analyses
The behaviour of the mites was videotaped with a Leica IC-A video module integrated in a Leica M50 microscope. To digitalise the videos, the video module was interfaced by the frame grabber HaSoTec FG-33-II (Indeo codec). EthoVision Pro® 3.1 [40] was used to analyse the behaviour of the larvae. The subtraction method was used for object detection and discrimination. To prevent electronic noise from being scored as genuine movement of the larvae by EthoVision Pro® [40], the calculation settings of the analysis module were adjusted to a minimum distance of 0.2 mm, which corresponds approximately to one larval body length, and a minimum velocity of 0.1 mm/s. Using EthoVision Pro® we analysed the inter-individual distances (mm), velocity (mm/s), distance moved (mm), moving time (s), absolute turning angle (°), absolute angular velocity (°/s), absolute meandering (°/mm) and relative proximity of the larvae. The absolute turning angle equals the average direction of an individual’s movement during one sample relative to the average direction of movement during the consecutive sample (sample rate: 5 samples/s). The absolute angular velocity (°/s) is calculated by dividing the turning angle by the sample interval and is an indicator of how fast an object is changing its direction. Meandering is the interrelation of the direction of an individual’s movement (turning angle) and the distance moved indicating the path tortuosity of the experimental organism. Relative proximity is a state parameter representing the percentage of time where two experimental organisms are together within a predefined distance. The “in proximity”-distance was predefined as two larval body lengths (∼0.4 mm). 43 familiar and 46 unfamiliar pairs out of 49 each videotaped could be successfully tracked and analysed with EthoVision Pro®. Personal video analysis additionally allowed for recording survival time (s), the number of encounters with A. andersoni survived and the reaction time of each larva when encountered by the predator. The predator was considered to encounter the larva when it came within a distance of approximately one larval body length (∼0.2 mm) to the larva. Larvae were considered to react to encounters with A. andersoni when they moved away from the predator after encounter. The reaction time was estimated by counting the number of single video frames between the encounter of the predator and the first observable movement of the larva away from the predator. For analysis, we only used the first two encounters of A. andersoni with each larva, within the first 10 minutes of each replicate, to avoid any obscuring effect of learning possibly caused by differing numbers of survived encounters. The videos were recorded with 25 progressive frames per second (PAL colour encoding system), which allowed to transform the number of frames into seconds.
Statistical Analyses
All statistical analyses were performed using SPSS 15.0.1 for Windows (SPSS Inc., Chicago, IL, USA, 2006). Separate generalized estimating equations (GEE) with normal distribution, identity link and autocorrelation structure between the two larvae of a pair were used to test the effects of familiarity on time, distance and path shape parameters within larval pairs. The reaction times were log-transformed before statistical analysis to normalize the data. The inter-individual distances between familiar and unfamiliar larvae were compared using t-tests for independent samples. Mann-Whitney U test was used to compare the proximity parameters within familiar and unfamiliar larvae due to non-normality.
Acknowledgments
We thank Daniela Hoffmann, Stefan Peneder, Andreas Walzer, Muluken Goftishu Muleta and Gernot Josef Zach for comments on a previous version of this manuscript. Sampling of the predatory mites complied with the Italian laws.
Funding Statement
This work was financially supported by the Austrian Science Fund (FWF): P20743-B17. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Lima SL (1998) Stress and decision making under the risk of predation: recent developments from behavioral, reproductive and ecological perspectives. Adv Study Behav 27: 215–290. [Google Scholar]
- 2. Lima SL, Dill LM (1990) Behavioral decisions made under the risk of predation: a review and prospectus. Can J Zool 68: 619–640. [Google Scholar]
- 3.Krause J, Ruxton GD (2002) Living in groups. Oxford, UK: Oxford University Press. 210 p.
- 4.Nonacs P, Blumstein DT (2010) Predation risk and behavioral life history. In: Westneat DF, Fox CW, editors. Evolutionary Behavioural Ecology. Oxford: Oxford University Press. 207–221.
- 5. Sillén-Tullberg B, Leimar O (1988) The evolution of gregariousness in distasteful insects as a defense against predators. Am Nat 132: 723–734. [Google Scholar]
- 6.Alcock J (2005) Animal behavior: An evolutionary approach. 8th edition. Sunderland, Massachusetts: Sinauer Associates. 564 p.
- 7. Dukas R (2002) Behavioural and ecological consequences of limited attention. Phil Trans R Soc Lond B 357: 1539–1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Clark CW, Dukas R (2003) The behavioral ecology of a cognitive constraint: limited attention. Behav Ecol 14: 151–156. [Google Scholar]
- 9. Blumstein DT (2010) Flush early and avoid the rush: a general rule of antipredator behavior? Behav Ecol 21: 440–442. [Google Scholar]
- 10. Sareen P, Wolf R, Heisenberg M (2011) Attracting the attention of a fly. PNAS 108: 7230–7235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. van Swinderen B (2007) Attention-like processes in Drosophila require short-term memory genes. Science 315: 1590–1593. [DOI] [PubMed] [Google Scholar]
- 12. Powell GVN (1974) Experimental analysis of the social value of flocking by starlings (Sturnus vulgaris) in relation to predation and foraging. Anim Behav 22: 501–505. [Google Scholar]
- 13. Ydenberg RC & Dill LM (1986) The economics of fleeing from predators. Adv Study Behav 16: 229–249. [Google Scholar]
- 14. Stankowich T, Blumstein DT (2005) Fear in animals: a meta-analysis and review of risk assessment. Proc R Soc Lond B 272: 2627–2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kelley JL, Magurran AE (2006) Learned defences and counterdefences in predator-prey interactions. In: Brown C, Laland K, Krause J, editors. Fish cognition and behavior. Oxford: Blackwell Publishing. 28–43.
- 16.Earley RJ, Dugatkin AA (2010) Behavior in groups. In: Westneat DF, Fox CW, editors. Evolutionary Behavioural Ecology. Oxford: Oxford University Press. 285–307.
- 17. Mateo JM (2004) Recognition systems and biological organization: the perception component of social recognition. Ann Zool Fenn 41: 729–745. [Google Scholar]
- 18.Griffiths SW, Ward A (2006) Learned recognition of conspecifics. In: Brown C, Laland K, Krause J, editors. Fish cognition and behavior. Oxford: Blackwell Publishing. 139–165.
- 19. Palphramand KL, White PCL (2007) Badgers, Meles meles, discriminate between neighbour, alien and self scent. Anim Behav 74: 429–436. [Google Scholar]
- 20. Chivers DP, Brown GE, Smith RJF (1995) Familiarity and shoal cohesion in fathead minnows (Pimephales promelas): implications for anti-predator behaviour. Can J Zoo 73: 955–960. [Google Scholar]
- 21. Griffiths SW, Brockmark S, Höjesjö J, Johnsson JI (2004) Coping with divided attention: the advantage of familiarity. Proc R Soc Lond B 271: 695–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Utne-Palm AC, Hart PJB (2000) The effects of familiarity on competitive interactions between threespine sticklebacks. Oikos 91: 225–232. [Google Scholar]
- 23. Höjesjö J, Johnsson JI, Pettersson E, Järvi T (1998) The importance of being familiar: individual recognition and social behaviour in sea trout (Salmo trutta). Behav Ecol 9: 445–451. [Google Scholar]
- 24. McMurtry JA, Croft BA (1997) Life-styles of phytoseiid mites and their roles in biological control. Annu Rev Entomol 42: 291–321. [DOI] [PubMed] [Google Scholar]
- 25. Walzer A, Schausberger P (2011) Threat-sensitive anti-intraguild predation behaviour: maternal strategies to reduce offspring predation risk in mites. Anim Behav 81: 177–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chant DA (1985) External anatomy. In: Helle W, Sabelis MW, editors. Spider mites. Their biology, natural enemies and control. Volume 1B. Amsterdam, Netherlands: Elsevier. 5–9.
- 27.Sabelis MW, Dicke M (1985) Long-range dispersal and searching behavior. In: Helle W, Sabelis MW, editors. Spider mites. Their biology, natural enemies and control. Volume 1B. Amsterdam, Netherlands: Elsevier. 141–160.
- 28. Janssen A, Bruin J, Jacobs G, Schraag R, Sabelis MW (1997) Predators use volatiles to avoid prey patches with conspecifics. J Anim Ecol 66: 223–232. [Google Scholar]
- 29. Walzer A, Paulus HF, Schausberger P (2006) Oviposition behavior of predatory mites: response to the presence of con-and heterospecific eggs. J Insect Behav 19: 305–320. [Google Scholar]
- 30. Schausberger P, Croft BA (2001) Kin recognition and larval cannibalism by adult females in specialist predaceous mites. Anim Behav 61: 459–464. [Google Scholar]
- 31. Schausberger P (2004) Ontogenetic isolation favours sibling cannibalism in mites. Anim Behav 67: 1031–1035. [Google Scholar]
- 32. Schausberger P (2007) Kin recognition by juvenile predatory mites: prior association or phenotype matching? Behav Ecol Sociobiol 58: 53–59. [Google Scholar]
- 33. Strodl MA, Schausberger P (2012) Social familiarity modulates group living and foraging behaviour of juvenile predatory mites. Naturwissenschaften 99: 303–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rowe C (1999) Receiver psychology and the evolution of multicomponent signals. Anim Behav 58: 921–931. [DOI] [PubMed] [Google Scholar]
- 35. Domenici P, Blagburn JM, Bacon JP (2011) Animal escapology I: theoretical issues and emerging trends in escape trajectories. J Exp Biol 214: 2463–2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Schausberger P (2003) Cannibalism among phytoseiid mites: a review. Exp Appl Acarol 29: 173–191. [DOI] [PubMed] [Google Scholar]
- 37.Sabelis MW (1985) Development. In: Helle W, Sabelis MW, editors. Spider mites. Their biology, natural enemies and control. Volume 1B. Amsterdam, Netherlands: Elsevier. 43–53.
- 38. Schausberger P, Croft BA (1999) Activity, feeding, and development among larvae of specialist and generalist phytoseiid mite species (Acari: Phytoseiidae). Environ Entomo 28: 322–329. [Google Scholar]
- 39. Schausberger P (1997) Inter-and intraspecific predation on immatures by adult females in Euseius finlandicus, Typhlodromus pyri and Kampimodromus aberrans (Acari: Phytoseiidae). Exp Appl Acarol 21: 131–150. [Google Scholar]
- 40.Noldus Information Technology (2005) EthoVision®. Video tracking system for automation of behavioral experiments. Reference manual version 3. Netherlands: Wageningen. 430 p.