Lipid bilayers compartmentalize eukaryotic cells into distinct organelles. This compartmentalization allows for specialization of diverse cellular processes, from DNA polymerization to zymogen proteolysis. While the specific complement of proteins present in each organelle defines its function, there is a dynamic flux between these organelles. The selective transport of proteins between organelles is the central process in the organization of membrane compartments. This process is largely mediated by the budding of transport vesicles from a donor compartment followed by the vectoral trafficking to and fusion with an acceptor compartment. Over the last several years considerable effort has been exerted to uncover the molecular mechanisms underlying this process. A set of protein families known as SNAREs [soluble N-ethylmaleimide-sensitive factor (NSF) attachment protein receptors] is at the crux of vesicle docking and/or fusion. Members of these families reside on transport vesicles (v-SNARES) and on target membranes (t-SNAREs) and bind to each other. While previously implicated in conferring specificity to vesicle trafficking, more recent studies have demonstrated that these proteins also may mediate the fusion process itself. In two papers in this issue of PNAS, Rothman and colleagues (1, 2) demonstrate that liposomes reconstituted with only v- and t-SNAREs can fuse and mix luminal contents with reasonably physiological kinetics. These results further implicate SNAREs as the core fusion machinery for intracellular vesicle trafficking.
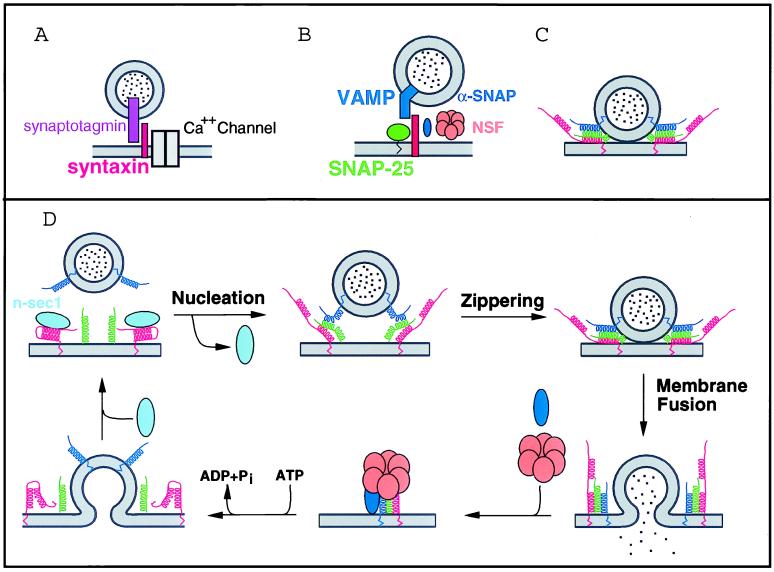
In the 1960s and 1970s with the advent of electron microscopy, the hypothesis of a secretory pathway was developed, in which proteins to be secreted initially are synthesized in the endoplasmic reticulum, moved to and then through the Golgi apparatus, packaged into secretory granules, and finally secreted (3). Further it was proposed that these various trafficking steps were mediated by transport vesicles. A molecular understanding of the role of SNAREs in this process began in the early 1990s with studies on the mammalian presynaptic nerve terminal. Immunoprecipitations with an antibody against the synaptic vesicle protein synaptotagmin yielded the protein syntaxin (a t-SNARE), which also was found to interact with Ca2+ channels. These interactions were hypothesized to bring vesicles into close proximity with the fusion signal Ca2+ and to possibly serve as a scaffold for the soluble membrane trafficking factors α-SNAP and NSF (see below) (4) (Fig. 1A).
Figure 1.
Historical hypothesized role of SNAREs in membrane trafficking. (A) The synaptic vesicle protein synaptotagmin was found to interact with the plasma membrane protein syntaxin, which also interacts with Ca++ channels. (B) Trans-SNARE complexes dock vesicles and provide a scaffold for the assembly of the fusion apparatus. (C) Parallel alignment of SNARES forces membranes into close apposition. (D) Current hypothesis that SNARE complex formation is regulated by proteins such as n-sec1. Membrane fusion occurs in concert with trans-SNARE complex formation. α-SNAP and NSF then break apart cis-SNARE complexes to reset the system for another round of fusion.
Concurrent with these studies, a cell-free assay system for Golgi membrane fusion was developed. With this system two proteins previously discovered as essential for secretion in yeast, α-SNAP (Sec17p) and NSF (Sec18p), were characterized from mammalian systems. It subsequently was found that these proteins interacted with the SNAREs syntaxin, SNAP-25, and VAMP (vesicle-associated membrane protein) (5). These three SNARE proteins were found to form a stable complex termed 7S for its size migration on glycerol gradients (6) (Fig. 1B). The central role of these proteins in a late stage of vesicle trafficking was solidified by several distinct genetic, biochemical, and cell-free assay systems. When it was found that these SNAREs were prototypes of protein families whose members were distributed throughout the secretory pathway (7), the hypothesis arose that these proteins mediated the specificity of vesicle trafficking by defining membranes that were compatible for docking and fusion. With the understanding that the cytosolic proteins α-SNAP and NSF could hydrolyze ATP and dissociate SNARE complexes, the specific docking hypothesis was further extended such that dissociation of the complex led to bilayer fusion.
This hypothesis then was modified by a series of experiments using an in vitro yeast vacuole fusion assay. One conclusion from these experiments was that α-SNAP and NSF act significantly before the fusion event, likely in a SNARE-priming role to dissociate cis-SNARE complexes (8). Follow-up experiments also confirmed that a v- and t-SNARE are required on opposite membranes before fusion (9). Thus while redefining the role of α-SNAP and NSF in vesicle trafficking as primarily SNARE primers, these experiments underscored in yet another system the central role of trans-SNARE complexes.
It is the nature of these trans-SNARE pairs that has been the focus of investigation over the last several years. At the time of the initial docking hypothesis, the orientation of SNAREs within the complex was not known. However, several structural studies, culminating in the crystal structure of the minimal interacting domains of the 7S complex, demonstrated that the SNAREs align in a parallel fashion (10–12). This finding implies that in a trans-SNARE state the vesicle and target membranes will be brought into close apposition (Fig. 1C). This realization sparked the hypothesis that the energy released from formation of the stable complex, in concert with the physical bringing together of lipid bilayers, might mediate the membrane fusion itself (Fig. 1D).
The ultimate test of this model is to faithfully reconstitute the process from purified components. In an attempt to accomplish this goal, a liposome fusion system dependent on SNARE proteins was developed (13). Syntaxin and SNAP-25 were reconstituted in one pool of liposomes, whereas VAMP was reconstituted in liposomes containing fluorophore-bound lipids, which at high concentrations allow fluorescent resonance energy transfer to occur. Fusion can be measured as the concentration of fluorescent lipids changes as bilayers mix. This system demonstrated that SNAREs could mediate lipid mixing, and the authors concluded that SNAREs indeed comprise a minimal fusion machinery. However, the slow kinetics and lack of an assay for luminal content mixing left lingering doubts about the physiological relevance of this claim.
To further address whether SNARE complex formation leads directly to fusion, a novel twist on a well-established cell permeabilized assay system was developed (14). The power of the new approach came from the use of botulinum neurotoxins, which specifically cleave members of the core 7S complex. By using these toxins in conjunction with rescuing peptides, those authors found, in contrast to the conclusions drawn from the vacuole fusion studies, that stable trans-SNARE complexes form only after Ca2+ is present. By making mutations in residues present in the core of the SNARE complex, they also demonstrated that the stability of the SNARE complex correlates with the extent of membrane fusion. In addition, both SNARE complex formation and Ca2+ triggering are closely coupled in time to vesicle fusion. Thus, these results support the model that membrane fusion occurs in concert with SNARE complex formation, and that stable, irreversible SNARE complex only exists in cis as the product of the fusion reaction itself (15).
Two papers in this issue of PNAS further address the mechanism of membrane fusion by extending the previously developed liposome fusion assay. Nickel et al. (1) use a novel complementary oligonucleotide assay system to clearly demonstrate that in the SNARE-mediated liposome fusion system the contents of v- and t-SNARE vacuoles merge, thus the SNAREs mediate complete bilayer fusion, rather than just hemifusion. In the second paper (2), when full-length SNAREs are incorporated into liposomes and then incubated at 37°C, fusion occurs with linear kinetics for at least 2 hr with the first-half round of fusion complete in 40 min. If the liposomes are preincubated at 4°C for 16 hr before incubation at 37°C, the initial kinetics are significantly faster, with the first round of fusion complete after 7 min. However, after 15 min this reaction follows the same, slower kinetics as the reaction without preincubation. The authors propose the preincubation allows trans-SNARE complexes to form, which permits a rapid first round of fusion. If the minimal SNARE interacting domain of syntaxin, the H3 domain, is used in place of the full-length protein with no preincubation, the kinetics are linear throughout the 2-hr experiment, with the first-half round of fusion occurring in 10 min. The N-terminal domain, which was cleaved away from syntaxin, has been previously shown to retard the binding of SNAP-25 to syntaxin, which is the rate-limiting step for SNARE complex formation. However, in this system syntaxin and SNAP-25 are purified and reconstituted into liposomes as a complex. Thus the N terminus of syntaxin must have an additional regulatory role in complex formation or fusion itself.
The conclusion from these papers is that SNAREs in an isolated system can fuse membranes, which contrasts with recent data using the vacuolar fusion assay. Experiments from this system suggest that while SNARE complex formation is an essential intermediate in vesicle docking and fusion, trans-SNARE pairing can be uncoupled from fusion itself (16). Thus these authors believe that trans-SNARE complex formation sends a signal to release calcium from the yeast vacuole, which in turn signals an as-yet-uncharacterized fusion machinery (16, 17). Obviously in the reconstituted liposome assay there are no proteins that could be downstream of SNAREs to mediate fusion. So either the SNARE-mediated liposome fusion is not translatable to what occurs in vivo or the vacuole studies are not interpreted correctly. Although other proteins such as NSF and annexins have been found to be fusogenic in a liposome system, it seems unlikely that SNAREs, which are present at the correct time and place for fusion in both cell-free and cell-permeabilized assays, are clearly necessary for a late step in vesicle trafficking, and are sufficient for fusion in vitro, do not carry out that function in vivo.
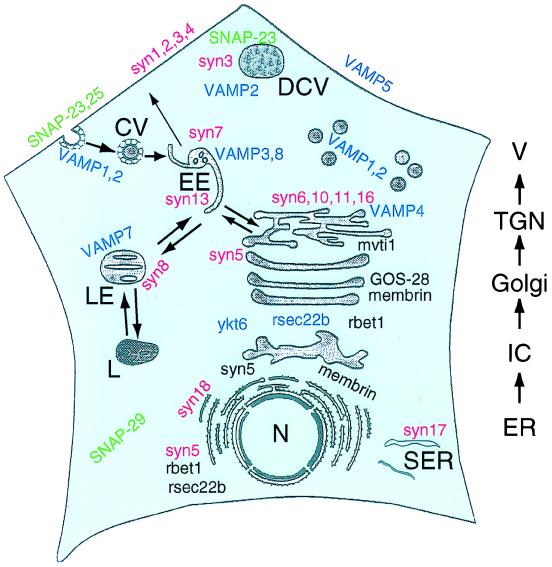
Thus while converging lines of evidence implicate SNAREs as the core fusion machinery, other recent studies address the role of SNAREs in underpinning the specificity of vesicle trafficking. One would expect three criteria to be fulfilled if SNAREs perform this function. First, there should be a relatively large number of SNAREs to distinguish between the various vesicle trafficking steps. Second, SNAREs should have distinct subcellular localizations. It appears these two criteria have been met, with many SNARES already identified and localized to specific subcellular locations (Fig. 2).
Figure 2.
Localization of SNARE proteins within the secretory pathway of a typical eukaryotic cell. Members of the syntaxin family are colored red. SNAP-25 family members are green, and VAMP family members are blue. N, nucleus; ER, endoplasmic reticulum; SER, smooth ER; IC, intermediate compartment; TGN, trans-Golgi network; V, vesicles; DCV, dense core vesicles; EE, early endosome; LE, late endosome; L, lysosome; CV, clathrin-coated vesicles.
The final and most important criterion is that SNAREs form selective complexes. Initial immunoprecipitations of SNAREs appeared to isolate specific sets of proteins. Antibodies against syntaxin 1 were able to precipitate SNAP-25 and VAMP, whereas syntaxin 5, present on the intermediate compartment and Golgi, was found to interact with endoplasmic reticulum and Golgi v-SNAREs. To examine these interactions in a more isolated system, in vitro binding studies using recombinant proteins were initiated (18, 19). In those experiments recombinant members of the syntaxin, VAMP, and SNAP-25 families were assayed for complex formation and complex stability. Surprisingly, almost any combination of SNAREs was able to form a complex that was SDS-resistant and had similar thermal stability. These results allow for three possibilities. One is that SNAREs are not involved in determining specificity of vesicle trafficking. The second is that thermal stability may not be an accurate barometer of intracellular events. Perhaps there is a significant kinetic difference in the formation of these SNARE complexes, which would be overlooked by a thermodynamic measurement. Third, the protein–protein interactions of the SNAREs themselves may not be specific in vitro, yet in concert with other layers of protein interactions, the overall fidelity of vesicle trafficking can be established. Thus the information for the specificity of membrane trafficking may not reside in the ability of the SNAREs to pair with each other, while in vivo specific pairing may indeed occur. It may well be that the translocation of vesicles from donor to acceptor sites via specific cytoskeletal and motor protein interactions, as well as rab-effector interactions, leads to specific SNARE complex formation, and thus fidelity in vesicle trafficking.
In summary the field of intracellular vesicle trafficking has become fascinatingly intricate from its beginnings with morphological descriptions from electron micrographs. With the recent convergence of cell-free, cell-permeabilized, and reconstituted in vitro assay systems, it appears we are approaching an understanding of the central players in vesicle trafficking. The next challenge lies in decoding the cross-talk between these central players and the bounty of other proteins associated with vesicle trafficking.
Footnotes
References
- 1.Nickel W, Weber T, McNew J A, Parlati F, Söllner T H, Rothman J E. Proc Natl Acad Sci. USA. 1999;96:12571–12576. doi: 10.1073/pnas.96.22.12571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Parlati F, Weber T, McNew J A, Westermann B, Söllner T H, Rothman J E. Proc Natl Acad Sci. USA. 1999;96:12565–12570. doi: 10.1073/pnas.96.22.12565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Palade G. Science. 1975;189:347–358. doi: 10.1126/science.1096303. [DOI] [PubMed] [Google Scholar]
- 4.Bennett M K, Calakos N, Scheller R H. Science. 1992;257:255–259. doi: 10.1126/science.1321498. [DOI] [PubMed] [Google Scholar]
- 5.Söllner T, Whiteheart S W, Brunner M, Erdjument-Bromage H, Geromanos S, Tempst P, Rothman J E. Nature (London) 1993;362:318–324. doi: 10.1038/362318a0. [DOI] [PubMed] [Google Scholar]
- 6.Söllner T, Bennett M K, Whiteheart S W, Scheller R H, Rothman J E. Cell. 1993;75:409–418. doi: 10.1016/0092-8674(93)90376-2. [DOI] [PubMed] [Google Scholar]
- 7.Hardwick K G, Pelham H R. J Cell Biol. 1992;119:513–521. doi: 10.1083/jcb.119.3.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mayer A, Wickner W, Haas A. Cell. 1996;85:83–94. doi: 10.1016/s0092-8674(00)81084-3. [DOI] [PubMed] [Google Scholar]
- 9.Nichols B J, Ungermann C, Pelham H R, Wickner W T, Haas A. Nature (London) 1997;387:199–202. doi: 10.1038/387199a0. [DOI] [PubMed] [Google Scholar]
- 10.Lin R C, Scheller R H. Neuron. 1997;19:1087–1094. doi: 10.1016/s0896-6273(00)80399-2. [DOI] [PubMed] [Google Scholar]
- 11.Hanson P I, Roth R, Morisaki H, Jahn R, Heuser J E. Cell. 1997;90:523–535. doi: 10.1016/s0092-8674(00)80512-7. [DOI] [PubMed] [Google Scholar]
- 12.Sutton R B, Fasshauer D, Jahn R, Brunger A T. Nature (London) 1998;395:347–353. doi: 10.1038/26412. [DOI] [PubMed] [Google Scholar]
- 13.Weber T, Zemelman B V, McNew J A, Westermann B, Gmachl M, Parlati F, Söllner T H, Rothman J E. Cell. 1998;92:759–772. doi: 10.1016/s0092-8674(00)81404-x. [DOI] [PubMed] [Google Scholar]
- 14.Chen Y A, Scales S J, Patel S M, Doung Y C, Scheller R H. Cell. 1999;97:165–174. doi: 10.1016/s0092-8674(00)80727-8. [DOI] [PubMed] [Google Scholar]
- 15.Xu T, Binz T, Niemann H, Neher E. Nat Neurosci. 1998;1:192–200. doi: 10.1038/642. [DOI] [PubMed] [Google Scholar]
- 16.Ungermann C, Sato K, Wickner W. Nature (London) 1998;396:543–548. doi: 10.1038/25069. [DOI] [PubMed] [Google Scholar]
- 17.Peters C, Mayer A. Nature (London) 1998;396:575–580. doi: 10.1038/25133. [DOI] [PubMed] [Google Scholar]
- 18.Yang B, Gonzalez L, Jr, Prekeris R, Steegmaier M, Advani R J, Scheller R H. J Biol Chem. 1999;274:5649–5653. doi: 10.1074/jbc.274.9.5649. [DOI] [PubMed] [Google Scholar]
- 19.Fasshauer D, Antonin W, Margittai M, Pabst S, Jahn R. J Biol Chem. 1999;274:15440–15446. doi: 10.1074/jbc.274.22.15440. [DOI] [PubMed] [Google Scholar]