This review discusses recent findings from clinical studies of antiangiogenic agents for metastatic breast cancer, as well as challenges facing clinicians as the role of antiangiogenics in metastatic breast cancer evolves.
Keywords: Angiogenesis, Metastatic breast cancer, Bevacizumab, Sunitinib, Sorafenib, Everolimus
Abstract
Angiogenesis has become an important target in the treatment of several solid tumors, including breast cancer. As monotherapy, antiangiogenic agents have demonstrated limited activity in metastatic breast cancer (MBC); therefore, they have generally been developed for use in combination with chemotherapies. Thus far, the experience with antiangiogenic agents for MBC has been mixed. The results from one study assessing addition of the monoclonal antibody bevacizumab to paclitaxel led to approval of bevacizumab for MBC. However, the modest improvement of progression-free survival rates in subsequent MBC studies has led to reappraisal of bevacizumab. Phase III studies have not produced evidence supporting use of the multikinase inhibitor sunitinib alone or in combination with MBC chemotherapy. Experience with sorafenib in a phase IIb program indicates potential when used in select combinations, particularly with capecitabine; however, phase III confirmatory data are needed. Although antiangiogenic therapies combined with chemotherapy have increased progression-free survival rates for patients with MBC, increases in overall survival times have not been observed. Some studies have tried to combine antiangiogenic agents such as bevacizumab and sunitinib or sorafenib, but that approach has been limited because of toxicity concerns. Sequential use of antiangiogenic agents with differing mechanisms of action may be an effective approach. Despite setbacks, angiogenesis will likely remain an important target of treatment for selected patients with MBC.
Introduction
More than 39,000 women died from breast cancer in the United States in 2011, with the vast majority having metastatic disease [1]. Approximately half of patients with metastatic breast cancer (MBC) will die from the disease within 2–3 years, and only 15% will survive beyond 5 years [2]. Although palliation of symptoms is the primary goal of treatment for many patients, small but clinically significant advances in treatment have been achieved and offer some survival gains. In the 1990s, incremental improvements in the survival of patients with MBC corresponded to the introduction of a number of novel chemotherapy agents (e.g., paclitaxel) and targeted therapies (e.g., trastuzumab) [3–6]. Nonetheless, strategies to further prolong survival have proved challenging.
Breast cancer is a heterogeneous disease, particularly in the metastatic setting, with interpatient as well as intrapatient (primary vs. metastatic tumors) differences in disease characteristics. Individualized treatment strategies consider the patient's age, performance status, prior therapies, and disease stage but rely primarily on HER2 and hormone receptor status. Thus, treatment is especially challenging for patients with advanced disease that is HER2-negative or hormone receptor–negative because there are fewer treatment options. Studies of combination chemotherapy have demonstrated modest improvements in survival compared with monotherapy but also greater toxicity [7], whereas other studies support the sequential use of agents over combination regimens [8, 9]. To advance MBC treatment, novel combinations are needed that improve symptom relief, disease control, and survival rates compared with monotherapy approaches, without negatively impacting a patient's quality of life (QOL).
Recent strategies have focused on combining targeted therapies with select chemotherapies. Generally, targeted therapies have demonstrated modest activity in MBC as monotherapies with acceptable toxicity [10–15], but data suggest that their addition to select therapies may potentiate antitumor activity through additive or synergistic effects or by mitigating treatment resistance [16–22]. The recent introduction of agents that target the DNA repair enzyme poly(ADP-ribose) polymerase (PARP) to overcome resistance to cytotoxic therapies has generated interest in a variety of tumor types, including breast cancer. Unfortunately, initial excitement generated by phase II data combining PARP inhibitors with chemotherapy for triple-negative MBC has met with more sobering results in the phase III setting [23–25]. Nonetheless, considerable interest remains for PARP inhibition in breast and other solid tumors.
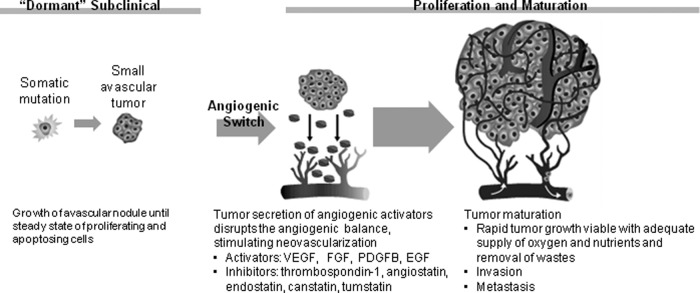
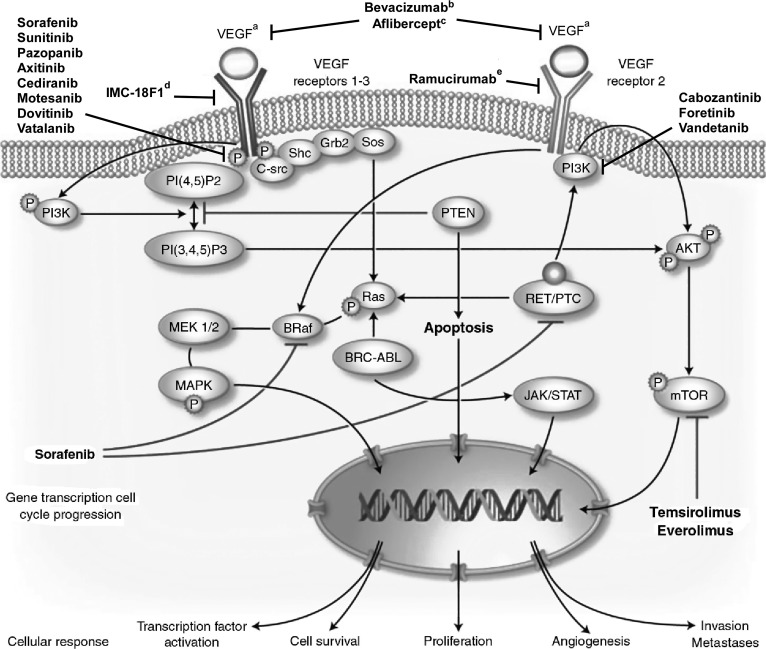
Angiogenesis has become an important target in the treatment of several solid tumors, including breast cancer (Fig. 1) [26–30]. In the microenvironment of a budding tumor, imbalances favoring angiogenic activators over inhibitors turn on the angiogenic switch at different stages of development, allowing for tumor vascularization, rapid proliferation, and facilitation of metastasis. Vascular endothelial growth factor (VEGF) and related factors and receptors form part of a primary pathway in tumor angiogenesis, with several antiangiogenic agents targeting various steps along this pathway (Fig 2.) [32–35].
Figure 1.
The angiogenic switch [29–31].
Abbreviations: EGF, epidermal growth factor; FGF, fibroblast growth factor; PDGFB, platelet-derived growth factor B; VEGF, vascular endothelial growth factor. Reprinted with permission from Brufsky AM. Expanding the targets in adjuvant therapy. Clinical Care Options Oncology. Available at: http://www.clinicaloptions.com/Oncology/Treatment%20Updates/Bisphosphonates%20and%20Targeted%20Agents.aspx. Accessed July 16, 2012.
Figure 2.
Intracellular vascular endothelial growth factor (VEGF) signal transduction pathway. Ligands bind to membrane receptors, which are phosphorylated, leading to activation of several cytoplasmic messengers, which activate transcription factors in the nucleus that involves target genes implicated in the proliferation, angiogenesis, apoptosis, and tumor invasion. Shown are some targeted therapies and their main inhibition targets [32–35]. aVEGF A–D ligands bind to VEGF receptors 1–3 with specific ligands preferentially binding to a specific receptor (e.g., VEGF A and C ligands bind to VEGF receptor 2); bmAb to VEGF A; ctargets VEGF A and B and placental growth factor (PlGF); dmAb to VEGF receptor 1; emAb to VEGF receptor 2.
Abbreviations: AKT, protein kinase B; BCR-ABL, Philadelphia chromosome; BRaf, B-type RAF kinase; C-src, cellular sarcoma; Grb, growth factor receptor–bound protein; JAK/STAT, Janus kinases/signal transducers and activators of transcription; MAPK, mitogen-activated protein kinase; MEK, MAPK kinase; mTOR, mammalian target of rapamycin; PI(4,5)P2, phosphatidylinositol (3,4) biphosphate; PI(3,4,5)P3, phosphatidylinositol (3,4,5) triphosphate; PI3K, phosphatidylinositol 3-kinase; PTC, papillary thyroid cancer; PTEN, phosphatase and tensin homologue; RET, rearranged during transfection; Shc, Src homology 2 domain-containing transforming protein; Sos, son of sevenless protein. Adapted from [32]. Copyright 2010 Springer. Reprinted with kind permission from Springer Science+Business Media B.V.
As with other targeted therapies, antiangiogenic agents have demonstrated limited activity as MBC monotherapies [10–13] and have generally been developed for use in combination with MBC chemotherapies. The monoclonal antibody bevacizumab has been the only antiangiogenic agent indicated for use in MBC when used in combination with first-line paclitaxel for HER2-negative disease. However, the failure of recent bevacizumab trials to reproduce the clinical benefit reported in the pivotal phase III trial has dramatically shifted the antiangiogenic paradigm in MBC. A number of small-molecule antiangiogenic agents, including multikinase inhibitors (MKIs), are being developed for MBC. This review will discuss recent findings from clinical studies with antiangiogenics and challenges facing clinicians as the role of antiangiogenics in MBC evolves.
Methods
Relevant studies were selected from a MEDLINE search on November 30, 2011 of “metastatic breast cancer” combined with “axitinib,” “bevacizumab,” “cediranib,” “everolimus,” “motesanib,” “pazopanib,” “sorafenib,” “sunitinib,” “vandetanib,” “varlitinib,” “foretinib,” “dovitinib,” “ramucirumab,” “IMC-18F1,” “aflibercept,” or “AMG 386.” Results were limited to English-language publications of clinical trials or randomized controlled trials. In addition, abstracts were selected from annual meetings of the American Society of Clinical Oncology, San Antonio Breast Cancer Symposium, and the European Society of Medical Oncology from January 1, 2008 to November 30, 2011.
Bevacizumab
Bevacizumab binds to VEGF directly, preventing its interaction with target receptors on the surface of endothelial cells and leading to regression of existing tumor vasculature and inhibition of new vasculature [36–39]. Phase III studies have shown that bevacizumab in combination with select chemotherapies improves disease control and progression-free survival (PFS) (Table 1).
Table 1.
Phase III trials of bevacizumab in combination with selected chemotherapies for metastatic breast cancer
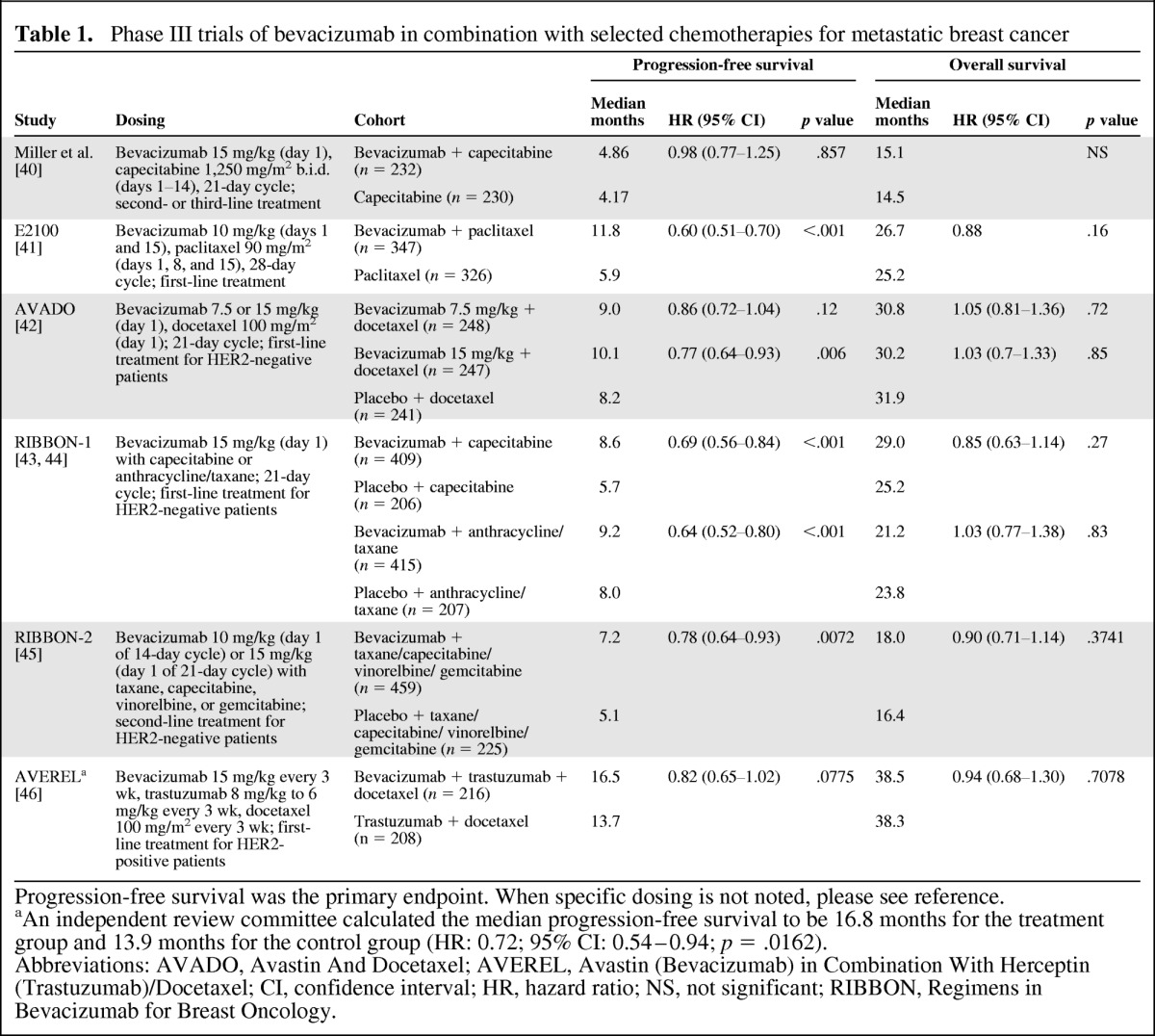
Progression-free survival was the primary endpoint. When specific dosing is not noted, please see reference.
aAn independent review committee calculated the median progression-free survival to be 16.8 months for the treatment group and 13.9 months for the control group (HR: 0.72; 95% CI: 0.54–0.94; p = .0162).
Abbreviations: AVADO, Avastin And Docetaxel; AVEREL, Avastin (Bevacizumab) in Combination With Herceptin (Trastuzumab)/Docetaxel; CI, confidence interval; HR, hazard ratio; NS, not significant; RIBBON, Regimens in Bevacizumab for Breast Oncology.
The conditional approval of bevacizumab in HER2-negative MBC was based on results of the E2100 study, which compared the combination of bevacizumab and first-line paclitaxel with paclitaxel alone [41]. The addition of bevacizumab to paclitaxel nearly doubled the primary endpoint of PFS (median: 11.8 vs. 5.9 months; p < .001), but did not significantly improve overall survival (OS) rates. As part of its conditional approval, the U.S. Food and Drug Administration (FDA) required follow-up studies with bevacizumab to better define the clinical benefit to support full approval. These follow-up studies, including Avastin And Docetaxel (AVADO) [42] and Regimens in Bevacizumab for Breast Oncology (RIBBON)-1 [43] for first-line treatment and RIBBON-2 [45] for second-line treatment, combined bevacizumab with MBC chemotherapies (e.g., docetaxel, capecitabine). Significant PFS benefit was associated with addition of bevacizumab in each of these follow-up studies with no improvement in OS, but the magnitude of the PFS benefit was notably more modest than that shown in E2100. In a metaregression analysis of five clinical trials (n = 3,841) of bevacizumab combined with chemotherapy in advanced breast cancer, PFS rates were significantly improved (hazard ratio [HR]: 0.68; 95% confidence interval [CI]: 0.56–0.81; p < .0001) for patients receiving first-line therapy but not for patients receiving second-line therapy (HR: 0.86; 95% CI: 0.69–1.07, p = .19) [47]. Similar results were reported in a separate meta-analysis of first-line trials only [48].
Infrequently, bevacizumab has been associated with clinically relevant adverse events (AEs), including bleeding, proteinuria, hypertension, and left ventricular dysfunction, when combined with breast cancer chemotherapy [49]. The risks associated with bevacizumab combination therapy, the lack of a survival benefit, and inconsistencies in the magnitude of PFS benefit across studies led the FDA to remove the MBC indication for bevacizumab in November 2011 [50]. As of November 2011, the Centers for Medicare and Medicaid Services and the National Comprehensive Cancer Network continued to support the use of bevacizumab for MBC [51]. In addition, the European Medicines Agency (EMA) chose to maintain the indication of bevacizumab with first-line paclitaxel and in 2011 expanded the MBC indication for bevacizumab based on data from the RIBBON-1 study to include the combination with first-line capecitabine for patients in whom other chemotherapy options are not appropriate [52]. In contrast, the EMA removed the MBC indication for bevacizumab in combination with docetaxel in 2010.
Despite these setbacks, a number of studies continue to explore the role of bevacizumab in breast cancer, attempting to identify patient populations and treatment combinations that will best exploit its potent, targeted activity. Although not definitive, a subgroup analysis of patients with triple-negative breast cancer enrolled in the RIBBON-2 study (n = 159) showed a significant improvement in PFS time, favoring the bevacizumab arm over the control arm (median: 6.0 vs. 2.7 months, HR: 0.49, 95% CI: 0.33–0.74; p = .0006), with a favorable trend in OS time (median: 17.9 vs. 12.6 months, HR: 0.62, 95% CI: 0.39–1.01; p = .053) [53]. A meta-analysis of patients with triple-negative disease (n = 621) enrolled in three first-line phase III studies (E2100, AVADO, and RIBBON-1) also showed a significant improvement in PFS time associated with the addition of bevacizumab to standard treatments (8.1 vs. 5.4 months, HR: 0.65, 95% CI: 0.54–0.78; p < .0001), but no apparent difference in OS time (18.9 vs. 17.5 months, HR: 0.96, 95% CI: 0.79–1.16; p = .673) [54].
Studies are also assessing bevacizumab as part of novel combinations [45, 55–57], as well as in the early disease stages [58–60]. Primary results were recently reported from the phase III Avastin (Bevacizumab) in Combination With Herceptin (Trastuzumab)/Docetaxel (AVEREL) study, which evaluated the addition of bevacizumab to the combination of trastuzumab and docetaxel in patients with locally recurrent metastatic HER2-positive disease [46]. The addition of bevacizumab improved PFS compared with the control arm, which was not statistically significant as assessed by investigators (median PFS time: 16.5 vs. 13.7 months; p = .0775) but was statistically significant in a secondary analysis by an independent review committee that was stratified and censored for nonprotocol therapy (16.8 vs. 13.9 months; p = .0162). An interim analysis of OS time showed no statistical difference between treatment arms (p = .7078).
Multikinase Inhibitors
In view of findings with bevacizumab, investigators began to explore the use of the MKIs sunitinib and sorafenib. Both agents have antiangiogenic activity that may be broader than that of bevacizumab by targeting multiple proangiogenic pathways. It has been speculated that a broader spectrum of activity might mitigate development of drug resistance [63]. Early studies in breast and other solid tumors demonstrated the feasibility of combining MKIs with widely used breast cancer therapies [64–66].
Sunitinib
Sunitinib malate, an oral type 1 MKI, binds directly to the ATP binding site. Studies indicate that sunitinib inhibits tumor angiogenesis, proliferation, and metastasis by targeting VEGF receptors (VEGFR) 1–3, platelet-derived growth factor receptor (PDGFR), stem cell factor receptor (KIT), Feline McDonough Sarcoma (FMS)-related tyrosine kinase 3 (Flt-3), colony-stimulating factor 1 receptor, and rearranged during transfection (RET) [33, 67]. Sunitinib is currently approved multinationally for use in gastrointestinal stromal tumors, advanced renal cell carcinoma, and pancreatic neuroendocrine tumors.
Early studies demonstrated clinical activity for sunitinib in previously treated patients with MBC. In a phase II study, 64 patients receiving sunitinib monotherapy achieved an overall response rate (ORR) of 11% and an additional 5% maintained stable disease for 6 months or more [11]. Treatment was tolerable. The most common grade 3/4 AEs were fatigue (14%), hand-foot skin reaction (HFSR; 9%), neutropenia (34%), thrombocytopenia (14%), and leukopenia (14%). Other studies that enrolled patients with MBC demonstrated the feasibility of combining sunitinib with paclitaxel [68], capecitabine [66], and docetaxel [69].
Based on phase II data, a phase III MBC program was developed to assess sunitinib monotherapy, as well as in combination with select chemotherapies, with PFS as the primary endpoint. Thus far, the program has not produced evidence to support use of sunitinib in MBC (Table 2). Sunitinib monotherapy was inferior to capecitabine for second-line treatment of HER2-negative MBC [70], and the addition of sunitinib to capecitabine did not improve PFS times compared with single-agent chemotherapy for patients with previously treated advanced breast cancer [72]. In a third trial, the addition of sunitinib to first-line docetaxel for HER2-negative advanced breast cancer provided a significant improvement in ORR compared with docetaxel alone (55% vs. 42%; p = .001), but this did not translate into a significant improvement in PFS time (median: 8.6 vs. 8.3 months; p = .265) or OS time (median: 24.8 vs. 25.5 months; p = .904) [71].
Table 2.
Phase III trials of sunitinib in advanced breast cancer
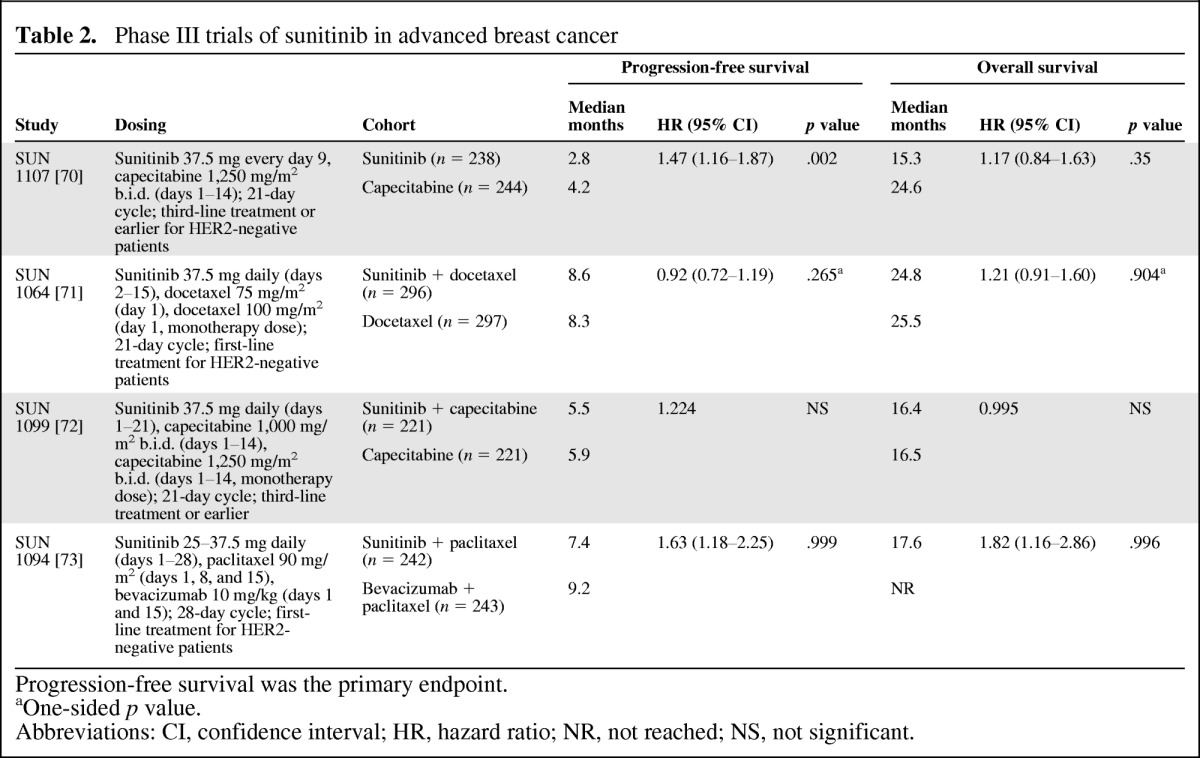
Progression-free survival was the primary endpoint.
aOne-sided p value.
Abbreviations: CI, confidence interval; HR, hazard ratio; NR, not reached; NS, not significant.
Finally, a phase III superiority study assessed the addition of sunitinib versus bevacizumab to paclitaxel [73]. The investigators concluded that the sunitinib-paclitaxel combination was inferior to the bevacizumab-paclitaxel combination because the bevacizumab arm was more favorable for both PFS and OS times. Although the ORR was 32% for both treatments, the duration of response was shorter in the sunitinib arm compared with that in the bevacizumab arm (median: 6.3 vs. 14.8 months). Generally, the safety profiles of the sunitinib combinations were consistent with previous studies of the individual agents with no unexpected AEs. The addition of sunitinib to standard therapies was associated with increases in the incidence of HFSR, neutropenia, and other hematologic toxicities [71–73].
Sorafenib
Sorafenib is an oral type 2 MKI that competes indirectly with ATP by occupying the hydrophobic pocket adjacent to the ATP binding site [33, 74–75]. Sorafenib targets cell surface kinases VEGFR 1–3, PDGFR, KIT, and Flt-3, and also the intracellular kinases C-rapidly accelerated fibrosarcoma (CRAF), BRAF, and mutant BRAF. Sorafenib inhibits tumor proliferation and angiogenesis in preclinical models. In breast cancer models, there is evidence that addition of sorafenib to other systemic therapies may provide additive or synergistic activity and chemosensitization [16–18]. Sorafenib is approved multinationally for unresectable hepatocellular and advanced renal cell carcinoma.
Initial clinical studies with sorafenib in MBC focused on its activity as a monotherapy and the feasibility of combining it with therapies for solid tumors, including MBC [10, 13, 64, 76]. As monotherapy, sorafenib demonstrated limited activity in heavily pretreated patients with MBC [10, 13]. In a phase II study of 54 patients who had received at least one prior chemotherapy regimen for MBC, 2% of patients achieved partial response, and the disease stabilized in an additional 37% of patients (≥6 months in 13%) [10]. The most common grade 3 AEs were rash/desquamation (6%), HFSR (4%), and fatigue (4%).
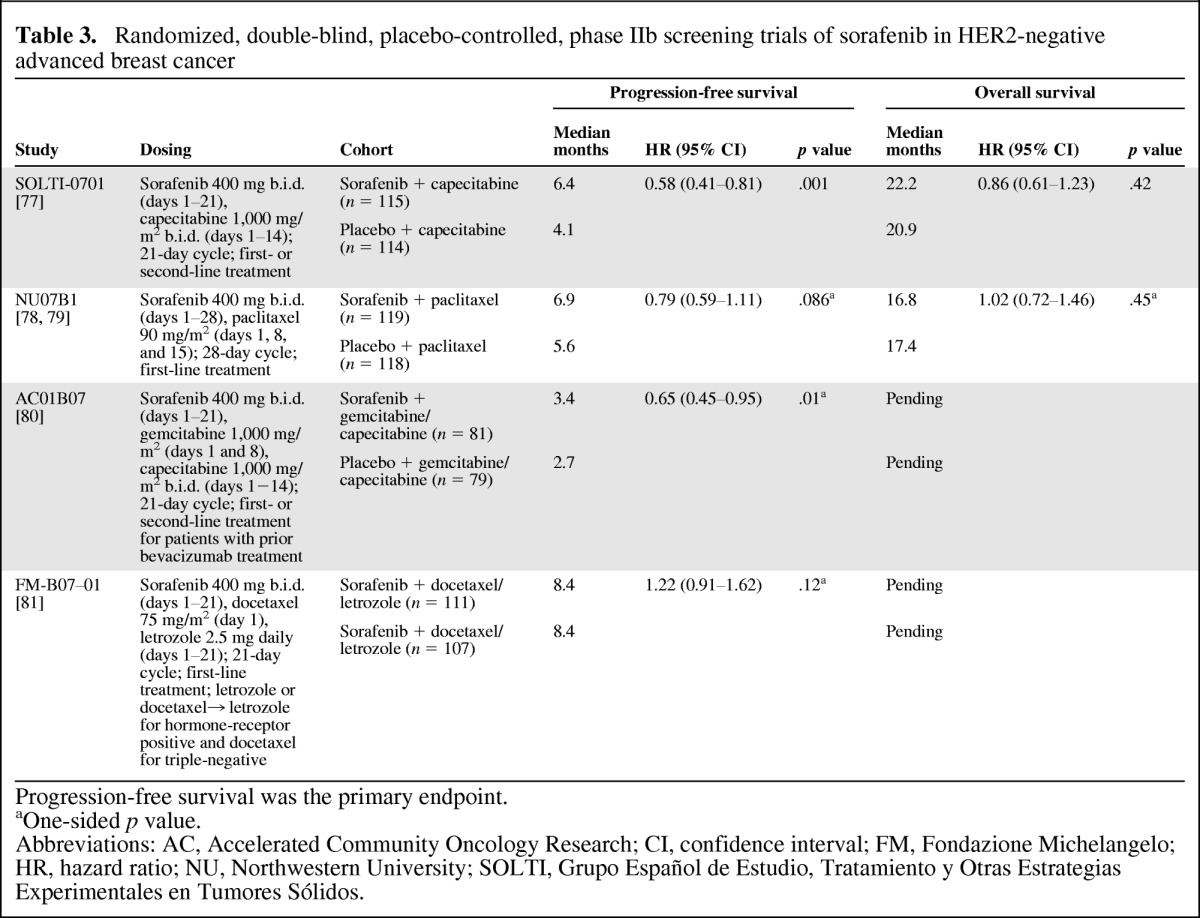
Based on results from early studies, the Trials to Investigate the Efficacy of Sorafenib (TIES) breast cancer program was initiated to assess sorafenib in combination with select systemic therapies for HER2-negative locally advanced or metastatic breast cancer (Table 3). TIES was a series of four double-blind, randomized, placebo-controlled, phase IIb screening studies designed to rapidly assess sorafenib in these combination regimens and determine whether any warranted a phase III trial [77]. The primary endpoint was PFS in all studies.
Table 3.
Randomized, double-blind, placebo-controlled, phase IIb screening trials of sorafenib in HER2-negative advanced breast cancer
Progression-free survival was the primary endpoint.
aOne-sided p value.
Abbreviations: AC, Accelerated Community Oncology Research; CI, confidence interval; FM, Fondazione Michelangelo; HR, hazard ratio; NU, Northwestern University; SOLTI, Grupo Español de Estudio, Tratamiento y Otras Estrategias Experimentales en Tumores Sólidos.
The addition of sorafenib to first- or second-line capecitabine in the SOLTI-0701 (Grupo Español de Estudio, Tratamiento y Otras Estrategias Experimentales en Tumores Sólidos) study provided a statistically significant improvement in PFS compared with placebo plus capecitabine (median: 6.4 vs. 4.1 months; p = .001) [77]. A modest but statistically significant improvement in PFS was also observed in the AC01B07 (Accelerated Community Oncology Research) study with addition of sorafenib to gemcitabine or capecitabine compared with the addition of placebo (median: 3.4 vs. 2.7 months; one-sided p = .01) in patients whose disease had progressed during or after a regimen that included bevacizumab [80]. There was no statistically significant improvement in PFS times when sorafenib was added to first-line paclitaxel in the NU07B1 (Northwestern University) study [78], or when it was added to first-line docetaxel and/or letrozole in the FM-B07-01 (Fondazione Michelangelo) study [81]. With regard to OS, a secondary endpoint of the TIES trials, there was no statistical difference between the treatment arms in either SOLTI-0701 or NU07B1 [77, 79]. OS data are pending for AC01B07 and FM-B07-01.
Addition of sorafenib to these therapies was manageable, although dose modifications were more common in the sorafenib arms than in the control arms. The most common grade 3/4 toxicity associated with sorafenib in these studies was grade 3 HFSR (associated with sorafenib) or hand-foot syndrome (HFS; associated with capecitabine) [77, 79–81]. In the sorafenib arm of SOLTI-0701, the rate of dose reductions was 53% for sorafenib and 78% for capecitabine, whereas corresponding values in the placebo arm were 14% and 33%, respectively [77]. Grade 3 HFSR/HFS developed in 44% of patients in the sorafenib arm and 14% in the placebo arm. Other AEs (any grade) that occurred more frequently in the sorafenib arm included rash (22% vs. 8%), diarrhea (58% vs. 30%), mucosal inflammation (33% vs. 21%), neutropenia (13% vs. 4%), and hypertension (18% vs. 12%), although rates of grade 3/4 events were generally low and did not differ between treatment arms. In the other TIES studies, the incidence of grade 3 HFSR/HFS ranged from 13% in FM-B07-01 to 39% in AC01B07 [79–81].
The PFS benefit observed in two studies will need to be confirmed in the phase III setting. These results should not be considered practice changing [77, 80]. A phase III trial comparing capecitabine in combination with sorafenib or placebo for treatment of locally advanced or metastatic HER2-negative breast cancer (RESILIENCE) was initiated in November 2010 [82]. Although similar in design to SOLTI-0701, the RESILIENCE study implemented important adjustments to the dosing schema and supportive care to mitigate toxicity associated with the phase II dosing, particularly HFSR/HFS, which was unacceptable for many patients.
mTOR Inhibitors
Everolimus is an oral inhibitor of the serine-threonine kinase mammalian target of rapamycin (mTOR), which is downstream of the phosphatidylinositol-3-kinase (PI3K)/AKT pathway. Everolimus has been shown to inhibit tumor cell proliferation, metabolism, and angiogenesis in preclinical models [83, 84]. Studies also indicate that the PI3K/AKT pathway may be involved in resistance to HER2 targeted therapy as well as hormone therapy; thus, inhibiting this pathway may improve the efficacy of these agents [19–21]. Everolimus is currently approved for use in advanced renal cell carcinoma, subependymal giant cell astrocytoma, and advanced pancreatic neuroendocrine tumors.
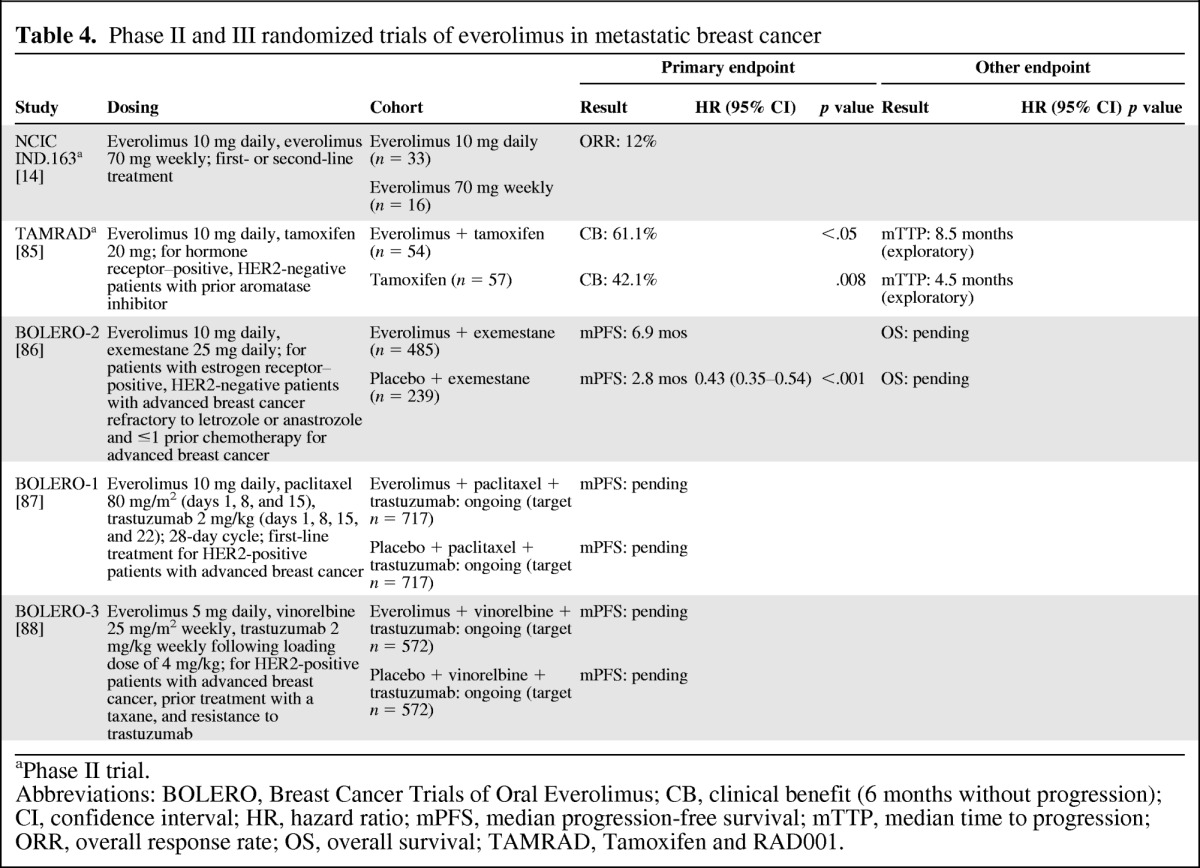
Early breast cancer studies with everolimus focused on its use as monotherapy and in combination with aromatase inhibitors or trastuzumab [14, 89–93] (Table 4). The encouraging results of these early studies supported the Breast Cancer Trials of Oral Everolimus (BOLERO) program, a series of phase III studies that assessed everolimus in combination with agents to treat hormone-receptor–positive or HER2-positive advanced disease. Initial results of one of these studies, BOLERO-2, were recently reported.
Table 4.
Phase II and III randomized trials of everolimus in metastatic breast cancer
aPhase II trial.
Abbreviations: BOLERO, Breast Cancer Trials of Oral Everolimus; CB, clinical benefit (6 months without progression); CI, confidence interval; HR, hazard ratio; mPFS, median progression-free survival; mTTP, median time to progression; ORR, overall response rate; OS, overall survival; TAMRAD, Tamoxifen and RAD001.
In BOLERO-2, the combination of everolimus and exemestane was assessed in patients with estrogen receptor–positive breast cancer refractory to letrozole or anastrozole [86]. The primary rationale for the use of everolimus in this combination was to overcome resistance to hormone therapy. The addition of everolimus to exemestane compared with exemestane alone resulted in a significant improvement in PFS by investigator assessment (median: 6.9 vs. 2.8 months; p < .001) with consistent PFS results across subgroups. ORR was 9.5% versus 0.4%, respectively (p < .001). OS data are pending. The most common grade 3/4 AEs associated with combination versus monotherapy were stomatitis (8% vs. 1%), anemia (6% vs. <1%), and dyspnea (4% vs. 1%). The BOLERO-1 and BOLERO -3 studies are ongoing and are assessing everolimus as part of combination regimens for HER2-positive disease.
Emerging Antiangiogenic Agents
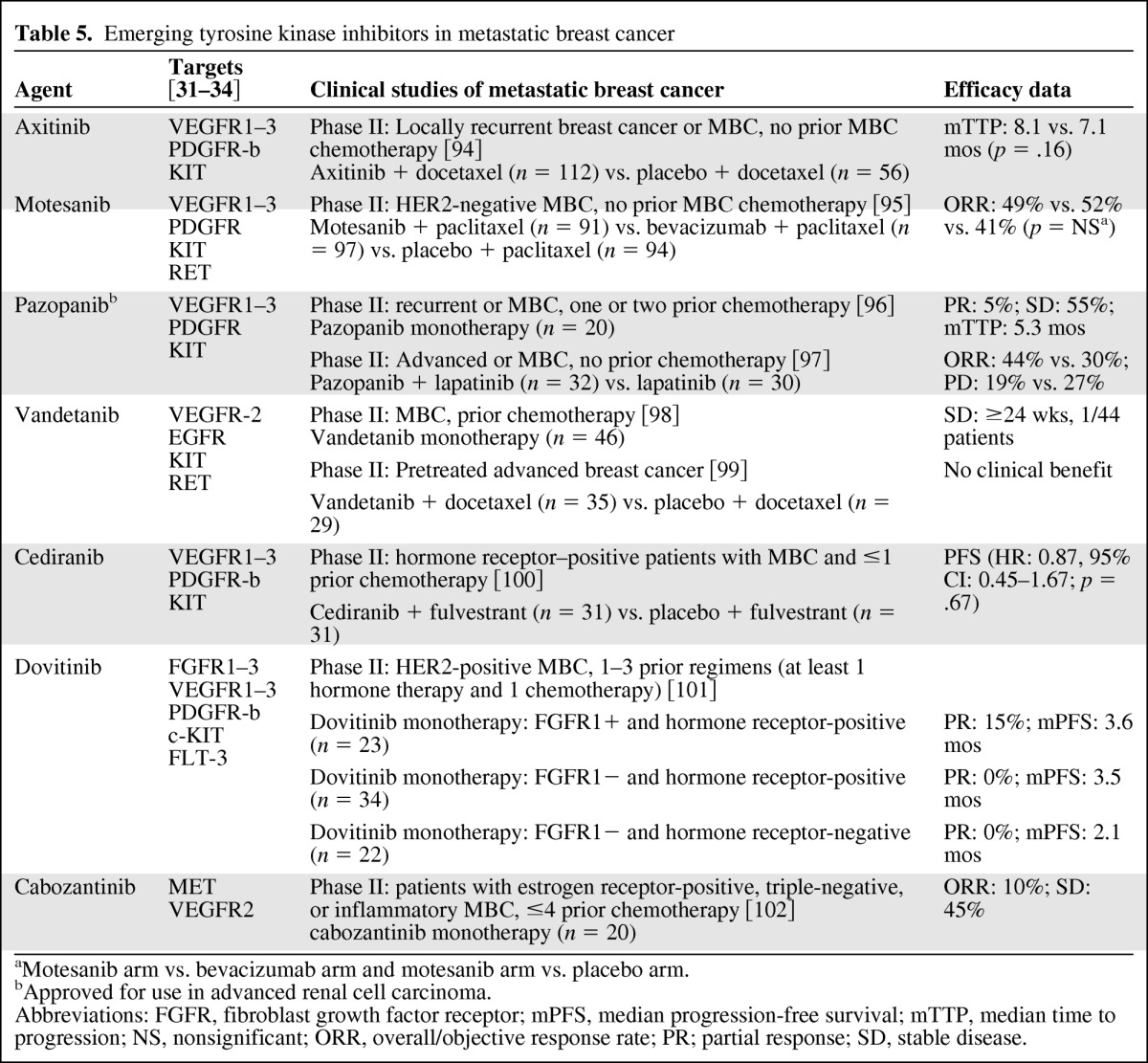
A number of novel antiangiogenic agents are being developed in MBC, including multikinase inhibitors (Table 5). Results have been encouraging. As with other antiangiogenics in MBC, monotherapy with these agents has shown modest activity, with a more clinically relevant potential benefit when used as part of combinations. In a phase II study of 20 patients with recurrent or MBC, pazopanib monotherapy provided a measure of disease stability with one patient achieving partial remission (5%) and 11 achieving stable disease (55%) [96]. In a separate randomized phase II study, an interim analysis of 62 patients with HER2-positive advanced or MBC receiving pazopanib plus lapatinib or lapatinib monotherapy reported response rates of 44% and 30% [97]. Other emerging MKIs being investigated in MBC include vatalanib [103, 104], foretinib [105], dovitinib [101], and cabozantinib [102]. The novel anti-VEGFR monoclonal antibodies ramucirumab (IMC-1121B) and IMC-18F1 [106–108] are also being developed for MBC, as well as the fusion proteins aflibercept (targets VEGF and placental growth factor) [109] and AMG 386 (targets angiopoietin) [110].
Table 5.
Emerging tyrosine kinase inhibitors in metastatic breast cancer
aMotesanib arm vs. bevacizumab arm and motesanib arm vs. placebo arm.
bApproved for use in advanced renal cell carcinoma.
Abbreviations: FGFR, fibroblast growth factor receptor; mPFS, median progression-free survival; mTTP, median time to progression; NS, nonsignificant; ORR, overall/objective response rate; PR; partial response; SD, stable disease.
Future Challenges with Antiangiogenics in MBC
Thus far, the experience with antiangiogenics in MBC has been mixed. The E2100 results assessing the bevacizumab-paclitaxel combination led to approval of the first antiangiogenic for MBC and generated considerable interest in this drug class. However, modest PFS benefits with bevacizumab combinations in subsequent studies have led to reappraisal of antiangiogenics [50–51, 111–114], and failure of the sunitinib MBC program has further dampened enthusiasm. However, there has been some encouraging news. Results from the TIES phase IIb studies were mixed but indicated a potential role for sorafenib in MBC, particularly in combination with capecitabine. However, the incidence of AEs with the sorafenib combinations indicates that the dosing strategy needs to be adjusted to improve the efficacy and tolerability ratio. In the BOLERO-2 study, addition of everolimus to exemestane provided a notable improvement in PFS with an acceptable toxicity profile, although this benefit likely has more to do with overcoming hormone resistance with everolimus rather than targeting angiogenesis. Nonetheless, OS data are cautiously awaited, as are results from BOLERO-1 and BOLERO-3.
As clinical development of antiangiogenics in MBC continues, it is important to understand the regulatory and clinical challenges that need to be overcome. The approval process for oncology drugs in the U.S. underwent significant changes over the past few decades. In the 1970s, the FDA used ORR as a standard measure for approval of oncology drugs [113, 115]. However, ORR does not always predict clinical benefit, so in the 1980s the FDA required an OS or QOL benefit or established surrogate measures as standard goals for regulatory approval. In the 1990s, recognizing that OS requires large sample sizes and long follow-up periods, which could delay access to treatment, the FDA accepted surrogate measures of clinical benefit (e.g., PFS) in select malignancies to accelerate access but with the provision of follow-up studies to better define clinical benefit in support of full regulatory approval.
PFS has now become a standard endpoint—and frequently, the primary endpoint—in MBC studies assessing antiangiogenics. It is likely that use of effective salvage therapies after disease progression confound OS data in MBC trials, particularly in the first- or second-line setting [111, 115–118]. Without standard strategies for salvage therapy, it is difficult to control for the impact of treatments after progression without designing trials that delineate subsequent therapies. PFS is not confounded by use of salvage therapies, and deaths unrelated to cancer have less impact compared with OS. On the other hand, PFS is limited by attrition bias and differences in assessment criteria such as interval between assessments, definition of progression, and centralized review versus investigator assessment. Furthermore, formal validation of PFS as a surrogate for OS has been unsuccessful in MBC thus far. Nonetheless, treatments that provide a clinically significant PFS benefit with acceptable toxicity or improvements in QOL are welcome additions to the MBC armamentarium.
There is some speculation that the discordance between PFS and OS findings is related to possible changes in tumor biology. Preclinical evidence suggests rapid rebound of angiogenesis after discontinuation of therapy and implies that antiangiogenic therapy may select for a resistant and more aggressive disease via hypoxia-induced upregulation of alternative angiogenic pathways or improved tumor survival in low oxygen environments [119–122]. Thus, the benefit of disease control with antiangiogenic therapy becomes short-lived at the time of progression; changes in tumor biology lead to a rapid decline and death that is comparable in time to that of having no antiangiogenic therapy. These preclinical phenomena have not been observed in the clinical setting to date [71, 123–124], but they do raise concerns.
Although angiogenesis is fundamental for development, growth, and metastasis of solid tumors, it has become increasingly evident that antiangiogenic therapy may have an inconsistent impact [125]. MBC is a heterogeneous disease with differences among patients and at the tumor level (e.g., primary tumor vs. metastasis). With bevacizumab, it appears that select patients may derive a clinical benefit, but this subgroup is yet to be characterized after five large trials [113, 126–127]. Moving forward, it is important to identify patients who will derive maximum benefit from antiangiogenic agents. This will likely require development of validated biomarkers, which in itself is a challenging task. Biomarker validation requires meticulous attention to methodology to identify potential candidates, to reduce the impact of false-positive and false-negative results, and to properly design pilot, definitive, and confirmatory studies [125].
A number of potential biomarkers for angiogenesis or antiangiogenesis have emerged in breast cancer research, but none have been validated. In patients with breast cancer receiving bevacizumab, correlations have been observed between outcomes and VEGF plasma levels, VEGF-2578 and -5411 AA genotypes, grade 3/4 hypertension, and other factors [32, 46, 127, 128]. In a subpopulation of 162 patients who volunteered for a translation research study during AVEREL, the benefit of adding bevacizumab to the trastuzumab and docetaxel combination compared with trastuzumab plus docetaxel was greater in patients with high levels of plasma VEGF-A at baseline (median PFS: 16.6 vs. 8.5 months, HR: 0.70, 95% CI: 0.43–1.14) compared with low levels of VEGF-A (median PFS: 16.5 vs. 13.6 months, HR: 0.83, 95% CI: 0.50–1.36) [46].
Conclusions
Over the past 5 years, the strategy for use of antiangiogenics in MBC has changed dramatically. Because angiogenesis plays a critical role in development and progression of solid tumors, early use of antiangiogenics in MBC followed a pattern similar to that of nonspecific cytotoxic therapy [125]. Antiangiogenic therapy has thus far demonstrated improvements in PFS, but prolonging OS remains elusive. MBC is a heterogeneous disease, and it appears that the effect of antiangiogenics may also be heterogeneous. Thus, a shift has occurred in the development of antiangiogenic agents for MBC, with greater emphasis on identifying the most favorable patient populations and combination therapies. Biomarkers for antiangiogenics are essential to identifying specific patient populations that would receive the most and least benefit from these combinations. More preclinical and clinical data are needed to better understand the impact of antiangiogenic therapy on tumor biology and whether there is an advantage to targeting a broader spectrum of angiogenic pathways. The ability to combine antiangiogenics such as bevacizumab and sunitinib or sorafenib has been limited because of toxicity concerns [129–131], but sequential use of antiangiogenics with differing mechanisms of action may be an effective approach [80, 132–133]. Despite setbacks, angiogenesis will likely remain an important target of treatment in selected patients with this devastating disease.
Acknowledgments
We thank the Lynn Sage Foundation and Dolores Knes Fund. This work was supported by Bayer HealthCare Pharmaceuticals and Onyx Pharmaceuticals.
Footnotes
- (C/A)
- Consulting/advisory relationship
- (RF)
- Research funding
- (E)
- Employment
- (H)
- Honoraria received
- (OI)
- Ownership interests
- (IP)
- Intellectual property rights/inventor/patent holder
- (SAB)
- Scientific advisory board
Author Contributions
Conception/Design: Sangeetha Reddy, Michael Raffin, Virginia Kaklamani
Provision of study material or patients: Virginia Kaklamani
Collection and/or assembly of data: Virginia Kaklamani
Data analysis and interpretation: Sangeetha Reddy, Michael Raffin, Virginia Kaklamani
Manuscript writing: Sangeetha Reddy, Michael Raffin, Virginia Kaklamani
Final approval of manuscript: Sangeetha Reddy, Michael Raffin, Virginia Kaklamani
References
- 1.American Cancer Society. Atlanta, GA: American Cancer Society; 2011. Cancer Facts and Figures 2011. [Google Scholar]
- 2.American Cancer Society. Breast cancer survival rates by stage. [Accessed November 16, 2011]. Available at: http://www.cancer.org/Cancer/BreastCancer/DetailedGuide/breast-cancer-survival-by-stage.
- 3.Chia SK, Speers CH, D'Yachkova Y, et al. The impact of new chemotherapeutic and hormone agents on survival in a population-based cohort of women with metastatic breast cancer. Cancer. 2007;110:973–979. doi: 10.1002/cncr.22867. [DOI] [PubMed] [Google Scholar]
- 4.Giordano SH, Buzdar AU, Smith TL, et al. Is breast cancer survival improving? Cancer. 2004;100:44–52. doi: 10.1002/cncr.11859. [DOI] [PubMed] [Google Scholar]
- 5.Arveux P, Grosclaude P, Reyrat E, et al. Breast cancer survival in France: A relative survival analysis based on 68,449 cases treated in the 20 French comprehensive cancer centers between 1980 and 1999. Proc Am Soc Clin Oncol. 2003;22 Abstract 3437. [Google Scholar]
- 6.Gennari A, Conte P, Rosso R, et al. Survival of metastatic breast carcinoma patients over a 20-year period: A retrospective analysis based on individual patient data from six consecutive studies. Cancer. 2005;104:1742–1750. doi: 10.1002/cncr.21359. [DOI] [PubMed] [Google Scholar]
- 7.Carrick S, Parker S, Thornton CE, et al. Single agent versus combination chemotherapy for metastatic breast cancer. Cochrane Database Syst Rev. 2009:CD003372. doi: 10.1002/14651858.CD003372.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Joensuu H, Holli K, Heikkinen M, et al. Combination chemotherapy versus single-agent therapy as first- and second-line treatment in metastatic breast cancer: A prospective randomized trial. J Clin Oncol. 1998;16:3720–3730. doi: 10.1200/JCO.1998.16.12.3720. [DOI] [PubMed] [Google Scholar]
- 9.Sledge GW, Neuberg D, Bernardo P, et al. Phase III trial of doxorubicin, paclitaxel, and the combination of doxorubicin and paclitaxel as front-line chemotherapy for metastatic breast cancer: An Intergroup trial (E1193) J Clin Oncol. 2003;21:588–592. doi: 10.1200/JCO.2003.08.013. [DOI] [PubMed] [Google Scholar]
- 10.Bianchi G, Loibl S, Zamagni C, et al. Phase II multicenter, uncontrolled trial of sorafenib in patients with metastatic breast cancer. Anticancer Drugs. 2009;20:616–624. [PubMed] [Google Scholar]
- 11.Burstein HJ, Elias AD, Rugo HS, et al. Phase II study of sunitinib malate, an oral multitargeted tyrosine kinase inhibitor, in patients with metastatic breast cancer previously treated with an anthracycline and a taxane. J Clin Oncol. 2008;26:1810–1816. doi: 10.1200/JCO.2007.14.5375. [DOI] [PubMed] [Google Scholar]
- 12.Cobleigh MA, Langmuir VK, Sledge GW, et al. A phase I/II dose-escalation trial of bevacizumab in previously treated metastatic breast cancer. Semin Oncol. 2003;30:117–124. doi: 10.1053/j.seminoncol.2003.08.013. [DOI] [PubMed] [Google Scholar]
- 13.Moreno-Aspitia A, Morton RF, Hillman DW, et al. Phase II trial of sorafenib in patients with metastatic breast cancer previously exposed to anthracyclines or taxanes: North Central Cancer Treatment Group and Mayo Clinic Trial N0336. J Clin Oncol. 2009;27:11–15. doi: 10.1200/JCO.2007.15.5242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ellard SL, Clemons M, Gelmon KA, et al. Randomized phase II study comparing two schedules of everolimus in patients with recurrent/metastatic breast cancer: NCIC Clinical Trials Group IND. 163. J Clin Oncol. 2009;27:4536–4541. doi: 10.1200/JCO.2008.21.3033. [DOI] [PubMed] [Google Scholar]
- 15.Burstein HJ, Storniolo AM, Franco S, et al. A phase II study of lapatinib monotherapy in chemotherapy-refractory HER2-positive and HER2-negative advanced or metastatic breast cancer. Ann Oncol. 2008;19:1068–1074. doi: 10.1093/annonc/mdm601. [DOI] [PubMed] [Google Scholar]
- 16.Ding Q, Huo L, Yang JY, et al. Down-regulation of myeloid cell leukemia-1 through inhibiting Erk/Pin 1 pathway by sorafenib facilitates chemosensitization in breast cancer. Cancer Res. 2008;68:6109–6117. doi: 10.1158/0008-5472.CAN-08-0579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Merz M, Komljenovic D, Zwick S, et al. Sorafenib tosylate and paclitaxel induce anti-angiogenic, anti-tumour and anti-resorptive effects in experimental breast cancer bone metastases. Eur J Cancer. 2011;47:277–286. doi: 10.1016/j.ejca.2010.08.019. [DOI] [PubMed] [Google Scholar]
- 18.Bonelli MA, Fumarola C, Alfieri RR, et al. Synergistic activity of letrozole and sorafenib on breast cancer cells. Breast Cancer Res Treat. 2010;124:79–88. doi: 10.1007/s10549-009-0714-5. [DOI] [PubMed] [Google Scholar]
- 19.Tokunaga E, Kataoka A, Kimura Y, et al. The association between Akt activation and resistance to hormone therapy in metastatic breast cancer. Eur J Cancer. 2006;42:629–635. doi: 10.1016/j.ejca.2005.11.025. [DOI] [PubMed] [Google Scholar]
- 20.Kirkegaard T, Witton CJ, McGlynn LM, et al. AKT activation predicts outcome in breast cancer patients treated with tamoxifen. J Pathol. 2005;207:139–146. doi: 10.1002/path.1829. [DOI] [PubMed] [Google Scholar]
- 21.Wang L, Zhang Q, Zhang J, et al. PI3K pathway activation results in low efficacy of both trastuzumab and lapatinib. BMC Cancer. 2011;11:248. doi: 10.1186/1471-2407-11-248. 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hastak K, Alli E, Ford JM. Synergistic chemosensitivity of triple-negative breast cancer cell lines to poly(ADP-Ribose) polymerase inhibition, gemcitabine, and cisplatin. Cancer Res. 2010;70:7970–7980. doi: 10.1158/0008-5472.CAN-09-4521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O'Shaughnessy J, Osborne C, Pippen JE, et al. Iniparib plus chemotherapy in metastatic triple-negative breast cancer. N Engl J Med. 2011;364:205–214. doi: 10.1056/NEJMoa1011418. [DOI] [PubMed] [Google Scholar]
- 24.Guha M. PARP inhibitors stumble in breast cancer. Nat Biotechnol. 2011;29:373–374. doi: 10.1038/nbt0511-373. [DOI] [PubMed] [Google Scholar]
- 25.O'Shaughnessy J, Schwartzberg LS, Danso MA, et al. A randomized phase III study of iniparib (BSI-201) in combination with gemcitabine/carboplatin (G/C) in metastatic triple-negative breast cancer (TNBC) J Clin Oncol. 2011;29(15 suppl) Abstract 1007. [Google Scholar]
- 26.Schneider BP, Miller KD. Angiogenesis of breast cancer. J Clin Oncol. 2005;23:1782–1790. doi: 10.1200/JCO.2005.12.017. [DOI] [PubMed] [Google Scholar]
- 27.Banerjee S, Dowsett M, Ashworth A, et al. Mechanisms of disease: Angiogenesis and the management of breast cancer. Nat Clin Pract Oncol. 2007;4:536–550. doi: 10.1038/ncponc0905. [DOI] [PubMed] [Google Scholar]
- 28.Fox SB, Generali DG, Harris AL. Breast tumour angiogenesis. Breast Cancer Res. 2007;9:216–226. doi: 10.1186/bcr1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407:249–257. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- 30.Bergers G, Benjamin LE. Tumorigenesis and the angiogenic switch. Nat Rev Cancer. 2003;3:401–410. doi: 10.1038/nrc1093. [DOI] [PubMed] [Google Scholar]
- 31.Folkman J. Seminars in medicine of the Beth Israel Hospital, Boston. Clinical applications of research on angiogenesis. N Engl J Med. 1995;333:1757–1763. doi: 10.1056/NEJM199512283332608. [DOI] [PubMed] [Google Scholar]
- 32.Alsina M, Ruiz-Echarri M, Capdevila J, et al. Biomarkers for therapies directed at angiogenesis. Curr Colorectal Cancer Rep. 2010;6:133–143. [Google Scholar]
- 33.Gotink KJ, Verheul HM. Anti-angiogenic tyrosine kinase inhibitors: What is their mechanism of action? Angiogenesis. 2010;13:1–14. doi: 10.1007/s10456-009-9160-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Broekman F, Giovannetti E, Peters GJ. Tyrosine kinase inhibitors: Multi-targeted or single-targeted? World J Clin Oncol. 2011;2:80–93. doi: 10.5306/wjco.v2.i2.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Giamas G, Man YL, Hirner H, et al. Kinases as targets in the treatment of solid tumors. Cell Signal. 2010;22:984–1002. doi: 10.1016/j.cellsig.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 36.Borgstrom P, Hillan KJ, Sriramarao P, et al. Complete inhibition of angiogenesis and growth of microtumors by anti-vascular endothelial growth factor neutralizing antibody: Novel concepts of angiostatic therapy from intravital videomicroscopy. Cancer Res. 1996;56:4032–4039. [PubMed] [Google Scholar]
- 37.Presta LG, Chen H, O'Connor SJ, et al. Humanization of an anti-vascular endothelial growth factor monoclonal antibody for the therapy of solid tumors and other disorders. Cancer Res. 1997;57:4593–4599. [PubMed] [Google Scholar]
- 38.Willett CG, Boucher Y, di Tomaso E, et al. Direct evidence that the VEGF-specific antibody bevacizumab has antivascular effects in human rectal cancer. Nat Med. 2004;10:145–147. doi: 10.1038/nm988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.O'Connor JP, Carano RA, Clamp AR, et al. Quantifying antivascular effects of monoclonal antibodies to vascular endothelial growth factor: Insights from imaging. Clin Cancer Res. 2009;15:6674–6682. doi: 10.1158/1078-0432.CCR-09-0731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miller KD, Chap LI, Holmes FA, et al. Randomized phase III trial of capecitabine compared with bevacizumab plus capecitabine in patients with previously treated metastatic breast cancer. J Clin Oncol. 2005;23:792–799. doi: 10.1200/JCO.2005.05.098. [DOI] [PubMed] [Google Scholar]
- 41.Miller K, Wang M, Gralow J, et al. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med. 2007;357:2666–2676. doi: 10.1056/NEJMoa072113. [DOI] [PubMed] [Google Scholar]
- 42.Miles DW, Chan A, Dirix LY, et al. Phase III study of bevacizumab plus docetaxel compared with placebo plus docetaxel for the first-line treatment of human epidermal growth factor receptor 2-negative metastatic breast cancer. J Clin Oncol. 2010;28:3239–3247. doi: 10.1200/JCO.2008.21.6457. [DOI] [PubMed] [Google Scholar]
- 43.Robert NJ, Dieras V, Glaspy J, et al. RIBBON-1: Randomized, double-blind, placebo-controlled, phase III trial of chemotherapy with or without bevacizumab for first-line treatment of human epidermal growth factor receptor 2-negative, locally recurrent or metastatic breast cancer. J Clin Oncol. 2011;29:1252–1260. doi: 10.1200/JCO.2010.28.0982. [DOI] [PubMed] [Google Scholar]
- 44.Robert NJ, Dieras V, Glaspy J, et al. RIBBON-1: Randomized, double-blind, placebo-controlled, phase III trial of chemotherapy with or without bevacizumab (B) for first-line treatment of HER2-negative locally recurrent or metastatic breast cancer (MBC) J Clin Oncol. 2009;27(15 suppl) doi: 10.1200/JCO.2010.28.0982. Abstract 1005. [DOI] [PubMed] [Google Scholar]
- 45.Brufsky AM, Hurvitz S, Perez E, et al. RIBBON-2: A randomized, double-blind, placebo-controlled, phase III trial evaluating the efficacy and safety of bevacizumab in combination with chemotherapy for second-line treatment of human epidermal growth factor receptor 2-negative metastatic breast cancer. J Clin Oncol. 2011;29:4286–4293. doi: 10.1200/JCO.2010.34.1255. [DOI] [PubMed] [Google Scholar]
- 46.Gianni L, Romieu G, Lichinitser M, et al. First results of AVEREL, a randomized phase III trial to evaluate bevacizumab (BEV) in combination with trastuzumab (H) + docetaxel (DOC) as first-line therapy for HER2-positive locally recurrent/metastatic breast cancer (LR/mBC). Presented at the 34th Annual San Antonio Breast Cancer Symposium; December 6–10, 2011; San Antonio, TX. [Google Scholar]
- 47.Cuppone FBE, Vaccaro V, Puglisi F, et al. Magnitude of risks and benefits of the addition of bevacizumab to chemotherapy for advanced breast cancer patients: Meta-regression analysis of randomized trials. J Exp Clin Cancer Res. 2011;30:54. doi: 10.1186/1756-9966-30-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.O'Shaughnessy J, Miles D, Gray RJ, et al. A meta-analysis of overall survival data from three randomized trials of bevacizumab (BV) and first-line chemotherapy as treatment for patients with metastatic breast cancer (MBC) J Clin Oncol. 2010;28(15 suppl) Abstract 1005. [Google Scholar]
- 49.Cortes J, Calvo V, Ramirez-Merino N, et al. Adverse events risk associated with bevacizumab addition to breast cancer chemotherapy: A meta-analysis. Ann Oncol. 2012;23(5):1130–1137. doi: 10.1093/annonc/mdr432. [DOI] [PubMed] [Google Scholar]
- 50.Lenzer J. FDA committee votes to withdraw bevacizumab for breast cancer. BMJ. 2011;343:d4244. doi: 10.1136/bmj.d4244. [DOI] [PubMed] [Google Scholar]
- 51.Burstein HJ. Bevacizumab for advanced breast cancer: All tied up with a RIBBON? J Clin Oncol. 2011;29:1232–1235. doi: 10.1200/JCO.2010.33.2684. [DOI] [PubMed] [Google Scholar]
- 52.European Medicines Agency. EPAR summary for the public: Avastin. [Accessed November 16, 2011]. Available at: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Summary_for_the_public/human/000582/WC500029260.pdf.
- 53.Brufsky A, Valero V, Tiangco B, et al. Second-line bevacizumab-containing therapy in patients with triple-negative breast cancer: Subgroup analysis of the RIBBON-2 trial. Breast Cancer Res Treat. 2012;133:1067–1075. doi: 10.1007/s10549-012-2008-6. [DOI] [PubMed] [Google Scholar]
- 54.O'Shaughnessy J, Romieu G, Dieras V, et al. Meta-analysis of patients with triple-negative breast cancer (TNBC) from three randomized trials of first-line bevacizumab (BV) and chemotherapy treatment for metastatic breast cancer (MBC) Cancer Res. 2011;70(24 suppl) P6–12-03. [Google Scholar]
- 55.Salvador J, Ciruelos E, Codes dVM, et al. AVALUZ study: First line with bevacizumab in combination with paclitaxel (P) and gemcitabine (G) in patients with HER-2 negative recurrent or metastatic BC: PFS analysis. Cancer Res. 2012;71(24 suppl) P1–14-03. [Google Scholar]
- 56.De la Haba-Rodriguez JR, von MG, Martin M, et al. Phase III trial evaluating the addition of bevacizumab to endocrine therapy as first-line treatment for advanced breast cancer: The LEA study. Cancer Res. 2012;71(24 suppl) doi: 10.1200/JCO.2014.57.2388. OT3–01-15. [DOI] [PubMed] [Google Scholar]
- 57.Lam S, de Groot S, Honkoop A, et al. Combination of paclitaxel and bevacizumab without or with capecitabine as first-line treatment of HER2-negative locally recurrent or metastatic breast cancer (LR/MBC): First results from a randomized, multicenter, open-label, phase II study of the Dutch Breast Cancer Trialists' Group (BOOG) Cancer Res. 2012;71(24 suppl) PD07–07. [Google Scholar]
- 58.Viens P, Petit T, Dalenc F, et al. P4–20-01: Multicentric phase II PACS 09/Beverly1 Trial: First efficacy and safety results of neoadjuvant chemotherapy combined with bevacizumab in HER2-negative patients with non-metastatic inflammatory breast cancer. Cancer Res. 2012;71(24 suppl) P4–20-01. [Google Scholar]
- 59.von Minckwitz G, Eidtmann H, Rezai M, et al. Neoadjuvant chemotherapy and bevacizumab for HER2-negative breast cancer. N Engl J Med. 2012;366:299–309. doi: 10.1056/NEJMoa1111065. [DOI] [PubMed] [Google Scholar]
- 60.Bear HD, Tang G, Rastogi P, et al. Bevacizumab added to neoadjuvant chemotherapy for breast cancer. N Engl J Med. 2012;366:310–320. doi: 10.1056/NEJMoa1111097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pierga JY, Petit T, Delozier T, et al. Neoadjuvant bevacizumab, trastuzumab, and chemotherapy for primary inflammatory HER2-positive breast cancer (BEVERLY-2): An open-label, single-arm phase 2 study. Lancet Oncol. 2012;13:375–384. doi: 10.1016/S1470-2045(12)70049-9. [DOI] [PubMed] [Google Scholar]
- 62.Mayer E, Ligibel J, Burstein H, et al. OT3–02-04: TBCRC 012: ABCDE, a phase II randomized study of adjuvant bevacizumab, metronomic chemotherapy (CM), diet and exercise after preoperative chemotherapy for breast cancer. Cancer Res. 2012;71(24 suppl) OT3–02-04. [Google Scholar]
- 63.Petrelli A, Giordano S. From single- to multi-target drugs in cancer therapy: When aspecificity becomes an advantage. Curr Med Chem. 2008;15:422–432. doi: 10.2174/092986708783503212. [DOI] [PubMed] [Google Scholar]
- 64.Dal Lago L, D'Hondt V, Awada A. Selected combination therapy with sorafenib: A review of clinical data and perspectives in advanced solid tumors. The Oncologist. 2008;13:845–858. doi: 10.1634/theoncologist.2007-0233. [DOI] [PubMed] [Google Scholar]
- 65.Takimoto CH, Awada A. Safety and anti-tumor activity of sorafenib (Nexavar) in combination with other anti-cancer agents: A review of clinical trials. Cancer Chemother Pharmacol. 2008;61:535–548. doi: 10.1007/s00280-007-0639-9. [DOI] [PubMed] [Google Scholar]
- 66.Sweeney CJ, Chiorean EG, Verschraegen CF, et al. A phase I study of sunitinib plus capecitabine in patients with advanced solid tumors. J Clin Oncol. 2010;28:4513–4520. doi: 10.1200/JCO.2009.26.9696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Faivre S, Demetri G, Sargent W, et al. Molecular basis for sunitinib efficacy and future clinical development. Nat Rev Drug Discov. 2007;6:734–745. doi: 10.1038/nrd2380. [DOI] [PubMed] [Google Scholar]
- 68.Kozloff M, Chuang E, Starr A, et al. An exploratory study of sunitinib plus paclitaxel as first-line treatment for patients with advanced breast cancer. Ann Oncol. 2010;21:1436–1441. doi: 10.1093/annonc/mdp565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Robert F, Sandler A, Schiller JH, et al. Sunitinib in combination with docetaxel in patients with advanced solid tumors: A phase I dose-escalation study. Cancer Chemother Pharmacol. 2010;66:669–680. doi: 10.1007/s00280-009-1209-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Barrios CH, Liu MC, Lee SC, et al. Phase III randomized trial of sunitinib versus capecitabine in patients with previously treated HER2-negative advanced breast cancer. Breast Cancer Res Treat. 2010;121:121–131. doi: 10.1007/s10549-010-0788-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bergh J, Bondarenko IM, Lichinitser MR, et al. First-line treatment of advanced breast cancer with sunitinib in combination with docetaxel versus docetaxel alone: Results of a prospective, randomized phase III study. J Clin Oncol. 2012;30:921–929. doi: 10.1200/JCO.2011.35.7376. [DOI] [PubMed] [Google Scholar]
- 72.Crown J, Dieras V, Staroslawska E, et al. Phase III trial of sunitinib (SU) in combination with capecitabine (C) versus C in previously treated advanced breast cancer (ABC) J Clin Oncol. 2010;28(7 suppl) Abstract LBA 1011. [Google Scholar]
- 73.Robert NJ, Saleh MN, Paul D, et al. Sunitinib plus paclitaxel versus bevacizumab plus paclitaxel for first-line treatment of patients with advanced breast cancer: A phase III, randomized, open-label trial. Clin Breast Cancer. 2011;11:82–92. doi: 10.1016/j.clbc.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wilhelm SM, Adnane L, Newell P, et al. Preclinical overview of sorafenib, a multikinase inhibitor that targets both Raf and VEGF and PDGF receptor tyrosine kinase signaling. Mol Cancer Ther. 2008;7:3129–3140. doi: 10.1158/1535-7163.MCT-08-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hasskarl J. Sorafenib: Recent results. Cancer Res. 2010;184:61–70. doi: 10.1007/978-3-642-01222-8_5. [DOI] [PubMed] [Google Scholar]
- 76.Isaacs C, Herbolsheimer P, Liu MC, et al. Phase I/II study of sorafenib with anastrozole in patients with hormone receptor positive aromatase inhibitor resistant metastatic breast cancer. Breast Cancer Res Treat. 2011;125:137–143. doi: 10.1007/s10549-010-1226-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Baselga J, Segalla JG, Roche H, et al. Sorafenib in combination with capecitabine: An oral regimen for patients with HER2-negative locally advanced or metastatic breast cancer. J Clin Oncol. 2012;30(13):1484–1491. doi: 10.1200/JCO.2011.36.7771. [DOI] [PubMed] [Google Scholar]
- 78.Gradishar W, Kaklamani V, Prasad Sahoo T, et al. A double-blind, randomized, placebo-controlled, phase 2b study evaluating the efficacy and safety of sorafenib (SOR) in combination with paclitaxel (PAC) as a first-line therapy in patients (pts) with locally recurrent or metastatic breast cancer (BC). Presented at the 32nd Annual San Antonio Breast Cancer Symposium; December 9–13, 2009; San Antonio, TX. [Google Scholar]
- 79.Bondarde S, Kaklamani V, Prasad Sahoo T, et al. Sorafenib in combination with paclitaxel as a first-line therapy in patients with locally recurrent or metastatic breast cancer: Overall survival results from a double-blind, randomized, placebo-controlled, phase 2b trial. Presented at the 33rd Annual San Antonio Breast Cancer Symposium; December 8–12, 2010; San Antonio, TX. [Google Scholar]
- 80.Hudis C, Tauer KW, Hermann RC, et al. Sorafenib (SOR) plus chemotherapy (CRx) for patients (pts) with advanced (adv) breast cancer (BC) previously treated with bevacizumab (BEV). Presented at the annual meeting of the American Society of Clinical Oncology; June 3–7, 2011; Chicago, IL. [Google Scholar]
- 81.Mariani G, Burdaeva O, Roman L, et al. A double-blind, randomized phase IIb study evaluating the efficacy and safety of sorafenib compared to placebo when administered in combination with docetaxel and/or letrozole in patients with metastatic breast cancer. Presented at the European Multidisciplinary Congress; September 23–27, 2011; Stockholm, Sweden. [Google Scholar]
- 82.Baselga J, Schwartzberg LS, Petrenciuc O, et al. Design of RESILIENCE: A phase (Ph) III trial comparing capecitabine (CAP) in combination with sorafenib (SOR) or placebo (PL) for treatment (tx) of locally advanced (adv) or metastatic HER2-negative breast cancer (BC). Presented at the annual meeting of the American Society of Clinical Oncology; June 3–7, 2011; Chicago, IL. [Google Scholar]
- 83.Houghton PJ. Everolimus. Clin Cancer Res. 2010;16:1368–1372. doi: 10.1158/1078-0432.CCR-09-1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Atkins MB, Yasothan U, Kirkpatrick P. Everolimus. Nat Rev Drug Discov. 2009;8:535–536. doi: 10.1038/nrd2924. [DOI] [PubMed] [Google Scholar]
- 85.Bachelot T, Bourgier C, Cropet C, et al. TAMRAD: A GINECO randomized phase ii trial of everolimus in combination with tamoxifen versus tamoxifen alone in patients (pts) with hormone-receptor positive, HER2 negative metastatic breast cancer (MBC) with prior exposure to aromatase inhibitors (AI) Cancer Res. 2011;70(24 suppl) Abstract S1–6. [Google Scholar]
- 86.Baselga J, Campone M, Piccart M, et al. Everolimus in postmenopausal hormone-receptor–positive advanced breast cancer. N Engl J Med. 2011;366:520–529. doi: 10.1056/NEJMoa1109653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.ClinicalTrials.gov. Everolimus in combination with trastuzumab and paclitaxel in the treatment of HER2 positive locally advanced or metastatic breast cancer (BOLERO-1) [Accessed November 8, 2011]. Available at: http://www.clinicaltrials.gov/show/NCT00876395.
- 88.ClinicalTrials.gov. Daily everolimus in combination with trastuzumab and vinorelbine in HER2/Neu positive women with locally advanced or metastatic breast cancer (BOLERO-3) [Accessed November 8, 2011]. Available at: http://www.clinicaltrials.gov/ct2/show/NCT01007942.
- 89.Morrow PK, Wulf GM, Ensor J, et al. Phase I/II study of trastuzumab in combination with everolimus (RAD001) in patients with HER2-overexpressing metastatic breast cancer who progressed on trastuzumab-based therapy. J Clin Oncol. 2011;29:3126–3132. doi: 10.1200/JCO.2010.32.2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Jerusalem G, Fasolo A, Dieras V, et al. Phase I trial of oral mTOR inhibitor everolimus in combination with trastuzumab and vinorelbine in pre-treated patients with HER2-overexpressing metastatic breast cancer. Breast Cancer Res Treat. 2011;125:447–455. doi: 10.1007/s10549-010-1260-x. [DOI] [PubMed] [Google Scholar]
- 91.Andre F, Campone M, O'Regan R, et al. Phase I study of everolimus plus weekly paclitaxel and trastuzumab in patients with metastatic breast cancer pretreated with trastuzumab. J Clin Oncol. 2010;28:5110–5115. doi: 10.1200/JCO.2009.27.8549. [DOI] [PubMed] [Google Scholar]
- 92.Awada A, Cardoso F, Fontaine C, et al. The oral mTOR inhibitor RAD001 (everolimus) in combination with letrozole in patients with advanced breast cancer: Results of a phase I study with pharmacokinetics. Eur J Cancer. 2008;44:84–91. doi: 10.1016/j.ejca.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 93.Dalenc F, Campone M, Hupperets P, et al. Everolimus in combination with weekly paclitaxel and trastuzumab in patients (pts) with HER2-overexpressing metastatic breast cancer (MBC) with prior resistance to trastuzumab and taxanes: A multicenter phase II clinical trial. J Clin Oncol. 2010;28(15 suppl) Abstract 1013. [Google Scholar]
- 94.Rugo HS, Stopeck AT, Joy AA, et al. Randomized, placebo-controlled, double-blind, phase II study of axitinib plus docetaxel versus docetaxel plus placebo in patients with metastatic breast cancer. J Clin Oncol. 2011;29:2459–2465. doi: 10.1200/JCO.2010.31.2975. [DOI] [PubMed] [Google Scholar]
- 95.Martin M, Roche H, Pinter T, et al. Motesanib, or open-label bevacizumab, in combination with paclitaxel, as first-line treatment for HER2-negative locally recurrent or metastatic breast cancer: A phase 2, randomised, double-blind, placebo-controlled study. Lancet Oncol. 2011;12:369–376. doi: 10.1016/S1470-2045(11)70037-7. [DOI] [PubMed] [Google Scholar]
- 96.Taylor SK, Chia S, Dent S, et al. A phase II study of pazopanib in patients with recurrent or metastatic invasive breast carcinoma: A trial of the Princess Margaret Hospital Phase II Consortium. The Oncologist. 2010;15:810–818. doi: 10.1634/theoncologist.2010-0081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Slamon D, Gomez H, Kabbinavar FF, Amit O, et al. Randomized study of pazopanib + lapatinib vs. lapatinib alone in patients with HER2-positive advanced or metastatic breast cancer. J Clin Oncol. 2008;26(15 suppl) Abstract 1016. [Google Scholar]
- 98.Miller KD, Trigo JM, Wheeler C, et al. A multicenter phase II trial of ZD6474, a vascular endothelial growth factor receptor-2 and epidermal growth factor receptor tyrosine kinase inhibitor, in patients with previously treated metastatic breast cancer. Clin Cancer Res. 2005;11:3369–3376. doi: 10.1158/1078-0432.CCR-04-1923. [DOI] [PubMed] [Google Scholar]
- 99.Boer K, Lang I, Llombart-Cussac A, et al. Vandetanib with docetaxel as second-line treatment for advanced breast cancer: A double-blind, placebo-controlled, randomized phase II study. Invest New Drugs. 2010;30:681–7. doi: 10.1007/s10637-010-9538-8. [DOI] [PubMed] [Google Scholar]
- 100.Hyams D, de Oliveira C, Snyder R, et al. Cediranib in combination with fulvestrant in hormone-sensitive metastatic breast cancer: a phase II randomized study. Clin Cancer Res. 2009;69(24 suppl) doi: 10.1007/s10637-013-9991-2. Abstract 204. [DOI] [PubMed] [Google Scholar]
- 101.Andre F, Bachelot TD, Campone M, et al. A multicenter, open-label phase II trial of dovitinib, a fibroblast growth factor receptor 1 (FGFR1) inhibitor, in FGFR1-amplified and nonamplified metastatic breast cancer (BC). Presented at the annual meeting of the American Society of Clinical Oncology; June 3–7, 2011; Chicago, IL. [Google Scholar]
- 102.Tolaney S, Nechushtan H, Berger R, et al. P1–17-10: Cabozantinib (XL184) in patients with metastatic breast cancer: Results from a phase 2 randomized discontinuation trial. Cancer Res. 2012;71(24 suppl) P1-17-10. [Google Scholar]
- 103.Schilsky R, Geary D, Skoog L, et al. Phase I and pharmacokinetic study of vatalanib plus capecitabine in patients with advanced cancer. Targeted Oncol. 2008;3:3–11. [Google Scholar]
- 104.Chiorean EG, Malireddy S, Younger AE, et al. A phase I dose escalation and pharmacokinetic study of vatalanib (PTK787/ZK 222584) in combination with paclitaxel in patients with advanced solid tumors. Cancer Chemother Pharmacol. 2010;66:441–448. doi: 10.1007/s00280-009-1179-2. [DOI] [PubMed] [Google Scholar]
- 105.Eder JP, Shapiro GI, Appleman LJ, et al. A phase I study of foretinib, a multi-targeted inhibitor of c-Met and vascular endothelial growth factor receptor 2. Clin Cancer Res. 2010;16:3507–3516. doi: 10.1158/1078-0432.CCR-10-0574. [DOI] [PubMed] [Google Scholar]
- 106.Mackey J, Gelmon K, Martin M, et al. TRIO-012: A multicenter, multinational, randomized, double-blind phase III study of IMC-1121B plus docetaxel versus placebo plus docetaxel in previously untreated patients with HER2-negative, unresectable, locally recurrent or metastatic breast cancer. Clin Breast Cancer. 2009;9:258–261. doi: 10.3816/CBC.2009.n.044. [DOI] [PubMed] [Google Scholar]
- 107.Spratlin JL, Cohen RB, Eadens M, et al. Phase I pharmacologic and biologic study of ramucirumab (IMC-1121B), a fully human immunoglobulin G1 monoclonal antibody targeting the vascular endothelial growth factor receptor-2. J Clin Oncol. 2010;28:780–787. doi: 10.1200/JCO.2009.23.7537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Vahdat LT, Miller K, Sparano JA, et al. Randomized phase II study of capecitabine with or without ramucirumab (IMC-1121B) or IMC-18F1 in patients with unresectable, locally advanced or metastatic breast cancer (mBC) previously treated with anthracycline and taxane therapy (CP20–0903/ NCT01234402) J Clin Oncol. 2011;29(15 suppl) Abstract TPS151. [Google Scholar]
- 109.Isambert N, Freyer G, Zanetta S, et al. Phase I dose-escalation study of intravenous aflibercept in combination with docetaxel in patients with advanced solid tumors. Clin Cancer Res. 2012;18:1743–1750. doi: 10.1158/1078-0432.CCR-11-1918. [DOI] [PubMed] [Google Scholar]
- 110.Dieras V, Jassem J, Dirix LY, et al. A randomized, placebo-controlled phase II study of AMG 386 plus bevacizumab (Bev) and paclitaxel (P) or AMG 386 plus P as first-line therapy in patients (pts) with HER2-negative, locally recurrent or metastatic breast cancer (LR/MBC) J Clin Oncol. 2011;29(15 suppl) Abstract 544. [Google Scholar]
- 111.Fojo T, Wilkerson J. Bevacizumab and breast cancer: The E2100 outlier. Lancet Oncol. 2010;11:1117–1119. doi: 10.1016/S1470-2045(10)70259-X. [DOI] [PubMed] [Google Scholar]
- 112.Kerbel RS. Reappraising antiangiogenic therapy for breast cancer. Breast. 2011;20:S56–S60. doi: 10.1016/S0960-9776(11)70295-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Carpenter D, Kesselheim AS, Joffe S. Reputation and precedent in the bevacizumab decision. N Engl J Med. 2011;365:e3. doi: 10.1056/NEJMp1107201. [DOI] [PubMed] [Google Scholar]
- 114.Sekeres MA. The Avastin story. N Engl J Med. 2011;365:1454–1455. doi: 10.1056/NEJMc1109550. [DOI] [PubMed] [Google Scholar]
- 115.Pazdur R. Endpoints for assessing drug activity in clinical trials. The Oncologist. 2008;13:19–21. doi: 10.1634/theoncologist.13-S2-19. [DOI] [PubMed] [Google Scholar]
- 116.Saad ED, Katz A, Hoff PM, et al. Progression-free survival as surrogate and as true end point: Insights from the breast and colorectal cancer literature. Ann Oncol. 2010;21:7–12. doi: 10.1093/annonc/mdp523. [DOI] [PubMed] [Google Scholar]
- 117.Dodd LE, Korn EL, Freidlin B, et al. Blinded independent central review of progression-free survival in phase III clinical trials: Important design element or unnecessary expense? J Clin Oncol. 2008;26:3791–3796. doi: 10.1200/JCO.2008.16.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.D'Agostino RB., Sr Changing end points in breast-cancer drug approval:the Avastin story. N Engl J Med. 2011;365:e2. doi: 10.1056/NEJMp1106984. [DOI] [PubMed] [Google Scholar]
- 119.Mancuso MR, Davis R, Norberg SM, et al. Rapid vascular regrowth in tumors after reversal of VEGF inhibition. J Clin Invest. 2006;116:2610–2621. doi: 10.1172/JCI24612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ebos JM, Lee CR, Cruz-Munoz W, et al. Accelerated metastasis after short-term treatment with a potent inhibitor of tumor angiogenesis. Cancer Cell. 2009;15:232–239. doi: 10.1016/j.ccr.2009.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Paez-Ribes M, Allen E, Hudock J, et al. Antiangiogenic therapy elicits malignant progression of tumors to increased local invasion and distant metastasis. Cancer Cell. 2009;15:220–231. doi: 10.1016/j.ccr.2009.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ebos JM, Kerbel RS. Antiangiogenic therapy: Impact on invasion, disease progression, and metastasis. Nat Rev Clin Oncol. 2011;8:210–221. doi: 10.1038/nrclinonc.2011.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Miles D, Harbeck N, Escudier B, et al. Disease course patterns after discontinuation of bevacizumab: Pooled analysis of randomized phase III trials. J Clin Oncol. 2011;29:83–88. doi: 10.1200/JCO.2010.30.2794. [DOI] [PubMed] [Google Scholar]
- 124.Allegra CJ, Yothers G, O'Connell MJ, et al. Phase III trial assessing bevacizumab in stages II and III carcinoma of the colon: Results of NSABP protocol C-08. J Clin Oncol. 2011;29:11–16. doi: 10.1200/JCO.2010.30.0855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Schneider BP, Sledge GW., Jr Anti-vascular endothelial growth factor therapy for breast cancer: Can we pick the winners? J Clin Oncol. 2011;29:2444–2447. doi: 10.1200/JCO.2011.34.9266. [DOI] [PubMed] [Google Scholar]
- 126.Chustecka Z. Avastin for breast cancer: FDA answer is still no. [Accessed November 20, 2011]. Available at: http://www.medscape.com/viewarticle/745590.
- 127.Schneider BP, Wang M, Radovich M, et al. Association of vascular endothelial growth factor and vascular endothelial growth factor receptor-2 genetic polymorphisms with outcome in a trial of paclitaxel compared with paclitaxel plus bevacizumab in advanced breast cancer: ECOG 2100. J Clin Oncol. 2008;26:4672–4678. doi: 10.1200/JCO.2008.16.1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Vidal M, Di Cosimo S, Torrejon D, et al. Survival outcome with bevacizumab: Activation of the phosphatidylinositol-3 kinase (PI3K) pathway due to PIK3CA mutations or PTEN loss makes a difference. Cancer Res. 2012;71(24 suppl) Abstract P5–13-01. [Google Scholar]
- 129.Rini BI, Garcia JA, Cooney MM, et al. A phase I study of sunitinib plus bevacizumab in advanced solid tumors. Clin Cancer Res. 2009;15:6277–6283. doi: 10.1158/1078-0432.CCR-09-0717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Feldman DR, Baum MS, Ginsberg MS, et al. Phase I trial of bevacizumab plus escalated doses of sunitinib in patients with metastatic renal cell carcinoma. J Clin Oncol. 2009;27:1432–1439. doi: 10.1200/JCO.2008.19.0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Azad NS, Posadas EM, Kwitkowski VE, et al. Combination targeted therapy with sorafenib and bevacizumab results in enhanced toxicity and antitumor activity. J Clin Oncol. 2008;26:3709–3714. doi: 10.1200/JCO.2007.10.8332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Rini BI, Michaelson MD, Rosenberg JE, et al. Antitumor activity and biomarker analysis of sunitinib in patients with bevacizumab-refractory metastatic renal cell carcinoma. J Clin Oncol. 2008;26:3743–3748. doi: 10.1200/JCO.2007.15.5416. [DOI] [PubMed] [Google Scholar]
- 133.Tamaskar I, Garcia JA, Elson P, et al. Antitumor effects of sunitinib or sorafenib in patients with metastatic renal cell carcinoma who received prior antiangiogenic therapy. J Urol. 2008;179:81–86. doi: 10.1016/j.juro.2007.08.127. [DOI] [PubMed] [Google Scholar]