Abstract
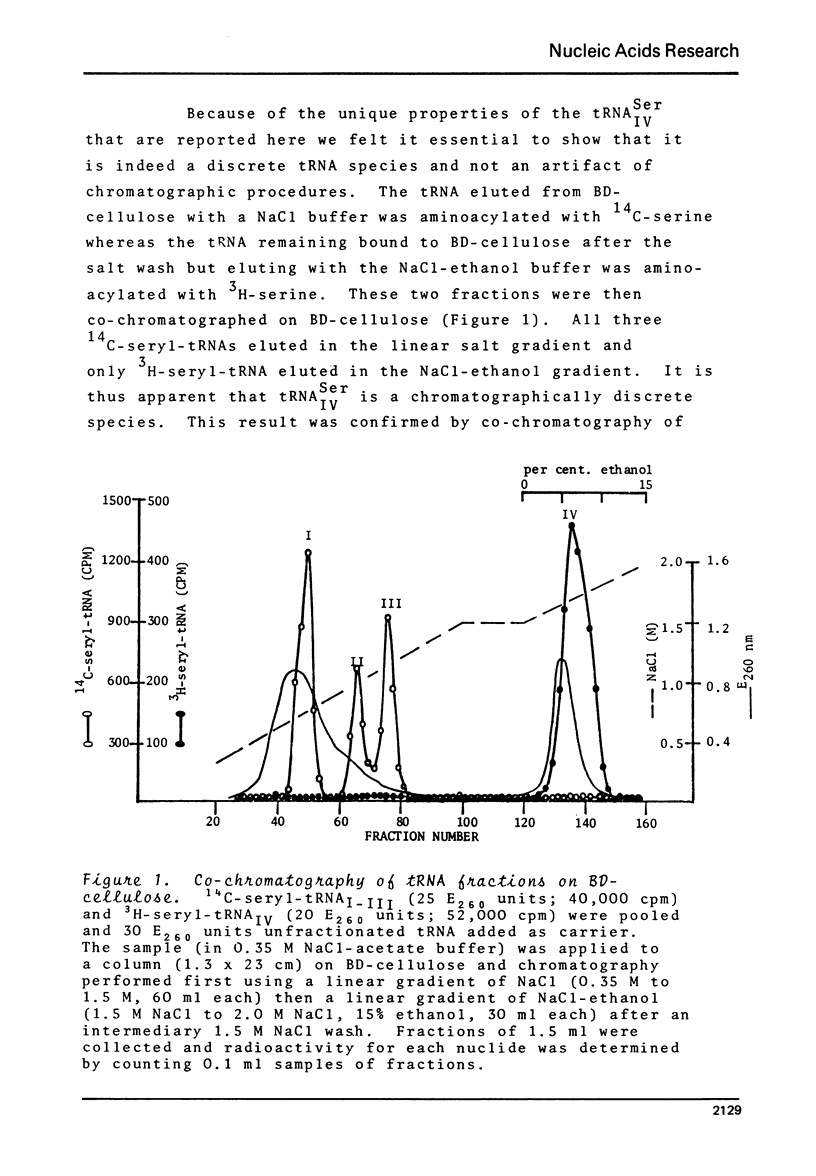
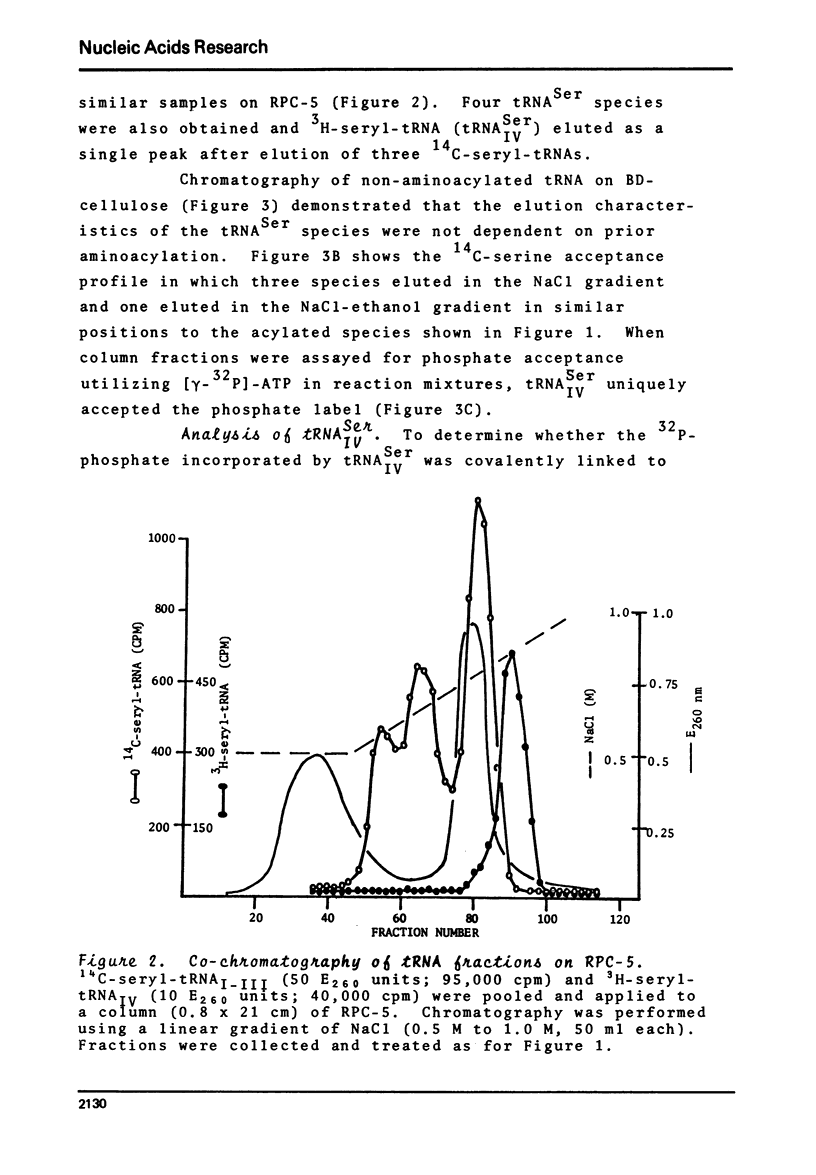
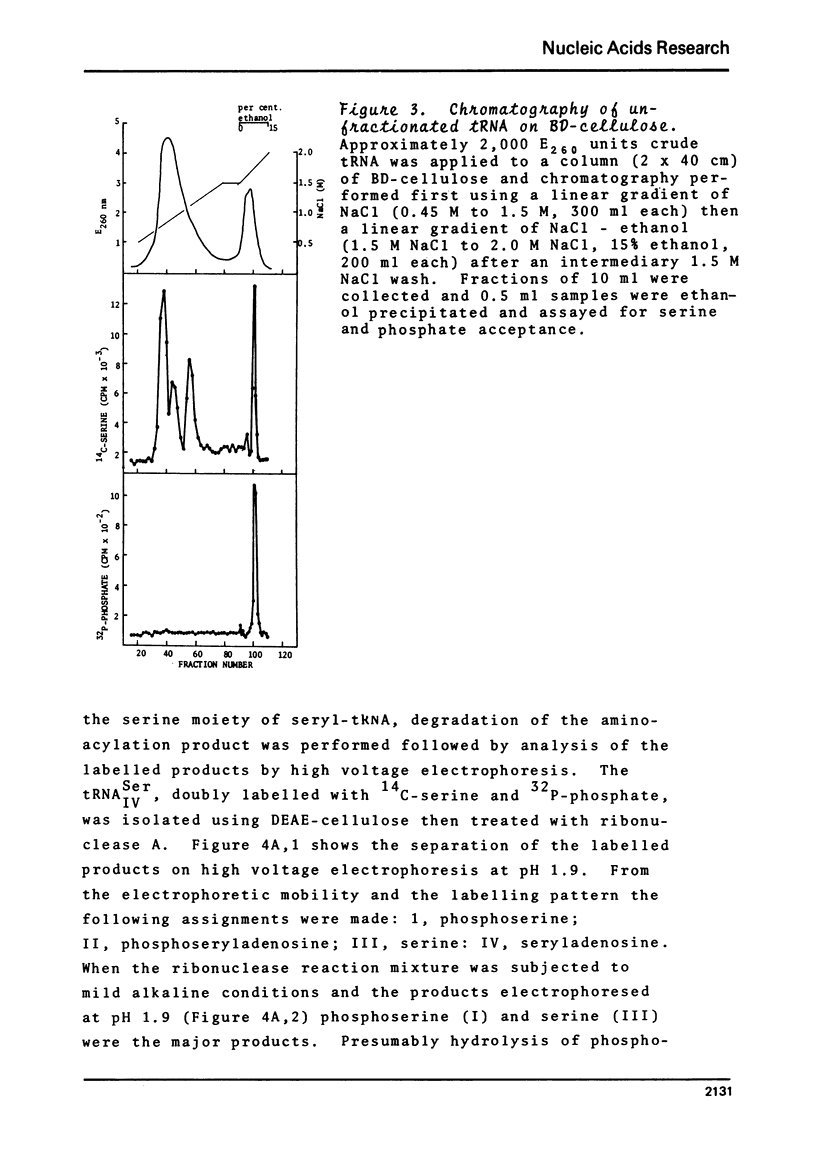
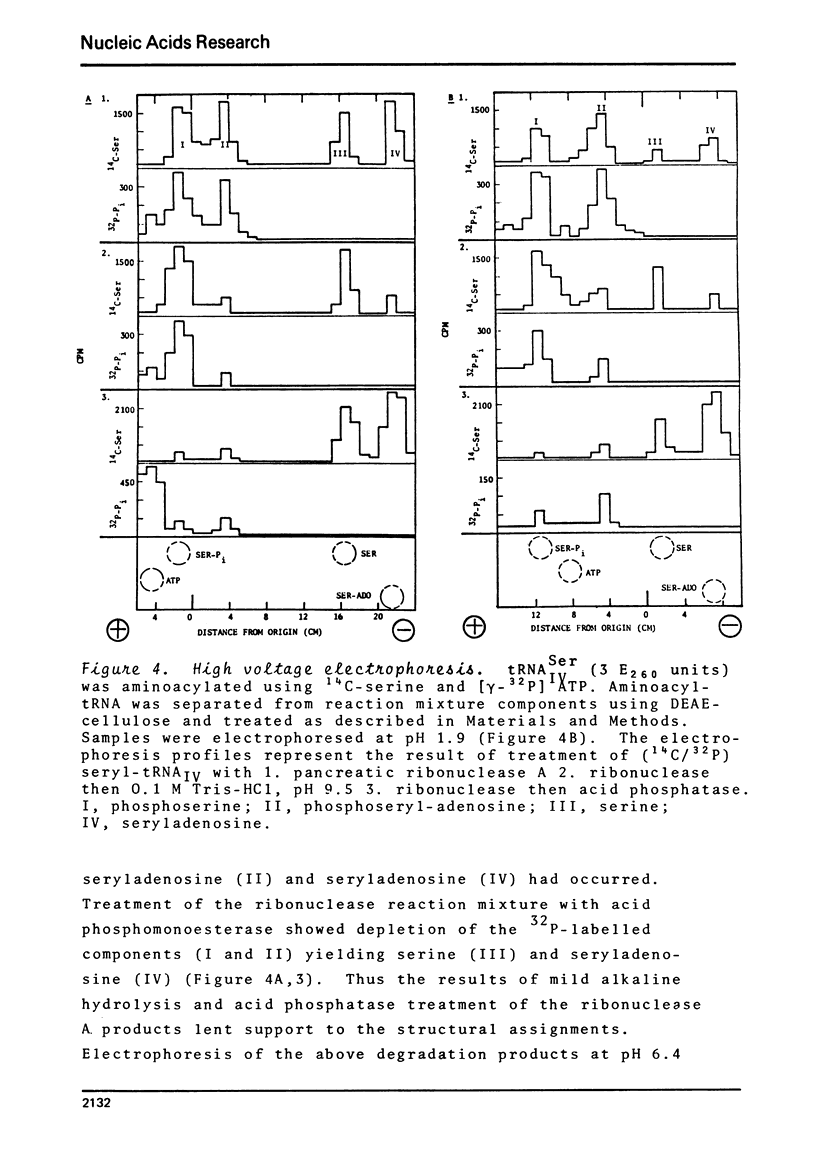
BD-cellulose and RPC-5 chromatography of tRNA isolated from lactating bovine mammary gland showed the presence of four seryl-tRNA isoacceptors. The species, tRNA IV Ser, with the strongest affinity for BD-cellulose (required ethanol in the elution buffer) could be phosphorylated in the presence of serine, [gamma-32 P]-ATP, seryl-tRNA synthetase and phosphotransferase activity from the same tissue. O-Phosphoserine was identified as the 32P-labelled product after mild alkaline hydrolysis of this aminoacylated tRNA. Pancreatic ribonuclease treatment of the aminoacylated tRNA yielded a labelled product which was identified as phosphoseryladenosine. These results indicated there is a specific phosphoseryl tRNA species in lactating bovine mammary gland. It appears that the formation of phosphoseryl-tRNA proceeds by enzymic phosphorylation of seryl-tRNA.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bingham E. W., Farrel H. M., Jr Casein kinase from the Golgi apparatus of lactating mammary gland. J Biol Chem. 1974 Jun 10;249(11):3647–3651. [PubMed] [Google Scholar]
- Bingham E. W., Farrell H. M., Jr, Basch J. J. Phosphorylation of casein. Role of the golgi apparatus. J Biol Chem. 1972 Dec 25;247(24):8193–8194. [PubMed] [Google Scholar]
- Bridgers W. F. Purification of mouse brain phosphoserine phosphohydrolase and phosphotransferase. Arch Biochem Biophys. 1969 Sep;133(2):201–207. doi: 10.1016/0003-9861(69)90446-9. [DOI] [PubMed] [Google Scholar]
- CARLSEN E. N., TRELLE G. J., SCHJEIDE O. A. TRANSFER RIBONUCLEIC ACIDS. Nature. 1964 Jun 6;202:984–986. doi: 10.1038/202984a0. [DOI] [PubMed] [Google Scholar]
- Chew L. F., Mackinlay A. G. Histone and casein kinases of lactating bovine mammary gland. Biochim Biophys Acta. 1974 Jul 7;359(1):73–82. doi: 10.1016/0005-2795(74)90133-0. [DOI] [PubMed] [Google Scholar]
- Gillam I., Millward S., Blew D., von Tigerstrom M., Wimmer E., Tener G. M. The separation of soluble ribonucleic acids on benzoylated diethylaminoethylcellulose. Biochemistry. 1967 Oct;6(10):3043–3056. doi: 10.1021/bi00862a011. [DOI] [PubMed] [Google Scholar]
- Glynn I. M., Chappell J. B. A simple method for the preparation of 32-P-labelled adenosine triphosphate of high specific activity. Biochem J. 1964 Jan;90(1):147–149. doi: 10.1042/bj0900147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould R. M., Thornton M. P., Liepkalns V., Lennarz W. J. Participation of aminoacyl transfer ribonucleic acid in aminoacyl phosphatidylglycerol synthesis. II. Specificity of alanyl phosphatidylglycerol synthetase. J Biol Chem. 1968 Jun 10;243(11):3096–3104. [PubMed] [Google Scholar]
- Kelmers A. D., Heatherly D. E. Columns for rapid chromatographic separation of small amounts of tracer-labeled transfer ribonucleic acids. Anal Biochem. 1971 Dec;44(2):486–495. doi: 10.1016/0003-2697(71)90236-3. [DOI] [PubMed] [Google Scholar]
- Mercier J. C., Grosclaude F., Ribadeau-Dumas B. Structure primaire de la caséine s1 -bovine. Séquence complète. Eur J Biochem. 1971 Nov 11;23(1):41–51. doi: 10.1111/j.1432-1033.1971.tb01590.x. [DOI] [PubMed] [Google Scholar]
- Mäenpä P. H., Bernfield M. R. A specific hepatic transfer RNA for phosphoserine. Proc Natl Acad Sci U S A. 1970 Oct;67(2):688–695. doi: 10.1073/pnas.67.2.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mäenpä P. H., Bernfield M. R. Quantitative variation in serine transfer ribonucleic acid during estrogen-induced phosphoprotein synthesis in rooster liver. Biochemistry. 1969 Dec;8(12):4926–4935. doi: 10.1021/bi00840a041. [DOI] [PubMed] [Google Scholar]
- Mäenpä P. H. Seryl transfer RNA alterations during estrogen-induced phosvitin synthesis. Quantitative assay of the hormone-responding species by ribosomal binding. Biochem Biophys Res Commun. 1972 May 26;47(4):971–974. doi: 10.1016/0006-291x(72)90588-8. [DOI] [PubMed] [Google Scholar]
- Nesbitt J. A., 3rd, Lennarz W. J. Participation of aminoacyl transfer ribonucleic acid in aminoacyl phosphatidylglycerol synthesis. I. Specificity of lysyl phosphatidylglycerol synthetase. J Biol Chem. 1968 Jun 10;243(11):3088–3095. [PubMed] [Google Scholar]
- Paoli A., Guiraud P., Brunel C. O-phosphoserine phosphatase from bovine brain and kidney. High molecular weight forms occurring during the purification. Biochim Biophys Acta. 1974 Dec 29;370(2):487–497. doi: 10.1016/0005-2744(74)90110-7. [DOI] [PubMed] [Google Scholar]
- Patton S., Keenan T. W. The milk fat globule membrane. Biochim Biophys Acta. 1975 Oct 31;415(3):273–309. doi: 10.1016/0304-4157(75)90011-8. [DOI] [PubMed] [Google Scholar]
- Ribadeau Dumas B., Brignon G., Grosclaude F., Mercier J. C. Structure primaire de la caséine beta bovine. Séquence complète. Eur J Biochem. 1972 Feb;25(3):505–514. doi: 10.1111/j.1432-1033.1972.tb01722.x. [DOI] [PubMed] [Google Scholar]
- Rosenberg E., Elson D. The formation of hydroxypyruvyl-tRNA. FEBS Lett. 1969 Aug;4(3):222–226. doi: 10.1016/0014-5793(69)80240-1. [DOI] [PubMed] [Google Scholar]
- Schirm J., Gruber M., Ab G. Post-translational phosphorylation of phosvitin. FEBS Lett. 1973 Mar 1;30(2):167–169. doi: 10.1016/0014-5793(73)80643-x. [DOI] [PubMed] [Google Scholar]
- Stewart T. S., Roberts R. J., Strominger J. L. Novel species of tRNA. Nature. 1971 Mar 5;230(5288):36–38. doi: 10.1038/230036a0. [DOI] [PubMed] [Google Scholar]
- Turkington R. W., Topper Y. J. Casein biosynthesis: evidence for phosphorylation of precursor proteins. Biochim Biophys Acta. 1966 Oct 31;127(2):366–372. doi: 10.1016/0304-4165(66)90391-6. [DOI] [PubMed] [Google Scholar]
- Wilcox M., Nirenberg M. Transfer RNA as a cofactor coupling amino acid synthesis with that of protein. Proc Natl Acad Sci U S A. 1968 Sep;61(1):229–236. doi: 10.1073/pnas.61.1.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W. K., Novelli G. D. Isoaccepting +RNA's in mouse plasma cell tumors that synthesize different myeloma protein. Biochem Biophys Res Commun. 1968 May 23;31(4):534–539. doi: 10.1016/0006-291x(68)90510-x. [DOI] [PubMed] [Google Scholar]



