The safety, efficacy, and potential biomarkers of activity of bevacizumab in patients with advanced hepatocellular carcinoma were assessed. Bevacizumab was active and well tolerated. The clinical value of circulating endothelial cells and interleukin-6 and -8 warrants further investigation.
Keywords: Hepatocellular carcinoma, Bevacizumab, Circulating endothelial cells, Prognosis, Biomarker
Abstract
Objective.
Hepatocellular carcinoma (HCC) is a highly vascularized tumor in which neoangiogenesis contributes to growth and metastasis. We assessed the safety, efficacy, and potential biomarkers of activity of bevacizumab in patients with advanced HCC.
Methods.
In this phase II trial, eligible patients received bevacizumab, 5 mg/kg or 10 mg/kg every 2 weeks. The disease-control rate at 16 weeks (16W-DCR) was the primary endpoint. Circulating endothelial cells (CECs) and plasma cytokines and angiogenic factors (CAFs) were measured at baseline and throughout treatment.
Results.
The 16W-DCR was 42% (95% confidence interval, 27%–57%). Six of the 43 patients who received bevacizumab achieved a partial response (objective response rate [ORR], 14%). Grade 3–4 asthenia, hemorrhage, and aminotransferase elevation occurred in five (12%), three (7%), and three (7%) patients, respectively. During treatment, placental growth factor markedly increased, whereas vascular endothelial growth factor (VEGF)-A dramatically decreased (p < .0001); soluble VEGF receptor-2 (p < .0001) and CECs (p = .03) transiently increased on day 3. High and increased CEC counts at day 15 were associated with the ORR (p = .04) and the 16W-DCR (p = .02), respectively. Lower interleukin (IL)-8 levels at baseline (p = .01) and throughout treatment (p ≤ .04) were associated with the 16W-DCR. High baseline IL-8 and IL-6 levels predicted shorter progression-free and overall survival times (p ≤ .04).
Conclusion.
Bevacizumab is active and well tolerated in patients with advanced HCC. The clinical value of CECs, IL-6, and IL-8 warrants further investigation.
Introduction
Hepatocellular carcinoma (HCC) is a major cause of cancer-related death, causing an estimated 42,600 deaths in the European Union [1]. Approximately 70% of cases are diagnosed at an advanced stage, not amenable to curative-intent treatments [2]. Systemic chemotherapy has been of limited success in the treatment of patients with advanced HCC, with no studies demonstrating a substantial benefit for single-agent or combination regimens [3]. HCC is a highly vascularized tumor, and aberrant expression of vascular endothelial growth factor (VEGF), the key mediator of tumor angiogenesis, has been implicated in its development and progression [4]. Tumor expression of VEGF correlates with vascular density, tumor invasiveness, and prognosis in patients with HCC [5]. The VEGF pathway therefore represents a promising therapeutic target in this disease. Sorafenib, a tyrosine kinase inhibitor (TKI) of VEGF receptor (VEGFR), platelet-derived growth factor receptor-β, and Raf, was shown to result in a superior disease-control rate (DCR) and overall survival (OS) outcome in patients with advanced HCC in two large randomized phase III trials, leading to its approval as the standard treatment in the first-line setting [6, 7].
Bevacizumab, a humanized recombinant monoclonal antibody that binds all isoforms of circulating VEGF-A, the main ligand of VEGFR, is approved for the treatment of several advanced solid tumors [8–10]. Preclinical studies demonstrated its activity in HCC, extending the time to progression of HCC xenografts in mouse models and significantly decreasing microvessel density [11]. The present study was undertaken to assess the efficacy and tolerability of bevacizumab monotherapy in patients with advanced HCC. Because no reliable biomarkers predicting the antitumor activity of bevacizumab have been validated so far, we took advantage of this single-agent phase II study to assess circulating endothelial cells (CECs) and plasma cytokines and angiogenic factors (CAFs) as potential biomarkers of bevacizumab activity.
Methods
Patient Selection
Patients were eligible if they had histologically confirmed advanced HCC that was not amenable to curative-intent therapies (e.g., resection, liver transplantation, or percutaneous ablation). The main inclusion criteria were: measurable disease according to the Response Evaluation Criteria in Solid Tumors (RECIST, version 1.0) [12], age ≥18 years, a World Health Organization performance status (WHO PS) score ≤2, Child-Pugh class A or compensated class B (≤7) liver cirrhosis (if any), Cancer of the Liver Italian Program (CLIP) score ≤3 [13], hemoglobin ≥8 g/dL, platelet count ≥75,000/mm3 adequate liver function with bilirubin ≤2× the upper limit of normal, and a prothrombin time within the normal range. One prior systemic therapy regimen (excluding antiangiogenic agents) and prior transarterial chemoembolization (TACE) were permitted. All patients provided written informed consent. Upper gastrointestinal endoscopy was required within 6 months of study entry. Propranolol had to be prescribed in cases of medium or large gastroesophageal varices (GEV). In cases of red wale marks, systematic preventive banding had to be performed before inclusion.
Exclusion criteria were: decompensated liver cirrhosis (Child–Pugh score >7), full-dose anticoagulation or platelet-inhibitory therapy, major surgical procedure in the 28 days prior to study entry, variceal bleeding in the previous 3 months, thromboembolic event or clinically significant cardiovascular disease (congestive heart failure, uncontrolled arterial hypertension, unstable angina, myocardial infarction, or severe cardiac arrhythmia) in the previous 6 months, previous or current other malignancies, and concomitant antitumor treatment including tamoxifen or somatostatin analogs.
Study Objectives
This study was designed as a single-center, nonrandomized, phase II study. The primary endpoint was the DCR at 16 weeks (16W-DCR), defined as the proportion of patients with a complete response, partial response (PR), or stable disease (SD) at 16 weeks after study entry, according to the RECIST (version 1.0). Secondary endpoints included a safety assessment, the objective response rate (ORR), the progression-free survival (PFS) interval, and the OS time. Exploratory objectives consisted of assessment of the associations between potential biomarkers of activity of bevacizumab and patient outcome. The study was approved by our institutional review board and an independent ethics committee.
Study Treatment
Bevacizumab, 5 mg/kg or 10 mg/kg, was administered as a 90-minute i.v. infusion every 2 weeks (see statistical analyses for details) until progressive disease (PD) or unacceptable toxicity. No dose reductions or escalations were allowed.
Safety
Patients were required to have undergone upper gastrointestinal endoscopy within 6 months of study entry. Propranolol was prescribed in cases of of medium or large GEV. A medical history, physical examination, WHO PS assessment, adverse event assessment, and measurement of biochemical and hematologic parameters were performed at baseline and before each treatment administration. Adverse events were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (version 3.0).
Tumor Assessments
Tumor assessments were performed at screening then every 8 weeks using the same imaging modality (three-phase computed tomography or magnetic resonance imaging), according to the RECIST (version 1.0). All tumor measurements were performed by the investigator. At the end of the study, tumor measurements were reviewed by an independent radiologist in our center who was blinded to the clinical and biological data.
Evaluation of Biomarkers
Peripheral blood sampling was performed at baseline before treatment initiation, then on day 3, day 15, and day 60 after the first administration of bevacizumab. After discarding the first 2 mL following venupunction, blood (3 mL) was collected in Cellsave™ tubes (Immunicon, Huttingdon Valley, PA), and CECs (CD45−CD31+CD146+ 7-amino-actinomycin-D [AAD]− cells) were measured in 1 mL whole blood by four-color flow cytometry according to a method we previously established [14]. Immunofluorescent staining was performed with CD31 fluorescein isothiocyanate (BD Pharmingen, Franklin Lakes, NJ), CD146 phycoerythrin (BD Pharmingen), and CD45 allophycocyanin (DakoCytomation, Glostrup, Denmark). Using this method, we previously found that the median CEC levels were 6.5/mL (range, 0–15/mL) in healthy adults and 16.0/mL (range, 0–179/mL) in patients with metastatic carcinoma of various types (p < .001) [14].
For CAF measurements, whole blood (10 mL) was collected in heparin tubes. Plasma levels of matrix metalloproteinase (MMP)-2, MMP-9, VEGF-A, soluble VEGFR-2 (sVEGFR-2), interleukin (IL)-6, IL-8, placental growth factor (PlGF), stromal derived factor (SDF)-1α, and tumor necrosis factor (TNF)-α were determined using commercial enzyme-linked immunosorbent assay (ELISA) kits (R&D Systems, Minneapolis, MN). Plasma samples were assayed in duplicate. Optical density values were considered significant if found to be at least twice as high as the background noise.
Statistical Analysis
Patients were enrolled using a two-stage Fleming design [15]. In stage 1, 25 patients had to be treated with bevacizumab at a dose of 5 mg/kg. If disease control at 16 weeks was observed in <11 patients, enrollment at that dose had to be stopped and 25 further patients had to be enrolled within stage 2 and treated with bevacizumab at a dose of 10 mg/kg. Otherwise, the next 25 patients enrolled in stage 2 were planned to receive 5 mg/kg bevacizumab. Thus, a maximum of 50 patients had to be included in two steps, giving a power of 0.96 (96% chance of demonstrating efficacy if the 16W-DCR was ≥65%). The α risk (one sided) was 0.03 (3% chance of demonstrating efficacy if the 16W-DCR was ≤40%).
Safety and activity analyses included all patients receiving at least one dose of bevacizumab. The 16W-DCR and ORR were reported with their 95% confidence intervals (CIs). PFS and OS curves were calculated using the Kaplan–Meier method.
Nonparametric tests (Wilcoxon or Kruskal–Wallis tests as appropriate) were used to compare biomarker values at baseline, day 3, day 15, and day 60, as well as changes from baseline to day, day 15, and day 60, according to patient prognostic characteristics (WHO PS score, Barcelona Clinic Liver Cancer [BCLC] classification [2], and CLIP score) and outcome (16W-DCR, ORR, and PFS and OS times). The log-rank test was used to compare survival curves.
The trial is registered in ClinicalTrials.gov (identifier, NCT00162669).
Role of the Funding Source
The funding source had no role in the initiation and design of the study, data collection, analysis, and interpretation, writing of the report, or the decision to submit for publication. The funding source did not have access to the raw data. The corresponding authors had full access to all data and the final responsibility to submit the manuscript for publication.
Results
Patient Population
Accrual was stopped before the inclusion of 50 patients because of the approval of sorafenib for the first-line systemic treatment of patients with advanced HCC. Of the 48 patients enrolled in May 2005 to December 2007, 43 received at least one dose of bevacizumab. Five patients did not receive treatment because of rapid liver failure (three patients), early disease progression (one patient), and stroke (one patient). Those five patients died between 23 and 74 days after registration and were excluded from all analyses.
More than half of the patients had extrahepatic metastases. Sixteen patients (37%) had a CLIP score ≥3, including two patients and one patient with CLIP scores of 4 and 5, respectively. Those three patients, although ineligible, were included in the analysis. Thirty-nine patients (91%) were classified as BCLC stage C. Half of the patients received one or more prior treatments (Table 1).
Table 1.
Patient baseline characteristics
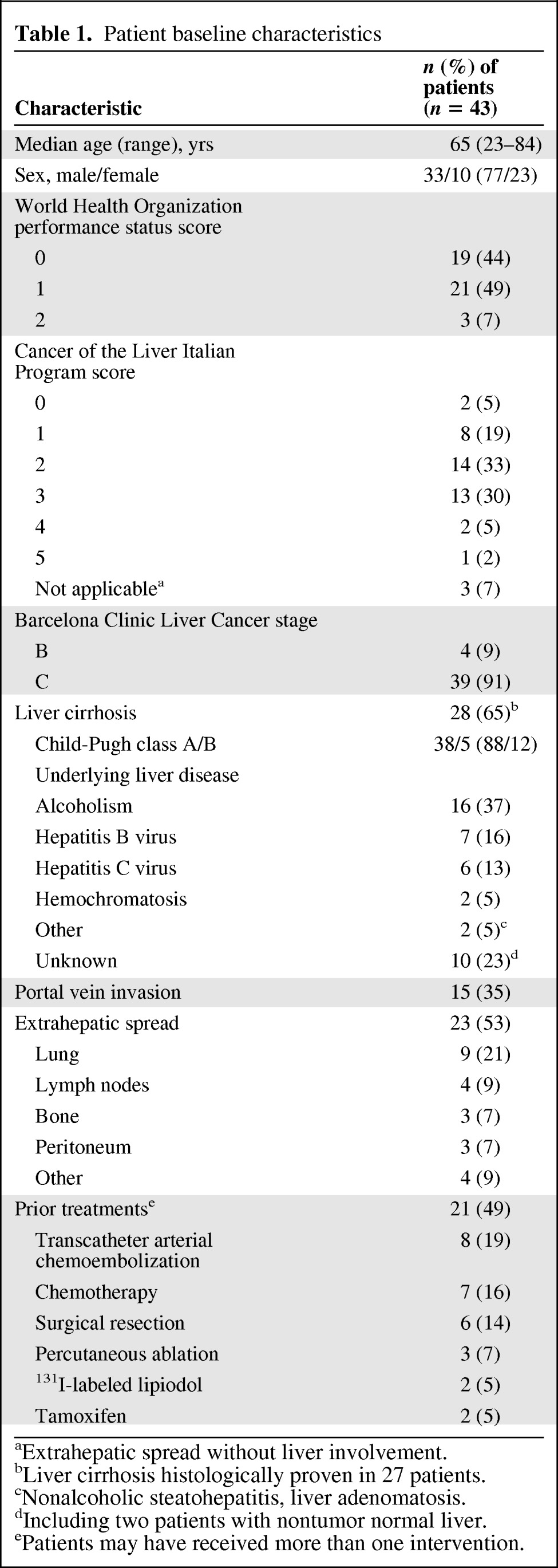
aExtrahepatic spread without liver involvement.
bLiver cirrhosis histologically proven in 27 patients.
cNonalcoholic steatohepatitis, liver adenomatosis.
dIncluding two patients with nontumor normal liver.
ePatients may have received more than one intervention.
Based on the investigator's assessment, the planned interim analysis following the inclusion of the first 25 patients showed that disease control at 16 weeks was observed in 10 patients treated with bevacizumab at the dose of 5 mg/kg. According to the study protocol, the decision to move to the second stage of the study was made following the inclusion of the first 25 patients because disease control at 16 weeks was observed in <11 patients. Therefore, the next patients were treated at the dose of 10 mg/kg. Retrospectively, based on an independent radiologist's review of tumor response performed at the end of the study, disease control at 16 weeks was actually observed in 11 of the first 25 patients.
Patients treated at the 5 mg/kg and 10 mg/kg dose levels received median numbers of eight (range, 2–38) and six (range, 1–52) treatment cycles, respectively. The main reasons for discontinuing treatment were PD (29 patients [67%], including four patients with concomitant liver failure and one patient with concomitant treatment-related toxicity), liver failure (three patients [7%], including one patient with concomitant treatment-related toxicity), and treatment-related toxicity (two patients [5%]).
Toxicity
Treatment toxicity was generally mild or moderate (Table 2). Sixteen (37%) patients experienced grade 3–4 toxicities. Grade 3–4 asthenia and grade 3–4 aminotransferase elevation were observed in five patients (12%) and three patients (7%), respectively, but none of these cases was considered as a serious adverse event. Four patients (9%) experienced gastrointestinal hemorrhage, including three cases of grade 2–3 GEV-related hemorrhage (bleeding peptic ulcer in the remaining case). There were no treatment-related deaths.
Table 2.
Toxicity
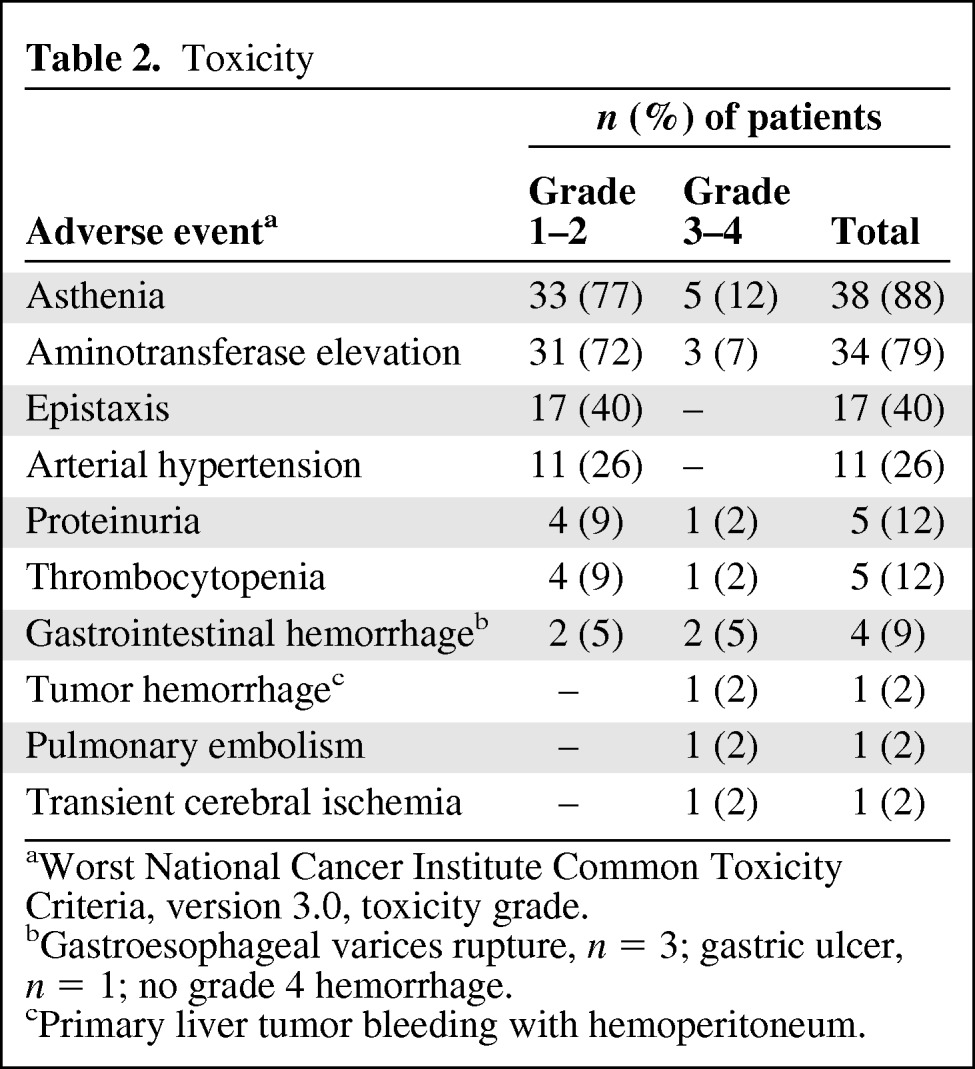
aWorst National Cancer Institute Common Toxicity Criteria, version 3.0, toxicity grade.
bGastroesophageal varices rupture, n = 3; gastric ulcer, n = 1; no grade 4 hemorrhage.
cPrimary liver tumor bleeding with hemoperitoneum.
Efficacy
Among the 38 patients evaluable for radiological response, six achieved a PR (intent-to-treat ORR, 14%; 95% CI, 4%–24%) of 148 days median duration (range, 55–362 days) and 18 patients had SD (DCR, 56%), including 12 patients who experienced SD for ≥16 weeks (16W-DCR, 42%; 95% CI, 27%–57%) (Fig. 1A). The 16W-DCRs were 39% (95% CI, 19%–59%) in patients treated with 5 mg/kg bevacizumab and 45% (95% CI, 23%–67%) in those treated at the 10-mg/kg dose.
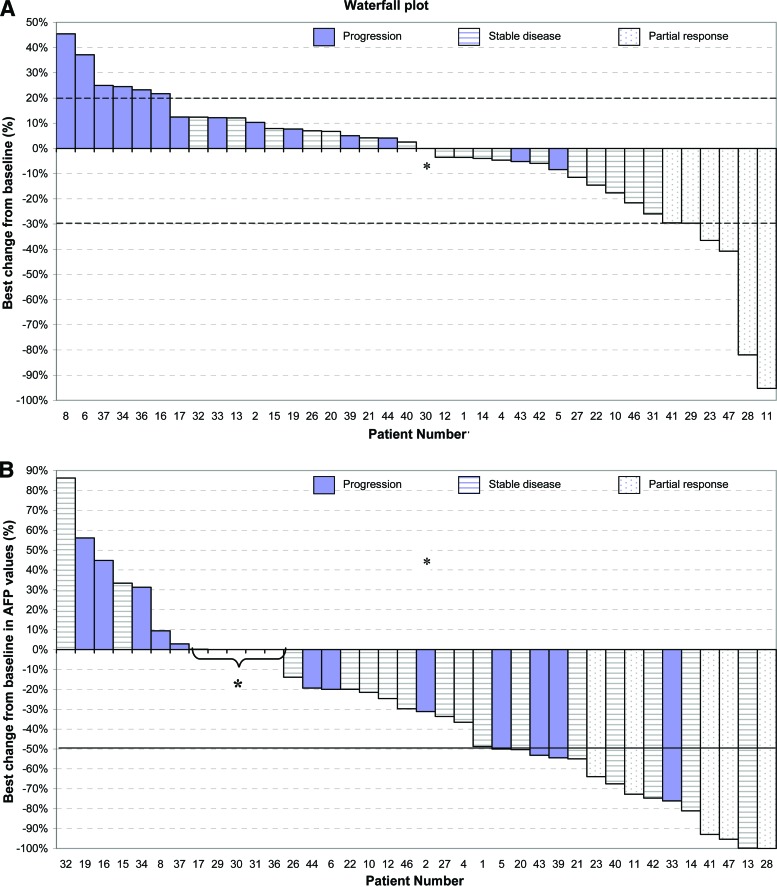
Figure 1.
Changes in tumor area and serum alphafoetoprotein level over time in patients with advanced hepatocellular carcinoma treated with bevacizumab. Waterfall plot of maximum percentage changes in tumor size of index lesions (A) and maximum changes in α-fetoprotein (AFP) levels from baseline (B). Evaluable patients (n = 38) are categorized according to Response Evaluation Criteria in Solid Tumors (version 1.0) as having an objective response (white bars), stable disease (dashed bars), or disease progression (purple bars). Eight patients with a <20% increase in the sum of the largest dimensions of the target lesions were categorized as having disease progression because of the appearance of new lesions.
*0% decrease or increase.
Baseline α-fetoprotein (AFP) levels were elevated in 36 patients (84%), including 23 patients (53%) with an AFP level ≥400 ng/mL. Among those 36 patients, 23 (63%) experienced a ≥50% decrease in AFP level (Fig. 1B).
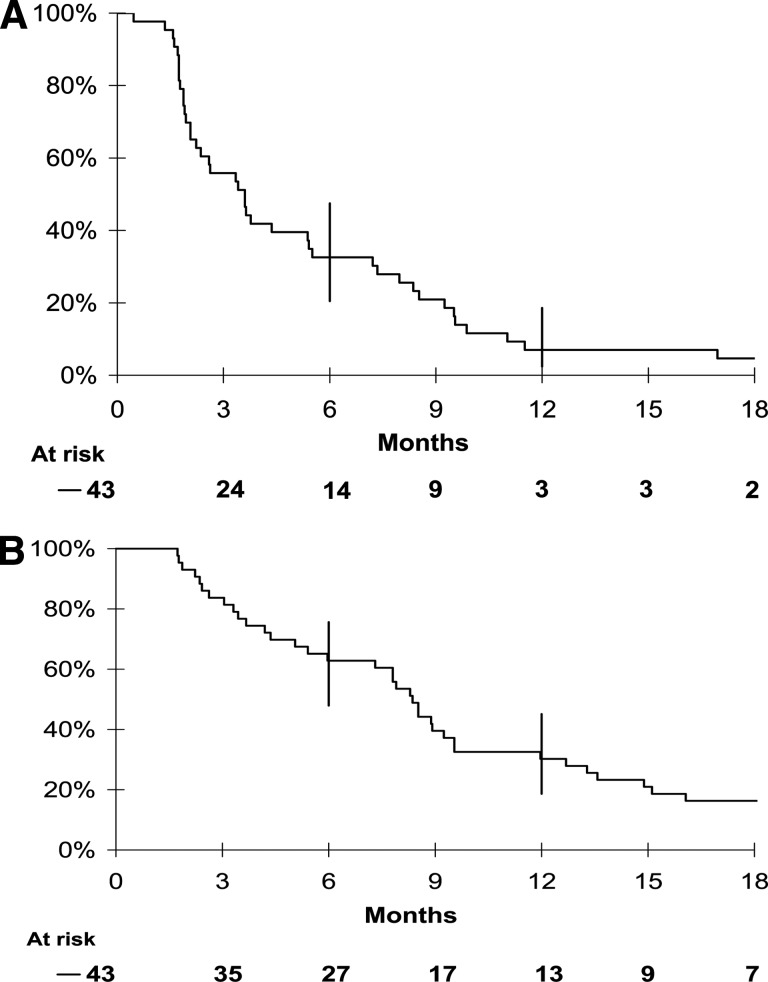
After a median follow-up of 27 months (range, 20–34 months), the median PFS interval was 3 months (95% CI, 2–4 months) and the median OS duration was 8 months (95% CI, 4–9 months) (Fig. 2). The 6- and 12-month PFS rates were 33% (95% CI, 20%–47%) and 7% (95% CI, 2%–19%), respectively. The 6- and 12-month OS rates were 63% (95% CI, 48%–76%) and 30% (95% CI, 19%–45%), respectively. At the cutoff date for analysis, three patients were still alive and in complete remission, two of whom received additional chemoembolization (n = 1) or orthotopic liver transplantation (n = 1). The latter patient experienced a complete remission of his multiple lung metastases after 18 months of bevacizumab therapy, which was stopped after 24 months according to the study recommendations. No disease relapse occurred within the 3 years after bevacizumab discontinuation.
Figure 2.
Kaplan-Meier estimates of overall survival and progression-free survival. (A): Progression-free survival probability. (B): Overall survival probability.
Biomarker Analyses
CEC and CAF concentrations at baseline, day 3, day 15, and day 60 were measured in 36 (84%) and 43 (100%) patients, respectively. Analysis of correlations between baseline biomarker values and prognostic patient characteristics showed that high IL-6 levels were correlated with a worse WHO PS score (p = .008).
Levels of IL-6, IL-8, MMP-2, MMP-9, VEGF, PlGF, and sVEGFR-2 were modulated during treatment (supplemental online Fig. S1, Table 3). SDF-1α and TNF-α values were lower than twice the background noise and were not considered significant. A transient increase in CEC levels was observed at day 3 (p = .03). The most significant changes in CAFs included a dramatic decrease in VEGF-A levels at all time points (p < .0001), a transient increase in sVEGFR-2 levels at day 3 (p < .0001), and an increase in PIGF levels at all time points (p < .0001), compared with baseline.
Table 3.
Levels of CECs and CAFs at baseline and day 3, day 15, and day 60
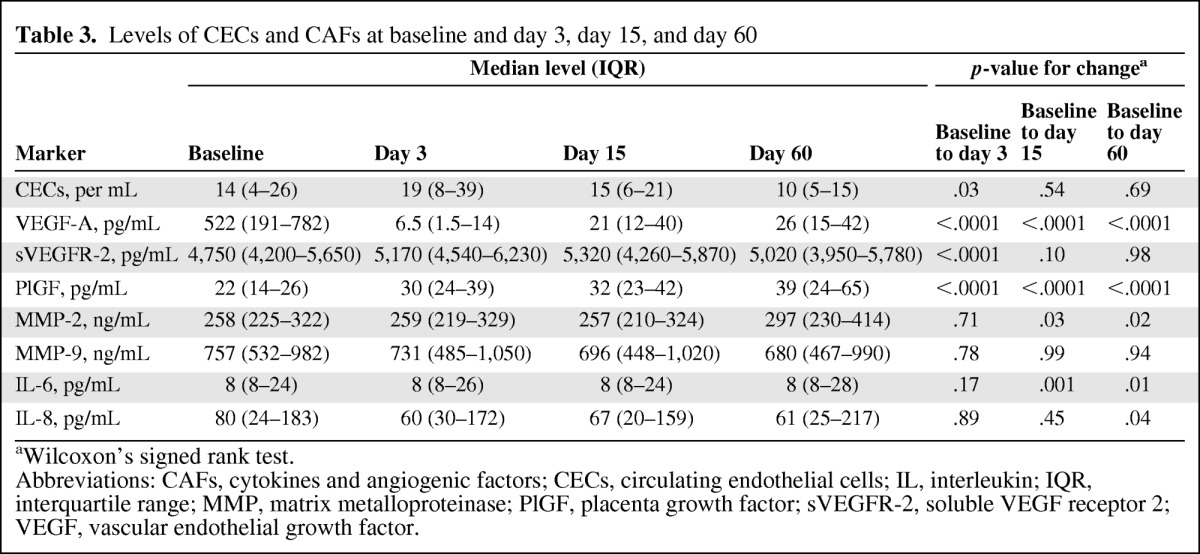
aWilcoxon's signed rank test.
Abbreviations: CAFs, cytokines and angiogenic factors; CECs, circulating endothelial cells; IL, interleukin; IQR, interquartile range; MMP, matrix metalloproteinase; PlGF, placenta growth factor; sVEGFR-2, soluble VEGF receptor 2; VEGF, vascular endothelial growth factor.
Correlations between CEC and CAF levels at each time point, as well as changes from baseline in CEC and CAF levels, and the ORR and 16W-DCR were examined. CEC level was the single marker associated with the ORR. Higher CEC levels at day 15 and an increase in CEC level from baseline to day 15 were associated with a better ORR (p = .04) and 16W-DCR (p = .02), respectively (Table 4). Furthermore, a lower IL-8 level at all time points was associated with a better 16W-DCR (p ≤ .04).
Table 4.
Association between CEC and IL-8 levels and response
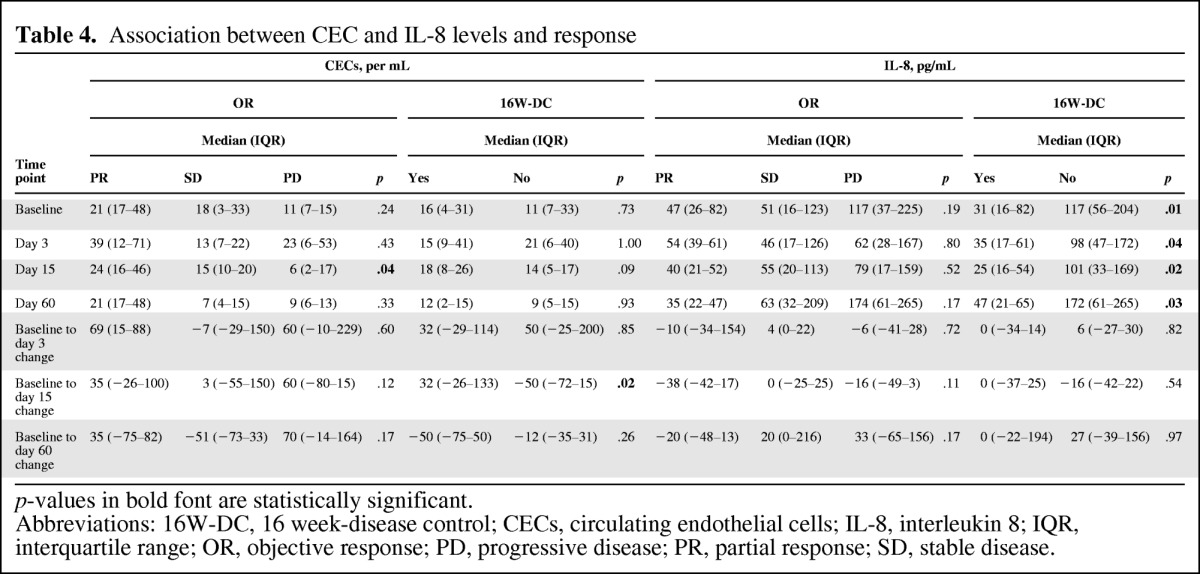
p-values in bold font are statistically significant.
Abbreviations: 16W-DC, 16 week-disease control; CECs, circulating endothelial cells; IL-8, interleukin 8; IQR, interquartile range; OR, objective response; PD, progressive disease; PR, partial response; SD, stable disease.
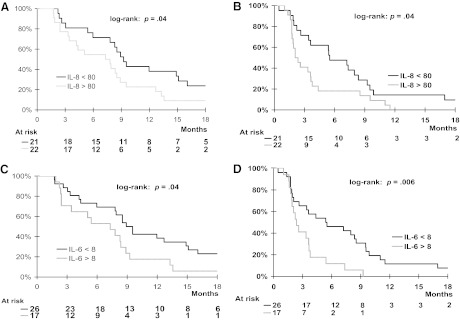
Correlations between baseline CEC and CAF levels and PFS and OS outcomes were examined. Elevated baseline IL-8 and IL-6 levels were correlated with both a shorter PFS interval (p = .04 and p = .006, respectively) and a shorter OS time (p = .04 and p = .04, respectively) (Fig. 3). None of the other biomarker levels were significantly correlated with patient outcome.
Figure 3.
Prognostic value of plasma interleukin (IL)-8 and IL-6 in patients with advanced hepatocellular carcinoma treated with bevacizumab. Overall (A) and progression-free (B) survival probabilities according to whether baseline plasma interleukin (IL)-8 levels were <80 pg/mL or ≥80 pg/mL. Overall (C) and progression-free (D) survival probabilities according to whether baseline plasma IL-6 levels were <8 pg/mL or ≥8 pg/mL.
Discussion
Our study supports the clinical and biological activity of bevacizumab as a single agent in patients with advanced HCC. The ORR of 14% compares favorably with those observed with VEGFR inhibitors (sorafenib, sunitinib) in both phase II and phase III trials (ORR <3%) [6, 16, 17] and may be of clinical value, notably for downstaging locally advanced tumors, which may allow subsequent curative-intent therapies. Encouraging ORRs, reaching 20%–25%, were obtained in phase II trials in which bevacizumab was combined with cytotoxic chemotherapy [18, 19] and with other targeted agents [20]. However, the relative contribution of bevacizumab in such trials remains to be defined in future randomized studies.
To date, only one previous study investigated the efficacy of single-agent bevacizumab in HCC patients. Siegel et al. [21] reported an ORR similar to ours (13%), but a somewhat longer PFS interval (median, 6.9 months), which is likely a result of the fact that no patient with overt extrahepatic metastasis or invasion of major blood vessels was included in their phase II study, whereas such patients accounted for 53% and 35% of patients, respectively, in ours. Additionally, they observed a similar activity of bevacizumab regardless of the dose of bevacizumab used (5 mg/kg or 10 mg/kg), as in our study. However, the statistical power of our analysis was insufficient to exclude a dose effect of bevacizumab in HCC patients.
Although cross-study comparisons should be made with caution, the 14% ORR and the 42% 16W-DCR observed in our study compare well with those observed with sorafenib in a phase II study in which three patients (2.2%) achieved a PR, eight patients (5.8%) had a minor response, and 46 patients (33.6%) had SD (≥16 weeks) [16]. Hence, our predefined endpoint of 65% might have been too ambitious. Additionally, the poor prognostic features of our population (Child-Pugh class B liver cirrhosis, 12%; extrahepatic metastases, 53%; prior TACE or systemic therapy, 47%; CLIP score ≥3, 37%) may account for this 16W-DCR as well as the median PFS and OS times of 3 months and 8 months, respectively. In the sorafenib phase II study [16], 28% of the patients had Child-Pugh class B liver cirrhosis, but the incidence of extrahepatic disease and CLIP score were not available. The fact remains that our survival data seem underwhelming in comparison with the sorafenib phase II and III data [6, 16], suggesting other alternative explanations such as a transient effect of bevacizumab on the tumor vasculature followed by rapid disease progression resulting from increased tumor hypoxia.
Bevacizumab monotherapy was generally safe and well tolerated. Despite systematic pretreatment esophagogastroscopy and primary prophylaxis of grade ≥2 GEV bleeding with β-blockers, three patients (7%) experienced grade 2–3 GEV-related hemorrhage, suggesting the possibility of a higher risk for bleeding with bevacizumab, particularly in patients with large GEV before starting therapy. Previous phase II trials of bevacizumab in HCC patients have reported severe GEV-related bleeding events [18–22]. However, this does not seem to be a specific side effect of bevacizumab because such events have also been described in phase II–III trials of anti-VEGFR TKIs [7, 17, 23]. Otherwise, patients who have cirrhosis, and consecutively portal hypertension, GEV, thrombocytopenia, and coagulopathy, have an estimated annual risk for variceal bleeding of 5%–15% [24, 25]. Therefore, careful patient selection for bevacizumab (or, more generally, antiangiogenic) therapy in HCC patients should be based on systematic pretreatment endoscopic screening for GEV and more aggressive prophylaxis of GEV bleeding (systematic banding and/or sclerotherapy if grade >1), as well as a follow-up endoscopy showing successful treatment of GEV (until grade ≤1) before allowing treatment administration.
As previously used in several phase II studies [21], we chose the 16W-DCR as the primary endpoint that was likely to be more accurate than the RECIST for assessing antiangiogenic therapies in HCC patients [26]. Indeed, the RECIST were recently shown to underestimate the true ORR and DCR because they do not account for antiangiogenic therapies that aim to achieve necrosis of the tumor, which may not be paralleled by tumor shrinkage [27]. Our study was designed before the publication of the American Association for the Study of Liver Disease–Journal of the National Cancer Institute guidelines for HCC that recommend ORR assessment be performed using the new proposed modified RECIST for HCC [28]. Finally, our ORR based on the RECIST version 1.0 should be considered as a conservative estimate and likely underoptimistic. The clinical value of a ≥50% decrease in AFP level (63% of patients in our study) remains to be demonstrated. In a substudy of this trial restricted to patients in whom at least one target liver lesion was evaluable by dynamic contrast-enhanced ultrasonography, changes in tumor perfusion as early as 3 days after bevacizumab administration were correlated with tumor response at 2 months, which may therefore represent potential surrogate measures of antiangiogenic activity in HCC patients [29].
Our study is the first to explore CECs and CAFs in relation to the activity of single-agent bevacizumab therapy in cancer patients [30]. CECs are mature differentiated cells shed from vessel walls, and high levels are observed in patients with vascular disorders [31] and with cancer [32–34]. Changes in CEC levels after bevacizumab therapy have been reported in patients with metastatic cancers [35, 36]. However, in most studies, bevacizumab was combined with chemotherapy, rendering the analysis of specific pharmacodynamic markers of bevacizumab impossible. We herein report that viable CEC levels increased as early as 48 hours after bevacizumab administration in most patients. CEC detachment from vessel walls could be caused by the withdrawal of free VEGF-A subsequent to antibody blockade. High CEC values on day 15 were correlated with the ORR, and the CEC level increase at day 15 from baseline was correlated with the 16W-DCR. Although exploratory in nature, these findings suggest that CEC counts may be potentially useful biomarkers of response to bevacizumab, and possibly to other antiangiogenic therapies for HCC. The extreme rarity of CECs and the lack of consensus on CEC surface markers have led to a considerable debate on the most appropriate techniques to be used for CEC enumeration—most commonly, flow cytometry and immunomagnetic separation followed by microscopic detection. Flow cytometry offers a high degree of flexibility with the possibility of combining multiple markers, allowing the identification of various CEC subsets. Flow cytometry is, however, currently not standardized, and achieving this goal is essential to clinically qualify CECs as biomarkers. The assay used herein was developed in accordance with recommendations for the identification of extremely rare events, including staining of a large volume of whole blood (1 mL), the use of a viability marker, accumulation of a large number of events (∼5 × 106) to ensure statistical analysis, and a multiple gating strategy [14]. We recently reported that CEC counts measured with the same cytometry assay at baseline and after the first administration of bevacizumab (plus chemotherapy) were correlated with the PFS time and ORR, respectively, in patients with advanced colorectal cancer [37]. Using flow cytometry, the shedding of nonviable CECs after antivascular treatment—particularly in response to metronomic chemotherapy known to induce antiangiogenic effects—has been reported as being associated with a favorable outcome [38, 39]. Using the semiautomated immunomagnetic separation-based CellSearch® technique (Veridex LLC, Raritan, NJ), Bidard et al. [36] showed that an increase in CEC levels during bevacizumab (plus chemotherapy) was correlated with outcome in advanced breast cancer patients. Collectively, these data suggest that CEC monitoring may help to predict patient outcome during bevacizumab-based therapy in patients with different tumor types. Obviously, our single-arm study precludes any definite conclusion on the predictive value of CECs. Furthermore, given the large number of analyses performed, these results should be confirmed in a larger population.
In accordance with phase I studies of bevacizumab [40], but in contrast to a report from Willett et al. [35], we observed that bevacizumab led to nearly complete trapping of free plasma VEGF-A. We cannot exclude the possibility that the methods used in our study to measure VEGF concentrations may have impacted our results (e.g., the ELISA test or collection tubes). These discrepancies could be elucidated soon by recently developed optimized ELISA tests that are able to detect different VEGF-A isoforms and require stringent isolation and storage procedures [41]. Nevertheless, this drop in free plasma VEGF-A level, along with a concomitant increase in sVEGFR-2 level, contrasts with changes observed with anti-VEGFR TKIs, which induce the opposite effects (i.e., an increase in plasma VEGF-A level and decrease in sVEGFR-2 level) [23]. These discrepancies suggest class-dependent effects of angiogenesis inhibitors on the kinetics of plasma VEGF-A and sVEGFR-2 levels (online supplement Fig. S2). Although not significant in multivariate models, plasma VEGF has been shown to be a prognostic factor in HCC patients [42]. We found no correlation between the baseline level and kinetics of VEGF-A and outcome, which is at variance with a phase II study of sunitinib in HCC patients [17] but consistent with data from patients with other cancers [43]. In addition to the different technologies used to measure VEGF discussed earlier, the small number of patients and, alternatively, the fact that this drop was observed in all our patients may explain these discrepancies.
We found an association between a high IL-8 level at any time point and a poor 16W-DCR. Furthermore, we found that high pretreatment levels of IL-8 and IL-6 were associated with shorter PFS and OS times, supporting the potential prognostic value of these biomarkers in HCC patients treated with antiangiogenic agents [23]. In a study of Zhu et al. [23], IL-6, IL-8, and CECs were not found to be modulated by sunitinib, but higher baseline plasma levels of IL-8 and IL-6 were correlated with worse outcomes. The prognostic value of baseline IL-8 and IL-6 levels was also reported in a very recent phase II study of thalidomide plus metronomic chemotherapy [44]. Conversely, IL-8 was the only serum cytokine not correlated with the PFS outcome in a study of 30 patients treated with sorafenib [45]. Although proposed mostly as a biomarker of HCC development [46, 47], IL-6 has been inconsistently found as a prognostic factor [48]. Emerging data have implicated inflammation and immune cells in promoting angiogenesis and carcinogenesis in HCC [49]. IL-8 is a proinflammatory CXC chemokine that promotes neutrophil chemotaxis and functions as a potent proangiogenic mediator within the tumor environment [50]. A recent report in both the preclinical and clinical settings demonstrated the implication of IL-8 in sunitinib therapy resistance in patients with renal cell carcinoma, supporting the concept that IL-8 levels might predict clinical response to this agent [51]. Collectively, these results and ours suggest the importance of proinflammatory pathways during anti-VEGF therapy in HCC patients and the potential role of IL-8 as an alternative angiogenic pathway capable of compensating anti-VEGF inhibition in HCC patients refractory to bevacizumab. Neuropilin and E-selectin, two markers of angiogenesis that were recently reported to correlate with outcome in randomized trials of chemotherapy alone or plus bevacizumab in breast cancer patients, should be investigated as potential predictive markers of response to VEGF inhibitors in HCC patients [52, 53].
In conclusion, bevacizumab has encouraging activity as a single agent in patients with advanced HCC and is relatively well tolerated. However, in light of a previous phase III study of sorafenib [6], our data do not allow us to claim that bevacizumab alone versus sorafenib should be the appropriate comparison in further phase III studies. Moreover, the recent failure of sunitinib [54] questions the relevance of blocking angiogenesis alone in advanced HCC treatment. Therefore, better survival outcomes resulting from dual inhibition of angiogenesis together with other pathways involved in HCC may be achieved using several promising combinations of targeted therapies. Some of these were assessed in a phase II trial [20], whereas others are currently under investigation in a phase III study. Furthermore, our data allow clarification of the roles of CAFs and CECs induced by single-agent bevacizumab therapy. Further investigation into the possible roles of CECs, IL-6, and IL-8 as biomarkers of bevacizumab activity is warranted. Finally, our data suggest that inhibiting IL-6 and IL-8 signaling might be of potential therapeutic interest in this disease.
See www.TheOncologist.com for supplemental material available online.
Supplementary Material
Acknowledgments
The study was funded by Roche. The trial is registered in ClinicalTrials.gov (identifier, NCT00162669).
Results were presented in part at the 33rd European Society for Medical Oncology Congress, Stockholm, Sweden, September 12–16, 2008 (Ann Oncol 2008;19(suppl), abstract 544P) and at the 45th Annual Meeting of the American Society of Clinical Oncology, May 29 to June 2, 2009, Orlando, Florida, (J Clin Oncol 2009;27(15 suppl), abstract 4597).
Footnotes
- (C/A)
- Consulting/advisory relationship
- (RF)
- Research funding
- (E)
- Employment
- (H)
- Honoraria received
- (OI)
- Ownership interests
- (IP)
- Intellectual property rights/inventor/patent holder
- (SAB)
- Scientific advisory board
Author Contributions
Conception/Design: Valérie Boige, Jean-Pierre Pignon, Michel Ducreux, Françoise Farace
Provision of study material or patients: Valérie Boige, David Malka, Thierry Debaere, Michel Ducreux
Collection and/or assembly of data: Valérie Boige, Abderrahmane Bourredjem, Clarisse Dromain, Charlotte Baey, Nathalie Jacques, Jean-Pierre Pignon, Nadege Vimond, Nathalie Bouvet-Forteau, Thierry Debaere, Françoise Farace
Data analysis and interpretation: Valérie Boige, David Malka, Abderrahmane Bourredjem, Clarisse Dromain, Charlotte Baey, Nathalie Jacques, Jean-Pierre Pignon, Nadege Vimond, Françoise Farace
Manuscript writing: Valérie Boige, David Malka, Abderrahmane Bourredjem, Clarisse Dromain, Jean-Pierre Pignon, Françoise Farace
Final approval of manuscript: Valérie Boige, David Malka, Abderrahmane Bourredjem, Clarisse Dromain, Charlotte Baey, Nathalie Jacques, Jean-Pierre Pignon, Nadege Vimond, Nathalie Bouvet-Forteau, Thierry Debaere, Michel Ducreux, Françoise Farace
References
- 1.Ferlay J, Autier P, Boniol M, et al. Estimates of the cancer incidence and mortality in Europe in 2006. Ann Oncol. 2007;18:581–592. doi: 10.1093/annonc/mdl498. [DOI] [PubMed] [Google Scholar]
- 2.Bruix J, Sherman M. Practice Guidelines Committee, American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208–1236. doi: 10.1002/hep.20933. [DOI] [PubMed] [Google Scholar]
- 3.Lopez PM, Villanueva A, Llovet JM. Systematic review: Evidence-based management of hepatocellular carcinoma—an updated analysis of randomized controlled trials. Aliment Pharmacol Ther. 2006;23:1535–1547. doi: 10.1111/j.1365-2036.2006.02932.x. [DOI] [PubMed] [Google Scholar]
- 4.Pang R, Poon RT. Angiogenesis and antiangiogenic therapy in hepatocellular carcinoma. Cancer Lett. 2006;242:151–167. doi: 10.1016/j.canlet.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 5.Tseng PL, Tai MH, Huang CC, et al. Overexpression of VEGF is associated with positive p53 immunostaining in hepatocellular carcinoma (HCC) and adverse outcome of HCC patients. J Surg Oncol. 2008;98:349–357. doi: 10.1002/jso.21109. [DOI] [PubMed] [Google Scholar]
- 6.Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 7.Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: A phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25–34. doi: 10.1016/S1470-2045(08)70285-7. [DOI] [PubMed] [Google Scholar]
- 8.Escudier B, Pluzanska A, Koralewski P, et al. Bevacizumab plus interferon alfa-2a for treatment of metastatic renal cell carcinoma: A randomised, double-blind phase III trial. Lancet. 2007;370:2103–2111. doi: 10.1016/S0140-6736(07)61904-7. [DOI] [PubMed] [Google Scholar]
- 9.Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 10.Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355:2542–2550. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 11.Finn RS, Bentley G, Britten CD, et al. Targeting vascular endothelial growth factor with the monoclonal antibody bevacizumab inhibits human hepatocellular carcinoma cells growing in an orthotopic mouse model. Liver Int. 2009;29:284–290. doi: 10.1111/j.1478-3231.2008.01762.x. [DOI] [PubMed] [Google Scholar]
- 12.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 13.A new prognostic system for hepatocellular carcinoma: A retrospective study of 435 patients: The Cancer of the Liver Italian Program (CLIP) investigators. Hepatology. 1998;28:751–755. doi: 10.1002/hep.510280322. [DOI] [PubMed] [Google Scholar]
- 14.Jacques N, Vimond N, Conforti R, et al. Quantification of circulating mature endothelial cells using a whole blood four-color flow cytometric assay. J Immunol Methods. 2008;337:132–143. doi: 10.1016/j.jim.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 15.Fleming TR. One-sample multiple testing procedure for phase II clinical trials. Biometrics. 1982;38:143–151. [PubMed] [Google Scholar]
- 16.Abou-Alfa GK, Schwartz L, Ricci S, et al. Phase II study of sorafenib in patients with advanced hepatocellular carcinoma. J Clin Oncol. 2006;24:4293–4300. doi: 10.1200/JCO.2005.01.3441. [DOI] [PubMed] [Google Scholar]
- 17.Faivre S, Raymond E, Boucher E, et al. Safety and efficacy of sunitinib in patients with advanced hepatocellular carcinoma: An open-label, multicentre, phase II study. Lancet Oncol. 2009;10:794–800. doi: 10.1016/S1470-2045(09)70171-8. [DOI] [PubMed] [Google Scholar]
- 18.Zhu AX, Blaszkowsky LS, Ryan DP, et al. Phase II study of gemcitabine and oxaliplatin in combination with bevacizumab in patients with advanced hepatocellular carcinoma. J Clin Oncol. 2006;24:1898–1903. doi: 10.1200/JCO.2005.04.9130. [DOI] [PubMed] [Google Scholar]
- 19.Sun W, Sohal D, Haller DG, et al. Phase 2 trial of bevacizumab, capecitabine, and oxaliplatin in treatment of advanced hepatocellular carcinoma. Cancer. 2011;117:3187–3192. doi: 10.1002/cncr.25889. doi: 10.1002/cncr.25889. [DOI] [PubMed] [Google Scholar]
- 20.Thomas MB, Morris JS, Chadha R, et al. Phase II trial of the combination of bevacizumab and erlotinib in patients who have advanced hepatocellular carcinoma. J Clin Oncol. 2009;27:843–850. doi: 10.1200/JCO.2008.18.3301. [DOI] [PubMed] [Google Scholar]
- 21.Siegel AB, Cohen EI, Ocean A, et al. Phase II trial evaluating the clinical and biologic effects of bevacizumab in unresectable hepatocellular carcinoma. J Clin Oncol. 2008;26:2992–2998. doi: 10.1200/JCO.2007.15.9947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hsu CH, Yang TS, Hsu C, et al. Efficacy and tolerability of bevacizumab plus capecitabine as first-line therapy in patients with advanced hepatocellular carcinoma. Br J Cancer. 2010;102:981–986. doi: 10.1038/sj.bjc.6605580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhu AX, Sahani DV, Duda DG, et al. Efficacy, safety, and potential biomarkers of sunitinib monotherapy in advanced hepatocellular carcinoma: A phase II study. J Clin Oncol. 2009;27:3027–3035. doi: 10.1200/JCO.2008.20.9908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Burroughs AK, Patch D. Primary prevention of bleeding from esophageal varices. N Engl J Med. 1999;340:1033–1035. doi: 10.1056/NEJM199904013401309. [DOI] [PubMed] [Google Scholar]
- 25.Bornman PC, Krige JE, Terblanche J. Management of oesophageal varices. Lancet. 1994;343:1079–1084. doi: 10.1016/s0140-6736(94)90186-4. [DOI] [PubMed] [Google Scholar]
- 26.Llovet JM, Di Bisceglie AM, Bruix J, et al. Panel of Experts in HCC-Design Clinical Trials. Design and endpoints of clinical trials in hepatocellular carcinoma. J Natl Cancer Inst. 2008;100:698–711. doi: 10.1093/jnci/djn134. [DOI] [PubMed] [Google Scholar]
- 27.Park JW, Finn RS, Kim JS, et al. Phase II, open-label study of brivanib as first-line therapy in patients with advanced hepatocellular carcinoma. Clin Cancer Res. 2011;17:1973–1983. doi: 10.1158/1078-0432.CCR-10-2011. [DOI] [PubMed] [Google Scholar]
- 28.Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30:52–60. doi: 10.1055/s-0030-1247132. [DOI] [PubMed] [Google Scholar]
- 29.Lassau N, Koscielny S, Chami L, et al. Advanced hepatocellular carcinoma: Early evaluation of response to bevacizumab therapy at dynamic contrast-enhanced US with quantification—preliminary results. Radiology. 2011;258:291–300. doi: 10.1148/radiol.10091870. [DOI] [PubMed] [Google Scholar]
- 30.Jubb AM, Harris AL. Biomarkers to predict the clinical efficacy of bevacizumab in cancer. Lancet Oncol. 2010;11:1172–1183. doi: 10.1016/S1470-2045(10)70232-1. [DOI] [PubMed] [Google Scholar]
- 31.Blann AD, Woywodt A, Bertolini F, et al. Circulating endothelial cells. Biomarker of vascular disease. Thromb Haemost. 2005;93:228–235. doi: 10.1160/TH04-09-0578. [DOI] [PubMed] [Google Scholar]
- 32.Goon PK, Lip GY, Boss CJ, et al. Circulating endothelial cells, endothelial progenitor cells, and endothelial microparticles in cancer. Neoplasia. 2006;8:79–88. doi: 10.1593/neo.05592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Taylor M, Rössler J, Geoerger B, et al. High levels of circulating VEGFR2+ bone marrow-derived progenitor cells correlate with metastatic disease in patients with pediatric solid malignancies. Clin Cancer Res. 2009;15:4561–4571. doi: 10.1158/1078-0432.CCR-08-2363. [DOI] [PubMed] [Google Scholar]
- 34.Jubb AM, Oates AJ, Holden S, et al. Predicting benefit from anti-angiogenic agents in malignancy. Nat Rev Cancer. 2006;6:626–635. doi: 10.1038/nrc1946. [DOI] [PubMed] [Google Scholar]
- 35.Willett CG, Duda DG, di Tomaso E, et al. Efficacy, safety, and biomarkers of neoadjuvant bevacizumab, radiation therapy, and fluorouracil in rectal cancer: A multidisciplinary phase II study. J Clin Oncol. 2009;27:3020–3026. doi: 10.1200/JCO.2008.21.1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bidard FC, Mathiot C, Degeorges A, et al. Clinical value of circulating endothelial cells and circulating tumor cells in metastatic breast cancer patients treated first line with bevacizumab and chemotherapy. Ann Oncol. 2010;21:1765–1771. doi: 10.1093/annonc/mdq052. [DOI] [PubMed] [Google Scholar]
- 37.Malka D, Boige V, Jacques N, et al. Clinical value of circulating endothelial cell levels in metastatic colorectal cancer patients treated with first-line chemotherapy and bevacizumab. Ann Oncol. 2012;23:919–927. doi: 10.1093/annonc/mdr365. [DOI] [PubMed] [Google Scholar]
- 38.Mancuso P, Colleoni M, Calleri A, et al. Circulating endothelial-cell kinetics and viability predict survival in breast cancer patients receiving metronomic chemotherapy. Blood. 2006;108:452–459. doi: 10.1182/blood-2005-11-4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dellapasqua S, Bertolini F, Bagnardi V, et al. Metronomic cyclophosphamide and capecitabine combined with bevacizumab in advanced breast cancer. J Clin Oncol. 2008;26:4899–4905. doi: 10.1200/JCO.2008.17.4789. [DOI] [PubMed] [Google Scholar]
- 40.Gordon MS, Margolin K, Talpaz M, et al. Phase I safety and pharmacokinetic study of recombinant human anti-vascular endothelial growth factor in patients with advanced cancer. J Clin Oncol. 2001;19:843–850. doi: 10.1200/JCO.2001.19.3.843. [DOI] [PubMed] [Google Scholar]
- 41.Miles DW, de Haas SL, Dirix L, et al. Plasma biomarker analyses in the AVADO phase III randomized study of first-line bevacizumab + docetaxel in patients with human epidermal growth factor receptor (HER) 2-negative metastatic breast cancer [abstract P2–16-04]. Presented at the 33rd Annual San Antonio Breast Cancer Symposium; December 8–12, 2010; San Antonio, TX. [Google Scholar]
- 42.Kaseb AO, Morris JS, Hassan MM, et al. Clinical and prognostic implications of plasma insulin-like growth factor-1 and vascular endothelial growth factor in patients with hepatocellular carcinoma. J Clin Oncol. 2011;29:3892–3899. doi: 10.1200/JCO.2011.36.0636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sessa C, Guibal A, Del Conte G, et al. Biomarkers of angiogenesis for the development of antiangiogenic therapies in oncology: Tools or decorations? Nat Clin Pract Oncol. 2008;5:378–391. doi: 10.1038/ncponc1150. [DOI] [PubMed] [Google Scholar]
- 44.Shao YY, Lin ZZ, Hsu C, et al. Efficacy, safety, and potential biomarkers of thalidomide plus metronomic chemotherapy for advanced hepatocellular carcinoma. Oncology. 2012;82:59–66. doi: 10.1159/000336126. [DOI] [PubMed] [Google Scholar]
- 45.Miyahara K, Nouso K, Tomoda T, et al. Predicting the treatment effect of sorafenib using serum angiogenesis markers in patients with hepatocellular carcinoma. J Gastroenterol Hepatol. 2011;26:1604–1611. doi: 10.1111/j.1440-1746.2011.06887.x. [DOI] [PubMed] [Google Scholar]
- 46.Porta C, De Amici M, Quaglini S, et al. Circulating interleukin-6 as a tumor marker for hepatocellular carcinoma. Ann Oncol. 2008;19:353–358. doi: 10.1093/annonc/mdm448. [DOI] [PubMed] [Google Scholar]
- 47.Wong VW, Yu J, Cheng AS, et al. High serum interleukin-6 level predicts future hepatocellular carcinoma development in patients with chronic hepatitis B. Int J Cancer. 2009;124:2766–2770. doi: 10.1002/ijc.24281. [DOI] [PubMed] [Google Scholar]
- 48.Parasole R, Izzo F, Perrone F, et al. Prognostic value of serum biological markers in patients with hepatocellular carcinoma. Clin Cancer Res. 2001;7:3504–3509. [PubMed] [Google Scholar]
- 49.de Visser KE, Eichten A, Coussens LM. Paradoxical roles of the immune system during cancer development. Nat Rev Cancer. 2006;6:24–37. doi: 10.1038/nrc1782. [DOI] [PubMed] [Google Scholar]
- 50.Waugh DJ, Wilson C. The interleukin-8 pathway in cancer. Clin Cancer Res. 2008;14:6735–6741. doi: 10.1158/1078-0432.CCR-07-4843. [DOI] [PubMed] [Google Scholar]
- 51.Huang D, Ding Y, Zhou M, et al. Interleukin-8 mediates resistance to antiangiogenic agent sunitinib in renal cell carcinoma. Cancer Res. 2010;70:1063–1071. doi: 10.1158/0008-5472.CAN-09-3965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Baar J, Silverman P, Lyons J, et al. A vasculature-targeting regimen of preoperative docetaxel with or without bevacizumab for locally advanced breast cancer: Impact on angiogenic biomarkers. Clin Cancer Res. 2009;15:3583–3590. doi: 10.1158/1078-0432.CCR-08-2917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jubb AM, Miller KD, Rugo HS, et al. Impact of exploratory biomarkers on the treatment effect of bevacizumab in metastatic breast cancer. Clin Cancer Res. 2011;17:372–381. doi: 10.1158/1078-0432.CCR-10-1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cheng A, Kang Y, Lin D, et al. Phase III trial of sunitinib versus sorafenib in advanced hepatocellular carcinoma [abstract 4000] J Clin Oncol. 2011;29(No 15) suppl:256S. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.