This review discusses the relevance of the Hedgehog pathway in cancers and summarizes the clinical experience thus far with Hedgehog inhibitors.
Keywords: Hedgehog, Cancer stem cells, PTCH, SMO, Arsenic, Cancer
Abstract
The Hedgehog (Hh) signaling pathway has been implicated in tumor initiation and metastasis across different malignancies. Major mechanisms by which the Hh pathway is aberrantly activated can be attributed to mutations of members of Hh pathway or excessive/inappropriate expression of Hh pathway ligands. The Hh signaling pathway also affects the regulation of cancer stem cells, leading to their capabilities in tumor formation, disease progression, and metastasis. Preliminary results of early phase clinical trials of Hh inhibitors administered as monotherapy demonstrated promising results in patients with basal cell carcinoma and medulloblastoma, but clinically meaningful anticancer efficacy across other tumor types seems to be lacking. Additionally, cases of resistance have been already observed. Mutations of SMO, activation of Hh pathway components downstream to SMO, and upregulation of alternative signaling pathways are possible mechanisms of resistance development. Determination of effective Hh inhibitor-based combination regimens and development of correlative biomarkers relevant to this pathway should remain as clear priorities for future research.
Introduction
Hedgehog (Hh) proteins were initially discovered in Drosophilia melanogaster, together with their signaling transduction pathway [1, 2]. This signaling pathway plays a critical role in embryonic development but is generally silenced in adults [3, 4]. However, this pathway is reactivated during tissue repair and regeneration [5–8]. In the last decade, there has been increasing evidence that the Hh pathway plays an important role in carcinogenesis; this knowledge has provided an attractive platform for the development of novel anticancer agents [9, 10]. In this review, we discuss the relevance of the Hh pathway in cancers and summarize the clinical experience thus far with Hh inhibitors.
Hedgehog Proteins and Signaling Transduction Pathway
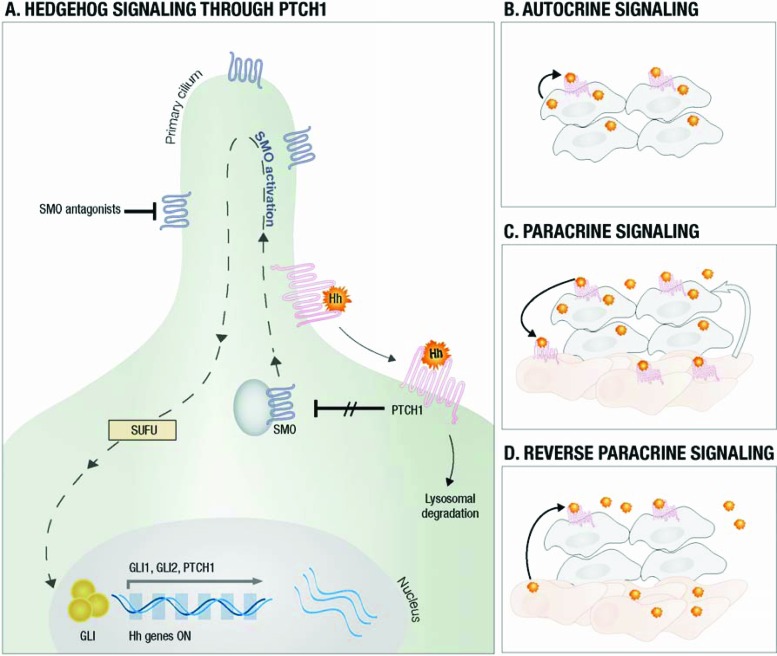
Three mammalian Hh proteins have been identified in humans; they are denoted by the prefixes Sonic, Indian, and Desert. Briefly, these proteins act as a ligand and activate the Hh signaling pathway by binding to Patched (PTCH1), a transmembrane protein present on the primary cilium of target cells. In the absence of an Hh ligand, PTCH1 represses the activity of Smoothened (SMO), which is now recognized to play an important role in the Hh signaling transduction pathway. Following Hh ligand binding to PTCH1, SMO repression is released and this subsequently results in the modulation of GLI transcription factors. There are three forms of GLI transcription factors: GLI1, GLI2, and GLI3. GLI1 has a transcriptional activator function, whereas GLI2 can either activate or repress gene expression depending on posttranscriptional/translational modifications. GLI3, converse to GLI1, yields a strong repressor effect on transcriptional activities [10, 11]. Primary cilia also play crucial roles in mammalian Hh signal transduction, as most of the key components for the Hh pathway (e.g., PTCH1, SMO and GLI transcription factors) are enriched within this structure [12]. Additionally, cilia play an important role for the trafficking of Hh pathway proteins, which is crucial for pathway activation [12]. Ultimately, the balance between the activating and repressive functions of GLIs results in the expression of target genes, among others GLI1, PTCH1, MYC, BCL-2, and Cyclin D1 [13–15].
Within this simplified description (Fig. 1A), there are numerous other cellular components engaged in the activation of Hh pathway, especially in the steps regulating GLI activity downstream from SMO. These components include suppressor of fused (SUFU), KIF7, protein kinase A (PKA), glycogen synthase kinase 3ß (GSK3ß), and casein kinase 1 (CK1) [13, 16–18]. SUFU is a negative regulator of this pathway; it achieves this effect via several mechanisms. Physically, SUFU sequesters GLI transcription factors, whereas functionally SUFU affects GLI transcription ability [19–21]. The kinase protein KIF7 acts as both a positive and negative regulator of Hh pathway [22, 23]. It interacts with GLI proteins and inhibits GLI-dependent transcriptional activation [22, 23]. Conversely, KIF7 may assume a positive role via its movement to cilia tip after pathway activation where it antagonizes the activity of SUFU [15]. However, the actual functions of most of these proteins are still subject to intensive studies and not fully understood [9, 10].
Figure 1.
Hedgehog signalling. (A) Hedgehog ligands (Hhl) bind to PTCH1 and unrepress SMO with activation of GLI and target genes. (B) The tumor produces Hhl and stimulates itself. (C) Tumor cells produce Hhl and activate signaling in nonmalignant cells. In turn, other signaling pathways are activated and stimulate tumor growth (arrow). (D) Stromal cells produce the Hhl required for tumor growth/survival.
Dysregulation of Hedgehog Pathway in Solid Tumors
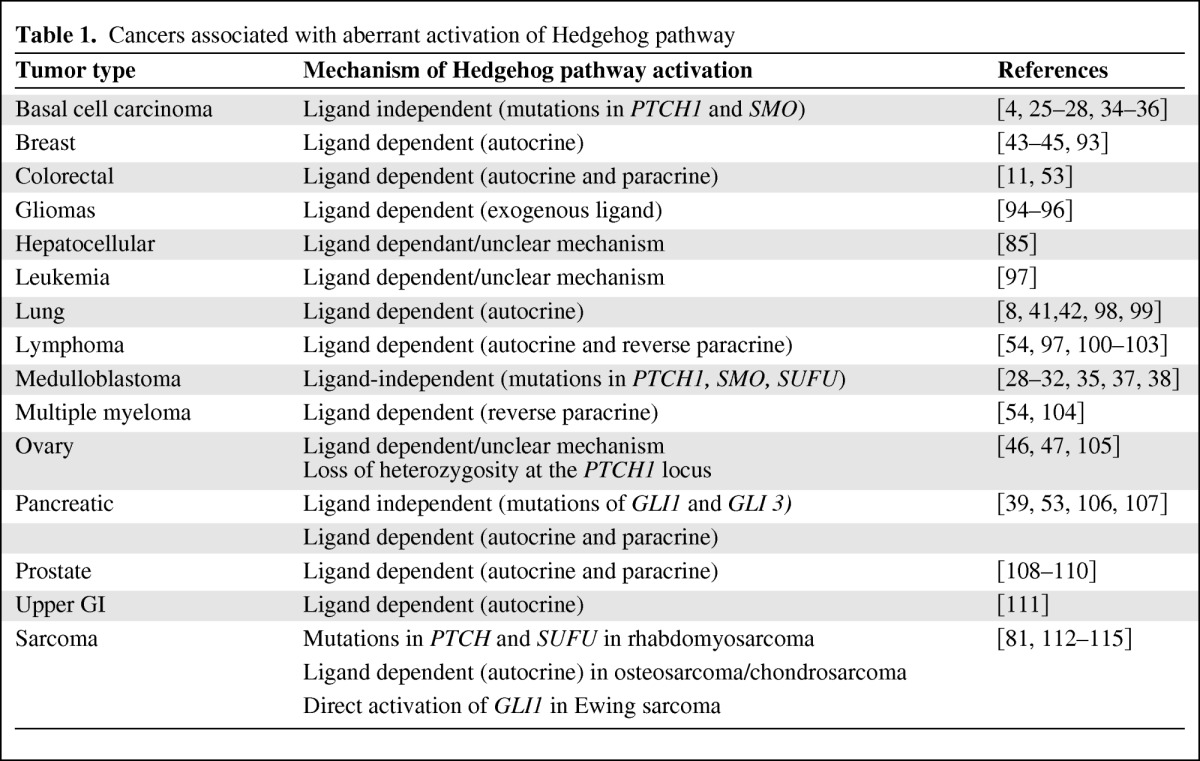
Aberrant activations of Hh pathway have been observed across a number of different malignancies (Table 1). The mechanisms by which aberrant activations of Hh signaling can lead to cancer are complex, but in general they include activating mutations of members in the Hh pathway (ligand-independent) and excessive/inappropriate expression of Hh ligands (ligand-dependent) [4, 10, 24].
Table 1.
Cancers associated with aberrant activation of Hedgehog pathway
Activating Mutations of Members in Hedgehog Pathway
Loss-of-function mutations in PTCH1 were initially identified in patients with basal cell nevus syndrome (BCNS; also known as Gorlin syndrome). These mutations lead to constitutive upregulation of the Hh pathway and patients are highly predisposed to the development of basal cell carcinomas (BCC) [4]. Further studies also showed that PTCH1 mutations occur in sporadic cases of BCC and medulloblastoma [4, 25–28]. PTCH1 mutations have been found in patients with central nervous system primitive neuroectodermal tumors or medulloblastomas [29–31]. More than 40 different PTCH1 mutations have been reported, which mostly result in truncated protein and are scattered throughout the gene. Although no mutational hot spots have been identified, exon 17 mutations have been seen more frequently in sporadic cases of medulloblastoma than BCNS. These clinical findings were supported by several preclinical reports that elegantly demonstrated the role of these mutations in carcinogenesis [32, 33]. In one study, spontaneous development of BCCs occurred when Hh was overexpressed in a transgenic mouse model; in another report, mice with heterozygous Ptch1 mutations went on to develop cerebellar medulloblastomas [32, 33].
Gain-of-function mutations in SMO are also present in some cases of sporadic BCCs [28, 34–36]. One mutation at base pair 1604 (G-to-T transversion) of exon 9 of the SMO gene changes codon 535 from tryptophan to leucine and has been reported in about 20% of sporadic BCCs [28, 35]. This mutation has resulted in constitutive SMO signaling and development of BCC-like tumors in transgenic mice [34, 36]. Additionally, the 1604 G-to-T mutation in SMO has also been described in medulloblastoma patients, albeit at much lesser frequency (1 out of 21 patients) [28]. Genetic alterations of other components of Hh pathway, such as GLI and SUFU mutations, have also been observed [37–39]. Inactivating germline mutations of SUFU have been reported in 3% of sporadic and >10% of desmoplastic medulloblastomas [37, 38]. Although alterations of GLI1 and GLI3 have been observed in global genomic analyses of pancreatic tumors, these are not thought to be activating, but rather are more likely to be passenger mutations [39].
Excessive/Inappropriate Expression of Hh Ligands
Aberrant activation of the Hh signaling pathway in cancers may also be ligand- dependent and has been reported in several malignancies [10, 40]. Ligand-dependent activation of the Hh pathway was initially described to occur in autocrine mode, but there is an increasing understanding that paracrine or reverse paracrine modes may also occur [10, 24].
Autocrine Stimulation
In the autocrine mode, tumor cells self-secrete Hh ligands to which they subsequently respond and culminate in activation of the signaling pathway. This mode has been previously described in a number of malignancies, as summarized in Table 1. In one study, 50% of small cell lung carcinomas (SCLCs) demonstrated overexpression of both Sonic hedgehog (SHH) and GLI1 in an autocrine fashion [8]. Moreover, the treatment of SCLC cell lines with cyclopamine (an SMO inhibitor) resulted in significant growth inhibition [8]. In another study, there was a marked increase in the expression of SHH in mice bearing human SCLC xenografts. Treatment of these mice with the SMO inhibitor LDE-225 following carboplatin and etoposide chemotherapy was highly effective in preventing recurrence of residual tumors [41]. Overexpression of SHH, GLI1, GLI2, and SMO has also been reported in tumor tissues of 80 patients with non-small cell lung carcinoma. In this series, elevated expression level of SMO correlated with the presence of nodal disease, implicating its role in metastasis and disease progression [42].
Autocrine activation of the Hh pathway has also been shown in breast cancer, both in cell lines and tumoral tissue studies [43–45]. In a study by Kubo et al., overexpression of SHH and GLI1 was detected in virtually all of the 52 breast cancer specimens examined [43]. In another series, the overexpression of SHH in breast cancers was shown to be secondary to hypomethylation of the ligand promoter as well as NF-κB upregulation [45]. Furthermore, akin to studies in lung cancers, inhibition of Hh pathway using cyclopamine led to suppression of proliferation in breast cancer cell lines in a dose-dependent manner [43]. Similar reports involving multiple ovarian cancer cell lines revealed upregulation in expression (more than fivefold) of several components of the Hh pathway, including GLI1 (9 of 19 cell lines), SMO (9 of 19 cell lines) and SHH (10 of 19 cell lines) [46].
Furthermore, dysregulation of PTCH1 in ovarian cancers has also been described [46, 47]. The treatment of ovarian cancer cell lines with the SMO inhibitors cyclopamine or KAAD-cyclopamine prevented their growth and migration [47, 48]. In addition to cell line data, one study involving 80 ovarian cancer specimens reported that components of the Hh signaling pathway were significantly increased and overexpression of PTCH1 and GLI1 conferred poor survival [47].
Despite the encouraging data described in this section, cyclopamine-based studies have inherent limitations which affect their interpretation. In many of the cell-line studies, the doses of cyclopamine used are now recognized to be associated with antiproliferative effects that are independent of Hh signaling [49, 50]. It has been shown that cyclopamine in high concentrations can induce apoptosis by increasing N-SMase2/ceramide and via generation of nitric oxide [49]. Moreover, it is difficult to reach systemic levels of cyclopamine in vivo because of its toxicity and relatively short elimination half-life [51]. The preclinical studies are further limited by the fact that there is no agreed-upon standard method to measure the Hh pathway activity and none of commercial antibodies against PTCH, SMO, or GLI have ever been shown to work specifically on fixed tissues [52].
Paracrine Stimulation
In the paracrine mode, Hh ligands are secreted by tumor cells and induce activity within infiltrating stromal cells. This in turn results in the production of unknown factors within the tumor environment, which ultimately support tumor growth [10, 40]. In a study by Yauch et al., paracrine activation of the Hh pathway was found in patient-derived xenografts of pancreatic and colon adenocarcinoma. Furthermore, inhibition of Hh pathway in the stroma resulted in tumor growth retardation and thus supports a paracrine mode of stimulation [53]. The exact mechanism by which Hh pathway activation in stromal cells can enhance tumor progression is unclear, but insulin-like growth factor-1 receptor (IGF-1R) and Wnt signaling pathways may play a role [53].
Reverse Paracrine Stimulation
Reverse paracrine stimulation occurs when Hh ligands produced by surrounding stromal cells activate the tumoral cell Hh pathway. Dierks et al. demonstrated that Hh ligands secreted by bone marrow, nodal, and splenic stromal cells represented survival factors for malignant B-cell lymphoma and plasmacytoma cells derived from a transgenic Eμ-Myc mouse model or from patients with these malignancies [54]. However, such paracrine stimulation still needs further confirmation.
The different modes of Hh pathway signaling are summarized in Figure 1B–1D.
Hedgehog Signaling in Cancer Stem Cells
Cancer stem cells (CSCs) are a subpopulation of cancer cells that are self-sustaining with the exclusive ability to self-renew and give rise to heterogeneous cell lineages within the tumor. They have been identified in a variety of cancers, and evidence is accumulating that CSCs may be responsible for treatment resistance and relapse [24]. Preclinical data have suggested a possible regulatory role of Hh pathway in CSCs across a number of malignancies, such as glioblastoma, breast cancer, pancreatic adenocarcinoma, and hematological malignancies [54–58]. Activation of Hh signaling has been reported in CD133-positive glioma CSCs and treatment of these cells with siRNA or cyclopamine led to a loss of tumorigenic drive [55, 59]. Similarly, in breast cancers, Hh pathway activation (using Hh ligands or manipulation of GLI1/2) or suppression (using cyclopamine) in CSCs resulted in altered expression of BMI1, a central regulator of stem cells. This in turn led to an increase or loss of tumorigenic potential, respectively, in both in vitro and in vivo settings [57]. In chronic myeloid leukemia, loss of SMO caused depletion of stem cells and use of cyclopamine led to reduction of stem cells in mouse model [58].
In addition to having tumorigenic properties, CSCs are also implicated in cancer progression and metastasis [60]. Hh signaling has been one of proposed pathways in this process [58, 61, 62]. Higher expression of PTCH1, GLI1, GLI2, and the target gene SNAIL1 has been reported in CD133-positive colon CSCs [63]. In vitro inhibition of Hh pathway activity in these cells using cyclopamine or siRNA resulted in decreased tumor cell proliferation and induced apoptosis [63]. Although these studies support the possible role of Hh pathway activation in CSCs, it remains pertinent to be reminded of the limitations posed by cyclopamine-based studies, as discussed previously.
Hh Inhibitors
The preclinical relevance of Hh signaling in cancers has resulted in the development of several targeted agents against this pathway. Most of these agents act by binding to and antagonizing SMO. At present, seven SMO inhibitors are being evaluated in the phase I or phase II clinical trial setting. These agents include vismodegib, BMS-833923, IPI-926, LDE-225, PF-04449913, LEQ 506, and TAK-441. Currently, most data concerning the clinical utility of these agents are based on trials of vismodegib. Although these data suggest clinical benefit in cancers driven by mutational activation, targeting the Hh pathway may be less likely to be successful in tumors in which aberrant ligand overexpression or signaling are not the oncogenic drivers, but rather are secondary to genetic changes in other signaling pathways [64–67].
Hh Inhibitors Antagonizing SMO
Vismodegib (GDC-0449)
Vismodegib is a potent orally bioavailable small molecule inhibitor of the Hh pathway that acts by binding and inhibiting SMO [68]. Antitumor activity of this drug was initially shown in preclinical models of medulloblastoma, colon, and pancreatic tumors [69]. A phase I clinical trial of this agent involved 68 patients with refractory, locally advanced, or metastatic solid tumors; it demonstrated an acceptable safety profile with no dose-limiting toxicity (DLT). The most frequently reported adverse events (AEs) have been muscle spasms, dysgeusia, fatigue, alopecia, and nausea. Seven grade 4 AEs (hyponatremia, fatigue, pyelonephritis, presyncope, resectable pancreatic adenocarcinoma, and paranoia with hyperglycemia) were reported in six patients (9%). Grade 3 AEs were observed in 28% of patients; they most commonly included hyponatremia (10%), abdominal pain (7%), and fatigue (6%) [69].
A phase 1 clinical trial of vismodegib has shown significant clinical activity in tumors with driver mutations of the Hh pathway [64, 65, 69]. The overall response rate (defined as both complete and partial responses) in advanced BCC was achieved in 19 out of 33 patients (58%). Complete response was achieved in two patients. The authors reported a median response duration of 12.8 months (range: 3.7–26.4 months) among evaluable patients [69]. The clinical benefit of vismodegib has been also reported in medulloblastoma. Treatment of a patient with refractory widespread metastatic medulloblastoma with a somatic mutation in PTCH1 (PTCH1-W844C) and loss of heterozygosity resulted in a rapid, although transient, regression of disease at all tumor sites and improvement of symptoms [64].
As the maximum tolerated dose was not reached, the recommended phase II dose was chosen as 150 mg p.o. daily based on the pharmacokinetic data showing saturable plasma concentrations of vismodegib. An attempt was made to reduce the frequency to 150 mg three times per week or once weekly following a loading dose of 150 mg daily for 11 days, but this maneuver failed to achieve unbound plasma concentration associated with efficacy in patients with BCC and medulloblastoma [70].
Two recently published clinical trials have further demonstrated the remarkable clinical benefit of this agent in BCC [71, 72]. In a study by Sekulic et al., vismodegib at a dose of 150 mg daily was associated with objective response rates of 30% and 43% in patients with locally advanced and metastatic BCC, respectively. In the group of locally advanced BCC, 13 out of 63 patients (21%) had a complete response. Median duration of response for both groups was 7.6 months [71]. In addition, vismodegib had promising results in a randomized placebo-controlled trial in patients with BCNS [72]. The primary endpoint of this study was reduction in the incidence of new BCCs that were eligible for surgical resection. Vismodegib at a daily dose of 150 mg significantly reduced the per-patient rate of new surgically eligible BCCs (2 vs. 29 cases per group per year). Although no tumors progressed during treatment with vismodegib, BCCs and palmar-plantar pits associated with BCNS both recurred after stopping the treatment [72]. Both studies reported notable drug-related toxicity profiles. Muscle spasms, alopecia, dysgeusia, nausea, decreased appetite, diarrhea, fatigue, and weight loss were the most commonly reported adverse events [71, 72]. Although most of these toxicities were low grade in nature, they led to treatment discontinuation in 54% of patients in the BCNS study. Furthermore, in the study by Sekulic et al., seven fatal events were reported [71]. Although all seven patients had coexisting conditions at baseline, it is relevant to note that three of the deaths were due to unknown causes [71].
A phase II randomized trial of vismodegib or placebo in combination with oxaliplatin, 5-fluorouracil, and leucovorin or irinotecan, 5-fluorouracil, and leucovorin plus bevacizumab in 195 patients with previously untreated metastatic colorectal cancer did not meet the primary endpoint of extending the progression-free survival [66]. Another phase II randomized placebo-controlled trial investigating vismodegib as maintenance therapy in patients with ovarian cancer in second or third complete remission did not demonstrate any significant improvement in progression-free survival [67]. These results underline the complex challenges in attaining clinical benefit from Hh pathway inhibition and also highlight the lack of utility for this class of agents to be used in cancer management when aberrance of Hh pathway is not the oncogenic driver.
At present, there are several phase II trials investigating the role of this agent in specific tumor types and different combinations with chemotherapy regimens (Table 2).
Table 2.
Hedgehog pathway inhibitors currently in clinical trials in cancer
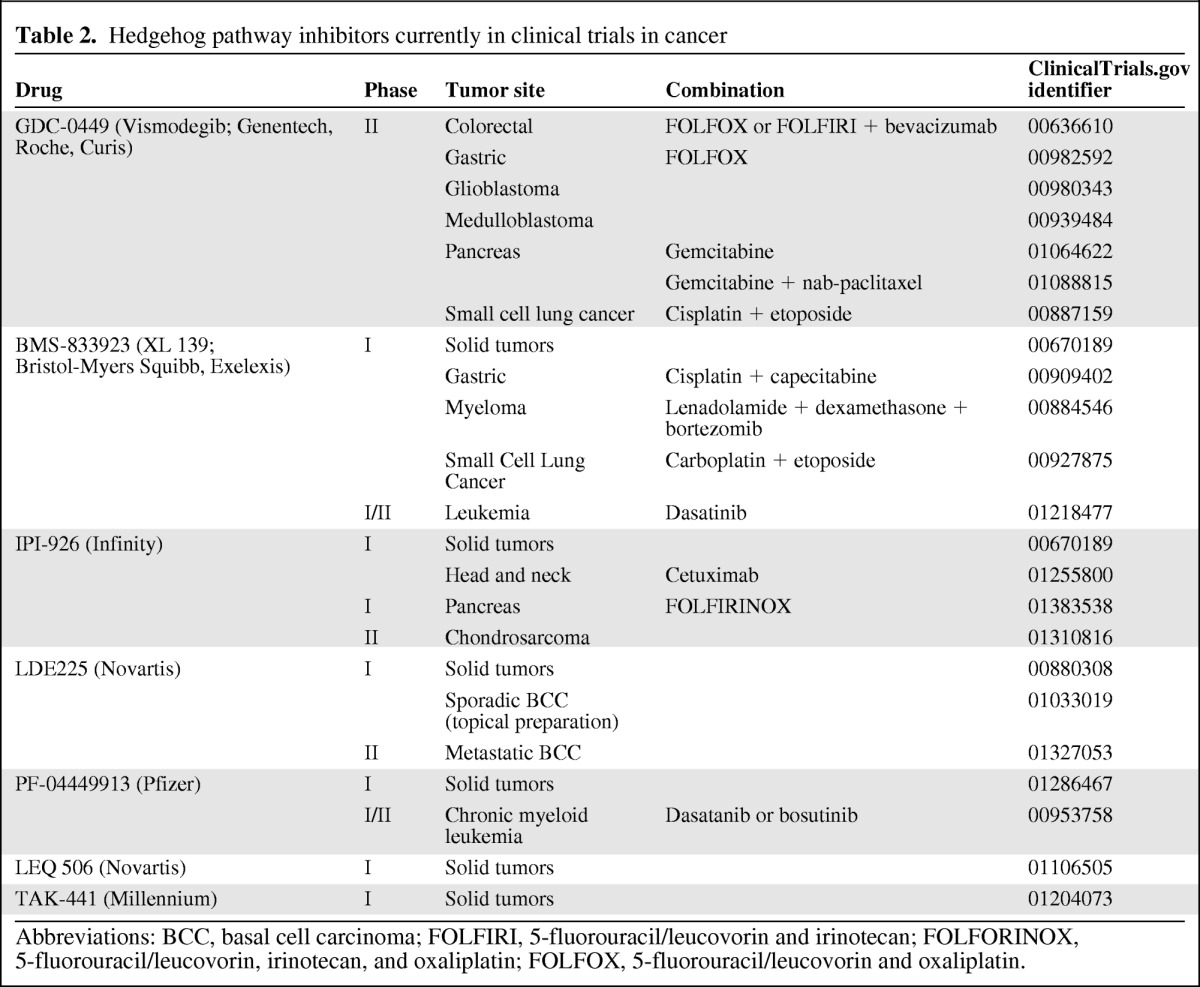
Abbreviations: BCC, basal cell carcinoma; FOLFIRI, 5-fluorouracil/leucovorin and irinotecan; FOLFORINOX, 5-fluorouracil/leucovorin, irinotecan, and oxaliplatin; FOLFOX, 5-fluorouracil/leucovorin and oxaliplatin.
BMS-833923 (XL139)
BMS-833923 is another potent, oral, small-molecule antagonist of SMO [73]. The inhibitory effect of this agent has been demonstrated in multiple cell lines, including engineered human medulloblastoma cell lines. A phase I trial of BMS-833923 has shown good clinical tolerance at doses up to 360 mg; evaluation of this agent in a phase I setting was still ongoing at the time of abstract reporting. The most common AEs include dysgeusia (44%), muscle spasms (44%), alopecia (15%), diarrhea (11%), myalgia (11%), dry mouth (11%), and nausea (11%). Grade 2 pancreatitis and lipase elevation occurred in one patient at a dose of 240 mg, whereas grade 3 hypophosphatemia was observed in one patient at 540 mg.
In this study, one patient with BCNS achieved complete response, one patient with non-small cell lung cancer had a partial response, and 21% (6 of 28 patients) remained on treatment longer than 100 days at the time of report [73]. Combination regimens of BMS-833923 with chemotherapy in several tumor types are currently underway; they are summarized in Table 2.
IPI-926 (Saridegib)
IPI-926 is an orally bioavailable, semisynthetic derivative of cyclopamine, which targets the Hh pathway by inhibiting SMO [74, 75]. When combined with gemcitabine, IPI-926 increases survival in a gemcitabine-resistant pancreatic cancer mouse model [76]. The potent antitumor activity of IPI-926 has been shown in medulloblastoma mouse models that are compound heterozygous for PTCH1 and HIC1 mutations [75]. In a phase I dose escalation trial conducted by Rudin et al., IPI-926 was well tolerated up to a dose of 160 mg daily. The most common AEs were fatigue (29% total; 3% were grade 3), alanine aminotransferase elevation (20%; 8% were grade 3), aspartate aminotransferase elevation (19%; 4% were grade 3); and nausea (19%; none were grade 3). Dose-limiting toxicities in this study were asymptomatic grade 3 increased transaminases and/or bilirubin (one patient at 160 mg, four patients at >160 mg), all of which resolved when drug was held. Clinical activity was observed in three patients with locally advanced BCC who received IPI-926 for longer than 12 months at the time of report [77].
The use of this agent in pancreatic cancer has also been evaluated. Initial preclinical studies demonstrated promising activity in a pancreatic cancer mouse model [76]. However, in a randomized, phase II, placebo-controlled study of gemcitabine plus IPI-926 versus gemcitabine plus placebo in patients with metastatic pancreatic cancer, the trial was stopped early due to a difference in survival favoring the placebo arm. Different combinations of IPI-926 with systemic agents as well as tumor specific trials are currently underway (Table 2).
LDE-225
LDE-225 is a selective orally bioavailable inhibitor of SMO. Phase I dose escalation of this agent in 72 patients with advanced solid tumors has shown an acceptable safety profile with a recommended phase II dose of 800 mg daily. The most frequently reported AEs were fatigue, nausea, vomiting, anorexia, muscle cramps, myalgia, and dysgeusia [78]. Topical preparation of LDE-225 has been also developed and studied in patients with BCNS. In a double-blind randomized study involving eight patients with 27 BCC tumors, of 13 BCCs treated with active compound, three showed complete responses, nine had partial responses, and one had no clinical response, in contrast to only one partial response out of 14 BCC tumors treated with the vehicle [79].
Clinical activity of LDE-225 was seen in medulloblastoma (partial response in one patient) and advanced BCC (complete response in one patient and partial responses in four patients). Disease stabilization (>4 months) was observed in five patients with lung adenocarcinoma, spindle cell sarcoma, breast cancer, and BCC [78].
PF-04449913, LEQ 506, and TAK-441
PF-04449913, LEQ 506, and TAK-441 are oral inhibitors of the Hh pathway that are currently undergoing evaluations in a phase I setting. A summary is detailed in Table 2.
Hh Inhibitors Antagonizing Components Downstream to SMO
Increased understanding of the Hh signaling pathway has led to new discoveries with previously known anticancer agents, such as arsenic. Recent data have shown that arsenicals antagonize the Hh pathway by targeting GLI transcriptional factors. Kim et al. [80] showed potent inhibitory effects of arsenic trioxide (ATO) and phenylarsine oxide on the Hh pathway in a cell line model (NIH 3T3). As arsenic treatment has been shown to affect the p38 MAPK and JNK pathways, the authors of this paper investigated whether the involvements of these two pathways are required for arsenic inhibition of Hh signaling. Interestingly, the inhibitory effect of the Hh pathway by arsenic was sustained even in the presence of a p38 MAP kinase (SB203580) or JNK (SP600125) inhibitors. This data suggest that the anti-Hh effect of arsenicals is independent of the p38 MAPK and JNK pathways.
In comparison with control specimens, ATO also significantly delayed the growth of medulloblastoma allografts derived from Ptch +/− p53 −/− mice [80]. Furthermore, ATO inhibited Hh activity mediated by SMO-D477G, a mutation that confers resistance to SMO antagonist. In another study by Beauchamp et al., ATO inhibited GLI1 activity in HepG2 cells co-transfected with GLI1 and pGL38×GLI binding element-driven luciferase reporter. This inhibitory effect was shown to be by direct interaction of ATO with GLI1 protein. These investigators also had previously established GLI1 as an important transcriptional target of the oncogenic fusion protein EWS/FLI1, an important driver of Ewing sarcomas [81]. Further observations corroborate this finding; for instance, treatment of Ewing sarcoma family of tumors (ESFT) cell lines with ATO led to marked cytotoxicity and the presence of higher GLI1/2 expression seems to indicate increased sensitivity to ATO. Additionally, tumor growth in ESFT xenografts was inhibited with ATO administration; this treatment was associated with an increased survival in constitutively activated SMO transgenic mouse model for medulloblastoma (ND2:SmoA1), with significantly decreased GLI target gene expression [82].
Novel inhibitors of GLI have also been evaluated in the preclinical setting, including GANT61, a small molecule which inhibits direct binding of GLI1 and GLI2 to the promoters of target genes HIP1, BCL-2, and the transcriptional activation of BCL-2 [11]. The use of this agent resulted in significant cell death across five different human colon carcinoma cell lines and was found to be more potent than cyclopamine [83]. Significant anti-tumor activity of GANT61 has been also noted in prostate cancer human xenografts [84].
A polymeric nanoparticle encapsulated formulation of a novel GLI1/GLI2 inhibitor, HPI-1 (NanoHHI), has also undergone early preclinical testing. This agent was shown to actively inhibit the proliferation and invasion of human HCC cell lines. NanoHHI also had a potent activity in HCC xenografts and resulted in decrease in the weight of the subcutaneous tumor xenografts. Additionally, it also significantly reduced the population of CD133-expressing HCC cells in orthotopic liver tumors [85].
Resistance to Hh Inhibitors Antagonizing SMO
The early data generated from a number of Hh inhibitors held promise for some tumor types, especially in patients with BCCs and medulloblastoma. However, akin to any targeted therapies that have been developed in cancer, the emergence of drug resistance is a distinct problem. Yauch et al. presented molecular profiling results at the time of resistance development from a patient with medulloblastoma taking vismodegib; the patient had an impressive clinical and radiological response at 2 months but relapsed at 3 months after treatment initiation. Although PTCH1 mutation was found in the original tumor, a novel mutation in SMO was detected after treatment initiation, explaining the acquired resistance [86].
Indeed, mutations at multiple sites in SMO can confer resistance to vismodegib and other SMO antagonists. A SMO mutation, heterozygous G-to-C mis-sense mutation at position 1697, that resulted in a change of codon 473 from Asp to His (D473H) has been described in Hh inhibitor resistance. Lack of specific binding of 14C-labeled GDC-0449 to SMO-D473H suggested deficiency in drug binding as a mechanism for developing resistance. Interestingly, a mutation altering the same amino acid also arose in a vismodegib-resistant mouse model [86].
Amplifications of GLI2 transcription factor and Hh target gene Cyclin D1 have been identified as alternative mechanisms for the development of resistance to Hh inhibitors [87]. Focal amplification of GLI2 conferred resistance to LDE225 in medulloblastoma mouse models. Additionally, a small number of resistant tumors also showed increased GLI2 mRNA expression in the absence of clear amplification, suggesting that upregulation may be secondary to alternative mechanisms [88].
Compensatory upregulation of the IGF-1R/PI3K pathway may also play a role in resistance development to SMO antagonists. This is based on the observation of increased upregulation of the IGF-1R/PI3K pathway in LDE225-resistant tumor samples. In keeping with this finding, the addition of the PI3K inhibitor BKM120 or the dual PI3K-mTOR inhibitor BEZ235 to the initial treatment with the SMO antagonist markedly delayed or even prevented the development of drug resistance in Ptch+/− Hic+/− mouse medulloblastomas [88].
Potential On-Target Adverse Effects of Inhibiting the Hh Pathway
The Hh pathway has a substantial role in endochondral ossification and bone homeostasis [89, 90]. This raises the possibility of adverse skeletal effects with Hh pathway inhibition. In one study using mouse models, the use of cyclopamine resulted in significantly lower bone mass and mineral density in comparison to the control group [91]. Moreover, transient inhibition of the Hh pathway in young mice has resulted in permanent bone defects and altered growth, which persisted after cessation of the Hh pathway inhibitor and restoration of pathway [92]. Given the fact that medulloblastomas and BCNS mostly present in pediatric patients, different Hh pathway inhibitors will be developed and studied in this patient population. Thus, special attention should be made to detect potential skeletal adverse effects, especially in pediatric patients.
Conclusion
Hh inhibitors currently represent an opportunity in the quest for novel anticancer therapies. However, the understanding of how this pathway affects different cancers is still under intense study and is not fully understood. This effort is further complicated by the limitations of earlier preclinical studies in which cyclopamine was used at a high dose.
For tumors in which Hh components are mutated, such as BCC, BCNS, and medulloblastoma, the proof of concept for this class of agents has been satisfied. Challenges still exist for the utility of these drugs in other tumor types. In such cancers, targeting Hh alone is unlikely to be effective and appropriate combinations with cytotoxic or other targeted agents need to be studied further. Reassuringly, a number of these initiatives are already in progress (Table 2). Moreover, the development of agents that target the Hh signaling pathway downstream to SMO will also provide further useful therapeutic strategies in this area.
Another challenging aspect in the development of Hh inhibitors is the development of acquired drug resistance, as it can substantially diminish the potential that these agents hold. Deeper understanding on the resistance mechanisms will enable the development of better strategies to overcome this problem. Currently, strategies may include concurrent combination with other targeted therapies such as PI3K-mTOR inhibitors or the development of second-generation Hh inhibitors that cotarget compensatory pathways conferring resistance. Finally, the development of correlative biomarker studies could also be informative in shedding light on the optimal patient populations to treat and should be undertaken with priority.
Footnotes
- (C/A)
- Consulting/advisory relationship
- (RF)
- Research funding
- (E)
- Employment
- (H)
- Honoraria received
- (OI)
- Ownership interests
- (IP)
- Intellectual property rights/inventor/patent holder
- (SAB)
- Scientific advisory board
Author Contributions
Conception/Design: Lillian L. Siu, Albiruni A. Razak
Provision of study material or patients: Solmaz Sahebjam, Albiruni A. Razak
Collection and/or assembly of data: Solmaz Sahebjam, Albiruni A. Razak
Data analysis and interpretation: Solmaz Sahebjam, Lillian L. Siu, Albiruni A. Razak
Manuscript writing: Solmaz Sahebjam, Lillian L. Siu, Albiruni A. Razak
Final approval of manuscript: Solmaz Sahebjam, Lillian L. Siu, Albiruni A. Razak
References
- 1.Varjosalo M, Bjorklund M, Cheng F, et al. Application of active and kinase-deficient kinome collection for identification of kinases regulating Hedgehog signaling. Cell. 2008;133:537–548. doi: 10.1016/j.cell.2008.02.047. [DOI] [PubMed] [Google Scholar]
- 2.Varjosalo M, Taipale J. Hedgehog: Functions and mechanisms. Genes Dev. 2008;22:2454–2472. doi: 10.1101/gad.1693608. [DOI] [PubMed] [Google Scholar]
- 3.Wicking C, Smyth I, Bale A. The Hedgehog signalling pathway in tumorigenesis and development. Oncogene. 1999;18:7844–7851. doi: 10.1038/sj.onc.1203282. [DOI] [PubMed] [Google Scholar]
- 4.Hahn H, Wicking C, Zaphiropoulous PG, et al. Mutations of the human homolog of drosophila patched in the nevoid basal cell carcinoma syndrome. Cell. 1996;85:841–851. doi: 10.1016/s0092-8674(00)81268-4. [DOI] [PubMed] [Google Scholar]
- 5.Wang Q, Huang C, Zeng F, et al. Activation of the Hh pathway in periosteum-derived mesenchymal stem cells induces bone formation in vivo: Implication for postnatal bone repair. Am J Pathol. 2010;177:3100–3111. doi: 10.2353/ajpath.2010.100060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beachy PA, Karhadkar SS, Berman DM. Tissue repair and stem cell renewal in carcinogenesis. Nature. 2004;432:324–331. doi: 10.1038/nature03100. [DOI] [PubMed] [Google Scholar]
- 7.Levi B, James AW, Nelson ER, et al. Human adipose-derived stromal cells stimulate autogenous skeletal repair via paracrine Hedgehog signaling with calvarial osteoblasts. Stem Cells Dev. 2011;20:243–257. doi: 10.1089/scd.2010.0250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Watkins DN, Berman DM, Burkholder SG, et al. Hedgehog signalling within airway epithelial progenitors and in small-cell lung cancer. Nature. 2003;422:313–317. doi: 10.1038/nature01493. [DOI] [PubMed] [Google Scholar]
- 9.Low JA, de Sauvage FJ. Clinical experience with Hedgehog pathway inhibitors. J Clin Oncol. 2011;28:5321–5326. doi: 10.1200/JCO.2010.27.9943. [DOI] [PubMed] [Google Scholar]
- 10.Merchant AA, Matsui W. Targeting Hedgehog–A cancer stem cell pathway. Clin Cancer Res. 2010;16:3130–3140. doi: 10.1158/1078-0432.CCR-09-2846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mazumdar T, Devecchio J, Agyeman A, et al. Blocking Hedgehog survival signaling at the level of the GLI genes induces DNA damage and extensive cell death in human colon carcinoma cells. Cancer Res. 2011;71:5904–5914. doi: 10.1158/0008-5472.CAN-10-4173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goetz SC, Anderson KV. The primary cilium: A signalling centre during vertebrate development. Nat Rev Genet. 2010;11:331–344. doi: 10.1038/nrg2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ruiz i Altaba A, Sanchez P, Dahmane N. GLI and Hedgehog in cancer: Tumours, embryos and stem cells. Nat Rev Cancer. 2002;2:361–372. doi: 10.1038/nrc796. [DOI] [PubMed] [Google Scholar]
- 14.Ruiz i Altaba A, Mas C, Stecca B. The GLI code: An information nexus regulating cell fate, stemness and cancer. Trends Cell Biol. 2007;17:438–447. doi: 10.1016/j.tcb.2007.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stecca B, Ruiz IAA. Context-dependent regulation of the GLI code in cancer by Hedgehog and non-Hedgehog signals. J Mol Cell Biol. 2010;2:84–95. doi: 10.1093/jmcb/mjp052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jiang J. Regulation of Hh/GLI signaling by dual ubiquitin pathways. Cell Cycle. 2006;5:2457–2463. doi: 10.4161/cc.5.21.3406. [DOI] [PubMed] [Google Scholar]
- 17.Pearse RV, 2nd, Collier LS, Scott MP, et al. Vertebrate homologs of drosophila suppressor of fused interact with the GLI family of transcriptional regulators. Dev Biol. 1999;212:323–336. doi: 10.1006/dbio.1999.9335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wong SY, Reiter JF. The primary cilium at the crossroads of mammalian Hedgehog signaling. Curr Top Dev Biol. 2008;85:225–260. doi: 10.1016/S0070-2153(08)00809-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barnfield PC, Zhang X, Thanabalasingham V, et al. Negative regulation of GLI1 and GLI2 activator function by suppressor of fused through multiple mechanisms. Differentiation. 2005;73:397–405. doi: 10.1111/j.1432-0436.2005.00042.x. [DOI] [PubMed] [Google Scholar]
- 20.Svard J, Heby-Henricson K, Persson-Lek M, et al. Genetic elimination of suppressor of fused reveals an essential repressor function in the mammalian hedgehog signaling pathway. Dev Cell. 2006;10:187–197. doi: 10.1016/j.devcel.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 21.Kogerman P, Grimm T, Kogerman L, et al. Mammalian suppressor-of-fused modulates nuclear-cytoplasmic shuttling of Gli-1. Nat Cell Biol. 1999;1:312–319. doi: 10.1038/13031. [DOI] [PubMed] [Google Scholar]
- 22.Cheung HO, Zhang X, Ribeiro A, et al. The kinesin protein Kif7 is a critical regulator of Gli transcription factors in mammalian Hedgehog signaling. Sci Signal. 2009;2:ra29. doi: 10.1126/scisignal.2000405. [DOI] [PubMed] [Google Scholar]
- 23.Liem KF, Jr., He M, Ocbina PJ, et al. Mouse Kif7/Costal2 is a cilia-associated protein that regulates sonic hedgehog signaling. Proc Natl Acad Sci U S A. 2009;106:13377–13382. doi: 10.1073/pnas.0906944106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ailles L, Siu LL. Targeting the Hedgehog pathway in cancer: Can the spines be smoothened? Clin Cancer Res. 2011;17:2071–2073. doi: 10.1158/1078-0432.CCR-11-0211. [DOI] [PubMed] [Google Scholar]
- 25.Gailani MR, Stahle-Backdahl M, Leffell DJ, et al. The role of the human Homologue of drosophila patched in sporadic basal cell carcinomas. Nat Genet. 1996;14:78–81. doi: 10.1038/ng0996-78. [DOI] [PubMed] [Google Scholar]
- 26.Dahmane N, Lee J, Robins P, et al. Activation of the transcription factor GLI1 and the sonic Hedgehog signalling pathway in skin tumours. Nature. 1997;389:876–881. doi: 10.1038/39918. [DOI] [PubMed] [Google Scholar]
- 27.Aszterbaum M, Rothman A, Johnson RL, et al. Identification of mutations in the human patched gene in sporadic basal cell carcinomas and in patients with the basal cell nevus syndrome. J Invest Dermatol. 1998;110:885–888. doi: 10.1046/j.1523-1747.1998.00222.x. [DOI] [PubMed] [Google Scholar]
- 28.Lam CW, Xie J, To KF, et al. A frequent activated Smoothened mutation in sporadic basal cell carcinomas. Oncogene. 1999;18:833–836. doi: 10.1038/sj.onc.1202360. [DOI] [PubMed] [Google Scholar]
- 29.Lee Y, Miller HL, Jensen P, et al. A molecular fingerprint for medulloblastoma. Cancer Res. 2003;63:5428–5437. [PubMed] [Google Scholar]
- 30.Raffel C, Jenkins RB, Frederick L, et al. Sporadic medulloblastomas contain PTCH mutations. Cancer Res. 1997;57:842–845. [PubMed] [Google Scholar]
- 31.Wolter M, Reifenberger J, Sommer C, et al. Mutations in the human homologue of the drosophila segment polarity gene patched (PTCH) in sporadic basal cell carcinomas of the skin and primitive neuroectodermal tumors of the central nervous system. Cancer Res. 1997;57:2581–2585. [PubMed] [Google Scholar]
- 32.Goodrich LV, Milenkovic L, Higgins KM, et al. Altered neural cell fates and medulloblastoma in mouse patched mutants. Science. 1997;277:1109–1113. doi: 10.1126/science.277.5329.1109. [DOI] [PubMed] [Google Scholar]
- 33.Oro AE, Higgins KM, Hu Z, et al. Basal cell carcinomas in mice overexpressing sonic Hedgehog. Science. 1997;276:817–821. doi: 10.1126/science.276.5313.817. [DOI] [PubMed] [Google Scholar]
- 34.Bale AE, Yu KP. The Hedgehog pathway and basal cell carcinomas. Hum Mol Genet. 2001;10:757–762. doi: 10.1093/hmg/10.7.757. [DOI] [PubMed] [Google Scholar]
- 35.Reifenberger J, Wolter M, Weber RG, et al. Missense mutations in smoh in sporadic basal cell carcinomas of the skin and primitive neuroectodermal tumors of the central nervous system. Cancer Res. 1998;58:1798–1803. [PubMed] [Google Scholar]
- 36.Xie J, Murone M, Luoh SM, et al. Activating smoothened mutations in sporadic basal-cell carcinoma. Nature. 1998;391:90–92. doi: 10.1038/34201. [DOI] [PubMed] [Google Scholar]
- 37.Slade I, Murray A, Hanks S, et al. Heterogeneity of familial medulloblastoma and contribution of germline PTCH1 and SUFU mutations to sporadic medulloblastoma. Fam Cancer. 2011;10:337–342. doi: 10.1007/s10689-010-9411-0. [DOI] [PubMed] [Google Scholar]
- 38.Taylor MD, Liu L, Raffel C, et al. Mutations in SUFU predispose to medulloblastoma. Nat Genet. 2002;31:306–310. doi: 10.1038/ng916. [DOI] [PubMed] [Google Scholar]
- 39.Jones S, Zhang X, Parsons DW, et al. Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science. 2008;321:1801–1806. doi: 10.1126/science.1164368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Caro I, Low JA. The role of the Hedgehog signaling pathway in the development of basal cell carcinoma and opportunities for treatment. Clin Cancer Res. 2010;16:3335–3339. doi: 10.1158/1078-0432.CCR-09-2570. [DOI] [PubMed] [Google Scholar]
- 41.Park KS, Martelotto LG, Peifer M, et al. A crucial requirement for Hedgehog signaling in small cell lung cancer. Nat Med. 2011;17:1504–1508. doi: 10.1038/nm.2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gialmanidis IP, Bravou V, Amanetopoulou SG, et al. Overexpression of Hedgehog pathway molecules and FOXM1 in non-small cell lung carcinomas. Lung Cancer. 2009;66:64–74. doi: 10.1016/j.lungcan.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 43.Kubo M, Nakamura M, Tasaki A, et al. Hedgehog signaling pathway is a new therapeutic target for patients with breast cancer. Cancer Res. 2004;64:6071–6074. doi: 10.1158/0008-5472.CAN-04-0416. [DOI] [PubMed] [Google Scholar]
- 44.Katano M. Hedgehog signaling pathway as a therapeutic target in breast cancer. Cancer Lett. 2005;227:99–104. doi: 10.1016/j.canlet.2004.11.030. [DOI] [PubMed] [Google Scholar]
- 45.Cui W, Wang LH, Wen YY, et al. Expression and regulation mechanisms of sonic Hedgehog in breast cancer. Cancer Sci. 2010;101:927–933. doi: 10.1111/j.1349-7006.2010.01495.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bhattacharya R, Kwon J, Ali B, et al. Role of Hedgehog signaling in ovarian cancer. Clin Cancer Res. 2008;14:7659–7666. doi: 10.1158/1078-0432.CCR-08-1414. [DOI] [PubMed] [Google Scholar]
- 47.Liao X, Siu MK, Au CW, et al. Aberrant activation of Hedgehog signaling pathway in ovarian cancers: Effect on prognosis, cell invasion and differentiation. Carcinogenesis. 2009;30:131–140. doi: 10.1093/carcin/bgn230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ray A, Meng E, Reed E, et al. Hedgehog signaling pathway regulates the growth of ovarian cancer spheroid forming cells. Int J Oncol. 2011;39:797–804. doi: 10.3892/ijo.2011.1093. [DOI] [PubMed] [Google Scholar]
- 49.Meyers-Needham M, Lewis JA, Gencer S, et al. Off-target function of the sonic-Hedgehog inhibitor cyclopamine in mediating apoptosis via nitric oxide-dependent neutral sphingomyelinase 2/ceramide induction. Mol Cancer Ther. 2012;11:1092–1102. doi: 10.1158/1535-7163.MCT-11-0705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Braun S, Oppermann H, Mueller A, et al. Hedgehog signaling in glioblastoma multiforme. Cancer Biol Ther. 2012;13:487–495. doi: 10.4161/cbt.19591. [DOI] [PubMed] [Google Scholar]
- 51.Lipinski RJ, Hutson PR, Hannam PW, et al. Dose- and route-dependent teratogenicity, toxicity, and pharmacokinetic profiles of the Hedgehog signaling antagonist cyclopamine in the mouse. Toxic Sci. 2008;104:189–197. doi: 10.1093/toxsci/kfn076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ng JM, Curran T. The Hedgehog's tale: Developing strategies for targeting cancer. Nat Rev Cancer. 2011;11:493–501. doi: 10.1038/nrc3079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yauch RL, Gould SE, Scales SJ, et al. A paracrine requirement for Hedgehog signalling in cancer. Nature. 2008;455:406–410. doi: 10.1038/nature07275. [DOI] [PubMed] [Google Scholar]
- 54.Dierks C, Grbic J, Zirlik K, et al. Essential role of stromally induced Hedgehog signaling in B-cell malignancies. Nat Med. 2007;13:944–951. doi: 10.1038/nm1614. [DOI] [PubMed] [Google Scholar]
- 55.Clement V, Sanchez P, de Tribolet N, et al. Hedgehog-GLI1 signaling regulates human glioma growth, cancer stem cell self-renewal, and tumorigenicity. Curr Biol. 2007;17:165–172. doi: 10.1016/j.cub.2006.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Feldmann G, Dhara S, Fendrich V, et al. Blockade of Hedgehog signaling inhibits pancreatic cancer invasion and metastases: A new paradigm for combination therapy in solid cancers. Cancer Res. 2007;67:2187–2196. doi: 10.1158/0008-5472.CAN-06-3281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu S, Dontu G, Mantle ID, et al. Hedgehog signaling and BMI-1 regulate self-renewal of normal and malignant human mammary stem cells. Cancer Res. 2006;66:6063–6071. doi: 10.1158/0008-5472.CAN-06-0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhao C, Chen A, Jamieson CH, et al. Hedgehog signalling is essential for maintenance of cancer stem cells in myeloid leukaemia. Nature. 2009;458:776–779. doi: 10.1038/nature07737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bar EE, Chaudhry A, Lin A, et al. Cyclopamine-mediated Hedgehog pathway inhibition depletes stem-like cancer cells in glioblastoma. Stem Cells. 2007;25:2524–2533. doi: 10.1634/stemcells.2007-0166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rasheed ZA, Yang J, Wang Q, et al. Prognostic significance of tumorigenic cells with mesenchymal features in pancreatic adenocarcinoma. J Natl Cancer Inst. 2010;102:340–351. doi: 10.1093/jnci/djp535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pannuti A, Foreman K, Rizzo P, et al. Targeting notch to target cancer stem cells. Clin Cancer Res. 2010;16:3141–3152. doi: 10.1158/1078-0432.CCR-09-2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Takahashi-Yanaga F, Kahn M. Targeting wnt signaling: Can we safely eradicate cancer stem cells? Clin Cancer Res. 2010;16:3153–3162. doi: 10.1158/1078-0432.CCR-09-2943. [DOI] [PubMed] [Google Scholar]
- 63.Varnat F, Duquet A, Malerba M, et al. Human colon cancer epithelial cells harbour active Hedgehog-GLI signalling that is essential for tumour growth, recurrence, metastasis and stem cell survival and expansion. EMBO Mol Med. 2009;1:338–351. doi: 10.1002/emmm.200900039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rudin CM, Hann CL, Laterra J, et al. Treatment of medulloblastoma with Hedgehog pathway inhibitor GDC-0449. New Engl J Med. 2009;361:1173–1178. doi: 10.1056/NEJMoa0902903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Von Hoff DD, LoRusso PM, Rudin CM, et al. Inhibition of the Hedgehog pathway in advanced basal-cell carcinoma. New Engl J Med. 2009;361:1164–1172. doi: 10.1056/NEJMoa0905360. [DOI] [PubMed] [Google Scholar]
- 66.Berlin J. A phase 2, randomized, double-blind, placebo-controlled study of Hedgehog pathway inhibitor (Hpi) GDC-0449 in patients with previously untreated metastatic colorectal cancer (mcrc) ESMO Meeting Abstracts. 2010;21:viii10. [Google Scholar]
- 67.Fehrenbacher L, Kaye S, Holloway R. A phase 2, randomized, placebo-controlled study of Hedgehog (Hh) pathway inhibitor GDC-0449 as maintenance therapy in patients with ovarian cancer in 2nd or 3rd complete remission (CR) ESMO Meeting Abstracts. 2010;21:viii11. [Google Scholar]
- 68.Robarge KD, Brunton SA, Castanedo GM, et al. GDC-0449: A potent inhibitor of Hedgehog pathway. Bioorg Med Chem Lett. 2009;19:5576–5581. doi: 10.1016/j.bmcl.2009.08.049. [DOI] [PubMed] [Google Scholar]
- 69.LoRusso PM, Rudin CM, Reddy JC, et al. Phase I trial of Hedgehog pathway inhibitor vismodegib (GDC-0449) in patients with refractory, locally advanced or metastatic solid tumors. Clin Cancer Res. 2011;17:2502–2511. doi: 10.1158/1078-0432.CCR-10-2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lorusso PM, Jimeno A, Dy G, et al. Pharmacokinetic dose-scheduling study of Hedgehog pathway inhibitor vismodegib (GDC-0449) in patients with locally advanced or metastatic solid tumors. Clin Cancer Res. 2011;17:5774–5782. doi: 10.1158/1078-0432.CCR-11-0972. [DOI] [PubMed] [Google Scholar]
- 71.Sekulic A, Migden MR, Oro AE, et al. Efficacy and safety of vismodegib in advanced basal-cell carcinoma. New Engl J Med. 2012;366:2171–2179. doi: 10.1056/NEJMoa1113713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tang JY, Mackay-Wiggan JM, Aszterbaum M, et al. Inhibiting the Hedgehog pathway in patients with the basal-cell nevus syndrome. New Engl J Med. 2012;366:2180–2188. doi: 10.1056/NEJMoa1113538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Siu LL, Papadopoulos K, Alberts SR, et al. A first-in-human, phase I study of an oral Hedgehog (Hh) pathway antagonist, BMS-833923 (XL139), in subjects with advanced or metastatic solid tumors. ASCO Meeting Abstracts. 2010;28:2501. [Google Scholar]
- 74.Tremblay MR, Nesler M, Weatherhead R, et al. Recent patents for Hedgehog pathway inhibitors for the treatment of malignancy. Expert Opin Ther Pat. 2009;19:1039–1056. doi: 10.1517/13543770903008551. [DOI] [PubMed] [Google Scholar]
- 75.Tremblay MR, Lescarbeau A, Grogan MJ, et al. Discovery of a potent and orally active Hedgehog pathway antagonist (IPI-926) J Med Chem. 2009;52:4400–4418. doi: 10.1021/jm900305z. [DOI] [PubMed] [Google Scholar]
- 76.Olive KP, Jacobetz MA, Davidson CJ, et al. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science. 2009;324:1457–1461. doi: 10.1126/science.1171362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rudin CM, Jimeno A, Miller WH, et al. A phase I study of IPI-926, a novel Hedgehog pathway inhibitor, in patients (pts) with advanced or metastatic solid tumors. ASCO Meeting Abstracts. 2011;29:3014. [Google Scholar]
- 78.Rodon Ahnert J, Baselga J, Tawbi HA, et al. A phase I dose-escalation study of LDE225, a Smoothened (SMO) antagonist, in patients with advanced solid tumors. ASCO Meeting Abstracts. 2010;28:2500. [Google Scholar]
- 79.Skvara H, Kalthoff F, Meingassner JG, et al. Topical treatment of basal cell carcinomas in nevoid basal cell carcinoma syndrome with a Smoothened inhibitor. J Invest Dermatol. 2011;131:1735–1744. doi: 10.1038/jid.2011.48. [DOI] [PubMed] [Google Scholar]
- 80.Kim J, Lee JJ, Gardner D, et al. Arsenic antagonizes the Hedgehog pathway by preventing ciliary accumulation and reducing stability of the GLI2 transcriptional effector. Proc Natl Acad Sci U S A. 2010;107:13432–13437. doi: 10.1073/pnas.1006822107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Beauchamp E, Bulut G, Abaan O, et al. GLI1 is a direct transcriptional target of EWS-FLI1 oncoprotein. J Biol Chem. 2009;284:9074–9082. doi: 10.1074/jbc.M806233200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Beauchamp EM, Ringer L, Bulut G, et al. Arsenic trioxide inhibits human cancer cell growth and tumor development in mice by blocking Hedgehog/GLI pathway. J Clin Inv. 2011;121:148–160. doi: 10.1172/JCI42874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mazumdar T, DeVecchio J, Shi T, et al. Hedgehog signaling drives cellular survival in human colon carcinoma cells. Cancer Res. 2011;71:1092–1102. doi: 10.1158/0008-5472.CAN-10-2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lauth M, Bergstrom A, Shimokawa T, et al. Inhibition of GLI-mediated transcription and tumor cell growth by small-molecule antagonists. Proc Natl Acad Sci U S A. 2007;104:8455–8460. doi: 10.1073/pnas.0609699104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Xu Y, Chenna V, Hu C, et al. A polymeric nanoparticle encapsulated hedgehog pathway inhibitor HPI-1 (nanoHHI) inhibits systemic metastases in an orthotopic model of human hepatocellular carcinoma. Clin Cancer Res. 2011;18:1291–1302. doi: 10.1158/1078-0432.CCR-11-0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yauch RL, Dijkgraaf GJ, Alicke B, et al. Smoothened mutation confers resistance to a Hedgehog pathway inhibitor in medulloblastoma. Science. 2009;326:572–574. doi: 10.1126/science.1179386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dijkgraaf GJ, Alicke B, Weinmann L, et al. Small molecule inhibition of GDC-0449 refractory smoothened mutants and downstream mechanisms of drug resistance. Cancer Res. 2011;71:435–444. doi: 10.1158/0008-5472.CAN-10-2876. [DOI] [PubMed] [Google Scholar]
- 88.Buonamici S, Williams J, Morrissey M, et al. Interfering with resistance to Smoothened antagonists by inhibition of the PI3K pathway in medulloblastoma. Sci Transl Med. 2010;2:51ra70. doi: 10.1126/scitranslmed.3001599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mackie EJ, Tatarczuch L, Mirams M. The skeleton: A multi-functional complex organ: The growth plate chondrocyte and endochondral ossification. J Endocrinol. 2011;211:109–121. doi: 10.1530/JOE-11-0048. [DOI] [PubMed] [Google Scholar]
- 90.Fuchs S, Dohle E, Kirkpatrick CJ. Sonic Hedgehog-mediated synergistic effects guiding angiogenesis and osteogenesis. Vitam Horm. 2012;88:491–506. doi: 10.1016/B978-0-12-394622-5.00022-5. [DOI] [PubMed] [Google Scholar]
- 91.Ohba S, Kawaguchi H, Kugimiya F, et al. Patched1 haploinsufficiency increases adult bone mass and modulates GLI3 repressor activity. Dev Cell. 2008;14:689–699. doi: 10.1016/j.devcel.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 92.Kimura H, Ng J, Curran T. Transient inhibition of the hedgehog pathway in young mice causes permanent defects in bone structure. Cancer Cell. 2008;13:249–260. doi: 10.1016/j.ccr.2008.01.027. [DOI] [PubMed] [Google Scholar]
- 93.Fiaschi M, Rozell B, Bergstrom A, et al. Development of mammary tumors by conditional expression of gli1. Cancer Res. 2009;69:4810–4817. doi: 10.1158/0008-5472.CAN-08-3938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Toftgard R. Hedgehog signalling in cancer. Cell Mol Life Sci. 2000;57:1720–1731. doi: 10.1007/PL00000654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Dahmane N, Sanchez P, Gitton Y, et al. The sonic Hedgehog-GLI pathway regulates dorsal brain growth and tumorigenesis. Development. 2001;128:5201–5212. doi: 10.1242/dev.128.24.5201. [DOI] [PubMed] [Google Scholar]
- 96.Ehtesham M, Sarangi A, Valadez JG, et al. Ligand-dependent activation of the Hedgehog pathway in glioma progenitor cells. Oncogene. 2007;26:5752–5761. doi: 10.1038/sj.onc.1210359. [DOI] [PubMed] [Google Scholar]
- 97.Irvine DA, Copland M. Targeting hedgehog in hematologic malignancy. Blood. 2012;119:2196–2204. doi: 10.1182/blood-2011-10-383752. [DOI] [PubMed] [Google Scholar]
- 98.Vestergaard J, Pedersen MW, Pedersen N, et al. Hedgehog signaling in small-cell lung cancer: Frequent in vivo but a rare event in vitro. Lung Cancer. 2006;52:281–290. doi: 10.1016/j.lungcan.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 99.Velcheti V, Govindan R. Hedgehog signaling pathway and lung cancer. J Thorac Oncol. 2007;2:7–10. doi: 10.1097/JTO.0b013e31802c0276. [DOI] [PubMed] [Google Scholar]
- 100.Hegde GV, Munger CM, Emanuel K, et al. Targeting of sonic Hedgehog-GLI signaling: A potential strategy to improve therapy for mantle cell lymphoma. Mol Cancer Ther. 2008;7:1450–1460. doi: 10.1158/1535-7163.MCT-07-2118. [DOI] [PubMed] [Google Scholar]
- 101.Lindemann RK. Stroma-initiated Hedgehog signaling takes center stage in B-cell lymphoma. Cancer Res. 2008;68:961–964. doi: 10.1158/0008-5472.CAN-07-5500. [DOI] [PubMed] [Google Scholar]
- 102.Singh RR, Cho-Vega JH, Davuluri Y, et al. Sonic hedgehog signaling pathway is activated in alk-positive anaplastic large cell lymphoma. Cancer Res. 2009;69:2550–2558. doi: 10.1158/0008-5472.CAN-08-1808. [DOI] [PubMed] [Google Scholar]
- 103.Singh RR, Kim JE, Davuluri Y, et al. Hedgehog signaling pathway is activated in diffuse large B-cell lymphoma and contributes to tumor cell survival and proliferation. Leukemia. 2010;24:1025–1036. doi: 10.1038/leu.2010.35. [DOI] [PubMed] [Google Scholar]
- 104.Peacock CD, Wang Q, Gesell GS, et al. Hedgehog signaling maintains a tumor stem cell compartment in multiple myeloma. Proc Natl Acad Sci U S A. 2007;104:4048–4053. doi: 10.1073/pnas.0611682104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Byrom J, Mudaliar V, Redman CW, et al. Loss of heterozygosity at chromosome 9q22–31 is a frequent and early event in ovarian tumors. Int J Oncol. 2004;24:1271–1277. [PubMed] [Google Scholar]
- 106.Tian H, Callahan CA, Kelly J, et al. Hedgehog signaling is restricted to the stromal compartment during pancreatic carcinogenesis. Proc Natl Acad Sci U S A. 2009;106:4254–4259. doi: 10.1073/pnas.0813203106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Thayer SP, di Magilano MP, Heiser PW, et al. Hedgehog is an early and late mediator of pancreatic cancer tumorigenesis. Nature. 2003;425:851–856. doi: 10.1038/nature02009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Karhadkar SS, Bova GS, Abdallah N, et al. Hedgehog signalling in prostate regeneration, neoplasia and metastasis. Nature. 2004;431:707–712. doi: 10.1038/nature02962. [DOI] [PubMed] [Google Scholar]
- 109.Sanchez P, Hernandez AM, Stecca B, et al. Inhibition of prostate cancer proliferation by interference with sonic Hedgehog-GLI1 signaling. Proc Natl Acad Sci U S A. 2004;101:12561–12566. doi: 10.1073/pnas.0404956101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Chen M, Tanner M, Levine AC, et al. Androgenic regulation of Hedgehog signaling pathway components in prostate cancer cells. Cell Cycle. 2009;8:149–157. doi: 10.4161/cc.8.1.7532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Berman DM, Karhadkar SS, Maitra A, et al. Widespread requirement for Hedgehog ligand stimulation in growth of digestive tract tumours. Nature. 2003;425:846–851. doi: 10.1038/nature01972. [DOI] [PubMed] [Google Scholar]
- 112.Tostar U, Toftgard R, Zaphiropoulos PG, et al. Reduction of human embryonal rhabdomyosarcoma tumor growth by inhibition of the Hedgehog signaling pathway. Genes Cancer. 2010;1:941–951. doi: 10.1177/1947601910385449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Tostar U, Malm CJ, Meis-Kindblom JM, et al. Deregulation of the hedgehog signalling pathway: A possible role for the PTCH and SUFU genes in human rhabdomyoma and rhabdomyosarcoma development. J Pathol. 2006;208:17–25. doi: 10.1002/path.1882. [DOI] [PubMed] [Google Scholar]
- 114.Zwerner JP, Joo J, Warner KL, et al. The EWS/FLI1 oncogenic transcription factor deregulates GLI1. Oncogene. 2008;27:3282–3291. doi: 10.1038/sj.onc.1210991. [DOI] [PubMed] [Google Scholar]
- 115.Hirotsu M, Setoguchi T, Sasaki H, et al. Smoothened as a new therapeutic target for human osteosarcoma. Mol Cancer. 2010;9:5. doi: 10.1186/1476-4598-9-5. [DOI] [PMC free article] [PubMed] [Google Scholar]