The radiobiologic, technical, and clinical aspects of stereotactic body radiation therapy are reviewed for various anatomical sites of oligometastases.
Keywords: Radiotherapy, Image-guided, Metastases, Neoplasm, Health care economics
Learning Objectives
After completing this course, the reader will be able to:
Assess stereotactic body radiation therapy (SBRT) as an emerging modality in the treatment of oligometastatic patients.
-
Discuss data on safety and efficacy of SBRT in the oligometastatic setting.
Evaluate SBRT as a competitive option in patients with a low burden of disease in the metastatic setting.
This article is available for continuing medical education credit at CME.TheOncologist.com
Abstract
In patients with proven distant metastases from solid tumors, it has been a notion that the condition is incurable, warranting palliative care only. The term “oligometastases” was coined to refer to isolated sites of metastasis, whereby the entire burden of disease can be recognized as a finite number of discrete lesions that can be potentially cured with local therapies. Stereotactic body radiation therapy (SBRT) is a novel treatment modality in radiation oncology that delivers a very high dose of radiation to the tumor target with high precision using single or a small number of fractions. SBRT is the result of technological advances in patient and tumor immobilization, image guidance, and treatment planning and delivery. A number of studies, both retrospective and prospective, showed promising results in terms of local tumor control and, in a limited subset of patients, of survival. This article reviews the radiobiologic, technical, and clinical aspects of SBRT for various anatomical sites.
Introduction
Improvements in the early detection of distant disease sites now frequently allow the diagnosis of single or limited organ metastases, defined as oligometastases [1]. In this setting, local treatments for oligometastases have been widely investigated and adopted for many cancers with the goal of improving disease control and survival outcomes. For several anatomical sites, surgical resection of metastases prolongs survival in selected patients [1, 2]. For example, surgical resection became the standard choice for patients with oligometastatic lung cancer, even though the benefits of resection and appropriate selection criteria in patients who develop metastases are poorly defined [1] and the ideal candidates for local therapy are difficult to select. It has been hypothesized that there may be a single target organ for metastases, and the rationale is that when primary cancer and regional nodes are controlled, the solitary or few metastases in that target metastatic organ can be cured [2].
In this scenario, radiotherapy could have a role in the local control of oligometastatic focal disease. Methods of morphological and functional imaging have improved enormously in the past decade and the oligometastatic situation occurs even more frequently during follow-up. As smaller foci of metastases are found, highly conformal radiation therapy, such as stereotactic body radiation therapy (SBRT), also known as stereotactic ablative radiotherapy (SABR), may well prove to be less invasive and more effective than surgery because of a lower rate of morbidity, lower costs, and the potential for delivering ablative treatments on an outpatient basis. Radiotherapy is currently in the midst of new developments in technology. High-tech improvements are refining the “ballistic” approach in order to deliver higher radiation doses to target volumes while sparing surrounding normal tissues of critical structures (organs at risk) by means of: (a) intensity-modulated radiation therapy, including volumetric modulation arc therapy and similar rotational approaches; (b) robotic arm delivery of radiation therapy, such as the CyberKnife® Robotic Radiosurgery System (Accuray Inc., Sunnyvale, CA); and (c) high linear energy transfer ionizing radiation delivery as represented by protons and other hadrons [3, 4]. Emerging data show that SBRT in its various treatment modalities is a safe and efficient way to locally control multiple metastatic sites. Preclinical data, clinical experience, and challenges are reviewed here and discussed.
Patient Selection
Selection criteria for SBRT in treating oligometastatic cancer remain crucial. In general, indications for SBRT are the same as those for metastasectomy, but without the limits regarding feasibility in patients unfit for surgery. In several reports, the eligibility criteria for SBRT for oligometastatic cancer were defined as follows: a limited number of metastases (one to five), a limited tumor diameter (<4 cm), a locally controlled primary tumor, and no other metastatic sites [5]. Other more specific and recently proposed selection criteria to offer SBRT to patients with various oligometastatic tumors include: a controlled primary, favorable histology, limited metastatic disease, the metachronous appearance of metastases, young age, and a good performance status (PS) of the patient [2, 6, 7]. In clinical practice, patients eligible for SBRT are essentially those for whom surgery is not feasible because of their age or PS and because of previous treatment with multiple lines of systemic therapy, when the toxicity of local treatments should be minimized.
Technical Aspects of SBRT
Regardless of the treatment delivery unit used, one feature in common is the image-guided therapy capability that enables verification of the location of the tumor or target volume before treatment delivery. This image-guided therapy can be performed using three-dimensional volume imaging, for example, cone beam computed tomography (CT). If two-dimensional imaging is used, invasive fiduciary markers positioned in the tumor or in close proximity to the tumor are required. These image-guidance procedures substantially reduce treatment setup error, using the tumor itself as a fiducial (frameless SBRT), and will in turn enable the planning target volume to be reduced. Currently, several commercially available integrated treatment units that use cone beam CT are on the market [8–10]. None of these treatment delivery units is superior to the others, with each system clearly having its strengths and weaknesses. The training and experience of the SBRT team are much more important than the treatment delivery unit used.
Radiobiology of SBRT
Historically, normal tissue effects are more greatly impacted by fraction sizes than acute effects are, which is why 1.8- to 2.0-Gy fractionation is considered the standard for conventional radiotherapy, resulting in longer treatment times. In fact, small doses per fraction result in a tumoricidal effect by means of mitotic death of cancer cells, allowing recovery of late sublethal damage of normal tissues at the same time. SBRT may add a novel mechanism of radiation-induced damage: at higher doses per fraction, emerging data suggest that, in addition to direct cytotoxicity, a different mechanism involving microvascular damage begins to have a substantial effect on the tumor cell kill [11, 12]. Endothelial apoptosis results in microvascular disruption and death of the tissue supplied by that vasculature [11]. Thus, even if hypofractionated irradiation may heighten the risks of late toxicity from a radiobiologic point of view, SBRT techniques substantially counteract this concern, reducing the volume of normal tissue exposed to high doses as a result of their precision.
SBRT by Site
A MEDLINE search was conducted in combination with reference checking for articles on arguments published in indexed journals. Studies excluded by our analysis were: (a) series with small numbers (fewer than seven) of patients, (b) series with a heterogeneous population, and (c) series with a relatively short follow-up duration (<10 months).
Lung Oligometastases
Lung metastases probably represent the paradigm of the potential benefit achievable by SBRT, which is able to produce high rates of tumor control with very limited toxicity (Table 1). For isolated or a few lung metastases (fewer than three or fewer than five, according to different selection criteria), the local control probability at 1 year is in the range of 70%–100%. In most series, the prescribed biologically effective doses are >100 Gy, with several fractionation schedules and different delivery techniques. In most of the studies, SBRT treatment was delivered in a few fractions (3–10), with a limited number of reports employing one single fraction. It is difficult to properly evaluate survival estimates and the real impact on patients' clinical outcomes using SBRT for lung metastases because there is an absence of randomized trials and because most of the phase I–II studies included patients with widely variable clinical characteristics. In a recent review by Siva et al. [13], the 2-year weighted overall survival (OS) rate estimate from the largest studies was 54.5%, ranging from higher rates in selected series such as the study by Norihisa et al. [6], which reported an OS rate at 2 years of 84%, to lower rates, such as the 39% reported from a multi-institutional trial conducted by Rusthoven et al. [14] in a population of nonsurgical unselected patients. In that study, 39.5% of patients presented with two metastases, 28.9% had received more than one previous line of chemotherapy, the median tumor volume was 4.2 cc, and roughly 30% of tumors had a volume >10 cc). Median survival times were not always reported, and ranged from 11.3 months in the pioneering experience of the Swedish group [15, 16] to 42.8 months in a cohort of 61 highly selected patients, the majority of whom were affected by a single metastasis from a primary lung tumor [17]. In most trials, the number of synchronous lung metastases was one to two or one to three, with some experience in patients with one to five lesions [18]. The predominant clinical presentation was a single lung metastasis.
Table 1.
Outcomes of stereotactic body radiation therapy for lung metastases from selected trials
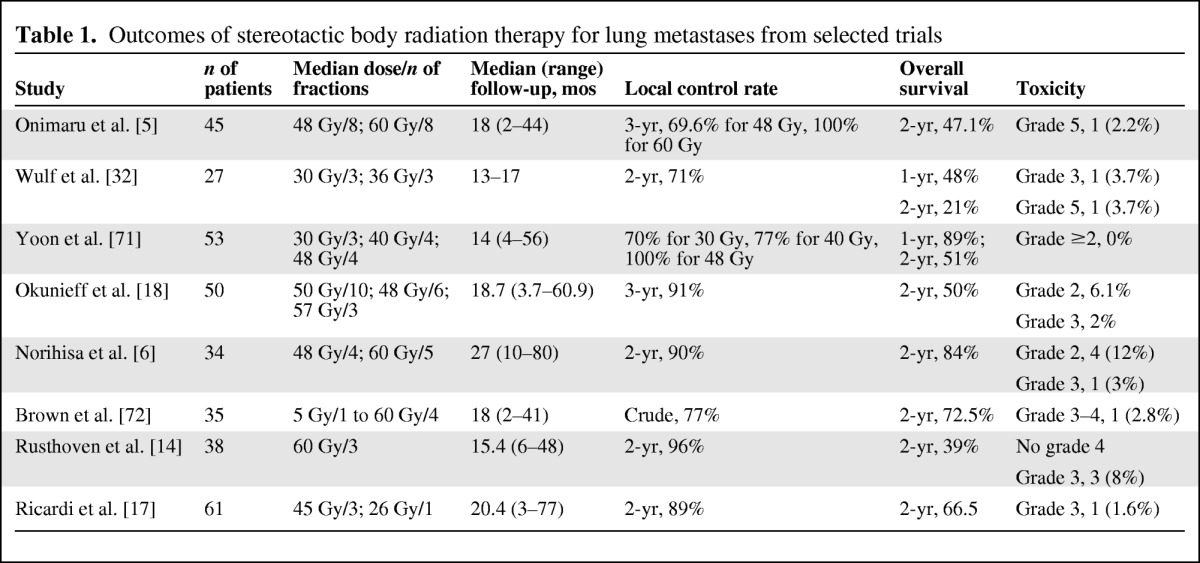
The available data suggest that the best results can probably be achieved in patients with a generally favorable presentation in terms of tumor volume, no or a few previous chemotherapy cycles, and the absence of extrathoracic metastatic disease. Moreover, in some studies, a relatively high percentage of patients received chemotherapy or other local treatments after SBRT, resulting in better disease control after systemic progression. In this setting, SBRT could play a major role in delaying progression, and the progression-free survival (PFS) interval appears to be a crucial endpoint in this patient population for future larger studies. Following early experience, it is natural to compare results using SBRT with results using surgical metastasectomy. Data from the International Registry of Lung Metastases [19] show OS rates of 70% at 2 years and 36% at 5 years in patients affected with a single metastasis. It is difficult to compare OS data using SBRT with data from historical surgical series for several reasons, with the main reason being the different clinical characteristics of the patients (most patients referred for SBRT are judged to be inoperable because of medical comorbidities that are able to significantly affect their OS outcome).
With regard to toxicity, the rates of acute toxicity with SBRT appear to be comparable with or even lower than those with any other alternative therapy. In a systematic review by Siva et al. [13], toxicity rates were very low. There was a 2.6% rate of grade ≥3 toxicity in a single fraction–radiosurgery series and a 4% rate of grade ≥3 toxicity in a hypofractionated radiotherapy series. The lack of grade 1–2 toxicity scores is likely a reflection of the retrospective nature of the majority of reports and incomplete toxicity recording. Generally higher rates of toxicity are reported when treating centrally located lesions (one death secondary to esophageal necrosis in a centrally located tumor). Late toxicity is difficult to evaluate because of the lack of published data. The lung is the major organ at risk, and radiation-induced lung injury (radiation pneumonitis and radiation fibrosis) represents a well-known radiological finding after SBRT, without an exact correlation with clinical or functional respiratory parameters. Currently, although there is still insufficient evidence to confirm the optimal tumor selection parameters, fractionation schedules, and radiation therapy techniques, in patients affected by a single or a few lung metastases (five or fewer), the high local control rates and potential survival benefits without significant side effects justify the use of SBRT as an alternative to surgery.
Among minimal ablation techniques, radiofrequency ablation (RFA) is the most frequently used method. In a prospective multicenter trial [20], RFA yielded a local control rate of 88% (in both the primary and metastatic lesions) and promising results in terms of OS and cancer-specific survival outcomes, even though the patient cohort was heterogeneous, making direct comparison of survival outcomes difficult. Peripheral and small-sized tumors (3–3.5 cm) are ideal for RFA treatment, and the most common complication is pneumothorax, with a reported incidence of 28%. Other toxicities include pleural effusion (14%) and pain (14%). Globally, RFA is a promising option in selected patients, but trials are needed in order to compare it with other alternative local ablative therapeutic modalities.
Liver Oligometastases
Much clinical experience has accumulated over the past 10–15 years reporting the efficacy and safety of SBRT in various patient populations with metastases localized to sites other than the lung [7, 13, 21–26]. The liver is one of the most common sites of metastatic spread from colorectal cancer (CRC). The data show that surgical resection of limited liver metastases can result in long-term survival in selected patients [27]. Surgery, however, is technically difficult, and only 10%–20% of metastatic CRC patients are candidates for surgical resection. In selected patients with a limited number of hepatic metastases who are not surgical candidates, a variety of ablative techniques have been developed. The most prominent in use are RFA, transarterial chemoembolization, and percutaneous ethanol injection. Although much less invasive than surgery, all have a certain grade of invasiveness and serious limitations. Retrospective analyses of RFA for liver metastases from CRC have shown broad variability in the 5-year survival rate in the range of 14%–55% [28]. Historically, the liver was thought to be a relatively radiosensitive organ, and it was difficult to achieve the radiation doses necessary to eradicate gross tumors without causing radiation-induced liver disease, which occurs ∼4–8 weeks following radiation therapy [29]. SBRT provides a noninvasive means of delivering a local ablative therapy for limited liver metastases, thereby providing optimal local tumor control as well as a limited dose to surrounding healthy tissue, and potentially lower complication rates. The local control rate achievable with SBRT varies in the range of 57%–100% according to several retrospective trials [30–35]. However, the follow-up times of most studies were relatively short, typically <18 months. A number of prospective studies on the use of SBRT for the treatment of liver metastases have been published (Table 2).
Table 2.
Summary of recent prospective trials with stereotactic body radiation therapy for liver metastases
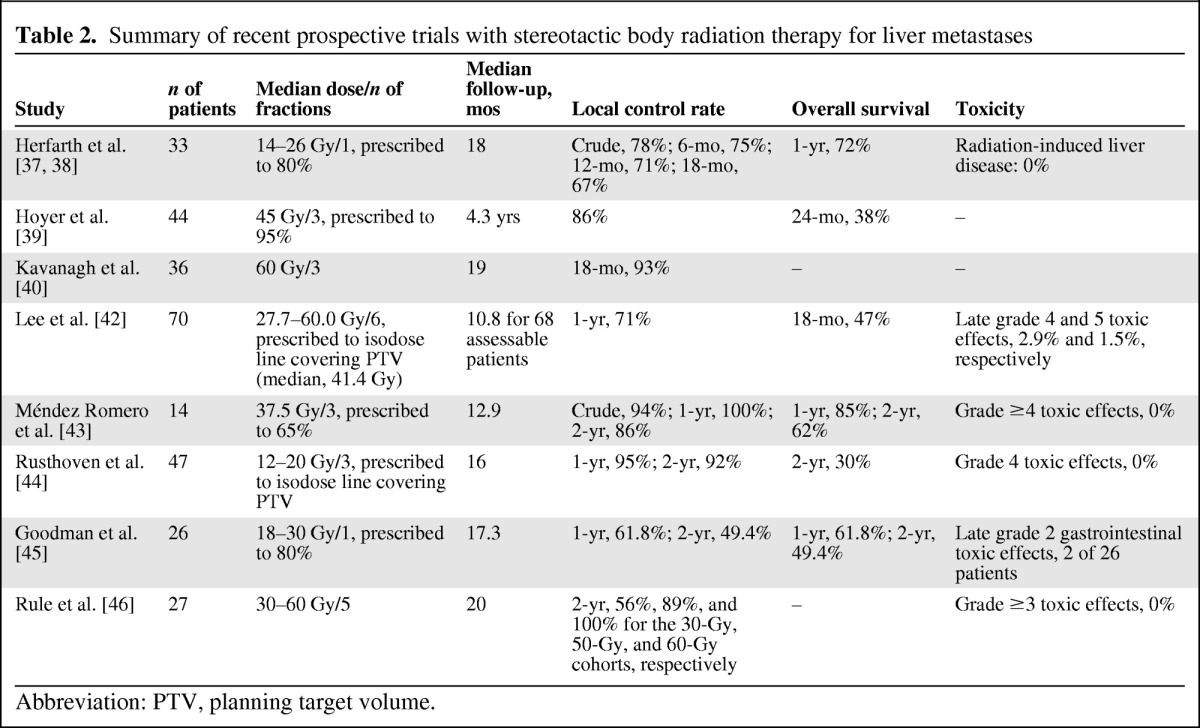
Abbreviation: PTV, planning target volume.
Notwithstanding the relatively short follow-up period, ≤18 months in the most series, data from published trials are promising and confirm that a small but significant fraction of oligometastatic patients may benefit from intensification of local therapy with higher radiation doses using SBRT [36–46].
Isolated Metastatic Lymph Nodes
Few published data exist on the local control rate using conventional radiotherapy in the context of isolated or limited lymph node metastases. SBRT does not replace chemotherapy but rather can augment its effects on focal areas of gross disease as well as metastatic lymph nodes. Although the dose and fractionation schedules have been extremely heterogeneous, early data from some recent series (Table 3) seem to be promising in terms of the local control rate [47–51]. Because small volumes are irradiated for metastatic lymph nodes, dose escalation might result in better efficacy without prohibitive toxicity.
Table 3.
Summary of published trials of stereotactic body radiation therapy for lymph node metastases
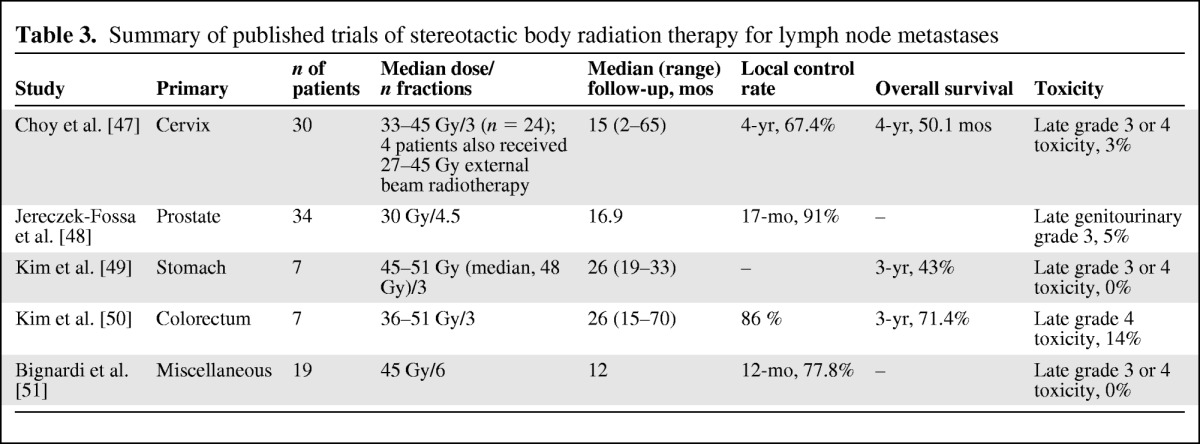
The poorer disease-free survival rates observed in several series may be explained by the substantial differences in the patient populations in several aspects, mainly concerning the primary tumor behavior and the burden of microscopic systemic disease outside the irradiated target [22, 51].
In summary, even though most patients treated locally using SBRT for lymph nodes metastases eventually fail at other sites, the local control provided by this initial experience may be potentially significant for preserving quality of life and delaying further systemic treatments [47–50]. Evaluation of tolerance doses to the vascular wall close to the lymph node target, especially with ablative doses, remains an interesting issue and will certainly involve long-term surviving patients.
Metastases in Adrenal Glands
Adrenal gland metastases can occur as a result of various types of extra-adrenal primary cancers, although the most frequent primary tumor is non-small cell lung cancer (NSCLC) [52]. In general, longer median survival and OS times have been demonstrated with resection of clinically isolated adrenal metastases when compared with nonsurgical therapy, including RFA, external beam radiotherapy, arterial embolization, radioembolization, blend embolization, chemical ablation, and cryoablation [53]. Table 4 depicts and compares the characteristics of the published studies on SBRT for adrenal metastases.
Table 4.
Summary of published trials of stereotactic body radiation therapy for adrenal metastases
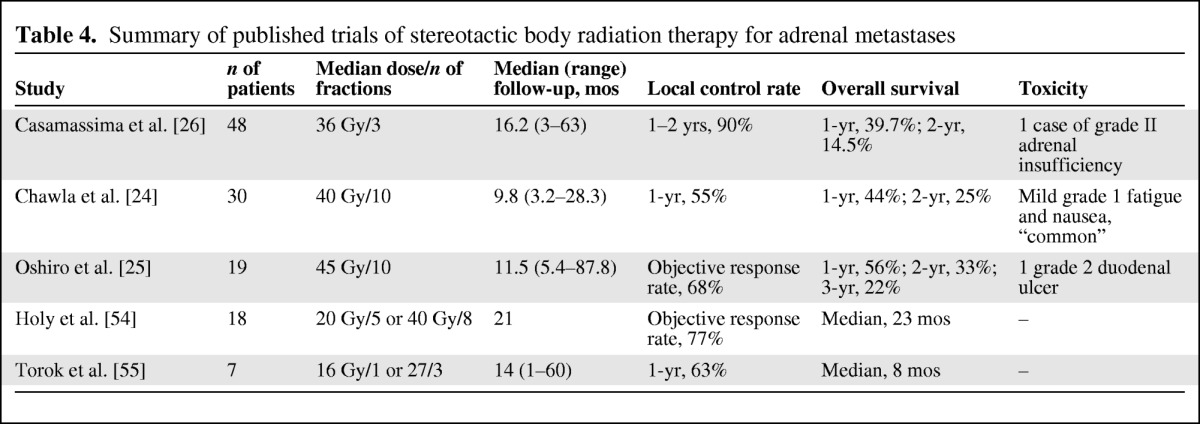
In summary, as of now, few studies have been published regarding the role of SBRT in treating patients with adrenal glands metastases, and several criticisms could arise regarding the lack of clear data on local control and on dose fractionation [24–26, 52–55]. Nevertheless, the good tolerability and the promising clinical results should stimulate the scientific community to further design clinical studies with the aim of optimizing local control and evaluating a potential PFS benefit.
Spinal Metastases
Spinal radiosurgery has been proven to be an option in the treatment of spinal metastases in properly selected patients, even though only retrospective and phase I–II studies are available. Local control based on imaging and/or pain control is achieved in ∼80% of presentations (given the follow-up of each study). As noted by Sahgal et al. [56] in a recent review, the lack of actuarial data overall makes it difficult to come to any firm conclusions other than that the rates of control are promising. There are no randomized trials comparing stereotactic radiotherapy with conventional radiotherapy with regard to pain control (another issue is that, for conventional treatments, local control is not typically assessed using imaging but based on clinical benefit). Stereotactic radiotherapy is also an option as retreatment for previously irradiated sites, with results on pain control that are comparable with those obtained in patients not previously treated [57]. Table 5 summarizes the results of several studies including stereotactic radiotherapy for spinal tumors. There are several dose prescription schedules and total doses or doses per fraction, making direct comparison difficult, with a follow-up time globally of a few months. The predominant pattern of failure after SBRT for spinal metastases is characteristic of the procedure because the principle of SBRT is to treat only the target region, and areas close to the spinal cord are frequently underdosed. Failure at the epidural space is commonly reported. Chang et al. [58] reported eight of 17 failures in this area occurring in 74 tumors. The posterior part of the vertebral body is at higher risk for recurrence. In some series, the whole vertebral body is contoured; in others, only the tumor is contoured using magnetic resonance imaging (the optimal target contouring is still debated). SBRT can also be safely applied in the postoperative setting, as reported by Sahgal et al. [56], with the intent of reducing the extent of surgery (which can be limited to epidural decompression and fixation). The available data suggest that SBRT is a promising technique for spinal metastases, but its application is still experimental and requires prospective controlled studies.
Table 5.
Summary of published trials of stereotactic body radiation therapy for spinal metastases
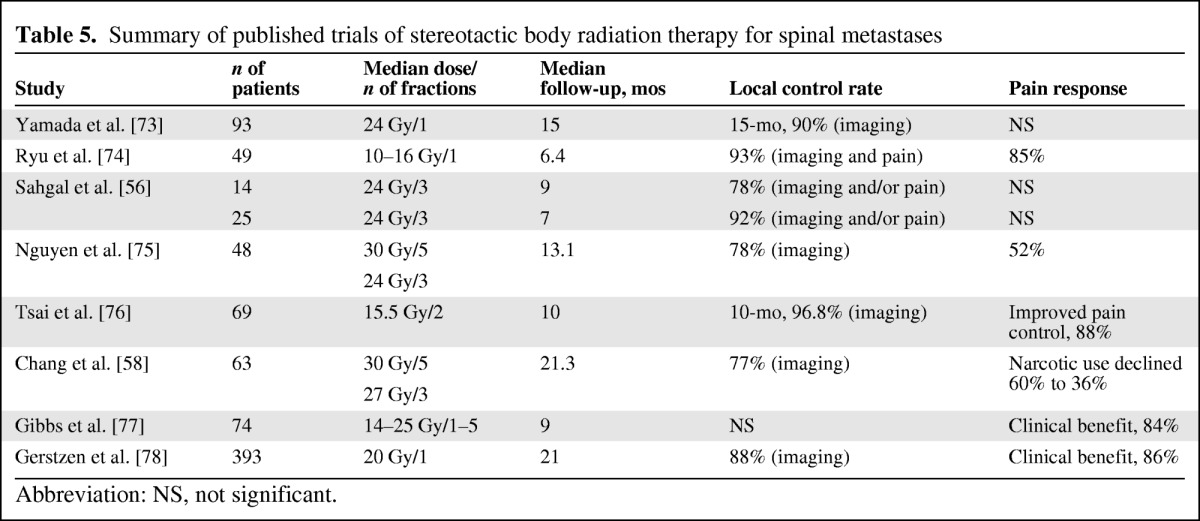
Abbreviation: NS, not significant.
Several other new effective ablation treatment strategies have recently been reported for the treatment of metastatic disease involving bone. These include the use of RFA, cryoablation, laser ablation, and microwave ablation. As shown in a recent review by Rosenthal and Callstrom [59], of these minimally invasive methods, RFA and cryoablation have been the most studied. The patient selection criteria are similar to those using SBRT. RFA is effective in reducing pain resulting from skeletal metastatic disease, as demonstrated in two multicentric trials [60, 61], even though the results of the two studies are different because of factors that include patient selection, the level of anesthesia, and the degree of tumor destruction. At the 3-month time point, there was a reduction in pain of 14 points in the American College of Radiology Imaging Network study and of 28 points in the Goetz et al. [60] study, wherein up to 95% of patients experienced pain relief. Toxicity included neuropathic pain, pain exacerbation, and bone fracture. Globally, grade 3 toxicity was reported in 5% of cases. These results need further evaluation, but in selected cases RFA could be comparable with SBRT. Cryoablation could be an alternative to RFA, but the currently available clinical data are very limited.
Cost-Effectiveness
Because SBRT is becoming used in more clinical situations, it is imperative to assess its cost-effectiveness as well as its efficacy. SBRT employing image guidance, high-precision dose delivery, more accurate target definition with better anatomical and biological imaging, and the possibility of dose verification during treatment via dose-adaptive radiation therapy permits a higher probability of tumor control. Such major technological progress certainly comes at a higher cost, and there are many concerns regarding the value of that progress. On the other hand, the higher equipment and resource costs associated with cutting-edge radiation oncology technologies can be partly mitigated by shorter treatment courses.
Additionally, better tumor control, less toxicity, and fewer treatment courses decrease the indirect costs of cancer care, including lost time and economic productivity secondary to treatment-related and cancer-related illness and death [62]. The U.S. National Institutes of Health estimate that 53% of the total costs of cancer care in 2010 was attributable to indirect mortality costs, and 8% was attributable to indirect morbidity costs [63]. Therefore, advances in radiation therapy can potentially result in substantial direct and indirect cost savings [64]. Beyond the given assumption that SBRT would be less expensive in most health systems than alternative options requiring anesthesia and/or hospitalization, few data in the literature explicitly address this issue. One of the most interesting studies was recently published by Sher et al. [65], who performed a cost-effectiveness analysis of SBRT versus RFA for patients with medically inoperable, early-stage NSCLC. Those authors concluded that, based on the currently available data, SBRT is the most cost-effective nonsurgical treatment for peripheral, early-stage lung cancers, rendering an incremental cost-effectiveness ratio for SBRT over RFA of $14,100/quality-adjusted life-year.
Conclusions
From preliminary published results, thanks to the more extensive prescription of SBRT and SABR, the role of radiation therapy for metastatic disease has evolved from palliating symptoms to a potentially curative purpose, as shown in specific patient settings, including promising data from oligometastases [66, 67].
A crucial criticism in oligometastatic patients remains the appropriateness of aggressive treatment, such as metastasectomy or SBRT, when the real survival advantages are not yet established. Excluding selected cases, usually weak or no evidence of longer survival times was shown when aggressive local treatments were used. An artifact of patient selection has also been denounced in several surgical series: clinicians rely on presumed benefit based on comparisons with poorly characterized survival estimates from other patients with advanced disease [68, 69].
In the subgroup of patients with a solitary metastasis, investigating SBRT dose escalation in order to optimize local control may be worthwhile. For cases with more than one metastasis, especially if more than one organ is involved, the selection criteria for SBRT should be evaluated with extreme attention to life expectancy and toxicity.
Crucial open issues are: (a) what is the real cutoff between pure palliative and hypothetical curative intent therapy in oligometastatic patients, (b) what is the correct timing with chemotherapy, and (c) what is the optimal target and how can the radiation oncologist define it as best as possible considering the risk for other potential microscopic foci of disease?
Considering the high propensity for distant progression in these patients, the combination of novel drugs and SBRT needs to be deeply explored. With this background and rationale, prospective trials of high-dose SBRT should be proposed to definitively assess its role in selected oligometastatic cancer patients. An international randomized phase II controlled trial called Comprehensive Treatment of Oligometastatic Tumors (ClinicalTrials.gov identifier, NCT01446744) is currently accruing patients. The intent of the investigation is to compare SBRT at ablative doses with the current approaches of chemotherapy and conventional radiotherapy. Clinicians are looking forward to finding out, from the results of this and other prospective randomized trials, the real impact of SBRT on OS and quality of life outcomes of oligometastatic patients [70].
Footnotes
- (C/A)
- Consulting/advisory relationship
- (RF)
- Research funding
- (E)
- Employment
- (H)
- Honoraria received
- (OI)
- Ownership interests
- (IP)
- Intellectual property rights/inventor/patent holder
- (SAB)
- Scientific advisory board
Author Contributions
Conception/Design: Filippo Alongi, Stefano Arcangeli, Andrea Riccardo Filippi, Umberto Ricardi, Marta Scorsetti
Provision of study material or patients: Filippo Alongi, Stefano Arcangeli, Andrea Riccardo Filippi, Umberto Ricardi, Marta Scorsetti
Collection and/or assembly of data: Filippo Alongi, Stefano Arcangeli, Andrea Riccardo Filippi, Umberto Ricardi, Marta Scorsetti
Data analysis and interpretation: Filippo Alongi, Stefano Arcangeli, Andrea Riccardo Filippi, Umberto Ricardi, Marta Scorsetti
Manuscript writing: Filippo Alongi, Stefano Arcangeli, Andrea Riccardo Filippi, Umberto Ricardi, Marta Scorsetti
Final approval of manuscript: Filippo Alongi, Stefano Arcangeli, Andrea Riccardo Filippi, Umberto Ricardi, Marta Scorsetti
References
- 1.Weichselbaum RR, Hellman S. Oligometastases revisited. Nat Rev Clin Oncol. 2011;8:378–382. doi: 10.1038/nrclinonc.2011.44. [DOI] [PubMed] [Google Scholar]
- 2.Rubin P, Brasacchio R, Katz A. Solitary metastases: Illusion versus reality. Semin Radiat Oncol. 2006;16:120–130. doi: 10.1016/j.semradonc.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 3.Yu CX, Amies CJ, Svatos M. Planning and delivery of intensity-modulated radiation therapy. Med Phys. 2008;35:5233–5241. doi: 10.1118/1.3002305. [DOI] [PubMed] [Google Scholar]
- 4.Welsh JS. Basics of particle therapy: Introduction to hadrons. Am J Clin Oncol. 2008;31:493–495. doi: 10.1097/COC.0b013e31816a6237. [DOI] [PubMed] [Google Scholar]
- 5.Onimaru R, Shirato H, Shimizu S, et al. Tolerance of organs at risk in small volume, hypofractionated, image-guided radiotherapy for primary and metastatic lung cancers. Int J Radiat Oncol Biol Phys. 2003;56:126–135. doi: 10.1016/s0360-3016(03)00095-6. [DOI] [PubMed] [Google Scholar]
- 6.Norihisa Y, Nagata Y, Takayama K, et al. Stereotactic body radiotherapy for oligometastatic lung tumors. Int J Radiat Oncol Biol Phys. 2008;72:398–403. doi: 10.1016/j.ijrobp.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 7.Nagata Y, Wulf J, Lax I, et al. Stereotactic radiotherapy of primary lung cancer and other targets: Results of consultant meeting of the International Atomic Energy Agency. Int J Radiat Oncol Biol Phys. 2011;79:660–669. doi: 10.1016/j.ijrobp.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 8.Boda-Heggemann J, Lohr F, Wenz F, et al. kV cone-beam CT-based IGRT: A clinical review. Strahlenther Onkol. 2011;187:284–291. doi: 10.1007/s00066-011-2236-4. [DOI] [PubMed] [Google Scholar]
- 9.Chang BK, Timmerman RD. Stereotactic body radiation therapy: A comprehensive review. Am J Clin Oncol. 2007;30:637–644. doi: 10.1097/COC.0b013e3180ca7cb1. [DOI] [PubMed] [Google Scholar]
- 10.Lo SS, Cardenes HR, Teh BS, et al. Stereotactic body radiation therapy for nonpulmonary primary tumors. Expert Rev Anticancer Ther. 2008;8:1939–1951. doi: 10.1586/14737140.8.12.1939. [DOI] [PubMed] [Google Scholar]
- 11.Fuks Z, Kolesnick R. Engaging the vascular component of the tumor response. Cancer Cell. 2005;8:89–91. doi: 10.1016/j.ccr.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 12.Hall EJ, Brenner DJ. The radiobiology of radiosurgery: Rationale for different treatment regimes for AVMs and malignancies. Int J Radiat Oncol Biol Phys. 1993;25:381–385. doi: 10.1016/0360-3016(93)90367-5. [DOI] [PubMed] [Google Scholar]
- 13.Siva S, MacManus M, Ball D. Stereotactic radiotherapy for pulmonary oligometastases: A systematic review. J Thorac Oncol. 2010;5:1091–1099. doi: 10.1097/JTO.0b013e3181de7143. [DOI] [PubMed] [Google Scholar]
- 14.Rusthoven KE, Kavanagh BD, Burri SH, et al. Multi-institutional phase I/II trial of stereotactic body radiation therapy for lung metastases. J Clin Oncol. 2009;27:1579–1584. doi: 10.1200/JCO.2008.19.6386. [DOI] [PubMed] [Google Scholar]
- 15.Lax I, Blomgren H, Larson D, et al. Extracranial stereotactic radiosurgery of localized targets. J Radiosurg. 1998;1:135–148. [Google Scholar]
- 16.Blomgren H, Lax I, Näslund I, et al. Stereotactic high dose fraction radiation therapy of extracranial tumors using an accelerator: Clinical experience of the first thirty-one patients. Acta Oncol. 1995;34:861–870. doi: 10.3109/02841869509127197. [DOI] [PubMed] [Google Scholar]
- 17.Ricardi U, Filippi AR, Guarneri A, et al. Stereotactic body radiation therapy for lung metastases. Lung Cancer. 2012;75:77–81. doi: 10.1016/j.lungcan.2011.04.021. [DOI] [PubMed] [Google Scholar]
- 18.Okunieff P, Petersen AL, Philip A, et al. Stereotactic body radiation therapy (SBRT) for lung metastases. Acta Oncol. 2006;45:808–817. doi: 10.1080/02841860600908954. [DOI] [PubMed] [Google Scholar]
- 19.The International Registry of Lung Metastases. Long-term results of lung metastasectomy: Prognostic analyses based on 5206 cases. J Thorac Cardiovasc Surg. 1997;113:37–49. doi: 10.1016/s0022-5223(97)70397-0. [DOI] [PubMed] [Google Scholar]
- 20.Lencioni R, Crocetti L, Cioni R, et al. Response to radiofrequency ablation of pulmonary tumours: A prospective, intention-to-treat, multicentre clinical trial (the RAPTURE study) Lancet Oncol. 2008;9:621–628. doi: 10.1016/S1470-2045(08)70155-4. [DOI] [PubMed] [Google Scholar]
- 21.Lo SS, Fakiris AJ, Chang EL, et al. Stereotactic body radiation therapy: A novel treatment modality. Nat Rev Clin Oncol. 2010;7:44–54. doi: 10.1038/nrclinonc.2009.188. [DOI] [PubMed] [Google Scholar]
- 22.Scorsetti M, Bignardi M, Alongi F, et al. Stereotactic body radiation therapy for abdominal targets using volumetric intensity modulated arc therapy with RapidArc: Feasibility and clinical preliminary results. Acta Oncol. 2011;50:528–538. doi: 10.3109/0284186X.2011.558522. [DOI] [PubMed] [Google Scholar]
- 23.Kavanagh BD, McGarry RC, Timmerman RD. Extracranial radiosurgery (stereotactic body radiation therapy) for oligometastases. Semin Radiat Oncol. 2006;16:77–84. doi: 10.1016/j.semradonc.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 24.Chawla S, Chen Y, Katz AW, et al. Stereotactic body radiotherapy for treatment of adrenal metastases. Int J Radiat Oncol Biol Phys. 2009;75:71–75. doi: 10.1016/j.ijrobp.2008.10.079. [DOI] [PubMed] [Google Scholar]
- 25.Oshiro Y, Takeda Y, Hirano S, et al. Role of radiotherapy for local control of asymptomatic adrenal metastasis from lung cancer. Am J Clin Oncol. 2011;34:249–253. doi: 10.1097/COC.0b013e3181dbb727. [DOI] [PubMed] [Google Scholar]
- 26.Casamassima F, Livi L, Masciullo S, et al. Stereotactic radiotherapy for adrenal gland metastases: University of Florence experience. Int J Radiat Oncol Biol Phys. 2012;82:919–923. doi: 10.1016/j.ijrobp.2010.11.060. [DOI] [PubMed] [Google Scholar]
- 27.Choti MA, Sitzmann JV, Tiburi MF, et al. Trends in long-term survival following liver resection for hepatic colorectal metastases. Ann Surg. 2002;235:759–766. doi: 10.1097/00000658-200206000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wong SL, Mangu PB, Choti MA, et al. American Society of Clinical Oncology 2009 clinical evidence review on radiofrequency ablation of hepatic metastases from colorectal cancer. J Clin Oncol. 2010;28:493–508. doi: 10.1200/JCO.2009.23.4450. [DOI] [PubMed] [Google Scholar]
- 29.Lawrence TS, Robertson JM, Anscher MS, et al. Hepatic toxicity resulting from cancer treatment. Int J Radiat Oncol Biol Phys. 1995;31:1237–1248. doi: 10.1016/0360-3016(94)00418-K. [DOI] [PubMed] [Google Scholar]
- 30.Lo SS, Fakiris AJ, Teh BS, et al. Stereotactic body radiation therapy for oligometastases. Expert Rev Anticancer Ther. 2009;9:621–635. doi: 10.1586/era.09.15. [DOI] [PubMed] [Google Scholar]
- 31.Blomgren H, Lax I, Göranson H, et al. Radiosurgery for tumors in the body: Clinical experience using a new method. J Radiosurg. 1998;1:63–74. [Google Scholar]
- 32.Wulf J, Hädinger U, Oppitz U, et al. Stereotactic radiotherapy of targets in the lung and liver. Strahlenther Onkol. 2001;177:645–655. doi: 10.1007/pl00002379. [DOI] [PubMed] [Google Scholar]
- 33.Sato M, Uematsu M, Yamamoto F. Feasibility of frameless stereotactic high-dose radiation therapy for primary and metastatic liver cancer. J Radiosurg. 1998;1:233–238. [Google Scholar]
- 34.Katz AW, Carey-Sampson M, Muhs AG, et al. Hypofractionated stereotactic body radiation therapy (SBRT) for limited hepatic metastases. Int J Radiat Oncol Biol Phys. 2007;67:793–798. doi: 10.1016/j.ijrobp.2006.10.025. [DOI] [PubMed] [Google Scholar]
- 35.Wada H, Takai Y, Nemoto K, et al. Univariate analysis of factors correlated with tumor control probability of three-dimensional conformal hypofractionated high-dose radiotherapy for small pulmonary or hepatic tumors. Int J Radiat Oncol Biol Phys. 2004;58:1114–1120. doi: 10.1016/j.ijrobp.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 36.Gunvén P, Blomgren H, Lax I, et al. Curative stereotactic body radiotherapy for liver malignancy. Med Oncol. 2009;26:327–334. doi: 10.1007/s12032-008-9125-4. [DOI] [PubMed] [Google Scholar]
- 37.Herfarth KK, Debus J, Lohr F, et al. Stereotactic single-dose radiation therapy of liver tumors: Results of a phase I/II trial. J Clin Oncol. 2001;19:164–170. doi: 10.1200/JCO.2001.19.1.164. [DOI] [PubMed] [Google Scholar]
- 38.Herfarth KK, Hof H, Bahner ML, et al. Assessment of focal liver reaction by multiphasic CT after stereotactic single-dose radiotherapy of liver tumors. Int J Radiat Oncol Biol Phys. 2003;57:444–451. doi: 10.1016/s0360-3016(03)00586-8. [DOI] [PubMed] [Google Scholar]
- 39.Hoyer M, Roed H, Traberg Hansen A, et al. Phase II study on stereotactic body radiotherapy of colorectal metastases. Acta Oncol. 2006;45:823–830. doi: 10.1080/02841860600904854. [DOI] [PubMed] [Google Scholar]
- 40.Kavanagh BD, Schefter TE, Cardenes HR, et al. Interim analysis of a prospective phase I/II trial of SBRT for liver metastases. Acta Oncol. 2006;45:848–855. doi: 10.1080/02841860600904870. [DOI] [PubMed] [Google Scholar]
- 41.Schefter TE, Kavanagh BD, Timmerman RD, et al. A phase I trial of stereotactic body radiation therapy (SBRT) for liver metastases. Int J Radiat Oncol Biol Phys. 2005;62:1371–1378. doi: 10.1016/j.ijrobp.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 42.Lee MT, Kim JJ, Dinniwell R, et al. Phase I study of individualized stereotactic body radiotherapy of liver metastases. J Clin Oncol. 2009;27:1585–1591. doi: 10.1200/JCO.2008.20.0600. [DOI] [PubMed] [Google Scholar]
- 43.Méndez Romero A, Wunderink W, Hussain SM, et al. Stereotactic body radiation therapy for primary and metastatic liver tumors: A single institution phase I–II study. Acta Oncol. 2006;45:831–837. doi: 10.1080/02841860600897934. [DOI] [PubMed] [Google Scholar]
- 44.Rusthoven KE, Kavanagh BD, Cardenes H, et al. Multi-institutional phase I/II trial of stereotactic body radiation therapy for liver metastases. J Clin Oncol. 2009;27:1572–1578. doi: 10.1200/JCO.2008.19.6329. [DOI] [PubMed] [Google Scholar]
- 45.Goodman KA, Wiegner EA, Maturen KE, et al. Dose-escalation study of single-fraction stereotactic body radiotherapy for liver malignancies. Int J Radiat Oncol Biol Phys. 2010;78:486–493. doi: 10.1016/j.ijrobp.2009.08.020. [DOI] [PubMed] [Google Scholar]
- 46.Rule W, Timmerman R, Tong L, et al. Phase I dose-escalation study of stereotactic body radiotherapy in patients with hepatic metastases. Ann Surg Oncol. 2011;18:1081–1087. doi: 10.1245/s10434-010-1405-5. [DOI] [PubMed] [Google Scholar]
- 47.Choi CW, Cho CK, Yoo SY, et al. Image-guided stereotactic body radiation therapy in patients with isolated para-aortic lymph node metastases from uterine cervical and corpus cancer. Int J Radiat Oncol Biol Phys. 2009;74:147–153. doi: 10.1016/j.ijrobp.2008.07.020. [DOI] [PubMed] [Google Scholar]
- 48.Jereczek-Fossa BA, Fariselli L, Beltramo G, et al. Linac-based or robotic image-guided stereotactic radiotherapy for isolated lymph node recurrent prostate cancer. Radiother Oncol. 2009;93:14–17. doi: 10.1016/j.radonc.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 49.Kim MS, Yoo SY, Cho CK, et al. Stereotactic body radiotherapy for isolated para-aortic lymph node recurrence after curative resection in gastric cancer. J Korean Med Sci. 2009;24:488–492. doi: 10.3346/jkms.2009.24.3.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim MS, Cho CK, Yang KM, et al. Stereotactic body radiotherapy for isolated paraaortic lymph node recurrence from colorectal cancer. World J Gastroenterol. 2009;15:6091–6095. doi: 10.3748/wjg.15.6091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bignardi M, Navarria P, Mancosu P, et al. Clinical outcome of hypofractionated stereotactic radiotherapy for abdominal lymph node metastases. Int J Radiat Oncol Biol Phys. 2011;81:831–838. doi: 10.1016/j.ijrobp.2010.05.032. [DOI] [PubMed] [Google Scholar]
- 52.Lam KY, Lo CY. Metastatic tumours of the adrenal glands: A 30-year experience in a teaching hospital. Clin Endocrinol (Oxf) 2002;56:95–101. doi: 10.1046/j.0300-0664.2001.01435.x. [DOI] [PubMed] [Google Scholar]
- 53.Duh QY. Resecting isolated adrenal metastasis: Why and how? Ann Surg Oncol. 2003;10:1138–1139. doi: 10.1245/aso.2003.10.916. [DOI] [PubMed] [Google Scholar]
- 54.Holy R, Piroth M, Pinkawa M, et al. Stereotactic body radiation therapy (SBRT) for treatment of adrenal gland metastases from non-small cell lung cancer. Strahlenther Onkol. 2011;187:245–251. doi: 10.1007/s00066-011-2192-z. [DOI] [PubMed] [Google Scholar]
- 55.Torok J, Wegner RE, Burton SA, et al. Stereotactic body radiation therapy for adrenal metastases: A retrospective review of a noninvasive therapeutic strategy. Future Oncol. 2011;7:145–151. doi: 10.2217/fon.10.165. [DOI] [PubMed] [Google Scholar]
- 56.Sahgal A, Bilsky M, Chang EL, et al. Stereotactic body radiotherapy for spinal metastases: Current status, with a focus on its application in the postoperative patient. J Neurosurg Spine. 2011:14.151–166. doi: 10.3171/2010.9.SPINE091005. [DOI] [PubMed] [Google Scholar]
- 57.Gagnon GJ, Henderson FC, Gehan EA, et al. Cyberknife radiosurgery for breast cancer spine metastases: A matched-pair analysis. Cancer. 2007;110:1796–1802. doi: 10.1002/cncr.22977. [DOI] [PubMed] [Google Scholar]
- 58.Chang EL, Shiu AS, Mendel E, et al. Phase I/II study of stereotactic body radiotherapy for spinal metastases and its pattern of failure. J Neurosurg Spine. 2007;7:151–160. doi: 10.3171/SPI-07/08/151. [DOI] [PubMed] [Google Scholar]
- 59.Rosenthal D, Callstrom MR. Critical review and state of the art in interventional oncology: Benign and metastatic disease involving bone. Radiology. 2012;262:765–780. doi: 10.1148/radiol.11101384. [DOI] [PubMed] [Google Scholar]
- 60.Goetz MP, Callstrom MR, Charboneau JW, et al. Percutaneous image-guided radiofrequency ablation of painful metastases involving bone: A multicenter study. J Clin Oncol. 2004;22:300–306. doi: 10.1200/JCO.2004.03.097. [DOI] [PubMed] [Google Scholar]
- 61.Dupuy DE, Liu D, Hartfeil D, et al. Percutaneous radiofrequency ablation of painful osseous metastases: A multicenter American College of Radiology Imaging Network trial. Cancer. 2010;116:989–997. doi: 10.1002/cncr.24837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yabroff KR, Davis WW, Lamont EB, et al. Patient time costs associated with cancer care. J Natl Cancer Inst. 2007;99:14–23. doi: 10.1093/jnci/djk001. [DOI] [PubMed] [Google Scholar]
- 63.American Cancer Society. Cancer Facts & Figures 2010. [accessed January 1, 2011]. Available at http://www.cancer.org/acs/groups/content/@epidemiologysurveilance/documents/document/acspc-026238.pdf.
- 64.Sullivan R, Peppercorn J, Sikora K, et al. Delivering affordable cancer care in high-income countries. Lancet Oncol. 2011;12:933–980. doi: 10.1016/S1470-2045(11)70141-3. [DOI] [PubMed] [Google Scholar]
- 65.Sher DJ, Wee JO, Punglia RS. Cost-effectiveness analysis of stereotactic body radiotherapy and radiofrequency ablation for medically inoperable, early-stage non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 2011;81:e767–e774. doi: 10.1016/j.ijrobp.2010.10.074. [DOI] [PubMed] [Google Scholar]
- 66.Thariat J, Marcy PY, Lagrange JL. [Trends in radiation therapy for the treatment of metastatic and oligometastatic disease in 2010.] Bull Cancer. 2010;97:1467–1476. doi: 10.1684/bdc.2010.1230. In French. [DOI] [PubMed] [Google Scholar]
- 67.Timmerman RD, Kavanagh BD, Cho LC, et al. Stereotactic body radiation therapy in multiple organ sites. J Clin Oncol. 2007;25:947–952. doi: 10.1200/JCO.2006.09.7469. [DOI] [PubMed] [Google Scholar]
- 68.Treasure T. Pulmonary metastasectomy: A common practice based on weak evidence. Ann R Coll Surg Engl. 2007;89:744–748. doi: 10.1308/003588407X232198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fiorentino F, Hunt I, Teoh K, et al. Pulmonary metastasectomy in colorectal cancer: A systematic review and quantitative synthesis. J R Soc Med. 2010;103:60–66. doi: 10.1258/jrsm.2009.090299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.ClinicalTrials.gov. Stereotactic Ablative Radiotherapy for Comprehensive Treatment of Oligometastatic Tumors (SABR-COMET) [accessed June 13, 2012]. Available at http://clinicaltrials.gov/ct2/show/NCT01446744.
- 71.Yoon SM, Choi EK, Lee SW, et al. Clinical results of stereotactic body frame based fractionated radiation therapy for primary or metastatic thoracic tumors. Acta Oncol. 2006;45:1108–1114. doi: 10.1080/02841860600812685. [DOI] [PubMed] [Google Scholar]
- 72.Brown WT, Wu X, Fowler JF, et al. Lung metastases treated by CyberKnife image-guided robotic stereotactic radiosurgery at 41 months. South Med J. 2008;101:376–382. doi: 10.1097/SMJ.0b013e318167ad8d. [DOI] [PubMed] [Google Scholar]
- 73.Yamada Y, Bilsky MH, Lovelock DM, et al. High-dose single-fraction image-guided intensity-modulated radiotherapy for metastatic spinal lesions. Int J Radiat Oncol Biol Phys. 2008;71:484–490. doi: 10.1016/j.ijrobp.2007.11.046. [DOI] [PubMed] [Google Scholar]
- 74.Ryu S, Fang Yin F, Rock J, et al. Image-guided and intensity-modulated radiosurgery for patients with spinal metastasis. Cancer. 2003;97:2013–2018. doi: 10.1002/cncr.11296. [DOI] [PubMed] [Google Scholar]
- 75.Nguyen QN, Shiu AS, Rhines LD, et al. Management of spinal metastases from renal cell carcinoma using stereotactic body radiotherapy. Int J Radiat Oncol Biol Phys. 2010;76:1185–1192. doi: 10.1016/j.ijrobp.2009.03.062. [DOI] [PubMed] [Google Scholar]
- 76.Tsai JT, Lin JW, Chiu WT, et al. Assessment of image-guided CyberKnife radiosurgery for metastatic spine tumors. J Neuroncol. 2009;94:119–127. doi: 10.1007/s11060-009-9814-7. [DOI] [PubMed] [Google Scholar]
- 77.Gibbs IC, Kamnerdsupaphon P, Ryu MR, et al. Image-guided robotic radiosurgery for spinal metastases. Radiother Oncol. 2007;82:185–190. doi: 10.1016/j.radonc.2006.11.023. [DOI] [PubMed] [Google Scholar]
- 78.Gerszten PC, Burton SA, Ozhasoglu C, et al. Radiosurgery for spinal metastases: Clinical experience in 500 cases from a single institution. Spine. 2007;32:193–199. doi: 10.1097/01.brs.0000251863.76595.a2. [DOI] [PubMed] [Google Scholar]


