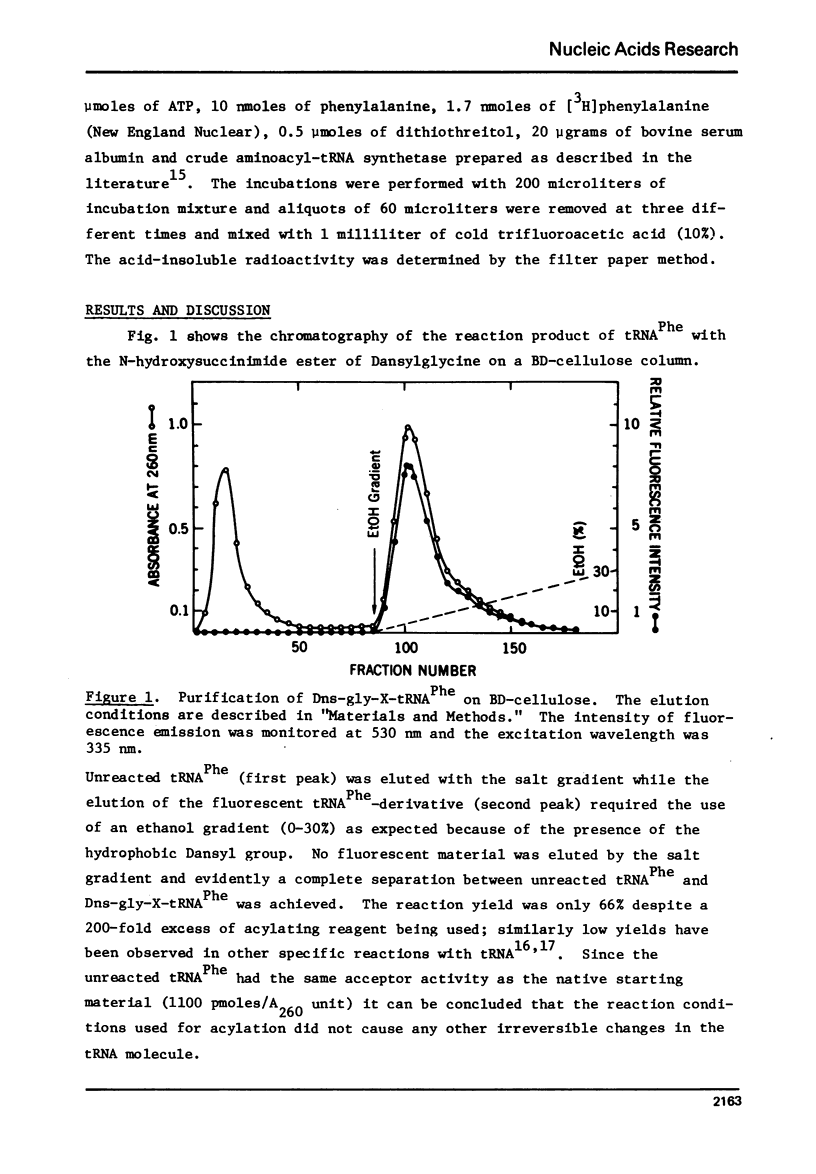
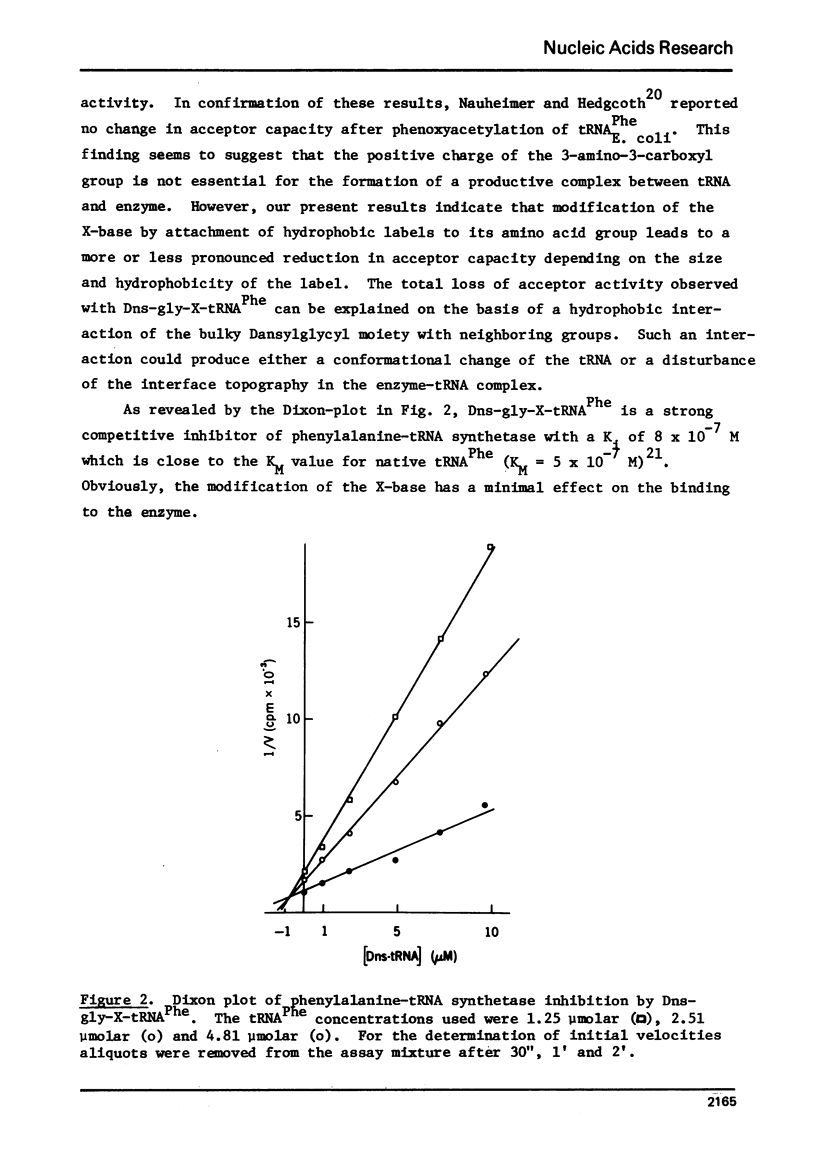
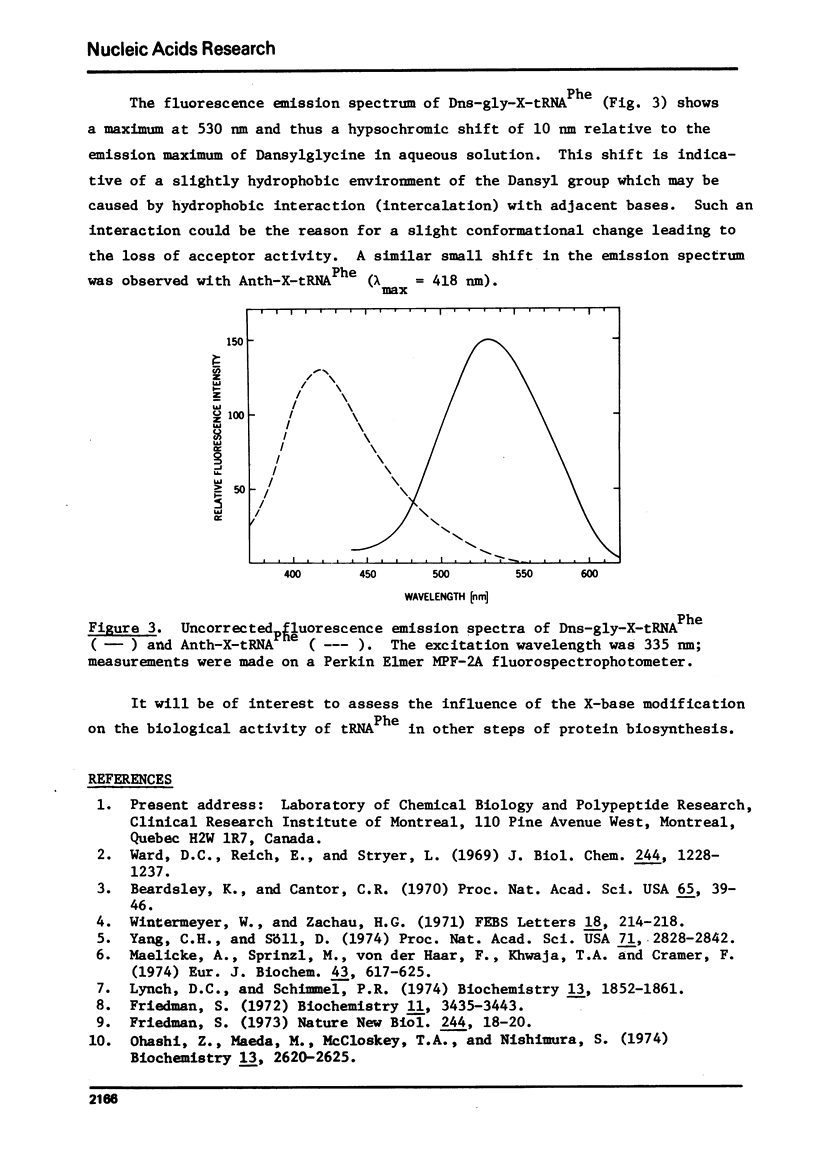
Abstract
tRNA PheE, coli was labeled with the N-hydroxysuccinimide esters of 1-dimethylaminonaphthalene-5-sulfonyl glycine and N-methylanthranilic acid through reaction with the amino acid moiety of its X-base, whereby yields of 66% and 24%, respectively, were obtained. The purified dimethylaminonaphthalene-sulfonate derivative could not be aminoacylated and was found to be a strong competitive inhibitor of phenylalanine-tRNA synthetase [Ki=8X10(-7) M]. The N-methylanthraniloyl derivative could be charged to an extent of 5% as compared to native tRNA Phe. The fluorescence emission spectra of the derivatives are indicative of a slightly hydrophobic environment for both fluorophores. The results suggest that the integrity of the polar amino acid group of the X-base is required for the maintenance of the biologically active conformation.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beardsley K., Cantor C. R. Studies of transfer RNA tertiary structure by singlet-singlet energy transfer. Proc Natl Acad Sci U S A. 1970 Jan;65(1):39–46. doi: 10.1073/pnas.65.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caron M., Dugas H. Specific spin-labeling of transfer ribonucleic acid molecules. Nucleic Acids Res. 1976 Jan;3(1):19–34. doi: 10.1093/nar/3.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman S. Acylation of transfer ribonucleic acid with the N-hydroxysuccinimide ester of phenoxyacetic acid. Biochemistry. 1972 Aug 29;11(18):3435–3443. doi: 10.1021/bi00768a017. [DOI] [PubMed] [Google Scholar]
- Friedman S., Li H. J., Nakanishi K., Van Lear G. 3-(3-amino-3-carboxy-n-propyl)uridine. The structure of the nucleoside in Escherichia coli transfer ribonucleic acid that reacts with phenoxyacetoxysuccinimide. Biochemistry. 1974 Jul 2;13(14):2932–2937. doi: 10.1021/bi00711a024. [DOI] [PubMed] [Google Scholar]
- Friedman S. Patterns of base modification in tRNA. Nat New Biol. 1973 Jul 4;244(131):18–20. doi: 10.1038/newbio244018a0. [DOI] [PubMed] [Google Scholar]
- Lynch D. C., Schimmel P. R. Effects of abnormal base ionizations on Mg2 plus binding to transfer ribonucleic acid as studied by a fluorescent probe. Biochemistry. 1974 Apr 23;13(9):1852–1861. doi: 10.1021/bi00706a013. [DOI] [PubMed] [Google Scholar]
- Maelicke A., Sprinzl M., von der Haar F., Khwaja T. A., Cramer F. Structural studies on phenylalanine transfer ribonucleic acid from yeast with the spectroscopic label formycin. Eur J Biochem. 1974 Apr 16;43(3):617–625. doi: 10.1111/j.1432-1033.1974.tb03449.x. [DOI] [PubMed] [Google Scholar]
- Nauheimer U., Hedgcoth C. Activation of several tRNAs of Escherichia coli by the phenoxyacetyl derivative of N-hydroxysuccinimide. Arch Biochem Biophys. 1974 Feb;160(2):631–642. doi: 10.1016/0003-9861(74)90440-8. [DOI] [PubMed] [Google Scholar]
- Ohashi Z., Maeda M., McCloskey J. A., Nishimura S. 3-(3-Amino-3-carboxypropyl)uridine: a novel modified nucleoside isolated from Escherichia coli phenylalanine transfer ribonucleic acid. Biochemistry. 1974 Jun 4;13(12):2620–2625. doi: 10.1021/bi00709a023. [DOI] [PubMed] [Google Scholar]
- Schofield P., Hoffman B. M., Rich A. Spin-labeling studies of aminoacyl transfer ribonucleic acid. Biochemistry. 1970 Jun 9;9(12):2525–2533. doi: 10.1021/bi00814a020. [DOI] [PubMed] [Google Scholar]
- Söll D., Cherayil J. D., Bock R. M. Studies on polynucleotides. LXXV. Specificity of tRNA for codon recognition as studied by the ribosomal binding technique. J Mol Biol. 1967 Oct 14;29(1):97–112. doi: 10.1016/0022-2836(67)90183-0. [DOI] [PubMed] [Google Scholar]
- Ward D. C., Reich E., Stryer L. Fluorescence studies of nucleotides and polynucleotides. I. Formycin, 2-aminopurine riboside, 2,6-diaminopurine riboside, and their derivatives. J Biol Chem. 1969 Mar 10;244(5):1228–1237. [PubMed] [Google Scholar]
- Wintermeyer W., Zachau H. G. Replacement of Y base, dihydrouracil, and 7-methylguanine in tRNA by artificial odd bases. FEBS Lett. 1971 Nov 1;18(2):214–218. doi: 10.1016/0014-5793(71)80447-7. [DOI] [PubMed] [Google Scholar]
- Yang C. H., Söll D. Covalent attachment of fluorescent groups to the 5'-end of transfer RNA. Arch Biochem Biophys. 1973 Mar;155(1):70–81. doi: 10.1016/s0003-9861(73)80010-4. [DOI] [PubMed] [Google Scholar]
- de Groot N., Lapidot Y., Panet A., Wolman Y. The synthesis of N-acetylphenylalanyl-sRNA. Biochem Biophys Res Commun. 1966 Oct 5;25(1):17–22. doi: 10.1016/0006-291x(66)90633-4. [DOI] [PubMed] [Google Scholar]


