Abstract
Animals can store learned information in their brains through a series of distinct memory forms. Short-lasting memory forms can be followed by longer-lasting, consolidated memory forms. However, the factors determining variation in memory consolidation encountered in nature have thus far not been fully elucidated. Here, we show that two parasitic wasp species belonging to different families, Cotesia glomerata (Hymenoptera: Braconidae) and Trichogramma evanescens (Hymenoptera; Trichogrammatidae), similarly adjust the memory form they consolidate to a fitness-determining reward: egg-laying into a host-insect that serves as food for their offspring. Protein synthesis-dependent long-term memory (LTM) was consolidated after single-trial conditioning with a high-value host. However, single-trial conditioning with a low-value host induced consolidation of a shorter-lasting memory form. For Cotesia glomerata, we subsequently identified this shorter-lasting memory form as anesthesia-resistant memory (ARM) because it was not sensitive to protein synthesis inhibitors or anesthesia. Associative conditioning using a single reward of different value thus induced a physiologically different mechanism of memory formation in this species. We conclude that the memory form that is consolidated does not only change in response to relatively large differences in conditioning, such as the number and type of conditioning trials, but is also sensitive to more subtle differences, such as reward value. Reward-dependent consolidation of exclusive ARM or LTM provides excellent opportunities for within-species comparison of mechanisms underlying memory consolidation.
Introduction
Rewards (e.g. food, hosts) of different value offered during associative conditioning are known to induce changes in the conditioned response, such as floral odor preference in honey- and bumblebees [1], [2], the duration of a feeding response to odors in parasitic wasps [3], win-shift tendency in birds [4], place preference in rats [5] and cache recovery preference in food-storing birds [6], as well as changes in the duration of memory for, e.g., odors in fruit flies [7]. The value of a rewarding reinforcer can vary in relation to its relative contribution to an animal's fitness and the reliability of the association between the learned information and the reward. We hypothesized that rewards of the same type but of different value may also induce consolidation of a different memory form. Consolidation of long-term memory may not be optimal when the reward value is relatively low, because it is energetically more costly than consolidation of shorter-lasting memory forms [8] and because instant consolidation of long-term memory would not be advantageous if the risk of making irrelevant associations is high [9].
In insects it has been shown that initial, labile anesthesia-sensitive memory (ASM) can be consolidated into ARM and/or more stable, protein synthesis-dependent LTM [10]–[14]. In contrast to ASM, the formation of both ARM and LTM is insensitive to retrograde amnesia applied immediately after conditioning [15]. Hence, both ARM and LTM are regarded as forms of “consolidated memory”. LTM differs from ARM in that only the consolidation of LTM is dependent on ‘de novo’ protein synthesis, which can be blocked by protein-synthesis inhibitors, and involves structural changes in the brain [10]. Molecular mechanisms of memory formation are highly conserved throughout the animal kingdom [16], although the nature of the disruptive treatments that are used to distinguish between memory forms may slightly differ. Also in vertebrates protein-synthesis inhibitors, such as anisomycin, are used to prevent the formation of LTM [17]. However, while cold-shock is often used in invertebrates to induce retrograde amnesia [18], [19], electroconvulsive shocks or CO2 are more widely used in homeothermic animals [20], [21]. Which memory form is consolidated is known to depend on the number of conditioning trials (single versus multiple trials spaced in time) and the type of conditioning (aversive conditioning using a punishing reinforcer versus appetitive conditioning using a rewarding reinforcer) [10]–[12]. Whether more subtle differences in the reinforcer can also affect memory consolidation is currently still unknown.
Parasitic wasps (parasitoids) are ideal model organisms for studying the effect of reward value on memory consolidation in an ecologically relevant context. These insects optimize searching efficiency for their inconspicuous hosts by learning to associate odor cues with host presence [13], a learning task that is intricately linked to fitness. The reward in this context is egg-laying into the host (Movies S1, S2). The odor cues that are learned differ between parasitoids of insect larvae and insect eggs: egg parasitoids can exploit pheromones emitted by adult mated female hosts and hitch-hike with them to egg-laying sites [22]–[24] (Movie S3), whereas parasitoids of insect larvae can respond to plant odors induced by feeding host larvae [25].
In this study, we investigated the effect of host-species (i.e. reward) on memory consolidation in two parasitic wasp species belonging to different families, Cotesia glomerata (Hymenoptera: Braconidae) and Trichogramma evanescens (Hymenoptera: Trichogrammatidae; Fig. 1, 2). These wasps parasitize the larvae and eggs, respectively, of two closely related cabbage white butterflies; Pieris brassicae, which lays egg clusters [26], and P. rapae, which deposits single eggs [27]. Both C. glomerata and T. evanescens wasps readily consolidate LTM after single-trial conditioning with P. brassicae [9], [23]. Pieris brassicae hosts offer a higher contribution to the maternal fitness of the two parasitic wasp species than P. rapae hosts, both for quantitative and qualitative reasons. First of all, a female parasitoid needs to find relatively few P. brassicae clusters to lay all her eggs, whereas she needs to locate a large number of single P. rapae hosts to do the same. Furthermore it has been shown for both parasitic species that one P. brassicae host (caterpillar or egg) gives rise to more and larger parasitoid offspring than one P. rapae host [28], [29]. We therefore hypothesized that single-trial conditioning with P. rapae will not result in LTM consolidation, but that instead only the shorter-lasting memory form ARM is consolidated.
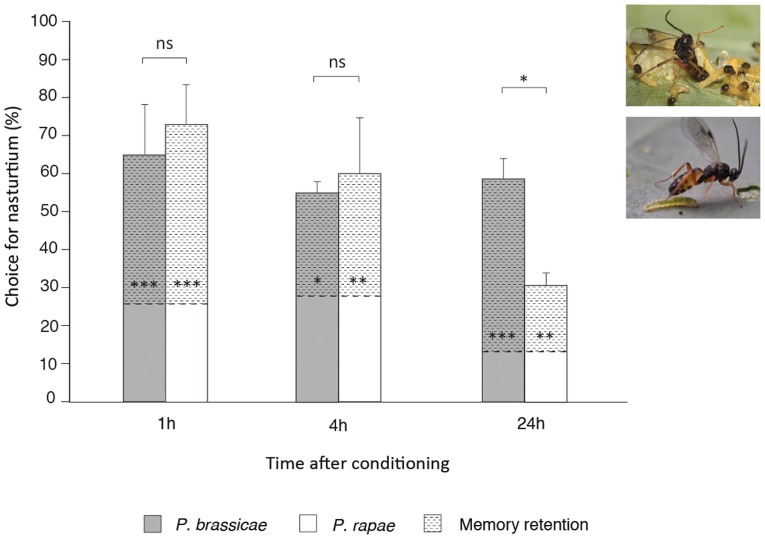
Figure 1. Cotesia glomerata memory retention after single-trial conditioning with a low- or high-value reward.
Mean percent choice (+ SE) of Cotesia glomerata for nasturtium (learned odor) at 1 h, 4 h and 24 h after single-trial conditioning with Pieris brassicae (high-value reward; dark grey bars) or P. rapae (low-value reward; white bars). Cotesia glomerata was conditioned by allowing each wasp to lay eggs into one newly-emerged Pieris caterpillar that was placed on either a host-damaged leaf of a nasturtium plant (Tropaeolum majus) or a Brussels sprouts plant (Brassica oleracea var. gemmifera) [41]. Wasps were tested in a two-choice wind tunnel set-up [42] in which they could fly towards, and land on, a nasturtium or a Brussels sprouts plant. Brussels sprouts odor is relatively more attractive to inexperienced wasps than nasturtium odor [35] and this preference cannot be further increased through conditioning with Brussels sprouts [15]. We used the percentage choice for nasturtium of wasps conditioned on Brussels sprouts as a reference (indicated by the dotted lines inside the bars), as these wasps have a higher flight response towards the plants than inexperienced wasps [9], [36], [41]. Mean reference values (± SE) are 25.7 (±14.12) for 1 h, 27.5 (±9.46) for 4 h and 13.3 (±4.64) for 24 h. Memory was defined as present when the percentage choice for nasturtium of wasps conditioned with the nasturtium odor was significantly higher than the reference (indicated by asterisks inside the bars), with a larger difference representing a higher memory retention level. Memory retention is indicated by the dashed pattern inside the bars. For each treatment we tested 10–15 C. glomerata wasps on at least 4 different days (n>40). Each wasp was tested only once. * = P<0.05; ** = P<0.01; *** = P<0.001; ns = not significant (GLM using the variable ‘memory retention’ for comparisons between bars (Table 2) and GLM with logit-link function using the variable ‘percentage choice for nasturtium’ for comparisons within bars (Table 1)). Photos represent a C. glomerata wasp parasitizing a P. brassicae caterpillar within a cluster (above) or a single P. rapae caterpillar (below). Photos courtesy of Hans M. Smid (www.bugsinthepicture.com).
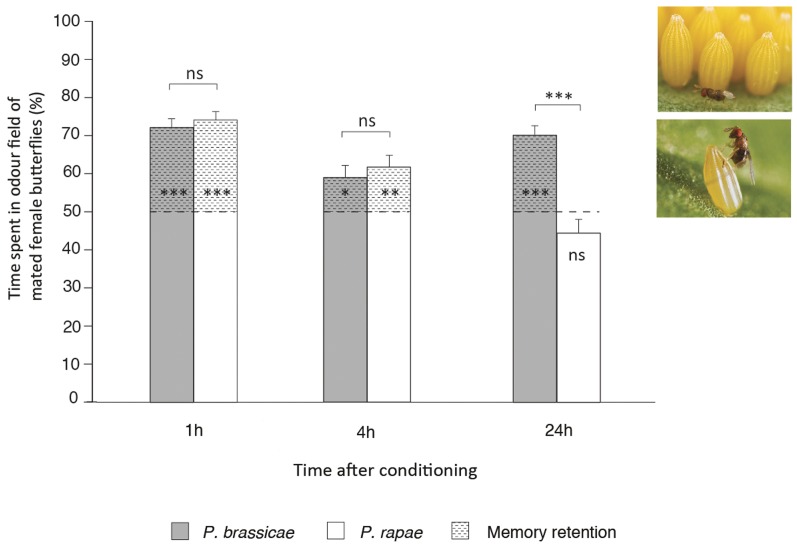
Figure 2. Trichogramma evanescens memory retention after single-trial conditioning with a low- or high-value reward.
Mean percent time (+ SE) spent in odor field of mated female butterflies (learned odor) of Trichogramma evanescens at 1 h, 4 h and 24 h after single-trial conditioning with Pieris brassicae (high-value reward; dark grey bars) or P. rapae (low-value reward; white bars). Trichogramma evanescens was conditioned by allowing each wasp to approach and mount a recently mated (and thus egg-laying) female butterfly, emitting an anti-aphrodisiac pheromone, followed by the reward: parasitizing one freshly laid single P. rapae egg or one P. brassicae egg within an egg cluster [23], [24]. Wasps were tested in a 2-chamber olfactometer [23], [24] with two mated female butterflies in one compartment and two males in the other. As these two odors are equally attractive to inexperienced wasps [23], [24], we used 50% of time spent in the odor field of mated female butterflies as a reference (indicated by the dotted line inside or above the bars). Memory was defined as present when the percentage of time spent in the odor field of mated female butterflies was significantly higher than the reference (indicated by asterisks inside the bars), with a larger difference representing a higher memory retention level. Memory retention is indicated by the dashed pattern inside the bars. For each treatment we tested a total of 40 T. evanescens wasps. Each wasp was tested only once. * = P<0.05; ** = P<0.01; *** = P<0.001; ns = not significant (GLM for comparisons between bars (Table 3) and WMPSRT for comparisons within bars). Photos represent a T. evanescens wasp parasitizing a P. brassicae egg within a cluster (above) or a single P. rapae egg (below). Photos courtesy of Nina E. Fatouros (www.bugsinthepicture.com).
Results
Memory Retention
We first tested for the presence of memory at different times after conditioning with either P. brassicae or P. rapae by comparing the percentage choice for nasturtium between wasps conditioned with the odor of a nasturtium plant and the reference group that was conditioned with the odor of a Brussels sprouts plant (C. glomerata; comparisons within bars in Fig. 1) or by assessing whether the difference in percentage of time spent in the odor field of mated female butterflies differs from 50% (T. evanescens; comparisons within bars in Fig. 2). Next, we tested whether the memory retention (MR) level changes with time after conditioning and whether this is dependent on the host-species used for conditioning (comparisons between bars in Fig. 1, 2). We found memory in C. glomerata following single-trial conditioning with either P. brassicae or P. rapae to be present at all times tested (Table 1; comparisons within bars in Fig. 1) However, we found a significant effect on MR level of the interaction between host-species and time after conditioning (F2,22 = 5.70, P = 0.010, Table 2; comparisons between bars in Fig. 1). Single-trial conditioning with the high-value host P. brassicae resulted in a significantly higher MR level than single-trial conditioning with the lower-value host P. rapae at 24 h after conditioning (Tukey-HSD, P = 0.016), while MR levels were comparable at 1 h (P = 0.960) and 4 h (P = 0.995) after conditioning. A similar difference in MR level was found for T. evanescens (interaction between host-species and time after conditioning (F2,234 = 15.29, P<0.001, Table 3; comparisons between bars in Fig. 2)). Also in T. evanescens was 24 h MR level significantly higher after single-trial conditioning with P. brassicae than after than single-trial conditioning with P. rapae (Tukey-HSD, P<0.001), whereas 1 h and 4 h MR levels were similar (P = 0.993 and P = 0.998, respectively) for both host-species. Memory was even completely absent in T. evanescens at 24 h after single-trial conditioning with P. rapae (percentage of time spent in the odor field of mated female butterflies = 44.3±3.54; Wilcoxon's matched-pairs signed-ranks test (WMPSRT), P = 0.116; comparison within 24 h bar in Fig. 2). These results indicate that both wasp species have longer-lasting memory after single-trial conditioning with P. brassicae than after single-trial conditioning with P. rapae.
Table 1. Cotesia glomerata; time series.
| Time after conditioning | Treatment | ||
| 1 h | Conditioning treatment | X2 2 = 16.25 | P<0.001 |
| • P. rapae on nasturtium | |||
| • P. brassicae on nasturtium | |||
| • P. brassicae on Brussels sprouts | |||
| Block (experimental day)* | X2 3 = 11.30 | P<0.001 | |
| 4 h | Conditioning treatment | X2 2 = 4.17 | P = 0.016 |
| • P. rapae on nasturtium | |||
| • P. brassicae on nasturtium | |||
| • P. brassicae on Brussels sprouts | |||
| Block (experimental day)* | X2 3 = 3.00 | P = 0.030 | |
| 24 h | Host-species | X2 1 = 2.41 | P = 0.121 |
| • P. rapae | |||
| • P. brassicae | |||
| Plant species used for conditioning | X2 1 = 43.79 | P<0.001 | |
| • nasturtium | |||
| • Brussels sprouts | |||
| Host-species * plant species used for conditioning | X2 1 = 6.49 | P = 0.011 | |
| Block (experimental day)* | X2 5 = 1.35 | P = 0.238 |
None of the interaction effects between block and treatment factors were significant (α = 0.05).
GLM with binomial distribution of error variance and a logit-link function. Response variable: Percentage choice for nasturtium (Fig. 1).
Table 2. Cotesia glomerata; time series.
| Treatment | ||
| Model | F5,22 = 3.77 | P = 0.013 |
| Host-species | F1,22 = 1.01 | P = 0.325 |
| • P. rapae | ||
| • P. brassicae | ||
| Time after conditioning | F2,22 = 2.32 | P = 0.122 |
| • 1 h | ||
| • 4 h | ||
| • 24 h | ||
| Host-species * time after conditioning | F2,22 = 5.70 | P = 0.010 |
GLM. Response variable: memory retention (results section).
Table 3. Trichogramma evanescens; time series.
| Treatment | ||
| Model | F5,234 = 14.09 | P<0.001 |
| Host-species | F1,234 = 9.27 | P = 0.003 |
| • P. rapae | ||
| • P. brassicae | ||
| Time after conditioning | F2,234 = 15.31 | P<0.001 |
| • 1 h | ||
| • 4 h | ||
| • 24 h | ||
| Host-species * time after conditioning | F2,234 = 15.29 | P<0.001 |
GLM. Response variable: Percentage of time spent in the odor field of mated female butterflies (Fig. 2). Data were arcsine square-root transformed to meet Levene's test for homogeneity of variance of treatment groups.
Subsequently we showed that both C. glomerata and T. evanescens are capable of forming 24 h-lasting memory with the lower-value reward P. rapae by comparing the choice behavior of wasps that received multiple conditioning trials spaced in time with P. rapae to the choice behavior of wasps that received a single conditioning trial with this host-species (X2 1 = 721.95, P<0.001, Table 4). Cotesia glomerata wasps that received three spaced conditioning trials with P. rapae had significantly higher 24 h memory (percentage choice for nasturtium = 79.0±4.20) than wasps that received a single conditioning trial with this host-species (percentage choice for nasturtium = 27.5±4.78), and T. evanescens wasps that were given two spaced conditioning trials with P. rapae formed memory that lasted at least 24 h (percentage of time spent in the odor field of mated female butterflies = 58.3±3.88; WMPSRT, P = 0.013).
Table 4. Cotesia glomerata; spaced conditioning.
| Treatment | ||
| Conditioning | X2 1 = 721.95 | P<0.001 |
| • Single conditioning trial with P. rapae | ||
| • Three conditioning trials with P. rapae spaced by 10 min intervals | ||
| Block (experimental day)* | X2 3 = 73.16 | P<0.001 |
The interaction effect between block and conditioning treatment was not significant (α = 0.05).
GLM with binomial distribution of error variance and a logit-link function. Response variable: Percentage choice for nasturtium (results section).
Memory Forms
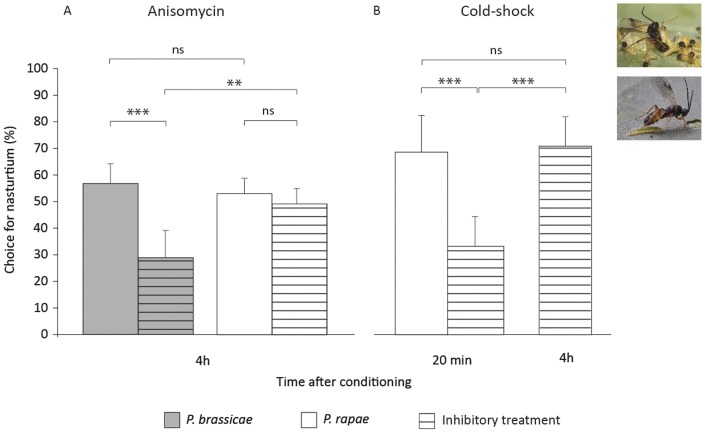
We further investigated for C. glomerata which memory form was consolidated after single-trial conditioning with P. rapae. We used the protein synthesis-inhibitor anisomycin to determine whether P. rapae-induced 4 h memory was protein synthesis-dependent. Since 4 h memory in C. glomerata following single-trial conditioning with P. brassicae is based on protein synthesis [15], we used wasps conditioned with P. brassicae to verify whether the anisomycin treatment was effective. We found a significant interaction between host-species and anisomycin treatment (X2 1 = 5.91, P = 0.015, Table 5, Fig. 3A). As expected, anisomycin caused a significant reduction in the percentage choice for nasturtium of wasps conditioned with P. brassicae on this plant species (P<0.001). However, it did not affect the percentage choice for nasturtium of wasps conditioned with P. rapae on this plant (P = 0.592). This shows that in C. glomerata 4 h memory following a single-trial conditioning with P. rapae is not LTM.
Table 5. Cotesia glomerata; disruptive treatments.
| Disruptive treatment | Treatment | ||
| Anisomycin | Host-species | X2 1 = 2.85 | P = 0.092 |
| • P. rapae | |||
| • P. brassicae | |||
| Treatment | X2 1 = 10.23 | P = 0.001 | |
| • Anisomycin | |||
| • Control | |||
| Host-species * treatment | X2 1 = 5.91 | P = 0.015 | |
| Block (experimental day)* | X2 3 = 3.17 | P = 0.023 | |
| Cold-shock | Treatment | X2 2 = 8.74 | P<0.001 |
| • P. rapae + cold-shock; test after 20 min | |||
| • P. rapae + cold-shock; test after 4 h | |||
| • P. rapae positive control; test after 20 min | |||
| Block (experimental day)* | X2 3 = 5.01 | P = 0.002 |
None of the interaction effects between block and treatment factors were significant (α = 0.05).
GLM with binomial distribution of error variance and a logit-link function. Response variable: Percentage choice for nasturtium (Fig. 3).
Figure 3. Cotesia glomerata memory dissection using inhibitory treatments.
Effect of the LTM consolidation-inhibitor anisomycin (A) or an ASM-inhibiting cold-shock treatment (B) on the percentage choice for the learned odor in Cotesia glomerata at 20 min and/or 4 h after single-trial conditioning with Pieris brassicae (high-value reward; dark grey bars) or P. rapae (low-value reward; white bars) on a nasturtium plant. The inhibitory treatments are indicated by horizontal stripes. Wasps were tested in a two-choice wind tunnel set-up in which they could fly towards, and land on, a nasturtium or a Brussels sprouts plant. Brussels sprouts emits an odor that is relatively more attractive to inexperienced wasps than nasturtium odor [35]. For each treatment 10–15 wasps were tested on at least 4 different days (n>40). Each wasp was tested only once. * = P<0.05; ** = P<0.01; *** = P<0.001; ns = not significant (GLM with logit-link function, Table 5).
Subsequently, we investigated whether P. rapae-induced 4 h memory in C. glomerata is anesthesia-sensitive (ASM) or anesthesia-resistant (ARM). Cooling wasps on ice for 2 min immediately following conditioning on nasturtium disrupts ASM without affecting ARM or LTM consolidation [15]. Such treatment, therefore, results in a temporal amnesia (around 20 min after single-trial conditioning) until consolidated memory is present after 2–3 h [15]. We therefore compared the wasps' choice between nasturtium and Brussels sprouts at 20 min and 4 h after the cold-shock treatment, using different groups of wasps for each time point. Cold-shock applied 10 min before conditioning should not induce amnesia [30] and was used as a positive control for 20 min memory. We found a significant effect of cold-shock treatment (X2 2 = 8.74, P<0.001, Table 5, Fig. 3B). As expected, the percentage choice for nasturtium of cold-shock treated wasps at 20 min after conditioning was reduced compared to the positive control treatment (P<0.001). However, the percentage choice for nasturtium of cold-shock treated wasps at 4 h after conditioning was not reduced (P = 0.812). This indicates that in C. glomerata 4 h memory following single-trial conditioning with P. rapae must be ARM.
Discussion
Our results clearly show that two parasitic wasp species that belong to different hymenopteran families adjust their memory for odors in a similar manner to different-value rewards, represented by the same two host-species. This similarity in memory adjustment, regardless of the fact that the two species i) lay their eggs in different host life stages, i.e. in the host egg or caterpillar, ii) use different host (induced) odors, and iii) require different conditioning and memory testing procedures, indicates that reward value-dependent memory formation is not specific for a single species or a certain group of closely related parasitic wasps, but is likely to be a more general phenomenon. In both species, egg-laying in P. brassicae resulted in LTM consolidation, whereas egg-laying in P. rapae resulted in a shorter-lasting memory, identified as ARM in C. glomerata. Pieris brassicae offers a higher contribution to the maternal fitness of the two parasitic wasp species than P. rapae. A female parasitoid needs to find relatively few clusters of P. brassicae to lay all her eggs, whereas she needs to locate a large number of single P. rapae hosts to do so. Trichogramma evanescens, which immediately stops its hosts' development after egg-laying (i.e. is an idiobiont), can parasitize a wide range of host-species in addition to the two cabbage white species [31]. It assesses reward value by “measuring” egg- and cluster size while walking over the egg (cluster) [32] (Movie S2). As a single P. brassicae egg is larger than a P. rapae egg, a P. brassicae egg allows for the deposition of more eggs (3.4±0.11 (n = 91) and 2.9±0.07 (n = 45), resp.; Mann-Whitney U test, P<0.001) and/or the development of larger offspring [29]. For a koinobiont parasitoid like Cotesia glomerata, whose host lives until the developing wasps reach maturity (Movie S1), host size is not indicative of host quality [28]. However, C. glomerata has consistently higher developmental rate and larger clutch- and adult size when developing from a P. brassicae caterpillar than when developing from a P. rapae caterpillar [28]. As this wasp species primarily specializes on cabbage whites [33], it has likely evolved to value P. brassicae as a greater reward than P. rapae.
Moreover, P. brassicae constitutes a relatively high value host for C. glomerata in terms of the reliability of the association between the learned plant odors and the reward, because this butterfly mostly oviposits its egg clusters in clumps of vegetation of the same species [26]. This increases the chance that C. glomerata will find another host cluster on a nearby plant of the same species. In contrast, P. rapae deposits single eggs on isolated and distant plants of various species [27], which increases the risk that C. glomerata learns an irrelevant plant odor from a single egg-laying experience into a P. rapae caterpillar. For T. evanescens the risk of making an irrelevant association is low for both host-species, as the learned cue, an anti-aphrodisiac pheromone, is a highly reliable indicator of future host egg availability.
It is very unlikely that the differences in memory pattern observed are influenced by differences in the conditioned stimuli (CS, in this case odors), as the wasps perceived the CS related to both host species equally well. The composition of the plant odor blend induced by feeding P. brassicae and P. rapae caterpillars is very similar [34]. It has previously been shown that neither inexperienced C. glomerata wasps [35], nor wasps that received a single experience with the plant-host complex [36], distinguish between P. brassicae- and P. rapae-damaged plants. Furthermore, we found inexperienced T. evanescens wasps to be equally attracted to the species-specific odor of mated female P. rapae butterflies compared to the odor of mated female P. brassicae butterflies (percentage of time spent in the odor field of the mated P. brassicae butterflies = 47.8±3.23; WMPSRT, P = 0.752). Trichogramma evanescens wasps could also learn to prefer the odor of both a P. rapae and a P. brassicae mated female over the odor of a mated female of the opposite species when tested at 1 h after single-trial conditioning (percentage of time spent in the field of the experienced odor = 57.2±3.51; WMPSRT, P = 0.027 when conditioned with P. rapae, and 62.0±3.45; WMPSRT, P = 0.002 when conditioned with P. brassicae), and the preference levels for the learned odors were similar for both host-species (GLM, P = 0.422).
In most animals for which memory forms have been described so far, long-lasting memory forms (such as ARM and LTM) can consolidate and occur as independent, parallel processes, which together give rise to the observed MR level [12], [37], but see [14]. The neural basis of these different memory forms seems highly conserved [38]. Nevertheless, our previous and current results show that parasitic wasps display a wealth of variation in the dynamics of memory formation. This natural variation offers some unique possibilities to address proximate and ultimate questions in multidisciplinary studies on learning and memory formation [13]. Such an approach, employing natural variation, is a novel contribution to current research on inbred lines, single gene mutants and transgenic animals derived from a few model species. The fact that mechanisms involved in memory formation are highly conserved, ensures that studies on parasitoids are highly relevant [39]. Unlike in most animals, including a closely related parasitoid species, Cotesia rubecula, memory transition from ASM to LTM in C. glomerata following a single or multiple, spaced egg-laying experiences into P. brassicae occurs without an intermediate ARM phase [9], [15]. This suggests that C. glomerata consolidates ARM or LTM in a mutually exclusive manner; only ARM after a single experience with P. rapae and only LTM after a single or after multiple, spaced experiences with P. brassicae. This finding provides excellent opportunities for within-species comparative research into the mechanisms underlying memory consolidation, because exclusive ARM or LTM consolidation processes can be studied after being induced by giving females one single egg-laying experience, either on a P. brassicae or on a P. rapae caterpillar. Such a comparison constitutes a natural, ecologically relevant approach, but also avoids pleiotropic effects [40], which can compromise the analysis of single-gene mutants and transgenic animals.
We conclude that the memory form that is consolidated does not only change in response to relatively large differences in conditioning, such as the number and type of conditioning trials [10]–[12], but is also sensitive to more subtle differences, such as reward value. Consolidation of specific memory forms should therefore be considered as a plastic trait that, rather than reflecting an animal's capability to learn a specific task, can be adjusted to differences in the perceived value of the reward.
Materials and Methods
Cotesia glomerata Experiments
Insects and Plants
Cotesia glomerata wasps (Hymenoptera: Braconidae; approximately 5.0 mm long) were obtained from a colony that originated from individuals collected in cabbage fields in the vicinity of Wageningen, the Netherlands. This laboratory colony, which passes through approximately 15 generations per year, was replaced each year by new field-collected individuals. Cotesia glomerata was reared in caterpillars of the large cabbage white butterfly Pieris brassicae L. (Lepidoptera: Pieridae). The two butterfly species Pieris brassicae and Pieris rapae were reared on Brussels sprouts plants (Brassica oleracea L. var. gemmifera cv. Cyrus, Brassicaceae). Insect rearing was performed in a climatic room at 20–22°C, 50–70% RH and a L16:D8 photoperiod. Upon emergence, male and female parasitoids were caged together to allow mating, and provided with water and honey. Only mated, 3–9 day-old inexperienced female C. glomerata wasps were used in the experiments. Brussels sprouts plants and nasturtium plants (Tropaeolum majus L. var. Glorious Gleam, Tropaeolaceae) used for the experiments were reared in a greenhouse at 20–25°C, 50–70% RH and a L16:D8 photoperiod.
Conditioning Procedure
Conditioning was performed as described previously [41]. A conditioning trial consisted of a single oviposition (egg-laying) experience into a newly emerged P. brassicae or P. rapae caterpillar that was placed on a damaged leaf of a host feeding-damaged nasturtium plant or a host feeding-damaged Brussels sprouts plant. The Brussels sprouts odor is relatively more attractive to inexperienced wasps than the nasturtium odor [35]. By using two plant species whose odors differ in attractiveness to inexperienced wasps we more closely mimic the natural situation, in which inexperienced wasps always have a strong preference for certain plant odors, and can shift this preference by learning. In our study system, a wasp can learn to increase its preference for nasturtium relative to Brussels sprouts during an oviposition experience with a caterpillar that is feeding on a nasturtium plant. However, a similar experience on a Brussels sprouts plant does not further increase the already existing preference for this plant odor [15]. As inexperienced wasps generally have a low flight response to the plants when tested in the wind tunnel [9], [36], [41], we used wasps conditioned on Brussels sprouts as a reference group. For the 24 h choice test two reference treatments were included, one consisting of wasps conditioned on Brussels sprouts with P. rapae and one consisting of wasps conditioned on Brussels sprouts with P. brassicae. Because we obtained similar preference levels for these two control treatments, we only included wasps conditioned with P. brassicae on Brussels sprouts as a reference group for the 1 h and 4 h choice tests. Two days before their use in the conditioning trials, plants were infested with 40 recently emerged caterpillars that were spread over two leaves. Shortly before conditioning all caterpillars were removed from the plants. Wasps were conditioned with newly emerged caterpillars in order to facilitate oviposition, as older caterpillars tend to defend themselves aggressively. For each conditioning trial a new caterpillar was placed on a damaged leaf. Unconditioned female wasps were individually placed in a glass tube, which was then brought in close proximity to a caterpillar on a damaged leaf. The wasps were released onto the leaf, ensuring direct contact of their antennae with a caterpillar and its products. This stimulation induced an immediate oviposition response, lasting approx. 10 s. Apart from single-trial conditioning, we also included a spaced conditioning treatment, in which C. glomerata wasps received three conditioning trials spaced by 10 min intervals. After each oviposition, the parasitized caterpillar was removed and the wasp was transferred to a cage with honey and water until the memory bioassay. Between multiple conditioning trials spaced in time, or when tested 1 h after conditioning, wasps were individually kept in a glass vial.
Choice Bioassay
Cotesia glomerata wasps were tested at 1 h, 4 h, and 24 h after conditioning in a two-choice wind tunnel set-up [42], in which wasps could fly towards and land on a nasturtium plant or a Brussels sprouts plant, both damaged by host-feeding. Each wasp was only tested once. We tested two six-week old nasturtium plants in a single pot against one eight-week old Brussels sprouts plant, in order to balance differences in size and frontal leaf density. Nasturtium and Brussels sprouts plants were each infested with resp. 20 or 10 newly emerged caterpillars spread over 2 leaves at resp. 2 and 1 day(s) before the choice test. Plants used in the wind tunnel for the 24 h choice test were infested either with Pieris brassicae or P. rapae, depending on the host species used for conditioning. Plants used for the 1 h and 4 h choice tests, for which only one reference treatment was included, were infested with Pieris brassicae, independent of the Pieris species used during conditioning. It has previously been shown that C. glomerata does not distinguish between P. brassicae- and P. rapae-damaged plants after a single experience with the plant-host complex [36]. Wasps were released two-by-two into the wind tunnel from the middle of an open glass cylinder that was placed at approximately 70 cm from the two odor sources. After half of the wasps of each conditioning treatment made a choice, plant position was changed in order to prevent bias towards one side of the wind tunnel. Wasps were given maximum 15 minutes to show a preference by flying towards, and landing on, one of the plants. Each experimental day we tested the preference of 10–15 wasps of all conditioning treatments belonging to the same time point or memory-inhibition assay (see below) using the same plant pair (except for the 24 h choice test, in which a different plant pair was used for wasps conditioned with P. rapae and for wasps conditioned with P. brassicae). For each time point/memory-inhibition assay, wasps were tested on at least 4 different days, resulting in n>40 wasps per conditioning treatment and at least 4 different plant pairs. Memory was defined as present when the percentage of wasps choosing for nasturtium was significantly higher for wasps conditioned on nasturtium compared to wasps conditioned on Brussels sprouts, with a larger difference between these two groups representing a higher memory retention level.
Long-Term Memory (LTM)-Inhibition Assay
We used the protein synthesis-inhibitor anisomycin (ANI) to determine whether 4 h memory in C. glomerata following single-trial conditioning with P. rapae is protein synthesis-dependent. The appropriate ANI concentration had been determined previously [9]. ANI treatment of C. glomerata wasps did not affect preference levels of unconditioned wasps, nor did it affect 1 h memory [9]. Female wasps were deprived of honey and water overnight, and fed 0.5 µL of a 3% sucrose solution containing 5 mM ANI the next morning, approximately 2 h before conditioning. Wasps serving as controls for the ANI-fed wasps were given the same treatment, but without ANI in the sucrose solution. Wasps were individually kept in vials for 1 h, and only those wasps that consumed the solution entirely were selected for subsequent conditioning. The selected wasps were transferred to a glass cage with access to water and honey until conditioning. Since 4 h memory in C. glomerata following single-trial conditioning with P. brassicae is based on LTM [15], we used the wasps conditioned on P. brassicae to verify whether the ANI treatment was effective.
Anesthesia-Sensitive Memory (ASM)-Inhibition Assay
We used cold-shock induced retrograde amnesia to investigate whether 4 h memory in C. glomerata following single-trial conditioning with P. rapae is based on ASM or ARM. Cooling wasps on ice for 2 min directly after conditioning disrupts ASM without affecting ARM or LTM consolidation [15]. Such cold-shock induced retrograde amnesia was prominent in Cotesia wasps at 20 min after single-trial conditioning [15]. Therefore we compared the wasps' choice distribution at 20 min and 4 h after cold-shock treatment, using different groups of wasps for each time point. Cold-shock applied 10 min before conditioning should not induce amnesia [30] and was used as a positive control for 20 min memory. The nature of our wind tunnel procedure (active oriented flight behavior) ensures that wasps were completely recovered from this treatment at the time of the choice test.
Data Analysis
To test for main and interaction effects of “host-species used during conditioning” and “time after conditioning” on memory, we calculated the level of memory retention for each replicate as the difference in the percentage of wasps landing on nasturtium between the group of wasps conditioned on nasturtium and the group of wasps conditioned on Brussels sprouts (the reference group). In this way we could control for the effect of plant pair, as each plant pair differed in relative attractiveness of the nasturtium plant and the cabbage plant, and only wasps belonging to the same time point, but not those belonging to different time points, were tested using the same plant pairs. Memory retention data were analyzed using the generalized linear model (GLM) procedure in SAS v. 8.02 (SAS, Inc., Chicago, IL; http://www.sas.com). A Levene test for homogeneity of variance of treatment groups was carried out, followed by comparisons of least square means of treatment groups that were corrected for type I errors with the Tukey-HSD method. To test whether memory was present at different times after conditioning with P. rapae or P. brassicae (i.e. whether the percentage choice for nasturtium of the wasps conditioned on nasturtium was higher than that of the reference group) we used a GLM procedure with binomial distribution of error variance and a logit-link function. For each treatment the number of individuals landing on nasturtium in the wind tunnel was used as the response variable, and the number of responding wasps as the binomial total. Data collected on different experimental days (representing different plant pairs) were considered as replicates, and experimental day was introduced as a block factor into the model. In case of overdispersal, the variance functions of the binomial distribution were allowed to have a multiplicative overdispersion factor by dividing the square root of the deviance of the model by the degrees of freedom [43]. Contrast statements were used to separate treatment groups when main effects or their interactions were significant. The same method was used to compare the percentage choice for nasturtium between treatment groups of the memory inhibition assays and between single- and multiple (spaced) conditioning trials. An α = 0.05 level of significance was used for all the comparisons.
Trichogramma evanescens Experiments
Insects
Trichogramma evanescens Westwood (Hymenoptera: Trichogrammatidae; approximately 0.5 mm long) (iso-female strain GD011) originated from a P. rapae egg collected in 2006 in a cabbage field in Wageningen, The Netherlands [23], [24]. Since then, it was reared in eggs of the moth Ephestia kuehniella under laboratory conditions (25±1°C, 50–70% rh, L16:D8). With approximately 26 laboratory generations per year and 3–4 years between collection and the execution of the experiments, T. evanescens had been in the laboratory for 78–104 generations at the time of the experiments. Only mated, 2-days-old inexperienced female T. evanescens wasps were used in the experiments. Mated female and male butterflies of P. brassicae and P. rapae were taken as copulating pairs the day before a conditioning trial or bioassay. Mated female Pieris butterflies are known to emit species-specific anti-aphrodisiac pheromones that are transferred by males during mating to render females less attractive to conspecific males [44].
Conditioning Procedure
Wasps were given a conditioning trial that mimics a successful ride on a mated female butterfly as described previously [23]. A trial consisted of first mounting a mated female butterfly, remaining on it during a short simulated flight (the butterfly was relocated with a pair of weak forceps), descending it, and finally laying eggs into one <24 h old butterfly egg of either P. rapae (single) or P. brassicae (present in a group of eggs) that was deposited on a Brussels sprouts leaf. Wasps carefully assessed egg (cluster) size by walking over – and drumming with their antennae on – one egg (or more eggs in the case of P. brassicae) before laying their own eggs. The conditioning procedure took place in a plastic container [23], [24]. Conditioned wasps were individually kept in glass vials with a drop of honey until the memory bioassay.
Apart from single-trial conditioning, we also included a spaced conditioning treatment, in which T. evanescens wasps received two conditioning trials spaced by a 1 h interval.
Choice Bioassay
We determined memory retention at 1 h, 4 h and 24 h after conditioning in T. evanescens by measuring the response of wasps to butterfly odors in an olfactory bioassay that we have developed previously [23], [24]. In a two-chamber olfactometer, two adult mated female butterflies were introduced as odor source in one chamber and two male butterflies in the other. The time spent by the wasps in one of the two odor fields was observed for 300 seconds. Because inexperienced T. evanescens do not distinguish between odors of male and mated female butterflies [23], [24], memory was defined as present when the percentage of time spent in the odor field of mated females was significantly higher than 50%, with a larger difference representing a higher memory retention level. We also investigated the possibility that the observed patterns in memory retention are not only related to differences in the reward value, but would also be influenced by differences in the perception of the conditioned stimulus, represented by the species-specific odors of mated female butterflies. We did this by testing the preference of inexperienced wasps, as well as the preference of wasps one hour after they received a single conditioning trial with one of the two butterfly species, in a two-chamber olfactometer with two mated female P. brassicae butterflies in one chamber and two mated female P. rapae butterflies in the other. Each day 10–15 wasps were tested, until a total of 40 wasps per combination was reached. Each wasp was only tested once. The olfactometer was rotated 180° after every third insect, to compensate for any unforeseen asymmetry in the setup. After each 3rd wasp tested, the butterflies were replaced with new ones.
Egg-laying Bioassay
In a no-choice situation, wasps were given the opportunity to parasitize P. rapae or P. brassicae eggs present on a Brussels sprouts leaf. We determined the number of wasp eggs allocated to a butterfly egg by observing a female wasp's abdominal movements after ovipositor insertion until withdrawal under a microscope as described previously [45]–[47].
Data Analysis
To test for main and interaction effects of “host-species used during conditioning” and “time after conditioning” we used a GLM procedure in SAS. The percentage of time that a female wasp spent in the odor field of mated female butterflies was the dependent response variable. Data were arcsine square-root transformed to meet Levene's test for homogeneity of variance of treatment groups. We made Tukey-HSD adjusted pairwise comparisons of least square means of treatment groups. A Wilcoxon's matched-pairs signed-ranks test (WMPSRT) was used to test whether the percentage of time spent in the odor field of mated female butterflies was significantly higher than 50%. To compare the number of eggs allocated to a P. brassicae with those allocated to a P. rapae egg we used a non-parametric Mann-Whitney U test for non-related samples. An α = 0.05 level of significance was used for all the comparisons.
Supporting Information
Oviposition behavior of female Cotesia glomerata wasps into first instar caterpillars of Pieris brassicae . These caterpillars of the large cabbage white butterfly P. brassicae are feeding gregariously on a leaf of Brussels sprouts, and are attacked by two C. glomerata females. The very fast insertion of the ovipositor into the caterpillars is clearly visible. The oviposition experience serves as a reward in associative learning, where plant odors are learned to predict the presence of suitable hosts [9], [15]. The parasitic wasp lays approximately 20 eggs in each caterpillar, after which the caterpillar will continue to develop until the fifth larval instar. When the parasitic wasp larvae are fully-grown they will emerge from the caterpillar, spin a cocoon and develop into adult parasitic wasps. The caterpillar will die before pupation. Movie (41 seconds) courtesy of Hans M. Smid.
(MOV)
Oviposition behavior of a female Trichogramma wasp into a Pieris brassicae egg. Eggs of the large cabbage white butterfly P. brassicae are normally present within a clutch of 20–50 eggs. For a better view the butterfly egg was isolated from the clutch. After the first contact a female wasp walks over - and drums with her antennae on - the butterfly egg to assess its size [32]. She then carefully adjusts the number and sex of the eggs that she oviposits into the butterfly egg accordingly [45]–[48]. After oviposition, the wasp eggs will develop into adult wasps inside the butterfly eggs at the cost of caterpillar development. Movie (41 seconds) courtesy of Nina E. Fatouros, Martinus E. Huigens and Urs Wyss (Institute of Phytopathology, University of Kiel, Germany).
(MOV)
Mounting behavior of female Trichogramma wasp on a mated Pieris brassicae female. Trichogramma wasp mounts a mated female of the large cabbage white butterfly Pieris brassicae to hitch a ride to the butterfly's oviposition sites where they parasitize the freshly laid eggs [22]–[24]. In case wasps try to climb onto the butterfly's leg, they can be kicked off. Therefore wasps mostly climb onto the butterfly's wings after which they tend to move towards the thorax just behind the butterfly's head. Movie (50 seconds) courtesy of Nina E. Fatouros, Martinus E. Huigens and Urs Wyss (Institute of Phytopathology, University of Kiel, Germany).
(MOV)
Acknowledgments
We thank Tibor Bukovinszky for statistical advice; Marian Joëls, Jeff Harvey, Kate Lessells and Koen Verhoeven for commenting on the manuscript; Joop Woelke and Loes Duivenvoorde for experimental assistance; Léon Westerd, Frans van Aggelen and André Gidding for insect rearing.
Funding Statement
This work was supported by The Netherlands Organisation for Scientific Research (NWO/ALW) VENI grants 863.05.020 (to MEH) and 863.09.002 (to NEF), NWO/ALW open competition grants 820.01.012 (to HMS) and 818.01.007 (to LEMV), and the German Research Foundation (DFG) grant FA 824/1-11 (to NEF).The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Cnaani J, Thomson JD, Papaj DR (2006) Flower choice and learning in foraging bumblebees: Effects of variation in nectar volume and concentration. Ethology 112: 278–285. [Google Scholar]
- 2. Wright GA, Choudhary AF, Bentley MA (2009) Reward quality influences the development of learned olfactory biases in honeybees. Proc R Soc Lond, Ser B: Biol Sci 276: 2597–2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wäckers F, Bonifay C, Vet L, Lewis J (2006) Gustatory response and appetitive learning in Microplitis croceipes in relation to sugar type and concentration. Anim Biol 56: 193–203. [Google Scholar]
- 4. Sulikowski D, Burke D (2007) Food-specific spatial memory biases in an omnivorous bird. Biol Lett 3: 245–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Camacho FJ, Portillo W, Quintero-Enriquez O, Paredes RG (2009) Reward value of intromissions and morphine in male rats evaluated by conditioned place preference. Physiol Behav 98: 602–607. [DOI] [PubMed] [Google Scholar]
- 6. Clayton NS, Dickinson A (1998) Episodic-like memory during cache recovery by scrub jays. Nature 395: 272–274. [DOI] [PubMed] [Google Scholar]
- 7. Burke CJ, Waddell S (2011) Remembering Nutrient Quality of Sugar in Drosophila . Curr Biol 21: 746–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mery F, Kawecki TJ (2005) A cost of long-term memory in Drosophila . Science 308: 1148–1148. [DOI] [PubMed] [Google Scholar]
- 9. Smid HM, Wang GH, Bukovinszky T, Steidle JLM, Bleeker MAK, et al. (2007) Species-specific acquisition and consolidation of long-term memory in parasitic wasps. Proc R Soc Lond, Ser B: Biol Sci 274: 1539–1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tully T, Preat T, Boynton SC, Delvecchio M (1994) Genetic dissection of consolidation memory in Drosophila . Cell 79: 35–47. [DOI] [PubMed] [Google Scholar]
- 11. Krashes MJ, Waddell S (2008) Rapid consolidation to a radish and protein synthesis-dependent long-term memory after single-session appetitive olfactory conditioning in Drosophila. . J Neurosci 28: 3103–3113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Isabel G, Pascual A, Preat T (2004) Exclusive consolidated memory phases in Drosophila . Science 304: 1024–1027. [DOI] [PubMed] [Google Scholar]
- 13. Hoedjes KM, Kruidhof HM, Huigens ME, Dicke M, Vet LEM, et al. (2011) Natural variation in learning rate and memory dynamics in parasitoid wasps: opportunities for converging ecology and neuroscience. Proc R Soc Lond, Ser B: Biol Sci 278: 889–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Eisenhardt D (2006) Learning and memory formation in the honeybee (Apis mellifera) and its dependency on the cAMP-protein kinase A pathway. Anim Biol 56: 259–278. [Google Scholar]
- 15. van den Berg M, Duivenvoorde L, Wang G, Tribuhl S, Bukovinszky T, et al. (2011) Natural variation in learning and memory dynamics studied by artificial selection on learning rate in parasitic wasps. Anim Behav 81: 325–333. [Google Scholar]
- 16. Kandel ER (2001) Neuroscience - The molecular biology of memory storage: A dialogue between genes and synapses. Science 294: 1030–1038. [DOI] [PubMed] [Google Scholar]
- 17. Bourtchouladze R, Abel T, Berman N, Gordon R, Lapidus K, et al. (1998) Different training procedures recruit either one or two critical periods for contextual memory consolidation, each of which requires protein synthesis and PKA. Learning & Memory 5: 365–374. [PMC free article] [PubMed] [Google Scholar]
- 18. Erber J (1976) Retrograde-amnesia in honeybees (Apis Mellifera Carnica). J Comp Physiol Phych 90: 41–46. [DOI] [PubMed] [Google Scholar]
- 19. Quinn WG, Dudai Y (1976) Memory phases in Drosophila . Nature 262: 576–577. [DOI] [PubMed] [Google Scholar]
- 20. McGaugh JL (2000) Neuroscience - Memory - a century of consolidation. Science 287: 248–251. [DOI] [PubMed] [Google Scholar]
- 21. Sara SJ, Hars B (2006) In memory of consolidation. Learning & Memory 13: 515–521. [DOI] [PubMed] [Google Scholar]
- 22. Fatouros NE, Huigens ME, van Loon JJA, Dicke M, Hilker M (2005) Chemical communication: butterfly anti-aphrodisiac lures parasitic wasps. Nature 433: 704–704. [DOI] [PubMed] [Google Scholar]
- 23. Huigens ME, Pashalidou FG, Qian MH, Bukovinszky T, Smid HM, et al. (2009) Hitch-hiking parasitic wasp learns to exploit butterfly antiaphrodisiac. Proc Natl Acad Sci USA 106: 820–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Huigens ME, Woelke JB, Pashalidou FG, Bukovinszky T, Smid HM, et al. (2010) Chemical espionage on species-specific butterfly anti-aphrodisiacs by hitchhiking Trichogramma wasps. Behav Ecol 21: 470–478. [Google Scholar]
- 25. Vet LEM, Dicke M (1992) Ecology of infochemical use by natural enemies in a tritrophic context. Annu Rev Entomol 37: 141–172. [Google Scholar]
- 26. Lemasurier AD (1994) Costs and benefits of egg clustering in Pieris brassicae . J Anim Ecol 63: 677–685. [Google Scholar]
- 27. Root RB, Kareiva PM (1984) The search for resources by cabbage butterflies (Pieris rapae) - ecological consequences and adaptive significance of Markovian movements in a patchy environment. Ecology 65: 147–165. [Google Scholar]
- 28. Harvey JA (2000) Dynamic effects of parasitism by an endoparasitoid wasp on the development of two host species: implications for host quality and parasitoid fitness. Ecol Entomol 25: 267–278. [Google Scholar]
- 29. Salt G (1940) Experimental studies in insect parasitism. VII. The effects of different hosts on the parasite Trichogramma evanescens Westw. (Hym. Chalcidoidea). Proc R Entomol Soc Lond A 15: 81–95. [Google Scholar]
- 30. Tempel BL, Bonini N, Dawson DR, Quinn WG (1983) Reward learning in normal and mutant Drosophila . Proc Natl Acad Sci USA 80: 1482–1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hase A (1925) Beiträge zur Lebensgeschichte der Schlupfwespe Trichogramma evanescens Westwood. Arb Biol Reichsan Land-Forstwirt Berl-Dahl 14: 171–224. [Google Scholar]
- 32. Schmidt JM, Smith JJB (1987) Short interval time measurement by a parasitoid wasp. Science 237: 903–905. [DOI] [PubMed] [Google Scholar]
- 33.Feltwell J, editor (1982) Large White Butterfly: The Biology, Biochemistry and Physiology of Pieris brassicae (Linneaeus). The Hague-Boston-London: Dr. W. Junk Publishers.
- 34. Geervliet JBF, Posthumus MA, Vet LEM, Dicke M (1997) Comparative analysis of headspace volatiles from different caterpillar-infested or uninfested food plants of Pieris species. J Chem Ecol 23: 2935–2954. [Google Scholar]
- 35. Geervliet JBF, Vet LEM, Dicke M (1996) Innate responses of the parasitoids Cotesia glomerata and C-rubecula (Hymenoptera: Braconidae) to volatiles from different plant-herbivore complexes. J Insect Behav 9: 525–538. [Google Scholar]
- 36. Geervliet JBF, Vreugdenhil AI, Vet LEM, Dicke M (1998) Learning to discriminate between infochemicals from different plant-host complexes by the parasitoids Cotesia glomerata and C. rubecula (Hymenoptera: Braconidae). Entomol Exp Appl 86: 241–252. [Google Scholar]
- 37. Izquierdo LA, Barros DM, Vianna MRM, Coitinho A, Silva TDE, et al. (2002) Molecular pharmacological dissection of short- and long-term memory. Cell Mol Neurobiol 22: 269–287. [DOI] [PubMed] [Google Scholar]
- 38.Dubnau J (2003) Neurogenetic dissection of conditioned behavior: Evolution by analogy or homology? J Neurogenet 17: : 295–+. [DOI] [PubMed] [Google Scholar]
- 39. Reaume CJ, Sokolowski MB (2011) Conservation of gene function in behaviour. Philos T Roy Soc B 366: 2100–2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Stearns FW (2011) One Hundred Years of Pleiotropy: A Retrospective (vol 186, pg 767, 2010). Genetics 187: 355–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bleeker MAK, Smid HM, Steidle JLM, Kruidhof HM, Van Loon JJA, et al. (2006) Differences in memory dynamics between two closely related parasitoid wasp species. Anim Behav 71: 1343–1350. [Google Scholar]
- 42. Geervliet JBF, Vet LEM, Dicke M (1994) Volatiles from damaged plants as major cues in long-range host-searching by the specialist parasitoid Cotesia rubecula . Entomol Exp Appl 73: 289–297. [Google Scholar]
- 43.McCullagh P, Nelder JA (1989) Generalized linear models. London: Chapman and Hall.
- 44. Andersson J, Borg-Karlson AK, Wiklund C (2003) Antiaphrodisiacs in pierid butterflies: A theme with variation!. J Chem Ecol 29: 1489–1499. [DOI] [PubMed] [Google Scholar]
- 45. Suzuki Y, Tsuji H, Sasakawa M (1984) Sex allocation and effects of superparasitism on secondary sex ratios in the gregarious parasitoid, Trichogramma chilonis (Hymenoptera: Trichogrammatidae). Anim Behav 32: 478–484. [Google Scholar]
- 46. Huigens ME, Luck RF, Klaassen RHG, Maas MFPM, Timmermans MJTN, et al. (2000) Infectious parthenogenesis. Nature 405: 178–179. [DOI] [PubMed] [Google Scholar]
- 47. Huigens ME, de Almeida RP, Boons PAH, Luck RF, Stouthamer R (2004) Natural interspecific and intraspecific horizontal transfer of parthenogenesis-inducing Wolbachia in Trichogramma wasps. Proc R Soc Lond, Ser B: Biol Sci 271: 509–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bai BR, Luck RF, Forster L, Stephens B, Janssen JAM (1992) The effect of host size on quality attributes of the egg parasitoid, Trichogramma pretiosum . Entomol Exp Appl 64: 37–48. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Oviposition behavior of female Cotesia glomerata wasps into first instar caterpillars of Pieris brassicae . These caterpillars of the large cabbage white butterfly P. brassicae are feeding gregariously on a leaf of Brussels sprouts, and are attacked by two C. glomerata females. The very fast insertion of the ovipositor into the caterpillars is clearly visible. The oviposition experience serves as a reward in associative learning, where plant odors are learned to predict the presence of suitable hosts [9], [15]. The parasitic wasp lays approximately 20 eggs in each caterpillar, after which the caterpillar will continue to develop until the fifth larval instar. When the parasitic wasp larvae are fully-grown they will emerge from the caterpillar, spin a cocoon and develop into adult parasitic wasps. The caterpillar will die before pupation. Movie (41 seconds) courtesy of Hans M. Smid.
(MOV)
Oviposition behavior of a female Trichogramma wasp into a Pieris brassicae egg. Eggs of the large cabbage white butterfly P. brassicae are normally present within a clutch of 20–50 eggs. For a better view the butterfly egg was isolated from the clutch. After the first contact a female wasp walks over - and drums with her antennae on - the butterfly egg to assess its size [32]. She then carefully adjusts the number and sex of the eggs that she oviposits into the butterfly egg accordingly [45]–[48]. After oviposition, the wasp eggs will develop into adult wasps inside the butterfly eggs at the cost of caterpillar development. Movie (41 seconds) courtesy of Nina E. Fatouros, Martinus E. Huigens and Urs Wyss (Institute of Phytopathology, University of Kiel, Germany).
(MOV)
Mounting behavior of female Trichogramma wasp on a mated Pieris brassicae female. Trichogramma wasp mounts a mated female of the large cabbage white butterfly Pieris brassicae to hitch a ride to the butterfly's oviposition sites where they parasitize the freshly laid eggs [22]–[24]. In case wasps try to climb onto the butterfly's leg, they can be kicked off. Therefore wasps mostly climb onto the butterfly's wings after which they tend to move towards the thorax just behind the butterfly's head. Movie (50 seconds) courtesy of Nina E. Fatouros, Martinus E. Huigens and Urs Wyss (Institute of Phytopathology, University of Kiel, Germany).
(MOV)