Summary
Chronically stressed rodents who are allowed to eat calorie-dense “comfort” food develop greater mesenteric fat, which in turn dampens hypothalamic-pituitary-adrenocortical (HPA) axis activity. We tested whether similar relations exist in humans, at least cross-sectionally. Fifty-nine healthy premenopausal women were exposed to a standard laboratory stressor to examine HPA response to acute stress and underwent diurnal saliva sampling for basal cortisol and response to dexamethasone administration. Based on perceived stress scores, women were divided into extreme quartiles of low vs. high stress categories. We found as hypothesized that the high stress group had significantly greater BMI and sagittal diameter, and reported greater eating after stressful events. In response to acute lab stressor, the high stress group showed a blunted cortisol response, lower diurnal cortisol levels, and greater suppression in response to dexamethasone. These cross-sectional findings support the animal model, which suggests that long-term adaptation to chronic stress in the face of dense calories result in greater visceral fat accumulation (via ingestion of calorie-dense food), which in turn modulates HPA axis response, resulting in lower cortisol levels.
Keywords: abdominal fat, cortisol, stress, stress eating, hypothalamic-pituitary-adrenal axis
Introduction
Obesity and obesity-related disease states such as metabolic syndrome are highly prevalent (Crawford, et al., 2010). Concurrently, the United States is faced with historically high levels of psychological stress (American Psychological Association, 2009). Both of these trends are taking place within a “toxic” food environment that promotes overeating – particularly overeating of calorie-dense, nutrient-poor foods (Wadden, Brownell, & Foster, 2002). There are robust and complex connections between obesity, psychological stress, and eating behavior (Adam & Epel, 2007; Dallman, 2010; Warne, 2009). The role of stress in promoting eating and obesity has been relatively well characterized. For example, stress has been shown to promote both obesity (Dallman, 2010; McEwen, 2008; Wardle, Chida, Gibson, Whitaker, & Steptoe, 2010) and food intake (Born, et al., 2010; E. Epel, R. Lapidus, et al., 2001; Pecoraro, Reyes, Gomez, Bhargava, & Dallman, 2004; Rutters, Nieuwenhuizen, Lemmens, Born, & Westerterp-Plantenga, 2009). In the former, abdominal obesity is most affected by stress due to the role of prolonged stress-induced glucocorticoid secretion in promoting abdominal fat deposition (Bjorntorp & Rosmond, 2000; Dallman, Pecoraro, & la Fleur, 2005). In the latter, also primarily driven by glucocorticoids, stress-induced eating tends to favor eating of highly palatable, nutrient-dense foods high in sugar and fat (Adam & Epel, 2007; Torres & Nowson, 2007; Warne, 2009). Further, acute and chronic stress can interact to exacerbate stress eating. For example, those who are under chronic stress tend to eat more under acute stress conditions (Gibson, 2006).
In the current study, we focus on the converse – eating and obesity affecting stress responses. Although this converse relationship is undoubtedly equally important, it has to date only been directly studied in non-human animal models (Dallman, 2010; Pecoraro, et al., 2004). In this model, termed the chronic stress response network model, rats exposed to repeated chronic restraint stress that are then given lard or sucrose demonstrate attenuated stress responses compared to those given no food. Specifically, the otherwise expected CRF expression and ACTH secretion in response to stress is reduced (Foster, et al., 2009; la Fleur, Houshyar, Roy, & Dallman, 2005; Pecoraro, et al., 2004). Similarly, rats given sucrose show attenuation of stress-induced activation of the lateral septum (Martin & Timofeeva, 2010). Early life stressors such as maternal separation in rats also appear to activate the chronic stress response network. A palatable cafeteria high-fat diet normalized the effects of prolonged maternal separation in rats, reversing increases in anxiety- and depression-like behaviors, increased cortisosterone, increased hypothalamic CRH, and increased hippocampal glucocorticoid receptor expression (Maniam & Morris, 2010). In other words, it appears that rats are “self-medicating” through the use of food to regulate their stress responses – specifically their hypothalamic-pituitary-adrenocortical (HPA) axis responses.
These rats, over time, develop greater mesenteric fat, and this mesenteric fat has been found over multiple studies to be negatively correlated with CRF mRNA expression in the paraventricular nucleus (Dallman, Akana, et al., 2003; Laugero, Bell, Bhatnagar, Soriano, & Dallman, 2001). This process is one purported mechanism explaining how, over time, chronically stressed humans appear to have hypocortisolism (Fries, Hesse, Hellhammer, & Hellhammer, 2005), but this has not yet been directly tested in humans. One study (Arce, Michopoulos, Shepard, Ha, & Wilson, 2009) found evidence of the chronic stress response network in rhesus monkeys: subordinate females consumed more calories, gained more weight, and subsequently showed lower diurnal cortisol responses and dampened cortisol responses to an acute social separation stressor.
In sum, greater mesenteric fat, likely developed through repeated consumption of palatable foods, dampens the activity of the HPA axis in chronically stressed rodents and appears to be conserved across species to monkeys. The chronic stress response network has to date only been tested in non-human animal species, and thus we test the potential relevance of this model to humans in the current study. Prior studies of eating, obesity, and stress responses have not directly tested for evidence of the chronic stress response network, and instead have focused on a main effects model whereby greater stress and cortisol is associated with greater obesity. Indeed, in non-stressed samples, there may be and have been documented (E. Epel, Battle, Hoffman-Goldberg, Kingston, & Brownell, 2004; Newman, O’Connor D, & Conner, 2006; Tataranni, et al., 1996) positive associations between abdominal fat and cortisol output in response to acute stress. There is reason to believe, however, that in highly stressed humans we might find the opposite relationship due to the chronic stress response network. These individuals likely have coped with high levels of stress by engaging in stress-eating, thereby developing blunted HPA axis responses like the rats given the opportunity to consume comfort food. Here, we isolate a very high stress group and test for evidence supporting the chronic stress response network.
Given that the prior studies show greater intake of comfort food during stress and recovery from stress, greater mesenteric fat pads, and the amount of the pad is directly related to lowered CRF in the brain and lowered HPA axis response to acute stress, we can make several hypotheses about what to expect in humans under stress who have recruited the chronic stress response network. Specifically, if the chronic stress response network is activated in humans, we would expect the following observations, cross-sectionally:
Those with high stress will have greater self-medication with palatable food, and thus will thus report higher scores on self-reported emotional eating.
Those with high stress should have greater abdominal fat distribution, as measured by sagittal diameter and overall adiposity as measured by BMI.
If those with high stress do tend to have greater abdominal fat distribution, they should also show dampened HPA axis activity.
Methods
Sample
Fifty-nine healthy premenopausal women aged 20 – 50 participated in this study. To capture a wide range of chronic psychological stress, this sample contained caregivers of chronically ill children (n = 40) and caregivers of healthy children (n = 19). Exclusion criteria included post-menopausal status, heavy drinking (7+ drinks per week), major depression, and chronic health conditions except controlled hypertension with beta blockers or ACE inhibitors (n = 2) and controlled hypothyroidism with Synthroid supplementation (n = 1). Smokers were included but were asked to refrain from smoking on the day of the lab session.
Procedures
All procedures were fully approved by the University of California, San Francisco Committee on Human Subjects Research. To control for menstrual cycle-related effects on cortisol reactivity, all women were tested within the first seven days of their follicular cycle. To control for diurnal rhythmicity of cortisol, all participants were run at the same time of day in the afternoon. After providing informed consent, participants completed the questionnaires described below.
Participants then underwent the Trier Social Stress Test (TSST; Kirschbaum et al., 1993). This is a standardized laboratory stressor designed to elicit psychological stress and cortisol responses. The TSST was 15 minutes long and consisted of a 5-minute speech preparation period, a 5-minute challenging serial subtraction task, and a videotaped 5-minute public speaking task in front of two evaluative, non-responsive audience members. Salivary cortisol samples were taken at baseline, 30 minutes after stressor onset, and 60 minutes after stressor onset. After the stressor, participants were asked to report on their negative emotions to measure psychological stress (see below).
On three consecutive days following the day of the lab session, participants conducted diurnal saliva sampling to measure cortisol. All three days followed the same sampling protocol: wakeup, wakeup + 30 minutes, and bedtime. At 2200h on the night of Day 1, participants ingested a low dose (0.5 mg) of dexamethasone, a synthetic glucocorticoid, to measure the extent to which participants suppressed endogenous cortisol in response to dexamethasone on Day 2. Day 1 and Day 3, therefore, were our measure of diurnal cortisol output and Day 2 was our measure of response to the dexamethasone suppression test.
Measures
Psychological measures
Perceived chronic psychological stress was measured using the Perceived Stress Scale (PSS; Cohen et al, 1983). This widely-used and extensively validated measure is designed to assess how unpredictable, uncontrollable, and overloaded respondents find their lives. A sample item is: “How often have you felt nervous and stressed?” Respondents are asked to rate how often they experienced stress in the past month on 5-point Likert-type scales from Never = 0 to Very Often = 4, and a total score is calculated such that higher score reflects higher perceived stress. Stress eating was measured using the Dutch Eating Behavior Questionnaire (DEBQ; Van Strien, Frijters et al. 1986) emotional eating subscale. A sample item is: “Do you have a desire to eat when you are irritated?” The DEBQ scales are well-validated and have high validity in terms of food consumption. A total score is calculated such that higher score reflects higher emotional eating. Psychological stress responses to the lab stressor were measured by asking participants to report how “worried,” “anxious,” and “fearful” they felt on a 5-point Likert-type scale from Never = 0 to Very Often = 4 immediately after the stressor. The Cronbach’s alpha for these three items was satisfactory, with α = .77.
Anthropometric measures
Body weight was assessed on a digital scale, with participants wearing light clothing. Body height was measured to the nearest 0.25 inch. Body mass index was calculated as weight (kg) divided by height squared (meters). Sagittal diameter, our measure of abdominal obesity, was measured as the horizontal length from the back to the belly, using an anthropometer measuring stick while the participant was standing.
Cortisol measures
Three indices of cortisol were examined in this study: (1) cortisol output in response to the TSST in the lab session; (2) diurnal cortisol output; and (3) cortisol suppression in response to dexamethasone. Cortisol output in response to the TSST was obtained by calculating the area-under-the-curve (AUC) according to the AUC with respect to ground formula outlined by Pruessner, Kirschbaum, Meinlschmid, and Hellhammer (2003). The same formula was applied to the diurnal cortisol measures using the average of Day 1 and Day 3 cortisol at each respective time point to calculate diurnal cortisol levels, and to the Day 2 cortisol values to calculate suppression in response to dexamethasone administration.
All data were normally distributed according to Q–Q plots with the exception of dexamethasone cortisol response and response to the acute lab stressor, which we natural log-transformed in the analyses.
Summary of Analytic Plan
To test our first hypothesis that the high stress group would report more emotional eating, we first divided women into quartiles of high versus low stress. We then examined whether the women high in chronic stress, when compared to the women low in chronic stress, reported greater emotional eating on the DEBQ using a one-way analysis of variance (ANOVA) controlling for age. To test our second hypothesis – that those with high stress should have higher abdominal fat distribution we – again conducted a one-way ANOVA, this time with sagittal diameter as the dependent variable again controlling for age. We also examined overall adiposity by using BMI as a dependent variable. To test our third hypothesis that those with high stress should show dampened HPA axis activity, we first tested whether the high stress group showed lower cortisol resopnses than the low stress group using one-way ANOVAs, controlling for age. Then, we examined correlations between sagittal diameter and (a) diurnal cortisol and (b) cortisol suppression to dexamethasone administration and (c) response to the lab stressor in the high and low stress groups. Because we had a priori predictions regarding directionality of these relationships, we use one-tailed tests of significance with an alpha level of p = .05.
Results
Descriptive statistics
Participants were on average 39 years old (SD = 6.03), with an average BMI of 25.04 (SD = 3.97) and sagittal diameter of 20.20 inches (SD = 4.92). The mean emotional eating score was 2.65 (SD = 1.05) and the mean perceived stress score was 15.70 (SD = 4.92). The women in the top quartile of perceived stress (n = 17) had an average score of 21.5, which is considered “high stress” according to normed values for adults older than 20 years from a poll of a representative U.S. sample (S. Cohen & Williamson, 1988). The women in the lowest quartile of perceived stress (n = 16) had an average of 10.5, considered “low stress” by the same norms. The high stress group was on average 41.13 years old (SD = 5.61), and the low stress group was on average 38.12 years old (SD = 5.86). The two groups were not statistically significantly different in age (p = .14). As might be expected, 94% of the high stress group were caregivers whereas 43% of the low stress group were caregivers. Caregivers had children who had a chronic condition for an average of 5.9 years (SD = 3.3), and the range was from 1 to 12 years. Controlling for caregiver status or years of caregiving, however, did not change the pattern of any results discussed below.
Main results
As hypothesized (H1), the high stress group reported higher levels of emotional eating versus the low-stress group (3.16 vs. 2.18; p = .05). Further, (H2) the high stress group also had greater sagittal diameter (20.92 vs. 18.24; p = .05) and BMI (25.97 vs. 23.89; p = .04) than the low stress group (see Table 1).
Table 1.
Main outcome measures and tests of differences between top vs bottom stress quartiles
| n | High Stress | Low Stress | p | |
|---|---|---|---|---|
|
|
||||
| Emotional eating (1–5 scale) | 19 | 3.16 (1.39) | 2.18 (0.95) | .05 |
| Saggital diameter (cm) | 31 | 20.92 (5.30) | 18.24 (4.09) | .05 |
| BMI | 32 | 25.97 (4.26) | 23.89 (3.24) | .04 |
| Reactivity to lab stressor | ||||
| Cortisol (mg/dL) | 29 | 51.15 (89.48) | 158.24 (183.13) | .03 |
| Psychological stress (1–4 scale) | 31 | 1.27 (0.46) | 0.61 (0.65) | .05 |
Note: Standard deviations appear in parentheses
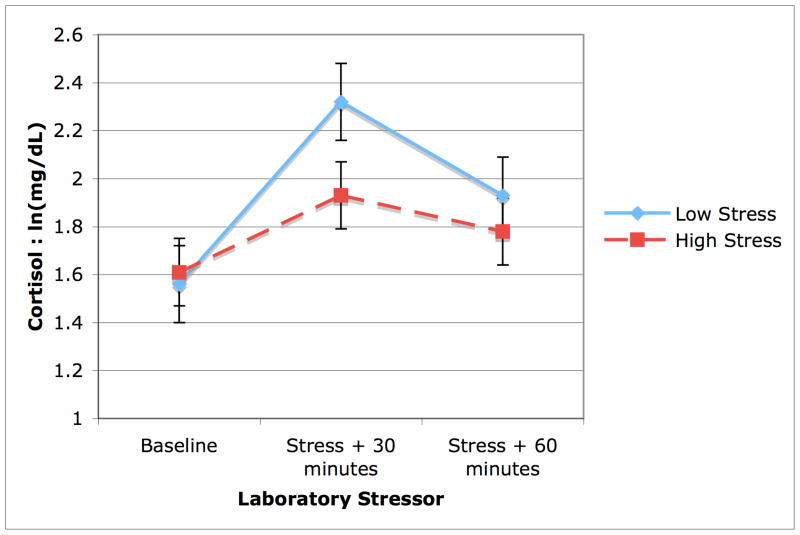
(H3) Compared to the low stress group, the high stress group also showed lower cortisol output in response to the lab stressor (51.13 vs.158.24; p = .03; Figure 1). We further tested using a one-way ANOVA whether the high stress group showed a similar psychological response to the stressor as the low stress group to see if their hypoactivity might be due to lack of psychological stress response or adrenal adaptation. We found that the high stress group in fact showed a greater psychological stress response to the stressor (1.27 vs. 0.61, F(1,30) = 2.87, p = .05), suggesting that they were not emotionally less stressed, but rather showed a comparatively lower HPA axis response to the stressor (see Table 1).
Figure 1.
Cortisol output in response to the laboratory stressor. Women with low perceived stress (solid line) show a characteristic increase and decrease in response to an acute laboratory stressor, whereas highly stressed women (dashed line) show a dampened response.
Although the high stress women had lower levels of both diurnal cortisol (high stress: M = 15.52, SD = 7.75 vs. low stress: M = 20.89, SD = 10.95) and cortisol response to dexamethasone (high stress: M = 1.23; SD = 1.50 vs. low stress: M = 1.48, SD = 1.23), the two groups were only marginally significantly different from one another (diurnal cortisol: F(1,32) = 2.6, p = .06; dexamethasone response: F(1,32) = 1.61, p = .10). However, as hypothesized (H3), in the high stress group, sagittal diameter was negatively correlated with diurnal basal cortisol levels (r = −.44, p = .05) and greater suppression of cortisol in response to the dexamethasone administration (r = −.55; p = .02). Figure 2 represents these correlations. These relationships did not emerge in the low stress group (see Table 2). The correlation between cortisol output in response to the stressor and sagittal diameter in the high stress group was, as hypothesized, negative (r = −.18, p > .05) but was not statistically significant.
Figure 2.
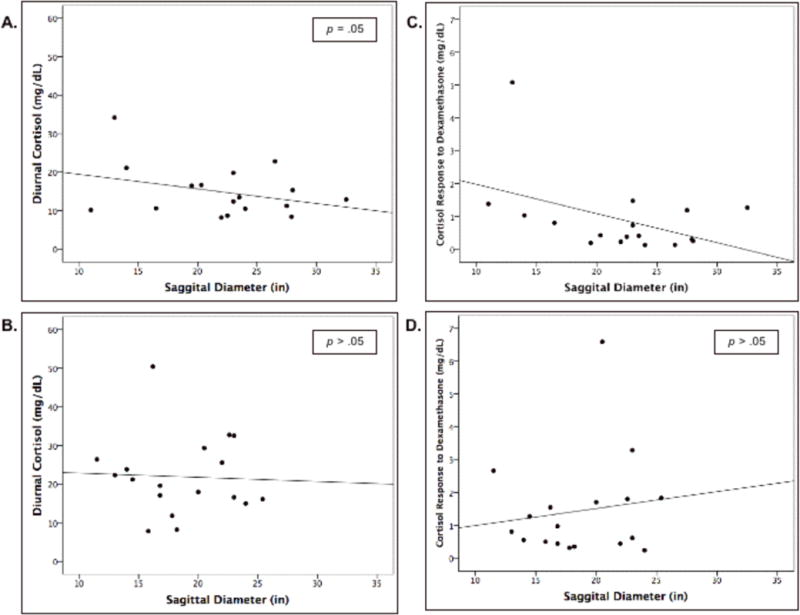
Correlations between sagittal diameter and A. diurnal cortisol in high-stress women; B. diurnal cortisol in low-stress women; C. cortisol response to the dexamethasone suppression test in high-stress women; D. cortisol response to the dexamethasone suppression test in low-stress women. Panels A and C represent statistically significant negative correlations. Note that the values in panels C and D are displayed as raw rather than log-transformed values.
Table 2.
Correlations between saggital diameter and cortisol outcomes
| Diurnal Cortisol | DEX suppression | Lab Stressor Response | ||
|---|---|---|---|---|
|
|
||||
| High stress | −0.44* (n = 15) | −0.55** (n = 15) | −0.18 (n = 13) | |
| Low stress | 0.02 (n = 16) | −0.02 (n = 16) | −0.06 (n = 16) | |
Note: Diurnal cortisol and lab stressor response are calculated as area-under the curve. All units are mg/dL.
p = .05;
p < .05
The chronic stress response network implicates abdominal rather than overall obesity, and thus we examined whether these correlations were unique to sagittal diameter rather than BMI. Sagittal diameter and BMI were correlated, as one might expect, r = .67, p < .001. Sagittal diameter remained correlated with dexamethasone response when partialling for BMI, r = −.23, p = .04. Both cortisol response to the acute stressor and diurnal cortisol remained negatively correlated with sagittal diameter, as expected, but were no longer statistically significant (cortisol response to acute stressor: r = −.11, p = .10; diurnal cortisol: r = −.11, p = .10). Of note, BMI did not statistically significantly correlate with any of the outcomes when controlling for sagittal diameter.
Discussion
Is comfort food truly comforting? Past findings show that in rats, chronic stress induces high cortisol output in response to acute stress, selective intake of “comfort food” (lard and sucrose), and preferential storage of abdominal fat. Consequently, in these rats, the greater the abdominal fat pad, the lower the subsequent HPA axis reactivity to acute stress. This has been labeled the chronic stress response network (Dallman, et al., 2004; Dallman, Pecoraro, et al., 2003; Dallman, et al., 2005). In this study, we tested whether relationships supporting such a network exist in highly stressed women. We found as hypothesized that highly stressed women reported greater stress eating, greater abdominal fat, and showed blunted output in response to acute stress, as well as other signs of a heightened sensitivity to cortisol (lower diurnal cortisol, and an enhanced negative feedback loop as indexed by dexamethasone response). This profile of HPA axis activity has been labeled “relative hypocortisolemia” (Fries, et al., 2005; Heim, Ehlert, & Hellhammer, 2000). Although cross-sectional, this study provides evidence consistent with the argument that, just as in rats, abdominal obesity in stressed humans may serve to attenuate both basal and acute cortisol indices.
Among the high stress women only, the greater the amount of abdominal fat, the lower the cortisol output and other signs of relative hypocortisolemia. Among the low stress women, who have higher cortisol than the highly stressed women, there were no relationships between abdominal fat and HPA axis function. This is the first direct demonstration of the potential existence of the chronic stress response network, as we understand it in rats, in humans.
This profile, while consistent across several indices of HPA activity, provides just a hint that the network exists. These relationships are cross sectional, and were found in a small sample. Further, the relation between abdominal fat and one of our cortisol outcomes (output in response to the lab stressor) did not reach statistical significance (although it was in the predicted direction). Did the stress and stress eating precede the changes in HPA axis function, as in rats? Or might the hypocortisolemia profile precede the eating behavior? These relationships clearly need to be tested experimentally, as much as possible, as well as longitudinally, in humans.
The pattern of results is at first glance at odds with some prior literature indicating higher cortisol levels in those who report more stress eating. For example, Epel and colleagues (2004) found that self-reported stress eaters had higher nocturnal urinary cortisol during exam periods. Newman, O’Connor, and Conner (2006) found that those who experienced more daily hassles ate a greater number of snacks but reacted to a laboratory stressor with more cortisol. The divergent findings are likely due to the intensity and chronicity of the stress experienced by the participants in this study compared to the students or general community members, respectively, in the prior studies. In this study, we purposely recruited a sample that contained very highly stressed participants (caregivers of chronically ill children), where we would expect a chronic stress response network to be most activated and observable.
Our characterization of the high-stress women’s response to the acute lab stressor as “blunted” implies that it is the high rather than low stress group that is deviant. A review of ten years of research with the Trier Social Stress test finds that 70–80% of subjects show increases in cortisol, similar to the pattern we observed in the low stress participants (Kudielka, Hellhammer, & Kirschbaum, 2007), but our data cannot conclude definitively one way or the other.
A putative mechanism for the accumulated abdominal obesity and activation of the chronic stress response network, according to the rat model, is eating in response to stress. In this study, however, eating in response to stress was not directly observed and we relied on a self-report measure of emotional eating. Future work should measure food consumption after acute stress and examine this in relation to cortisol outcomes.
Past studies have observed inconsistencies in the direction of the effect of stress on HPA responses, with some finding higher cortisol responses and others finding lower. The existence of a high stress-relative hypocortisolism is not a well-identified syndrome, and may have multiple etiologies. For example, this profile has been related to stress sensitivity, history of trauma, and chronic pain (Fries, et al., 2005; Heim, et al., 2000). It may be that an independent pathway to this profile is stress eating and abdominal fat deposition, Alternatively, it may be that the stress eating is part of cause of the stress syndrome seen in clinical states, at least in people who have developed excessive adiposity, but is not causally driving the hypocortisolemia. Regardless, the knowledge from rat studies and the current data suggest it is vitally important to consider the role of comfort food and abdominal fat when trying to understand HPA axis profiles in states of stress. Examining the role of stress eating may help untangle the observed inconsistencies among highly stressed populations and their responses to stress.
Acknowledgments
We gratefully acknowledge Drs. Eli Puterman and Aoife O’Donovan for their comments on the manuscript. We also acknowledge Drs. Melvin Heyman, Bryna Siegel, and Paul Harmatz for help recruiting clinic participants, and the busy mothers for donating their time.
Role of Funding Source: This research was supported by the following: Robert Wood Johnson Foundation (RWJF) Health and Society Scholars Program, National Institute of Mental Health Award K08 MH64110–01A1; RWJF and NIMH had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Footnotes
Contributors: A. J. Tomiyama managed the literature searches, conducted statistical analyses, and wrote the first draft of the manuscript. E. S. Epel and M. F. Dallman contributed to subsequent drafts. E. S. Epel designed the study and wrote the protocol. M. F. Dallman contributed to the literature search. All authors contributed to and have approved the final manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adam TC, Epel ES. Stress, eating and the reward system. Physiol Behav. 2007;91(4):449–458. doi: 10.1016/j.physbeh.2007.04.011. [DOI] [PubMed] [Google Scholar]
- American Psychological Association. Stress in America. Washington, DC: 2009. [Google Scholar]
- Arce M, Michopoulos V, Shepard KN, Ha QC, Wilson ME. Diet choice, cortisol reactivity, and emotional feeding in socially housed rhesus monkeys. Physiol Behav. 2009;101(4):446–455. doi: 10.1016/j.physbeh.2010.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorntorp P, Rosmond R. Neuroendocrine abnormalities in visceral obesity. Int J Obes Relat Metab Disord. 2000;24(Suppl 2):S80–85. doi: 10.1038/sj.ijo.0801285. [DOI] [PubMed] [Google Scholar]
- Born JM, Lemmens SG, Rutters F, Nieuwenhuizen AG, Formisano E, Goebel R, et al. Acute stress and food-related reward activation in the brain during food choice during eating in the absence of hunger. Int J Obes (Lond) 2010;34(1):172–181. doi: 10.1038/ijo.2009.221. [DOI] [PubMed] [Google Scholar]
- Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. Journal of Health & Social Behavior. 1983;24(4):385–396. [PubMed] [Google Scholar]
- Cohen S, Williamson G. Perceived stress in a probability sample of the United States. In: Oskamp SSS, editor. The social psychology of health: Claremont Symposium on applied social psychology. Newbury Park, CA: Sage; 1988. [Google Scholar]
- Crawford AG, Cote C, Couto J, Daskiran M, Gunnarsson C, Haas K, et al. Prevalence of obesity, type II diabetes mellitus, hyperlipidemia, and hypertension in the United States: findings from the GE centricity electronic medical record database. Popul Health Manag. 2010;13(3):151–161. doi: 10.1089/pop.2009.0039. [DOI] [PubMed] [Google Scholar]
- Dallman MF. Stress-induced obesity and the emotional nervous system. Trends Endocrinol Metab. 2010;21(3):159–165. doi: 10.1016/j.tem.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallman MF, Akana SF, Laugero KD, Gomez F, Manalo S, Bell ME, et al. A spoonful of sugar: feedback signals of energy stores and corticosterone regulate responses to chronic stress. Physiol Behav. 2003;79(1):3–12. doi: 10.1016/s0031-9384(03)00100-8. [DOI] [PubMed] [Google Scholar]
- Dallman MF, Akana SF, Strack AM, Scribner KS, Pecoraro N, La Fleur SE, et al. Chronic stress-induced effects of corticosterone on brain: direct and indirect. Ann N Y Acad Sci. 2004;1018:141–150. doi: 10.1196/annals.1296.017. [DOI] [PubMed] [Google Scholar]
- Dallman MF, Pecoraro N, Akana SF, La Fleur SE, Gomez F, Houshyar H, et al. Chronic stress and obesity: a new view of “comfort food”. Proc Natl Acad Sci U S A. 2003;100(20):11696–11701. doi: 10.1073/pnas.1934666100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallman MF, Pecoraro NC, la Fleur SE. Chronic stress and comfort foods: self-medication and abdominal obesity. Brain Behav Immun. 2005;19(4):275–280. doi: 10.1016/j.bbi.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Epel E, Battle K, Hoffman-Goldberg J, Kingston S, Brownell K. Stress-induced cortisol and eating in healthy young women. 2004. manuscript in progress. [Google Scholar]
- Epel E, Jimenez S, Brownell K, Stroud L, Stoney C, Niaura R. Are stress eaters at risk for the metabolic syndrome? Ann N Y Acad Sci. 2004;1032:208–210. doi: 10.1196/annals.1314.022. [DOI] [PubMed] [Google Scholar]
- Epel E, Lapidus R, et al. Stress may add bite to appetite in women: A laboratory study of stress-induced cortisol and eating behavior. Psychoneuroendocrinology. 2001;26:37–49. doi: 10.1016/s0306-4530(00)00035-4. [DOI] [PubMed] [Google Scholar]
- Foster MT, Warne JP, Ginsberg AB, Horneman HF, Pecoraro NC, Akana SF, et al. Palatable foods, stress, and energy stores sculpt corticotropin-releasing factor, adrenocorticotropin, and corticosterone concentrations after restraint. Endocrinology. 2009;150(5):2325–2333. doi: 10.1210/en.2008-1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries E, Hesse J, Hellhammer J, Hellhammer DH. A new view on hypocortisolism. Psychoneuroendocrinology. 2005;30(10):1010–1016. doi: 10.1016/j.psyneuen.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Gibson LE. Emotional influences on food choice: Sensory, physiological and psychological pathways. Physiol Behav. 2006 doi: 10.1016/j.physbeh.2006.01.024. [DOI] [PubMed] [Google Scholar]
- Heim C, Ehlert U, Hellhammer D. The potential role of hypocortisolism in the pathophysiology of stress-related bodily disorders. Psychoneuroendocrinology. 2000;25:1–35. doi: 10.1016/s0306-4530(99)00035-9. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Pirke K, Hellhammer D. The “Trier Social Stress Test”--A tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 1993;28:76–81. doi: 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- Kudielka BM, Hellhammer DH, Kirschbaum C. Ten years of research with the Trier Social Stress Test (TSST) - revisited. In: H-JE, WP, editors. Social neuroscience: Integerating biological and psychological explanations of social behavior. New York: The Guilford Press; 2007. pp. 56–83. [Google Scholar]
- la Fleur SE, Houshyar H, Roy M, Dallman MF. Choice of lard, but not total lard calories, damps adrenocorticotropin responses to restraint. Endocrinology. 2005;146(5):2193–2199. doi: 10.1210/en.2004-1603. [DOI] [PubMed] [Google Scholar]
- Laugero KD, Bell ME, Bhatnagar S, Soriano L, Dallman MF. Sucrose ingestion normalizes central expression of corticotropin-releasing-factor messenger ribonucleic acid and energy balance in adrenalectomized rats: a glucocorticoid-metabolic-brain axis? Endocrinology. 2001;142(7):2796–2804. doi: 10.1210/endo.142.7.8250. [DOI] [PubMed] [Google Scholar]
- Maniam J, Morris MJ. Palatable cafeteria diet ameliorates anxiety and depression-like symptoms following an adverse early environment. Psychoneuroendocrinology. 2010;35(5):717–728. doi: 10.1016/j.psyneuen.2009.10.013. [DOI] [PubMed] [Google Scholar]
- Martin J, Timofeeva E. Intermittent access to sucrose increases sucrose-licking activity and attenuates restraint stress-induced activation of the lateral septum. Am J Physiol Regul Integr Comp Physiol. 2010 doi: 10.1152/ajpregu.00371.2009. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Central effects of stress hormones in health and disease: Understanding the protective and damaging effects of stress and stress mediators. Eur J Pharmacol. 2008;583(2–3):174–185. doi: 10.1016/j.ejphar.2007.11.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman E, O’Connor DB, Conner M. Daily hassles and eating behaviour: The role of cortisol reactivity status. Psychoneuroendocrinology. 2006 doi: 10.1016/j.psyneuen.2006.11.006. [DOI] [PubMed] [Google Scholar]
- Pecoraro N, Reyes F, Gomez F, Bhargava A, Dallman MF. Chronic stress promotes palatable feeding, which reduces signs of stress: Feedforward and feedback effects of chronic stress. Endocrinology. 2004 doi: 10.1210/en.2004-0305. [DOI] [PubMed] [Google Scholar]
- Pruessner J, Kirschbaum C, Meinlschmidt G, Hellhammer DH. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology. 2003;28:916–931. doi: 10.1016/s0306-4530(02)00108-7. [DOI] [PubMed] [Google Scholar]
- Rutters F, Nieuwenhuizen AG, Lemmens SG, Born JM, Westerterp-Plantenga MS. Acute stress-related changes in eating in the absence of hunger. Obesity (Silver Spring) 2009;17(1):72–77. doi: 10.1038/oby.2008.493. [DOI] [PubMed] [Google Scholar]
- Tataranni PA, Larson D, Snitker S, Young J, Flatt J, Ravussin E. Effects of glucocorticoid on energy metabolism and food intake in humans. American Journal of Physiology. 1996;271:E317–E325. doi: 10.1152/ajpendo.1996.271.2.E317. [DOI] [PubMed] [Google Scholar]
- Torres SJ, Nowson CA. Relationship between stress, eating behavior, and obesity. Nutrition. 2007;23(11–12):887–894. doi: 10.1016/j.nut.2007.08.008. [DOI] [PubMed] [Google Scholar]
- Van Strien T, Frijters JGR, Bergers GPA, Defares PB. The Dutch Eating Behavior Questionnaire (DEBQ) for assessment of restrained, emotional and external eating behavior. Int J Eat Disord. 1986;5:747–755. [Google Scholar]
- Wadden TA, Brownell KD, Foster GD. Obesity: responding to the global epidemic. J Consult Clin Psychol. 2002;70(3):510–525. doi: 10.1037//0022-006x.70.3.510. [DOI] [PubMed] [Google Scholar]
- Wardle J, Chida Y, Gibson EL, Whitaker KL, Steptoe A. Stress and Adiposity: A Meta-Analysis of Longitudinal Studies. Obesity (Silver Spring) 2010 doi: 10.1038/oby.2010.241. [DOI] [PubMed] [Google Scholar]
- Warne JP. Shaping the stress response: interplay of palatable food choices, glucocorticoids, insulin and abdominal obesity. Mol Cell Endocrinol. 2009;300(1–2):137–146. doi: 10.1016/j.mce.2008.09.036. [DOI] [PubMed] [Google Scholar]