Abstract
Radiolabeled ZM 241385 (4-(2-[7-amino-2-{furyl){1,2,4)triazolo{2,3-a}{1,3,5}triazin-5-ylaminoethyl)phenol), has previously been used as a high affinity radioligand for the labeling of A2A adenosine receptors in cell membranes. Anoiher subtype, the A2B receptor, is the least well-defined subtype of adenosinc receptors and lacks selective pharmacological probes. In the present study, we have used [3H]ZM 241385 as a radioligand to label recombinant human A2B adenosine receptors in HEK-293 cell membranes, that do not express A2A adenosine receptors, and found that the phannacological profile is consislent with the SAR of A2B receplors. Saturable, specific binding (Kd 33.6 nM, Bmax 4.48 pmol/mg protein) that was best described by a one-site model was found, and specific binding was approximately 75% of total binding. [3H]ZM 241385 binding was displaceable by a large number of compounds known to interact with A2B receptors; thus, this method has promise as a tool in the search for agonists and antagonists selective for this subtype. Xanthine analogs, which are antagonists, proved to be the most potent displacers. The Ki of XAC, xanthine amine congener, was 12.3 nM, while CPX (8-cyclopcmyl-1,3-dipropylxanthine) was less potent. The non-selective triazoloquinazoline antagonist CGS 15943 (Ki 16.4 nM), which is similar in structure to ZM 241385, was slightly less potent than XAC, The non-xanthine A2B-antagonist alloxazine displaced [3H]ZM 241385-binding with a Ki of 462 nM, similar to its affinity in funct ional assays. Adenosine derivatives known to activate this receptor subtype, such as NECA (5′-N-ethylcarboxamidoadcnosine) and R-PIA (N6-phenylisopropyladenosine), were considerably less potent than the 8-substituted xanthines examined.
Keywords: G protein-coupled receptors, radioligand binding, purines, xanthines, adenosine analogues
INTRODUCTION
Four extracellular G protein-coupled receptors for adenosine have been identified: A1, A2A, A2B, and A3 receptors [1], Like A2A receptors, the A2B receptors are coupled to stimulation of adenylylcyclase [2,3] and furthermore are associated with a rise in intracellular calcium [4]. The adenosine A2B receptor is the poorest characterized subtype of adenosine receptors, and is thought to be involved in the control of vascular tone, cell growth and gene expression, mast cell degranulation, and intestinal secretion [5]. Whereas for the other subtypes selective agonists, antagonists, and rad iolabeled ligands are available [1], no such compounds are known for the A2B rcceptor. For pharmacological studies of the role of A2B receptors, both selective agonists and antagonisls are needed. Selective antagonists of the A2B receptor are potentially useful in the treatment of asthma [5,6] and intestinal disorders [7]. Although an adenosine A2B receptor-mediated stimulation of adenylyl cyclase in the brain has been known for nearly two decades [2], the role of this subtype in the brain is largely unknown.
The potencies of various adenosine agonists and antagonisls have been measured at A2B receptors in functional assays [8-13] but nOt generally in binding assays [14, 15]. For example, agonist-induced cyclic AMP production by over 100 adenosine derivatives was measured in Chinese hamster ovary (CHO) cells stably transfected with human A2B rcceptor cDNA [11]. The order of potency at the A2B receptor is NECA (5′-N-ethylcarboxamidoadenosine) > N6-benzyl adenosine analogues > N6-cycloalkyJ adenosine analogues, and no agonists more potent than NECA were found. Antagonists have been studied in VA13 fibroblasts and other cell lines of human and mouse origin [8,12]. In the original study in which the rat A2B receptor was cloned [16]. [3H]NECA was used as a radioligand; however, this binding assay appears to be unacceptable for widespread screening due to low affinity. [3H]1,3-Diethyl-8-phenylxanthine ([3H]DPX) [6] and 125I-ABOPX (125I-3-(4-amino-3-iodobenzyl)-8-(phenyl-4-oxyacetate)-1-propylxanthine) [14] have also been used as radioligands of moderate affinity at recombinant A2B receptors in membranes. [3H]CPX has also been used in binding assays in whole cells [15], but the marginally high affinity of this xanthine does not permit binding to the human A2B receptor in cell membranes, and thus a radioligand of higher affinity is still sought.
The SAR at the human A2B receptor of 5-acylated derivatives of the non-selective adenosine amagonist 9-chloro-2-(2-furanyl)[1,2,4]triazolo[1,5-c]quinazolin-5-amine (CGS 15943) has been Studied [13]. A series of straight chain N5-aminoalkylacyl derivatives demonstrated that for A2B receptors the optional chain length occurs with three methylene groups, i.e. the N5-γ-aminobutyryl derivative. MRS 1224, which had a pA2 value of 8.0 but was not selective for A2B receptors. A molecular model of the triazoloq uinazoline/A2B receptor complex, based on the structure of rhodopsin utilizing a “cross-docking” procedure was developed in order to visualize the environment of the ligand binding site [13]. IJzerman and colleagues reported the high affinity of the related heterocyclic derivative 2M 241385 [15], a triazolotriazine derivative, at human A2B receptors. The Ki value for displacement or binding of [3H]CPX by ZM 241385 in CHO cells expressing recombinant A2B receptors was 21 nM. This compound was previously introduced as an antagonist with high affinity and selectivity at A2A receptors [17]. Radiolabeled forms of ZM 241385 [18,24] have previously been used as a radioligand ror the labeling of A2A adenosine receptors in brain membranes, thus, for the binding assay at A2B receptors il is critical to use cell membranes in which the A2A receptor is not expressed.
RESULTS
The non-xanthine antagonist radioligand [3H]ZM 241385 was found to bind specifically to the human A2B receptor expressed in HEK-293 cells, using CPX 10 defi ne non-specific binding. The percent of specific binding using 30 nM of the radioligand was initially in the range of 50–70%. In order to optimize the experimental conditions, the amount of protein required in each assay tube (Figure 2) and the association kinetics of binding (Figure 3) were studied. The specific binding reached a maximum of 75% of total binding with 30 μg protein and increased no further (Figure 2). Thus, it was necessary for the amount of protein present in each assay tube to be at least 25 μg. In the association experiment (Figure 3), a plateau existed between 30 and 90 min of incubation at 25 °C; therefore, 1 hour was selected for as the time of incubation for subsequent experiments.
FIGURE 2.
Dependence of the percent specific binding of [3H]ZM 241385 to the human A2B receptor on the amount of protein present in each assay tube. [3H]ZM 241385 (30 nM) was incubated with membranes for 60 min at 25 °C. Non-specific binding was delcnnined with 20 μM CPX.
FIGURE 3.
Time plot of association of [3H]ZM 241385 to HEK-293 cell membranes expressing the human A2B receptor. Total binding (○). non-specific binding (▲), and specific binding (●) are shown. [3H]ZM 241385 (30 nM) was incubated wilh 18 μg of membranes at 25 °C. Non-specific binding was determined with 20 μM CPX.
The effects of cations, chelators and temperature changes on specific [3H]ZM 241385 binding to human A2B receptors expressed in HEK-293 cell membranes were studied. The presence of the monovalent cation Na+ (at a concentration of 10 or 100 mM) or the divalent cations Ca2+ or Mg2+ (lor 10 mM), caused a 20–30% reduction in the amount of specific binding of 30 nM [3H]ZM 241385. Addition of the chelating agents in excess of the concentration of Ca2+ (EGTA or EDTA) or Mg2+ (EDTA) restored the full degree of binding (data not shown). Curiously, the presence of the divalent cations Zn2+ or Mn2+ (1 mM) caused a ~5% increase in the amount of specific binding of 30 nM [3H]ZM 241385. Nevertheless. neither Zn2+ nor Mn2+ was added during standard saturation or competition experiments.
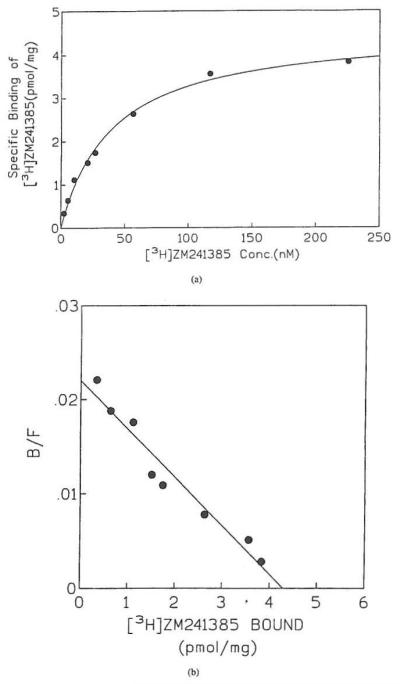
Under optimized conditions (60 min incubation at 25 °C with 30 μg protein/tube, in Ca2+-free medium), binding of [3H]ZM 241385 to membranes of HEK-293 cells expressing the human A2B receptor was salurable and was best described by a one-site model (as computed with the Prism program). The fraction of specific binding was approximately 75% of total binding. A Kd value of 33.6 ± 2.8 nM and a Bmax of 4.48 ± 0.15 pmol/mg were detennined in 4 independent experiments. A representative saturation isotherm and a Scatchard transfonnation of the same data are shown in Figures 4A and 4B.
FIGURE 4.
(a) Saturation isotherm of specific [3H]ZM 241385 binding 10 human A2B receptors expressed in HEK-293 cell membranes and (b) Scatchard analysis of the same data. [3H]ZM 241385 (2–225 nM) was incubated with 30 μg of membranes for 60 min. at 25 °C. Non-specific binding was determined with 20 μM CPX. Four experiments were carried out; data from one representative experiment (each data point measured in duplicate) are shown.
The binding of [3H]ZM 241385 was inhibited by a number of compounds, both an tagonists and agonists, known to interact with A2B receptOrs (Table 1). A typical set of displacement curves is shown in Figure 5. The most potent displacers of [3H]ZM 241385 binding were the positively-charged xanthine derivative, XAC, and the triazoloquinazoline, cas 15943, with Ki values of 12.3 and 16.4 nM, respectively. ZM 241385, itself, was fairly potent (28.3 nM), while CPX. which has been used as a tritiated radioligand at this subtype [15,25]. had a slightly lower affinity (50.9 nM). A negatively-charged xanthine derivative, XCC [10], and its ethyl ester, XCC-OEt [10], had Ki values of 31.8 and 18,7 nM, respectively. Consistent with its previously reported affinity at A2B receptors [6]. the xanthine enprofyUine (3-propylxanthine) was 2.5-fold more potent than theophylline in displacing binding of [3H]ZM 241385. Alloxazine, which has been reported to be moderately selective (10-fold) for A2B vs, Al and A2A receptors [12], had a Ki value of 462 nM, The Hill coefficients (nH) in the competition experiments were generally in the range of 0,7 – 1.0 for antagonists and agonists. Competition by NECA, resulted in an nH value of 0.67, The A3 receptor-selective antagonists [26] MRS 1191 (3-ethyl 5-benzyl 2-methyl-6-phenyl-4-phenylethynyl-1,4-(±)-dihydropyridine-3,5-dicarboxylate) and MRS 1523 (2,3-ethyl-4,5-dipropyl-6-phenylpyridine-3-thiocarboxylate-5-carboxylate) did not appreciably displace specific binding of [3H]ZM 241385 at human A2B receptors, and thus have very low affinity at this subtype.
TABLE I.
Ki values for displacement of [3H]ZM 241385 binding to human A2B receptors expressed in HEK-293 cell membranes. Specific binding was approximately 75% of total binding. Values are means (±S.E.M.) of 3-5 separate experiments.
| Compound1 | Ki ± S.E.M. (nM) |
|---|---|
| Antagonists | |
| XAC | 12.3 ± 4.6 |
| CGS 15943 | 16.4 ± 3.6 |
| XCC-OEt | 18.7 ± 0.5 |
| ZM 241385 | 28.3 ± 3.3 |
| XCC | 31.8 ± 3.8 |
| CPX | 50.9±6.1 |
| alloxazine | 462 ± 78 |
| enprofylline | 6160 ± 450 |
| theophylline | 15.200 ± 8800 |
| MRS 1523 | 30% displacement at 25 μM |
| MRS 1191 | 0% displacement at 30 μM |
| Agonists | |
| NECA | 361 ± 123 |
| R-PIA | 3830 ± 1700 |
| SPA | 13, 800 ± 5500 |
| CPA | 21, 100±4300 |
| CGS 21680 | <10% displacement at 100 μM |
FIGURE 5.
Effects of the antagonists XAC (●), CPX (○), and enprofylline (▲) and the agonist R-PIA (solid ▲) on radioligand binding to human A2B receptors expressed in HEK-293 cell membranes. Membranes (30 μg) were incubated for 60 min at 25 °C with 30 nM [3H]ZM 241385. Non-specific binding was determined with 20 μM CPX, Foor experiments were carried out; data from one representative experiment (each data point measured in duplicate) are shown.
The non-selective agonist NECA, which has also been reponed as a tritiated radioligand at this subtype [16], was a considerably less potent competitor of binding (Ki value 398 nM) than the antagonists ZM 241385 and CPX. Among other agonists, i.e. adenosine derivatives, the affinities tended to be weak in comparison to the xanthine antagonists, consistent with functional assays [11,12]. Also as in functional assays (Figure 1), CPA is relatively weak among N6-substituted analogues. Finally, the A2A-selective agonist CGS 21680 (2-[4-[(2-carboxyethyl)phenylJethylaminol-5′-N-clhylcarbamoyladenosine) did not significantly displace [3H]ZM 241385 binding, even at a concentration of 100 μM, which is consistent with functional studies showing this agonist to be inactive at A2B-receptors and selective for the A2A-receptor SUbtype [3].
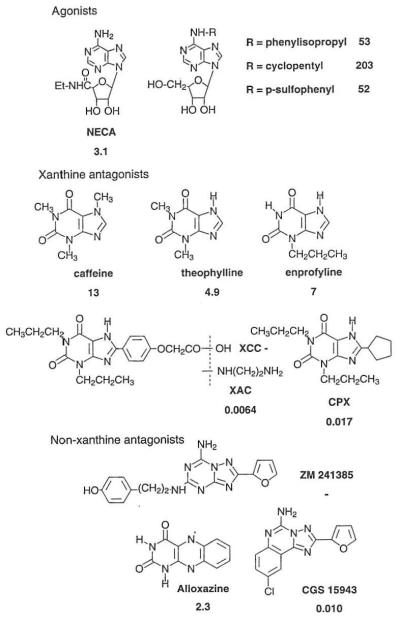
FIGURE 1.
Structures and potencies at A2B adenosine receptors of agonists and antagonists. Agonist EC50 and antagonist KB values, when given are expressed in μM and are from functional assays at A2B receptors in fibroblasts (either stimulation of adenylyl cyclase or antagonism of agonist-stimulated adenyly1 cyclase) [8,9,12] or in mammalian tells expressing the recombinant human A2B adenosine receptor [5,12,13].
DISCUSSION
[3H]ZM 241385 binds with high affinity to a single class of binding sites in HEK-293 cell membranes expressing the human A2B receptor. The pharmacological characteristics of this binding site resemble functional studies of A2B receptors carried out so far [10-12,14,15,20]. For use in membrane systems, [3H]ZM 241385 is preferable to [3H]CPX as a radioligand, which is only satisfactory with whole cells [15]. Both of these antagonists are preferable to [3H]NECA. Another antagonist used as an iodinated radioligand for recombinant A2B receptors, 125I-IABOPX [14], was not compared in this study. [3H]ZM 241385 is not selective for the A2B receptor, due to the demonstrated high affinity of this compound at A2A receptors from a variety of species [17,18]. In the transfected HEK-293 cell membranes used in this study, A2A receptors are not detectable. Curiously, Palmer et al. [18] found that 125I-ZM 241385 did not bind delectably to rat A2B receptors. Thus, the affinity of such triazolotriazines at A2B receptors may be highly dependent on species and/or subtle ligand structural differences.
The xanthine enprofylline [6], which is efficacious as an anti-asthmatic agent, was earlier thought to act through a non-adenosine receptor-mediated mechanism. However, the discovery that enprofylline has greater than anticipated affinity and partial selectivity at the A2B subtype [6], supports the hypothesis that A2B receptor antagonism may contribute to anti-asthmatic activity of xanthines [5]. Thus, the search for more potent and/or selective A2B receptor antagonists may provide new therapeutic agents. In the present study we have confirmed that several 8-phenyl-substituted xanthine derivatives (XAC, XCC, and XCC-OEt) were highly potent at human A2B receptors, as originally indicated in a functional assay (cyclic AMP accumulation) in ral brain slices [10]. Thus, this high affinity was independent of the presence or absence of a charged group on the 8-phenyl-linked chain.
In conclusion, [3H]ZM 241385 binding to the recombinant human A2B receptor in membranes is a practical method for characterization of these receptors and their ligands in systems where the A2A receptor is not co-expressed [19,20], The development of binding assays for this subtype of adenosine receptors that are useful with cell membranes will aid in the elucidation of the SAR of A2B receptor agonists and antagonists, which are under development [11,13,23].
MATERIALS AND METHODS
Binding of [3H]-4-(2-[7-amino-2-{furyl}{1,2,4)triazolo{2,3-a}{1,3,5}triazin-5-ylaminoethyl)phenol ([3H]ZM 241385, 17 Ci/mmol, Tocris Cookson, Bristol, UK) to membranes prepared from HEK-293 cells stably expressing the human A2B receptor (Batch 1365, Receptor Biology, Inc., Beltsville MD) was studied. The assay medium consisted of a Mg2+-free buffer containing 50 mM Tris, and 1 mM EDTA, at pH 6.5. The glass incubation tubes contained a total volume of 100 μL, consisting of 25 μL of the membrane suspension containing 30 μg protein (stored at −80°C), [3H]ZM 241385 (final concentration 30 nM), and a solution of the proposed displacer, where applicable. Nonspecific binding was detennined in the presence of 20 μM CPX. All non-radioactive compounds were initially dissolved in DMSO, and diluted with buffer to the final concentration, where the amount of DMSO never exceeded 1 %.
Incubations of 1hr aI room temperature were terminated by rapid filtration over Whatman GF/B filters, which had been presoaked in 0.5% polyethyleneimine, using a Brandell cell harvester (Brandell, Gaithersburg, MO). The tubes were rinsed three times with 2 mL ice-cold TRIS buffer (pH 6.5). To insure that the wash buffer remained ice-cold, the reservoir was immersed in an ice bath.
For saturation studies, the concentration of [3H]ZM 24 1385 ranged from 2 to 280 nM (10 points in curve). For competition experiments, at least five different concentrations of competitor, spanning 3 orders of magnitude adjusted appropriately for the IC50 of each compound, were used. IC50 values, calculated with the nonlinear regression method implemented in the Prism program (GraphPAO, San Diego, CA), were converted to apparent Ki values using the Cheng-Prusoff equation [21] and a Kd value of 33.6 nM.
Acknowledgmellts
We thank Dr. Joel Linden (University of Virginia) and Dr. A. IJzerman (LACDR, Leiden, The Netherlands) for helpful discussions.
ABBREVIATIONS
- ABOPX
3-(4-amino-3-iodobenzyl)-8-oxyacetate-1-propyl-xanthine
- COS 15943
9-chloro-2-(2-furanyl)[1,2,4]triazoloL I,5-c]quinazolin-5-amine
- CGS
21680 2-[4-[(2-carboxyethyl)phenyl]ethylamino]-5′-N-ethylcarbamoyladenosine
- CHO cells
Chinese hamster ovary cells
- CPA
N6-cyclopentyladenosine
- CPX
8-cyclopentyl-1,3-dipropylxanthine
- DMSO
dimethylsulfoxide
- EGTA
ethylene glycol-bis-(β-aminoethylether)-N,N,N′,N′-tetraacetic acid
- EDTA
ethylenediaminetetraacetic acid
- HEK cells
human embryonic kidney cells
- Ki
equilibrium inhibition constant
- MRS 1224
5-(4-aminobutyryl)amino-9-chloro-2-(2-furanyl)[1,2,4)triazolo[1,5-c)quinazoline
- MRS 1191
3-ethyl 5-benzyl 2-methyl-6-phenyl-4-phenylethynyl-1,4-(±)-dihydropyridine-3,5-dicarboxylate
- MRS 1523
2,3-ethyl-4,5-dipropy 1-6-phenylpyridine-3-thiocarboxylate-5-carboxylate
- NECA
5′-N-ethylcarboxamidoadenosine
- R-PIA
R-N6-phenylisopropyladenosine
- SAR
structure-activity relationship
- SPA
N6-p-sulfophenyladenosine
- Tris
tris(hydroxymethyl)aminomethane
- XAC
8-[4-[[[[(2-aminoethy I)amino]carbonyl]methyl]oxy]phenyl]-1,3-dipropylxanthine
- XCC
8-[4-[[carboxymethyl]oxy]phenyl]-1,3-dipropylxanthine
- ZM 241385
4-(2-[7-amino-2-{furyl}{1,2,4}triazolo{2,3-a}{1,3,5}triazin-5-ylaminoethyl}phenol
References
- [1].Linden J, Jacobson KA. Molecular biology of recombinant adenosine receptors. In: Burnstock G, Dobson JG, Liang BT, Linden J, editors. Cardiovascular Bioloy of Purines. Kluwer; 1998. pp. 1–20. [Google Scholar]
- [2].Daly JW, Buus-Lamb P, Padgett W. Subclasses of adenosine receptors in the central nervous system: interaction with caffeine and related methylxanthines. cell. Mol. Neurobiol. 1983;41:69–80. doi: 10.1007/BF00734999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Hide I, Padgeu WL, Jacobson KA, Daly IW. A2A-Adenosine reccptors from rat striatum and rat pheochromocytoma PC12 cells: Characterization with radioligand binding and by activation of adenylate cyclase. Mol. Pharmacol. 1992;41:352–359. [PMC free article] [PubMed] [Google Scholar]
- [4].Feoktistov I, Murray JJ, Biaggioni I. Positive modulation of intracellular Ca2+ by adenosine A2b, prostacyclin and prostaglandin E2 reeeptors via a cholera toxin-sensitive mechanism in human erythroleukemia cells. Mol. Pharmalcol. 1994;45:1160–1167. [PubMed] [Google Scholar]
- [5].Feoktistov I, Ind Bilggioni I. Adenosine A2B ReceptOrs. Pharmacol Rev. 1997;49:381–402. [PubMed] [Google Scholar]
- [6].Robeva AS, Woodard R, Jin X, Gao Z, Bhattacharya S, Taylor HE, Rosin DL, Linden J. Molecular characterization of recombinant human adenosine receptors. Drug Dev Res. 1996;39:243–252. [Google Scholar]
- [7].Strohmeier GR, Reppert SM, Lencer WI, Madara JL. The A2b adenosine receptor mediates cAMP responses to adenosine receptor agonists in human intestinal epithelia. J. Bioi. Chem. 1995;270:2387–2394. doi: 10.1074/jbc.270.5.2387. [DOI] [PubMed] [Google Scholar]
- [8].Bruns RF. Adenosine antagonism by purines. pteridines and benzopteridines in human fibroblasts. Biochem. Pharmacol. 1981;30:325–333. doi: 10.1016/0006-2952(81)90062-9. [DOI] [PubMed] [Google Scholar]
- [9].Bruns RF. Adenosine receptor activation in human fibroblasts: nucleoside agonists and antagonists. Can. J. Physiol. Pharmacol. 1980;58:673–691. doi: 10.1139/y80-110. [DOI] [PubMed] [Google Scholar]
- [10].Jacobson KA, Kirk KL, Padgett WL, Daly JW. Functionalized congeners of 1,3-dialkylxamhines: preparation of analogues with high affinity for adenosine receplors. J. Med. Chem. 1985;28:1334–1340. doi: 10.1021/jm00147a038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].de Zwart M, Link R, von Frijtag Drabbe Künzel JK, Cristali G, Jacobson KA, Townsend-Nicholson A, JJzerman AP. A screening of adenosine analogues on the human adenosine A2B receptor as part of a search for potent and selective agonists. Nucleos. Nucleotid. 1998;17:969–986. doi: 10.1080/07328319808004215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Brackett LE, Daly JW. Functional characterization of the A2B adenosine receptor in NIH 3T3 fibroblasts. Biochem. Pharmacol. 1994;47:801–814. doi: 10.1016/0006-2952(94)90480-4. [DOI] [PubMed] [Google Scholar]
- [13].Kim Y-C, de Zwart M, Chang L, Moro S, von Frijtag Drabbe Künzel JK, Melman N, IJzerman AP, Jacobson KA. Derivatives of the triazoloquinazoline adenosine antagonist (CGS 15943) having high potency at the human A2B and A3 receptor subtypes. J. Med. Chem. 1998;41:2835–2841. doi: 10.1021/jm980094b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Linden J. Molecular characterization of A2A and A2B receptors. Drug Devel. Rea. 1998;43:2. [Google Scholar]
- [15].de Zwart M, Vollinga RC, von Frijtag Drabbe Künzel JK, Beukers M, Slecgers D, IJzerman AP. Potent antagonists for the A2B receptor. II. Triazolotiazines with high affinity. Drug Devel. Res. 1998;43:28. [Google Scholar]
- [16].Stehle JH, Rivkees SA, Lee JJ, Weaver DR, Deeds JD, Reppert SM. Molecular Cloning and Expression of the cDNA for a Novel A2-Adenosine Receptor Subtype. Mol Emdocrinol. 1992;6:384–393. doi: 10.1210/mend.6.3.1584214. [DOI] [PubMed] [Google Scholar]
- [17].Poucher SM, Keddie JR, Singh P, Stoggall SM, Caulkett PWR, Jones G, Collis MG. The in-vitro pharmacology of ZM241385, a potent nonxanthine, A2a selective adeoosine receptor antagonist. Br. J. Pharmacol. 1995;115:1096–1102. doi: 10.1111/j.1476-5381.1995.tb15923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Palmer TM, Poucher SM, Jacobson KA, Stiles GL. 125I-4-(2-(7-Amino-2-{furyl}{l,2,4}triazolo{2, 3-a}{1,3,5 }triazin-5-ylaminoethyl)phenol (125I-ZM241385). a high affinity antagonist radioligand Selective for the A2a adenosine receptor. Mol. Pharmacol. 1996;48:970–974. [PMC free article] [PubMed] [Google Scholar]
- [19].Cooper J, Hill SJ, Alexander SP. An endogenous A2B adenosine receplor coupled to cyclic AMP generation in human embryonic kidney (HEK 293) cells. Br. J. Pharmacol. 1997;122:546–550. doi: 10.1038/sj.bjp.0701401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Alexander SPH, Cooper J, Shine J, Hill SJ. Characterization of the human brain putative A2B adenosine receptor expressed in Chinese hamster ovary (CHO.A2B.4) cells. Br. J. Pharmacology. 1996;119:1286–1290. doi: 10.1111/j.1476-5381.1996.tb16035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Cheng YC, Prusoff WH. Relationship between the inhibition constant (Ki) and the concentration of inhibition which causes 50 percent inhibition (IC50) of an enzyme reaction. Biochemical Pharmacology. 1973;22:3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- [22].Auchampach JA, Jin J, Wan TC, Caughey GH, Linden J. Canine mast cell adenosine receptors: cloning and expression of the A3 receptors and evidence that degranulation is mediated by the A2B receptor. Mol Pharmacol. 1998;52:846–860. doi: 10.1124/mol.52.5.846. [DOI] [PubMed] [Google Scholar]
- [23].Cristalli G. Synthesis and charaterization of potent ligands at human recombinant adenosine receptors. Drug Divel. Res. 1998;43:23. [Google Scholar]
- [24].Alexander SPH. Binding of the antagonist radioligand [3H]-ZM241385 to A2A adenosine rceeptors in rat brain homogenates. Br. J. Pharmacol. 1998;125:27P. [Google Scholar]
- [25].Hook J, Max SI, Farr MF, Baumgold J. Cloning and phannacological characterization of a human A2B adenosine receptor expressed in HEK293 cells. Soc. for Neurosci. 1998;24:2056. [Google Scholar]
- [26].Li A-H, Moro S, Melman N, Ji X.-d., Jacobson KA. Structure activity relationships and molecular modeling of 3,5-diacyl-2,4-dialkylpyridine derivatives as selective A3 adenosine receptor antagonists. J. Med. Chem. 1998;41:3186–3201. doi: 10.1021/jm980093j. [DOI] [PMC free article] [PubMed] [Google Scholar]