Abstract
The locomotor effects in mice of selective A1 and A2 adenosine agonists, antagonists and combinations of agonists were investigated using a computerized activity monitor. The A2-selective agonist 2-[(2-aminoethylamino)carbonylethylphenylethylamino]-5'-N-ethylcarboxamidoadenosine (APEC), an amine derivative of 2-(carboxyethylphenylethylamino)adenosine-5'-carboxamide, was a more potent locomotor depressant than its amide conjugates. The rank order of potency after i.p. injection for adenosine agonists was 5'-N-ethylcarboxamidoadenosine (NECA) (ED50, 5.8 nmol/kg) > APEC (ED50, 25 nmol/kg) > N6-cyclohexyladenosine (CHA) (ED50, 270 nmol/kg). An A1-selective, centrally acting, adenosine antagonist, 8-cyclopentyltheophylline (10 mg/kg), completely reversed the locomotor depressant effects of CHA (A1-selective) and NECA (nonselective) at doses of agonists as high as twice the ED50, and shifted the dose-response curves to the right, suggesting a primary involvement of A1 receptors. 8-cyclopentyltheophylline did not affect the depressant effects of APEC at the ED50, consistent with the A2-selectivity of APEC. The locomotor effects of APEC and CHA were completely reversed by theophylline, but not by the peripherally active 8-p-sulfophenyltheophylline, indicating central action of the adenosine agonists. The depressant effects of APEC, but not of NECA or CHA, were reversed significantly by an A2-selective adenosine receptor antagonist, 4-amino-8-chloro-1-phenyl-[1,2,4]triazol[4,3-a]quinoxaline. Low or subthreshold doses of CHA potentiated the depressant effects of APEC. A subthreshold dose of CHA did not alter the depressant effect of NECA, whereas a subthreshold dose of APEC increased the depressant effects of low doses of NECA. Thus, it appears that A1- and A2-selective adenosine agonists have separate central depressant effects, which can be potentiative. The relatively high potency of NECA in vivo could be due to a synergism between central A1 and A2receptor activation by this nonselective agonist.
Adenosine is a modulator of many physiological functions. In the CNS adenosine depresses neuronal activity and causes behavioral depression (Snyder, 1985; Dunwiddie, 1985; Dunwiddie et al., 1986; Phillis et al., 1986; Fredholm and Dunwiddie, 1988; Durcan and Morgan, 1989a). At least two classes of adenosine receptors have been defined: A1-adenosine receptors inhibit, whereas A2-adenosine receptors stimulate adenylate cyclase (Van Calker et al., 1979; Hamprecht and Van Calker, 1985). A1 receptors also can inhibit calcium fluxes (Cerbai et al., 1988) and stimulate potassium fluxes (Belardinelli and Isenberg, 1983). Effects of A1 receptors on phosphoinositide breakdown also have been reported (Linden and Delahunty, 1989). The relevance of A1 and A2 receptors to CNS function is under active investigation.
A1-selective agonists such as CHA and R-PIA, and the non-selective agonist NECA, are potent locomotor depressants in rodents (Snyder et al., 1981; Seale et al., 1988; Bruns et al., 1988; Heffner et al., 1989). Alkylxanthines, such as theophylline and caffeine, which act as CNS stimulants, are adenosine antagonists and reverse the behavioral depression elicited by adenosine analogs (Snyder et al., 1981; Barraco et al., 1983, 1984; Katims et al., 1983; Glowa et al., 1985). The locomotor depressant actions of adenosine agonists appear to be centrally mediated, because they are reversed by theophylline, but not by xanthines such as 8-PST that poorly penetrate the blood-brain barrier (Katims et al., 1983; Seale et al., 1988; Nikodijevic et al., 1990; Durcan and Morgan, 1989b). The depressive effects of N6-cyclopentyladenosine, a close analog of CHA, are reversed by highly A1-selective antagonists such as CPT, indicating that A1 receptors activated by N6-cycloalkyladenosines subserve behavioral depression (Bruns et al., 1988). However, the potencies of adenosine agonists in locomotor depression were recently found to correlate to the potencies of the analogs at A2 adenosine receptors and not to potencies at A1 adenosine receptors (Durcan and Morgan, 1989a), leading to the proposal that primarily A2 receptors are involved in these effects. Spealman and Coffin (1986) also concluded that A2 receptors were involved in disrupting schedule-controlled behavior in monkeys. However, in similar studies in rats, the 100- to 300-fold greater potency of R-PIA relative to S-PIA is more consonant with the involvement of A1 receptors (Goldberg et al., 1985).
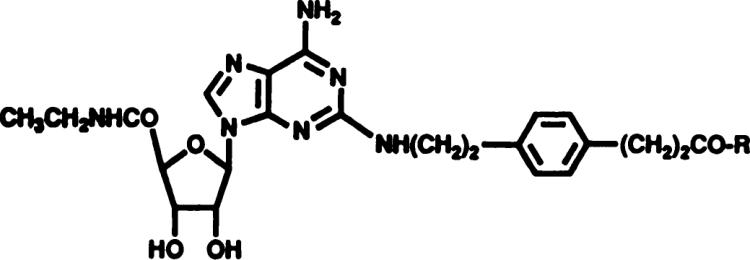
Although A1-selective agonists have been developed, adenosine agonists or antagonists truly selective for A2 adenosine receptors for use as physiological probes have been difficult to identify. CGS21680 is A2-selective in competitive binding experiments at central A1 (measured in cortex) and A2 (measured in striatum) adenosine receptors by a factor of 140, and was shown to be A2-selective in the cardiovascular system (Hutchison et al., 1989; Jarvis et al., 1989). CGS21680 contains a carboxylic acid functionality, which is expected to limit its passage across the blood-brain barrier. Using a functionalized congener approach, a series of long chain derivatives of CGS21680 that retain A2 potency and selectivity and do not contain the carboxylic functionality was synthesized (Jacobson et al., 1989). An amine derivative, APEC (table 1, compound 1) had a Ki value of 6 nM vs. [3H]NECA at central A2 receptors compared to 235 ± 54 nM vs. [3H]R-PIA at central A1 adenosine receptors in rat brain (unpublished data). APEC served as a synthetic intermediate for molecular probes for A2 adenosine receptors (Jacobson et al., 1989; unpublished data). Recently, we reported that APEC is a potent locomotor depressant in mice and that the pharmacological profile of this in vivo action suggests activation of A2 adenosine receptors (Nikodijevic et al., 1990).
TABLE 1.
Summary of locomotor depression in mice elicited by various 2-substitiited-5′-carboxamidoadenosine analogsa
| ||||
|---|---|---|---|---|
| Compound R | A2 selectivityb | Dose | Horizontl Activity | Total Distance Traveled |
| nmol/kg | ||||
| 1. −NH(CH2)2NH2 (APEC) | 20 | 30 | 2420 ± 121 (↓62%) | 1600 ± 96 (↓52%) |
| 300 | 320 ± 22 (↓95%) | 170 ± 11 (↓95%) | ||
| 2. −NH(CH2)2NHCO(CHL2)2-ϕ-OH | 73 | 23 | 5220 ± 605 (↓18%) | 2346 ± 431 (↓30%) |
| 230 | 3568 ± 572 (↓44%) | 1985 ± 338 (↓40%) | ||
| 3. NH(CH2)2NHCOCH2-2-thiophene | 93 | 24 | 6756 ± 565 (↓6%) | 3351 ± 249 (↑1%) |
| 240 | 3248 ± 620 (↓49%) | 1686 ± 298 (↓91%) | ||
| 2400 | 569 (↓91%) | 283 (↓91%) | ||
| 4.c NH(CH2)2NHCSNH-ϕ-SO3Na | 145 | 21 | 4764 ± 528 (↓25%) | 2466 ± 204 (↓38%) |
| 210 | 3756 ± 460 (↓41%) | 2068 ± 233 (↓38%) | ||
| 5. −NH(CH2)4NHCO-ϕ-CH2F | 77 | 23 | 3786 ± 395 (↓40%) | 2140 ± 255 (↓36%) |
| 230 | 3170 ± 281 (↓50%) | 1436 ± 126 (↓57%) | ||
Locomotor activity was indicated by counts, expressed as the mean ± S.E.M. for horizontal activity and total distance in a Digiscan activity monitor (control values, n = 35, were 6370 ± 478 and 3340 ± 218, respectively). Percent depression relative to vehicle control is given in parentheses. Adenosine derivatives were injected i.p. at the dose indicated in mg/kg b.wt., in a vehicle consisting of 20:80 v/v mixture of Emulphor EL-620 and phosphate-buffered saline and administered i.p. in a volume of 5 ml/kg b.wt. Monitoring was initiated 10 min after injection, and carried out for 30 min (n = 6–7, except where noted).
A2 selectivity ratios are equal to the ratio of K1 values at A1/A2 receptors from binding studies using [3H]N6-phenyliSopropyladenosine and [3H]CGS21680 (Jarvis et al., 1989) in the rat brain (values from Jacobson et al., 1990). The K1 values at A2 receptors range from 12 (APEC) to 26 nM (compound 4).
Combined with a 10 mg/kg dose of the peripheral antagonist 8-PST, the locomotor activity folowing a dose (21 nmol/kg) of compound 4 was 4733 ± 560 counts (↓25%)for horizontal activity and 2339 ± 220 counts (↓30%) for total distance traveled (n = 4).
Few antagonists with even modest selectivity for A2 receptors have been developed. However, from a series of triazoloquinoxalines, CP-66,713 proved to be aselective A2 antagonist (Sarges et al., 1990). CP-66,713 has IC50 values of 21 nM vs. [3H]NECA and 270 nM vs. [3H]R-PIA at rat brain A2 and A1 adenosine receptors, respectively. The purpose of the present study is to characterize the A1 and A2 receptor-related components of locomotor depression induced by adenosine analogs, using CHA as an A1-selective agonist, CPT as an A1-selective antagonist, NECA as a nonselective agonist, APEC as an A2-selective agonist and CP-66,713 as an A2-selective antagonist.
Materials and Methods
Chemicals
NECA, CHA, 8-PST, CPT, and hydroxypropyl-γ-cyclodextrin were obtained from Research Biochemicals, Inc. (Natick, MA). CGS 21680C (Na salt) was the generous gift of Dr. A. Hutchison of CIBA-Geigy Corp. (Summit, NJ). APEC and derivatives 2 through 5 (table 1) were synthesized as described (Jacobson et al., 1989). CP-66,713 was prepared by Pfizer Central Research (Groton, CT) according to the procedure of Sarges et al. (1990).
Animal Studies
Subjects
Adult male mice of the NIH (Swiss) strain weighing 25 to 30 g were housed in groups of 12 animals per cage. In the animal room and in the experimental room, mice were kept on a 12/12 h dim lighting/dark cycle. The animals were given free access to standard pellet food and water and were habituated for 24 h in laboratory conditions before testing. Each animal was used only once in the activity monitor.
Locomotor activity
The behavioral effects in mice elicited by selective A1 and A2 adenosine agonists and antagonists administered peripherally were investigated. Individual animals were studied in a Digiscan activity monitor (Omnitech Electronics Inc., Columbus, OH) equipped with an IBM-compatible computer. The monitor included multiple activity monitor cages (40 × 40 × 30.5 cm), each of which is surrounded by horizontal and vertical sensors, which were not detectable by the rodent. The cages were connected to a Digiscan Analyzer and printer, which collects, sorts and prints out the locomotor activity information collected over a specified time period. The data were automatically written to disk at the end of the period, and group data were tabulated. The measurements made by the monitor represent multivariate locomotor analysis with specific measures, such as simultaneous measurements of ambulatory, rearing, stereotypical and rotational behaviors. Two nonequivalent parameters (Sanberg et al., 1985) were analyzed: 1) horizontal activity, which represents the total number of beam interruptions in the horizontal direction and 2) total distance traveled, which indicated the distance in cm traveled by the animal. The latter is dependent on the path taken. For most purposes in this study, only total distance traveled is presented, because changes in horizontal activity were highly correlated with total distance traveled. Mice were tested individually, in a sound- (white noise) and light- (low level fluorescent light from above) controlled area. Data were collected between 9 A.M. and 1 P.M. for three consecutive intervals of 10 min each and analyzed as a group for a 30-min sampling period, except for figure 1. Statistical analysis was performed using the Student t test. The results are reported as mean ∓ S.E. for each point. The number of mice in each experimental group was n = 6 to 33, except for the control group, where n = 35.
Fig. 1.
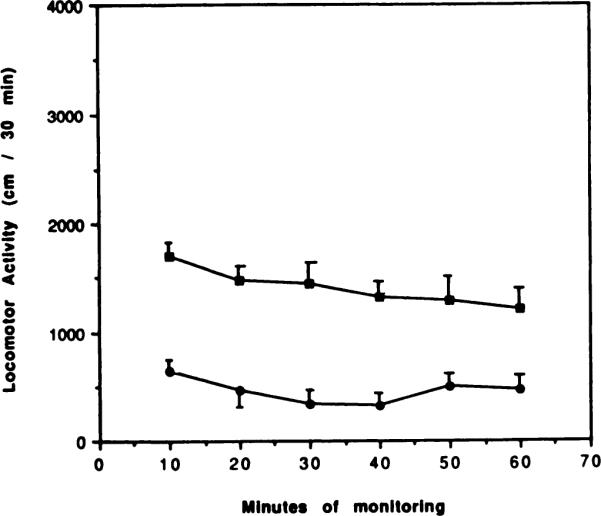
Locomotor activity over 60 min in control animals (∎) and for animals injected i.p. with APEC (30 nmol/kg), an A2selective adenosine agonist (●). Activity counts for total distance traveled are shown in relation to time elapsed since the beginning of monitoring in a Digiscan activity monitor, initiated 10 min after injection i.p. (n = 6). This dose of APEC in other experiments (see fig. 7) corresponded to the ED50 dose.
ED50 values (figs. 3–7) were determined by fitting the data with nonlinear regression to sigmoidal curves using the computer program GraphPad (ISI) and represent the dose which produced half the maximal effect observed (100% value for all curves corresponds to the control in the absence of drug and 0% is the maximum response elicited by agonist). Due to a limited number of points on the curves for a combination of theophylline and agonist (figs. 3 and 4), it was not possible to calculate a precise ED50. ED50 values varied somewhat between experimental sets. Control values were determined separately for each experiment.
Fig. 3.
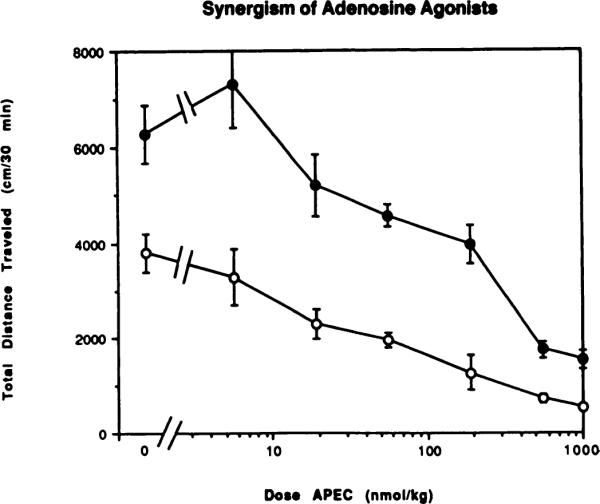
Reversal by theophylline (10 mg/kg) of locomotor depression elicited by the A2-selective adenosine agonist APEC. The antagonist or vehicle was injected 10 min before the agonist. The activity reflects the total distance traveled (mean ± S.E.) over a 30-min period, begun 10 min after the agonist injection in the absence (◯) or presence (●) of theophylline (n = 6–12).
Fig. 7.
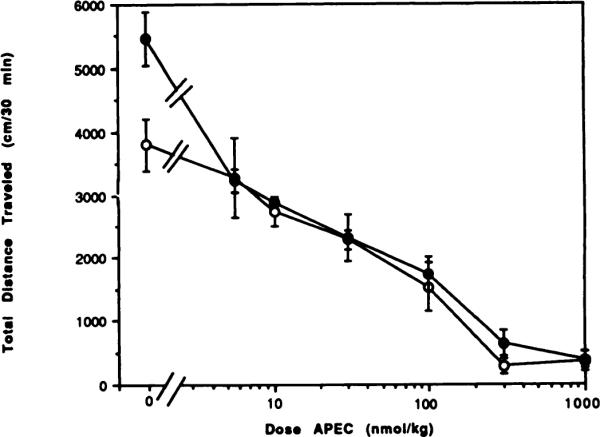
Dose-response curves for locomotor depression in mice by the adenosine agonist APEC in the absence (◯) or presence (●) of the A1-selective antagonist CPT (10 mg/kg). The antagonist or vehicle was injected 10 min before the agonist. The activity reflects the total distance traveled (mean ± S.E.) over a 30-min period (n = 6–12).
Fig. 4.
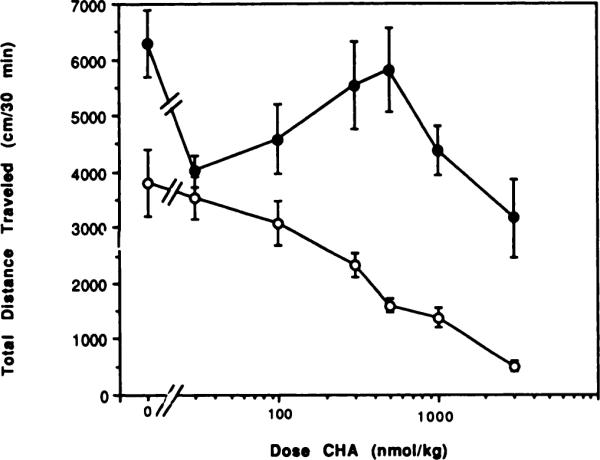
Reversal by theophylline (10 mg/kg) of locomotor depression elicited by the A1-selective adenosine agonist CHA. The antagonist or vehicle was injected 10 min before the agonist. The activity reflects the total distance traveled (mean ± S.E.) over a 30-min period, begun 10 min after the agonist injection in the absence (◯) or presence (●) of theophylline (n = 6–12).
Drug administration
All drugs were dissolved in a 20:80 v/v mixture of Emulphor EL-620 (GAF Chemicals Corp., Wayne, NJ) and phosphate-buffered saline and administered i.p. in a volume of 5 ml/kg body weight. Warming and sonication aided in dissolving the drugs. CP-66,713 was of very low aqueous solubility, and this necessitated increasing the volume injected to 10 ml/kg. Where applicable, the antagonist was injected 10 min before the agonist. When a combination of agonists was administered, there was a 5-min delay between injections. Immediately after the final injection, the mouse was placed in the activity monitor cage, and data collection was begun after a delay of 10 min.
Isobolographic Analysis
To determine whether the locomotor depressant effects of CHA and APEC are additive or greater than additive (synergistic), three subseries of experimental groups were used. First, three dose-effect curves were determined: two with APEC or CHA given alone, and a third with a combination of approximately equipotent doses of CHA and APEC. Five points (n = 6–12 for each) were used to determine the dose-response curve in each subseries of experiments. In the subseries for the combination, the ratio of doses of APEC and CHA was between 1:4 and 1:6, because in preliminary experiments it was found that APEC was approximately 4 to 6 times as potent as CHA in locomotor depression. The construction of the dose-response curves and the determination of ED50 values were based on the Probit procedure (Finney, 1971; Kissin et aL., 1990). The resulting ED50 values were then plotted in the form of an isobologram (fig. 9). The ED50 value for each drug alone was plotted on the x and y axes, and the combined ED50 value was placed in the dose field.
Fig. 9.
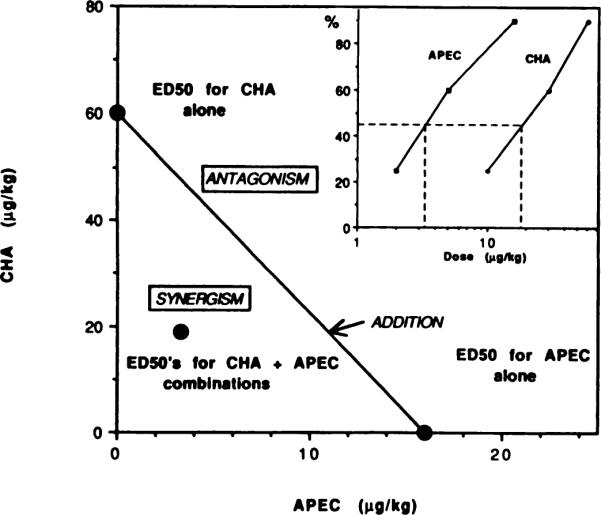
An isobotogram depicting the effects of combinations of adenosine agonists at various doses, which indicates that the interaction of A1 and A2 agonists is synergistic. The construction of the dose-effect curves and determination of ED50 values was based on the probit procedure (Kissin et al., 1990; Finney, 1971). A CHA-APEC subseries, in which the ratio of doses was between 4:1 and 6:1 (combinations of approximately equieffective doses), was administered. The ED50 values for the combination were 6.1 nmol/kg for APEC and 54 nmol/kg for CHA. The inset shows the data from which the ED50 values were determined. Percent locomotor depression is plotted as a function of the dose of each agonist in the combination subseries.
Algebraic (fractional) analysis (Kissin et al., 1990) was based on the relationship of ED50 doses for APEC and CHA in combination (Ac and Cc, respectively) to the doses of these drugs that produce the same effect when given separately (As and Cs, respectively). The value of the sum of fractional doses (Ac/As + Cc/Cs) was interpreted to indicate synergism (<1.0), antagonism (>1.0) or additivity (=1.0), based on a previous application of this method (Berenbaum, 1977).
Results
Locomotor depression elicited by novel adenosine analogs
APEC and other 2-substituted-amino-adenosine-5′-carboxamide derivatives, including conjugates of APEC, were examined for locomotor effects in varying doses. Table 1 shows the results for APEC, compound 1, and conjugates of APEC (Jacobson et al., 1989), in which the amino group has been condensed with the following: p-hydroxyphenylpropionic acid (compound 2) or 2-thienylacetic acid (compound 3), both of which are iodinatable for potential radiolabeling, and p-sulfophenylisothiocyanate (compound 4), which is charged for potentially restricting activity to the periphery. Another derivative, consisting of a higher homolog of APEC coupled to the p-fluoromethylbenzoyl group, a prosthetic group for radiofluorination (compound 5), is included. All of these conjugates, which were reported to be A2-selective in binding assays in the range of 50- to 350-fold (Jacobson et al., 1989), were found to depress locomotor activity at low doses indicating in vivo potency. We did not determine ED50 values for each compound, except for APEC (compound 1). Nevertheless, for each compound there was a definite trend of the dose dependence of the locomotor depression. At a dose of 30 nmol/kg, APEC was the most potent, followed in order of potency by compounds 5 > 4 > 2 > 3. At a 10-fold higher dose, the conjugates were nearly equipotent, yet still less potent than APEC. The locomotor depression induced by derivative 4 was not reversible by a high dose of the nonselective peripherally acting antagonist, 8-p-sulfophenyltheophylline, suggesting that the action of the agonist was not limited to the periphery in spite of the presence of the charged sulfonate group. This might be a result of in vivo hydrolysis of derivative 4. We previously reported that the precursor of APEC, CGS21680, was only very weakly active as a locomotor depressant when administered peripherally (Nikodijevic et al., 1990).
Locomotor depression elicited by an A2-selective adenosine agonist, APEC
Mice were injected i.p. with 30 nmol/kg (found previously to be the ED50) of APEC, or with vehicle, and locomotor activity was measured for six successive periods of 10 min beginning 10 min after the administration of the drug or a vehicle. Figure 1 indicates that the depressant effects of APEC on the total distance traveled were intense and remained constant during the 1-h monitoring period. Depression elicited by APEC, in both parameters, significantly differed from vehicle controls at all time points (P < .001). In vehicle-treated mice, locomotor activity decreased gradually by approximately 20% between 10 and 60 min after the beginning of monitoring.
Effects of theophylline on locomotor activity in mice
Initially, the nonselective centrally active adenosine antagonist, theophylline, was used to demonstrate that APEC was acting in vivo at adenosine receptors. It was first necessary to determine the effect of theophylline alone, injected i.p. in mice, as a behavioral stimulant. Figure 2 shows a dose-dependent stimulation of total distance traveled at doses ranging from 1 to 30 mg/kg (P < .005). However, at the highest dose tested (100 mg/kg), theophylline produced a significant depressant effect on locomotor activity compared to the control value (P < .05). A dose of 10 mg/kg theophylline was used to investigate reversal of adenosine agonist-induced behavioral depression.
Fig. 2.
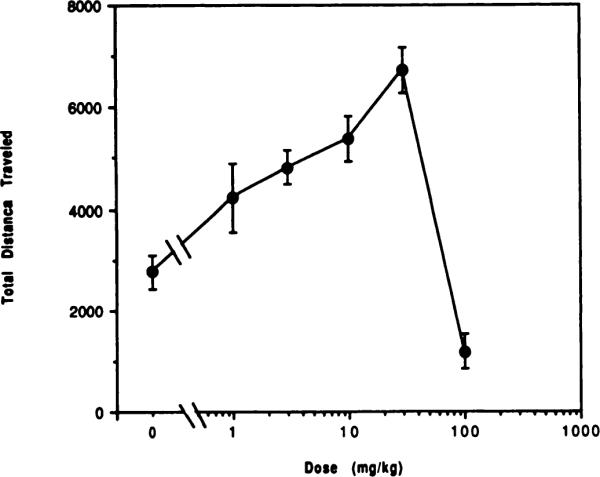
Dose-response curves for locomotor stimulation and depression in mice by the adenosine antagonist theophylline. Activity counts for total distance traveled (mean ± S.E.) over a 30-min period are shown as a function of dose of theophylline injected i.p (n = 6–8).
Effects of theophylline on locomotor depression elicited by selective adenosine agonists
Reversal of the centrally elicited locomotor depressant effects of adenosine agonists by the nonselective adenosine antagonist theophylline, a well-documented phenomenon (Snyder et al., 1981; Barraco et al., 1983; Glowa et al., 1985), was examined. Locomotor depressant effects of adenosine agonists selective for A1 or A2 adenosine receptors, CHA and APEC, respectively, were measured in the presence and absence of theophylline (10 mg/kg), injected i.p. Figures 3 and 4 show the effect of a fixed dose of theophylline on the dose-response curves for locomotor depression by CHA and APEC. The curve for APEC (fig. 3) was clearly shifted to the right (EC50 shifted from 25.2 nmol/kg to approximately 266 nmol/kg) in the presence of theophylline, suggesting a pharmacological antagonism, as would be predicted from the large body of biochemical experiments with theophylline/caffeine and adenosine analogs carried out previously in other laboratories. However, the stimulant effects of theophylline complicate interpretation of interactions of the two agents. The curve for CHA (fig. 4) was shifted markedly to the right in the presence of theophylline. The EC50 was shifted from 268 nmol/kg in the absence of theophylline to an undetermined greater value. Theophylline completely blocked the depressant effect of CHA relative to control at doses up to 1000 nmol/kg, as well as the effect of APEC at doses up to 25 nmol/kg. Again the stimulant effects of theophylline complicated interpretation of the interactions of theophylline and the adenosine analogs. At the highest dose of CHA tested (3000 nmol/kg) there was a statistical difference (P < .001) between the values for locomotor activity with agonist alone or agonist and theophylline combined. Remarkably, at the lowest dose of CHA there was no longer any locomotor stimulation elicited by theophylline.
Effects of an A1-selective antagonist (CPT) on adenosine agonist-induced locomotor depression
The rank order of potency (figs. 5–7) after i.p. injection for adenosine analogs was NECA (ED50, 5.8 nmol/kg) > APEC (ED50, 25 nmol/kg) > CHA (ED50, 270 nmol/kg)
Fig. 5.
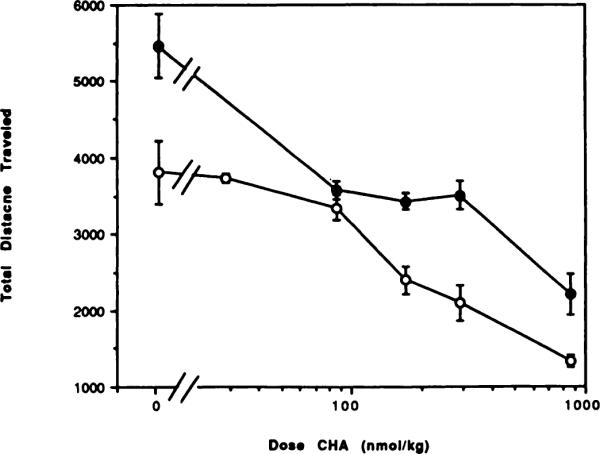
Dose-response curves for locomotor depression in mice by the adenosine agonist CHA in the absence (◯) or presence (●) of the A1-selective antagonist CPT (10 mg/kg). The antagonist or vehicle was injected 10 min before the agonist. The activity reflects the total distance traveled (mean ± S.E.) over a 30-min period (n = 6–12).
CPT, an A1-selective antagonist, has been reported to antagonize the centrally induced locomotor depressant effects of adenosine agonists, such as N6-cyclopentyladenosine (Bruns et al., 1988). To further explore this finding, we tested a single dose of CPT with different doses of the adenosine agonists CHA, NECA and APEC. At a dose of 10 mg/kg (at its maximum aqueous solubility), CPT, like theophylline, caused a behavioral stimulation (figs. 5–7). At this dose CPT significantly reversed the dose-related depressant effects of both CHA and NECA, but there was no reversal of the effects of APEC administered at a dose equal to the previously determined ED50. Dose-response curves for CHA- and NECA-treated mice were shifted to the right in the presence of CPT. Reversal elicited by the antagonist CPT was complete for both CHA (fig. 5) and NECA (fig. 6) at doses of agonists up to twice the ED50 dose. The EC50 for CHA was shifted from 172 to 620 nmol/kg in the presence of CPT. At the highest dose of CHA tested (1000 nmol/kg) there was a stastical difference (P < .001) between the values for locomotor activity with agonist alone or agonist and CPT combined. The EC50 for NECA was shifted from 5.76 to 43.8 nmol/kg in the presence of CPT. These results might suggest that the locomotor depressant effects of CHA and NECA are primarily the result of the stimulation of central A1 adenosine receptors. In contrast, the locomotor depressant effects of an A2 receptor-selective adenosine analog, APEC (Nikodijevic et al., 1990), indicated that activation of A2 adenosine receptors also could result in locomotor depression. Consistent with this conclusion, the A1-selective antagonist CPT was unable to reverse the locomotor depression induced by APEC (fig. 7).
Fig. 6.
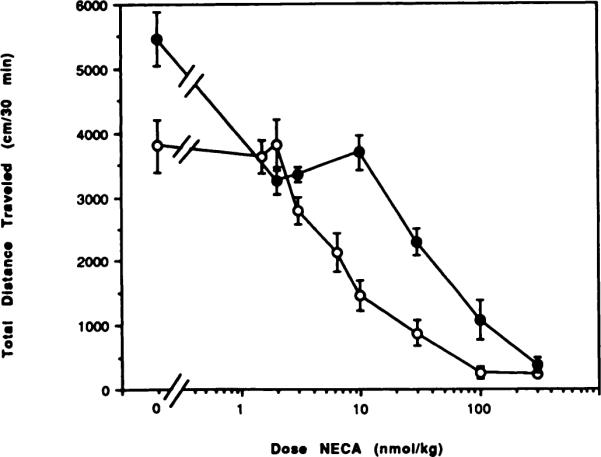
Dose-response curves for locomotor depression in mice by the adenosine agonist NECA in the absence (◯) or presence (●) of the A1-selective antagonist CPT (10 mg/kg). The antagonist or vehicle was injected 10 min before the agonist. The activity reflects the total distance traveled (mean ± S.E.) over a 30-min period (n = 6–12).
The effect of an A2-selective adenosine antagonist (CP-66,713) on adenosine agonist-induced locomotor depression
An A2-selective adenosine antagonist, CP-66,713, was recently developed (Sarges et al., 1990). CP-66,713 has a 13-fold selectivity for A2 receptors in binding studies in rat brain and has a Ki value of 12 nM at rat brain A2 receptors (vs. binding of [3H]CGS21680, unpublished data). CP-66,713 was used as an A2-selective antagonist for further characterization of the locomotor depressant effects of APEC. Results presented in figure 8 show a reversal by CP-66,713 of the locomotor depression in mice induced by APEC. CP-66,713, at a dose of 1.5 mg/kg administered i.p., significantly reversed the dose-dependent depressant effects of APEC at doses as high as twice the ED50 dose (fig. 8). The ED50 of APEC in this experiment shifted from 8.47 to 66.4 nmol/kg in combination with CP-66,713. However, at higher doses of APEC (≥180 nmol/kg), CP-66,713 failed to diminish the locomotor depression. Higher doses of CP-66,713 could not be administered because of a low solubility of approximately 1 μM in aqueous buffers. In the Emulphor injection medium the maximum solubility of CP-66,713 was found by ultraviolet analysis to be 0.4 mM. Use of a 40% solution of the solubilizer hydroxypropyl-γ-cyclodextrin as a cosolvent failed to increase the solubility of CP-66,713 significantly. CP-66,713 dissolved in dimethylsulfoxide/aqueous mixtures could not be used, because dimethylsulfoxide alone, present in the vehicle at a concentration of only 15%, elicited a locomotor depressant effect in mice (data not shown). At a dose of 1.5 mg/kg, CP-66,713 alone did not cause a significant stimulation of behavior (fig. 8).
Fig. 8.
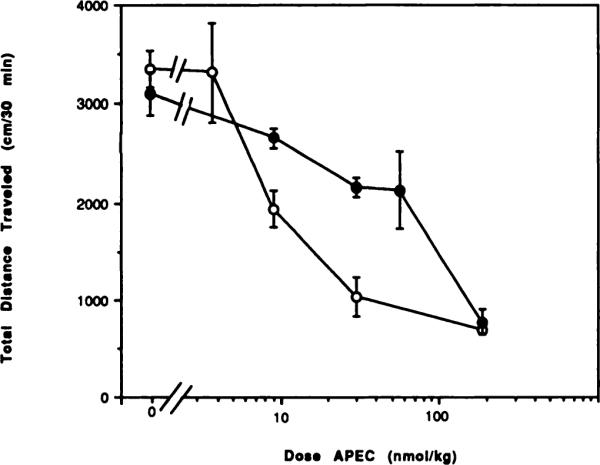
Dose-response curves for locomotor depression in mice by the adenosine agonist APEC in the absence (◯) or presence (●) of an A2-setective adenosine antagonist, CP-66,713 (1.5 mg/kg). The antagonist was injected 10 min before the agonist. The activity reflects the total distance traveled (mean ± S.E.) over a 30-min period (n = 8). In this set of experiments and those of table 2, APEC was more potent than in others (see fig. 7, tables 1 and 3).
Peripherally administered CP-66,713 appears to be selective as a central A2 adenosine receptor antagonist in the mouse. Thus, CP-66,713, at a dose of 1.5 mg/kg. did not block the locomotor depression induced by doses equal to the previously determined ED50 doses of the A1-selective agonist CHA or of the nonselective agonist NECA (table 2).
TABLE 2.
Locomotor effects of an A2-selective adenosine antagonist, CP-66,713, alone or in combination with CHA, NECA, or APEC
| Drug(s)a | n | Locomotor Activityb | Depression(↓) |
|---|---|---|---|
| % | |||
| Noneb | 27 | 3344 ± 184 | 0 |
| CP-66,713 | 10 | 3104 ± 218 | 6↓ |
| CHA | 8 | 2073 ± 145 | 38↓ |
| CHA + CP-66,713 | 7 | 2006 ± 120 | 40↓ |
| NECA | 20 | 1806 ± 99 | 46↓ |
| NECA + CP-66,713 | 9 | 1538 ± 92 | 54↓ |
| APEC | 16 | 1237 ± 87 | 63↓ |
| APEC + CP-66,713 | 8 | 2408 ± 72 | 28↓ |
Dose of CP-66,713 was 1.5 mg/kg i.p. The antagonist was injected 10 min before each agonist. The doses of CHA (170 nmol/kg), NECA (6.5 nmol/kg) and APEC (30 nmol/kg) were chosen to approximate the ED50.
Vehicle control.
Mean total distance traveled (cm/30 min) ± S.E.M., and depression relative to vehicle control, are shown.
Locomotor effects of combinations of adenosine agonists
Combinations of adenosine agonists, both CHA and APEC (selective A1 and A2 agonists, respectively) as well as NECA (a nonselective adenosine agonist), were administered in order to explore possible facilitatory effects of A2 receptor activation on A1 receptor-elicited depression and vice versa. Results from this study are presented in figure 9 and tables 4 through 6.
TABLE 4.
Algebraic fractional analysis of locomotor depression elicited by combinations of CHA and APEC. Fractions represent an expression of the doses of the drugs in combination that produce the same effect when given separately (i.e., equieffective). Antagonism is indicated if the sum of the fractions is more than 1.0, whereas synergism is indicated if the sum of the fractions is less than 1.0 (see Kissin et al., 1990). With the combination of APEC and CHA, the sum of the fractions indicated a degree of synergism of 1.89.
| Subseries | Fraction |
Sum of Fractionsa | |
|---|---|---|---|
| CHA Component | APEC Component | ||
| ED50 in nmol/kg | |||
| CHA alone | 1.00/170 | 0.00 | 1.00 |
| APEC alone | 0.00 | 1.00/30 | 1.00 |
| CHA + APEC combination | 0.32/54 | 0.21/6.1 | 0.53 |
The sum of the fractions is defined as [(ED50 APEC, in combination/ED50 APEC, alone) + (ED50 CHA, in combination/ED50 CHA, alone)].
TABLE 6.
Locomotor effects of combinations of NECA and APEC at various doses
| Dose |
Locomotor Activity | Depression | |
|---|---|---|---|
| APEC | NECA | ||
| nmol/kg | % | ||
| 0 | 0 | 3400 ± 211 | 0 |
| 3.7 | 0 | 3468 ± 35 | 2↑ |
| 0 | 3.2 | 2380 ± 71 | 30↓** |
| 0 | 6.5 | 2142 ± 86 | 37↓** |
| 3.7 | 3.2 | 2006 ± 99 | 41↓** |
| 3.7 | 6.5 | 1972 ± 59 | 42↓** |
a The activity reflects the mean total distance traveled (cm) ± S.E.M. over a 30-min period, begun 10 min after the last injection (APEC). Injections of agonists in the same animal were separated by a 5-min interval (n = 8–14).
P < .01 compared to vehicle control or 3.7 nmol/kg APEC.
The A1-selective agonist CHA, at a dose of 29 nmol/kg, which by itself has only a small effect on locomotor activity in mice, resulted in a significant potentiation of the depressant effect of APEC (table 3) when administered together with APEC at either a noneffective dose (3.7 nmol/kg) or at the previously determined ED50 dose. In both cases, the effect of the combination was greater than additive (P < .001).
TABLE 3.
Potentiative depressant effects of adenosine agonists in combinationa
| Dose |
Locomotor Activity | Depression | |
|---|---|---|---|
| APEC | CHA | ||
| nmol/kg | % | ||
| 0 | 0 | 3400 ± 184 | 0 |
| 3.7 | 0 | 3468 ± 35 | 2↑ |
| 30 | 0 | 2142 ± 129 | 37↓** |
| 0 | 29 | 3264 ± 68 | 4↓ |
| 0 | 170 | 1972 ± 122 | 42↓** |
| 3.7 | 29 | 2414 ± 221 | 29↓** |
| 30 | 29 | 1632 ± 85 | 52↓** |
| 30 | 170 | 204 ± 8 | 94↓** |
The activity reflects the mean total distance traveled (cm) ± S.E.M. over a 30-min period, begun 10 min after the last injection (CHA). Injections of agonists in the same animal were separated by a 5-min interval, n = 6–33.
P < .01 compared to vehicle control.
The potentiation of locomotor depressant effects was also observed in mice treated with a combination of CHA and APEC, administered at their respective, previously determined ED50 doses. The isobolographic analysis comparing APEC and CHA ED50 doses when the drugs were given in combination vs. separately is presented in figure 9. The ED50 values for the combination were 6.1 nmol/kg for APEC and 54 nmol/kg for CHA. The point corresponding to the ED50 doses for the combination deviates to the left of the line of additivity, clearly indicating statistically valid synergism. An algebraic (fractional) analysis of these data is presented in table 4. With the combination, the sum of the fractions (0.53) indicated a degree of synergism of 1.89.
A combination of CHA at a subthreshold dose with a minimum effective dose (3.2 nmol/kg) or previously determined ED50 (6.5 nmol/kg) dose of NECA did not potentiate the depressant effect of NECA (table 5).
TABLE 5.
Depressant effects of NECA and CHA in combination
| Dose |
Locomotor Activity | Depression | |
|---|---|---|---|
| CHA | NECA | ||
| nmol/kg | % | ||
| 0 | 0 | 3400 ± 211 | 0 |
| 0 | 3.2 | 2380 ± 102 | 30↓** |
| 0 | 6.5 | 2142 ± 128 | 37↓** |
| 29 | 0 | 3264 ± 68 | 4↓** |
| 29 | 3.2 | 2244 ± 187 | 34↓** |
| 29 | 6.5 | 1904 ± 133 | 44↓** |
a The activity reflects the mean total distance traveled (cm) ± S.E.M. over a 30-min period, begun 10 min after the last injection (CHA). Injections of agonists in the same animal were separated by a 5-min interval (n = 9–12).
P < .01 compared to vehicle control.
In order to see if the A2-selective adenosine agonist APEC could potentiate the locomotor depression induced by NECA, further experiments were conducted using a combination of APEC (3.7 nmol/kg, a dose that was without effect on locomotor activity) and NECA (at two different doses: at 3.2 nmol/kg, nearly the lowest effective dose, or at 6.5 nmol/kg, the previously determined ED50 for NECA). Results of these experiments are presented in table 6. APEC at a noneffective dose potentiated the locomotor depression in mice induced by 3.2 nmol/kg of NECA. The effect of the combination on total distance traveled was greater than additive (P < 0.001). There was no potentiative effect of the same dose of APEC in combination with the previously determined ED50 dose of NECA.
Discussion
Adenosine agonists elicit a profound behavioral depression in mammals (Snyder et al., 1981; Glowa et al., 1985; Durcan and Morgan, 1989a; Yarbrough and McGuffin-Clineschmidt, 1981). Similar locomotor depressions are elicited after either parenteral or intracerebroventricular administration and appear to be centrally mediated. The main evidence for a central site of action is that adenosine antagonists, such as 8-PST, that do not cross the blood-brain barrier do not reverse the behavioral effects of adenosine analogs (Katims et al., 1983; Seale et al., 1988; Durcan and Morgan, 1989b). Our preliminary study (Nikodijevic et al., 1990) also supported an assumption that locomotor depressant effects of adenosine analogs are not peripheral because they could not be blocked by 8-PST or 1,3-dipropyl-p-sulfophenylxanthine, two potent, but nonselective, peripherally acting adenosine antagonists. A 10 mg/kg dose of the standard peripheral antagonist 8-PST was chosen because it has been shown to effectively block the peripheral actions of adenosine agonists in other studies (see above).
The nature of the receptors subserving the centrally mediated depression has not been resolved. Selective A1 agonists such as CHA and N6-cyclopentyladenosine elicit behavioral depression, and selective A1 antagonists do reverse the effects of adenosine analogs in mice. However, the in vivo potencies of several adenosine analogs did not correlate with potencies in binding at a rat brain A1 receptor, but did correlate with potencies at a rat striatal A2 receptor (Durcan and Morgan, 1989a). The lack of behavioral effects of R-PIA in one strain of mice was attributed to low levels of brain A1 receptors (Fredholm et al., 1985), but levels of A2 receptors were not ascertained. In two mouse strains, a selective increase in sensitivity to NECA, but not to CHA was observed (Seale et al., 1986). In one strain, NECA was more potent than CHA, suggesting that A2 receptors were important in this strain, whereas in the other strain NECA and CHA were equipotent. Recently we reported that the selective A2 agonist APEC can elicit a centrally mediated locomotor depression (Nikodijevic et al., 1990). Preliminary data on behavioral depression elicited by a somewhat A2-selective agonist, 2-phenylaminoadenosine (CV 1808), indicated that A2 receptors were involved (Brans et al., 1988). Thus, it appears that either activation of A1 or A2 receptors can lead to locomotor depression. The importance and possible interactions of such receptor pathways to the effects of very potent nonselective agonists, such as NECA, remain unknown.
Our previous report of behavioral effects of a recently developed A2-selective adenosine agonist (APEC) strongly suggested possible involvement of central A2 receptors in the locomotor depression that is induced by adenosine and certain active analogs. The results in screening other A2-selective agonists (table 1) in locomotor depression have revealed that APEC is highly potent in vivo in relation to other analogs and, therefore, an appropriate choice for the present study. The Ki values at A2 receptors from binding studies using [3H]CGS21680 (Jarvis et al., 1989) in the rat brain range from 12 for APEC to 26 nM for compound 4 (values from Jacobson et al., 1989). Some of these analogs are more A2-selective than APEC and still cause behavioral depression. CGS21680 was relatively weak as a locomotor depressant. Doses of 30 nmol/kg and 1000 nmol/kg resulted in 13 and 62% decreases, respectively, in total distance traveled. This likely reflects poor penetration of the blood-brain barrier by CGS21680, which contains a carboxylate group.
The present study provides evidence that potentiative interactions between A1 and A2 adenosine receptor components can contribute to the locomotor depressant effects of adenosine and its analogs. The potency of CPT (an A1-selective adenosine antagonist) in inhibiting the locomotor depression elicited by CHA or NECA (A1-selective and nonselective adenosine agonists, respectively) provided strong evidence for an A1 receptor involvement in locomotor depression induced by these two adenosine agonists. Similarly, the lack of reversal by CPT of the locomotor depression elicited by APEC (a 41-fold A2-selective adenosine agonist) indicates that an A1 receptor is not involved in the action of this adenosine agonist. Theophylline did block depressant effects of CHA at doses up to 1000 nmol/kg and APEC at doses up to 25 nmol/kg. The pronounced right shift in the depressant effects of CHA and APEC by theophylline indicates that adenosine receptors are involved. However, theophylline has behavioral stimulant activity alone (fig. 2), and this complicates interpretation of its antagonistic activity vs. adenosine analogs. Curiously, we have observed that a low dose of CHA abolishes the stimulatory effect of theophylline (fig. 3b).
The present dose of theophylline (10 mg/kg) was chosen to avoid possible contributions from the depressant effects of this xanthine that begin to manifest themselves at doses >30 mg/kg (fig. 2). The depressant effect of methylxanthines at very high doses has been noted previously with caffeine and other xanthines (Thithapandha et al., 1972; Kaplan et al., 1989).
The A2-selective adenosine antagonist CP-66,713, a triazoloquinoxaline derivative, is selective for A2 receptors by a factor of 13 in binding assays (Sarges et al., 1990). It was used to further investigate the nature of adenosine receptors involved in behavioral depression. CP-66,713 blocked locomotor depression in mice induced by APEC at twice the ED50 dose. It was not possible to block higher depressant doses of APEC with CP-66,713, presumably because the low solubility of the antagonist precluded administration of higher dosages. Pretreatment of mice with CP-66,713 did not block the depressant effects of NECA and CHA at previously determined ED50 doses (table 2). This is consistent with our previous report (Nikodijevic et al., 1990), which suggested that primarily A1 receptors are involved in the depression elicited by both CHA and NECA. Antagonism of the locomotor depressant effects of APEC by CP-66,713 further supports the conclusion that the depressant effects of APEC are due to the stimulation of A2 adenosine receptors in the brain.
The relative potencies of NECA and CHA did not correlate to their A1 receptor affinities even though both were antagonized by the A1-selective antagonist CPT. NECA was much more potent than CHA as a locomotor depressant, whereas the Ki values for a rat brain A1 receptor are 6.3 nM and 1.3 nM, respectively. At rat striatal A2 receptors, the Ki values were 10.3 and 514 nM, respectively (Bruns et al., 1986). The better correlation of locomotor effects with A2 receptor affinities have led to the proposal that A2 receptors are involved (Durcan and Morgan, 1989a), although the antagonism by selective A1 antagonists, such as CPT, would appear to contradict such a conclusion. Pharmacokinetics might play a role in the relatively high potency of NECA. However, it is possible that the high potency of NECA compared to CHA is due to the fact that NECA is a potent nonselective agonist and may elicit depression through summation of both A1 and A2 receptor activation, with A1 activation being essential and A2 activation potentiative. Therefore, we have experimented with combinations of A1- and A2-selective adenosine agonists in the same mouse. Injection of a noneffective dose of CHA together with subthreshold or previously determined ED50 doses of APEC, increased locomotor depression in mice in a potentiative manner (table 3). However, the same dose of CHA did not potentiate the locomotor depressant effects induced by NECA. Administration of CHA and APEC at these ED50 doses to the same animal produced a greater than additive depressant effect in mice. When a combination of NECA and APEC (at a noneffective dose) was used, a significant potentiation of depressant effects was observed at the lowest effective dose of NECA, but not at its previously determined ED50 dose (table 3).
These results indicate that either A1- or A2-selective adenosine agonists are central depressants and that they can act in a synergistic (potentiative) manner through activation of A1 and A2 adenosine receptors in the brain. This synergism between A1 and A2 agonists may account for the high potency of NECA as a locomotor depressant, observed in this study and in others (Seale et al., 1988; Durcan and Morgan, 1989b), in relation to its affinity for either A1 or A2 adenosine receptors. It appears likely that the depression due to activation of A1 receptors is of primary importance, and that A2 receptor activation is mainly potentiative. However, the lack of effect of the A2 selective antagonist CP-66,713 on the depression elicited by NECA (table 2) seems inconsistent with this interpretation. It could be that doses of NECA that activate the A1 receptors are fully saturating the A2 receptors and it is, therefore, difficult to block NECA activation of A2 receptors with CP-66,713. Higher doses of CP-66,713 could not be used because of low solubility. Certainly, at the previously determined ED50 dose for NECA, APEC could not further enhance the depressant effect of NECA (table 6). In summary, the present results indicate that both A1and A2 receptors can be involved in the regulation of behavioral states by adenosine agonists. The results with APEC indicate that a clearly A2-selective agonist can elicit full behavioral depression.
The nature of interaction of A1 and A2 receptor activation in vivo is unknown. Synergistic interactions of A1 and A2 receptors have not been demonstrated in vitro with cells or membranes. It likely represents interactions at the level of different neuronal pathways involved in behavior, some of which are regulated by A1 receptors, others of which are regulated by A2 receptors.
ABBREVIATIONS
- APEC
2-[(2-aminoethylamino)carbonylethylphenylethylamino]-5′-N-ethylcarboxamicloadenosine
- CGS21680
2-(carboxyethyl-phenylethylamino)adenosine-5′-carboxamide
- CHA
N6-cyclohexyladenosine
- CNS
central nervous system
- CPT
8-cyclopentyltheophylline
- CP-66
713, 4-aminc-8-chloro-1-phenyl-[1,2,4]triazolo[4,3-a]quinoxaline
- NECA
5′-N-ethylcarboxamidoadenosine
- 8-PST
8-para-sulfophenyltheophylline
- R-PIA
N6-R-(phenylisopropyl)adenosine.
References
- Barraco RA, Coffin VL, Altman HJ, Phillis JW. Central effects of adenosine analogs on locomotor activity in mice and antagonism of caffeine. Brain Res. 272;272:392. doi: 10.1016/0006-8993(83)90591-7. [DOI] [PubMed] [Google Scholar]
- Barraco RA, Aggarwal AK, Phillis JW, Moron MA, Wu PH. Dissociation of the locomotor and hypertensive effects of adenosine analogues in the rat. Neurosci. Lett. 1984;48:139–144. doi: 10.1016/0304-3940(84)90009-0. [DOI] [PubMed] [Google Scholar]
- Belardinelli L, Isenberg G. Isolated atrial myocytes: Adenosine and acetylcholine increase potassium conductance. Am. J. Physiol. 1983;244:H734–H737. doi: 10.1152/ajpheart.1983.244.5.H734. [DOI] [PubMed] [Google Scholar]
- Berenbaum MC. Synergy, additivism and antagonism in immunosuppression. Clin. Exp. Immunol. 1977;28:1–18. [PMC free article] [PubMed] [Google Scholar]
- Bruns RF, Lu GH, Pugsley TA. Characterization of the A2adenosine receptor labeled by [3H]NECA in rat striatal membranes. Mol. Pharmacol. 1986;29:331–346. [PubMed] [Google Scholar]
- Bruns RF, Davis RE, Ninteman FW, Poschel BPH, Wiley JN, Heffner TG. Adenosine antagonists as pharmacological tools. In: Paton DM, editor. Physiology and Pharmacology of Adenosine and Adenine Nucleotides. Taylor and Francis; London, England: 1988. pp. 39–49. [Google Scholar]
- Cerbai E, Klockner U, Isenberg G. Ca-antagonistic effects of adenosine in guinea pig atrial cells. Am. J. Physiol. 1988;255:H872–H878. doi: 10.1152/ajpheart.1988.255.4.H872. [DOI] [PubMed] [Google Scholar]
- Dunwiddie TV. The physiological role of adenosine in the CNS. Int. Rev. Neurobiol. 1985;27:103–124. doi: 10.1016/s0074-7742(08)60556-5. [DOI] [PubMed] [Google Scholar]
- Dunwiddie TV, Worth TS, Olsson RA. Adenosine analogs mediating depressant effects on synaptic transmission in rat hippocampus: Structure-activity relationships for the N6-subregion. Naunyn-Schmiedeberg's Arch. Pharmacol. 1986;334:77–85. doi: 10.1007/BF00498743. [DOI] [PubMed] [Google Scholar]
- Durcan MJ, Morgan PF. Evidence for adenosine A2 receptor involvement in the hypomobility effects of adenosine analogs in mice. Eur. J. Pharmacol. 1989a;168:285–290. doi: 10.1016/0014-2999(89)90789-9. [DOI] [PubMed] [Google Scholar]
- Durcan MJ, Morgan PF. NECA-induced hypomotility in mice: Evidence for a predominantly central site of action. Pharmacol. Biochem. Behav. 1989b;32:487–490. doi: 10.1016/0091-3057(89)90185-8. [DOI] [PubMed] [Google Scholar]
- Finney DJ. Probit Analysis. 3rd ed. Cambridge University Press; Cambridge, England: 1971. [Google Scholar]
- Fredholm BB, Zahniser NR, Weiner GR, Proctor WR, Dunwiddie TV. Behavioural sensitivity to PIA in selective bred mice is related to a number of A1 adenosine receptors but not to cyclic AMP accumulation in brain slices. Eur. J. Pharmacol. 1985;111:133–136. doi: 10.1016/0014-2999(85)90123-2. [DOI] [PubMed] [Google Scholar]
- Fredholm BB, Dunwiddie TV. How does adenosine inhibit transmitter release? Trends Pharmacol. Sci. 1988;9:130. doi: 10.1016/0165-6147(88)90194-0. [DOI] [PubMed] [Google Scholar]
- Glowa JR, Sobel E, Malaspina S, Dews PB. Behavioral effects of caffeine, (−)N-(R)-1-methyl-2-phenylethyl)-adenosine (PIA) and their combination in the mouse. Psychopharmacology. 1985;87:421–424. doi: 10.1007/BF00432506. [DOI] [PubMed] [Google Scholar]
- Goldberg SR, Prada JA, Katz JL. Stereoselective behavioral effects of N6-phenylisopropyl-adenosine and antagonism by caffeine. Psychopharmacology. 1985;87:272–277. doi: 10.1007/BF00432706. [DOI] [PubMed] [Google Scholar]
- Hamprecht B, Van Calker D. Nomenclature of adenosine receptors. Trends Pharmacol. Sci. 1985;6:153. [Google Scholar]
- Heffner TG, Wiley JN, Williams AE, Bruns RF, Coughenour LL, Downs DA. Comparison of the behavioral effects of adenosine agonists and dopamine antagonists in mice. Psychopharmacol. 1989;98:31–37. doi: 10.1007/BF00442002. [DOI] [PubMed] [Google Scholar]
- Hutchison AJ, Webb RL, Oei HH, Ghai GR, Williams M. CGS 21680C, an A2 selective adenosine receptor agonist with preferential hypotensive activity. J. Pharmacol. Exp. Ther. 1989;251:47–55. [PubMed] [Google Scholar]
- Jacobson KA, Barrington WW, Pannell LK, Jarvis MF, Ji X-D, Hutchison AJ, Stiles GL. Agonist-derived molecular probes for A2-adenosine receptors. J. Mol. Recogn. 1989;2:170–178. doi: 10.1002/jmr.300020406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis MF, Schutz R, Hutchison AJ, Do E, Sills MA, Williams M. [3H]CGS 21680, an A2 selective adenosine receptor agonist directly labels A2 receptors in rat brain tissue. J. Pharmacol. Exp. Ther. 1989;251:888–893. [PubMed] [Google Scholar]
- Kaplan GB, Greenblatt DJ, Leduc BW, Thompson ML, Shader RI. Relationship of plasma and brain concentrations of caffeine and metabolites to benzodiazepine receptor binding and locomotor activity. J. Pharmacol. Exp. Ther. 1989;248:1078–1083. [PubMed] [Google Scholar]
- Katims JJ, Annau Z, Snyder SH. Interactions in the behavioral effects of methylxanthines and adenosine derivatives. J. Pharmacol. Exp. Ther. 1983;227:167–173. [PubMed] [Google Scholar]
- Kissin I, Brown PT, Bradley EL. Morphine and fentanyl anesthetic interactions with diazepam: Relative antagonism in rats. Anesth. Analg. 1990;71:236–241. [PubMed] [Google Scholar]
- Linden J, Delahunty TM. Receptors that inhibit phospho-inositide breakdown. Trends Pharmacol. Sci. 1989;10:114–120. doi: 10.1016/0165-6147(89)90209-5. [DOI] [PubMed] [Google Scholar]
- Nikodijevic O, Daly JW, Jacobson KA. Characterization of the locomotor depression produced by an A2-selective adenosine agonist. FEBS Lett. 1990;261:67–70. doi: 10.1016/0014-5793(90)80638-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillis JW, Barraco RA, DeLong RE, Washington DC. Behavioral characteristics of centrally administered adenosine analogs. Pharmacol. Biochem. Behav. 1986;24:263. doi: 10.1016/0091-3057(86)90349-7. [DOI] [PubMed] [Google Scholar]
- Sanberg PR, Hagenmeyer SH, Henault MA. Automated measurement of multivariate locomotor behavior in rodents. Neurobehav. Toxicol. Teratol. 1985;7:87–94. [PubMed] [Google Scholar]
- Sarges R, Howard HR, Browne RG, Lebel LA, Seymour PA, Koe BK. 4-Amino[1,2,4]triazolo[4,3-a]quinoxalines—a novel class of potent adenosine receptor antagonists and potential rapid onset anti-depressants. J. Med. Chem. 1990;33:2240–2254. doi: 10.1021/jm00170a031. [DOI] [PubMed] [Google Scholar]
- Seale T, Abla KA, Cao W, Parker KM, Rennert OM, Carney JM. Inherent hyporesponsiveness to methylxanthine induced behavioral changes associated with supersensitivity to 5′-N-ethylcarboxamidoadenosine (NECA) Pharm. Biochem. Behav. 1986;25:1271–1277. doi: 10.1016/0091-3057(86)90122-x. [DOI] [PubMed] [Google Scholar]
- Seale T, Abla KA, Shamim MT, Carney JM, Daly JW. 3,7-Dimethyl-1-propargylxanthine: a potent and selective in vivo antagonist of adenosine analogs. Life Sci. 1988;43:1671–1684. doi: 10.1016/0024-3205(88)90478-x. [DOI] [PubMed] [Google Scholar]
- Snyder SH, Katims JS, Annau Z, Bruns RF, Daly JW. Adenosine receptors and the actions of the methylxanthines. Proc. Natl. Acad. Sci. USA. 1981;78:3260–3264. doi: 10.1073/pnas.78.5.3260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder SH. Adenosine as a neuromodulator. Annu. Rev. Neurosci. 1985;8:103–124. doi: 10.1146/annurev.ne.08.030185.000535. [DOI] [PubMed] [Google Scholar]
- Spealman RD, Coffin VL. Behavioral effects of adenosine analogs in squirrel monkeys: Relation to adenosine A2 receptors. Psychopharmacology. 1986;90:419–421. doi: 10.1007/BF00179203. [DOI] [PubMed] [Google Scholar]
- Thithapandha A, Maling HM, Gillette JR. Effects of caffeine and theophylline on activity of rats in relation to brain xanthine concentrations. Proc. Soc. Exp. Biol. Med. 1972;139:582–586. doi: 10.3181/00379727-139-36191. [DOI] [PubMed] [Google Scholar]
- Van Calker D, Muller M, Hamprecht B. Adenosine regulates via two different types of receptors: the accumulation of cyclic AMP in cultured brain cells. J. Neurochem. 33;33:999. doi: 10.1111/j.1471-4159.1979.tb05236.x. [DOI] [PubMed] [Google Scholar]
- Yarbrough GG, McGuffin-Clineschmidt TC: In vivo behavioral assessment of central nervous system purinergic receptors. Eur. J. Pharmacol. 1981;76:137–144. doi: 10.1016/0014-2999(81)90495-7. [DOI] [PubMed] [Google Scholar]


