Abstract
Selective agonists for A3 adenosine receptors (ARs) could potentially be therapeutic agents for a variety of disorders, including brain and heart ischemic conditions, while partial agonists may have advantages over full agonists as a result of an increased selectivity of action. A number of structural determinants for A3AR activation have recently been identified, including the N6-benzyl group, methanocarba substitution of ribose, 2-chloro and 2-fluoro substituents, various 2’- and 3’-substitutions and 4’-thio substitution of oxygen. The 2-chloro substitution of CPA and R-PIA led to A3 antagonism (CCPA) and partial agonism (Cl-R-PIA). 2-Chloroadenosine was a full agonist, while 2-fluoroadenosine was a partial agonist. Both 2’- and 3’- substitutions have a pronounced effect on its efficacy, although the effect of 2’-substitution was more dramatic. The 4-thio substitution of oxygen may also diminish efficacy, depending on other substitutions. Both N6-methyl and N6-benzyl groups may contribute to the A3 affinity and selectivity; however, an N6-benzyl group but not an N6-methyl group diminishes A3AR efficacy. N6-benzyl substituted adenosine derivatives have similar potency for human and rat A3ARS while N6-methyl substitution was preferable for the human A3AR. The combination of 2-chloro and N6-benzyl substitutions appeared to reduce efficacy further than either modification alone. The A2AAR agonist DPMA was shown to be an antagonist for the human A3AR. Thus, the efficacy of adenosine derivatives at the A3AR appears to be more sensitive to small structural changes than at other subtypes. Potent and selective partial agonists for the A3AR could be identified by screening known adenosine derivatives and by modifying adenosine and the adenosine derivatives.
INTRODUCTION
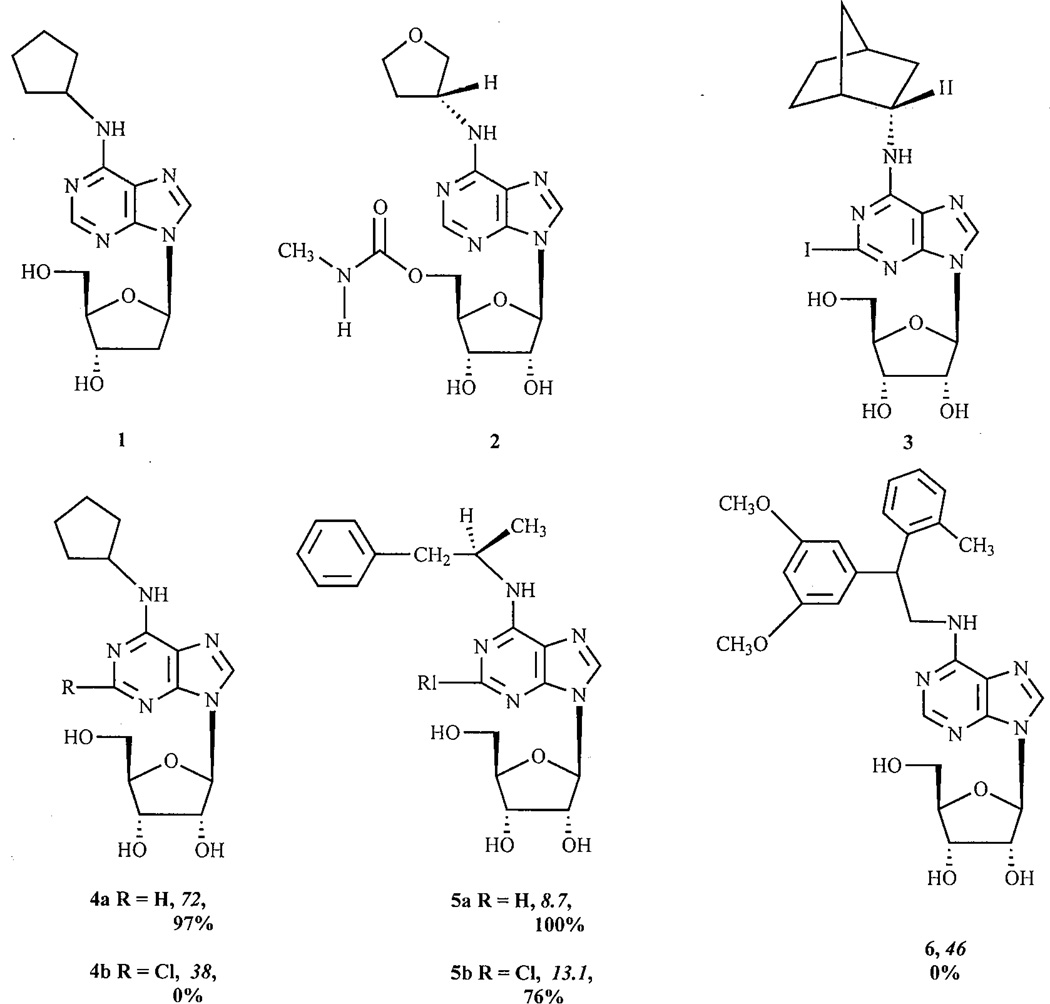
Four adenosine receptor (AR) subtypes have been cloned and pharmacologically characterized; they are termed A1, A2A, A2B and A3 [1]. The A1 and A3 ARs preferentially couple to the Gi/o family of G proteins and A2A and A2B ARs to Gs proteins. Adenosine is an endogenous agonist for all of these receptors. All four subtypes of the AR are attractive targets for drug development. Agonists for A1 and A3 ARs could, for example, be used for both brain and heart protection, however, severe cardiovascular side effects may be expected due to the strong hypotensive effects of a series of A1 AR agonists [2]. The use of full agonists of the A1 AR as antiarrhythmic agents is limited by their causing of high-grade atrioventricular (AV) block, profound bradycardia, atrial fibrillation, and vasodilation. These side effects were major drawbacks in the clinical application of AR agonists. Partial agonists could, however, produce less pronounced cardiovascular effects and may act more selectively; e.g. the partial agonist 2-deoxyCPA 1 produced a less dramatic effect on heart rate than the full agonist CPA [3]. The A1 AR partial agonist, 5-{6-[((3R)oxolan-3-yl)amino]purin-9-yl}(3S,2R,4R,5R)-3,4-dihydroxyoxolan-2-yl)methoxy]-N-methyl-carboxamide (CVT-2759 2)*, selectively slows AV conduction in guinea pig hearts. Partial agonists may also be useful for slowing AV nodal conduction, and thereby ventricular rate, without causing AV block, bradycardia, atrial arrhythmias, or vasodilation [4]. Another advantage to partial agonists is that they are less likely to cause receptor desensitization and downregulation than full agonists. Partial agonists for β-adrenergic receptors, for example, have met with considerable success as therapeutic agents [5].
Modification of adenosine and its analogues leading to partial agonists for A1 ARs has been well documented [6–8]. In addition, partial agonists for A1 ARs have clearly envisioned therapeutic applications. Isolated examples of partial agonists for A2A ARs have been reported [9–11]. Hutchinson et al. [12] reported that 2-iodo-N6-(2-S-endo-norborn-2-yl)adenosine 3 was a full agonist at the A1AR but have no detectable agonist activity at the A2AAR. Recent publications from our laboratory and others have made an extensive evaluation of both the known adenosine derivatives and those containing novel modification of both the ribose and adenine moieties [13–18, 10, 19]. A number of structural determinants for A3 AR activation have been identified, and lead to the conclusion that the efficacy of adenosine derivatives appears to be more dependent on smaller structural changes at A3 than at other subtypes. For example, 2-chloro-N6-cyclopentyladenosine (CCPA 4b) and 2-chloro- N6-R-phenylisopropyladenosine Cl-R-PIA 5b are full agonists at A1 ARs, but at the human A3AR they are an antagonist and a partial agonist, respectively. N6-[2-(3.5-dimethoxyphenyl)-2- (2-methylphenylethyl)] adenosine (DPMA 6), an agonist at A2AARs, is an antagonist at the human A3AR [15].
Partial agonists for A3 ARs could potentially be therapeutic agents for brain and heart ischemic conditions. It may be productive to explore simple and small substitutions that diminish efficacy and enhance affinity leading to selectivity. In this review, we describe the substitutions at the adenosine and adenosine derivatives that led to a number of high affinity and selective partial agonists showing a great variation in degree of agonism.
SUBSTITUTION OF THE ADENINE MOIETY AT 2- AND 8-POSITION
Analogues of the A1-selective agonist N6-cyclopentyl-adenosine (CPA 4a) having 8-alkyl substituents displayed both reduced affinity and efficacy at the A1AR [20–21]. At the A3 AR, 8-substitution greatly reduces affinity [22].
Both CCPA 4b and its 2-H analogue, CPA 4a, are potent agonists for human A1 ARs while having moderate binding affinities at the human A3AR. We investigated the activation by CPA and CCPA of the human A3AR stably transfected in Chinese hamster ovary (CHO) cells [14]. CPA inhibited forskolin-stimulated cyclic AMP production in CHO cells in a dose-dependent manner, corresponding to an EC50 value of 240 nM, but CCPA had no such effect, suggesting that CCPA might be an antagonist for the human A3AR. This was further demonstrated through the antagonism, by CCPA, of the effect of the potent A3 AR agonist Cl-IB-MECA 7b. CCPA shifted the Cl-IB-MECA dose-response curve to the right in a concentration-dependent manner. The differential effects of CPA and CCPA on the human A3AR were further demonstrated in a functional assay of phospholipase C (PLC) activity. CPA, but not CCPA, induced phosphoinositide turnover in intact CHO cells expressing the human A3AR in a concentration-dependent manner. Thus, the 2-substituted adenosine analogue, CCPA, was a moderately potent antagonist (Ki=38 nM) at the human A3AR.
Similar to CPA, R-PIA 5a was also a potent and selective agonist for A1 ARs; the 2-substituted analogue, 2-Cl-R-PIA 5b, was demonstrated to be less efficacious than its 2-H analogue [15]. 2-Chloroadenosine 9 was fully efficacious, however, 2-fluoroadenosine 8 displayed approximately 30% of the efficacy of the full agonist 2-chloroadenosine. IJzerman and coworkers also reported that the introduction of 2-chloro might decrease the efficacy of both N6- and N6,5’-di-substituted adenosine derivatives; this is in line with the above findings [18].
Previously, it was found that 2-fluoroadenosine acted as a partial agonist at the A2A AR [9]. The finding that 2-fluoroadenosine was a partial agonist for A3 ARs is consistent with, and an extension of that report. It was interesting that the 2-halo substitution was generally thought to enhance only the affinity of the ligands, little attention has been paid to the dramatic effects of 2-substitution to diminish the A3AR efficacy. However, it remains to be determined if most of the other adenosine derivatives currently used are full agonists for one subtype while at the same time being partial agonists, full agonists or antagonists for other AR subtypes. Also, it should be noted that a compound might be classified as a nearly full agonist, a partial agonist, or an antagonist in different tissues; the classification will also depend on the tissue in which the measurement is made [23].
N6-SUBSTITUTION OF THE ADENINE MOIETY
Actually, the first partial agonists for A3 ARs, such as N6-benzyladenosine, have existed long before they were recognized as such. These compounds might have been regarded as full agonists for the A3 AR because they are full agonists at other subtypes and N6-benzyl NECA 11 and other N6-benzyl-5’-N-alkylamide adenosine derivatives are known to be full agonists at A3 ARs [22, 19].
Recently, the activation of the human A3AR by a wide range of N6-substituted adenosine derivatives was studied in intact CHO cells stably expressing this receptor. A number of substitutions at the N6-position of the adenine moiety, including benzyl and large cycloalkyl groups, were found to decrease the maximum agonist efficacy at the A3AR. A chloro substituent was demonstrated to either increase or decrease the efficacy, depending on the position of substitution at the benzyl group [15]. Interestingly, DPMA 6, a potent agonist for the A2AAR, was demonstrated to be a moderately potent antagonist for the human A3AR (Ki=106 nM).
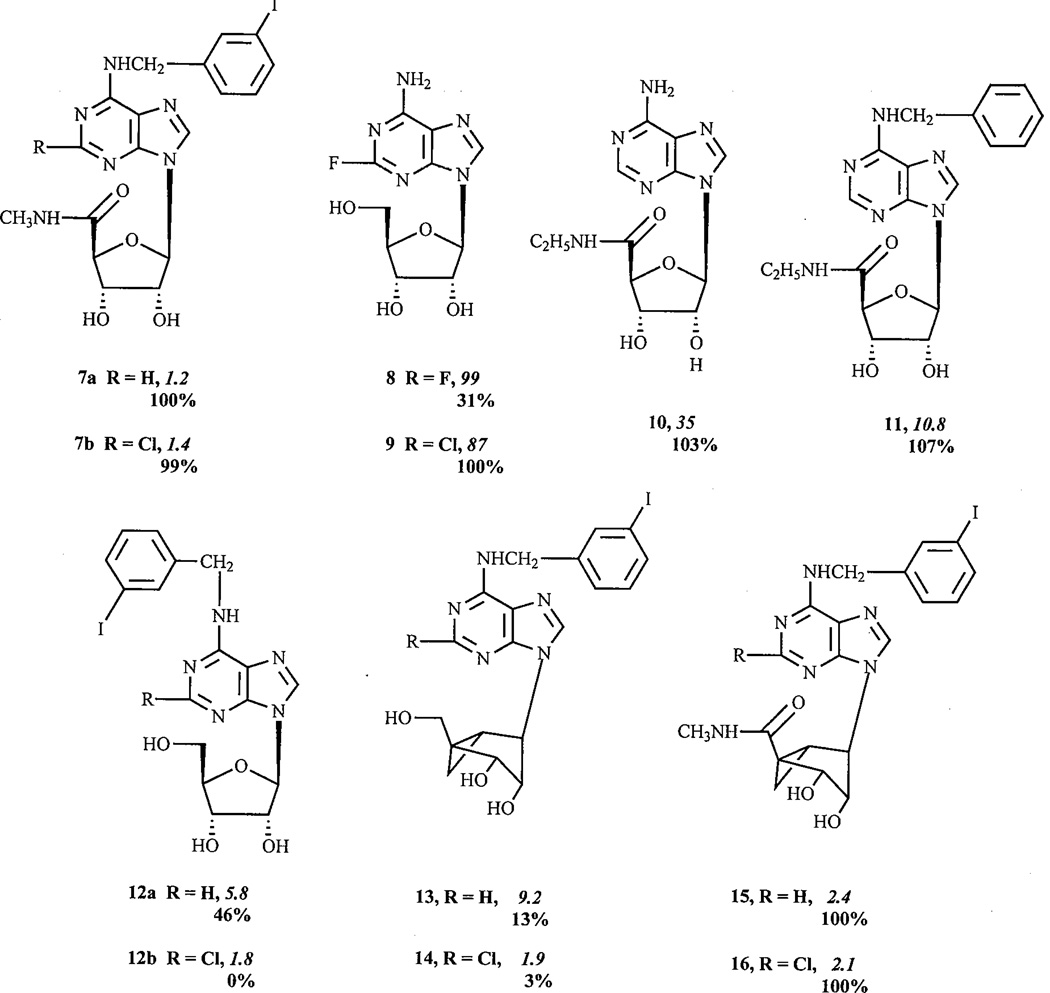
The N6-(3-iodobenzyl) group, which enhances the affinity for A3 ARs [22], may lead to a reduction of efficacy even in the absence of multiple substitutions of adenosine [17, 14, 13]. Thus, MRS 541 12a was a low-efficacy, partial agonist. The combination of 2-chloro and N6-substitution appears to reduce efficacy further than either modification alone. Thus, MRS 542 12b was demonstrated to be an antagonist with little, if any, detectable agonist activity.
The N6-cyclopentyl group alone did not reduce efficacy, however the combination of 2-chloro and N6-cyclopentyl for 4b in the present pharmacological system appeared to abolish agonism entirely [14].
The above-mentioned effects of reduction of efficacy are subject to reversal upon, at least certain, flexible 5’-substitutions of the molecule. The 5 ’-alkylamide group (either N-ethyl as in NECA 10, or N-methyl) appeared to restore efficacy. Comparison of pairs of compounds differing only in the presence of the 5’-alkylamide group demonstrates that the efficacy-preserving effect of this substituent takes precedence over other modifications that may reduce efficacy. Thus, IB-MECA 7a was more efficacious than MRS 541 12a (N6-(3-iodobenzyl)), Cl-IB-MECA 7b was more efficacious than MRS 542 12b (2-chloro and N6-(3-iodobenzyl)), MRS 1939 15 was more efficacious than MRS 1743 13 (N6-(3-iodobenzyl) and (N)-methanocarba), and MRS 1898 16 was more efficacious than MRS 1760 14 (2-chloro, N6-(3-iodobenzyl), and (N)-methanocarba). Also, the full agonism of MRS 1898 was consistent with the above patterns, since although this compound is a 2-chloro-(N)-methanocarba derivative, it also contains the 5’-N-methylamide group.
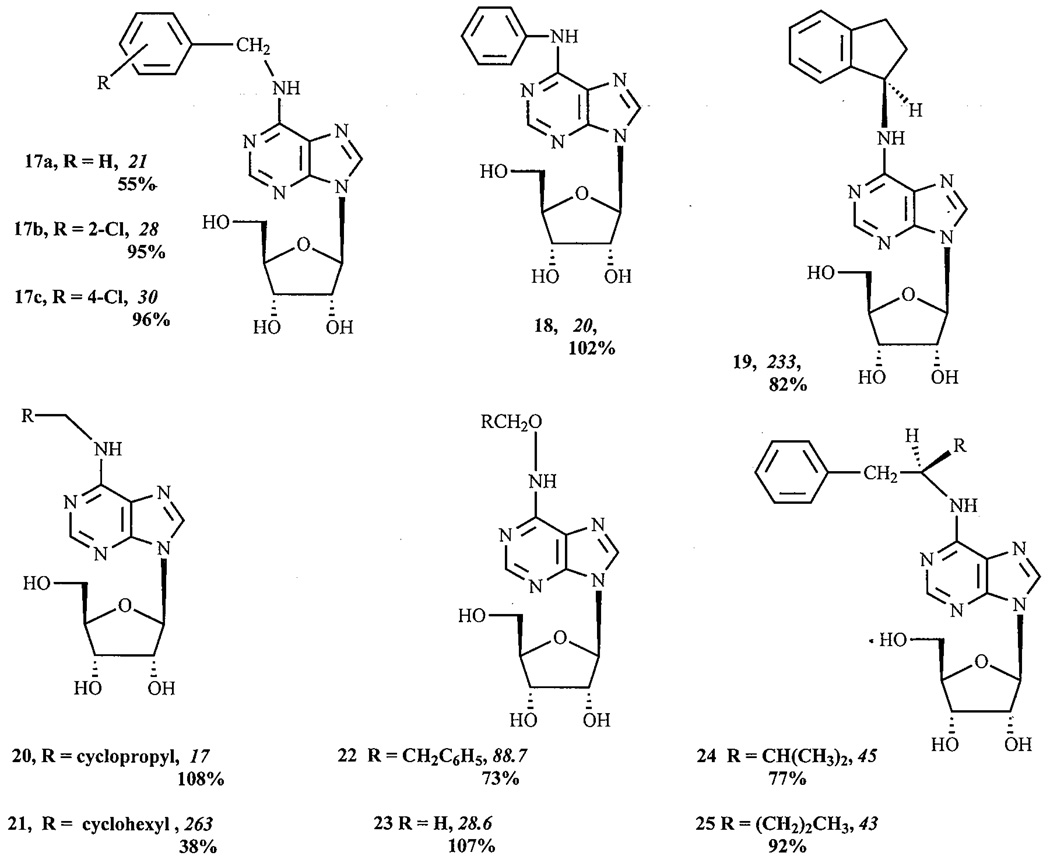
As described above, 2-chloro substitution of the adenine ring may both increase the affinity and decrease the efficacy of the adenosine derivatives for the human A3AR [18, 14, 13]. A further study demonstrated that a chloro substituent might alternately decrease, increase, or have no effect on the efficacy of the adenosine derivatives, depending on the position of substitution [15]. In contrast, a chloro substituent at the 2- or 4- positions (17b,c) of the benzyl group of N6-benzyladenosine 17a (itself having 55% of maximal efficacy) significantly increased the efficacy, converting the partial agonist N6-benzyladenosine into full agonists. Chloro substitution at the 3-position of the benzyl group also significantly increased the efficacy, although to a lesser extent. Similarly, a fluoro substituent at the 2-position of the benzyl ring also induced a modest increase in the efficacy. In contrast to the N6-benzyl group, which decreased the efficacy, the N6-phenyl group did not significantly influence the efficacy, thus N6-phenyladenosine 18 was still a full agonist. Also, similarly to Cl-IB-MECA 7b [13], the benzyl group did not influence the efficacy of NECA 10, and thus, N6-benzyl-NECA 11 [22] was a full agonist.
The degree of steric bulk present on the N6-substituent was correlated with loss of A3 efficacy. The appending of an additional cyclopropyl group to N6-cyclopropylmethyl adenosine 20 caused a slight change of its affinity and dramatically diminished its maximal A3AR efficacy. Similarly, bridging the methylene group in N6-(2-methylbenzyl) adenosine (the nonselective, full agonist metrifudil) to give N6-R-l-indanyl-adenosine 19, thus introducing a ring constraint, also reduced A3 efficacy. A comparison of the compounds N6-cyclopropylmethyl 20 and N6-cyclohexylmethyl 21 suggested that the enlargement of a ring attached to the N6-methyl group appeared to lower both affinity and efficacy at the human A3AR, while lengthening the chain between N6 and the phenyl group seemed to mainly decrease efficacy, with minimum efficacy observed with N6-benzyladenosine 17a. Compared with N6-(2-phenylethyl) adenosine, the introduction of an oxygen (hydroxylamine linkage; 2-phenylethoxy 22) seemed to induce a larger decrease in its affinity for all three AR subtypes and a smaller decrease of its maximal A3AR efficacy. This was in contrast to N6-methoxyadenosine 23, which showed enhanced potency at the rat A3AR as a result of the oxygen inclusion [24]. Branching al the terminal alkyl position (R-1-phenyl-isopentyl 24 versus R-1-phenyl-2-pentyl 25) did not change the A3AR affinity but diminished the A3AR efficacy.
Hence, within a narrow series of adenosine derivatives, careful examination of the structure activity relationship (SAR) of the N6-substituted group shows that a great variation in degree of agonism may be produced. From our studies, this series of adenosine derivatives displayed the entire range from 0% to 100% agonism.
SUBSTITUTIONS OF THE RIBOSE MOIETY
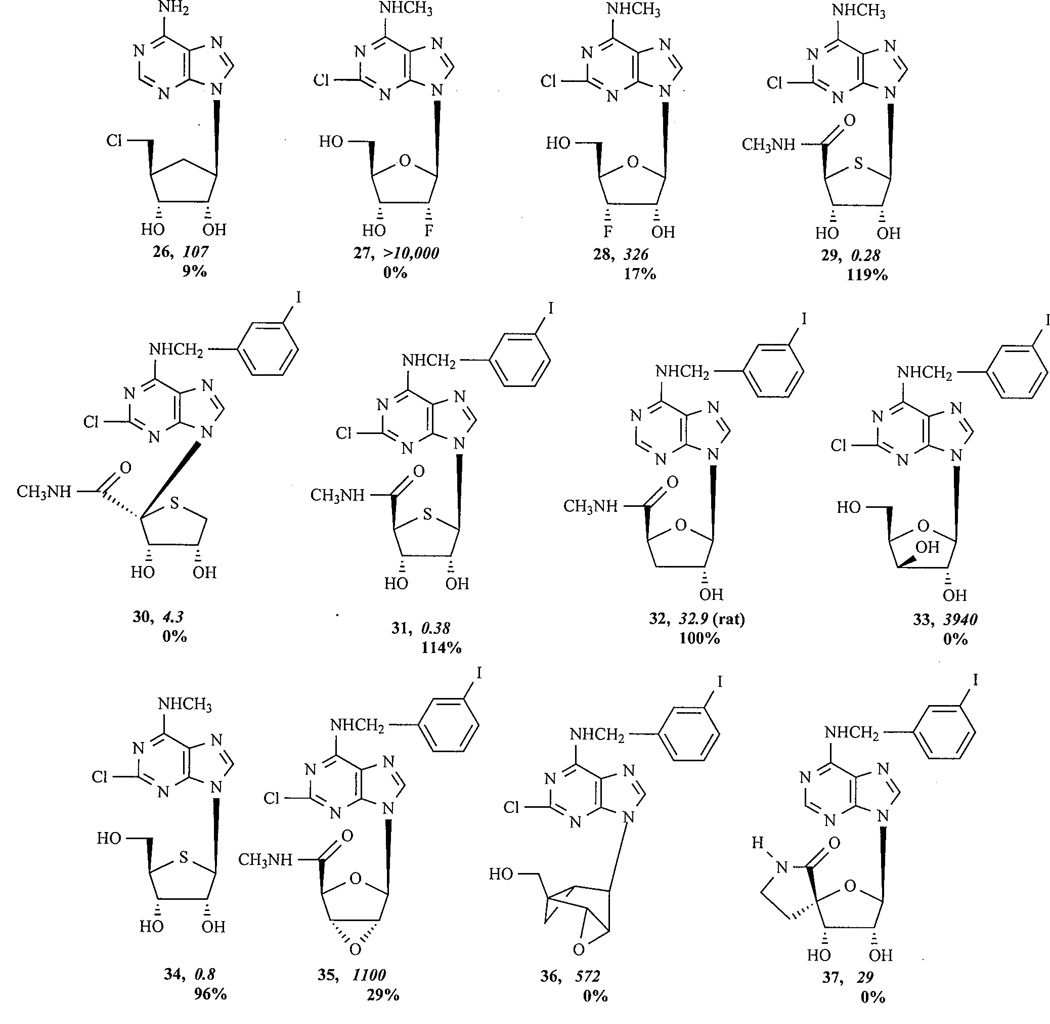
A number of strategies have been pursued to develop partial agonists for ARs by modifying the ribose moiety. A 5’-chloro-5’-deoxy substitution 26, already reported for adenosine agonists [24, 10], greatly reduced A3AR efficacy. Modifications at the ribose moiety of adenosine resulted in a number of A1-selective compounds with reduced A1AR agonist activity. Removal of the 2’- or 3’-hydroxyl group of full agonists resulted in a reduction of both affinity and efficacy [25]. The 2’-and 3’-hydroxy groups of adenosine and its derivatives are required for agonist activity and high affinity binding to the A1AR [26]. The respective 5’-modifications (including 5’-thioethers), in combination with N6-benzyl substituents known to increase A3AR affinity, resulted in partial agonists with high affinity at the human A3AR [17, 10]. Increasing the size of the 5’-substituents reduced the efficacy at A3 ARs [18, 10].
Consistent with earlier findings, it has recently been found that both 2'- and 3'-hydroxyl groups in the ribose moiety are essential for agonist binding and activation, with the 2'-hydroxyl being more critical [27, 16]. Thus, the 2'-fluoro substitution, as in 27, eliminated both binding and activation, while a 3'-fluoro substitution, as in 28, led to only a partial reduction of potency and efficacy at the A3AR. The diminished efficacy caused by 3'-fluoro could not be overcome with a 5'-amide [16]. The 4’-thio substitution generally enhanced potency [28]. The combined 4'-thio and 5’-amide substitution led to 29, the most potent and selective A3AR agonist (N6-methyl) yet reported [28]. The 4’-thio substitution enhanced or diminished the efficacy, depending on other substitutions. Interestingly, the shifting of the N6-(3-iodobenzyl)adenine moiety from the 1'- to 4'-position 30 had only a minor influence on its selectivity for the A3AR, but transformed the potent agonist 4’-thio substituted Cl-IB-MECA into a potent antagonist 31 (Ki=4.3 nM). This unusual nucleoside analogue antagonized agonist-induced inhibition of cyclic AMP production in A3AR-expressing CHO cells (KB=3.0 nM). However, a 3’-deoxyadenosine derivative 32 was still a full agonist for the A3AR with no affinity change [29].
The stereochemistry and substitution of the ribose hydroxyl groups greatly affected the intrinsic efficacy [16]. The arabino adenosine derivative 33 displayed greatly reduced A3AR binding affinity due to inversion of stereochemistry at the 3’-carbon. Like its stereoisomer, MRS 542 12b, it did not display agonist action at the human A3AR. The 2’-fluoro substitution of MRS 542 eliminated human A3AR binding. The 3’-fluoro substitution of MRS 542 allowed a greater degree of agonism than at the 2’-position. The binding affinity of the corresponding 3’-fluoro analogue was weaker than for MRS 542 12b itself, although it remained an antagonist. The partial agonism of 3’-fluoro analogues could not be overcome (i.e., full agonism restored) with the 5’-amide modification, unlike previous findings with partial agonists having both 2’- and 3’-hydroxyl groups [13]. The effect of unsurmountable diminished agonism at the A3AR in 3’-fluoro analogues was even greater when the N6-(3-iodobenzyl) group was present, as in the antagonist 2-chloro-N6-(3-iodobenzyl)-5'-N-methylcarbamoyl-3’-fluoro-3’-deoxyadenosine [16].
The 4’-thio modification generally enhanced potency, yet decreased efficacy. The N6-methyl group counteracted the potency-reducing effect of a 2-chloro group; thus, 2-chloro-4’-thio-N6-methyl adenosine 34 was a very potent full agonist. The 4’-thio group increased efficacy in comparison to its oxygen analogue, MRS 542. Consistent with the ability of a flexible 5’-amide group to overcome various efficacy-reducing structural changes [13], the 4’-thio substituted Cl-IB-MECA 31 was still a full agonist. 2’,3’-Epoxide derivatives, in both riboside 35 and (N)-methanocarba 36 series, and a cyclized 4’,5’-uronamide derivative MRS 1292 37 were antagonists [13]. Although the 5’-uronamide structure is present in MRS 1292, it is cyclic and, therefore, sterically rigid and does not restore efficacy.
REPLACEMENT OF THE RIBOSE RING SYSTEM
A carbocyclic modification of the ribose moiety incorporating ring constraints is a general approach for the design of A1 and A3 AR agonists having favorable pharmacodynamic properties. While simple carbocyclic substitution of adenosine agonists greatly diminishes potency, methanocarba-adenosine analogues (e.g. 13 – 16 and 38, tend to have greater potency. Such analogues having conformationally constrained bicyclic rings) have defined the role of sugar puckering in stabilizing the active AR-bound conformation and thereby have allowed identification of a favored isomer. In such analogues a fused cyclopropane moiety constrains the pseudosugar ring of the nucleoside to either a Northern (N) or Southern (S) conformation, as defined in the pseudorotational cycle. In binding assays at A1, A2A, and A3 ARs, (N)-methanocarba-adenosine was of higher affinity than the (S)-analogue, particularly at the human A3 AR (N/S affinity ratio of 150). We have studied the affinity and efficacy of (N)-methanocarba analogues of various N6-substituted adenosine derivatives, including cyclopentyl and 3-iodobenzyl, in which the parent compounds are potent agonists at either A1 or A3 ARs, respectively [30].
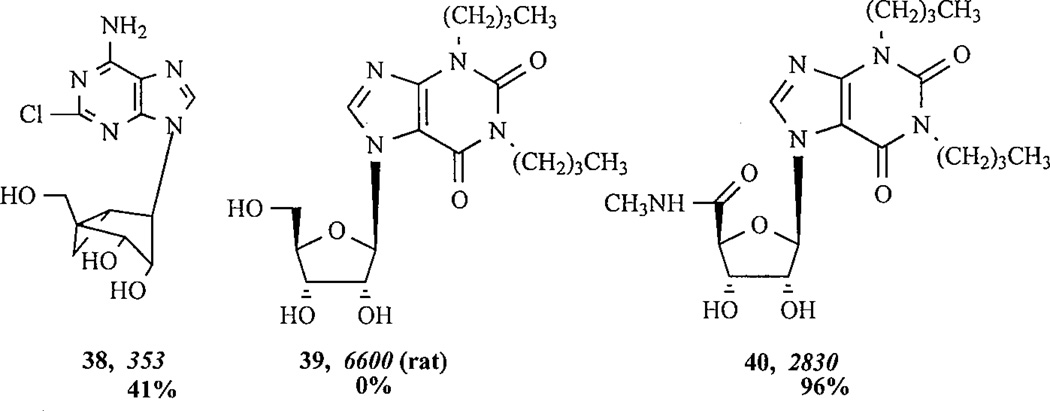
The (N)-methanocarba substitution of the ribose ring, which results in a rigidification of an A3-AR preferred conformation of this moiety [31], while preserving and/or enhancing binding selectivity, also appears to reduce efficacy except when a 5’-uronamide was also present. Thus, the 2-H analogue, (N)-methanocarba-N6-(3-iodobenzyl) adenosine (MRS 1743 13), was less efficacious than the corresponding riboside, MRS 541 12a. Similarly, in the simple 2-chloro case, MRS 1873 38 was less efficacious than CADO 9. The 5’-uronamides MRS 1898 16 and MRS 1939 15 are fully efficacious. MRS 1743 13 and its 2-chloro derivative 14 had Ki values of 9.2 and 1.9 nM at the human A3AR, respectively, and were highly selective partial agonists [31,13]. The 5'-uronamide modification preserved, i.e. N6-(3-iodobenzyl), or enhanced, i.e. N6-methyl, affinity at the human A3AR, while the 2'-deoxy modification reduced affinity and efficacy in a functional assay [32].
SUBSTITUTION OF ADENINE MOIETY WITH XANTHINE
Adenosine is the endogenous agonist for all four subtypes of ARs, and ribose is the basic structure of the adenosine and adenosine derivatives. In an effort to design partial agonists for ARs, several xanthine derivatives (antagonists) have been used to replace the adenine moiety of adenosine and adenosine derivatives.
Theophylline-7-riboside was one of the first partial agonists identified for ARs [6]. Later, 7-β-D-ribofuranosylxanthine was found to have higher affinity and greater selectivity for the A1 AR than previously reported xanthine nucleosides, and to be a partial agonist [33].
1,3-Dibutylxanthine-7-riboside 39 has been found to be a partial agonist at rat A3 ARs [22] with moderate potency (Ki=6 µM). l,3-Dialkylxanthine-7-riboside analogues modified at the 1-, 3-, and 8-purine positions and at the ribose 5'-position were synthesized and examined for affinity at A3 ARs stably expressed in CHO cells [34]. The affinity of xanthine 7-ribosides at A3 ARs depended on the 1,3-dialkyl substituents in the order: Pent > or = Bu ≫ Hx > Pr, Me. 1,3-Dipentylxanthine 7-riboside was slightly selective for A3 ARs (2-fold versus A1 and 10-fold versus A2A). 8-Methoxy substitution was tolerated at A3 ARs. 2-Thio versus 2-oxo substitution increased potency at all three subtypes and slightly increased A3 versus A1 selectivity. The 5'-uronamide modification, which was previously found to enhance A3 selectivity in N6-benzyladenosine derivatives, was also incorporated into the xanthine 7-ribosides, with similar results. The affinity of 1,3-dialkylxanthine 7-riboside 5'-uronamides at A3 ARs depended on the N-alkyluronamide substituent in the order: MeNH > EtNH ≫ NH2 ≫ Me2N. Thus, 1,3-dibutylxanthine 7-riboside 5'-N-methylcarboxa-mide (DBXRM 40) was the most potent and A3-selective in the series. Affinity of the 5'-uronamides at A3 ARs was dependent on the 1,3-dialkyl substitution in the order: Bu > Pent > Hex. DBXRM, with a Ki, value of 229 nM at A3 ARs, was 160-fold selective for rat A3 versus A1 ARs and > 400-fold selective versus A2A ARs. This derivative acted as a full agonist in the rat A3AR-mediated inhibition of adenylate cyclase [34]. Consistent with this study, it was later demonstrated that DBXRM was a full agonist for the human A3AR [13], thus there was no species difference in its observed efficacy.
CONCLUSION
The identification of critical structural determinants for A3AR activation should prove useful for further understanding the mechanism of receptor activation and development of more potent and selective full agonists, partial agonists and antagonists for A3ARS. Novel and unique ligands may be identified by optimizing the selectivity, efficacy and binding affinity of the adenosine derivatives at different AR subtypes.
Chart 2.
Chart 3.
Chart 4.
ACKNOWLEDGEMENTS
We thank Joshua Blaustein (NIDDK) for carefully reading and correcting this manuscript.
Biography

CHARTS 1 – 5: CHEMICAL STRUCTURES OF ADENOSINE DERIVATIVES DESCRIBED IN THE TEXT
Chart 1.
Chart 5.
After each structure number are given pharmacological parameters at the human A3AR, i.e. the Ki value (nM) in binding experiments (italics) and the % maximal efficacy observed at a concentration of 10 µM.
Footnotes
Zablocki et al., this volume.
REFERENCES
- 1.Fredholm BB, IJzerman AP, Jacobson KA, Klotz KN, Linden J. International Union of Pharmacology. XXV. Nomenclature and classification of ARs. Pharmacol. Rev. 2001;53:527–552. [PMC free article] [PubMed] [Google Scholar]
- 2.Mathot RA, van Schaick EA, Langemeijer MW, Soudijn W, Breimer DD, IJzerman AP, Danhof M. Pharmacokinetic-pharmacodynamic relationship of the cardiovascular effects of adenosine A1 receptor agonist N6-cyclopentyladenosine in the rat. J. Pharmacol. Exp. Ther. 1994;268:616–624. [PubMed] [Google Scholar]
- 3.Danhof M, Mandema JW, Hoogerkamp A, Mathot RA. Pharmacokinetic-pharmacodynamic modeling in pre-clinical investigations: principles and perspectives. Eur. J. Drug. Metab. Pharmacokinet. 1993;18:41–47. doi: 10.1007/BF03220007. [DOI] [PubMed] [Google Scholar]
- 4.Wu L, Belardinelli L, Zablocki JA, Palle V, Shryock JC. A partial agonist of the A1-AR selectively slows AV conduction in guinea pig hearts. Am. J. Physiol. Heart Circ. Physiol. 2001;280:H334–H343. doi: 10.1152/ajpheart.2001.280.1.H334. [DOI] [PubMed] [Google Scholar]
- 5.Barlow JJ, Main BG, Snow HM. Beta-adrenoceptor stimulant properties of amidoalkylamino-substituted l-aryl-2-ethanols and 1-(aryloxy)-2-propanols. J. Med. Chem. 1981;24:315–322. doi: 10.1021/jm00135a015. [DOI] [PubMed] [Google Scholar]
- 6.IJzerman AP, van der Wenden EM, von Frijtag Drabbe Künzel JK, Mathot RA, Danhof M, Borea PA, Varani K. Partial agonism of theophylline-7-riboside on adenosine receptors. Naunyn Schmiedebergs Arch. Pharmacol. 1994;350:638–645. doi: 10.1007/BF00169369. [DOI] [PubMed] [Google Scholar]
- 7.Mathot RA, Van der Wenden EM, Soudijn W, IJzerman AP, Danhof M. Deoxyribose analogues of N6-cyclopentyladenosine (CPA): partial agonists at the adenosine A1 receptor in vivo. Br. J. Pharmacol. 1995;116:1957–1964. doi: 10.1111/j.1476-5381.1995.tb16398.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van der Wenden EM, Carnielli M, Roelen HC, Lorenzen A, von Frijtag Drabbe Künzel JK, IJzerman AP. 5'-substituted adenosine analogs as new high-affinity partial agonists for the adenosine A1 receptor. J. Med. Chem. 1998;41:102–108. doi: 10.1021/jm970508l. [DOI] [PubMed] [Google Scholar]
- 9.Daly JW, Padgett W. Agonist activity of 2- and 5’-substituted adenosine analogs and their N6-cycloalkyl derivatives at A1 and A2-ARs coupled to adenylate cyclase. Biochem. Pharmacol. 1992;43:1089–1093. doi: 10.1016/0006-2952(92)90616-q. [DOI] [PubMed] [Google Scholar]
- 10.van Tilburg EW, Gremmen M, von Frijtag Drabbe Künzel JK, de Groote M, IJzerman AP. 2,5'-Disubstituted adenosine derivatives: evaluation of selectivity and efficacy for the adenosine A1, A2A, and A3 receptor. J. Med. Chem. 2002;45:420–429. doi: 10.1021/jm010952v. [DOI] [PubMed] [Google Scholar]
- 11.van Tilburg EW, Gremmen M, von Frijtag Drabbe Künzel JK, de Groote M, IJzerman AP. 2,8-Disubstituted adenosine derivatives as partial agonists for the adenosine A2A receptor. Bioorg. Med. Chem. 2003;11:2183–2192. doi: 10.1016/s0968-0896(03)00123-8. [DOI] [PubMed] [Google Scholar]
- 12.Hutchinson SA, Baker SP, Scammells PJ. New 2, N6-disubstituted adenosines: potent and selective A1 adenosine receptor agonists. Bioorg. Med. Chem. 2002;10:1115–1122. doi: 10.1016/s0968-0896(01)00384-4. [DOI] [PubMed] [Google Scholar]
- 13.Gao ZG, Kim SK, Biadatti T, Chen W, Lee K, Barak D, Kim SG, Johnson CR, Jacobson KA. Structural determinants of A3 adenosine receptor activation: Nucleoside ligands at the agonist/antagonist boundary. J. Med. Chem. 2002;45:4471–4484. doi: 10.1021/jm020211+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gao ZG, Jacobson KA. 2-Chloro-N6-cyclopentyladenosine, adenosine A1 receptor agonist, antagonizes the adenosine A3 receptor. Eur. J. Pharmacol. 2002;443:39–42. doi: 10.1016/s0014-2999(02)01552-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gao ZG, Blaustein JB, Gross AS, Melman N, Jacobson KA. N6-Substituted adenosine derivatives: selectivity, efficacy, and species differences at A3 adenosine receptors. Biochem. Pharmacol. 2003;55:1675–1684. doi: 10.1016/s0006-2952(03)00153-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gao ZG, Jeong LS, Moon HR, Kim HO, Choi WJ, Shin DH, Elhalem E, Comin MJ, Melman N, Mamedova L, Gross AS, Rodriguez JB, Jacobson KA. Structural determinants of efficacy at A3 adenosine receptors: Modification of the ribose moiety. Biochem. Pharmacol. 2004;67:893–901. doi: 10.1016/j.bcp.2003.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Tilburg EW, von Frijtag Drabbe Künzel J, de Groote M, Vollinga RC, Lorenzen A, IJzerman AP. N6,5'-Disubstituted adenosine derivatives as partial agonists for the human adenosine A3 receptor. J. Med. Chem. 1999;42:1393–1400. doi: 10.1021/jm981090+. [DOI] [PubMed] [Google Scholar]
- 18.van Tilburg EW, van der Klein PA, von Frijtag Drabbe Künzel JK, de Groote M, Stannek C, Lorenzen A, IJzerman AP. 5'-O-alkyl ethers of N,2-substituted adenosine derivatives: partial agonists for the adenosine A1 and A3 receptors. J. Med. Chem. 2001;44:2966–2975. doi: 10.1021/jm001114o. [DOI] [PubMed] [Google Scholar]
- 19.Kim HO, Ji XD, Siddiqi SM, Olah ME, Stiles GL, Jacobson KA. 2-Substitution of N6-benzyladenosine-5'-uronamides enhances selectivity for A3- adenosine receptors. J. Med. Chem. 1994;37:3614–3621. doi: 10.1021/jm00047a018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roelen H, Veldman N, Spek AL, von Frijtag Drabbe Künzel JK, Mathot RA, IJzerman AP. N6,C8-distributed adenosine derivatives as partial agonists for adenosine A1 receptors. J. Med. Chem. 1996;39:1463–1471. doi: 10.1021/jm950267m. [DOI] [PubMed] [Google Scholar]
- 21.van Schaick EA, Tukker HE, Roelen HC, IJzerman AP, Danhof M. Selectivity of action of 8-alkylamino analogues of N6-cyclopentyladenosine in vivo: haemodynamic versus anti-lipolytic responses in rats. Br. J. Pharmacol. 1998;124:607–618. doi: 10.1038/sj.bjp.0701868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Van Galen PJM, van Bergen AH, Gallo-Rodriguez C, Melman N, Olah ME, IJzerman AP, Stiles GL, Jacobson KA. A binding site model and structure-activity relationships for the rat A3 adenosine receptor. Mol. Pharmacol. 1994;45:1101–1111. [PMC free article] [PubMed] [Google Scholar]
- 23.Kenakin TP. The Pharmacologic Analysis of Drug-Receptor Interactions. Raven, Philadelphia: LIPPINCOTT; 1997. [Google Scholar]
- 24.Mogensen JP, Roberts SM, Bowler AN, Thomsen C, Knutsen LJ. The synthesis of new adenosine A3 selective ligands containing bioisosteric isoxazoles. Bioorg. Med. Chem. Lett. 1998;8:1767–1770. doi: 10.1016/s0960-894x(98)00302-3. [DOI] [PubMed] [Google Scholar]
- 25.van der Wenden EM, von Frijtag Drabbe Künzel JK, Mathot RA, Danhof M, IJzerman AP, Soudijn W. Robose-modified adenosine analogues as potential partial agonists for the AR. J. Med. Chem. 1995;38:4000–4006. doi: 10.1021/jm00020a014. [DOI] [PubMed] [Google Scholar]
- 26.Lohse MJ, Klotz KN, Schwabe U, Cristalli G, Vittori S, Grifantini M. 2-Chloro- N6-cyclopentyladenosine: a highly selective agonist at A1 adenosine receptors. Naunyn Schmiedebergs Arch. Pharmacol. 1988;337:687–689. doi: 10.1007/BF00175797. [DOI] [PubMed] [Google Scholar]
- 27.Lim MH, Kim HO, Moon HR, Lee SJ, Chun MW, Gao ZG, Melman N, Jacobson KA, Kim JH, Jeong LS. Design, synthesis and binding affinity of 3'-fluoro analogues of Cl-IB-MECA as adenosine A3 receptor ligands. Bioorg. Med. Chem. Lett. 2003;13:817–820. doi: 10.1016/s0960-894x(03)00027-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jeong LS, Jin DZ, Moon HR, Kim HO, Chun MW, Melman N, Gao ZG, Jacobson KA. N6-Substituted D-4'-thioadenosine-5'-methyluronamides: Potent and selective agonists at the human A3 adenosine receptor. J. Med. Chem. doi: 10.1021/jm034098e. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jacobson KA, Siddiqi SM, Olah ME, Ji XD, Melman N, Bellamkonda K, Meshulam Y, Stiles GL, Kim HO. Structure-activity relationships of 9-alkyladenine and ribose-modified adenosine derivatives at rat A3 adenosine receptors. J. Med. Chem. 1995;35:1720–1735. doi: 10.1021/jm00010a017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jacobson KA, Ji X, Li AH, Melman N, Siddiqui MA, Shin KJ, Marquez VE, Ravi RG. Methanocarba analogues of purine nucleosides as potent and selective AR agonists. J. Med. Chem. 2000;43:2196–2203. doi: 10.1021/jm9905965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jacobson KA, Ji X, Li AH, Melman N, Siddiqui MA, Shin KJ, Marquez VE, Ravi RG. Methanocarba analogues of purine nucleosides as potent and selective AR agonists. J. Med. Chem. 2000;43:2196–2203. doi: 10.1021/jm9905965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee K, Ravi G, Ji XD, Marquez VE, Jacobson KA. Ring-Constrained (N)-methanocarba nucleosides as adenosine receptor agonists: independent 5'-uronamide and 2'-deoxy modifications. Bioorg. Med. Chem. Lett. 2001;11:1333–1337. doi: 10.1016/s0960-894x(01)00213-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bridson PK, Lin X, Melman N, Ji XD, Jacobson KA. Synthesis and AR affinity of 7-β-D-ribofuranosylxanthine. Nucleosides Nucleotides. 1998;17:759–768. doi: 10.1080/07328319808004673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim HO, Ji XD, Melman N, Olah ME, Stiles GL, Jacobson KA. Selective ligands for rat A3 adenosine receptors: structure-activity relationships of 1,3-dialkylxanthine 7-riboside derivatives. J. Med. Chem. 1994;37:4020–4030. doi: 10.1021/jm00049a021. [DOI] [PMC free article] [PubMed] [Google Scholar]