Abstract
The penetration of the adenosine antagonists 8-cyclopentyl-1,3-dimethylxanthine (CPT), 8-cyclopentyl-1,3-dipropylxanthine (CPX), 8(p-sulfophenyltheophylline (8-PST), and 8-[4-[[[[(2-aminoethyl) amino]carbonyl]methyl]oxy]phenyl]-l,3-dipropylxanthine (XAC) into mouse brain was determined using ex vivo binding and locomotor studies. CPT and CPX (25 and 0.25 mg/kg, respectively) both penetrated into brain in substantial amounts: 49 and 17% of theoretical levels assuming free penetration throughout the body, 10 min after i.p. injection, respectively. Brain levels of CPT decreased rapidly, declining to undetectable levels by 30 min post-injection, whereas levels of CPX declined much more slowly. As expected, no detectable brain levels of 8-PST were found following i.p. injection of 50 mg/kg. XAC (20 mg/kg) penetrated into brain poorly: 1.6% after 10 min and 3.2% 20 min post-injection. The ability of CPT to stimulate locomotor activity paralleled the brain levels, i.e. it was similar to theophylline at short times and the effect rapidly diminished. These studies demonstrate the usefulness of ex vivo binding in determining CNS penetration of adenosine receptor ligands.
Caffeine, theophylline, and other alkylxanthines act as behavioral stimulants [1–3] and have been characterized biochemically as antagonists of adenosine, acting at A1 and A2 adenosine receptors. Xanthines have also been shown to reverse the behavioral depression elicited by adenosine agonists. The potency of xanthine derivatives in locomotor assays, both as stimulants and as antagonists of agonist-induced depression, is often not commensurate with their potency as adenosine antagonists. Although synthetic analogues of theophylline and various non-xanthine; heterocycles that have 104-fold or greater potency than theophylline in adenosine receptor affinity have been reported [4–6], the relationship between behavioral stimulation and adenosine receptor antagonism is unclear. To adequately characterize this relationship in vivo, we have measured the blood-brain barrier penetrability and half-life in the CNS of peripherally administered adenosine antagonists.
Ex vivo binding has been used to measure the ability of a variety of drugs to penetrate into the CNS following intraperitoneal injection [7–9]. Despite its ease and rapidity, this technique has yet to be used in assessing the CNS penetration of adenosine antagonists. The following report describes the time course for CNS penetration of four adenosine antagonists, 8-cyclopentyl-1,3-dimethylxanthine(CPT§),8-cyclopentyl-l,3-dipropylxanthine (CPX), 8-p-sulfophenyltheophylline (8-PST), and 8-[4-[[[[(2-aminoethyl)amino]carbonyl]methyl]oxy]-phenyl]-1,3-dipropylxanthine (XAC). XAC, CPT, and CPX are A1-selective in the rat brain by factors of 53, 130, and 460, respectively [5, 6]. CPX and XAC are of nanomolar affinity at rat and mouse A1 receptors, and have been characterized as radioligands [10, 11]. 8-PST is of lesser potency and selectivity, but has been utilized as a peripherally-selective xanthine [12, 13].
MATERIALS AND METHODS
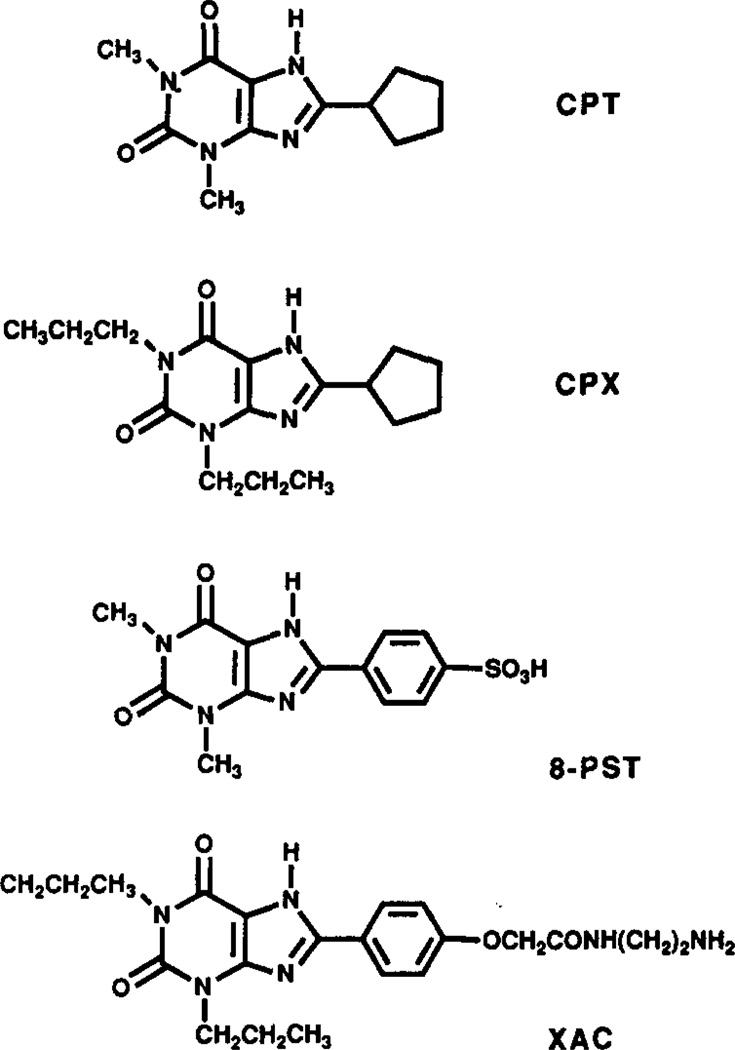
Compounds were obtained from the following sources: CPX, CPT, and 8-PST were from Research Biochemicals, Inc. (Natick, MA). Their structures are shown in Fig. 1. R-[3H]Methyl-2-phenylethyladenosine ([3H]PIA, 42.5 Ci/mmol), [3H]-8-cyclopentyl-1,3-dipropylxanthine ([3H]CPX, 109 Ci/mmol), and [methyl-14C] bovine serum albumin (BSA) (0.024 mCi/mg) were from NEN-DuPont (Boston, MA). XAC was synthesized as described [5]. Before injection in the mice, XAC was repurified by isocratic reverse-phase HPLC to eliminate potential minor contaminants not detected by thin-layer chromatography. Aliquots of a saturated solution of XAC (0.1 mL, 125/mL) in dimethylformamide were applied to a Vydac C4-protein column (1 × 25 cm), using a mobile phase of 30% acetonitrile in water, containing 0.1% trifluoroacetic acid, at a flow rate of 3 mL/min. The combined purified XAC trifluoroacetate fractions (retention time 7 min) were reduced in volume by evaporation and lyophilized to provide a 78% recovery.
Fig. 1.
Adenosine antagonists structurally related to theophylline.
Male Swiss-Webster mice (20–25 g) were used in these studies. Mice were injected intraperitoneally with 100 µL of test compound. Basic stock solutions of compounds were made in 0.01 N NaOH and then diluted as needed with 0.9% NaCl. After the indicated time, mice were decapitated following ether anesthesia, and the brains were removed rapidly. The cerebellum and hindbrain (pons and medula) of each were discarded, and the remainder of the brain was weighed and homogenized in a teflon-glass homogenizer in 8 vol. of 50 mM Tris-HCl, pH 7.4. Adenosine deaminase (ADA; Sigma, Type VI from calf intestine) was added (6 U/mL of homogenate), and the homogenate was incubated at 37° for 45–60min. Aliquots of this homogenate were used for binding studies.
Binding studies were performed by incubating a 50-µL aliquot of homogenate with 2 nM [3H]PIA and 50 mM Tris buffer (pH 7.4) in a total volume of 250 µL for 60 min at 37°. Following rapid filtration over GF/B filters, the filters were washed three times with the Tris buffer, then equilibrated with scintillation counting fluid and counted. Non-specific binding was determined by co-incubation with 20 µM 2-chloroadenosine. This amounted to less than 4% of the total [3H]PIA bound and was routinely subtracted. Ki values for the xanthine derivatives were determined using the Cheng-Prusoff equation [14]. Standard curves were constructed by incubating brain homogenate from untreated mice with various concentrations of inhibitor using the identical conditions as above. Each determination was performed in quadruplicate using at least three different animals.
The percent brain penetration of test compounds was determined by comparing the amount of inhibition of [3H]PIA binding in homogenates from treated and control brain. By comparing this percent inhibition with that obtained from the standard curve, the concentration of compound entering the brain was determined. One hundred percent brain penetration was defined as the amount of compound reaching the brain in the absence of any permeability barrier, assuming that the injected dose distributed uniformly throughout the mouse. Each determination was performed in quadruplicate and data are expressed as means ± SD from at least three different animals.
Individual adult male mice of NIH (Swiss) strain between 5 and 6 weeks of age weighing at least 25 g were studied in a Digiscan activity monitor (Omnitech Electronics Inc., Columbus, OH) with computerized data analysis (ILAM software). Animals were placed in the cage immediately after injection, and monitoring for three intervals of 10 min each was initiated after 10 min. Total distance travelled was used as a measure of locomotor activity. Xanthines were dissolved in a 20:80 (v/v) mixture of Emulphor EL-620 (GAF Chemicals Corp., Wayne, NJ) and phosphate-buffered saline and injected i.p. in a volume of 5 mL/kg body weight. Control values for vehicle-injected animals were determined for each experiment.
RESULTS
Scatchard analysis of [3H]PIA binding to membranes from crude homogenates treated with 6 U/mL of ADA revealed two affinities: a high-affinity site with a Kd of 1.5 ± 0.2 nM (N = 3) and a Bmax of 398 ± 29 fmol/mg protein, as well as a site of much lower affinity. The high affinity observed for R-PIA at A1-adenosine receptors compares favorably with values previously published [6].
Omission of ADA resulted in a higher Kd (4.5 nM) and lower Bmax (250fmol/mg), consistent with the assumption that endogenous adenosine, present even in the membrane preparation, partially occupies the receptor. To demonstrate that this difference was not an artifact reduced by the presence of ADA (for example, [3H]PIA binding hypothetically to the enzyme, which may be immobilized in the cell membrane), we treated membranes from PC12 cells, that do not contain A1-adenosine receptors, with ADA (1.5 mg of membranes in 0.5 mL buffer treated with 6 U of enzyme for 60 min at 37°) and found no resulting increase in binding. These results further substantiate the idea that the added ADA is removing endogenous adenosine and not providing an additional binding site for the radioligand.
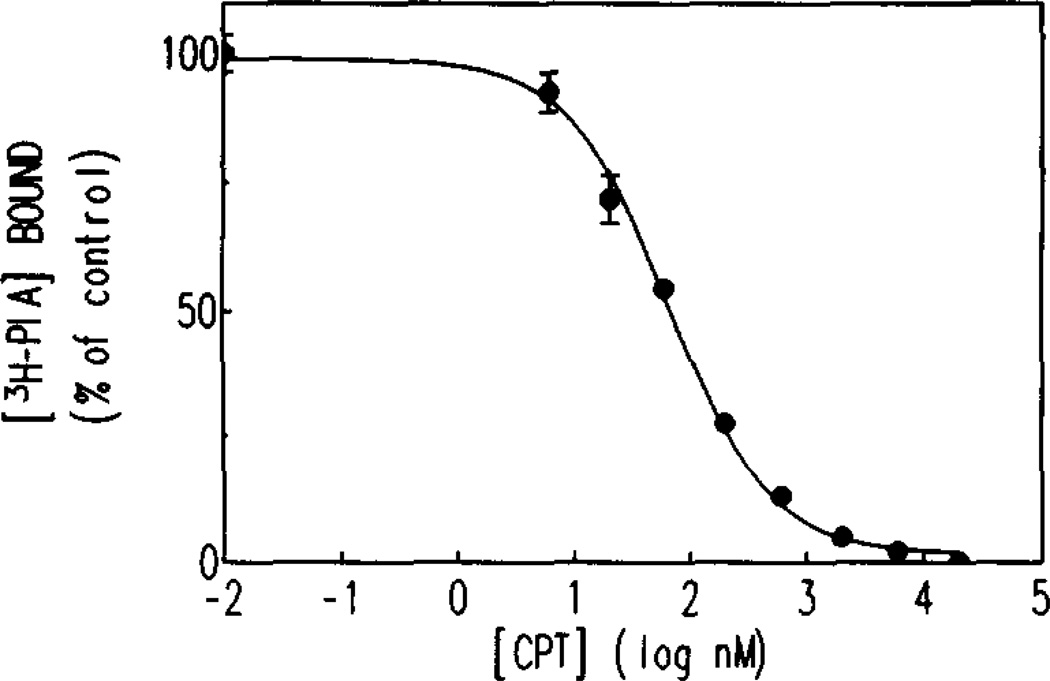
Inhibition of [3H]PIA binding to brain homogenates by xanthine derivatives occurred with Ki values consistent with A1-adenosine receptor affinities (8-PST, 2.6 µM; CPT, 28 nM; CPX, 4.1 nM; XAC, 8.2 nM). An example of a competition curve for CPT is shown in Fig. 2. The detection limits for ex vivo binding are related to these Ki values. Since brain tissue was diluted a total of 40-fold by homogenization and dilution for binding studies, a concentration of compound 40 times its Ki value would be readily detectable in this ex vivo assay. The detection limits of this method depend on the brain penetrability of the compound being measured: a highly permeable compound will have a detection limit of around four times its Ki value, at which concentration a 10% inhibition in binding would be observed. In this connection, under control conditions, total [3H]PIA binding amounted to around 5500cpm per 50-µL aliquot tested, with about 150cpm of this being non-specific binding. Since the maximal inhibition of binding caused by injection of 25 mg/kg of CPT (93% inhibition) still left about 350 cpm of specific binding, sufficient counts remained to allow for reasonably accurate estimates of drug concentration.
Fig. 2.
Competition curve for CPT inhibition of [3H]PIA binding to homogenate of mouse brain. Homogenate was treated with ADA (6 U/mL), and then incubated with 2nM [3H]PIA in 50 mM Tris-HCl buffer (pH 7.4) for 60 min at 37°. Data points represent averages of duplicate values and the line is a non-linear regression analysis of the data. In the absence of CPT, specific binding amounted to about 5500 cpm (100% of control).
To demonstrate that the xanthines detected in brain homogenate of treated animals were primarily from brain rather than from the brain vasculature, the following experiments were undertaken to measure the approximate contribution of brain vasculature to total brain volume. Mice were injected i.v. (tail vein) with [14C]BSA (0.1 µCi/animal) and after 15 min, radioactivity levels in serum and in forebrain were determined. At this time, 100 µL of serum contained 11,041 ± 832 cpm(average ± range, N = 2), whereas the entire forebrain (approximate mass 0.32 g) contained only 411 ± 48 cpm (average ± range, N = 2), indicating that the vascular space is extremely small (roughly 1%) compared to brain volume. Therefore, the levels of xanthines detected by the ex vivo binding procedure (>l% of theoretical) represent primarily those from brain rather from vasculature.
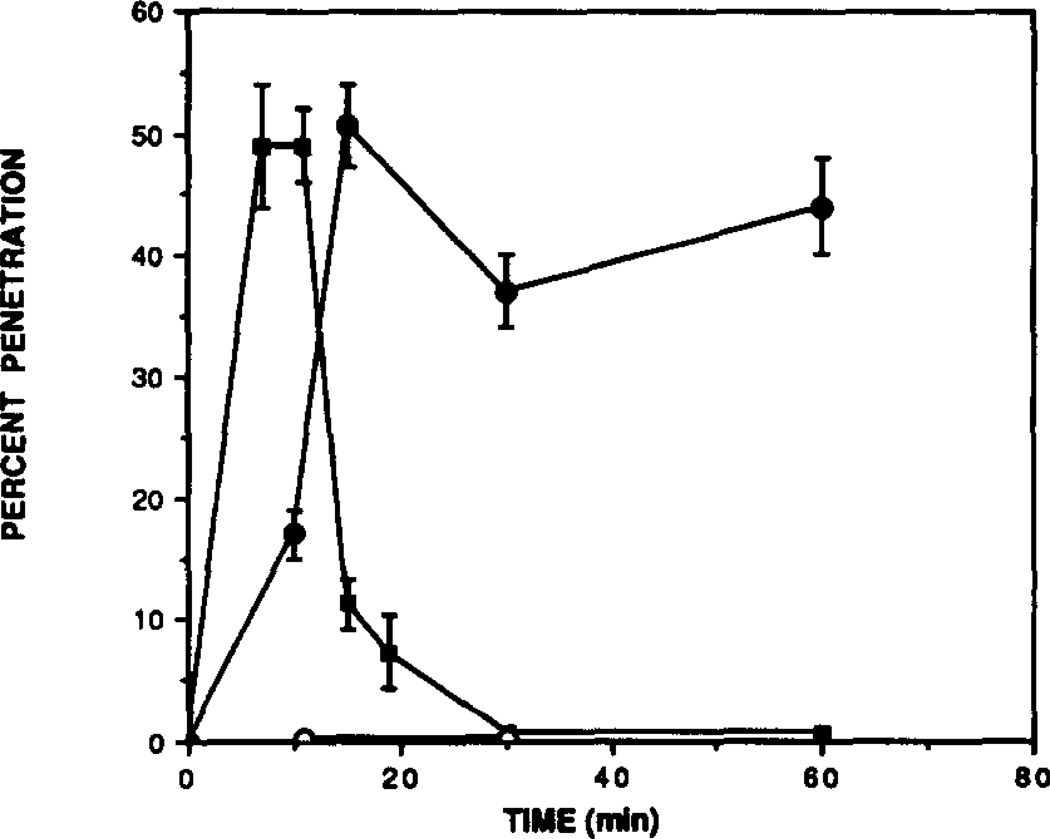
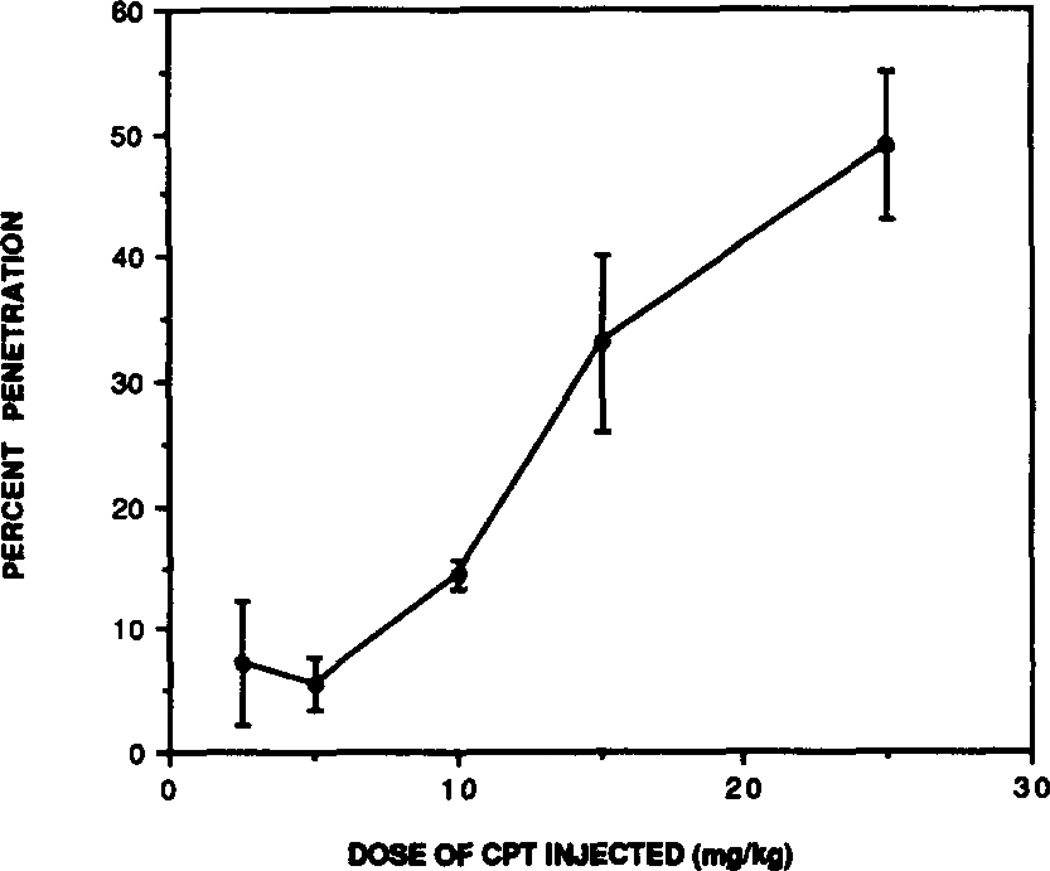
Intraperitoneal administration of 25 mg/kg CPT resulted in a rapid accumulation of CPT in brain that reached maximal levels by 7 min after injection (Fig. 3). At this time, the concentration of CPT in brain was 49 µM which represents 49% of theoretical penetration. One hundred percent penetration is defined here as the concentration of compound expected in brain without any diffusion barrier. This level of CPT was maintained in brain for about 4 min, after which it declined markedly. By 19 min following injection, it had declined to 7.3 µM, or 7.3% theoretical penetration assuming uniform tissue distribution of the injected dose. As shown in Fig. 4, the percent penetration of CPT was dose dependent.
Fig. 3.
Time course for penetration of xanthines in the mouse brain. Mice were injected i.p. with: (■] CPT, 25 mg/ kg; (●) CPX, 0.25 mg/kg; and (○) 8-PST, 50 mk/kg. After the indicated time, their forebrains were removed, homogenized in 8 vol. of Tris-HCl, pH 7.4, and, after treatment with ADA (6 U/mL) used directlfy for [3H]PIA binding. Concentrations of xanthines in brain were determined by comparing [3H]PIA binding to that from a standard curve. One hundred percent brain penetration is defined as the amount of compound reaching the brain in the absence of any permeability barrier assuming that the injected dose distributes uniformly throughout the mouse. Each determination is the mean ± SD from at least three different animals.
Fig. 4.
Dose-dependency for CPT penetration into brain relative to each injected dose. The indicated doses of CPT were injected i.p., and ex vivo binding was performed 10 min after injection. The error bars represent average ± range of duplicate animals.
Levels of CPT in the plasma were also measured at various time points following i.p. injection of 25 mg/kg doses. These levels decreased from 50.8 µM at 7 min following injection, to 45.9 µM, 19.3 µM and 10.5 µM at 11, 19 and 30 min following injection of this compound. This rapid decrease in serum levels parallels the rapid decline in brain and may reflect either elimination or metabolic breakdown of this compound.
In contrast to the rapid accumulation and rapid decline of CPT levels in brain, i.p. administration of CPX (0.25 mg/kg) resulted in a somewhat slower brain penetration, reaching maximal levels by 15 min. At this time, the brain concentration of CPX was 344 nM, representing 50.6% penetration. This compound cleared the brain much more slowly than CPT (see Fig. 3), and significant levels (252 nM) remained in brain 30 and 60 min following injection.
We next measured the brain penetration of 8-PST, a compound that should not penetrate the blood-brain barrier because of its high charge [15]. Injection of 50mg/kg of 8-PST resulted in no detectable accumulation in brain either 10 or 30 min post-injection. This injected dose would have resulted in a brain concentration of 200 µM had it been 100% permeable into brain. Since the Ki of this compound was 2.6 µM, we conclude that less than 4.8% of it penetrated into brain.
To account for the possibility that the CPX observed in the brain extracts was from brain and not some other peripheral compartment expressing A1 receptors, [3H ]CPX (1 µCi) was administered (i.p.) into each of six animals. Three of these mice were co-injected with 50mg/mL of the peripheral antagonist 8-PST. After 15 min, the animals were killed, and brains were removed, homogenized in Tris buffer, and processed for scintillation counting. The 8-PST failed to displace any of the observed radioactivity from the brain extract, demonstrating that the CPX had indeed penetrated into brain rather than simply bound to A1 receptors in some other compartment. By this time, 1.1 ± 0.1% of this injected dose had accumulated in the brain: since brain is approximately 1.3% of the weight of the whole animal, this represents approximately 85% of theoretical penetration. The difference between this value and the lower value (50.6%) determined by ex vivo binding may be due to tracer amounts used in this experiment compared to the larger doses used for the ex vivo binding.
XAC (xanthine amine congener) is mainly but not entirely charged at physiological pH, by virtue of the primary amino group. Injection of 20mg/kg XAC resulted in 1.6% (N = 2, range 1.4 to 1.8, corresponding to 600 nM) penetration 10 min following i.p. injection, and 3.2% (N = 2, range 2.7 to 3.7, corresponding to 1.2 µM) penetration 20 min following i.p. injection.
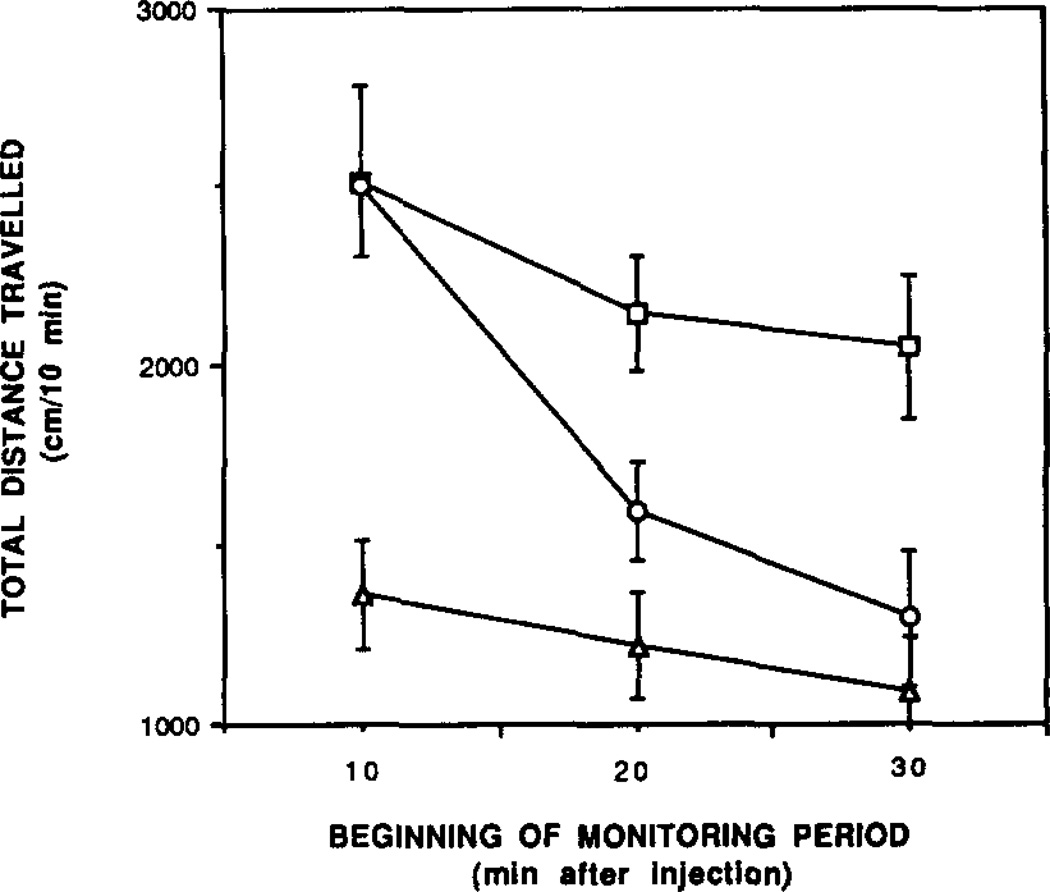
We carried out locomotor studies in mice using a computerized monitoring apparatus, based on previous studies of the locomotor depressant effects of adenosine agonists [12, 16]. Doses of CPT or theophylline (both 10 mg/kg) were injected intraperitoneally, and locomotor activity as indicated by total distance travelled was measured in 10-min intervals, beginning 10 min post-injection. From the ex-vivo binding data, this injected dose of CPT resulted in brain concentrations of 5.5 and 1 µM, 10 and 20 min respectively, following injection. Both xanthines were found to stimulate locomotor activity (Fig. 5). In the first 10-min interval measured, the degree of stimulation relative to vehicle control by theophylline (42%) or CPT (47%) was nearly identical. At longer times, the stimulation by theophylline persisted, but the effect of CPT disappeared, and at 35-min post-injection the level of locomotor activity was similar to that of the control mice. Thus, CPT stimulates locomotor behavior, but only within the first 20min post-injection. These findings are consistent with the pharmacokinetics of this compound (Fig. 3) which show that brain concentrations decline rapidly by 20 min, and suggest that the locomotor activity is centrally mediated. In contrast to CPT, injection of 0.25 mg/kg of CPX failed to elicit a statistically significant elevation in locomotor activity (N = 6, data not shown), despite the previous findings that CPX penetrates rapidly into brain.
Fig. 5.
Time course of locomotor activity. Mice were treated with 10 mg/kg CFT (○) or 10 mg/kg theophylline (□) compared to vehicle control (△). The total distance travelled (cm/30min) in an Omnitech Activity analyser (dimensions of plexiglass cage: 40 × 40 cm, 30 cm high) was calculated using ILAM software. Animals were placed in the cage immediately after injection, and monitoring for three intervals of 10 min each was initiated after 10 min. Error bars shown represent the standard error of the mean of six to eight animals.
DISCUSSION
These studies demonstrate quantitatively that the A1-selective adenosine analogues CPT and CPX penetrate into brain, and have considerably different pharmacokinetics. 8-PST, a polar compound by virtue of an aryl sulfonate group that is permanently charged at physiological pH, was found not to penetrate into brain. XAC, which bears an amino group, was found to enter the mouse brain to a small degree, consistent with its reported proconvulsant effects at very high intravenous doses [17], which are presumably centrally-mediated. The brain penetrability of these compounds is summarized in Table 1. Given the recent interest in the behavioral effects of methylxanthines, such studies are especially important. CPT and CPX have been reported to be centrally active in the reversal of adenosine agonist induced locomotor depression [12, 18]. [11C] CPT has been proposed as an in vivo receptor imaging ligand for central adenosine receptors.* 8-PST has been utilized as a peripherally selective adenosine antagonist by Evoniuk et al. [19], Seale et al. [16], von Lubitz and Marangos [13], and others, based on indirect evidence [1] for exclusion from the brain.
Table 1.
Brain penetration of adenosine antagonists
| Compound | Dose (mg/kg) |
Max. brain concentration µM) |
Detection limit (nM) |
% Penetration |
|---|---|---|---|---|
| CPT | 25 | 49 ± 25* | 272 | 49 |
| CPX | 0.25 | 0.34 ± 0.02 | 32 | 50.6 |
| 8-PST | 50 | <9.6 | 9600 | <4.8 |
| XAC | 20 | 1.2 ± 0.1 | 80 | 3.2 |
Compounds were injected i.p. at the indicated doses, and the mice were killed at various times to measure ex vivo binding. Maximum brain concentrations occurred at varying times as shown in Fig. 3. The detection limit refers to the concentration of compound in brain that would cause 10 ± 5% inhibition of binding. Percent penetration is calculated by dividing the maximum brain concentration by the theoretical brain concentration of compound in the absence of any blood-brain barrier.
Mean ± SD, N = 3–4.
Concentrations of simple xanthines in the CNS following peripheral administration have been measured. Stahle et al. [20] used microdialysis to measure the concentration of theophylline in rat brain following subcutaneous administration. They found that a dose of 20–24mg of theophylline resulted in a brain-free extracellular concentration of 60–90 µM. The injected doses and the resulting concentrations of xanthine in the brain were comparable to those with CPT administration in the present study. It is interesting to note, however, that both theophylline in the study by Stahle et al. [20] and CPX in the present study remained elevated in brain for much longer times than CPT. Similarly, Kaplan et al. [21] found that the levels of caffeine in the plasma and brains of mice after parenteral administration were maintained up to several hours post-injection (a 20 mg/kg dose had half-lives of 1.25 and 0.93 hr in the brain and plasma, respectively). In the current studies, the rapid decrease in CPT concentration in brain was paralleled by decreasing CPT concentrations in serum, and may be caused by either elimination or metabolism of this compound.
Although a correlation between the time course of locomotor stimulation by CPT and its measured levels in the brain was observed, no such correlation was observed for CPX. The reason for the absence of locomotor stimulation by CPX is not clear: the ex vivo binding studies clearly indicate that this compound readily penetrates into brain. It is interesting to note that Bruns et al. [18] reported an absence of locomotor stimulation by CPT at doses up to 30 mg/kg. Possibly this might be related to the rapid decrease in CPT from the CNS. This absence of correlation, however, demonstrates the difficulty in interpreting adenosine receptor-mediated locomotor stimulation. Because of its relatively high Ki, ex vivo binding of theophylline could not be reliably measured, so no such correlations could be made for theophylline.
Acknowledgements
We thank Dr. Raymond Bartus for providing the impetus for these studies, and Cortex Pharmaceuticals, Inc. for support. Dr. Robin Barraco of Wayne State University, Detroit. MI. offered helpful suggestions concerning adenosine deaminase. We thank Dr. John Daly of NIH for providing membranes from PC12 cells and for helpful discussions.
Footnotes
Abbreviations: CPT, 8-cyclopentyl-1,3-dimethylxanthine; CPX, 8-cyclopentyl-1,3-dipropylxanthine; 8-PST, 8-p-sulfophenyltheophylline; XAC, 8-[4[[[[(2-aminoethyl)-amino]carbonyl]methyl]oxy]phenyl]-1,3-dipropylxanthine; ADA, adenosine deaminase; and [3H]PIA R-[3H]methyl-2-phenylethyladenosine.
Yorke JC, Prenant C and Crouzel C, Synthesis of carbon-11 labelled cyclopentyltheophylline: A radioligand for PET studies of adenosine receptors. Eighth International Symposium on Radiopharmaceutical Chemistry, Princeton NJ, Abstracts, p. 269, 1990.
REFERENCES
- 1.Snyder SW, Katims JJ, Annau Z, Bruns RF, Daly JW. Adenosine receptors and the actions of methylxanthines. Proc Natl Acad Sci USA. 1981;78:3260–3264. doi: 10.1073/pnas.78.5.3260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barraco RA. Behavioral actions of adenosine and related substances. In: Phillips JW, editor. Adenosine and Adenine Nucleotides as Regulators of Cellular Function. Boca Raton, FL: CRC Press; 1991. pp. 339–366. [Google Scholar]
- 3.Daly JW, Bruns RF, Snyder SH. Adenosine receptors in the central nervous system: Relationship to the central actions of methylxanthines. Life Sci. 1981;28:2083–2097. doi: 10.1016/0024-3205(81)90614-7. [DOI] [PubMed] [Google Scholar]
- 4.Daly JW, Padgett W, Shamim MT, Butts-Lamb P, Waters J. 1,3-Dialkyl-8-(p-sulfophenyl)xanthines: Potent water-soluble antagonists for A1 and A2 adenosine receptors. J Med Chem. 1985;28:487–492. doi: 10.1021/jm00382a018. [DOI] [PubMed] [Google Scholar]
- 5.Jacobson KA, Kirk KL, Padgett WL, Daly JW. Functionalized congeners of 1,3-dialkylxanthines: Preparation of analogues with high affinity for adenosine receptors. J Med Chem. 1985;28:1334–1340. doi: 10.1021/jm00147a038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bruns RF, Lu GH, Pugsley TA. Characterization of the A2 adenosine receptor labeled by [3H]NECA in rat striatal membranes. Mol Pharmacol. 1986;29:331–346. [PubMed] [Google Scholar]
- 7.Carroll JA, Forrest JD, Shaw JS. Ex-vivo binding: A measure of drug penetration into the CNS. Br J Pharmacol. 1987;92(Suppl):689P. [Google Scholar]
- 8.Heffez DS, Nowak TS, Passoneau JV. Nimodipine levels in gerbil brain following parenteral drug administration. J Neurosurg. 1985;63:589–592. doi: 10.3171/jns.1985.63.4.0589. [DOI] [PubMed] [Google Scholar]
- 9.Freedman SB, Harley EA, Pate1 S. Direct measurement of muscarinic agents in the central nervous system of mice using ex vivo binding. Ear J Pharmacol. 1989;174:253–260. doi: 10.1016/0014-2999(89)90317-8. [DOI] [PubMed] [Google Scholar]
- 10.Bruns RF, Fergus JH, Badger EW, Bristol JA, Santay LA, Hartman JD, Hays SJ, Huang CC. Binding of the A1-selective adenosine antagonist 8-cyclopentyl-1,3-dipropylxanthine to rat brain membranes. Naunyn Schmiedebergs Arch Pharmacol. 1987;335:59–63. doi: 10.1007/BF00165037. [DOI] [PubMed] [Google Scholar]
- 11.Jacobson KA, Kirk KL, Padgett WL, Daly JW. [3H]Xanthine amine congener of 1,3-dipropyl-8-phenylxanthine: An antagonist radioligand for adenosine receptors. Proc Natl Acad Sci USA. 1986;83:4089–4093. doi: 10.1073/pnas.83.11.4089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nikodijevic O, Sarges R, Daly JW, Jacobson KA. Behavioral effects of A1- and A2-selective adenosine agonists and antagonists: Evidence for synergism and antagonism. J Pharmacol Exp Ther. 199l;259:286–294. [PMC free article] [PubMed] [Google Scholar]
- 13.von Lubitz DKJE, Marangos PJ. Cerebral ischemia in gerbils: Postischemic administration of cyclohexyl adenosine and 8-sulphophenyl-theophylline. J Mol Neurosci. 2:53–59. doi: 10.1007/BF02896926. 190. [DOI] [PubMed] [Google Scholar]
- 14.Cheng Y-C, Prusoff WH. Relationship between the inhibition constant (Ki) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol. 1973;22:3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- 15.Heller LJ, Olsson RA. Inhibition of rat ventricular automaticity by adenosine. Am J Physiol. 1985;248:H907–H913. doi: 10.1152/ajpheart.1985.248.6.H907. [DOI] [PubMed] [Google Scholar]
- 16.Scale TW, Abla KA, Shamim MT, Carney JM, Daly JW. 3,7-Dimethyl-1-proparglyxanthine: A potent and selective in vivo antagonist of adenosine analogs. Life Sci. 1988;43:1671–1684. doi: 10.1016/0024-3205(88)90478-x. [DOI] [PubMed] [Google Scholar]
- 17.Morgan PF, Deckert J, Jacobson KA, Marangos PJ, Daly JW. Potent convulsant actions of the adenosine receptor antagonist, xanthine amine convener (XAC) Life Sci. 1989;45:719–728. doi: 10.1016/0024-3205(89)90091-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bruns RF, Davis RE, Ninteman FW, Poschel BPH, Wiley JN, Heffner TG. Adenosine antagonists as pharmacological tools. In: Paton DM, editor. Adenosine and Adenine Nucleotides: Physiology and Pharmacology. London: Taylor & Francis; 1988. pp. 39–50. [Google Scholar]
- 19.Evoniuk G, von Borstel RW, Wurtman RJ. Antagonism of the cardiovascular effects of adenosine by caffeine or 8-(p-sulphophenyl)theophylline. J Pharmacol Exp Ther. 1987;242:428–432. [PubMed] [Google Scholar]
- 20.Stahle L, Segersvard S, Ungerstedt U. Theophylline concentration in the extracellullar space of the rat brain: Measurement by microdialysis and relation to behaviour. Eur J Pharmacol. 1990;185:187–193. doi: 10.1016/0014-2999(90)90639-n. [DOI] [PubMed] [Google Scholar]
- 21.Kaplan GB, Tai NT, Greenblatt DJ, Shader RI. Caffeine-induced behavioural stimulation is dose- and concentration-dependent. Br J Pharmacol. 1990;100:435–440. doi: 10.1111/j.1476-5381.1990.tb15824.x. [DOI] [PMC free article] [PubMed] [Google Scholar]