Abstract
Background
Estrogens act on estrogen receptors distributed in articular cartilages, synovial membrane, and ligaments, which are thought to be related with degenerative changes. Meanwhile, progesterone is known to have a weak anabolic action on bone formation This study evaluates the effects of estrogen and progesterone hormone on bone/cartilage turnover in ovariectomized (OVX) rats.
Methods
Thirty-five 7-month-old female Sprague-Dawley rats were randomly divided into 5 groups and then ovariectomized bilaterally except the sham control group. The first and the second group acting as controls did not receive hormonal therapy, the third group received estrogen, the fourth group received progesterone, and the fifth group received combination of both hormones 10 weeks after surgery. Evaluations were done using the serum levels of cartilage oligomeric matrix protein (COMP) for cartilage turnover, collagen type I C-telopeptide (CTX-1) and osteocalcin (OC) for bone turnover at 11, 15, 19 weeks after OVX and histology using the Osteoarthritis Research Society International (OARSI) osteoarthritis (OA) cartilage histopathology assessment system.
Results
Significantly less cartilage degradation (decreased levels of COMP) was found in the combined hormone treated group in comparison with OVX group. Similarly, both hormonal treatment resulted in increased bone formation and decreased bone resorption i.e., a low overall bone turnover status (decrease in the serum OC and CTX-1 levels).
Conclusions
Combined estrogen and progesterone therapy was found to be convincing in terms of reducing the severity of OA in this experimental model.
Keywords: Estrogen, Progesterone, Cartilage oligomeric matrix protein, Collagen type I C-telopeptide, Osteocalcin
Osteoarthritis (OA) is degenerative changes leading to serious disability in joints by affecting and areas around soft tissue and subchondral bone as well as hyaline cartilage. Risk factors of OA are age,1) gender,2) obesity,1) and malalignment3) of the lower limb. Additionally, estrogen deficiency is known to be related with the occurrence and progress of OA. Under the age 50, male patients4,5) tend to have more OA than females. However, from the beginning of menopause stage, female patients suffering from OA dramatically increase.6,7)
The association between estrogen and OA had been verified in a murine model. Researches on deficiency and complement of estrogen in articular cartilages were conducted in some of animal model studies.8) In many experimental animal studies, ovariectomy (OVX) induced OA while, complement of estrogen delayed degenerations in cartilages.9-11) Estrogens act on estrogen receptors distributed in articular cartilages, synovial membrane, and ligaments,12-14) which are thought to be related with degenerative changes.
The positive effect of injections of estrogen on the characteristics and changes in articular cartilages is widely recognized.15-17) Progesterone has its receptor in osteoblast in bone tissue and acts positively on the severity of OA directly.18) But, in contrast to estrogen, whose principle action of increasing and preserving cancellous bone volume is clearly due to an inhibition of cartilage and bone resorption, progesterone had a weak anabolic action on bone formation.19) This study evaluated the association between the cartilage of the OA and bone changes affected by sex hormones using the biochemical markers and histological findings of cartilage and bone turnover by performing OVX on the OA induced rats. The hypothesis was that combined estrogen and progesterone therapy would be more effective than administering estrogen alone in terms of suppressing the progression of OA.
METHODS
Experimental Animals and Research Design
Thirty-five 7-month-old female Sprague-Dawley rats (Charles River Lab., Wilmington, MA, USA) were used in the experiment. The entire procedure of the experiment was authorized by Institute for Experimental Animals of Konkuk University (KU09024). The animals were maintained at the Laboratory Animal Research Center of Konkuk University (KULARC) for 1 week before experiments. They were housed, two per cage, in a room maintained at 23 ± 1℃ with a 12-hour/12-hour light/dark cycle and given food (FOMULA-M07, E-Joeun Feed Co., Jeongeup, Korea) and Reverse Osmosis water ad libitum.
Experimental Groups
Sprague-Dawley rats were randomly divided into 5 groups, composed of seven rats in each group.
Cluster I: The control group of female rats treated the same (F-sham control)
Cluster II: Ovariectomized (OVX) group
Cluster III: β-estradiol administered group after ovariectomy(OVX-E)
Cluster IV: Progesterone administered group after ovariectomy (OVX-P)
Cluster V: β-estradiol and progesterone combination group after ovariectomy (OVX-E-P)
Cluster III, IV, V were administered with hormones ten weeks after OVX.
Surgery: Ovariectomy
Each Sprague-Dawley rat was anesthetized with 2% Forane (Isoflurane, Abbott Lab., Abbott Park, IL, USA) in N2O : 0.4 L/min and O2 : 1 L/min (Vaporizer V720100 & Tabletop/Wall Mopunt V701002, SurgiVet, Waukesha, WI, USA). The skin of rats was sterilized with Povidone Iodine Solution (10 g/100 mL, Hyundai Pharm Co. Ltd, Seoul, Korea). A 3 cm long incision was made at the center area of lower half of the body and tail end and bidirectional incision was made in the muscle. Once the abdominal cavity was opened, ovaries surrounded by body fats were found. The points where the fallopian tubes and the uterine horns meet were incised and ovaries were removed. Muscle approximation and skin closure were performed with catgut sutures. Sham-control groups were operated using the same incision and approach, but without removing ovaries. Right after the surgery, gentamycin (6 mg/kg, Kukje Pharm., Seongnam, Korea) was administered by intramuscular injection. After the surgery, all of the rats were allowed to move freely inside the plastic cage.
Hormone Administration
Hormones were administered by using a method of osmotic pump effect.20) Hormones were administered in groups III, IV, and V. A dose of 5.3 mg/kg β-estradiol (Sigma, St. Louis, MO, USA) was injected through silastic tube (3 cm) while a dose of 133.3 mg/kg progesterone (Sigma) was injected through two silastic tubes (5 cm). Silastic tubes were cut into appropriate lengths and hormones were injected. Afterwards, the other end of silastic tube was blocked (Silastic Medical Adhesive, Type A, Dow Corning Co., Midland, MI, USA) and it was left to dry. Subcutaneous insertion of silastic tubes containing hormones were done in the lateral mid-dorsal region under 2% Forane anesthesia. Insertion of silastic tubes were performed 10 weeks after OVX and were changed every four weeks.
Biochemical Markers of Cartilage/Bone
Serum samples were collected in tail vein using needle catheter (IV catheter 24 GA 0.75IN, 0.7 × 19 mm, BD, Franklin Lakes, NJ, USA). The serum samples were collected 11, 15, and 19 weeks after OVX and those samples were frozen at a temperature of -70℃ until analyzed using an enzyme linked immunosorbent assay (ELISA) technique. Cartilage oligomeric matrix protein (COMP) was used as a marker for cartilage turnover. Serum levels of collagen type I C-telopeptide (CTX-1) and osteocalcin (OC) were assessed for bone turnover.
COMP low-polymer
Serum level was investigated using animal COMP (MD Biosciences, St. Paul, MN, USA). Bovine COMP was used to coat the micro titer plates and serum from rats as calibrators. Polyclonal antisera were used as a primary antibody for the COMP of rats. The sample and calibrator were cultured together in microtiter plates and those were washed off to put in a secondary antibody. The cultured microtiter plates were assessed using a microtiter plate at 450 nm. Blood samples were collected through venipuncture and coagulated to separate serums using a centrifuge. After serums were eliminated, the assessment was immediately performed. Samples were needed to be diluted at least 10 times the sample buffer. The level of serum samples were expressed in units per liter (U/L), based on the plotting of a fourth degree polynomial fit calibration curve, using the standard concentration samples.
CTX-1
Rat CTX-1 was analyzed according to manufacturer's instructions. RatLaps (Immunodiagnostic Systems Nordic A/S, Herlev, Denmark) ELISA assesses degradation products using a special monoclonal antibody in a competitive ELISA (C-ELISA). This research evaluated serum samples. An investigation was performed by incubating a biotinylated form of a synthetic peptide representing the C-telopeptide epitope EKSQDGGR. This was followed by addition of sample and primary antibody. Concentration of samples was determined by the standard assessment of synthetic peptide based on calibration curve construction. Absorbance was measured at 450 nm with a 650 nm reference filter. Serum sample level used standard concentration samples and was expressed in a unit of ng/mL based on the 4th order polynomial fit calibration curve plotting.
OC
The ELISA (Rat-MID Osteocalcin ELISA, Immunodiagnostic Systems Nordic A/S) kit having high specificity to rats was used to measure the serum OC level. All required reagents were included in the kit and the experimental procedure was performed according to the instructions of the manual. The absorbance was measured at 450 nm with a 650 nm filter as reference. The levels of serum samples were expressed in nanograms per milliliter (ng/mL), based on the plotting of a fourth degree polynomial fit calibration curve, using the standards concentration samples.
Autopsy
The rats in 5 groups were sacrificed under CO2 (30% to 70%) at 10 weeks post commencement of hormonal therapy i.e., 20 weeks after OVX. Muscles and other soft tissues were removed by making incisions at the upper two-thirds of the thigh and the lower two-thirds of the tibia. Consequently, medial femoral condyle was obtained to be used at a microscopic examination.
Histological Analysis
Histological assessment was conducted on the whole-layer of cartilage sagittal section in medial femoral condyle of weight-bearing site. Samples including articular cartilage and its lower bone were fixed in 50 volumes of 10% neutral buffered formalin for 72 hours. The samples were placed in formic acid solution inside a shaker which rotates at a frequency of 150 times per minute for decalcification. The formic acid solution was replaced every day until decalcification process was completed (1 to 2 weeks). The samples were completely washed off until the neutral-pH state after the decalcification. Those tissues were dehydrated using alcohol with different concentration and cleared in a xylene to make samples transparent. Afterwards, they were embedded into paraffin using a tissue processor with fixation period of 58 hours. Each paraffin tissue lump was cut into 5 µm and then dyed with Safranin O. Corresponding slides were recorded using a digital camera (original magnification, ×400, Olympus DP71, Olympus, Tokyo, Japan). Histopathological classification of arthritic lesion pains was classified according to the Osteoarthritis Research Society International (OARSI) cartilage OA histopathology grading system.21) The OARSI grading system the semi-quantitative methodology for evaluating the cartilage OA histopathology. The study used recommended OA grading scale (combined index of grades and stages, on a 0-24 scale). Two independent and blinded observers, who were not informed on any of compositions or brand names, conducted histological assessment.
Statistical Analysis
The SPSS ver. 17.0 (SPSS Inc., Chicago, IL, USA) was used in statistical analysis. Biochemical markers among different groups were compared using the ANOVA test. When a significant difference was observed, repeated Duncan's multiple range analysis was carried on for comparison. A difference was considered significant when the p-value was less than 0.05.
RESULTS
Changes in Serum Levels of Biochemical Markers
Changes in serum COMP levels
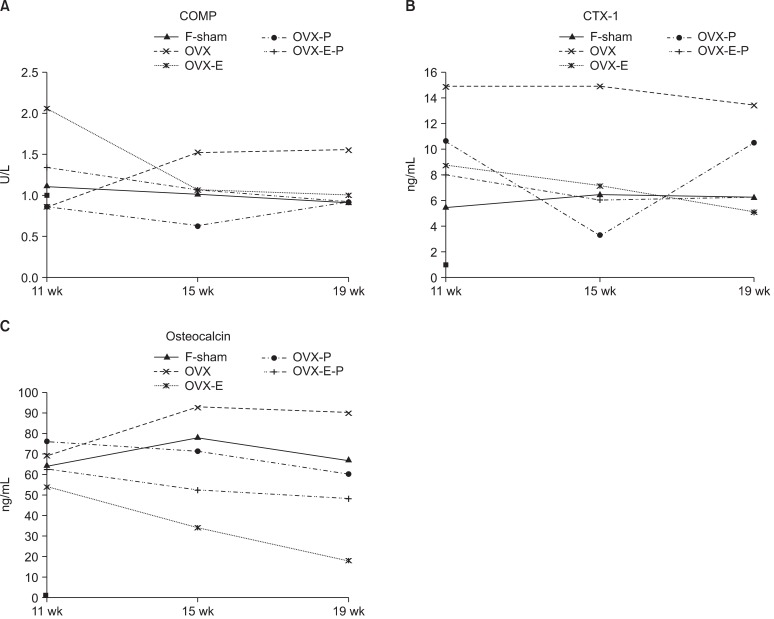
The sham operated and OVX group showed a fairly stable COMP levels throughout the study period (Fig. 1A). The OVX group had a higher absolute COMP levels than the sham group, this was not statistically significant (p > 0.05). In case of the estrogen administered group (OVX-E), serum COMP level was decreased from the beginning after administering the estrogen. The level was drastically decreased 9 weeks after the hormone administration (p = 0.004). The OVX-P group also exhibited low level of COMP 9 weeks after the hormone administration (p = 0.03). A combination of estrogen and progesterone (OVX-E-P) was found to be more beneficial with significantly lowered levels early at 7 weeks post-hormone treatment (p = 0.02). A combination of estrogen and progesterone provided a more effective and early protection from cartilage degradation.
Fig. 1.
Changes in serum levels in biochemical markers of bone and cartilage turnover in the cohort study. Cartilage turnover was assessed using cartilage low polymer substrate (COMP) (A) as a marker. The serum levels of collagen type I C-telopeptide (CTX-1) (B) and osteocalcin (OC) (C) were measured for bone turnover. Assessments were made 11, 15, and 19 weeks after OVX (equivalent to 1, 5, and 9 weeks after the hormone administration). Data show the average serum levels. F-sham: control group treated the same, OVX: ovariectomy, OVX-EL: estrogen group, OVX-P: progesterone group, OVX-E-P: estrogen and progesterone combination group.
Changes in serum CTX-1 levels
A similar trend was also seen in the levels of serum CTX-1 levels of sham operated and OVX groups (Fig. 1B). Subsequent to hormonal administration, a fall in serum CTX-1 levels in estrogen alone (OVX-E) and in combination with progesterone (OVX-E-P) suggest of reduced bone resorption in subchondral bone. A combination therapy was shown to provide an early protection with respect to lowering of the bone resorption marker levels (5 weeks post hormonal treatment, p = 0.02). At 9 weeks post-hormone treatment, estrogen (OVX-E) and in combination (OVX-E-P) resulted in significant fall in serum levels of CTX-1 (p = 0.003).
Changes in serum OC levels
A reduced bone turnover as suggested by lowering of levels of OC in response to treatment with estrogen was seen as early as 1 week post-hormone administration (p = 0.006) (Fig. 1C). Similar effect was also shown by combination therapy, but at a later stage (5 weeks post-hormone, p = 0.04). Thus in reducing the bone turnover rate, estrogen alone is potent than combination with progesterone. This effect was supported by a significant difference found in OVX-E and OVX-E-P groups at 1 and 9 weeks post-hormonal treatment (p = 0.008 and 0.01, respectively).
Results of Histological Analysis
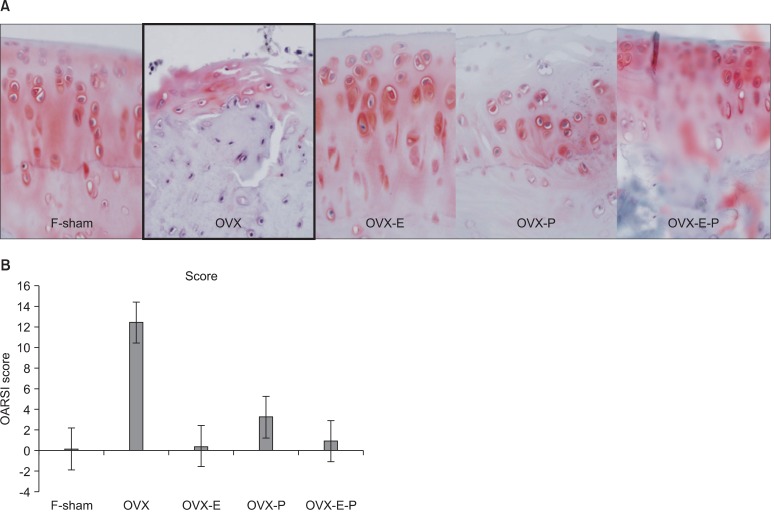
When the 20-week scarified groups were compared, the OVX group exhibited the most severe OA appearance (p = 0.0258). However, no statistical significance was found among the hormone administered groups. OA grades of each group are as follows: F-sham, 2; OVX, 13.00 ± 5.62 (mean ± SD); OVX-E, 0.80 ± 0.45; OVX-P, 6; OVX-E-P, 2.20 ± 0.84 (Fig. 2).
Fig. 2.
Findings on tissue examination. (A) The OVX group showed severe OA to a level of statistically significance (Safranin O stain, ×400). (B) Osteoarthritis Research Society International (OARSI) cartilage OA histopathology grading assessment graph of each group. OVX: ovariectomy, OA: osteoarthritis, F-sham: control group treated the same, OVX-E: estrogen group, OVX-P: progesterone group, OVX-E-P: estrogen and progesterone combination group.
DISCUSSION
In this study, the effect of combined sex hormone therapy was observed in biological markers of cartilage and bone turnover and histological findings. Combined estrogen and progesterone therapy exhibited suppression of the OA progress in the COMP and CTX-1 factors similar to the F-sham control group. Moreover, the OC levels, the bone formation marker, showed highly statistically significant. As predicted by the hypothesis, combined estrogen and progesterone therapy was more effective in suppressing cartilage and bone turnover compare to the estrogen alone treatment. However, no significant difference was shown in terms of histological findings since all groups exhibited similar findings except for the OVX group.
The ovariectomized Sprague-Dawley rats are widely used in examining the OA diseases related to cartilage degeneration and changes in subchondral bone22) and also commonly used in determining treatment efficacy.15,23) Hormone replacement therapy (HRT) has shown substantial effect in preventing cartilage degeneration and bone turnover.24) This research used 7-month-old "retired breeder rats"25) unlike generally used ten-weeks-old Sprague-Dawley rats. Those "retired breeder rats" are Sprague-Dawley rats induced with hormone deficiency in bone metabolism accompanied with menopause after OVX.
Adverse effects of hormone deficiency on bone homeostasis are experienced before and after menopause and those are more evidently shown when the menstrual periods have stopped. The estrogen alone or combined estrogen and progesterone therapies are known to be effective in treating osteoporosis after menopause.15,26,27) According to the data of HRT affecting knee cartilage volumes and bone compliance of subchondral bone, patients receiving long-term HRT exhibited 10% greater effectiveness compare to ordinary woman of the same age.28) Moreover, patients receiving long-term HRT are known to be less threatening from the risk of knee and hip OA according to X-rays compare to women who do not.29,30) Those results raised estrogen deficiency as one of the causes resulting the OA and invigorated more studies on the effect of estrogen on cartilage and bone.
Otterness et al.31) initially proposed a theory of complex analysis of biochemical markers representing synthesis and degradation that accuracy of diagnosing the degree of cartilage degeneration could be enhanced. Since then, many clinical studies were able to predict the consequences of the OA progression the HRT by using the biomarkers in more detail. Bio-markers are mainly classified into the following three groups according to a dual action of bone turnover: 1) markers that reflect bone resorption; 2) markers that reflect osteoclast number, and 3) markers that reflect bone formation. Bone resorption markers are different matrix-derived fragments that are generated during the osteoclastic resorption activity, such as the C-terminal cross-linked CTX-1 fragment and the N-terminal telopeptide of type I collagen fragment.32,33) Markers that reflect osteoclast number are tartrate-resistant acid phosphatase and, potentially, cathepsin K, which are enzymes produced and released into the circulation by the osteoclasts. 34) The bone formation markers can be divided into two categories: 1) proteins, which reflect increased osteoblast differentiation, and thereby indicate increased bone formation, such as bone-specific alkaline phosphatase and OC,35) or 2) fragments of pro-collagen, which are released during collagen incorporation into the newly formed bone matrix, and thereby directly reflect bone formation, such as the N-terminal and C-terminal pro-fragments of type I collagen.36)
COMP is a non-collagenous protein isolated from articular cartilage.37) It is localized in articular cartilage and the proliferative and hypertrophic zones of the epiphyseal growth plate.38) COMP has been implicated in the maintenance of chondrocyte phenotype, cell growth and matrix development.39) It has also been reported that elevated serum levels of COMP have been found in patients with knee OA.40) Therefore, it has been a potential biomarker for monitoring the progression of cartilage destruction and cartilage damage in arthritis.41) This study was based on COMP level evaluation for cartilage turnover evaluation.
This research had limitations. Combined estrogen and progesterone therapy showed a statistically significant difference in cartilage turnover, bone formation and histological findings. However, the synergic effect of estrogen and progesterone in bone resorption markers that could verify the probability was not shown in the OVX-E and OVX-E-P groups. More distinct effects could be proved by conducting additional studies by adjusting dosage of combined hormones and performing bone mineral density and micro CT exams by increasing the size of samples.
ACKNOWLEDGEMENTS
This study was supported by a grant of Konkuk University in 2011. Authors thank Ms. Min-Jung Lee for her statistical analysis and illustrations of this work.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Schouten JS, van den Ouweland FA, Valkenburg HA. A 12 year follow up study in the general population on prognostic factors of cartilage loss in osteoarthritis of the knee. Ann Rheum Dis. 1992;51(8):932–937. doi: 10.1136/ard.51.8.932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Felson DT, Zhang Y, Hannan MT, et al. The incidence and natural history of knee osteoarthritis in the elderly: the Framingham Osteoarthritis Study. Arthritis Rheum. 1995;38(10):1500–1505. doi: 10.1002/art.1780381017. [DOI] [PubMed] [Google Scholar]
- 3.Brouwer GM, van Tol AW, Bergink AP, et al. Association between valgus and varus alignment and the development and progression of radiographic osteoarthritis of the knee. Arthritis Rheum. 2007;56(4):1204–1211. doi: 10.1002/art.22515. [DOI] [PubMed] [Google Scholar]
- 4.Wilson MG, Michet CJ, Jr, Ilstrup DM, Melton LJ., 3rd Idiopathic symptomatic osteoarthritis of the hip and knee: a population-based incidence study. Mayo Clin Proc. 1990;65(9):1214–1221. doi: 10.1016/s0025-6196(12)62745-1. [DOI] [PubMed] [Google Scholar]
- 5.Lawrence JS, Bremner JM, Bier F. Osteo-arthrosis. Prevalence in the population and relationship between symptoms and x-ray changes. Ann Rheum Dis. 1966;25(1):1–24. [PMC free article] [PubMed] [Google Scholar]
- 6.Oliveria SA, Felson DT, Reed JI, Cirillo PA, Walker AM. Incidence of symptomatic hand, hip, and knee osteoarthritis among patients in a health maintenance organization. Arthritis Rheum. 1995;38(8):1134–1141. doi: 10.1002/art.1780380817. [DOI] [PubMed] [Google Scholar]
- 7.McAlindon TE, Snow S, Cooper C, Dieppe PA. Radiographic patterns of osteoarthritis of the knee joint in the community: the importance of the patellofemoral joint. Ann Rheum Dis. 1992;51(7):844–849. doi: 10.1136/ard.51.7.844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sniekers YH, Weinans H, Bierma-Zeinstra SM, van Leeuwen JP, van Osch GJ. Animal models for osteoarthritis: the effect of ovariectomy and estrogen treatment-a systematic approach. Osteoarthritis Cartilage. 2008;16(5):533–541. doi: 10.1016/j.joca.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 9.Calvo E, Castaneda S, Largo R, Fernandez-Valle ME, Rodriguez-Salvanes F, Herrero-Beaumont G. Osteoporosis increases the severity of cartilage damage in an experimental model of osteoarthritis in rabbits. Osteoarthritis Cartilage. 2007;15(1):69–77. doi: 10.1016/j.joca.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 10.Hoegh-Andersen P, Tanko LB, Andersen TL, et al. Ovariectomized rats as a model of postmenopausal osteoarthritis: validation and application. Arthritis Res Ther. 2004;6(2):R169–R180. doi: 10.1186/ar1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ham KD, Loeser RF, Lindgren BR, Carlson CS. Effects of long-term estrogen replacement therapy on osteoarthritis severity in cynomolgus monkeys. Arthritis Rheum. 2002;46(7):1956–1964. doi: 10.1002/art.10406. [DOI] [PubMed] [Google Scholar]
- 12.Ushiyama T, Ueyama H, Inoue K, Ohkubo I, Hukuda S. Expression of genes for estrogen receptors alpha and beta in human articular chondrocytes. Osteoarthritis Cartilage. 1999;7(6):560–566. doi: 10.1053/joca.1999.0260. [DOI] [PubMed] [Google Scholar]
- 13.Arts J, Kuiper GG, Janssen JM, et al. Differential expression of estrogen receptors alpha and beta mRNA during differentiation of human osteoblast SV-HFO cells. Endocrinology. 1997;138(11):5067–5070. doi: 10.1210/endo.138.11.5652. [DOI] [PubMed] [Google Scholar]
- 14.Dietrich W, Haitel A, Holzer G, Huber JC, Kolbus A, Tschugguel W. Estrogen receptor-beta is the predominant estrogen receptor subtype in normal human synovia. J Soc Gynecol Investig. 2006;13(7):512–517. doi: 10.1016/j.jsgi.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 15.Anderson GL, Limacher M, Assaf AR, et al. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women's Health Initiative randomized controlled trial. JAMA. 2004;291(14):1701–1712. doi: 10.1001/jama.291.14.1701. [DOI] [PubMed] [Google Scholar]
- 16.Rasanen T, Messner K. Articular cartilage compressive stiffness following oophorectomy or treatment with 17beta-estradiol in young postpubertal rabbits. Acta Obstet Gynecol Scand. 1999;78(5):357–362. [PubMed] [Google Scholar]
- 17.Turner AS, Athanasiou KA, Zhu CF, Alvis MR, Bryant HU. Biochemical effects of estrogen on articular cartilage in ovariectomized sheep. Osteoarthritis Cartilage. 1997;5(1):63–69. doi: 10.1016/s1063-4584(97)80032-5. [DOI] [PubMed] [Google Scholar]
- 18.Yoshioka T, Sato B, Matsumoto K, Ono K. Steroid receptors in osteoblasts. Clin Orthop Relat Res. 1980;(148):297–303. [PubMed] [Google Scholar]
- 19.Schmidt IU, Wakley GK, Turner RT. Effects of estrogen and progesterone on tibia histomorphometry in growing rats. Calcif Tissue Int. 2000;67(1):47–52. doi: 10.1007/s00223001096. [DOI] [PubMed] [Google Scholar]
- 20.Zhou H, Shen V, Dempster DW, Lindsay R. Continuous parathyroid hormone and estrogen administration increases vertebral cancellous bone volume and cortical width in the estrogen-deficient rat. J Bone Miner Res. 2001;16(7):1300–1307. doi: 10.1359/jbmr.2001.16.7.1300. [DOI] [PubMed] [Google Scholar]
- 21.Pritzker KP, Gay S, Jimenez SA, et al. Osteoarthritis cartilage histopathology: grading and staging. Osteoarthritis Cartilage. 2006;14(1):13–29. doi: 10.1016/j.joca.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 22.Sniekers YH, Intema F, Lafeber FP, et al. A role for subchondral bone changes in the process of osteoarthritis; a micro-CT study of two canine models. BMC Musculoskelet Disord. 2008;9:20. doi: 10.1186/1471-2474-9-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bendele AM. Animal models of osteoarthritis. J Musculoskelet Neuronal Interact. 2001;1(4):363–376. [PubMed] [Google Scholar]
- 24.Radin ER, Paul IL, Rose RM. Pathogenesis of primary osteoarthritis. Lancet. 1972;1(7765):1395–1396. doi: 10.1016/s0140-6736(72)91130-0. [DOI] [PubMed] [Google Scholar]
- 25.Sherman SS, Smith JC, Jr, Tobin JD, Soares JH., Jr Ovariectomy, dietary zinc, and bone metabolism in retired breeder rats. Am J Clin Nutr. 1989;49(6):1184–1191. doi: 10.1093/ajcn/49.6.1184. [DOI] [PubMed] [Google Scholar]
- 26.Effects of hormone therapy on bone mineral density: results from the postmenopausal estrogen/progestin interventions (PEPI) trial: the Writing Group for the PEPI. JAMA. 1996;276(17):1389–1396. [PubMed] [Google Scholar]
- 27.Torgerson DJ, Bell-Syer SE. Hormone replacement therapy and prevention of nonvertebral fractures: a meta-analysis of randomized trials. JAMA. 2001;285(22):2891–2897. doi: 10.1001/jama.285.22.2891. [DOI] [PubMed] [Google Scholar]
- 28.Wluka AE, Davis SR, Bailey M, Stuckey SL, Cicuttini FM. Users of oestrogen replacement therapy have more knee cartilage than non-users. Ann Rheum Dis. 2001;60(4):332–336. doi: 10.1136/ard.60.4.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nevitt MC, Felson DT, Williams EN, Grady D. The effect of estrogen plus progestin on knee symptoms and related disability in postmenopausal women: The Heart and Estrogen/Progestin Replacement Study, a randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2001;44(4):811–818. doi: 10.1002/1529-0131(200104)44:4<811::AID-ANR137>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 30.Spector TD, Nandra D, Hart DJ, Doyle DV. Is hormone replacement therapy protective for hand and knee osteoarthritis in women?: the Chingford Study. Ann Rheum Dis. 1997;56(7):432–434. doi: 10.1136/ard.56.7.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Otterness IG, Swindell AC, Zimmerer RO, Poole AR, Ionescu M, Weiner E. An analysis of 14 molecular markers for monitoring osteoarthritis: segregation of the markers into clusters and distinguishing osteoarthritis at baseline. Osteoarthritis Cartilage. 2000;8(3):180–185. doi: 10.1053/joca.1999.0288. [DOI] [PubMed] [Google Scholar]
- 32.Sorensen MG, Henriksen K, Schaller S, Karsdal MA. Biochemical markers in preclinical models of osteoporosis. Biomarkers. 2007;12(3):266–286. doi: 10.1080/13547500601070842. [DOI] [PubMed] [Google Scholar]
- 33.Karsdal MA, Hjorth P, Henriksen K, et al. Transforming growth factor-beta controls human osteoclastogenesis through the p38 MAPK and regulation of RANK expression. J Biol Chem. 2003;278(45):44975–44987. doi: 10.1074/jbc.M303905200. [DOI] [PubMed] [Google Scholar]
- 34.Halleen JM, Alatalo SL, Suominen H, Cheng S, Janckila AJ, Vaananen HK. Tartrate-resistant acid phosphatase 5b: a novel serum marker of bone resorption. J Bone Miner Res. 2000;15(7):1337–1345. doi: 10.1359/jbmr.2000.15.7.1337. [DOI] [PubMed] [Google Scholar]
- 35.Gundberg CM. Biochemical markers of bone formation. Clin Lab Med. 2000;20(3):489–501. [PubMed] [Google Scholar]
- 36.Suvanto-Luukkonen E, Risteli L, Sundstrom H, Penttinen J, Kauppila A, Risteli J. Comparison of three serum assays for bone collagen formation during postmenopausal estrogen-progestin therapy. Clin Chim Acta. 1997;266(2):105–116. doi: 10.1016/s0009-8981(97)00140-x. [DOI] [PubMed] [Google Scholar]
- 37.DiCesare PE, Morgelin M, Carlson CS, Pasumarti S, Paulsson M. Cartilage oligomeric matrix protein: isolation and characterization from human articular cartilage. J Orthop Res. 1995;13(3):422–428. doi: 10.1002/jor.1100130316. [DOI] [PubMed] [Google Scholar]
- 38.Ekman S, Reinholt FP, Hultenby K, Heinegard D. Ultrastructural immunolocalization of cartilage oligomeric matrix protein (COMP) in porcine growth cartilage. Calcif Tissue Int. 1997;60(6):547–553. doi: 10.1007/s002239900278. [DOI] [PubMed] [Google Scholar]
- 39.Shen Z, Heinegard D, Sommarin Y. Distribution and expression of cartilage oligomeric matrix protein and bone sialoprotein show marked changes during rat femoral head development. Matrix Biol. 1995;14(9):773–781. doi: 10.1016/s0945-053x(05)80020-4. [DOI] [PubMed] [Google Scholar]
- 40.Clark AG, Jordan JM, Vilim V, et al. Serum cartilage oligomeric matrix protein reflects osteoarthritis presence and severity: the Johnston County Osteoarthritis Project. Arthritis Rheum. 1999;42(11):2356–2364. doi: 10.1002/1529-0131(199911)42:11<2356::AID-ANR14>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 41.Vilim V, Olejarova M, Machacek S, Gatterova J, Kraus VB, Pavelka K. Serum levels of cartilage oligomeric matrix protein (COMP) correlate with radiographic progression of knee osteoarthritis. Osteoarthritis Cartilage. 2002;10(9):707–713. doi: 10.1053/joca.2002.0819. [DOI] [PubMed] [Google Scholar]