Abstract
Background
Large conductance Ca2+-activated K+ (BK) channels regulate smooth muscle tone. The BK channel β1-subunit increases Ca2+ sensitivity of the α-subunit in smooth muscle. We studied β1-subunit knockout (KO) mice to determine if gastrointestinal (GI) motility was altered.
Methods
Colonic and intestinal longitudinal muscle reactivity to bethanechol and colonic migrating motor complexes (CMMCs) were measured in vitro. Gastric emptying and small intestinal transit were measured in vivo. Colonic motility was assessed in vivo by measuring fecal output and glass bead expulsion time. Myoelectric activity of distal colon smooth muscle was measured in vitro using intracellular microelectrodes.
Main findings
Bethanechol-induced contractions were larger in the distal colon of β1-subunit KO compared to WT mice; there were no differences in bethanechol reactivity in the duodenum, ileum or proximal colon of WT vs. β1-subunit KO mice. There were more retrogradely propagated CMMCs in the distal colon of β1-subunit KO compared to WT mice. GI transit was unaffected by β1-subunit KO. Fecal output was decreased and glass bead expulsion times were increased in β1-subunit KO mice. Membrane potential of distal colon smooth muscle cells from β1-subunit KO mice was depolarized with higher action potential frequency compared to WT mice. Paxilline (BK channel blocker) depolarized smooth muscle cells and increased action potential frequency in WT distal colon.
Conclusions and inferences
BK channels play a prominent role in smooth muscle function only in the distal colon of mice. Defects in smooth muscle BK channel function disrupt colonic motility causing constipation.
Keywords: Potassium channels, colonic motility, constipation
INTRODUCTION
Large-conductance Ca2+-activated K+ (BK) channels are expressed by many cell types including neurons1 skeletal2 cardiac3 smooth muscle4 and immune cells.5 In all cell types, BK channels contain 4 α-subunits that form the channel pore and each α-subunit contains several domains that participate in channel activation.6 BK channel activation is voltage- and Ca2+-dependent and voltage-dependent activation is regulated by the α-subunit.6 Accessory β-subunits modulate Ca2+ sensitivity of the α-subunit and there are four β-subunit subtypes (β1–β4).7 The β1-subunit is smooth muscle specific.8,9 β1-subunits contribute to regulation of smooth muscle tone in the trachea, blood vessels and urinary bladder.10–13 Ca2+-dependent activation of BK channels requires Ca2+ influx through L-type Ca2+ channels.14 In smooth muscle cells, Ca2+ activates ryanodine receptors (RyRs) on the smooth endoplasmic reticulum to cause Ca2+ sparks.4,15 Ca2+ sparks initiate spontaneous transient outward K+ currents (STOCs) carried by BK channels. STOCs cause membrane hyperpolarization that closes L-type Ca2+ channels subsequently resulting in smooth muscle relaxation.4,15 Together, Ca2+ sparks coupled with STOCs regulate smooth muscle tone.
The importance of the β1-subunit in regulating smooth muscle tone has been illustrated by studies using β1-subunit knockout (KO) mice. Myocytes taken from β1-subunit KO mice show a reduction in the open probability of the BK channel in urinary bladder13, arterial12 and tracheal myocytes16 as well as a reduced coupling of Ca2+ sparks and STOCs in arterial and colonic myocytes.12,17 In colonic smooth muscle cells from β1-subunit KO mice, Ca2+ sparks were normal but STOCs were smaller and less frequent indicative of a separation between Ca2+ sparks and STOCs.17 Although Ca2+ spark-STOC uncoupling occurs in colonic smooth muscle cells of β1-subunit KO mice, the integrative functional consequences of this uncoupling are unknown. In the present study, we tested the hypothesis that the absence of the β1-subunit in mice would disrupt gut motility in vitro and in vivo.
MATERIALS and METHODS
Mice
All animal use protocols were approved by the Institutional Animal Care and Use Committee at Michigan State University. Homozygous breeder β1-subunit KO mice were obtained from Dr. Robert Brenner, University of Texas Health Science Center at San Antonio.10,12 Homozygous BK β1-subunit KO mice were then bred in house. The β1-subunit KO mice are congenic as a result of inbreeding to the C57BL/6 strain. Control mice were C57BL/6 (WT) mice from Jackson Laboratories (Bar Harbor, ME). β1-subunit KO mice were weaned at 3 wk. All mice were fed normal diet and were studied at 10–12 wk of age (male, 25–30 g). Mice were euthanized using isoflurane anesthesia followed by cervical dislocation. We have used BK channel β1-subunit KO mice in a number of previously published studies of smooth muscle function in vitro and in vivo.11,18,19
In vitro studies
Cholinergic reactivity of small intestinal and colonic longitudinal muscle
The small intestine and colon of WT and β1-subunit KO mice were dissected into proximal and distal segments. Each segment (~1cm) was suspended in individual jacketed tissue baths (25 mL volume) filled with warmed (37 °C), oxygenated (95% O2, 5% CO2) Krebs buffer solution of the following composition (millimolar): 117 NaCl, 4.7 KCl, 2.5 CaCl2, 1.2 MgCl2, 1.2 NaH2PO4, 25 NaHCO3, and 11 glucose. The tissue was attached to a tissue holder and a force-displacement transducer (FT03C, Grass Instruments, Inc.) using silk thread. After a 20 minute incubation period, the optimal initial tension in which the tissue displayed the maximum force in response to acetylcholine (ACh, 100 μmol L−1) was determined by increasing passive tension in stepwise increments until the maximum ACh-induced contraction was achieved. Small intestinal and colonic segments were placed under 0.5 g and 0.8g initial tension, respectively. All tissue segments were allowed to equilibrate for 20 min. The muscarinic cholinergic receptor agonist, bethanechol (0.3–100 μmol L−1) was then tested to determine muscle reactivity in colonic and small intestinal segments. Individual bethanechol concentrations were added at 10 min intervals for 1 min to ensure sufficient time for maximum contraction to occur. Bethanechol was washed out after 1 min. Data were recorded and analyzed using a Powerlab system and Chart software (ADI Instruments, Colorado Springs, CO http://www.adinstruments.com/). After each experiment, tissues were weighed and results are expressed as force (g) per tissue weight (mg).
Intracellular recordings of smooth muscle electrical activity
Conventional intracellular electrophysiological techniques were used to obtain recordings of smooth muscle electrical activity in the distal colon of WT and β1-subunit KO mice. Small segments of distal colon were placed in a petri dish containing Krebs buffer solution. The segment was cut open along the mesenteric border and pinned flat. The mucosa and submucosa were removed using fine forceps. A 5 mm2 section was cut out and transferred to a silastic elastomer-lined recording chamber. The section was stretched lightly and pinned to the chamber floor using small stainless steel pins. The chamber was placed on the stage of an inverted microscope and the chamber was then perfused with oxygenated (95% O2, 5% CO2) Krebs buffer solution (37 °C) at a flow rate of 3 mL min−1. Drugs were added in known concentrations to the flowing Krebs solution using a system of 3-way stopcocks. Drug concentrations reached steady state in the recording chamber within 4 min.
Smooth muscle cells were impaled with glass microelectrodes (filled with 2 M KCl, tip resistance = 60 – 90 MΩ). Although the tissue was anchored to the floor of the recording chamber, there was still substantial muscle movement throughout the recording periods. Despite this complication, it was possible to maintain impalements for periods of 2 – 60 min. Membrane potential was recorded using an Axoclamp 2A amplifier, a Digidata 1322A analog-digital converter and Axoscope 9.2 software (all from Molecular Devices, Sunnyvale, CA USA). Data were stored on a computer hard drive for later analysis. Data were only collected and analyzed from individual recordings that lasted ≥ 2 min.
Colonic Migrating Motor Complex (CMMC) Studied In Vitro
Preparation of colonic segments for recordings of the CMMC was performed as described previously.(20) Briefly, the entire colon was removed from euthanized mice and placed into Krebs buffer solution. Luminal contents were gently flushed out with Krebs buffer solution. A stainless-steel rod was inserted into the lumen and the tissue was secured at each end with surgical silk. Metal clips (Fine Science Tools, Foster City, CA USA) were attached to the oral and anal end of the tissue 2 cm apart and connected by surgical silk to separate force transducers. The rod holding the tissue was secured in a bath (30 mL) containing oxygenated Krebs buffer solution (37 °C) and stretched to an initial tension of 0.5 g. The tissue was allowed to equilibrate for 1 h during which a regular pattern of propagating contractions was established (CMMC). The CMMC was recorded for an additional hour and a 20 min segment was selected for analysis. During the entire experiment, resting tension was monitored and the Krebs buffer solution was changed every 20 min. Frequency, amplitude, and duration of CMMCs were measured as described previously.(20) Propagated CMMCs were determined as a complex in which a contraction occurred first at the oral recording site followed by a contraction at the anal site. Contractions propagated between the two recording sites at a velocity of ~2 mm sec−1.(20) Retrogradely propagated contractions (anal to oral) were identified as those complexes in which a contraction occurred first at the anal recording site and after several seconds, a contraction occurred at the oral recording site. Recordings were obtained using 2 Grass Instruments CP122A strain gauge amplifiers. The output of these amplifiers was fed to an analog/digital converter (Minidigi 1A, Molecular Devices, Sunnyvale, CA; http://www.moleculardevices.com/) and Axoscope software 9.2 (Molecular Devices).
Motility Studies In Vivo
Fecal pellet output was evaluated in WT and β1-subunit KO mice. Mice were individually housed and food and water were removed for 1 h between 10 and 11 AM. Fecal pellets were collected from each mouse during this time and pellets were counted and weighed. Fecal pellets were placed into a 60 °C oven overnight and dry weight was then determined. This protocol was repeated on 3 consecutive days. Fecal pellet wet and dry weight from each animal was averaged over the 3 day period and that average value was used for each mouse. These values were averaged for statistical comparisons.
Colonic motility was assessed in vivo by measuring time to expulsion of a glass bead inserted 2 cm into the colon. A single bead (3 mm) was inserted 2 cm into the distal colon of WT and β1-subunit KO mice that were fasted 12 h prior to experimentation. Expulsion time was then determined for each mouse.21
Small intestinal transit was assessed in vivo. WT and β1-subunit KO mice were fasted 12 h prior to experimentation. Mice were gavaged with 0.1 mL of 10 mg mL−1 FITC-dextran. After 30 min, mice were euthanized. The gastrointestinal tract from the stomach to the ileum was removed. The small intestine was sectioned into 6 segments (5 cm). Segments were flushed with 1.5 mL PBS and the content of each segment was placed in a microfuge tube and quickly centrifuged. FITC fluorescence activity was measured (488 nm excitation) in a 200 μL aliquot of each sample using a plate reader (Fluoroskan Ascent/FL, Thermo Scientific, Rockford, IL). The percentage of total FITC fluorescence in the stomach and each intestinal segment was determined and the geometric center (GC) of the fluorescence distribution was calculated.22
Reagents and drugs
All drugs and reagents were obtained from Sigma Aldrich Chemical Company (St. Louis, MO USA).
Statistical Analysis
Two group comparisons were made using paired or unpaired Student’s t-test or Fisher’s exact test. Comparisons of bethanechol concentration response curves in tissues from WT and β1-subunit KO mice were made using a two way ANOVA and Bonferoni’s post hoc test. This was done across all tissues because bethanechol concentration response curves in distal colon preparations did not reach a maximum response at the highest concentration tested of 100 μmol L−1. Therefore, calculation of accurate half maximal effective concentrations (EC50) was not possible. For all comparisons P < 0.05 was considered statistically significant. Data are reported as mean ± S.E.M.
RESULTS
Reduced distal colonic propulsive motility in β1-subunit KO mice in vivo
To investigate gut function in vivo, fecal pellet output was measured. The number of fecal pellets produced in 1 h was substantially less in β1-subunit KO compared to WT mice (Fig. 1A). This result was quantitated by measuring fecal pellet wet and dry weights, which revealed significant reductions from β1-subunit KO compared to WT mice (Fig. 1B). Further evidence for reductions in propulsive distal colonic motility in β1-subunit KO mice was obtained in the glass bead expulsion time assay. These studies revealed significantly longer expulsion times in the β1-subunit KO compared to WT mice (Fig. 1C).
Figure 1.

Reduced propulsive colonic motility in β1-subunit KO mice. (A) Fecal pellet output was measured over a 1 h period for 3 consecutive days in each mouse. The 3 day average for each mouse was used as a measure of output for that animal. Fecal pellet wet and dry weight were lower in β1-subunit KO (n=13) compared to WT (n=13) mice (*P < 0.05). (B) The fecal pellet wet/dry ratio was also lower for β1-subunit KO compared to WT mice (*P < 0.05). (C) Expulsion time for a glass bead inserted into the rectum was increased in β1-subunit KO (n= 10) compared to WT (n = 7) mice (*P < 0.05).
The results above suggested that impaired BK channel function is associated with reduced propulsive distal colonic motility. We next measured gastric emptying and small intestinal transit to determine if BK channel deficiencies caused reductions in upper GI propulsive motility. FITC-conjugated dextran was administered by gastric gavage and progression of the marker was measured 30 min later. There were no differences in the progression of the marker (as measured by the geometric center of marker distribution) in WT vs. β1-subunit KO mice (Fig. 2).
Figure 2.
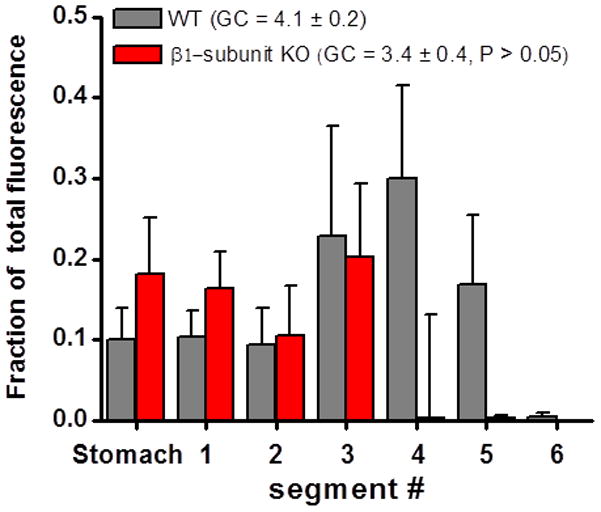
Gastrointestinal transit is not altered in β1-subunit KO mice. Gastrointestinal transit was measured by calculating the geometric center (GC) of the distribution of dextran-FITC in the stomach and 6 small intestinal segments. There was no difference in the mean GC in β1-subunit KO (n=8) and WT mice (n=8)(P > 0.05).
Increased cholinergic reactivity in vitro in distal colon of β1-subunit KO mice
Bethanechol, a muscarinic cholinergic receptor agonist, was used to elicit longitudinal muscle contractions of duodenal, ileal, proximal colon and distal colon segments maintained in vitro. There were no differences in bethanechol concentration response curves in the duodenum, ileum or proximal colon of β1-subunit KO compared to WT mice (Fig. 3A,B,C). However, bethanechol-induced contractions were increased in amplitude in the distal colon of β1-subunit KO compared to WT mice (Fig. 3D). It was not possible to compared bethanechol potency in WT and β1-subunit KO tissues as maximum responses were not achieved in the bethanechol concentration range used in this study.
Figure 3.
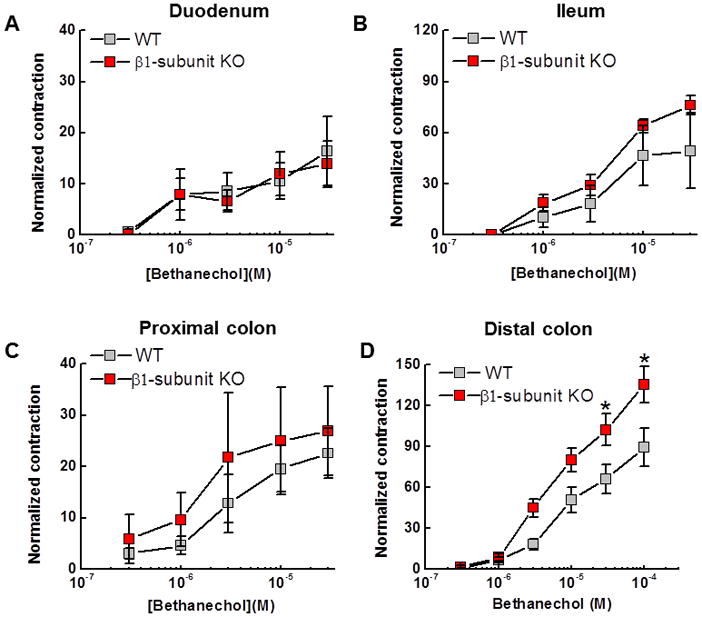
Comparison of in vitro cholinergic reactivity in the small intestine and colon of WT and β1-subunit KO mice. (A, B) Bethanechol-induced contractions of the longitudinal muscle in the duodenum and ileum from WT (n=5) and β1-subunit KO (n=5) mice were similar. (C) Bethanechol-induced contractions in the proximal colon of WT (n=7) and β1-subunit KO (n=8) mice were also similar. (D) There was an increase in contraction amplitude [O1]in the bethanechol concentration response curve in the distal colon of β1-subunit KO (n=8) compared to WT (n=8) mice (*P < 0.05).
Alteration in the CMMC in the distal colon of β1-subunit KO mice
The CMMC is a spontaneous pattern of propagating contractions that occurs in the mouse colon in vitro (Fig. 4A). There were no differences in the amplitude of contractions, frequency of the CMMC or contraction propagation speed when colonic segments from WT and β1-subunit KO mice were compared (data not shown). It was also found that almost 80% of the CMMCs that began at the oral end of the segment propagated to the distal recording site in segments from both WT and β1-subunit KO mice (Fig. 4B). Colonic segments from β1-subunit KO mice exhibited significantly more retrogradely (anal to oral) propagating contractions compared to segments from WT mice (Fig. 4B).
Figure 4.
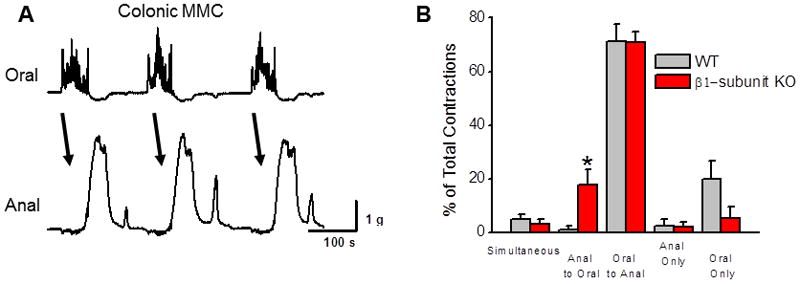
In vitro recordings of CMMC in conic segments from β1-subunit KO and WT mice. (A) Representative recording of CMMC in the colon from a WT mouse. Recording shows contractions propagate regularly in an oral to anal direction. (B) Summary of propagation patterns in colon segments from WT (n=7) and β1-subunit KO (n=9) mice. Almost 80% of CMMCs propagate in an oral to anal direction in tissues from both types of mice. However, there was a significant increase in the percentage of retrogradely (anal to oral) propagated contractions in colonic segments from β1-subunit KO compared to WT mice (*P < 0.05).
Increased action potential activity in distal colonic smooth muscle from β1-subunit KO mice
Intracellular recordings were obtained from circular smooth muscle cells in vitro in the distal colon of WT and β1-subunit KO mice. Recordings were obtained from 42 cells in distal colonic tissues from 11 WT mice. Action potential firing occurred in short bursts with a 10.4 ± 0.6 s interburst interval in 30 cells while action potentials occurred continuously in the remaining 12 cells (n = 9 mice)(Fig. 5A). Recordings were obtained from 29 cells in distal colonic tissues from 7 β1-subunit KO mice. Action potential firing occurred continuously in 23 cells while bursting occurred in the remaining 6 cells (Fig. 5B). The proportion of bursting cells was significantly higher in WT compared to β1-subunit KO mice (P < 0.05, Fisher’s exact test). The average action potential firing frequency was also greater in tissues from β1-subunit KO compared to WT mice (Fig. 5C). The resting membrane potential in circular smooth muscle cells from the distal colon of β1-subunit KO mice was significantly more depolarized compared to that in WT preparations (Fig. 5D). The L-type Ca2+ channel antagonist nifedipine (1 μmol L−1) completely blocked action potential firing in distal colon circular smooth muscle cells from WT and β1-subunit KO mice (Fig. 6). Nifedipine depolarized the membrane potential in distal colon circular smooth muscle cells from WT mice (Fig. 6A, mean depolarization after 5 min nifedipine application = 4.4 ± 2.2 mV). Conversely, nifedipine did not change the membrane potential of distal colon smooth muscle cells from β1-subunit KO mice (Fig. 6B, mean depolarization after 5 min nifedipine application = 2.6 ± 2.3 mV).
Figure 5.
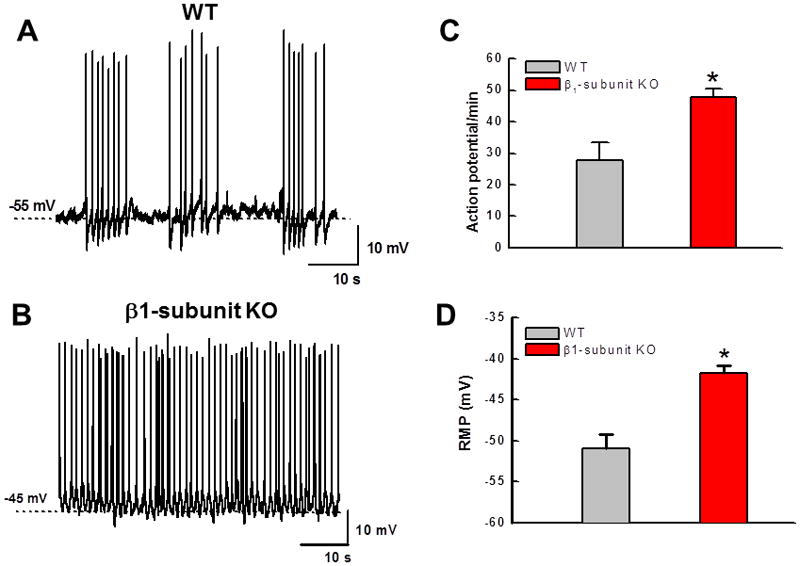
Altered action potential firing and decreased resting membrane potential in distal colon circular smooth muscle cells in vitro from β1-subunit KO compared to WT mice. (A) Representative recording of action potential firing pattern in the distal colon of a WT mice. Action potentials occur in short bursts with a ~10 s interburst interval. (B) Recording of continuous action potential firing in the distal colon of a β1-subunit KO mouse. (C) Mean action potential firing rate is greater in distal colon of β1-subunit KO (n=31 cells from 7 mice) compared to WT (n=42 cells from 11 mice) mice (*P < 0.05). (D) Reduced resting membrane potential in distal colon circular smooth muscle cells from β1-subunit KO compared to WT mice (*P < 0.05).
Figure 6.
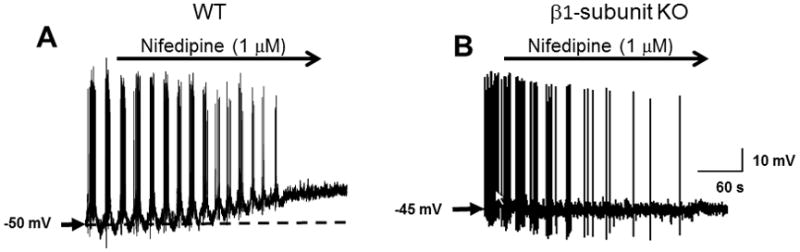
Nifedipine blocks action potential firing in tissues from WT (A) and BK β1-subunit KO mice (B). Nifedipine caused a small depolarization in tissues from WT but not BK β1-subunit KO mice.
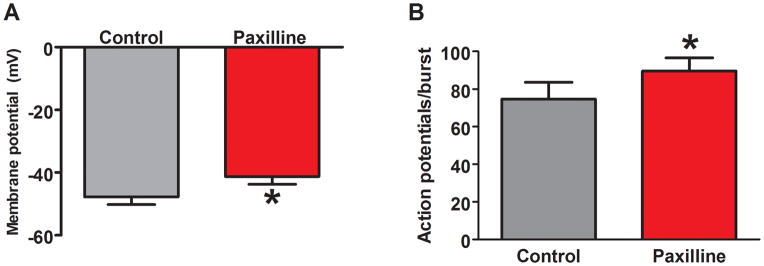
We next tested the effects of paxilline (0.5 μmol L−1), a BK channel blocker, on membrane potential and action potential firing patterns in the distal colon of WT mice. It would be expected that paxilline treatment should mimic the effects of β1-subunit KO. Paxilline treatment for a minimum of 4 minutes caused a membrane potential depolarization (Fig. 7, top trace, Fig. 8A). Paxilline did not change the bursting action potential firing pattern but it did cause an increase in the number of action potentials during each burst (Fig. 7 bottom traces, Fig. 8B).
Figure 7.
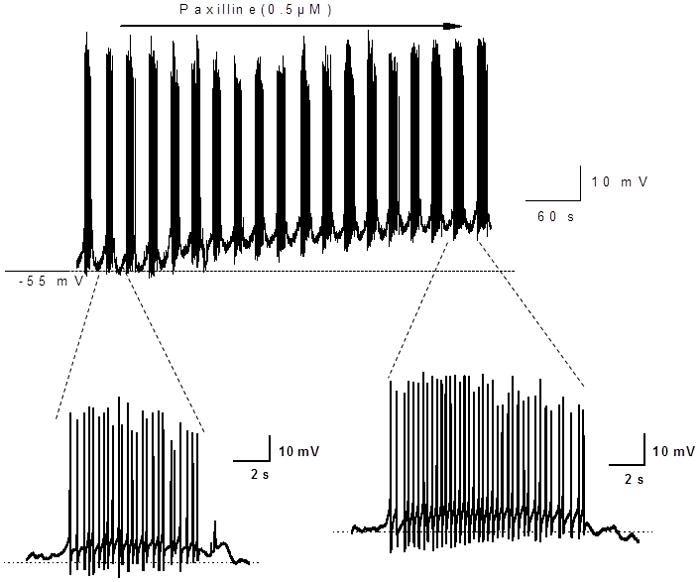
Representative recordings of membrane potential and action potential bursting in smooth muscle cells from the distal colon of a WT mouse and the effects of the BK channel blocker, paxilline. Upper trace shows a slow time base recording of action potential bursting and membrane potential. Paxilline causes a membrane depolarization but action potential bursting persists. Lower traces show expanded views of action potential bursting in the absence (left) and presence (right) of paxilline. Paxilline caused a prolongation of the bursts of action potentials.
Figure 8.
Paxilline caused a significant decrease in resting membrane potential of distal colon smooth muscle cells from WT mice (recordings from 9 cells from 3 mice)(*P< 0.05, paired t-test). Paxilline also caused an increase in the number of action potentials per burst (*P<0.05 vs. control, paired t-test).
DISCUSSION
The results of this study show that compromised BK channel function in the gastrointestinal tract leads to reduced propulsive motility particularly in the distal colon. This is caused by an increase in the excitability of distal colonic smooth muscle cells and a disruption of normal action potential firing patterns.
BK channels and colonic motility
We assessed colonic motility in vivo indirectly by measuring fecal pellet output and glass bead expulsion time. Both techniques are used routinely in mice as it is difficult to make more direct in vivo assessments of colon motility.21,23,24 There are limited studies in which in vivo assessments of colon motility have been made. Intracolonic pressure was assessed at a single site in the distal colon using a radiotelemeter typically used for blood pressure measurements.25 This approach allows measurements of intestinal contractions in unanesthetized and unrestrained mice. However, this technique does not provide information about contraction propagation or patterns. In another study, propagation patterns and intracolonic pressures were determined using miniature manometry catheters and pressure transducers in unanesthetized but restrained mice. Propagated pressure waves were recorded but restraint stress was shown to influence colonic motility patterns.26 Our results indicate fecal pellet output by β1-subunit KO was reduced compared to WT mice. This could result from decreased propulsive motility or from water absorption changes by colonic epithelial cells. The latter is supported by the reduced fecal pellet wet/dry weight ratio from β1-subunit KO mice suggesting increased colonic water absorption. However, glass bead expulsion time was increased in β1-subunit KO mice compared to WT mice suggesting propulsive colonic motility was reduced. Longer colonic transit times would allow for more water absorption accounting for the reduced fecal pellet wet/dry weight ratio from β1-subunit KO mice. In addition, deletion of the β1-subunit in gastrointestinal smooth muscle would alter contractility and therefore propulsive motility, which is consistent with the known function of the BK channel and β1-subunit in smooth muscle cells.
Our data suggest BK channel function plays a more prominent role in controlling colonic motility than either gastric or small intestinal motility. This conclusion is supported by our data showing gastric emptying and small intestinal propulsion of FITC labeled dextran sulfate were not different in WT vs. β1-subunit KO mice. We measured liquid emptying and propulsion in the stomach and small intestine, respectively. BK channel function would contribute to gastric accommodation and small intestinal tone regulation and this may be more critical for solid emptying and propulsion (as occurs in the distal colon). However, our in vitro data indicate BK channel function is more prominent in the distal colon vs. the small intestine (see below). Selective impairment of distal colon transit (as shown by our fecal output and glass bead expulsion data suggest that delayed distal colon transit could lead to a reduction in whole gut transit in the absence of detectable changes in motor function in more proximal regions including the stomach and small intestine.
Alterations in smooth muscle function in vitro in the distal colon of β1-subunit KO mice
Our in vivo data indicate BK channel function is more prominent in the distal colon than in the proximal colon and small intestine. We conducted in vitro studies in an effort to identify possible mechanisms responsible for impairment of propulsive colonic motility. Because acetylcholine is an important stimulant of smooth muscle contraction throughout the gut, we investigated cholinergic reactivity in small intestinal and colonic segments maintained in vitro. We used the muscarinic cholinergic receptor agonist, bethanechol, to minimize activation of enteric neurons that could release modulatory neurotransmitters confounding interpretations of any genotypic differences in cholinergic reactivity. Cholinergic reactivity was similar in small intestinal and proximal colon segments from WT and β1-subunit KO mice. However, cholinergic reactivity was increased significantly in distal colon segments from β1-subunit KO vs. those from WT mice as indicated by the increase in contraction amplitude in the bethanechol concentration response curve. This increase in cholinergic reactivity would be consistent with the known function of BK channels and the β1-subunit in smooth muscle. BK channels are activated by Ca2+ released from intracellular stores and the β1-subunit modulates channel function by increasing Ca2+ sensitivity.7,15,16 Absence of the β1-subunit would reduce Ca2+ sensitivity and therefore reduce channel opening. Reductions in outward K+ currents would increase muscle excitability and reactivity to drugs that cause muscle contraction.10,13
BK channel function may be more prominent in the distal colon compared to the small intestine or proximal colon because of the different functional roles of these tissues. The distal colon is primarily a storage organ while the small intestine and proximal colon function to absorb nutrients and water from the gut lumen. Hence, the need for smooth muscle relaxation (which would be facilitated by BK channel activity) is less critical in the small intestine and proximal colon compared to the distal colon.
The conclusion that BK channels function to regulate smooth muscle excitability is supported by our electrophysiological data showing that action potential firing is increased as seen by continuous action potential firing in distal colon smooth muscle cells from β1-subunit KO mice. Conversely, action potentials occurred in short bursts with an interburst interval of ~10 s in WT cells. Action potentials in all smooth muscle cells were Ca2+-dependent because action potentials were blocked by the L-type Ca2+ channel antagonist, nifedipine, and were tetrodotoxin resistant. Bursting behavior in WT cells would result from Ca2+ entry during action potential generation subsequently activating BK channels and bringing the membrane potential below action potential threshold. Firing would cease until intracellular Ca2+ levels fell below those needed to activate BK channels allowing the membrane to depolarize again leading to another burst of action potentials. In β1-subunit KO cells, the BK channel would be less sensitive to Ca2+ and would not open during intracellular Ca2+ increases that would occur during action potential bursts. Post-burst hyperpolarization would be absent and therefore smooth muscle cells could fire action potentials continuously. Continuous firing would also be driven by the depolarized membrane potential we measured in β1-subunit KO smooth muscle cells. The BK channel blocker, paxilline partially mimicked the effects of β1-subunit KO. Paxilline caused a depolarization of smooth muscle membrane potential supporting the role of the BK channel in regulating membrane potential. However, paxilline did not change the action potential firing pattern from bursting to continuous. In the presence of paxilline, bursting behavior was sustained but the duration of each burst was prolonged. These data suggest that blocking the BK channel has different effects on smooth muscle activity compared to deletion of a molecular component of the channel. Alternatively, there may be other molecular changes in the mechanisms controlling smooth muscle excitability in response to β1-ubunit KO that are not mimicked by acute pharmacological blockade of the BK channel.
Nifedipine caused a depolarization in distal colon smooth muscle cells from WT but not β1-subunit KO mice. This is consistent with Ca2+ entry through L-type Ca2+ channels driving BK channel activation and hyperpolarization of the membrane potential. In the absence of the β1-subunit, the α-subunit is less sensitive to Ca2+ activation and therefore the membrane potential of smooth muscle cells is less polarized. As stated above, nifedipine was able to block action potentials in both WT and β1-subunit KO cells. In β1-subunit KO cells, action potentials occur continuously until the addition of nifedipine. Nifedipine acts to reduce gradually the action potential firing frequency as nifedipine concentrations slowly reached a steady state level. Firing frequency decreased but we did not detect bursting in β1-subunit KO cells as action potentials fired continuously but at a progressively lower frequency.
CMMCs are disrupted in the distal colon of β1-subunit KO mice
In the colon, fecal material is propelled by giant migrating contractions that occur periodically and move colonic content along the length of the colon. The CMMC in the mouse colon is the human equivalent of these giant migrating contractions and the CMMC occurs spontaneously in the mouse colon in vitro. This provides an opportunity for detailed studies of the mechanisms responsible for these propulsive contractions. The CMMC occurs in regular intervals with contractions that begin at the oral end of colonic segments and propagate to the anal end in > 80% of cases. We found that contractions occurred at the anal end of the segment before a contraction at the oral end more often in segments from β1-subunit KO compared to WT mice. This disruption in the normal propagation pattern could be a result of the increase in smooth muscle excitability described above in tissues from β1-subunit KO mice. Disruption of oral to anal propagation would also be responsible for reduced fecal pellet output and prolonged glass bead expulsion time detected in our in vivo studies. Hagen et al.17 studied the electrophysiological properties of colon smooth muscle cells from β1-subunit KO mice. Although they did not study integrative colonic function, they did report that colons of β1-subunit KO mice had loose fecal material that was not formed into pellets typically seen in the colon from WT mice. These observations differ from our own data where we found that β1-subunit KO mice produce hard dry fecal pellets that might have been caused by reduced propulsive colonic motility. The previous study did not provide any specific data on this issue so it is difficult to compare the data from the previous work with our study.
Summary and conclusions
The β1-subunit of the BK channel is smooth muscle specific and regulates Ca2+ sensitivity of the pore forming α-subunit. Knockout of the gene encoding the β1-subunit in mice causes impaired colonic propulsive motility with little effect on upper GI motility. Disruption of propagated propulsive contractions is due to a change in action potential firing patterns in circular smooth muscle from a bursting pattern to continuous action potential firing.
Slow transit constipation can occur in different segments of the colon. Our data suggest that the β1-subunit KO mouse may be a model of segmental slow transit constipation in humans in which colonic dysmotility affects the left-side (descending colon). Slow transit constipation is associated with altered propulsive pressure waves at least in a subset of patients. In addition, there are neuronal, myogenic and Interstitial cell of Cajal (ICC) based alterations associated with slow transit constipation in humans.27–29 These changes include loss of ICC and myenteric nerve fibers containing substance P and VIP.30 Myogenic deficiencies in human slow transit constipation have been identified using histopathological assessments but it is also possible that functional changes not revealed by histology could be responsible for impaired muscle function. Altered function or expression of ion channels in smooth muscle could disrupt propulsive motility patterns. Our data suggest that impaired BK channel function would result in slow transit constipation. These data also suggest that the β1-subunit KO mouse may be a suitable animal model for testing prokinetic drugs that could be used for treatment of slow transit constipation due to alteration in distal or descending colon motor function.
Acknowledgments
This work was supported by a grant from the National Institutes of Health, USA (DKR5650738 to JJG).
The authors thank Dr. Robert Brenner (University of Texas, San Antonio USA) for the gift of the BK β1-subunit KO breeder mice.
Footnotes
The authors have no financial or other conflicts to disclose.
MF and HX performed the in vitro and in vivo motility studies, and analyzed these data. MF wrote the first draft of the manuscript. YB performed the intracellular electrophysiology studies and analyzed these data. JJG prepared the final draft of the manuscript and provided funding for the work.
References
- 1.Salkoff L, Butler A, Ferreira G, Santi C, Wei A. High-conductance potassium channels of the SLO family. Nat Rev Neurosci. 2006;7:921–931. doi: 10.1038/nrn1992. [DOI] [PubMed] [Google Scholar]
- 2.Tricarico D, Mele A, Conte Camerino D. Phenotype-dependent functional and pharmacological properties of BK channels in skeletal muscle: effects of microgravity. Neurobiol Dis. 2005;20:296–302. doi: 10.1016/j.nbd.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 3.Ko JH, Ibrahim MA, Park WS, et al. Cloning of large-conductance Ca2+-activated K+ channel alpha-subunits in mouse cardiomyocytes. Biochem Biophys Res Commun. 2009;389:74–79. doi: 10.1016/j.bbrc.2009.08.087. [DOI] [PubMed] [Google Scholar]
- 4.Wellman GC, Nelson MT. Signaling between SR and plasmalemma in smooth muscle: sparks and the activation of Ca2+-sensitive ion channels. Cell Calcium. 2003;34:211–229. doi: 10.1016/s0143-4160(03)00124-6. [DOI] [PubMed] [Google Scholar]
- 5.Essin K, Gollasch M, Rolle S, et al. BK channels in innate immune functions of neutrophils and macrophages. Blood. 2009;113:1326–1331. doi: 10.1182/blood-2008-07-166660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Latorre R, Morera FJ, Zaelzer C. Allosteric interactions and the modular nature of the voltage- and Ca2+-activated (BK) channel. J Physiol. 2010;588:3141–3148. doi: 10.1113/jphysiol.2010.191999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sweet TB, Cox DH. Measuring the influence of the BKCa β1 subunit on Ca2+ binding to the BKCa channel. J Gen Physiol. 2009;133:139–150. doi: 10.1085/jgp.200810129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Knaus HG, Folander K, Garcia-Calvo M, et al. Primary sequence and immunological characterization of β-subunit of high conductance Ca2+-activated K+ channel from smooth muscle. J Biol Chem. 1994;269:17274–17278. [PubMed] [Google Scholar]
- 9.Garcia-Calvo M, Knaus HG, McManus OB, Giangiacomo KM, Kaczorowski GJ, Garcia ML. Purification and reconstitution of the high-conductance, calcium-activated potassium channel from tracheal smooth muscle. J Biol Chem. 1994;269:676–682. [PubMed] [Google Scholar]
- 10.Semenov I, Wang B, Herlihy JT, Brenner R. BK channel β1 subunits regulate airway contraction secondary to M2 muscarinic acetylcholine receptor mediated depolarization. J Physiol. 2011;589:1803–1817. doi: 10.1113/jphysiol.2010.204347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu H, Kandlikar SS, Westcott EB, Fink GD, Galligan JJ. Requirement for functional BK channels in maintaining oscillation in venomotor tone revealed by species differences in expression of the beta1 accessory subunits. J Cardiovasc Pharmacol. 2012;59:29–36. doi: 10.1097/FJC.0b013e318233614c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brenner R, Peréz GJ, Bonev AD, et al. Vasoregulation by the beta1 subunit of the calcium-activated potassium channel. Nature. 2000;407:870–876. doi: 10.1038/35038011. [DOI] [PubMed] [Google Scholar]
- 13.Petkov GV, Bonev AD, Heppner TJ, Brenner R, Aldrich RW, Nelson MT. Beta1-subunit of the Ca2+-activated K+ channel regulates contractile activity of mouse urinary bladder smooth muscle. J Physiol. 2001;537:443–452. doi: 10.1111/j.1469-7793.2001.00443.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vandael DH, Marcantoni A, Mahapatra S, et al. Ca(v)1. 3 and BK channels for timing and regulating cell firing. Mol Neurobiol. 2010;42:185–198. doi: 10.1007/s12035-010-8151-3. [DOI] [PubMed] [Google Scholar]
- 15.Ohi Y, Yamamura H, Nagano N, et al. Local Ca2+ transients and distribution of BK channels and ryanodine receptors in smooth muscle cells of guinea-pig vas deferens and urinary bladder. J Physiol. 2001;534:313–326. doi: 10.1111/j.1469-7793.2001.t01-3-00313.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Semenov I, Wang B, Herlihy JT, Brenner R. BK channel β1-subunit regulation of calcium handling and constriction in tracheal smooth muscle. Am J Physiol. 2006;291:L802–L810. doi: 10.1152/ajplung.00104.2006. [DOI] [PubMed] [Google Scholar]
- 17.Hagen BM, Bayguinov O, Sanders KM. Beta 1-subunits are required for regulation of coupling between Ca2+ transients and Ca2+-activated K+ (BK) channels by protein kinase C. Am J Physiol. 2003;285:C1270–C1280. doi: 10.1152/ajpcell.00153.2003. [DOI] [PubMed] [Google Scholar]
- 18.Xu H, Wang Y, Garver H, Galligan JJ, Fink GD. Vascular BK Channel Deficiency Exacerbates Organ Damage and Mortality in Endotoxemic Mice. J Cardiovasc Pharmacol. 2012;59:207–214. doi: 10.1097/FJC.0b013e31823b493b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu H, Garver H, Galligan JJ, Fink GD. Large-conductance Ca2+-activated K+ channel beta1-subunit knockout mice are not hypertensive. Am J Physiol. 2011;300:H476–H485. doi: 10.1152/ajpheart.00975.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Devries MP, Vessalo M, Galligan JJ. Deletion of P2X2 and P2X3 receptor subunits does not alter motility of the mouse colon. Front Neurosci. 2010;4:1–7. doi: 10.3389/fnent.2010.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Raffa RB, Mathiasen JR, Jacoby HI. Colonic bead expulsion time in normal and mu-opioid receptor deficient (CXBK) mice following central (ICV) administration of mu- and delta-opioid agonists. Life Sci. 1987;41:2229–2234. doi: 10.1016/0024-3205(87)90520-0. [DOI] [PubMed] [Google Scholar]
- 22.Miller MS, Galligan JJ, Burks TF. Accurate measurement of intestinal transit in the rat. J Pharmacol Methods. 1981;6:211–217. doi: 10.1016/0160-5402(81)90110-8. [DOI] [PubMed] [Google Scholar]
- 23.Stengel A, Goebel-Stengel M, Wang L, Larauche M, Rivier J, Taché Y. Central somatostatin receptor 1 activation reverses acute stress-related alterations of gastric and colonic motor function in mice. Neurogastroenterol Motil. 2011;23:e223–236. doi: 10.1111/j.1365-2982.2011.01706.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fichna J, Dicay M, Hirota SA, et al. Differential effects of salvinorin A on endotoxin-induced hypermotility and neurogenic ion transport in mouse ileum. Neurogastroenterol Motil. 2011;23:583–e212. doi: 10.1111/j.1365-2982.2011.01699.x. [DOI] [PubMed] [Google Scholar]
- 25.Hoogerwerf WA, Shahinian VB, Cornélissen G, et al. Rhythmic changes in colonic motility are regulated by period genes. Am J Physiol. 2010;298:G143–G150. doi: 10.1152/ajpgi.00402.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gourcerol G, Wang L, Adelson DW, Larauche M, Taché Y, Million M. Cholinergic giant migrating contractions in conscious mouse colon assessed by using a novel noninvasive solid-state manometry method: modulation by stressors. Am J Physiol. 2009;296:G992–G1002. doi: 10.1152/ajpgi.90436.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kashyap P, Gomez-Pinilla PJ, Pozo MJ, Cima RR, Dozois EJ, Larson DW, Ordog T, Gibbons SJ, Farrugia G. Immunoreactivity for Ano1 detects depletion of Kit-positive interstitial cells of Cajal in patients with slow transit constipation. Neurogastroenterol Motil. 2011;23:760–765. doi: 10.1111/j.1365-2982.2011.01729.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Knowles CH, Farrugia G. Gastrointestinal neuromuscular pathology in chronic constipation. Best Pract Res Clin Gastroenterol. 2011;25:43–57. doi: 10.1016/j.bpg.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dinning PG, Zarate N, Hunt LM, et al. Pancolonic spatiotemporal mapping reveals regional deficiencies in, and disorganization of colonic propagating pressure waves in severe constipation. Neurogastroenterol Motil. 2010;22:e340–e349. doi: 10.1111/j.1365-2982.2010.01597.x. [DOI] [PubMed] [Google Scholar]
- 30.King SK, Sutcliffe JR, Ong SY, et al. Substance P and vasoactive intestinal peptide are reduced in right transverse colon in pediatric slow-transit constipation. Neurogastroenterol Motil. 2010;22:883–e234. doi: 10.1111/j.1365-2982.2010.01524.x. [DOI] [PubMed] [Google Scholar]