Abstract
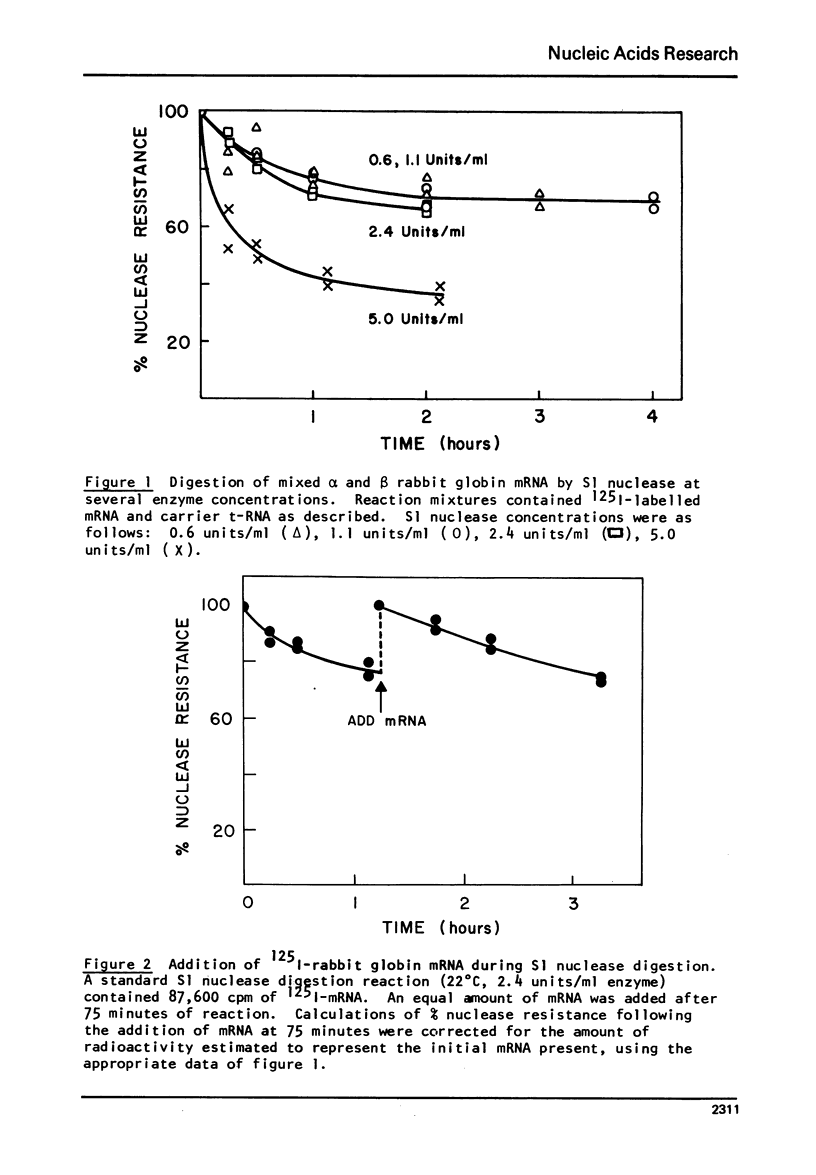
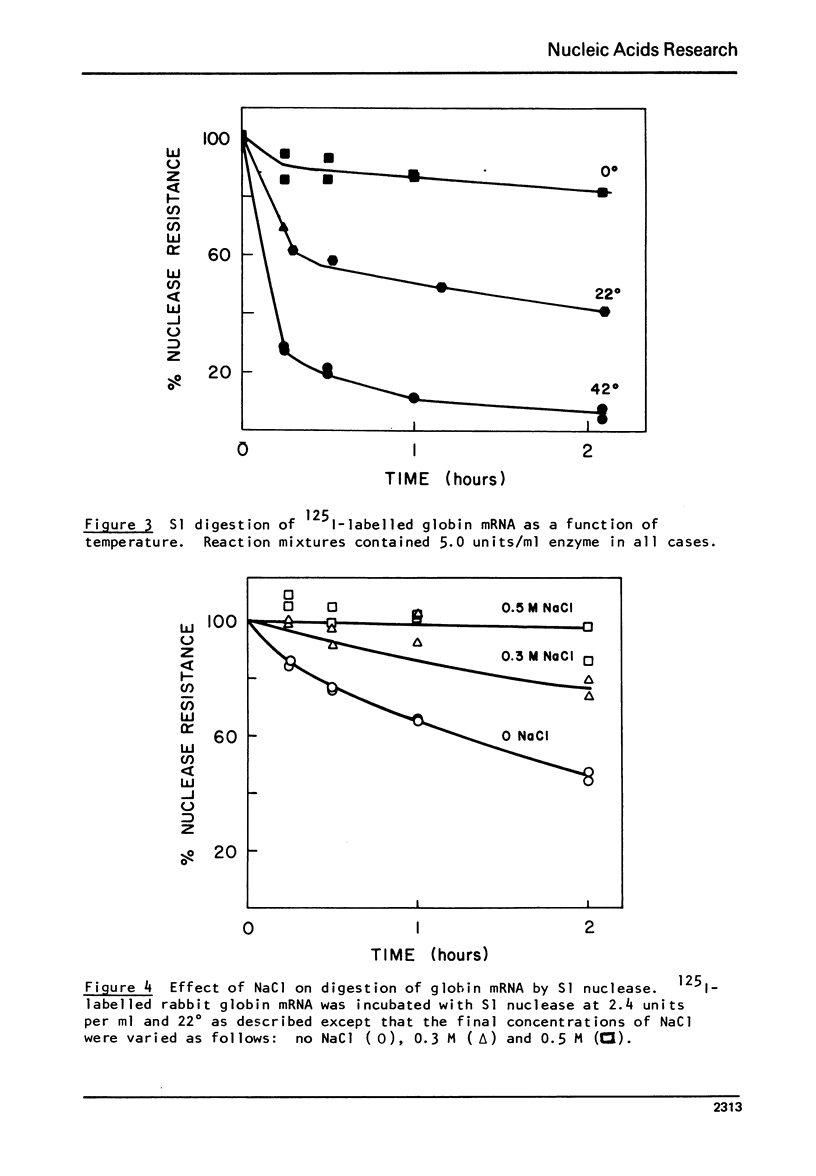
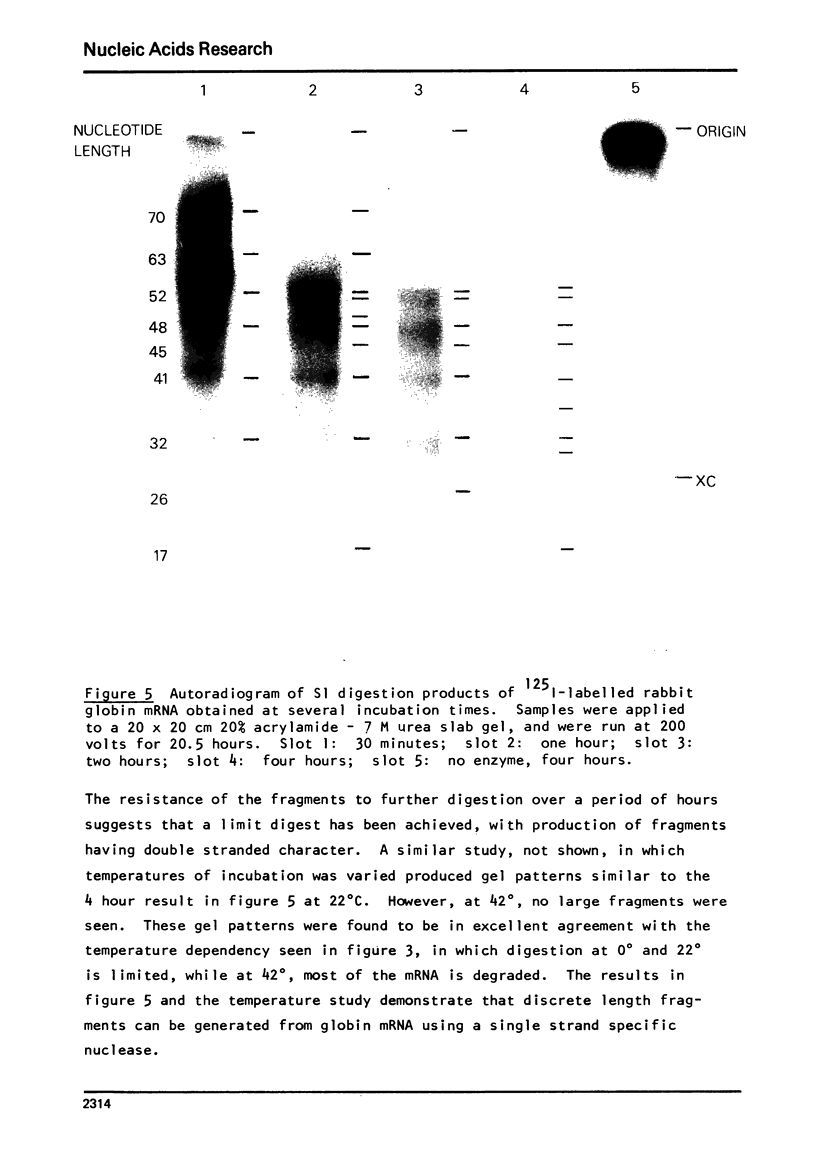
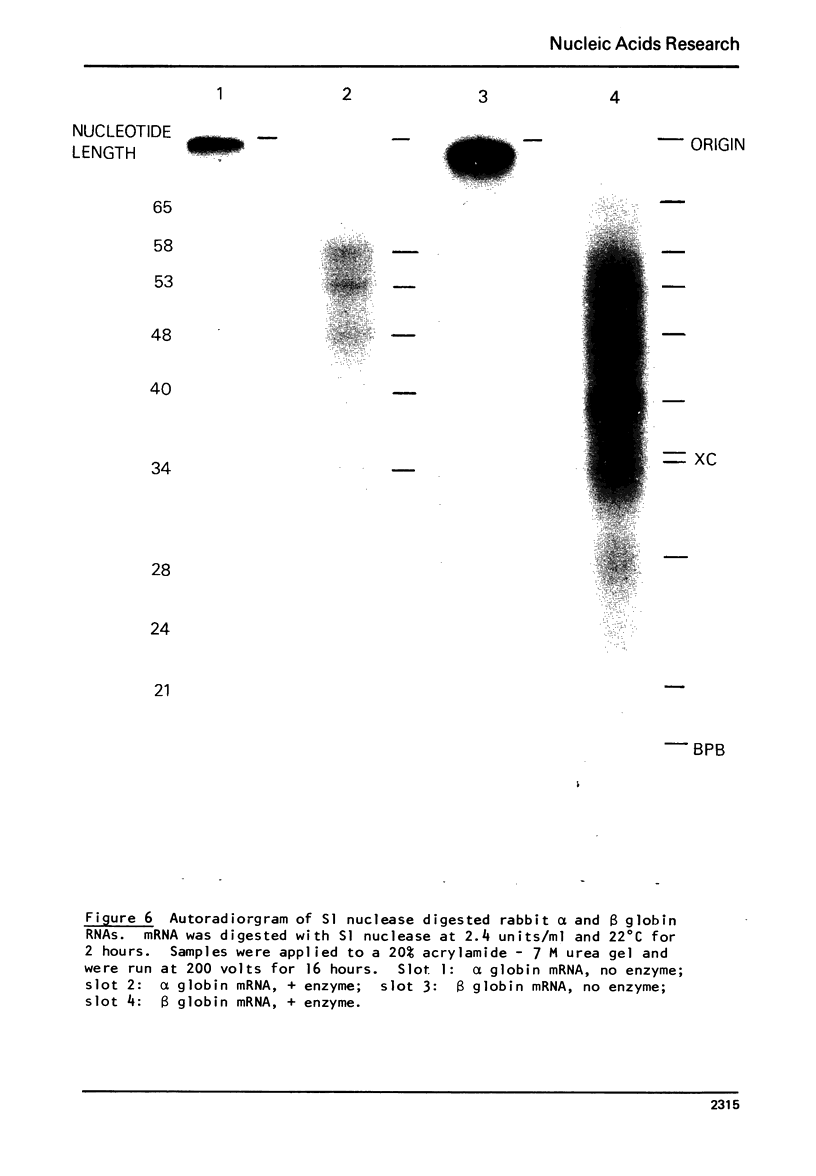
S1 nuclease isolated from Aspergillus oryzae has been used to investigate the secondary structure of rabbit globin messenger RNA (mRNA). The enzyme, which is specific for single stranded nucleotides, digests globin mRNA to a limited extent, with 65-75% of the mRNA nucleotides resistant to digestion under mild conditions. This limited digestion is not due to enzyme inactivation, but rather to the normal activity of the single-strand nuclease. The reaction was studied as a function of temperature, salt and enzyme concentration. Analysis of the products of digestion on 20% acrylamide- 7M urea slab gels reveals a stable pattern of unique fragments ranging in size from 9 to 71 nucleotides. Separated alpha and beta globin mRNAs show similar, but not identical gel patterns, indicating strong structural similarities between the two species. The high degree of nuclease resistance, along with the fragment patterns seen on polyacrylamide gels, gives evidence to support a model of rabbit globin mRNA which contain specific, rather than random, helical structure.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams J. M., Cory S. Modified nucleosides and bizarre 5'-termini in mouse myeloma mRNA. Nature. 1975 May 1;255(5503):28–33. doi: 10.1038/255028a0. [DOI] [PubMed] [Google Scholar]
- Ando T. A nuclease specific for heat-denatured DNA in isolated from a product of Aspergillus oryzae. Biochim Biophys Acta. 1966 Jan 18;114(1):158–168. doi: 10.1016/0005-2787(66)90263-2. [DOI] [PubMed] [Google Scholar]
- Beard P., Morrow J. F., Berg P. Cleavage of circular, superhelical simian virus 40 DNA to a linear duplex by S1 nuclease. J Virol. 1973 Dec;12(6):1303–1313. doi: 10.1128/jvi.12.6.1303-1313.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourguignon G. J., Tattersall P. J., Ward D. C. DNA of minute virus of mice: self-priming, nonpermuted, single-stranded genome with a 5'-terminal hairpin duplex. J Virol. 1976 Oct;20(1):290–306. doi: 10.1128/jvi.20.1.290-306.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brawerman G. Eukaryotic messenger RNA. Annu Rev Biochem. 1974;43(0):621–642. doi: 10.1146/annurev.bi.43.070174.003201. [DOI] [PubMed] [Google Scholar]
- Commerford S. L. Iodination of nucleic acids in vitro. Biochemistry. 1971 May 25;10(11):1993–2000. doi: 10.1021/bi00787a005. [DOI] [PubMed] [Google Scholar]
- Efstratiadis A., Maniatis T., Kafatos F. C., Jeffrey A., Vournakis J. N. Full length and discrete partial reverse transcripts of globin and chorion mRNAs. Cell. 1975 Apr;4(4):367–378. doi: 10.1016/0092-8674(75)90157-9. [DOI] [PubMed] [Google Scholar]
- Favre A., Morel C., Scherrer K. The secondary structure and poly(A) content of globin messenger RNA as a pure RNA and in polyribosome-derived ribonucleoprotein complexes. Eur J Biochem. 1975 Sep 1;57(1):147–157. doi: 10.1111/j.1432-1033.1975.tb02285.x. [DOI] [PubMed] [Google Scholar]
- Furuichi Y., Morgan M., Shatkin A. J., Jelinek W., Salditt-Georgieff M., Darnell J. E. Methylated, blocked 5 termini in HeLa cell mRNA. Proc Natl Acad Sci U S A. 1975 May;72(5):1904–1908. doi: 10.1073/pnas.72.5.1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germond J. E., Vogt V. M., Hirt B. Characterization of the single-strand-specific nuclease S1 activity on double-stranded supercoiled polyoma DNA. Eur J Biochem. 1974 Apr 16;43(3):591–600. doi: 10.1111/j.1432-1033.1974.tb03446.x. [DOI] [PubMed] [Google Scholar]
- Gould H. J., Hamlyn P. H. The molecular weight of rabbit globin messenger RNA's. FEBS Lett. 1973 Mar 15;30(3):301–304. doi: 10.1016/0014-5793(73)80674-x. [DOI] [PubMed] [Google Scholar]
- Harada F., Dahlberg J. E. Specific cleavage of tRNA by nuclease S1. Nucleic Acids Res. 1975 Jun;2(6):865–871. doi: 10.1093/nar/2.6.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holder J. W., Lingrel J. B. Determination of secondary structure in rabbit globin messenger RNA by thermal denaturation. Biochemistry. 1975 Sep 23;14(19):4209–4215. doi: 10.1021/bi00690a009. [DOI] [PubMed] [Google Scholar]
- Holder J. W., Lingrel J. B. Determination of secondary structure in rabbit globin messenger RNA by thermal denaturation. Biochemistry. 1975 Sep 23;14(19):4209–4215. doi: 10.1021/bi00690a009. [DOI] [PubMed] [Google Scholar]
- Kazazian H. H., Jr Separation of alpha- and beta-globin messenger RNAs. Nat New Biol. 1972 Aug 9;238(84):166–169. doi: 10.1038/newbio238166a0. [DOI] [PubMed] [Google Scholar]
- Kazazian H. H., Jr, Snyder P. G., Cheng T. C. Separation of alpha- and beta-globin messenger RNAs by formamide gel electrophoresis. Biochem Biophys Res Commun. 1974 Aug 5;59(3):1053–1061. doi: 10.1016/s0006-291x(74)80086-0. [DOI] [PubMed] [Google Scholar]
- Leong J. A., Garapin A. C., Jackson N., Fanshier L., Levinson W., Bishop J. M. Virus-specific ribonucleic acid in cells producing rous sarcoma virus: detection and characterization. J Virol. 1972 Jun;9(6):891–902. doi: 10.1128/jvi.9.6.891-902.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., van deSande H. Chain length determination of small double- and single-stranded DNA molecules by polyacrylamide gel electrophoresis. Biochemistry. 1975 Aug 26;14(17):3787–3794. doi: 10.1021/bi00688a010. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Kee S. G., Efstratiadis A., Kafatos F. C. Amplification and characterization of a beta-globin gene synthesized in vitro. Cell. 1976 Jun;8(2):163–182. doi: 10.1016/0092-8674(76)90001-5. [DOI] [PubMed] [Google Scholar]
- Milstein C., Brownlee G. G., Cartwright E. M., Jarvis J. M., Proudfoot N. J. Sequence analysis of immunoglobulin light chain messenger RNA. Nature. 1974 Nov 29;252(5482):354–359. doi: 10.1038/252354a0. [DOI] [PubMed] [Google Scholar]
- Méchali M., de Recondo A. M., Girard M. Action of the S1 endonuclease from Aspergillus oryzae on simian virus 40 supercoiled component I DNA. Biochem Biophys Res Commun. 1973 Oct 15;54(4):1306–1320. doi: 10.1016/0006-291x(73)91130-3. [DOI] [PubMed] [Google Scholar]
- Nienhuis A. W., Falvey A. K., Anderson W. F. Preparation of globin messenger RNA. Methods Enzymol. 1974;30:621–630. doi: 10.1016/0076-6879(74)30060-2. [DOI] [PubMed] [Google Scholar]
- Prensky W., Steffensen D. M., Hughes W. L. The use of iodinated RNA for gene localization. Proc Natl Acad Sci U S A. 1973 Jun;70(6):1860–1864. doi: 10.1073/pnas.70.6.1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proudfoot N. J. Sequence analysis of the 3' non-coding regions of rabbit alpha- and beta-globin messenger RNAs. J Mol Biol. 1976 Nov 15;107(4):491–525. doi: 10.1016/s0022-2836(76)80080-0. [DOI] [PubMed] [Google Scholar]
- Rushizky G. W., Shaternikov V. A., Mozejko J. H., Sober H. A. S1 nuclease hydrolysis of single-stranded nucleic acids with partial double-stranded configuration. Biochemistry. 1975 Sep 23;14(19):4221–4226. doi: 10.1021/bi00690a011. [DOI] [PubMed] [Google Scholar]
- St John T., Johnson J. D., Bonner J. Degradation of duplex DNA by S1 nuclease from Aspergillus. Biochem Biophys Res Commun. 1974 Mar 15;57(1):240–247. doi: 10.1016/s0006-291x(74)80382-7. [DOI] [PubMed] [Google Scholar]
- Tal J. The cleavage of transfer RNA by a single strang specific endonuclease from Neurospora crassa. Nucleic Acids Res. 1975 Jul;2(7):1073–1082. doi: 10.1093/nar/2.7.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt V. M. Purification and further properties of single-strand-specific nuclease from Aspergillus oryzae. Eur J Biochem. 1973 Feb 15;33(1):192–200. doi: 10.1111/j.1432-1033.1973.tb02669.x. [DOI] [PubMed] [Google Scholar]
- Vournakis J. N., Efstratiadis A., Kafatos F. C. Electrophoretic patterns of deadenylylated chorion and globin mRNAs. Proc Natl Acad Sci U S A. 1975 Aug;72(8):2959–2963. doi: 10.1073/pnas.72.8.2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vournakis J. N., Flashner M. S., Katopes M., Kitos G. A., Vamvakopoulos N. C., Sell M. S., Wurst R. M. Structural studies on intact and deadenylylated rabbit globin mRNA. Prog Nucleic Acid Res Mol Biol. 1976;19:233–252. doi: 10.1016/s0079-6603(08)60922-8. [DOI] [PubMed] [Google Scholar]