Abstract
Purpose
A type 2 recombinant human bone morphogenetic protein (rhBMP2) and Masquelet’s procedure were used in three children presenting with congenital pseudarthrosis of the tibia (CPT). Recent studies on CPT suggested the presence in situ of pathologic tissues promoting pseudarthrosis. The authors hypothesized that large segmental resection of pseudarthrosis could improve prognosis of the CPT. Masquelet’s procedure and rhBMP2 have been advocated for the treatment of long bone defect.
Method
The authors report three cases of CPT in children treated with Masquelet’s procedure and application of rhBMP2. They analyzed all published cases of CPT similarly treated.
Results
In the present study, Masquelet’s procedure did not improve the results in the treatment of CPT, but segmental bone reconstruction was possible. Bone healing was obtained in three out of the five applications of rhBMP2. In one case, the patient’s parents asked for leg amputation. Analysis of the 33 published cases with the application of BMP in CPT points to a 62 % healing rate in this pathology.
Conclusion
The authors confirmed that segmental bone reconstruction is possible in CPT using Masquelet’s procedure. In the literature, the success rate of the application of rhBMP in CPT appears to be lower than the healing rate usually reported without BMP. Nevertheless, the strict selection of patients, limited number of cases, and their heterogeneity make interpreting the results difficult. However, the theoretical risk which the children are exposed to during the use of BMP makes rigorous selection of the indications necessary. Finally, the interest of rhBMP2 application in Masquelet’s procedure remained to be proven.
Keywords: Bone morphogenetic proteins, Induce membrane, Congenital pseudarthrosis, Neurofibromatosis, Child
Introduction
Bone morphogenetic proteins (BMPs) have been in clinical use in adults for several years in clearly defined indications: long bones pseudarthrosis, treatment of fresh open fractures, and anterior intersomatic spine arthrodesis or lumbosacral arthrodesis [1, 2]. BMPs are members of the large family of the transforming growth factors (TGFβ). They induce mesenchymal cells proliferation and differentiation into osteoblasts [3–6]. Two of the numerous identified BMPs are used for clinical applications: rhBMP2 and rhBMP7 (human recombinant BMP2 and human recombinant BMP7, respectively). They have been studied in animals and their healing improvement activity is well documented [7, 8]. Nevertheless, little is known about these proteins implicated in the tumor process (especially for long-term follow-up), and, in the absence of specific studies, the principle of caution applies [9, 10]. The use of BMPs in children is, as yet, not allowed, and reported cases are compassionate. Such a lot is at stake in congenital pseudarthrosis of the tibia (CPT) that the outcome could be an amputation [11]. The results obtained using BMPs in adults, particularly in the case of pseudarthrosis of long bones, have opened up the perspective of applying these proteins to children in certain highly complex situations. A few authors have reported their experience in compassionate indications, especially CPT [12–18].
CPT in children is one of the most challenging pathologies in pediatric orthopedics. Numerous procedures have been described in this treatment, with varying degrees of success [19–29]. Moreover, the knowledge of the phenomena altering the physiological bone tissue or the periosteum in neurofibromatosis type 1 (NF1) is not yet complete and has improved even more recently. The studies of Cho et al. [30], Schindeler and Little [31], and Ippolito et al. [32] led us to make the hypothesis that a large resection of pathologic bone and periosteum is required in this disease to improve results. The procedure described by Masquelet et al. [33, 34] (also call “induced membrane procedure”) seemed to be an appropriate procedure for bone reconstruction in this disease. This study reports on and analyses the results of the application of rhBMP2 in addition to or following Masquelet’s procedure in three clinical cases of CPT in children. Two patients were treated twice with BMP. We also analyzed the results of all published cases of CPT in children treated with the application of BMP.
Patients and methods
The limited number of observations made on children led us to envisage analyzing the total number of cases already published and treated with other procedures. To our knowledge, few authors have reported experience (Table 1). We have tried to analyze all the available published cases whilst adding our own experience.
Table 1.
Reported cases of applications of bone morphogenetic proteins (BMPs) in children in the literature
| Authors | Year | Number of cases | Indications | BMP type |
|---|---|---|---|---|
| Fabeck et al. [12] | 2006 | 1 | Cg Ps T | rhBMP7 |
| Anticevic et al. [13] | 2006 | 1 | Cg Ps T | rhBMP7 |
| Lee et al. [14] | 2006 | 5 | Cg Ps T | rhBMP7 |
| Kujala et al. [15] | 2008 | 1 | Cg Ps T | Native bovine BMP |
| Burkhart and Rommens [45] | 2008 | 1 | Tibial Ps after tumoral resection | rhBMP7 |
| Dohin et al. [16] | 2009 | 10 | Cg Ps T | rhBMP7 |
| Richards et al. [17] | 2010 | 7 | Cg Ps T | rhBMP2 |
| Spiro et al. [18] | 2011 | 5 | Cg Ps T | rhBMP2 |
Ps pseudarthrosis, Cg congenital, T tibia, rhBMP7 human recombinant bone morphogenetic protein 7, rhBMP2 human recombinant bone morphogenetic protein 2
The criteria studied were: indication of the use of BMP, type of BMP used, number of bone sites treated, treatment technique used, number of healed bone sites, time taken to heal, length of follow-up, and possible complication or relapse (fracture or pseudarthrosis). In the cases presented by the authors, radiological healing was considered to be reached when at least three out of four bone cortexes of the tibia presented evidence of bone bridging on the two orthogonal X-rays.
The BMPs used in the presented cases and published cases were the two available types: rhBMP2 (InductOs®, Wyeth Europa Ltd™, Metronic™, France) and rhBMP7 (Osigraft®, Stryker Biotech™, Hopkinton, MA, USA). The application technique for rhBMP2 used a sponge of a purified bovine collagen soaked in a reconstituted solution of the active drug. The sponge was then applied to the bone site (with or without a bone graft). In the present cases, only rhBMP2 was used. For rhBMP7, the application technique was somewhat different: the base consisted of powdered purified bovine collagen which was soaked in a reconstituted solution of the active drug and had the consistency of dank sandy dough which was applied to the bone site.
Masquelet’s procedure consists of two surgical procedures [33, 34]. First, a large resection of pathologic tissue was performed and a spacer made of prosthetic cement was put in place of pathologic bone and stabilized with osteosynthesis. Second, 6 weeks later, the spacer was removed, exposing a bone defect surrounded by a new vascularized membrane. The defect was filled with cancellous bone graft and a sponge soaked in a reconstituted solution of rhBMP2 was additionally laid on the bone graft in one case (case 3). The membrane was closed on the bone graft.
In the presented cases, parental consent was requested for application of the rhBMP2, as well as for the exploitation of the medical charts for scientific ends.
There was no external funding source for the study or the authors.
Clinical cases
Case 1—Q.R. was born in June 2000 and had a congenital bow (CB) of the right tibia (Crawford type II). He presented NF1 with a pseudo-tumoral glioma of the optic nerve. In December 2003, he broke his right leg and presented a pseudarthrosis after orthopedic treatment (Fig. 1b); an “induce membrane” (Masquelet’s procedure) was carried out in June 2004 (Fig. 1c). The bone graft was subsequently partially and spontaneously reduced. Finally, a pseudarthrosis developed distally. A second operation, adding a bone graft, some rhBMP2, and an osteosynthesis with a plate, was carried out in January 2005. Healing was achieved in 14 weeks (Fig. 1e) but the plate snapped 1 year later. Yet another graft was carried out in March 2006 with a second application of rhBMP2 and a new osteosynthesis with a plate (Fig. 1g). During post operative care, the surgical area became swollen and severely inflamed with phlyctena and osteolysis of the graft (Fig. 1h). A deep infection with identification of Pseudomonas aeroginosa surrounded and the plate was removed. At the request of the parents, a below-knee amputation was performed in December 2006 (Fig. 1i). At final follow-up (May 2011), Quentin was in good health, using a leg prosthesis.
Fig. 1.
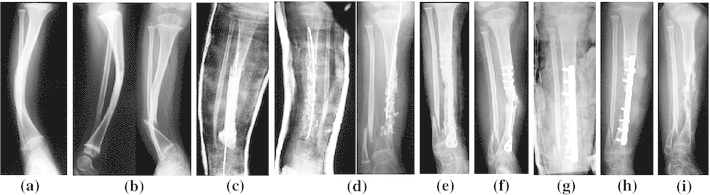
Observation 1, Quentin: a Congenital bow of the tibia in neurofibromatosis type 1 (NF1), b fracture, orthopedic treatment with casting failed, c Masquelet’s procedure, d pseudarthrosis relapses, e first application of human recombinant bone morphogenetic protein 2 (rhBMP2), healing within 14 weeks (distal screws were removed early in order to protect the growth plate), f 1 year follow-up, fracture of the plate, g second application of rhBMP2 and bone grafting, h in situ infection with bone absorption, i radiological result when parents asked for amputation
Case 2—L.K. was born in May 2000 with a cyst shape congenital pseudarthrosis of the left tibia (CPT) (Crawford type III) (Fig. 2a). He walked aged 1, but despite the use of a brace, his leg gradually became misshapen (Fig. 2b). Surgery was necessary with excision of the pseudarthrosis, followed by an autogenous vascularized fibular graft (Fig. 2c) and osteosynthesis using a mono-lateral external fixator. Healing did not occur and the pseudarthrosis set in again (Fig. 2d). In February 2004, an operation using Masquelet’s procedure was performed, but pseudarthrosis continued on the distal part (Fig. 2e). A further excision followed by a bone graft, osteosynthesis with a plate, and application of rhBMP2 was carried out in September 2005. Union was obtained in 28 weeks (Fig. 2f, g). In March 2010, he presented a midshaft fracture of the tibia (Fig. 2h). The orthopedic treatment led to healing and the tibia was still sound at the final follow-up in May 2011 (Fig. 2i).
Fig. 2.
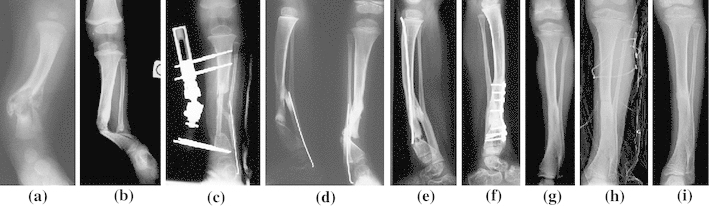
Observation 2, Loris: cystic congenital pseudarthrosis of the tibia (CPT), a 2 months old, b worse evolution despite orthopedic treatment, c first treatment with vascularized autogenous fibula graft and external frame, d pseudarthrosis relapses, e third pseudarthrosis despite performing a Masquelet’s procedure, f third treatment with the application of rhBMP2 leading to bone consolidation, g result in July 2008, h fracture in March 2010 with orthopedic treatment, i result at final follow-up in May 2011
Case 3—A.C. was born in October 2003 with a CB of the tibia (Fig. 3a) in NF1 (Crawford type III). She broke her leg in September 2006 (Fig. 3b). A Masquelet’s procedure was applied in November 2006; the second procedure used a tibial cortical bone graft and an iliac cancellous bone graft associated with rhBMP2 (Fig. 3c, d). In June 2007, a pseudarthrosis was still present proximally to the bone graft (Fig. 3e). When excision of the pseudarthrosis was done (September 2007), a second application of rhBMP2 was made with an iliac cortico-cancellous bone graft (Fig. 3f). Later, a gradual curve appeared in April 2008 and the bone broke underneath the plate with relapse pseudarthrosis (Fig. 3g). In May 2008, another graft with Fassier–Duval nailing was carried out and, by October 2008, finally, the tibia had healed without complication (Fig. 3h, i). At the final follow-up in May 2011, the tibia was still sound (Fig. 3j, k).
Fig. 3.
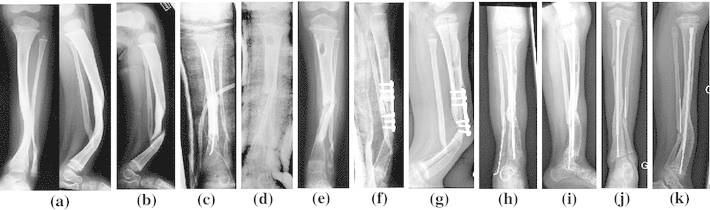
Observation 3, Alicia: a congenital bow of the tibia in NF1, b fracture at 3 years old, c, d first procedure (Masquelet’s) and application of rhBMP2, e 7 months follow-up, pseudarthrosis relapses, f second application of rhBMP2 and bone grafting, g pseudarthrosis relapses, h third procedure with intramedullary nailing (Fassier–Duval nail) and bone grafting (May 2008), i bone consolidation (October 2008), j, k result at final follow-up (May 2011)
Results
The results obtained for these three patients are presented in Table 2. Masquelet’s procedure allowed bone rebuilding after large resection of the pathologic tissues in the three patients. Nevertheless, we noted a pseudarthrosis in every case at one’s extremity of the grafted area. Three of the five pseudarthroses treated with rhBMP2 healed up. In the successful cases, the average healing time was 18 weeks, whereas the average follow-up lasted 24 months. It is to be noted that one of the healed bones broke again after a year, leading to infection after he was re-operated and, eventually, at the parents’ request, to amputation.
Table 2.
Summary of the three cases reported in this study: three procedures used Masquelet’s procedure, five anatomic sites were treated with the application of human recombinant bone morphogenetic protein 2 (rhBMP2), and three anatomic sites healed
| Patients | Number of previous surgeries | Number of treated anatomic sites | Result of Masquelet’s procedure | Number of healed anatomic sites after rhBMP application | Surgical technique of rhBMP application | Follow-up (months)/complications | Delay for consolidation (weeks) |
|---|---|---|---|---|---|---|---|
| 1A | 1 (Masquelet’s procedure) | 1 | Healing proximally and pseudarthrosis distally Total bone rebuilding |
1 | Screwed plate and bone grafting | 12 (fracture) | 14 |
| 1B | 2 | 1 | – | 0 | Screwed plate and bone grafting | 9 (infection and resorption of bone graft) | – (amputation) |
| 2 | 2 (1 Masquelet’s procedure) | 1 | Healing proximally and pseudarthrosis distally Total bone rebuilding |
1 | Screwed plate and bone grafting | 65 | 28 |
| 3A | 0 | 1 (Masquelet’s procedure) | Healing distally and pseudarthrosis proximally Total bone rebuilding |
1 (1 extremity) | Bone grafting and cast immobilization | 4 (pseudarthrosis at 1 out of 2 extremities) | – (1 extremity: 12) |
| 3B | 1 | 1 | – | 0 | Screwed plate and bone grafting | 31 (fracture) | – (pseudarthrosis: nailing and bone grafting, healed 24 later) |
| Total/mean | 5 | Three bone rebuilding Three pseudarthrosis |
3 | 24 months | 18 weeks |
Analysis of cases reported in the literature (Table 3) highlight the fact that most of the observations concern patients who, despite numerous operations, have not got better. Surgical treatment used internal or external devices in the three presented cases. We performed three procedures described by Masquelet et al. [33, 34]. The similarity of the severe condition and the good results reported in this technique encouraged us to adapt the procedure in CPT. In the three present cases, bone segmental resection was totally rebuilt, but we noted persistence of a pseudarthrosis at one out of the two extremities of the segmental bone resection. Osteosynthesis techniques used in the literature were various; nevertheless, nailing and external osteosynthesis with an Ilizarov device have been preferred by several authors, sometimes in combination (Table 4; Fig. 4). Nailing appears to be the procedure with which consolidation has been obtained in most of the cases.
Table 3.
Summary of the published cases and mean delay for consolidation and complications reported by the authors
| Delay for consolidation (average)/follow-up | Number of cases and successful cases | BMP | Surgical technique | Complications and outcome | |
|---|---|---|---|---|---|
| Fabeck et al. [12] | 112 weeks (first radiological signs from 5 weeks)/ns | 1 case, 1 success | rhBMP7 | Nailing and bone grafting | – |
| Anticevic et al. [13] | 24 weeks/45 months | 1 case, 1 success (6 applications, 1 success) | rhBMP7 | Ilizarov | – |
| Lee et al. [14] | 24 weeks/14.5 months (12–18 months) | 5 cases, 1 success | rhBMP7 | External devices associated with nailing | 1 local sterile discharge |
| Richards et al. [17] | 29 weeks (17–37 weeks)/72 months (48–108 months) | 7 cases, 5 successes (8 applications and 6 successes) | rhBMP2 | Nailing and bone grafting | 1 fracture (1 amputation) |
| Kujala et al. [15] | 24 weeks/24 months | 1 case, 1 success | Native bovine BMP | Ilizarov and biocoral | – |
| Dohin et al. [16] | 20.9 weeks (8–60 weeks)/29.5 months (3.5–74 months) | 10 cases, 9 successes (16 applications and 10 successes) | rhBMP7 | 4 external devices, 8 nailing and 1 cast (5/13 procedures without bone grafting) | 1 recurring tibia bow, 1 fracture, 2 pins infections |
| Spiro et al. [18] | 14.2 weeks (12–16 weeks)/31 months (10–48 months) | 5 cases, 5 successes (6 applications and 6 successes) | rhBMP2 | Nailing with or without Ilizarov | 1 pins infection , 1 compartment syndrome, 2 fractures |
| Present study 2012 | 18 weeks/24 months (4–40 months) | 3 cases, 3 successes (5 applications) | rhBMP2 | 3 Masquelet’s procedures, 4 plates with grafting, 1 cast with grafting | 1 local inflammatory reaction, 1 infection (1 amputation), 1 fracture |
| 33 weeks (22 weeks except for the case reported by Fabeck et al.)/average 34 months | 33 cases and 26 successes (44 applications and 29 successes) |
ns not specified
Table 4.
Analysis of the literature regarding results and osteosynthesis
| Total of cases | Total of procedures | Total of applications | Successes | Failures | Cast | Plate | Nail | Ilizarov | Nail + Ilizarov | Monolateral external device | Nail + monolateral external device | Elastic nailing | Ilizarov + vascularized bone graft | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fabeck et al. [12] | 1 | 1 | 1 | 1 | 0 | 1/1 | ||||||||
| Anticevic et al. [13] | 1 | 1 | 1 | 1 | 0 | 1/1 | ||||||||
| Lee et al. [14] | 5 | 6 | 6 | 1 | 5 | 0/1 | 0/1 | 0/1 | 1/3 | |||||
| Richards et al. [17] | 7 | 8 | 8 | 6 | 2 | 6/8 | ||||||||
| Kujala et al. [15] | 1 | 1 | 1 | 1 | 0 | 0/1 | ||||||||
| Dohin et al. [16] | 10 | 13 | 16 | 10 | 6 | 1/1 | 6/7 (+2) | 1/2 | 1/1 | 0/1 | 1/1 (+1) | |||
| Spiro et al. [18] | 5 | 6 | 6 | 6 | 0 | 1/1 | 5/5 | |||||||
| Dohin-Kohler (present study) | 3 | 5 | 5 | 3 | 2 | 1/1 | 2/4 | |||||||
| Total | 33 | 41 | 44 | 29 | 15 | 2/2 | 2/4 | 14/18 (+2) | 2/5 | 6/6 | 0/1 | 1/3 | 0/1 | 1/1 (+1) |
a/b number of healed cases/total number of procedures, (+n) number of cases with two healed sites
Fig. 4.
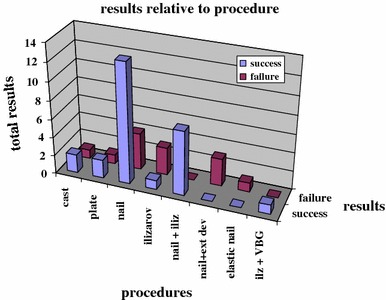
Results presented relative to the procedure used for stabilization after the application of bone morphogenetic protein (BMP)
About the use of BMP in CPT, all reported cases and these three present cases are characterized by heterogeneous types of surgical procedure after the application of BMP, depending, in most cases, on the authors’ preference. Furthermore, in most of the cases, procedures were performed for re-operations. These facts must be taken into account when interpreting results. The BMP used varied from one author to another, and the criteria for this choice were not specified. Thirty-three cases of the use of BMP in pseudarthrosis of the tibia in children have been reported to date. Out of a total of 44 applications, 19 were rhBMP2 and 24 were rhBMP7. In one case, the authors used native bovine BMP [15]. The overall success rate (26/33) was 79 % (Table 3). The success rate falls to 66 % when only successful applications of BMP are considered (29/44). The rate of positive results depending on the type of BMP used revealed a significant difference for rhBMP2 (Yates’ Chi-square test, p < 0.2): 13 successes out of 24 applications (54 %) for rhBMP7 and 15 out of 19 (79 %) for rhBMP2. Overall, the average follow-up lasted 34 months. The time taken to heal in successful cases was 22 weeks (with the exception of Fabeck et al.’s observation, in which the healing time was not clearly specified [12]).
Apart from the deep infection which we reported in this series, there was another serious complication (Spiro et al. [18): compartment syndrome. No other author witnessed any local swelling. There were no secondary effects or complications directly linked to the use of BMP in the cases reported. From a purely orthopedic point of view, four repeated fractures were observed and there was one recurrence of a bow.
Discussion
CPT is a rare pathology occurring in between 1/140,000 and 1/250,000 births and is one of the most complex orthopedic situations in pediatrics. In more than 50 % of cases, this occurs in a context of NF1 [19]. The numerous treatment approaches proposed in the literature goes to show the difficulties encountered to obtain healing. The results reported in the literature in the treatment of congenital pseudarthrosis with usual techniques noted a healing rate of 60–85 %. However, in most of the cases, numerous operations were needed, either because of frequent non-unions either or repeated fractures in a short delay. Nowadays, the most commonly used techniques applied either individually or in combination are nailing, vascularized bone graft, and Ilizarov-type circular external frame [20, 22, 23, 25–29, 35–42].
Technique
The surgical technique initially used in the three patients was in accord with the literature. Plates were used in re-operation in the three cases after the first failed procedures with the aim to obtain tibiofibular fusion. This type of osteosynthesis could be open to criticism regarding the three other options recommended in the literature; nevertheless, the results could be argued: in case 1, we preserved ankle mobility with a plate, but the first plate broke when bone reconstruction was obtained, and then infection surrounded; in case 2, we preserved ankle mobility with a plate and obtained bone healing; in case 3, bone grafting healed but pseudarthrosis recurred beneath the plate. We considered that all therapeutic options must be discussed in these dramatic situations. Such therapeutic difficulties go to show why additional treatments such as electromagnetic stimulation, bisphosphonates, and, more recently, BMP have been tried out [43, 44].
Application of BMP
First described by Urist [3, 4] in 1950, the proteins isolated from a demineralized bone matrix known as BMPs are part of the large family of TGFβ. They are secreted by the bone marrow cells, bone, muscle, periosteum, and endosteum cells, and stoked in the mineralized bone matrix. Their defining principle activity is osseous-induction; that is to say, the capacity to induce cellular differentiation in order to synthesize the mineral matrix, even aside of the bone tissue itself or on a heterotypical site. They ensure the differentiation of the mesenchymal stem cells, which are recruited, multiplied, and shunted toward the cartilaginous stock (chondroblasts) and bone stock (osteoblasts). They play an inductive and regulatory role. A second important activity is their angiogenesis capacity. There are over 30 of them, but only two have been developed for application in mankind and produced by genetic engineering: the recombining BMP2 and BMP7. The use of rhBMP in pseudarthrosis of the tibia seems promising in the light of initial observations published about children [12–18, 45] and the results obtained in adults [1, 2]. In children, the question arises as to the early and long-term side effects. The potential influence of these proteins on growth has not been documented. The possibility of triggering or development of tumors (one BMP was identified in certain tumorous pathologies [9]) remains unconfirmed for several authors and no cases of malignant tumors have been reported when safety recommendations are followed [9, 46, 47]. Steib et al. [10] reported a case of malignant tumor when rhBMP was used in an NF1 patient (who presented with scoliosis), but the authors could not follow the safety recommendations for the use of the drug (contraindication in the presence of tumor process) because of the severity of the case. The appearance of heterotypical calcifications may have been at the origin of complications, which are closely monitored nowadays. The possibility of a paradoxical effect of bone resorption has been reported in spine surgery. The local overdosage of the drug could play a role in resorption. The induction of antibodies observed has, so far, not led to any description of complications and seems to be transitory [1, 2, 12, 14, 48–50]. Despite these uncertainties and in the absence of any serious complications described in the previously published cases, we considered the benefit/risk ratio to be favorable and put forward a few indications for exceptional cases. We used rhBMP2 in the three presented cases because of its earlier activity in the bone formation process and its lower cost compared to rhBMP7. The first application we carried out in 2005 seemed encouraging and led us to continue using this drug. Two patients had repeated application and one patient had application of rhBMP2 from the onset. We justified these indications by patients’ status as challenging diseases. The analysis of the three cases reported in this article points to a low success rate. Although only two cases gave good results in the long term, nevertheless, the relatively short initial healing delay must be underlined (18 weeks).
Literature analysis brings up other questions and comments: the conditions in which reported procedures were carried out are often unclear and most cases described are complex and dramatic clinical situations. BMP is only used as an associated treatment and its efficacy could depend on the osteosynthesis, the procedure itself, and the time the rhBMP is applied (carrier, dosage, half-life of the drug, associated bone graft). Finally, the type of BMP used could play a role. In this analysis of the literature, we noted a significant difference between a 54 % success rate for rhBMP7 (13 out of 24) and 79 % for rhBMP2 (15 out of 19 applications) (Yates’ Chi-square test, p < 0.2). Nevertheless, this result must be analyzed regarding the small size of the studied groups.
The use of BMP in children raises an important ethical problem. The theoretical risks involved in using BMP for a growing person and the uncertainty of the long-term effects of these products are still not yet documented. The classical adverse effects observed and described in our patients (local swelling [18, 51], heterotopic calcifications [52–54]) are rare, but already reported in children. We observed in one case a severe local inflammatory reaction without complication. Swelling could be consecutive to excessive bone grafting (in volume) or absence of drainage to prevent suction of the drug during the immediate post operative period; conversely when there was an absence of logical cause, the drug could be in question. We did not note any heterotopic calcification; among the explanations, the use of the drug in a stuffy area could limit drug spreading in surrounding soft tissues. The presence of antibodies developed against the BMP or the carrier seems to be transitory and with no further effect: for this reason, we did not check for the presence of antibodies in our patients. Out of the 44 applications of BMP in children with CPT reported in the literature, we noted 14 incidents or complications (31 %). The cases which could possibly be directly connected with the use of BMP were early local inflammatory reactions (case 1) in the present series and compartment syndrome reported by Spiro et al. [18]. Nevertheless, fracture or recurrent bow leg are part of the possible incidents following the treatment of CPT, more in relation with poor mechanical conditions than default for consolidation. Three fractures are reported in the literature and one underwent treatment in the presented cases. If these fractures healed without surgical procedure, one can consider delayed fractures as benign complications. Analysis must take into account that this pathology presents a high potential risk for repeated fractures. Regarding this risk, the question remains as to whether another fracture occurring after healing obtained by BMP can be considered as a failure of this drug and not a failure of the procedure (i.e., osteosynthesis technique or resection of the pathological tissues).
Otherwise, the infection reported in this study cannot be clearly linked to the use of BMP and could have surrounded without the use of the drug. It was the only failure we observed, the severe infection leading to resorption of the bone graft (case 1). In this case, the parents were discouraged and asked for amputation. To date, this child is well and has settled well with his leg prosthesis. Even if, to date, no long-term effect has been reported, all these risks call for extreme caution in using BMP in children and its application must be limited to rare or exceptional situations with a severe prognosis, as in the case of CPT. It is to be remembered that, up till now, the use for BMPs for children is not officially allowed, their application coming under compassionate indications.
Finally, it is by clinical analogy with pseudarthrosis that other authors have tried using BMP in children, in particular for CPT. The reasons for choosing a particular type of rhBMP were not reported by the authors. An overall analysis of the results obtained in the literature (Table 3) showed a success rate of 78 % (26 cases out of 33), which is not higher than other treatment described so far in this pathology. Moreover, the success rate related to the number of drug applications has decreased to 66 % (26/44), which could be considered as the real efficacy rate of rhBMP. The time to consolidation is not mostly improved following the application of BMP (22 weeks).
Osteosynthesis and Masquelet’s procedure
This disease brings up the question of the local conditions required to obtained healing. Several parameters can, indeed, come into play, such as the vascular or tissue state of the soft surrounding area or the absence or presence of residual pathological bone tissue at the interfaces with the bone graft. However, certain observations showed good results even in the absence of bone excision or in multi-operated patients whose surrounding soft scar tissue had been previously modified by surgery on numerous occasions. It must also be underlined that most of the operations included a bone graft, which could influence the result obtained. Moreover, certain patients were treated right from the first operation with BMP without a bone graft, and some of these cases gave a positive result. Encouraged by previously published successes in Masquelet’s procedure and believing in the responsibility of the status of the local soft tissues (either soft scar tissue or pathologic hamartoma tissue) [30, 31, 33, 34, 55, 56], we decided to apply the procedure to congenital pseudarthrosis. Moreover, in one patient, we used Masquelet’s procedure in association with rhBMP2 application (case 3). Bone rebuilding was obtained but pseudarthrosis persisted even in the presence of rhBMP2. We cannot make any conclusions about the efficacy of Masquelet’s procedure in congenital pseudarthrosis, but we demonstrated that such a procedure allowed a large resection of pathologic bone and reconstruction of the wide long bone diaphyseal defect in this disease. We believe that Masquelet’s procedure must take a place in the therapeutic solutions in the treatment of congenital pseudarthrosis as reported previously [55]. To our knowledge, it is the first time Masquelet’s procedure is reported in CPT.
Overall, the surgical techniques described in the literature were very varied, depending largely on the teams’ habits, and while the results could be influenced by the choice of technique, it was difficult to draw any conclusion on this score (Table 4). It should be highlighted that, under no circumstances, can BMP make up for poor surgical techniques and that their usage must respect the principles of a stable osteosynthesis and as favorable an environment as possible from a vascular and bone point of view. Analyzing the results regarding osteosynthesis (Table 4; Fig. 4) is delicate. The heterogeneity of the procedures used following the application of rhBMP and the high heterogeneity of cases’ histories with previous treatments make conclusions hazardous. Nevertheless, as previously noted [20–22], intramedullary nailing (isolated or associated with an Ilizarov device) appears to be the preferred method with the highest rate of success.
Limitations
This study was limited by the small number of patients, relatively short follow-up, and retrospective nature. Moreover, the literature review is based on reports of individual experiences with a low level of evidence. Further studies are mandatory, but the heterogeneity of surgical procedures and patient populations will represent a great difficulty in the evaluation of BMP in CPT.
Conclusion
It is not easy to know how innocuous bone morphogenetic proteins (BMPs) are and to draw conclusions about the interest of BMPs in children. Conversely, the clinical use of BMPs in children in the case of congenital pseudarthrosis of the tibia (CPT) seems to be a logical indication, in theory at least. An analysis of the literature and reported cases do not provide conclusive evidence but only a possible benefit. A better understanding of the way different BMPs act should make it possible to progress in the method for the application of these drugs and especially in CPT. Today, it appears essential to explore this therapeutic process in optimal analytical conditions and security for young patients.
The knowledge of the phenomena altering the physiological bone tissue or the periosteum in neurofibromatosis type 1 (NF1) hamartoma is not yet complete. Even if consolidation could be obtained without large resection of pathologic tissues, we advocate Masquelet’s procedure in order to improve local conditions in the treatment of CPT. The first results are encouraging, despite the fact that re-operations were needed. Interest in the association of Masquelet’s procedure and human recombinant BMP2 (rhBMP2) application remains to be proven.
Conflict of interest
None.
References
- 1.Friedlaender GE, Perry CR, Cole JD, Cook SD, Cierny G, Muschler GF, et al. Osteogenic protein-1 (bone morphogenetic protein-7) in the treatment of tibial nonunions. J Bone Joint Surg Am. 2001;83-A Suppl 1(Pt 2):S151–S158. [PMC free article] [PubMed] [Google Scholar]
- 2.Govender S, Csimma C, Genant HK, Valentin-Opran A, Amit Y, Arbel R, et al. Recombinant human bone morphogenetic protein-2 for treatment of open tibial fractures: a prospective, controlled, randomized study of four hundred and fifty patients. J Bone Joint Surg Am. 2002;84-A(12):2123–2134. doi: 10.2106/00004623-200212000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Urist MR. Bone: formation by autoinduction. Science. 1965;150(3698):893–899. doi: 10.1126/science.150.3698.893. [DOI] [PubMed] [Google Scholar]
- 4.Urist MR, Strates BS. Bone morphogenetic protein. J Dent Res. 1971;50(6):1392–1406. doi: 10.1177/00220345710500060601. [DOI] [PubMed] [Google Scholar]
- 5.Kloen P, Di Paola M, Borens O, Richmond J, Perino G, Helfet DL, et al. BMP signaling components are expressed in human fracture callus. Bone. 2003;33(3):362–371. doi: 10.1016/S8756-3282(03)00191-1. [DOI] [PubMed] [Google Scholar]
- 6.Chen D, Zhao M, Mundy GR. Bone morphogenetic proteins. Growth Factors. 2004;22(4):233–241. doi: 10.1080/08977190412331279890. [DOI] [PubMed] [Google Scholar]
- 7.Salkeld SL, Patron LP, Barrack RL, Cook SD. The effect of osteogenic protein-1 on the healing of segmental bone defects treated with autograft or allograft bone. J Bone Joint Surg Am. 2001;83-A(6):803–816. doi: 10.2106/00004623-200106000-00001. [DOI] [PubMed] [Google Scholar]
- 8.Ripamonti U, Van Den Heever B, Sampath TK, Tucker MM, Rueger DC, Reddi AH. Complete regeneration of bone in the baboon by recombinant human osteogenic protein-1 (hOP-1, bone morphogenetic protein-7) Growth Factors. 1996;13(3–4):273–289. doi: 10.3109/08977199609003228. [DOI] [PubMed] [Google Scholar]
- 9.Obert L, Deschaseaux F, Garbuio P. Critical analysis and efficacy of BMPs in long bones non-union. Injury. 2005;36(Suppl 3):S38–S42. doi: 10.1016/j.injury.2005.07.033. [DOI] [PubMed] [Google Scholar]
- 10.Steib JP, Bouchaïb J, Walter A, Schuller S, Charles YP. Could an osteoinductor result in degeneration of a neurofibroma in NF1? Eur Spine J. 2010;19(Suppl 2):S220–S225. doi: 10.1007/s00586-010-1416-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McCarthy RE. Amputation for congenital pseudarthrosis of the tibia. Indications and techniques. Clin Orthop Relat Res. 1982;166:58–61. [PubMed] [Google Scholar]
- 12.Fabeck L, Ghafil D, Gerroudj M, Baillon R, Delincé P. Bone morphogenetic protein 7 in the treatment of congenital pseudarthrosis of the tibia. J Bone Joint Surg Br. 2006;88(1):116–118. doi: 10.1302/0301-620X.88B1.16619. [DOI] [PubMed] [Google Scholar]
- 13.Anticevic D, Jelic M, Vukicevic S. Treatment of a congenital pseudarthrosis of the tibia by osteogenic protein-1 (bone morphogenetic protein-7): a case report. J Pediatr Orthop B. 2006;15(3):220–221. doi: 10.1097/01.bpb.0000194439.75378.ac. [DOI] [PubMed] [Google Scholar]
- 14.Lee FY, Sinicropi SM, Lee FS, Vitale MG, Roye DP, Jr, Choi IH. Treatment of congenital pseudarthrosis of the tibia with recombinant human bone morphogenetic protein-7 (rhBMP-7). A report of five cases. J Bone Joint Surg Am. 2006;88(3):627–633. doi: 10.2106/JBJS.D.02201. [DOI] [PubMed] [Google Scholar]
- 15.Kujala S, Vähäsarja V, Serlo W, Jalovaara P. Treatment of congenital pseudarthrosis of the tibia with native bovine BMP: a case report. Acta Orthop Belg. 2008;74(1):132–136. [PubMed] [Google Scholar]
- 16.Dohin B, Dahan-Oliel N, Fassier F, Hamdy R. Enhancement of difficult nonunion in children with osteogenic protein-1 (OP-1): early experience. Clin Orthop Relat Res. 2009;467(12):3230–3238. doi: 10.1007/s11999-009-0967-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Richards BS, Oetgen ME, Johnston CE. The use of rhBMP-2 for the treatment of congenital pseudarthrosis of the tibia: a case series. J Bone Joint Surg Am. 2010;92(1):177–185. doi: 10.2106/JBJS.H.01667. [DOI] [PubMed] [Google Scholar]
- 18.Spiro AS, Babin K, Lipovac S, Stenger P, Mladenov K, Rupprecht M, et al. Combined treatment of congenital pseudarthrosis of the tibia, including recombinant human bone morphogenetic protein-2: a case series. J Bone Joint Surg Br. 2011;93(5):695–699. doi: 10.1302/0301-620X.93B5.25938. [DOI] [PubMed] [Google Scholar]
- 19.Hefti F, Bollini G, Dungl P, Fixsen J, Grill F, Ippolito E, et al. Congenital pseudarthrosis of the tibia: history, etiology, classification, and epidemiologic data. J Pediatr Orthop B. 2000;9(1):11–15. doi: 10.1097/01202412-200001000-00003. [DOI] [PubMed] [Google Scholar]
- 20.Charnley J. Congenital pseudarthrosis of the tibia treated by intramedullary nail. J Bone Joint Surg Am. 1956;38-A(2):283–290. [PubMed] [Google Scholar]
- 21.Dobbs MB, Rich MM, Gordon JE, Szymanski DA, Schoenecker PL. Use of an intramedullary rod for treatment of congenital pseudarthrosis of the tibia. A long-term follow-up study. J Bone Joint Surg Am. 2004;86-A(6):1186–1197. doi: 10.2106/00004623-200406000-00010. [DOI] [PubMed] [Google Scholar]
- 22.Grill F, Bollini G, Dungl P, Fixsen J, Hefti F, Ippolito E, et al. Treatment approaches for congenital pseudarthrosis of tibia: results of the EPOS multicenter study. European Paediatric Orthopaedic Society (EPOS) J Pediatr Orthop B. 2000;9(2):75–89. doi: 10.1097/01202412-200004000-00002. [DOI] [PubMed] [Google Scholar]
- 23.Ilizarov GA, Gracheva VI. Bloodless treatment of congenital pseudarthrosis of the crus with simultaneous elimination of shortening using dosed distraction. Ortop Travmatol Protez. 1971;32(2):42–46. [PubMed] [Google Scholar]
- 24.Keret D, Bollini G, Dungl P, Fixsen J, Grill F, Hefti F, et al. The fibula in congenital pseudoarthrosis of the tibia: the EPOS multicenter study. European Paediatric Orthopaedic Society (EPOS) J Pediatr Orthop B. 2000;9(2):69–74. doi: 10.1097/01202412-200004000-00001. [DOI] [PubMed] [Google Scholar]
- 25.Ohnishi I, Sato W, Matsuyama J, Yajima H, Haga N, Kamegaya M, et al. Treatment of congenital pseudarthrosis of the tibia: a multicenter study in Japan. J Pediatr Orthop. 2005;25(2):219–224. doi: 10.1097/01.bpo.0000151054.54732.0b. [DOI] [PubMed] [Google Scholar]
- 26.Romanus B, Bollini G, Dungl P, Fixsen J, Grill F, Hefti F, et al. Free vascular fibular transfer in congenital pseudoarthrosis of the tibia: results of the EPOS multicenter study. European Paediatric Orthopaedic Society (EPOS) J Pediatr Orthop B. 2000;9(2):90–93. doi: 10.1097/01202412-200004000-00003. [DOI] [PubMed] [Google Scholar]
- 27.Thabet AM, Paley D, Kocaoglu M, Eralp L, Herzenberg JE, Ergin ON. Periosteal grafting for congenital pseudarthrosis of the tibia: a preliminary report. Clin Orthop Relat Res. 2008;466(12):2981–2994. doi: 10.1007/s11999-008-0556-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Toh S, Harata S, Tsubo K, Inoue S, Narita S. Combining free vascularized fibula graft and the Ilizarov external fixator: recent approaches to congenital pseudarthrosis of the tibia. J Reconstr Microsurg. 2001;17(7):497–508. doi: 10.1055/s-2001-17752. [DOI] [PubMed] [Google Scholar]
- 29.Weiland AJ, Daniel RK. Congenital pseudarthrosis of the tibia: treatment with vascularized autogenous fibular grafts. A preliminary report. Johns Hopkins Med J. 1980;147(3):89–95. [PubMed] [Google Scholar]
- 30.Cho TJ, Seo JB, Lee HR, Yoo WJ, Chung CY, Choi IH. Biologic characteristics of fibrous hamartoma from congenital pseudarthrosis of the tibia associated with neurofibromatosis type 1. J Bone Joint Surg Am. 2008;90(12):2735–2744. doi: 10.2106/JBJS.H.00014. [DOI] [PubMed] [Google Scholar]
- 31.Schindeler A, Little DG. Recent insights into bone development, homeostasis, and repair in type 1 neurofibromatosis (NF1) Bone. 2008;42(4):616–622. doi: 10.1016/j.bone.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 32.Ippolito E, Corsi A, Grill F, Wientroub S, Bianco P. Pathology of bone lesions associated with congenital pseudarthrosis of the leg. J Pediatr Orthop B. 2000;9(1):3–10. doi: 10.1097/01202412-200001000-00002. [DOI] [PubMed] [Google Scholar]
- 33.Masquelet AC, Fitoussi F, Begue T, Muller GP. Reconstruction of the long bones by the induced membrane and spongy autograft. Ann Chir Plast Esthet. 2000;45(3):346–353. [PubMed] [Google Scholar]
- 34.Masquelet AC, Begue T. The concept of induced membrane for reconstruction of long bone defects. Orthop Clin North Am. 2010;31(1):27–37. doi: 10.1016/j.ocl.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 35.Dobbs MB, Rich MM, Gordon JE, Szymanski DA, Schoenecker PL. Use of an intramedullary rod for the treatment of congenital pseudarthrosis of the tibia. Surgical technique. J Bone Joint Surg Am. 2005;87 Suppl 1(Pt 1):33–40. doi: 10.2106/JBJS.D.02764. [DOI] [PubMed] [Google Scholar]
- 36.Dormans JP, Krajbich JI, Zuker R, Demuynk M. Congenital pseudarthrosis of the tibia: treatment with free vascularized fibular grafts. J Pediatr Orthop. 1990;10(5):623–628. doi: 10.1097/01241398-199009000-00010. [DOI] [PubMed] [Google Scholar]
- 37.El-Gammal TA, El-Sayed A, Kotb MM. Telescoping vascularized fibular graft: a new method for treatment of congenital tibial pseudarthrosis with severe shortening. J Pediatr Orthop B. 2004;13(1):48–56. doi: 10.1097/00009957-200401000-00010. [DOI] [PubMed] [Google Scholar]
- 38.Gilbert A, Brockman R. Congenital pseudarthrosis of the tibia. Long-term followup of 29 cases treated by microvascular bone transfer. Clin Orthop Relat Res. 1995;314:37–44. [PubMed] [Google Scholar]
- 39.Kanaya F, Tsai TM, Harkess J. Vascularized bone grafts for congenital pseudarthrosis of the tibia. Microsurgery. 1996;17(8):459–469. doi: 10.1002/(SICI)1098-2752(1996)17:8<459::AID-MICR9>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 40.Kim HW, Weinstein SL. Intramedullary fixation and bone grafting for congenital pseudarthrosis of the tibia. Clin Orthop Relat Res. 2002;405:250–257. doi: 10.1097/00003086-200212000-00032. [DOI] [PubMed] [Google Scholar]
- 41.Paley D, Catagni M, Argnani F, Prevot J, Bell D, Armstrong P. Treatment of congenital pseudoarthrosis of the tibia using the Ilizarov technique. Clin Orthop Relat Res. 1992;280:81–93. [PubMed] [Google Scholar]
- 42.Weiland AJ, Weiss AP, Moore JR, Tolo VT. Vascularized fibular grafts in the treatment of congenital pseudarthrosis of the tibia. J Bone Joint Surg Am. 1990;72(5):654–662. [PubMed] [Google Scholar]
- 43.Paterson DC, Simonis RB. Electrical stimulation in the treatment of congenital pseudarthrosis of the tibia. J Bone Joint Surg Br. 1985;67(3):454–462. doi: 10.1302/0301-620X.67B3.3873458. [DOI] [PubMed] [Google Scholar]
- 44.Schindeler A, Ramachandran M, Godfrey C, Morse A, McDonald M, Mikulec K, et al. Modeling bone morphogenetic protein and bisphosphonate combination therapy in wild-type and Nf1 haploinsufficient mice. J Orthop Res. 2008;26(1):65–74. doi: 10.1002/jor.20481. [DOI] [PubMed] [Google Scholar]
- 45.Burkhart KJ, Rommens PM. Intramedullary application of bone morphogenetic protein in the management of a major bone defect after an Ilizarov procedure. J Bone Joint Surg Br. 2008;90(6):806–809. doi: 10.1302/0301-620X.90B6.20147. [DOI] [PubMed] [Google Scholar]
- 46.Termaat MF, Den Boer FC, Bakker FC, Patka P, Haarman HJ. Bone morphogenetic proteins. Development and clinical efficacy in the treatment of fractures and bone defects. J Bone Joint Surg Am. 2005;87(6):1367–1378. doi: 10.2106/JBJS.D.02585. [DOI] [PubMed] [Google Scholar]
- 47.Vaibhav B, Nilesh P, Vikram S, Anshul C. Bone morphogenic protein and its application in trauma cases: a current concept update. Injury. 2007;38(11):1227–1235. doi: 10.1016/j.injury.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 48.Geesink RG, Hoefnagels NH, Bulstra SK. Osteogenic activity of OP-1 bone morphogenetic protein (BMP-7) in a human fibular defect. J Bone Joint Surg Br. 1999;81(4):710–718. doi: 10.1302/0301-620X.81B4.9311. [DOI] [PubMed] [Google Scholar]
- 49.Vaidya R, Carp J, Sethi A, Bartol S, Craig J, Les CM. Complications of anterior cervical discectomy and fusion using recombinant human bone morphogenetic protein-2. Eur Spine J. 2007;16(8):1257–1265. doi: 10.1007/s00586-007-0351-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wong DA, Watters WC., 3rd To err is human: quality and safety issues in spine care. Spine. 2007;32(11 Suppl):S2–S8. doi: 10.1097/BRS.0b013e318053d4cd. [DOI] [PubMed] [Google Scholar]
- 51.Smucker JD, Rhee JM, Singh K, Yoon ST, Heller JG. Increased swelling complications associated with off-label usage of rhBMP-2 in the anterior cervical spine. Spine. 2006;31(24):2813–2819. doi: 10.1097/01.brs.0000245863.52371.c2. [DOI] [PubMed] [Google Scholar]
- 52.Axelrad TW, Steen B, Lowenberg DW, Creevy WR, Einhorn TA. Heterotopic ossification after the use of commercially available recombinant human bone morphogenetic proteins in four patients. J Bone Joint Surg Br. 2008;90(12):1617–1622. doi: 10.1302/0301-620X.90B12.20975. [DOI] [PubMed] [Google Scholar]
- 53.Shields LB, Raque GH, Glassman SD, Campbell M, Vitaz T, Harpring J, et al. Adverse effects associated with high-dose recombinant human bone morphogenetic protein-2 use in anterior cervical spine fusion. Spine. 2006;31(5):542–547. doi: 10.1097/01.brs.0000201424.27509.72. [DOI] [PubMed] [Google Scholar]
- 54.Wysocki RW, Cohen MS. Ectopic ossification of the triceps muscle after application of bone morphogenetic protein-7 to the distal humerus for recalcitrant nonunion: a case report. J Hand Surg Am. 2007;32(5):647–650. doi: 10.1016/j.jhsa.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 55.Kohler R, Solla F, Pinson S, Romana C, Chau E, Dohin B. Congenital pseudarthrosis of the forearm in a neurofibromatosis patient: case report and review of the literature. Rev Chir Orthop Reparatrice Appar Mot. 2005;91(8):773–781. doi: 10.1016/S0035-1040(05)84489-7. [DOI] [PubMed] [Google Scholar]
- 56.Pelissier P, Masquelet AC, Bareille R, Pelissier SM, Amedee J. Induced membranes secrete growth factors including vascular and osteoinductive factors and could stimulate bone regeneration. J Orthop Res. 2004;22(1):73–79. doi: 10.1016/S0736-0266(03)00165-7. [DOI] [PubMed] [Google Scholar]


