Abstract
Purpose
The active or aggressive character in certain localisations of aneurysmal bone cysts in children requires either curettage with a considerable recurrence rate or a radical segmental excision, raising complex reconstructive challenges. Cyst maturation with subsequent ossification may be observed either spontaneously or after incisional biopsy.
Patients
Five new cases of active aneurysmal bone cysts (ABCs) with healing of the cyst after biopsy alone are reported. All patients had no treatment of the cyst after the biopsy.
Results
In two cases, the lesion initially increases in size immediately after the biopsy, and it is only secondarily that the lesion decreases in size. Four out of five cases of the spontaneous healing occurred in pelvic bone. The cysts healed after, respectively, 36, 24, 12, 32 and 12 months.
Conclusions
The emergence of these new cases of spontaneous healing encourages promoting clinical and radiological supervision after biopsy in selected cases. Unfortunately, it is impossible to predict a possible aggressive behaviour in ABCs. Then, if the lesion is quickly aggressive with clinically and radiologically increasing size after biopsy, it would be illogical and dangerous to let this ABC evolve. It would be necessary to treat it without delay. On the other hand, if the lesion moderately increased after the biopsy, it is possible to wait and observe the patient during a period of 5 months for a possible healing, if the ABC localisation is not dangerous. Of course, if the lesion does not increase in size after biopsy, there is no delay to treat it.
Keywords: Aneurysmal bone cyst, Spontaneous healing, Benign bone tumour, Adolescent
Introduction
Aneurysmal bone cyst (ABC) is a benign cystic lesion of bone composed of blood-filled spaces separated by connective tissue septa containing fibroblasts, osteoclast-type giant cells and reactive woven bone [1]. The term “aneurysmal” refers to both the radiographic appearance of the bones, which have the distended appearance of an aneurysm, and also to the large cystic blood-filled spaces found at operation. The term “aneurysmal bone cyst” has been accepted throughout the world, although the lesion to which it refers is neither an aneurysm nor a bone cyst [2, 3]. ABC is a rare lesion with an incidence of 0.14 per 105 individuals (about 1 % of benign bone tumours) [4]. Although ABC may be observed at any age, it distinctly predominates in patients from 10 to 20 years of age [5]. Among the demographic data on 411 children with primary ABCs, the femur (22 %), tibia (17 %), spine (15 %), humerus (10 %), pelvis (9 %) and fibula (9 %) were the most common locations [6].
The lesions were staged according to Capanna et al.’s classification [7]. Inactive cysts have a complete periosteal shell with defined sclerotic bone limits. Active cysts have an incomplete periosteal shell and defined bone limits. Aggressive cysts have an indefinite margin and show uniform osteolysis. The active or aggressive character in certain localisations of ABCs in children requires either curettage with a considerable recurrence rate or a radical segmental excision raising complex reconstructive challenges. Cyst maturation with subsequent ossification may be observed either spontaneously or after incisional biopsy [8–14]. These cases are rare and some of them occurred in adults or in inactive lesions. The authors report their experience of five healings after biopsy of active ABCs in children.
Case reports
Case 1
A boy aged 12 years and 4 months presented with a two-month history of moderate pain in the right groin, which developed after a bicycle fall. Gradually, the pain became more severe, followed by limp and painful mass in the right pubic area. Radiological investigation showed an expanding active lytic lesion of the right superior pubic ramus and the adjacent pubic symphisis (Fig. 1). Technetium bone scan revealed an increased uptake in this area. Magnetic resonance imaging (MRI) showed a lytic lesion expanding to the right anterior column of the acetabulum, ilio-pubic ramus and soft tissues. This mass was relatively limited, with periphery lobulated contours pushing back the muscles and the bladder. Internal septa with multiple fluid–fluid levels were identified. The pattern was typical of the “blow-out” appearance of ABC. A surgical biopsy was carried out and the histological features were those of an ABC. No treatment was instituted. Five months later, technetium bone scan revealed a central decrease of fixation and the radiographs showed that the expansion of the lesion was increasing in size but the wall appeared slightly more delimited and calcified. At 9 months, expansion seemed to have stopped and bone formation had started within the tumour. At 3 years, radiological follow-up showed complete stabilisation and healing. At 8 years follow-up, X-ray revealed a practically normal pelvis with only a thickening of the right superior pubic ramus (Fig. 2).
Fig. 1.
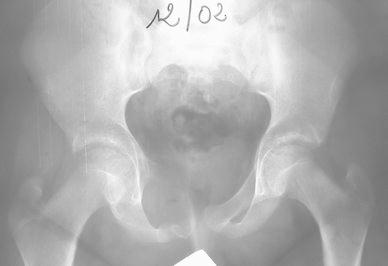
Initial X-ray of an active lesion in the right pubis area
Fig. 2.
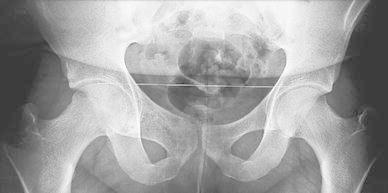
Radiological aspect of the lesion at 8 years follow-up
Case 2
A girl, aged 13 years and 6 months, complained of pain in the right hip when walking and running for a month and a half. Clinical examination showed limp and painful limitation of mobility of the hip. Radiological examination showed active cystic lesion with septa inside involving the posterior wall and the depth of the acetabulum. Bone scintigraphy revealed hyperactivity of this lesion. Computed tomography (CT) scans showed a multi-geodic lesion encircled by trabeculations and cortical erosion with articular rupture (Fig. 3). A biopsy was carried out and the histological features were those of an ABC. The only therapeutic instruction was the prohibition of weight-bearing for 6 months until the beginning of the radiological healing of the lesion. At a follow-up of 2 years, healing and re-ossification of the lesion was complete. At a follow-up of 4 years, CT scans showed a normal and regular articular line space and an osteocondensation of the entire acetabulum with some small residual geodes (Fig. 4). At a follow-up of 11 years, the hip was clinically asymptomatic with a normal mobility.
Fig. 3.
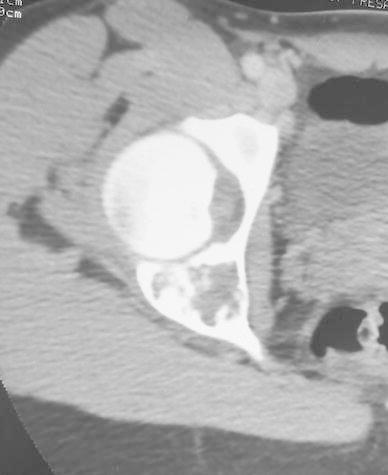
Active cystic lesion involving the posterior wall and the depth of the acetabulum. Multi-geodic lesion with articular effraction
Fig. 4.
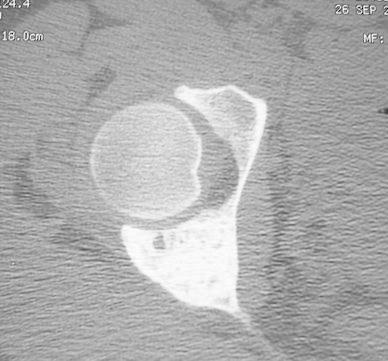
Computed tomography (CT) scan at 4 years follow-up showed a normal and regular articular line space and an osteocondensation of the entire acetabulum with some small residual geodes
Case 3
A 14-year-old boy presented with a 3-month history of left painful hip. The radiological feature was an active lytic lesion of the ischium. Bone scintigraphy revealed an increased uptake. MRI showed a lytic lesion (2 × 3 cm) of the ischium. A biopsy under CT scan was performed and confirmed the histological result of an ABC. No treatment has been proposed. The painful symptoms disappeared 3 months later and the lesion was completely healed 1 year later. At a follow-up of 3 years, no recurrence occurred.
Case 4
A girl, aged 13 years, presented with a 1-month history of spontaneous pain in the right elbow. Clinical examination revealed swelling in the posterior part of the proximal forearm. Radiological investigation at first attendance showed an active expanding lesion of the upper ulna (Fig. 5). Bone scintigraphy showed increased uptake in the lesion. A surgical biopsy was carried out and the histological features were those of an ABC. No treatment was instituted, as her parents refused further treatment. Over the next 4 months, a swelling of the proximal forearm developed. On radiological and CT scan investigations, expansion of this lytic lesion had progressed, blowing the cortex with partitions inside (Fig. 6). At 9 months, expansion seemed to have stopped and bone formation developed within the lesion. At 2 years and 8 months follow-up, CT scans noted a complete stabilisation followed by tumour regression and new ossification. After a 16-year follow-up, the patient revealed to be entirely asymptomatic, but X-ray investigation showed a persistent deformation of the bone, with large residual geodes (Fig. 7). In fact, the lesion moved into the latent stage with good cortices and does not require additional treatment. This does not rule out the need for further follow-up down the road.
Fig. 5.
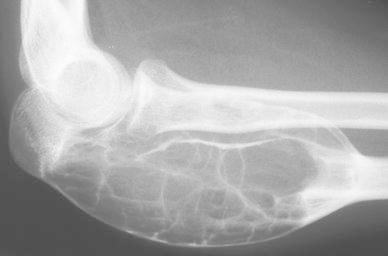
Lateral view of an active expanding lesion of the upper ulna
Fig. 6.
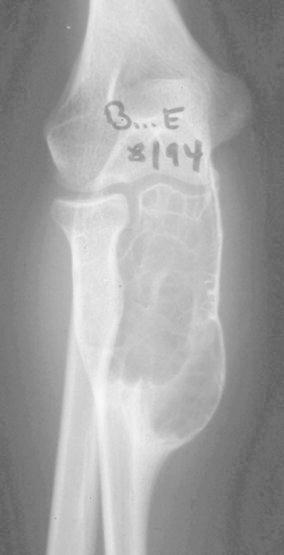
Lateral view of the expansion of this lytic lesion
Fig. 7.
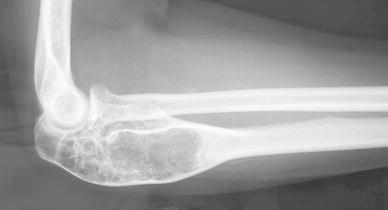
Lateral view of the lesion with large residual geodes at 16 years follow-up
Case 5
A girl, aged 11 years, presented with a 10-month history of spontaneous pain in the left hip. Radiological investigation revealed an active lytic lesion of the pubic ramus. MRI showed a lytic lesion (6 × 3 × 2.5 cm) of the ilio-pubic ramus extending to the anterior part of the acetabulum. A biopsy was performed, which confirmed the histological result of an ABC. No treatment has been proposed. The painful symptoms disappeared 3 months later and the lesion was completely healed 1 year later. At a follow-up of 3 years, no recurrence occurred.
Discussion
The radiological features of ABC vary according of the phase of development. Four main stages are distinguished [15]. In the initial lytic phase, a well-defined area of bone resorption with no distinctive features is observed. In the phase of active development, there is the typical sub-periosteal “blow-out” expansile appearance. In the stabilisation phase, there is a distinct peripheral bony shell with internal septa and trabeculations, resulting in the so-called “soap bubble” appearance. Finally, the healing phase is characterised by progressive ossification of the cyst, resulting in a dense bony mass of irregular structure. At this stage, recurrence is not seen [15].
Several hypotheses concerning the physiopathology of ABC have been proposed. Lichtenstein [16, 17] proposed that ABC is related to a circulatory disturbance causing increased venous pressure due to thrombosis of a sizeable vein or the formation of arteriovenous fistulas. The evidence supporting the haemodynamic hypothesis includes: (1) the often marked and occasionally rapid extension of some of these cysts; (2) the common operative finding of a blood-filled cyst, the blood often welling up, occasionally to the extent of giving rise to some concern and (3) angiography may sometimes show changes suggesting a vascular lesion and the presence of an arteriovenous shunt.
For Biesecker et al. [18], a primary lesion of bone initiates an osseous, arteriovenous malformation and, thereby, creates, via its haemodynamic forces, a secondary reactive lesion of bone, which we know as an ABC. It appears that, in some way, breaking up the existing equilibrium in the blood-filled cavities (e.g. simple biopsy or incomplete curettage) may be sufficient to cause involution of the lesion [10, 19, 20]. It is important to notice that there is sometimes little difference between a large open biopsy and a mild curettage [14]. The lesion sometimes initially increases in size immediately after the biopsy, as in our cases 1 and 4, and it is only secondarily that the lesion decreases in size. McQueen et al. [10] noticed the same evolution with the tumour increasing in size both clinically and radiologically over the following 2 months for the first case and over the following 5 months for the second case. Malghem et al. [11] also noticed the same evolution but only in one patient (aged 19 years) out of three, over the following 4.5 months. It is not possible to know if this regression is the consequence of the biopsy or the natural evolution of the cyst. However, this decrease of size occurred 4–5 months after the biopsy. It could be hypothesised that the biopsy was an accelerating element of the natural evolution of the cyst. Several authors [8, 20] noticed that ABCs often healed with small static residual cysts. In case 4, the ABC healed with large static residual cysts. These residual geodes cause concern on healing and possible recurrence (case 4 with 16 years follow-up), but after periods of observation, the final state of these children can be considered as close to recovery (healing manifested by increased cortical and septal thickening) [14, 20]. It is, therefore, important to avoid excessively early repeat surgical procedure to treat a possible recurrence when faced with a residual geode. Most of the recurrences occur within 12–18 months [9, 18, 21, 22]. For this reason, it is not logical to operate on residual geodes of an ABC with more than 2 years follow-up, except if the lesion continues to enlarge. Evaluation of residual geodes based on plain radiographs certainly underestimates geodes because they are mostly not seen on plain radiographs but only with CT imaging [14].
We treated (not only operated), respectively, 49 and 52 ABCs in our two institutions in the same period of the discussed five patients (16 years follow-up). Biopsy alone is not a treatment in our departments. ABCs were treated in a classic way (e.g. curettage, resection, alcoholic solution of zein). For some cases, treatment was not immediately commenced after the biopsy, either because their parents refused further treatment or because the surgeon held out for surgery. We then observed cyst maturation with subsequent ossification. Healing following biopsy in children is rarely described in the literature. Two ABCs of the pelvis in two boys aged 10 and 11 years was described in 1985 by McQueen et al. [10]. Malghem et al. [11] described in 1989 three cases but in young adults. In Capanna et al.’s study [23], two children with a pelvic ABC, aged 13 and 15 years, had only incisional biopsies, as their parents refused further treatment. Although both lesions were aggressive, with expansion into the soft tissues, the lesions became quiescent with reconstitution of a radiographic bony shell. Cottalorda et al. [14], in a multi-centre study of pelvic ABC, noticed the same evolution in four ABCs (but with one inactive). Most of the spontaneous healings in the literature occurred in pelvic bone (four out of five cases in our study; of course, the reported group of 80 % pelvic involvement does not represent the 9 % rate in the total ABCs). Healing can also be observed in other bones (spine, tibia, femur, upper ulna) [8, 11]. The reasons for the apparent difference in the behaviour of long bone and pelvic cysts have not been determined [9]. The real incidence of spontaneous healing is difficult to evaluate because there are no series considering the natural history of ABCs without treatment.
In most cases, when an active or aggressive ABC is diagnosed, a curative treatment is rapidly performed [20]. If the patients of Capanna et al.’s series [23] and Cottalorda et al.’s series [14] had been rapidly operated on, it would not have been possible to observe the spontaneous healing after biopsy. It might be possible that spontaneous healing could not be as rare as that described in the literature because most of them are treated rapidly after diagnosis [14].
The authors’ policy to wait and see for pelvic ABCs, even if symptomatic, is based on previous random observations of similar cases in the authors’ respective institutions and on the assumption that active histologically certified ABCs, even in long bones, could behave as other benign cystic lesions, i.e. move either to the latent phase or aggressive phase. Further progression over the next few months will not dramatically change the final outcome following treatment if the latter is needed, taking into consideration the possible high morbidity of surgical treatment of some pelvic ABCs in balance with no treatment at all if spontaneous resolution or stabilisation happens to occur. In some cases, the localisation and extent of the cysts are such that operative treatment is extremely hazardous. Resection is sometimes impossible and curettage can be extremely difficult, with considerable blood loss. The emergence of a few cases of spontaneous healing (even in active or aggressive lesions) encourages promoting a clinical and radiological supervision after biopsy in selected cases.
Three evolutions are likely to be observed:
The lesion does not increase after biopsy. In this case, there is no time deadline to treat it.
The lesion increases moderately in volume during the first months after biopsy, then decreases in size within 4 or 5 months, as described in cases 1 and 4 and in some cases reported in the literature [10, 11]. In such cases, supervision must be continued and surgery might be avoided. However, in some potentially dangerous localisations (spine), this therapeutic attitude must not be advised.
Sometimes, the size and vascularisation of the lesion do not allow waiting for the natural evolution of the tumour, especially since spontaneous healing is not systematically observed [20]. When the lesion quickly becomes aggressive with a clinically and radiologically increasing size after biopsy, it would be illogical and dangerous to let this ABC evolve. A treatment must then be proposed without delay.
In the literature, a minimum of a 2-year review was selected for most of the recurrences occurring within 12–18 months [9, 18, 21, 22]. In our four cases, the minimum follow-up was 3 years. It is difficult to evaluate when a cyst completely healed after biopsy because, in the literature, some authors mentioned only the longest follow-up and not the moment of complete healing. In our five cases, the cysts healed after, respectively, 36, 24, 12, 32 and 12 months. This is may be fate but the healing of these cysts occurred at the end of juvenile growth. McQueen et al. [10], investigating two cases of spontaneous healing after biopsy, noticed that, respectively, 2 and 3 years after diagnosis, healing was complete.
Conclusion
The possibility of healing after biopsy alone encourages the consideration of a conservative therapeutic approach in selected cases. Unfortunately, it is impossible to predict a possible aggressive behaviour in active aneurysmal bone cysts (ABCs). Then, if the lesion is quickly aggressive with clinically and radiologically increasing size after biopsy, it would be illogical and dangerous to let this ABC evolve. It would be necessary to treat it without delay. On the other hand, if the lesion moderately increased after the biopsy, it is possible to wait and observe the patient during a period of 5 months for a possible healing, if the ABC localisation is not dangerous. Of course, if the lesion does not increase in size after biopsy, there is no delay to treat it.
Footnotes
Conflict of interest
None.
References
- 1.Rosenberg AE, Nielsen GP, Fletcher JA. Aneurysmal bone cyst. In: Fletcher CDM, Unni KK, Mertens F, editors. World Health Organization: classification of tumours: pathology and genetics of tumours of soft tissue and bone. Lyon: International Agency for Research on Cancer Press; 2002. pp. 338–339. [Google Scholar]
- 2.de Kleuver M, van der Heul RO, Veraart BEEMJ. Aneurysmal bone cyst of the spine: 31 cases and the importance of the surgical approach. J Pediatr Orthop B. 1998;7:286–292. doi: 10.1097/01202412-199810000-00006. [DOI] [PubMed] [Google Scholar]
- 3.Bonakdarpour A, Levy WM, Aegerter E. Primary and secondary aneurysmal bone cyst: a radiological study of 75 cases. Radiology. 1978;126:75–83. doi: 10.1148/126.1.75. [DOI] [PubMed] [Google Scholar]
- 4.Leithner A, Windhager R, Lang S, Haas OA, Kainberger F, Kotz R. Aneurysmal bone cyst. A population based epidemiologic study and literature review. Clin Orthop Relat Res. 1999;363:176–179. [PubMed] [Google Scholar]
- 5.Cottalorda J, Bourelle S. Modern concepts of primary aneurysmal bone cyst. Arch Orthop Trauma Surg. 2007;127:105–114. doi: 10.1007/s00402-006-0223-5. [DOI] [PubMed] [Google Scholar]
- 6.Cottalorda J, Kohler R, Sales de Gauzy J, Chotel F, Mazda K, Lefort G, Louahem D, Bourelle S, Dimeglio A. Epidemiology of aneurysmal bone cysts in children: a multicenter study and literature review. J Pediat Orthop B. 2004;13:389–394. doi: 10.1097/01202412-200411000-00008. [DOI] [PubMed] [Google Scholar]
- 7.Capanna R, Bettelli G, Biagini R, Ruggieri P, Bertoni F, Campanacci M. Aneurysmal cysts of long bones. Ital J Orthop Traumatol. 1985;11:409–417. [PubMed] [Google Scholar]
- 8.Arlet V, Rigault P, Padovani JP, Mallet JF, Finidori G, Touzet P. Le kyste anévrysmal des os chez l’enfant. Rev Chir Orthop Reparatrice Appar Mot. 1987;73:337–348. [PubMed] [Google Scholar]
- 9.Cole WG. Treatment of aneurysmal bone cysts in childhood. J Pediatr Orthop. 1986;6:326–329. doi: 10.1097/01241398-198605000-00012. [DOI] [PubMed] [Google Scholar]
- 10.McQueen MM, Chalmers J, Smith GD. Spontaneous healing of aneurysmal bone cysts. A report of two cases. J Bone Joint Surg Br. 1985;67:310–312. doi: 10.1302/0301-620X.67B2.3980546. [DOI] [PubMed] [Google Scholar]
- 11.Malghem J, Maldague B, Esselinckx W, Noel H, De Nayer P, Vincent A. Spontaneous healing of aneurysmal bone cysts. A report of three cases. J Bone Joint Surg Br. 1989;71:645–650. doi: 10.1302/0301-620X.71B4.2768314. [DOI] [PubMed] [Google Scholar]
- 12.Tillman BP, Dahlin DC, Lipscomb PR, Stewart JR. Aneurysmal bone cyst: an analysis of ninety-five cases. Mayo Clin Proc. 1968;43:478–495. [PubMed] [Google Scholar]
- 13.van Loon CJM, Veth RPH, Pruszczynski M, Lemmens JAM, van Horn JR. Aneurysmal bone cyst. Long-term results and functional evaluation. Acta Orthop Belg. 1995;61:199–204. [PubMed] [Google Scholar]
- 14.Cottalorda J, Chotel F, Kohler R, de Gauzy JS, Louahem D, Lefort G, Dimeglio A, Bourelle S. Aneurysmal bone cysts of the pelvis in children: a multicenter study and literature review. J Pediatr Orthop. 2005;25:471–475. doi: 10.1097/01.bpo.0000158002.30800.8f. [DOI] [PubMed] [Google Scholar]
- 15.Dabska M, Buraczewski J. Aneurysmal bone cyst. Pathology, clinical course and radiologic appearances. Cancer. 1969;23:371–389. doi: 10.1002/1097-0142(196902)23:2<371::AID-CNCR2820230213>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 16.Lichtenstein L. Aneurysmal bone cyst. A pathological entity commonly mistaken for giant-cell tumor and occasionally for hemangioma and osteogenic sarcoma. Cancer. 1950;3:279–289. doi: 10.1002/1097-0142(1950)3:2<279::AID-CNCR2820030209>3.0.CO;2-F. [DOI] [Google Scholar]
- 17.Lichtenstein L. Aneurysmal bone cyst; observations on fifty cases. J Bone Joint Surg Am. 1957;39-A:873–882. [PubMed] [Google Scholar]
- 18.Biesecker JL, Marcove RC, Huvos AG, Miké V. Aneurysmal bone cysts. A clinicopathologic study of 66 cases. Cancer. 1970;26:615–625. doi: 10.1002/1097-0142(197009)26:3<615::AID-CNCR2820260319>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 19.Papavasiliou VA, Sferopoulos NK. Aneurysmal bone cyst: a preliminary report on a new surgical approach. J Pediatr Orthop. 1990;10:362–364. doi: 10.1097/01241398-199005000-00013. [DOI] [PubMed] [Google Scholar]
- 20.Cottalorda J, Bourelle S. Current treatments of primary aneurysmal bone cysts. J Pediatr Orthop B. 2006;15:155–167. doi: 10.1097/01.bpb.0000210588.50899.29. [DOI] [PubMed] [Google Scholar]
- 21.Clough JR, Price CHG. Aneurysmal bone cyst: pathogenesis and long term results of treatment. Clin Orthop Relat Res. 1973;97:52–63. doi: 10.1097/00003086-197311000-00009. [DOI] [PubMed] [Google Scholar]
- 22.Vergel de Dios AM, Bond JR, Shives TC, McLeod RA, Unni KK. Aneurysmal bone cyst. A clinicopathologic study of 238 cases. Cancer. 1992;69:2921–2931. doi: 10.1002/1097-0142(19920615)69:12<2921::AID-CNCR2820691210>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 23.Capanna R, Bertoni F, Bettelli G, Present D, Biagini R, Ruggieri P, Mancini I, Campanacci M. Aneurysmal bone cysts of the pelvis. Arch Orthop Trauma Surg. 1986;105:279–284. doi: 10.1007/BF00449926. [DOI] [PubMed] [Google Scholar]


