Abstract
The centrosome is a subcellular organelle that is responsible for the majority of microtubule organization. Through this ability, the centrosome is involved in cell division, migration, and polarization. Recent studies have revealed intriguing asymmetries between mother and daughter centrioles as well as between mother and daughter centrosomes, and the involvement of such asymmetries in multiple cellular and developmental processes. This review aims to summarize recent discoveries on such asymmetries in centrioles/centrosomes and the potential implication of their inheritance patterns during cell division and development.
Centrosome biogenesis in animal cells
The centrosome is a complex molecular assembly that functions as the major microtubule-organizing center (MTOC) in animal cells. As such, it plays an instrumental role in a plethora of cellular processes including cell motility, intracellular transport, assembly of the bipolar spindle apparatus and cell division. Centrosomes are composed of a pair of barrel-shaped centrioles of 9-fold symmetry, surrounded by a proteinaceous matrix collectively referred to as pericentriolar material (PCM), which contains microtubule-nucleating complexes like the γ-TuRCs. The centrosome is thought to be composed of over 100 proteins in human cells and we are only beginning to understand the precise role of individual components in the biogenesis of this fascinatingly complex organelle [1]. Centrosomes duplicate once and only once per cell cycle. The tight regulation of centrosome number ensures that two centrosomes are present during mitosis, each organizing one of the two spindle poles. Prior to mitosis, centrosomes need to increase in size and microtubule nucleation capacity, a process known as centrosome maturation [2]. The failure to correctly regulate centrosome number and size has been linked to aneuploidy and cancer formation [3].
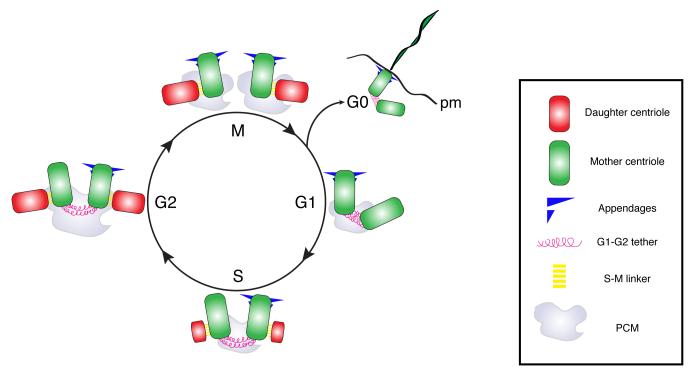
Centrosome number is largely regulated through the control of centriole duplication. Centrioles mark the site where PCM is recruited and therefore define the number of centrosomes present in the cell. Centriole duplication initiates at the M/G1 stage of the cell cycle where the mother and daughter centrioles lose their orthogonal orientation, a process referred to as centriole disengagement, which requires Seperase activity on the S-pahse to M-phase linker (S-M linker, Fig. 1)[4,5]. During S-phase, the sequential recruitment and assembly of a core set of centriole components leads to the formation of new, daughter centriole, in the vicinity of the pre-existing mother centriole which is often referred to as a procentriole [6,7]. The daughter centrioles continue to grow during the G2 and M phase of the cell cycle but the cellular mechanism underlying the control of centriole growth remain elusive. Once centriole duplication is complete, the two older, mother centrioles remain tethered through fibrous connections that are severed at the S/G2 transition. These fibrous connections, also called G1-G2 tether, are composed at least in part of c-NAP1 and Rootletin and their disassembly is regulated in a cell cycle dependent manner by NEK2 kinase [8]. This allows centrosome to separate and position themselves on opposite sides of the nuclease upon entry into mitosis where they will participle in the assembly of the mitotic spindles. Several factors contribute to the accurate positioning of the mitotic spindle, in particular during asymmetric cell division. How various centrosome-related asymmetries can contribute to accurate spindle positioning will be discussed in the following sections.
Fig. 1. Centrosome biogenesis and the cell cycle in animal cells.
Schematic representation of the centrosome/centriole duplication cycle in animal cells. The centrosome consists of mother (green) and daughter centrioles (red), that are interconnected by S-M tethers and embedded in pericentriolar material (PCM), which anchors microtubules. The cycle can be separated in four slightly overlapping phases. i) Centriole disengagement, ii) centriole duplication, iii) centrosome maturation and iv) centrosome separation followed by spindle assembly. The mother centriole can be distinguished by the presence of appendages. During disengagement centrioles lose their orthogonal arrangement through the removal of the S-M linker. During S phase, procentrioles forms perpendicular to each mother centriole. During S-G2 phases, the daughter centrioles continue to elongate. In late S-phase G2, the centrosome increases in size and the newly formed centriole pairs disconnect through the disassembly of the G1-G2 tether, so that at the onset of G2/M the two centrosomes move to opposite sides of the cell and establish the two mitotic spindle poles. At the end of the cycle, the daughter centrioles acquire appendages and behave as a mother centriole during the subsequent cycle. When cell exit the cell cycle and enter G0, centrioles move to the plasma membrane (pm) and assemble cilia.
Inherent centrosome asymmetries
As described in the previous section, the centrioles are inherently asymmetric cellular structures, both in terms of morphology and age. One intrinsic asymmetry is defined by the polarity of the centriole MTs, their minus ends pointing towards their proximal ends and their plus ends towards the distal end [9]. The mother centriole is also longer than the daughter centriole and possesses two distinct sets of projections at their distal ends called subdistal and distal appendages (Fig. 1). While subdistal appendages are implicated in the anchoring of MTs, distal appendages have been implicated in cilia formation and docking of the basal body at the plasma membrane. Primary cilia are immotile cellular appendages that protrude from the plasma membrane of most vertebrate cells necessary for the efficient transduction of extracellular cues during animal development and in the maintenance of tissue homeostasis. Defects in ciliogenesis have been like to several devastating diseases including polycystic kidney disease and Bardet-Biedl and orofaciodigital syndromes.
During cell division, one of the two daughter cells receives the older of the two mother centrioles. This centriole was formed prior to the last cell cycle. The other daughter cell receives the newer mother centriole that was most recently assembled in the previous cell cycle. It was elegantly shown by Anderson and Stearns that even though both mother centrioles can readily assemble primary cilia after mitotic cell division, the cell inheriting the older of the two mother centrioles have been shown to assemble primary cilia considerably faster, correlating with a differential response to hedgehog signal, which requires cilium for efficient signaling [10]. This striking observation that centriole age could be associated with pronounced phenotypic variation and differential response to various signaling cues including morphogens raised the tantalizing possibility that the inherent asymmetry of centrioles could contribute to the determination of cell fate during mitotic division.
Asymmetric centrosome inheritance during stem cell division
As described above, two centrosomes are distinct from each other, in part due to the age difference in their centrioles. While the presence of proteins that are specific to either the mother or daughter centrioles has provoked researchers’ interest in its meaning, the first example of asymmetric centrosomal inheritance during cell division came with the realization that some stem cells inherit the mother centrosome upon division.
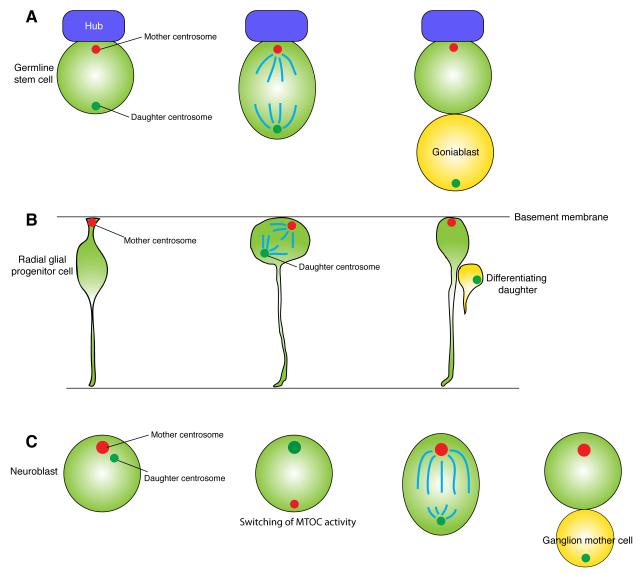
The Drosophila male germline stem cell (GSC) was the first example of stereotypical centrosome inheritance [11]. Male GSCs reside in the stem cell niche that specifies stem cell identity; at the apical tip of the testis, GSCs attach to the hub cells and are encapsulated by cyst stem cells, which together create the stem cell niche for GSCs [12-14]. Thus, under normal conditions, maintenance of the hub attachment specifies GSC identity [15]. Male GSCs divide asymmetrically by placing each of two daughter cells into distinct microenvironments (inside and outside of the niche) producing one stem cell and one differentiating cell (Fig. 2A). During this asymmetric stem cell division, the mother centrosome always remains within the stem cell side, while the daughter centrosome is always sent into the differentiating daughter [11]. The higher microtubule organizing center (MTOC) activity of the mother centrosome presumably enables it to be continuously anchored to the adherens junction that is formed between the GSCs and hub cells (Fig. 2A). Subsequently, a similar observation was made in the mouse neural cortex, in which the mother centrosome remains in the neural glial progenitor cells (i.e., stem cells) (Fig. 2B) [16], suggesting that the stereotypical centrosome inheritance during asymmetric stem cell division is a widespread phenomenon. These observations provoked the speculation that the mother (or daughter) centrosomes might bear fate determinants, or other characteristics that contribute to cell fates. However, at the same time, an alternative explanation may be that the higher MTOC activity passively allows the mother centrosome to remain at a certain location such as near the adherens junction.
Fig. 2. Asymmetric stem cell division and centrosome inheritance.
A. Drosophila male germline stem cells (GSCs) inherit the mother centrosome upon division. The mother centrosome is anchored to the interface between the GSC and the hub cells. The hub secretes the signaling ligand (Unpaired, Upd) to specify GSC identity.
B. Mouse radial glial progenitor cells inherit the mother centrosome upon division. The mother centrosome is located near the basal membrane throughout the cell cycle, even during interkinetic nuclear migration. This mother centrosome is also involved in primary cilia formation of radial glial progenitor cells.
C. Drosophila neuroblasts inherit the daughter centrosome upon division. During each cell cycle, the mother centrosome is inactivated, while the daughter acquires robust microtubule organizing activity.
The surprise came when researchers found that the daughter centrosome is consistently inherited by the stem cells in Drosophila neuroblasts, which function as stem cells for brain development in Drosophila [17,18] (Fig. 2C). Previously, the asymmetric behavior of the two centrosomes was observed during the neuroblast cell cycle [19-21]. The centrosome with high MTOC activity stays in the neuroblast and the other with minimal MTOC activity is destined to the differentiating daughter, ganglion mother cells. At this point, many people assumed that the centrosome that has higher MTOC activity and stays in the neuroblast would be the mother centrosome. However, subsequent studies revealed that it is actually the daughter centrosome that is inherited by the neuroblast [17,18]. During the neuroblast cell cycle, the mother centrosome, which had a robust MTOC activity immediately following the previous mitosis, is always inactivated, while the daughter acquires the high MTOC activity and is eventually inherited by the neuroblast. This phenomenon clearly reveals the presence of a precise regulatory mechanism that ensures the accurate inheritance of certain centrosomes by certain cells, making it more likely that cells indeed have “reasons” to segregate the mother or daughter centrosome to particular cells, instead of the mother centrosome being passively inherited by stem cells due to its high MTOC activity.
Why do stem cells inherit mother or daughter centrosomes stereotypically?
Although not yet molecularly dissected, the existence of precise regulation on which the centrosome is inherited by stem cells indicates that cells do have reason(s) to do so. Here we summarize a few possibilities indicated by available observation. The possibilities are mainly categorized into two: 1) the mother or daughter centrosome is associated with fate determinants, and/or 2) the mother or daughter centrosome is capable of regulating cellular phenomena associated with the cell fate.
1) Is the mother or daughter centrosome associated with the fate determinants?
It has been shown that, in mollusk embryos, fate-determining mRNA is associated with one pole of the spindle, resulting in asymmetric inheritance of such mRNA [22]. Although it is not known whether the difference between mother and daughter centrosomes is used to set up an asymmetric spindle pole with distinct mRNA contents, it is tempting to speculate that stem cells indeed regulate the localization of fate-determining factors by associating them with the mother or daughter centrosomes.
2) Does the mother or daughter centrosome regulate asymmetry in cytokinesis?
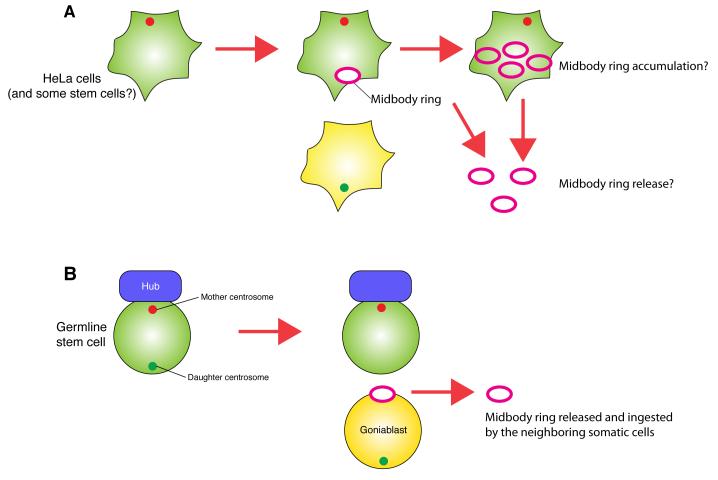
Recent studies have revealed that the difference between mother and daughter centrosomes contributes to asymmetric cytokinesis. Upon completion of cytokinesis, the midbody ring (MR; remnant of the cytokinetic contractile ring) is inherited only by one daughter of the division, imposing inherent asymmetry to cell division even in apparently symmetrically dividing cells. Interestingly, using various cancer cell lines and pluripotent cell lines (ES and iPS cells), the cell that contains the mother centrosome was shown to inherit the MR [23,24] (Fig. 3A). They further found that MR inheritance has a strong correlation with cell fate such as maintenance of pluripotency or stem cell identity [23]. However, Ettinger et al. recently reported that midbody release (instead of accumulation) correlates with stem cell identity in various cell lines [25] (Fig. 3A). This correlates their earlier findings that radial glial progenitor cells releases the MRs, which are intriguingly enriched with a stem cell marker CD133 [26]. Future investigation is needed to understand this apparent controversy. Using the Drosophila germline as a model system, we recently found that the MR is always inherited by the differentiating cell that inherits the daughter centrosome in Drosophila male GSCs (Salzmann et al., submitted) (Fig. 3B), contrasting to the report by Kuo et al., in the directionality of the MR inheritance regarding the centrosome age as well as stem cell fate. Such a MR inherited by the differentiating daughter (gonialblast) is eventually released and ingested by neighboring somatic cells, where it is degraded. Furthermore, we found that in Drosophila female GSCs, the mother centrosome is inherited by the differentiating daughter, like the Drosophila neuroblast, and the MR is inherited by the daughter centrosome-containing stem cells (Salzmann et al., submitted). These clarify that the mother or daughter centrosomes is unlikely to have inherent information associated with stem cell identity. Instead, certain information is likely to be transmitted via the centrosome (or the MR, the inheritance of which is determined by the centrosome age) in a context-dependent manner.
Fig. 3. Midbody ring inheritance and the centrosome.
A. The cell with the mother centrosome inherits the mother centrosome and midbody ring. Stem cells and cancer cells were reported to accumulate and/or release the midbody ring (see the text for detail).
B. Drosophila male GSCs, which inherit the mother centrosome, exclude the midbody ring upon division. The midbody ring that is inherited by the differentiating gonialblast is eventually released and ingested by neighboring somatic cells.
3) Is the mother or daughter centrosome associated with other factors related to stem cell identity?
While not being fate determinants, other factors have been proposed to be inherited asymmetrically by association with the centrosome. The immortal strand hypothesis (ISH) was proposed decades ago that stem cells might inherit the template copy of genomic DNA to avoid accumulation of replication-induced mutations. The mother centrosome has been speculated to provide some anchorage to retain the template copy of the genome [27,28], although immortal strand segregation does not appear to occur in Drosophila male GSCs, which stereotypically inherit the mother centrosome [29]. The ISH is still under debate and will require further technical advancement [30] to obtain definitive answers. Another factor that is reported to be inherited asymmetrically by association with the centrosome is the aggrosome, the aggregates of non-degradable proteins [31]. Certain stem cells were proposed to be protected from accumulation of damaged proteins by segregating them into short-lived, differentiating daughters.
Concluding remarks
As reviewed here, asymmetries in centrosome structure and function have attracted researchers’ interest for decades, and now their functional relevance in many cellular and developmental contexts, particularly in the context of stem cell behavior, has begun to emerge. While the notion that centrosome asymmetry can contribute to asymmetric cell division is fascinating, it actually imposes a new problem as well: how would symmetrically dividing cells overcome this inherent asymmetry? Can cells turn on and off the asymmetry associated with the centrosome depending on the context? We foresee exciting years to come addressing these new lines of questioning regarding centrosome asymmetry and its inheritance pattern.
Acknowledgment
The research in the Pelletier laboratory is supported by operating grants from the Canadian Cancer Society (019562), Natural Sciences and Engineering Research Council of Canada (RGPIN-355644-2008) and a grant-in-aid from the Krembil Foundation. L.P. holds a Canada Research Chair (Tier 2) in Centrosome Biogenesis and Function. The research in the Yamashita laboratory is supported by NIH (R01GM086481, R21HD067692) and the MacArthur Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References cited
- 1.Andersen JS, Wilkinson CJ, Mayor T, Mortensen P, Nigg EA, Mann M. Proteomic characterization of the human centrosome by protein correlation profiling. Nature. 2003;426:570–574. doi: 10.1038/nature02166. [DOI] [PubMed] [Google Scholar]
- 2.Palazzo RE, Vogel JM, Schnackenberg BJ, Hull DR, Wu X. Centrosome maturation. Curr Top Dev Biol. 2000;49:449–470. doi: 10.1016/s0070-2153(99)49021-0. [DOI] [PubMed] [Google Scholar]
- 3.Bettencourt-Dias M, Hildebrandt F, Pellman D, Woods G, Godinho SA. Centrosomes and cilia in human disease. Trends in genetics: TIG. 2011;27:307–315. doi: 10.1016/j.tig.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tsou MF, Stearns T. Mechanism limiting centrosome duplication to once per cell cycle. Nature. 2006;442:947–951. doi: 10.1038/nature04985. [DOI] [PubMed] [Google Scholar]
- 5.Tsou MF, Stearns T. Controlling centrosome number: licenses and blocks. Curr Opin Cell Biol. 2006;18:74–78. doi: 10.1016/j.ceb.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 6.Pelletier L, O’Toole E, Schwager A, Hyman AA, Muller-Reichert T. Centriole assembly in Caenorhabditis elegans. Nature. 2006;444:619–623. doi: 10.1038/nature05318. [DOI] [PubMed] [Google Scholar]
- 7.Nigg EA, Stearns T. The centrosome cycle: Centriole biogenesis, duplication and inherent asymmetries. Nature cell biology. 2011;13:1154–1160. doi: 10.1038/ncb2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bahe S, Stierhof YD, Wilkinson CJ, Leiss F, Nigg EA. Rootletin forms centriole-associated filaments and functions in centrosome cohesion. The Journal of cell biology. 2005;171:27–33. doi: 10.1083/jcb.200504107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bornens M. Centrosome composition and microtubule anchoring mechanisms. Current opinion in cell biology. 2002;14:25–34. doi: 10.1016/s0955-0674(01)00290-3. [DOI] [PubMed] [Google Scholar]
- 10.Anderson CT, Stearns T. Centriole age underlies asynchronous primary cilium growth in mammalian cells. Current biology: CB. 2009;19:1498–1502. doi: 10.1016/j.cub.2009.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yamashita YM, Mahowald AP, Perlin JR, Fuller MT. Asymmetric inheritance of mother versus daughter centrosome in stem cell division. Science. 2007;315:518–521. doi: 10.1126/science.1134910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fuller MT, Spradling AC. Male and female Drosophila germline stem cells: two versions of immortality. Science. 2007;316:402–404. doi: 10.1126/science.1140861. [DOI] [PubMed] [Google Scholar]
- 13.Leatherman JL, Dinardo S. Zfh-1 controls somatic stem cell self-renewal in the Drosophila testis and nonautonomously influences germline stem cell self-renewal. Cell Stem Cell. 2008;3:44–54. doi: 10.1016/j.stem.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leatherman JL, Dinardo S. Germline self-renewal requires cyst stem cells and stat regulates niche adhesion in Drosophila testes. Nat Cell Biol. 2010;12:806–811. doi: 10.1038/ncb2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yamashita YM, Jones DL, Fuller MT. Orientation of asymmetric stem cell division by the APC tumor suppressor and centrosome. Science. 2003;301:1547–1550. doi: 10.1126/science.1087795. [DOI] [PubMed] [Google Scholar]
- 16.Wang X, Tsai JW, Imai JH, Lian WN, Vallee RB, Shi SH. Asymmetric centrosome inheritance maintains neural progenitors in the neocortex. Nature. 2009;461:947–955. doi: 10.1038/nature08435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Januschke J, Llamazares S, Reina J, Gonzalez C. Drosophila neuroblasts retain the daughter centrosome. Nature communications. 2011;2:243. doi: 10.1038/ncomms1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Conduit PT, Raff JW. Cnn dynamics drive centrosome size asymmetry to ensure daughter centriole retention in drosophila neuroblasts. Curr Biol. 2010;20:2187–2192. doi: 10.1016/j.cub.2010.11.055. [DOI] [PubMed] [Google Scholar]
- 19.Rusan NM, Peifer M. A role for a novel centrosome cycle in asymmetric cell division. J Cell Biol. 2007;177:13–20. doi: 10.1083/jcb.200612140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rebollo E, Sampaio P, Januschke J, Llamazares S, Varmark H, Gonzalez C. Functionally Unequal Centrosomes Drive Spindle Orientation in Asymmetrically Dividing Drosophila Neural Stem Cells. Dev Cell. 2007;12:467–474. doi: 10.1016/j.devcel.2007.01.021. [DOI] [PubMed] [Google Scholar]
- 21.Rebollo E, Roldan M, Gonzalez C. Spindle alignment is achieved without rotation after the first cell cycle in Drosophila embryonic neuroblasts. Development. 2009;136:3393–3397. doi: 10.1242/dev.041822. [DOI] [PubMed] [Google Scholar]
- 22.Lambert JD, Nagy LM. Asymmetric inheritance of centrosomally localized mRNAs during embryonic cleavages. Nature. 2002;420:682–686. doi: 10.1038/nature01241. [DOI] [PubMed] [Google Scholar]
- 23.Kuo TC, Chen CT, Baron D, Onder TT, Loewer S, Almeida S, Weismann CM, Xu P, Houghton JM, Gao FB, et al. Midbody accumulation through evasion of autophagy contributes to cellular reprogramming and tumorigenicity. Nature cell biology. 2011 doi: 10.1038/ncb2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gromley A, Yeaman C, Rosa J, Redick S, Chen CT, Mirabelle S, Guha M, Sillibourne J, Doxsey SJ. Centriolin anchoring of exocyst and SNARE complexes at the midbody is required for secretory-vesicle-mediated abscission. Cell. 2005;123:75–87. doi: 10.1016/j.cell.2005.07.027. [DOI] [PubMed] [Google Scholar]
- 25.Ettinger AW, Wilsch-Brauninger M, Marzesco AM, Bickle M, Lohmann A, Maliga Z, Karbanova J, Corbeil D, Hyman AA, Huttner WB. Proliferating versus differentiating stem and cancer cells exhibit distinct midbody-release behaviour. Nature communications. 2011;2:503. doi: 10.1038/ncomms1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Farkas LM, Huttner WB. The cell biology of neural stem and progenitor cells and its significance for their proliferation versus differentiation during mammalian brain development. Curr Opin Cell Biol. 2008;20:707–715. doi: 10.1016/j.ceb.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 27.Tajbakhsh S. Stem cell identity and template DNA strand segregation. Curr Opin Cell Biol. 2008;20:716–722. doi: 10.1016/j.ceb.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 28.Tajbakhsh S, Gonzalez C. Biased segregation of DNA and centrosomes - moving together or drifting apart? Nat Rev Mol Cell Biol. 2009;10:804–810. doi: 10.1038/nrm2784. [DOI] [PubMed] [Google Scholar]
- 29.Yadlapalli S, Cheng J, Yamashita YM. Drosophila male germline stem cells do not asymmetrically segregate chromosome strands. J Cell Sci. 2011;124:933–939. doi: 10.1242/jcs.079798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Steinhauser ML, Bailey AP, Senyo SE, Guillermier C, Perlstein TS, Gould AP, Lee RT, Lechene CP. Multi-isotope imaging mass spectrometry quantifies stem cell division and metabolism. Nature. 2012;481:516–519. doi: 10.1038/nature10734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rujano MA, Bosveld F, Salomons FA, Dijk F, van Waarde MA, van der Want JJ, de Vos RA, Brunt ER, Sibon OC, Kampinga HH. Polarised Asymmetric Inheritance of Accumulated Protein Damage in Higher Eukaryotes. PLoS Biol. 2006;4:e417. doi: 10.1371/journal.pbio.0040417. [DOI] [PMC free article] [PubMed] [Google Scholar]