Abstract
Two new and five known stilbenes and one new alkylresorcinol were isolated from the orchid Phragmipedium calurum during a screen for new anticancer compounds. The compounds were evaluated for antiproliferative activity against multiple human cancer cell lines. Two of the compounds (1 and 7) displayed moderate activity against several cell lines.
Keywords: Phragmipedium calurum, Orchidaceae, stilbene, alkylresorcinol, cytotoxicity, cancer
1. Introduction
Cancer comprises over 100 different diseases and affects more than 13 million people in the US alone (Malakoff, 2011; Mariotto et al., 2011). Great strides have been made in combating cancer over the last 40 years, and newer targeted therapies have extended survival times and avoided the toxicity of earlier chemotherapy drugs. Unfortunately, many types of cancer do not yet have adequate treatments, and many tumors that initially respond to targeted therapy eventually become resistant (Kaiser, 2011). Thus, it is clear that there is still a need for new cancer drugs.
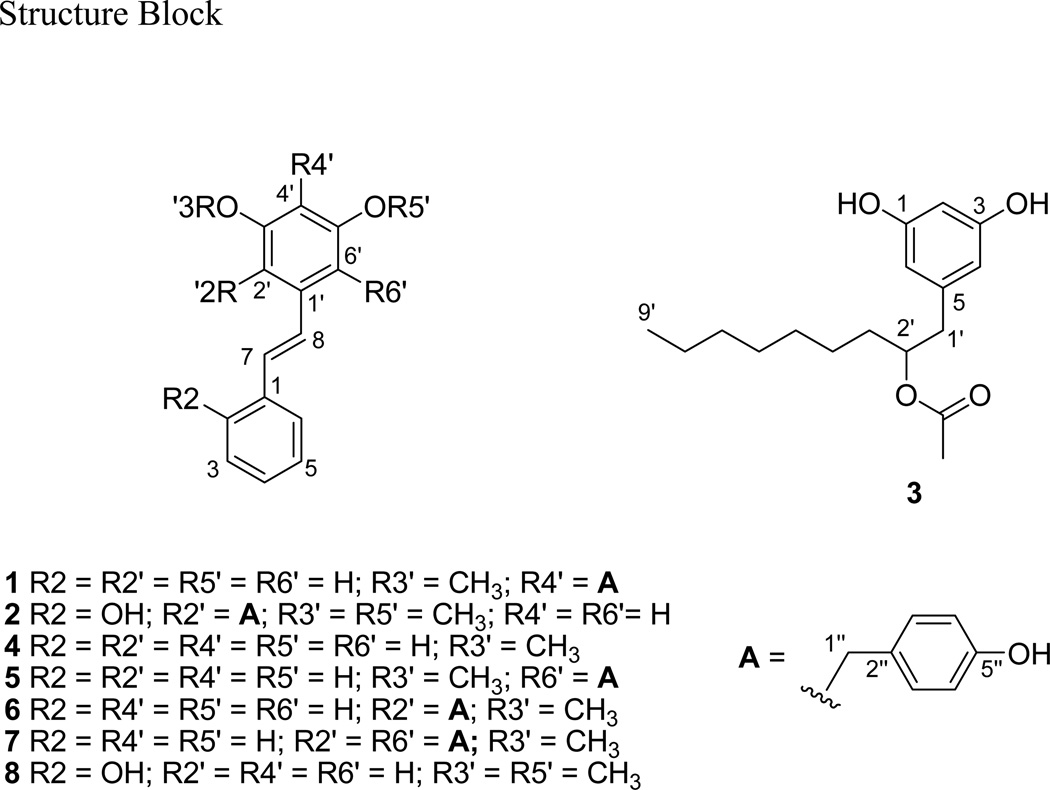
As part of an effort to discover new compounds as leads for cancer drugs, we screened a portion of our existing library of purified plant natural products for antiproliferative activity against NCI-H460 lung cancer cells. One of the hits from this screen was the stilbene 7, which we had previously isolated from orchid species of the genus Phragmipedium (Garo et al., 2007). A related compound that had been isolated from Phragmipedium at the same time was also found to be active in this assay, but lack of material prohibited full structure determination. In order to further examine the bioactivity of these compounds, we carried out a larger scale isolation. This larger isolation, along with more sensitive NMR technology, allowed us to establish the structure of the minor compound as the new compound 1. The re-isolation also yielded two more new compounds (2, 3) as well as additional material for compounds 4–8. Structure determination of the new compounds and assessment of all the compounds’ antiproliferative activity is presented here.
2. Results and discussion
The molecular formula of 1 was determined to be C22H20O3 based on HRESIMS. The 1H NMR spectrum of 1 (Table 1) matched that of the previously isolated active compound, confirming the re-isolation. Like its isomer 5 (Garo et al., 2007), it displayed resonances for an unsubstituted phenyl ring (δH 7.52, 7.33, 7.22), a 4-hydroxybenzyl moiety (δH 7.06, 6.61, 3.84), two meta-coupled aromatic protons (δH 6.66, 6.67), a methoxyl group (δH 3.83), and a trans-double bond (δH 7.04, 7.08; J = 16 Hz). Compound 1 thus appeared to have the same chemical moieties as 5 but with different connectivity. Because only a small amount of 1 (120 µg) was available, obtaining a complete 2D NMR data set was facilitated by using the Bruker BioSpin TCI 1.7 mm MicroCryoProbe. HMBC correlations from the H-1″ methylene protons (δH 3.84) to two oxygenated carbons C-5′ (δC 157.0) and C-3′ (δC 160.0) placed the 4-hydroxybenzyl group at the 4′-position on the central aromatic ring. Chemical shifts and COSY and HMBC correlations (Table 1) indicated that the rest of the molecule was the same as 5. In particular, the chemical shifts of C-2′ (δC 101.8) and C-6′ (δC 107.4) indicated that each of these carbons was adjacent to an oxygenated carbon. HMBC correlations from H-2′ and H-6′ to C-8 (δC 130.0) supported the placement of the stilbene linkage. Chemical shifts of C-3′ and C-5′ were assigned based on HMBC correlations from H-2′ to C-3′ (δC 160.0) and from H-6′ to C-5′ (δC 157.0). The methoxyl group was then placed at C-3′ based on an HMBC correlation from the methoxyl protons to C-3′.
Table 1.
NMR Spectroscopic Data (600 MHz, CD3OD) for 1 and 2
| 1 | 2 | |||||
|---|---|---|---|---|---|---|
| position | δC | δH (mult, J in Hz) | HMBCa | δC | δH (mult, J in Hz) | HMBCa |
| 1 | 138.5 | 3/5, 8 | 125.5 | 8 | ||
| 2 | 127.4 | 7.52 (d, 7.7) | 4, 7 | 156.0 | 4, 6, 7 | |
| 3 | 129.6 | 7.33 (t, 7.5) | 3/5 | 116.3 | 6.79 (d)b | 5 |
| 4 | 128.4 | 7.22 (t, 7.3) | 2/6 | 129.0 | 7.05 (td, 7.8, 1.3) | 6 |
| 5 | 129.6 | 7.33 (t, 7.5) | 3/5 | 120.4 | 6.79 (t)b | 3 |
| 6 | 127.4 | 7.52 (d, 7.7) | 4, 7 | 127.4 | 7.35 (dd, 8.1, 1.3) | 4, 7 |
| 7 | 129.5 | 7.08 (d, 16.0) | 2/6 | 126.4 | 7.26 (d, 16.2) | 6 |
| 8 | 130.0 | 7.05 (d, 16.0) | 2•, 6• | 127.2 | 7.41 (d, 16.2) | 7, 6• |
| 1• | 138.5 | 7 | 139.9 | 7, 1•• | ||
| 2• | 101.8 | 6.67 (br s) | 6• | 121.4 | 8, 4•, 6•, 1•• | |
| 3• | 160.0 | 2•, 1••, | 159.8 | 1••, | ||
| OCH3-3• | OCH3-3• | |||||
| 4• | 117.7 | 2•, 6•, 1•• | 98.5 | 6.50 (d, 2.5) | 6• | |
| 5• | 157.0 | 6•, 1•• | 160.2 | 6•, | ||
| OCH3-5• | ||||||
| 6• | 107.5 | 6.66 (br s) | 8, 2• | 102.2 | 6.83 (d, 2.5) | 8, 4• |
| 1•• | 28.6 | 3.84 (s) | 3••/7•• | 30.3 | 3.99 (s) | 3••/7•• |
| 2•• | 133.9 | 1••, 4••/6•• | 133.7 | 1••, 4••/6•• | ||
| 3••/7•• | 130.5 | 7.06b | 1••, 3••/7•• | 129.8 | 6.97 (d, 8.4) | 1••, 3••/7•• |
| 4••/6•• | 115.5 | 6.61 (d, 8.5) | 4••/6•• | 115.5 | 6.63 (d, 8.4) | 4••/6•• |
| 5•• | 155.6 | 3••/7•• | 155.8 | 3••/7•• | ||
| OCH3-3• | 56.0 | 3.83 (s) | 55.7 | 3.81 (s) | ||
| OCH3-5• | 55.3 | 3.85 (s) | ||||
HMBC correlations are from the proton(s) listed to the indicated carbon.
Signal partially obscured.
HRESIMS indicated a molecular formula of C23H22O4 for 2. Its 1H NMR spectrum (Table 1) also resembled that of 5. The most notable difference was the loss of symmetry in the phenyl ring; the coupling pattern for the protons of this ring indicated substitution at the 2-position. HMBC correlations from H-3 (δH 6.79), H-6 (δH 7.35), and H-7 (δH 7.26) to C-2 (δC 156.0) confirmed oxygenation at this position. HMBC correlations from H-1′ (δH 3.99) to C-1′ and C-3′ indicated attachment of the 4-hydroxybenzyl group to C-2′ in the central ring, between the stilbene linker and an oxygenated position. The meta-coupling of H-4′ and H-6′ (J = 2.5 Hz) and the chemical shifts of C-4′ (δC 95.5) and C-6′ (δC 102.2) required that the remaining oxygen on the central ring be placed at C-5′. Finally, the 1H NMR spectrum indicated that 2 contains two methoxyl groups (δH 3.81, 3.85). These were placed at C-3′ and C-5′, respectively, based on HMBC correlations from the methoxyl protons to C-3′ (δC 159.8) and C-5′ (δC 160.2).
The 1H NMR spectrum of 3 (Table 2) was markedly different than those of the other compounds. The aromatic region contained only two signals: δH 6.15 (2H, H-4 and H-6) and δH 6.12 (1H, H-2). The chemical shifts, integration, and coupling constants (J = 2.1 Hz) of these protons, as well as the chemical shifts of the corresponding carbons (δC 108.8 and 101.5, respectively) indicated a symmetric phenyl ring oxygenated at positions 3 and 5 (according to the atom numbering shown for 3). The 1H spectrum also indicated the presence of an alkyl chain, an oxygenated methine (δH 5.00, H-2′), and an acetate (δH 1.98). HMBC correlations from the H-1′ methylene protons at δH 2.63 and 2.69 to C-5 and C-4/6 placed this methylene adjacent to the phenyl ring. The COSY and HMBC NMR spectra were used to trace the remainder of the alkyl chain, and placed the oxygenated carbon at C-2′. Placement of the acetate at C-2′ was confirmed by HMBC correlations from the acetate protons and H-2′ to the ester carbonyl at δC 172.6. The length of the alkyl chain is consistent with the molecular formula of C17H26O4 established by HRESIMS.
Table 2.
NMR Spectroscopic Data (600 MHz, CD3OD) for 3
| 3 | |||
|---|---|---|---|
| position | δC | δH (mult, J in Hz) | HMBCa |
| 1/3 | 159.0 | 2, 4/6 | |
| 2 | 101.5 | 6.12 (t, 2.1) | 4/6 |
| 4/6 | 108.8 | 6.15 (d, 2.1) | 2, 4/6, 1• |
| 5 | 141.1 | 1• | |
| 1• | 41.3 | 2.63 (dd, 13.5, 6.2) | 2•, 3• |
| 2.69 (dd, 13.5, 7.0) | |||
| 2• | 76.2 | 5.00 (m) | 1•, 3• |
| 3• | 34.3 | 1.54 (m) | 1•, 2• |
| 4• | 25.9 | 1.30 (m) b | 2•, 3• |
| 5• | 30.0 | 1.30 (m) b | |
| 6• | 30.0 | 1.30 (m) b | |
| 7• | 32.7 | 1.30 (m) b | 9• |
| 8• | 23.5 | 1.27 (m) b | 9• |
| 9• | 14.2 | 0.89 (t, 7.0) | |
| OAc-2• | 20.7 | 1.98 (s) | |
| 172.6 | 2•, OAc-2• | ||
HMBC correlations are from the proton(s) listed to the indicated carbon.
Signal partially obscured.
Compound 3 is classified as an alkylresorcinol. Alkylresorcinols have been found in bacteria, fungi, and plants, and have been shown to have antimicrobial, antiparasitic, and cytotoxic activities (Kozubek and Tyman, 1999). Plant alkylresorcinols tend to have longer aliphatic chains (13–27 carbons) with one or more unsaturations (Baerson et al., 2010); however, short, saturated alkylresorcinols similar to 3 have been identified from Ononis natrix (Fabaceae) (Cañedo et al., 1997). Alkylresorcinol synthases (ARS) and stilbene synthases (STS) are closely related Type III polyketide synthase enzymes (Cook et al., 2010). Each catalyzes the successive addition of multiple two-carbon units to a starter molecule, followed by intramolecular cyclization and aromatization of the resulting ketide chain. ARS and STS differ in their starting molecules; STS uses p-coumaroyl-CoA as a starter, while ARS enzymes use a fatty acid-derived CoA starter. The structure of 3 is consistent with a heptanoyl-CoA starter condensed with five two-carbon units and then cyclized; in this scenario, the oxygen at the 2′-position is derived from the ketone of the first two-carbon unit added (Funa et al., 2007).
Each of the isolated compounds was assayed for antiproliferative activity against a small panel of human cancer cell lines. The resulting IC50 values are listed in Table 3. Compounds 1 and 7 displayed moderate activity against several of the cell lines.
Table 3.
IC50s against human cancer cell lines (µM)
| Compound | NCI-H460 | NCI-H226 | NCI-H522 | A549 | M14 | PC3 |
|---|---|---|---|---|---|---|
| 1 | 1.3 ± 0.7 | 8.5 ± 0.8 | 8.5 ± 0.9 | 5.5 ± 0.4 | 2.2 ± 0.2 | 4.1 ± 0.4 |
| 2 | > 10 | > 10 | 9 ± 1 | > 10 | > 10 | > 10 |
| 3 | > 10 | > 10 | > 10 | 9 ± 1 | > 10 | > 10 |
| 4 | > 10 | > 10 | > 10 | >10 | > 10 | > 10 |
| 5 | 7.8 ± 0.4 | > 10 | > 10 | > 10 | > 10 | > 10 |
| 6 | > 10 | > 10 | > 10 | > 10 | > 10 | > 10 |
| 7 | 2.0 ± 0.8 | > 10 | > 10 | > 10 | 3 ± 1 | 5 ± 2 |
| 8 | 8.1 ± 0.7 | > 10 | > 10 | > 10 | > 10 | > 10 |
| camptothecin | 0.003 | 0.05 | 0.2 | 0.01 | 0.01 | 0.05 |
2.1. Concluding remarks
In this study, we explored the antiproliferative activity of seven stilbenes and one alkylresorcinol from the orchid Phragmipedium calurum. Five of the stilbenes are decorated with 4-hydroxybenzyl moieies, a somewhat unusual substituent for stilbenes (Garo et al., 2007). Compounds 1 and 7 were moderately active against one or more human cancer cell lines, and provide a starting point for medicinal chemistry efforts and mechanistic studies.
3. Experimental
3.1. General experimental procedures
NMR spectra were acquired at 600 MHz on a Bruker Avance 600 MHz spectrometer equipped with a Bruker BioSpin TCI 1.7 mm MicroCryoProbe. For each compound, 1H, gCOSY, ROESY, gHSQC, and gHMBC spectra were acquired; 13C chemical shifts were obtained from the HSQC and HMBC spectra. Spectra were recorded in CD3OD, and chemical shifts are reported with respect to the residual non-deuterated solvent signal. HRESIMS was done on a Waters LCT time-of-flight mass spectrometer with an electrospray interface and polyethylene glycol as the internal standard. The amount of each compound isolated was determined using HPLC/ELSD as previously described (Hu et al., 2005). Semipreparative HPLC was performed on a Beckman HPLC system including a Beckman 168 diode array UV detector, a Sedere Sedex 75 ELSD detector, and an ISCO Foxy Jr. fraction collector. A splitter was used to divert 10% of the eluent to the ELSD detector, while the rest passed through a diode array UV detector and was then collected. UV λmax values were taken from the diode array detector during semipreparative HPLC purification in 45–50% CH3CN with 0.05% TFA. Optical rotation was measured on Jasco P-1010 polarimeter using a 100 µL cell with a 0.1 dm path length.
3.2. Plant material
P. calurum was grown in the greenhouse at the Missouri Botanical Garden (USA), and sent frozen to Sequoia, where it was lyophilized. A voucher specimen of P. calurum (Naibi 23) is kept at the Missouri Botanical Garden.
3.3 Extraction and isolation
P. calurum (60 g dry weight) was extracted with EtOH/EtOAc (1:1, v/v) to obtain 8 g of dry extract. The extract was then subjected to silica gel CC (5 × 28 cm) with the following eluents (500 ml each): hexane-EtOAc (3:1), hexane-EtOAc (1:1), EtOAc, EtOAc-MeOH (7:3), and EtOAc-MeOH (1:1). Three to four fractions were collected during elution with each solvent. The second fraction from the hexane-EtOAc (1:1) elution was dried down and then further purified using a C18 solid phase extraction (SPE) column (5 g) equilibrated in 50% MeOH and eluted with MeOH (30 ml). The SPE product (250 mg) was then further fractionated using preparative HPLC on a Betasil C18 column (Thermo Scientific, 21.2 × 100 mm, 5 µm, 3 × 83 mg injections) with a flow rate of 20 ml/min. The column was eluted with 60% CH3CN for 2 min, followed by a 34 min gradient to 85% CH3CN, then 85% CH3CN for 6 minutes; the HPLC solvents contained 0.05% TFA. Fractions (one minute each) were collected from 2–42 min.
Preparative HPLC fraction 3 from the three runs was pooled and fractionated by semipreparative HPLC on an Atlantis C18 column (Waters, 10 × 250 mm, 5 µm) eluted at 3 ml/min with 45% CH3CN for 5 min, followed by a gradient to 50% CH3CN over 60 min. Twenty-five injections afforded 5.4 mg of 4 (tR = 44.3 min), 2.1 mg of 6 (tR = 48.1 min), 152 µg of 7 (tR = 56.2 min), and 169 µg of 2 (tR = 57.3 min). Preparative HPLC fraction 4 from the three runs was pooled and fractionated using the same semipreparative HPLC column using an isocratic elution with 47% CH3CN for 64 min. Seven injections afforded 476 µg of 8 (tR = 40.4 min), 202 µg of 5 (tR = 44.0 min), 347 µg of 3 (tR = 47.7 min), and 120 µg of 1 (tR = 59.2 min). All semipreparative HPLC solvents contained 0.05% (v/v) TFA.
3.4 Cytotoxicity assay
NCI-H460 (large cell lung carcinoma), NCI-H226 (lung squamous cell carcinoma), NCI-H522 (non-small lung adenocarcinoma), and PC-3 (prostate adenocarcinoma) cells were obtained from ATCC. M14 (amelanotic melanoma) and A549 (non-small lung adenocarcinoma) cells were obtained from the National Cancer Institute. Cells were grown in RPMI-1640 with 10% FBS supplemented with L-glutamine and HEPES. Cells were seeded into 96-well plates at 5 × 102 to 5 × 104 cells/well and allowed to adhere overnight; the medium was then removed. A stock solution of test compound in DMSO was diluted in medium to generate a series of working solutions. Aliquots (100 µl) of the working solutions were added to the appropriate test wells to expose cells to the final concentrations of compound in a total volume of 100 µl. Eight different concentrations were tested, with 2–5 wells per concentration.
Camptothecin was used as a positive control; wells containing vehicle without compound were used as negative controls. Plates were kept for 72 h in a 37° C, 5% CO2 incubator. After incubation, viable cells were detected with the CellTiter 96 AQueous Non-Radioactive Cell Proliferation Assay (Promega). Dose-response curves were generated and IC50 values were determined using GraphPad Prism 5 software.
3.5 (E)-5′-hydroxy-4′-(4-hydroxybenzyl)-3′-methoxystilbene (1)
UV λmax 223, 317 nm; 1H and 13C NMR: see Table 1; HR-ESIMS m/z 333.1502 [M+H]+ (calcd for C22H21O3: 333.1491).
3.6 (E)-2-hydroxy-2′-(4-hydroxybenzyl)-3′,5′-dimethoxystilbene (2)
UV λmax 217, 235, 286, 324 nm; 1H and 13C NMR: see Table 1; HR-ESIMS m/z 363.1567 [M+H]+ (calcd for C23H23O4: 363.1596).
3.7 5-(2-acetoxynonyl)resorcinol (3)
[α]D25 −223° (c 0.10, CHCl3); UV λmax 209, 223, 279 nm; 1H and 13C NMR: see Table 2; HR-ESIMS m/z 295.1916 [M+H]+ (calcd for C17H27O4: 295.1909).
Highlights.
Two stilbenes and an alkylresorcinol were isolated from Phragmipedium calurum.
These compounds were found during a screen for new anticancer agents.
Two of the compounds (1 and 7) displayed moderate antiproliferative activity.
Acknowledgements
We acknowledge the scientific collaboration with Advanced Chemistry Development, Inc., (ACD Labs) and the use of the ACD/SpecManager and ACD/Structure Elucidator. We acknowledge the staff of the Missouri Botanical Garden providing plant material. The project described was partially supported by Award Number R43CA141944 from the National Cancer Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baerson SR, Schroder J, Cook D, Rimando AM, Pan Z, Dayan FE, Noonan BP, Duke SO. Alkylresorcinol biosynthesis in plants: new insights from an ancient enzyme family? Plant Signaling Behav. 2010;5:1286–1289. doi: 10.4161/psb.5.10.13062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cañedo LM, Miguel del Corral JM, San Feliciano A. 5-Alkylresorcinols fromOnonis natrix . Phytochemistry. 1997;44:1559–1563. [Google Scholar]
- Cook D, Rimando AM, Clemente TE, Schroder J, Dayan FE, Nanayakkara NPD, Pan Z, Noonan BP, Fishbein M, Abe I, Duke SO, Baerson SR. Alkylresorcinol synthases expressed inSorghum bicolor root hairs play an essential role in the biosynthesis of the allelopathic benzoquinone sorgoleone. Plant Cell. 2010;22:867–887. doi: 10.1105/tpc.109.072397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funa N, Awakawa T, Horinouchi S. Pentaketide Resorcylic Acid Synthesis by Type III Polyketide Synthase fromNeurospora crassa. J.Biol. Chem. 2007;282:14476–14481. doi: 10.1074/jbc.M701239200. [DOI] [PubMed] [Google Scholar]
- Garo E, Hu J-F, Goering M, Hough G, O’Neil-Johnson M, Eldridge G. Stilbenes from the OrchidPhragmipedium sp . J. Nat. Prod. 2007;70:968–973. doi: 10.1021/np070014j. [DOI] [PubMed] [Google Scholar]
- Hu JF, Garo E, Yoo HD, Cremin PA, Zeng L, Goering MG, O’Neil-Johnson M, Eldridge GR. Application of capillary-scale NMR for the structure determination of phytochemicals. Phytochem. Anal. 2005;16:127–133. doi: 10.1002/pca.831. [DOI] [PubMed] [Google Scholar]
- Kaiser J. Combining Targeted Drugs to Stop Resistant Tumors. Science. 2011;331:1542–1545. doi: 10.1126/science.331.6024.1542. [DOI] [PubMed] [Google Scholar]
- Kozubek A, Tyman JHP. Resorcinolic Lipids, the Natural Non-isoprenoid Phenolic Amphiphiles and Their Biological Activity. Chem. Rev. (Washington DC) 1999;99:1–25. doi: 10.1021/cr970464o. [DOI] [PubMed] [Google Scholar]
- Malakoff D. Can Treatment Costs Be Tamed? Science. 2011;331:1545–1547. doi: 10.1126/science.331.6024.1545. [DOI] [PubMed] [Google Scholar]
- Mariotto AB, Robin Yabroff K, Shao Y, Feuer EJ, Brown ML. Projections of the Cost of Cancer Care in the United States: 2010–2020. Journal of the National Cancer Institute. 2011;103:117–128. doi: 10.1093/jnci/djq495. [DOI] [PMC free article] [PubMed] [Google Scholar]