Abstract
A powerful Fe(III)/NaBH4-mediated free radical hydrofluorination of unactivated alkenes is disclosed using Selectfluor as a source of fluorine and resulting in exclusive Markovnikov addition. In contrast to the traditional and unmanageable free radical hydrofluorination of alkenes, the Fe(III)/NaBH4-mediated reaction is conducted under exceptionally mild reaction conditions (0 °C, 5 min, CH3CN/H2O). The reaction can be conducted open to the air and with water as a cosolvent and demonstrates an outstanding substrate scope and functional group tolerance.
The growing presence of fluorine in pharmaceuticals and agrochemicals1 as well as the use of 18F as a positron emitter for positron emission tomography (PET) molecular imaging2 have stimulated a renewed interest in methods for the introduction of fluorine under mild reaction conditions often using transition metals.3 In spite of this recent interest, the hydrofluorination of alkenes has remained a challenging transformation. Olah and coworkers developed conditions using HF/pyridine or HF with other amines that provide the hydrofluorination of alkenes in good yields. However, this method has limitations with respect to substrate stability and functional group tolerance under the relatively harsh reaction conditions.4 The complementary free radical hydrofluorination of alkenes is typically regarded as an unmanageable reaction. Fluorine (F2) and related sources of atomic fluorine (F·) including hypofluorites exhibit an uncontrollable reactivity that precludes their use.5 In part, the hazards of such reactions may be addressed using the more manageable, but still highly reactive XeF26 or potentially perfluoroalkanes7 although the reagent availability (XeF2) and scope of their behavior as free radical traps remain to be fully established. Neither is adaptable to 18F labeling procedures. Recently, we described an Fe(III)/NaBH4-mediated free radical oxidation of a trisubstituted alkene for introduction of the vinblastine C20′ tertiary alcohol,8 that proved general for the oxidation of unactivated alkenes.9 In the latter studies, we defined the alkene substrate scope, established the exclusive Markovnikov addition regioselectivity, introduced the use of alternative free radical traps, and examined the Fe(III) salt and initiating hydride source. Due to the excellent functional group tolerance and its mild reaction conditions (0 °C, 5-30 min) that are relatively insensitive to the reaction parameters, including the use of water as a cosolvent, we sought to develop a method for the free radical incorporation of fluorine using this strategy. This method would introduce the hydrogen and fluorine from separate reagent sources, effecting a Markovnikov free radical hydrofluorination of alkenes.
Examination and optimization of the reaction parameters using Fe(III) oxalate (Fe2(ox)3) and NaBH4 were first explored with the hydrofluorination of 9-decen-1-ol in conjunction with various sources of potential fluorine radical donors (Table 1). Selectfluor and N-fluorobenzene-sulfonimide (NFSI) were screened as sources of fluorine for this reaction, because their 18F derivatives are available and used to incorporate 18F labels.10,11 Although typically regarded as sources of electrophilic fluorine (F+), a recent study demonstrated that they may also serve as effective sources of atomic fluorine (F·).12 Selectfluor and NFSI provided comparable and modest yields of 1a in a THF/H2O (1:1) solvent mixture, whereas tosyl fluoride was ineffective as a source of a radical fluorine trap under these conditions (Table 1, entries 1-3). Adjusting the solvent mixture to CH3CN/H2O (1:1) to improve the solubility of Selectfluor substantially improved the yield (entry 4). The solvent mixture was found to be critical to providing good yields of the desired products since each solvent alone was ineffective (0-14%) compared to the mixture (entries 5-7). Remarkably, the reaction time could be reduced to only 5 min (entry 8), an important consideration for 18F radiolabeling because of the 110 min half-life of 18F. Compared to prior studies where the companion reagent coordination to Fe(III) may compete,9 the Fe(III)/NaBH4-mediated hydrofluorination reaction proved relatively insensitive to the Fe(III) source (Fe2(ox)3, 75% = Fe(NO3)3, 75%, Fe(acac)3, 71% > Fe2(SO4)3, 55%, > FeCl3, 25% + 66% chloride > FeF3 (insoluble), 0%). Omission of the Fe(III) reagent from the reaction mixture provided only recovered starting alkene substrate confirming its essential role. Additional control experiments revealed that the reaction was surprisingly insensitive to air, so all reactions were run open to an atmosphere of air. Sodium and lithium borohydride (NaBH4 and LiBH4) were effective as the initiating hydride source, while PhSiH3, Bu3SnH, NaCNBH3, and NaB(OAc)3H did not support the desired reactivity (NaBH4, 75% = LiBH4, 73% > BH3, 45% > NaCNBH3, NaB(OAc)3H, 0%). Aside from its availability, cost, and ease of use, NaBH4 is especially attractive as a formal hydrogen radical source because the availability of NaBD4 and NaBT4 provides the opportunity for the introduction of additional isotopic and radiolabels using this method. Although this was not investigated in detail, related systems (Co(acac)2/PhSiH3, Selectfluor) used for the oxidation or functionalization of unactivated alkenes did not support the hydrofluorination reaction.13,14 Finally, the reaction proved sensitive to the reaction concentration (0.05 M < 0.025 M < 0.0125 M > 0.00625 M for 1a, see Supporting Information) and reductions in the stoichiometric reagent amounts below those indicated (2 equiv of Fe2(ox)3, 3.2-6.4 equiv of NaBH4, see Supporting Information) led to progressively lower conversions.
Table 1.
Optimization of Reaction Parameters.

| ||||
|---|---|---|---|---|
| entry | Fe2(ox)3 (equiv) | F• source (equiv) | Solvent | Yield (%) |
| 1 | 4 | Tosyl fluoride (4) | THF/H2O | 0 |
| 2 | 4 | NFSI (4) | THF/H2O | 23 |
| 3 | 4 | Selectfluor (4) | THF/H2O | 20 |
| 4 | 4 | Selectfluor (4) | CH3CN/H2O | 66 |
| 5 | 2 | Selectfluor (2) | CH3CN/H2O | 75 |
| 6 | 2 | Selectfluor (2) | CH3CN | 0 |
| 7 | 2 | Selectfluor (2) | H2O | 14 |
| 8a | 2 | Selectfluor (2) | CH3CN/H2O | 75 |
| 9b | 2 | Selectfluor (2) | CH3CN/H2O | 70 |
Reaction time was 5 min.
NaBH4 (3.2 equiv).
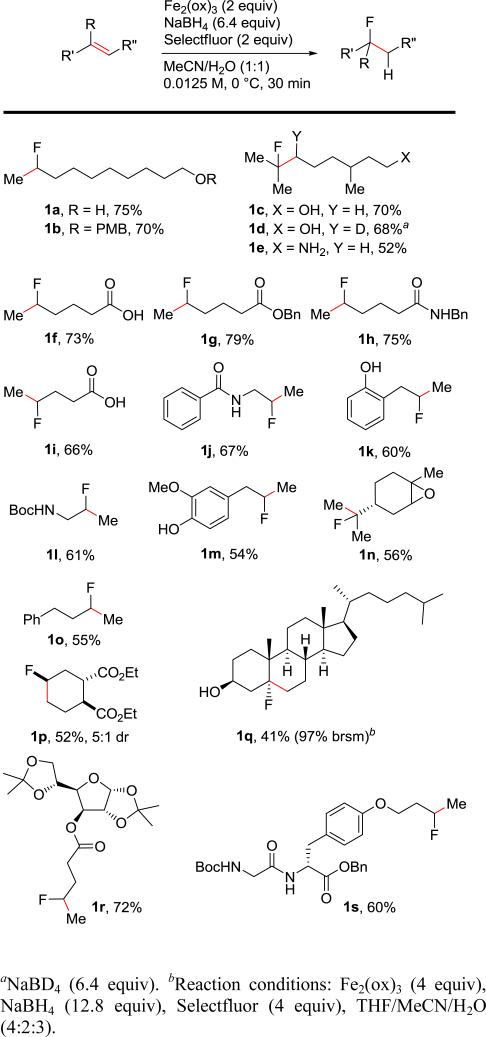
The alkene substrate scope proved quite general with unactivated terminal alkenes, as well as di- and trisubstituted alkenes participating in the hydrofluorination reaction effectively (Scheme 1). A wide range of substrates and functional groups are tolerated including unprotected and protected alcohols, protected and free amines, phenols, epoxides, ketals, acetals, carboxylic acids, carbamates, amides, esters, peptides, and carbohydrates. Notably, the free radical hydrofluorination is so fast that the competitive fluorolactonization of 4-pentenoic acid with Selectfluor is not observed,15 instead providing product 1i in 66% yield. This result also provides mechanistic insight into this reaction, indicating that it does not proceed through a carbocation intermediate. In fact, a series of substrates capable of proximal nucleophilic participation in an alkene functionalization reaction (1f, 1h-1l) failed to display this competitive behavior, providing only the free radical hydrofluorination products in good yields. Eugenol provided the desired hydrofluorination product 1m instead of the known Selectfluor oxidation of 4-substituted phenols to the corresponding 4-fluorocyclohexadienones.16 Diethyl trans-4-cyclohexene-1,2-dicarboxylate provided a 52% yield of the fluorinated product 1p (5:1 dr). The 5:1 axial/equatorial diastereoselectivity of this reaction is consistent with previously reported radical additions to cyclohexenes.17 The sterically hindered trisubstituted alkene in cholesterol was fluorinated to provide 1q in 41% yield (97% brsm) as a single diastereomer. Only styrenes proved to be ineffective alkene substrates, instead yielding the benzylic alcohols and corresponding dimers of the benzylic radicals under the reaction conditions.18
Scheme 1.
Scope of Hydrofluorination of Unactivated Alkenes.
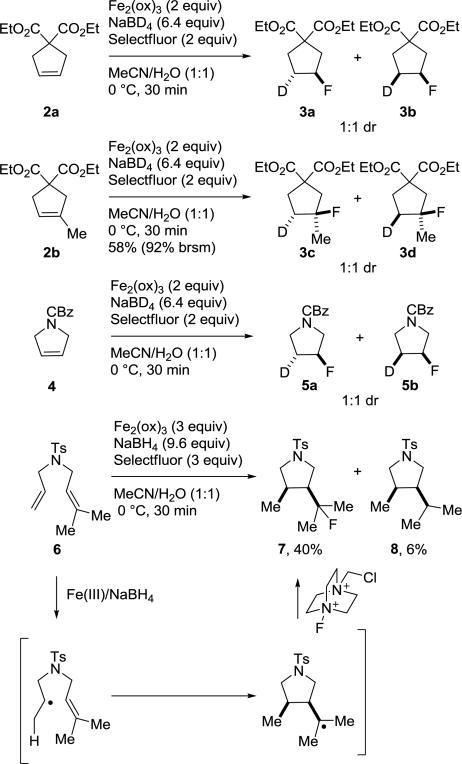
Complementary to prior mechanistic studies,8,9 we further probed the mechanism of the reaction using the substrates 2, 4 and 6 (Scheme 2). Substrates 2 and 4 provided a 1:1 mixture of product diastereomers, indicating that the addition of hydrogen and fluorine across the double bond is nondiastereospecific, consistent with the previously established free radical mechanism of Fe(III)/NaBH4-mediated reactions.8,9 The reactions of 2 and 4, like that of 1d, also confirm that NaBD4(NaBH4) serves as the reagent source of the hydrogen atom and not solvent, highlighting the additional reagent labeling potential (D,T) of the reaction, and produced the pharmaceutically important pyrrolidine 5 in the case of 4. Substrate 6 served to further probe the presence of a radical intermediate in this hydrofluorination reaction. Under the reaction conditions (Fe(III)/NaBH4) in the presence of Selectfluor, the cyclized product 7 was observed in a 40% yield, along with the cyclized, but reduced byproduct 8 (6%). In previously reported mechanistic studies on the electrophilic fluorination of alkenes with Selectfluor, no analogous cyclization onto a pendant alkene was observed and was indicative of reactions that proceed through an ionic mechanism. In contrast, the reaction of 6 provided the cyclized products 7 and 8, inconsistent with an ionic mechanism and further supportive of free radical intermediates generated under these reaction conditions.19 It is not yet clear whether the reaction involves generation of an initiating Fe–H and its unusual Markovnikov migratory insertion onto the alkene followed by subsequent homolytic cleavage of the resulting C–Fe bond for alkyl radical generation, or whether an Fe–H or oxidized borane/borohydride (e.g., BH3·/BH4·) simply serve as an initiating hydrogen atom donor.
Scheme 2.
Finally, subjecting the alkyne 9 to the reaction conditions resulted in the generation of the monofluorinated product 1e in 34% yield, with no vinyl fluoride or difluorinated product observed (eq 1). The isolation of benzyl hex-5-enoate (10) from the reaction mixture suggests the alkene is an intermediate en route to formation of the monofluorinated product 1e and that reduction of the initial vinyl radical occurs faster and in preference to fluorination. Previously, Olah has reported that the ionic reaction of alkynes with HF/pyridine provides the difluorinated products4 and more recently, Sadighi and coworkers reported that a Au-catalyzed reaction of alkynes with Et3N·3HF provides the vinyl fluoride products.20 Our complementary results with 9 are consistent with an Fe/NaBH4-mediated free radical reduction of the alkyne followed by addition of a hydrogen atom to the alkene to form an alkyl radical which is then trapped with a fluorine atom from Selectfluor.21
 |
(1) |
An initial examination of an Fe(III)/NaBH4-mediated reaction for the hydrofluorination of unactivated alkenes is reported. Complementary to the more traditional approaches for the introduction of fluorine into organic substrates using nucleophilic (F-) or electrophilic (F+) reagents and reactivity, the studies detailed herein provide the first general method for the effective free radical fluorination (F·) of unactivated alkene substrates. The broad alkene scope of the reaction, the exclusive Markovnikov addition regioselectivity, the facile nature of this method, its insensitivity to air and moisture, and the excellent functional group tolerance make this reaction an attractive method for the hydrofluorination of alkenes. Because of the availability of labeled reagents,12 the well-defined and broad substrate scope, the superb functional group compatibility required for late stage utilization, the technically non-demanding reaction protocol, and especially the rapid reaction times (5 min, 0 °C), the extension of this method to 18F introduction for PET imaging should prove especially useful.
Supplementary Material
ACKNOWLEDGMENT
We gratefully acknowledge the financial support of the National Institute of Health (CA042056, CA115526). T.J.B. is a NIH postdoctoral fellow (CA165303).
Footnotes
Supporting Information. Experimental procedures, additional optimization studies, and analytical data and spectra for all products and new compounds. This material is available free of charge via the Internet at http://pubs.acs.org.
The authors declare no competing financial interests.
REFERENCES
- 1.Müller K, Faeh C, Diederich F. Science. 2007;317:1881. doi: 10.1126/science.1131943. [DOI] [PubMed] [Google Scholar]
- 2.a Phelps ME. Proc. Natl. Acad. Sci. U.S.A. 2000;97:9226. doi: 10.1073/pnas.97.16.9226. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Ametamey SM, Honer M, Schubiger PA. Chem. Rev. 2008;108:1501. doi: 10.1021/cr0782426. [DOI] [PubMed] [Google Scholar]
- 3.For a review, see: Furuya T, Hamlet AS, Ritter T. Nature. 2011;473:470. doi: 10.1038/nature10108. See also selected examples: Watson DA, Su M, Teverovskiy G, Zhang Y, Garcia-Fortanet J, Kinzel T, Buchwald SL. Science. 2009;325:1661. doi: 10.1126/science.1178239.Lee E, Kamlet AS, Powers DC, Neumann CN, Boursalian GB, Furuya T, Choi DC, Hooker JM, Ritter T. Science. 2011;334:639. doi: 10.1126/science.1212625.Katcher MH, Doyle AG. J. Am. Chem. Soc. 2010;132:17402. doi: 10.1021/ja109120n.Hollingworth C, Hazari A, Hopkinson MN, Tredwell M, Benedetto E, Huiban M, Gee AD, Brown JM, Gouverneur V. Angew. Chem., Int. Ed. 2011;50:2613. doi: 10.1002/anie.201007307.Grushin VV. Acc. Chem. Res. 2010;43:160. doi: 10.1021/ar9001763.Chan KSL, Wasa M, Wang XS, Yu J-Q. Angew. Chem., Int. Ed. 2011;50:9081. doi: 10.1002/anie.201102985.Wang XS, Mei TS, Yu J-Q. J. Am. Chem. Soc. 2009;131:7520. doi: 10.1021/ja901352k.Fier PS, Hartwig JF. J. Am. Chem. Soc. 2012;134:10795. doi: 10.1021/ja304410x.
- 4.a Olah GA, Nojima M, Kerekes I. Synthesis. 1973:779. [Google Scholar]; b Olah GA, Watkins M. Org. Synth. 1978;58:75. [Google Scholar]
- 5.Rosen S. Acc. Chem. Res. 1988;21:307. [Google Scholar]
- 6.Tius MA. Tetrahedron. 1995;51:6605. [Google Scholar]
- 7.a Yamada S, Garvryushin A, Knochel P. Angew. Chem., Int. Ed. 2010;49:2215. doi: 10.1002/anie.200905052. [DOI] [PubMed] [Google Scholar]; b Dobele M, Vanderheiden S, Jung N, Brase S. Angew. Chem., Int. Ed. 2010;49:5986. doi: 10.1002/anie.201001507. [DOI] [PubMed] [Google Scholar]
- 8.a Ishikawa H, Colby DA, Boger DL. J. Am. Chem. Soc. 2008;130:420. doi: 10.1021/ja078192m. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Ishikawa H, Colby DA, Seto S, Va P, Tam A, Kakei H, Rayl TJ, Hwang I, Boger DL. J. Am. Chem. Soc. 2009;131:4904. doi: 10.1021/ja809842b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leggans EK, Barker TJ, Duncan KK, Boger DL. Org. Lett. 2012;14:1428. doi: 10.1021/ol300173v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.a Teare H, Robins EG, Kirjavainen A, Forsback S, Sandford G, Solin O, Luthra SK, Gouverneur V. Angew. Chem., Int. Ed. 2010;49:6821. doi: 10.1002/anie.201002310. [DOI] [PubMed] [Google Scholar]; b Teare H, Robins EG, Årstad E, Luthra SK, Gouverneur V. Chem. Commun. 2007:2330. doi: 10.1039/b701177f. [DOI] [PubMed] [Google Scholar]
- 11.Nyffeler PT, Durón SG, Burkart MD, Vincent SP, Wong C-H. Angew. Chem., Int. Ed. 2005;44:192. doi: 10.1002/anie.200400648. [DOI] [PubMed] [Google Scholar]
- 12.For the introduction of N-fluorobenzenesulfonimide and Selectfluor in radical fluorinations, see: Rueda-Becerril M, Sazepin CC, Leung JCT, Okbinoglu T, Kennepohl P, Paquin J-F, Sammis GM. J. Am. Chem. Soc. 2012;134:4026. doi: 10.1021/ja211679v.
- 13.Isayama S, Mukaiyama T. Chem. Lett. 1989:1071. [Google Scholar]
- 14.For recent Co/RSiH3-catalyzed functionalizations of unactivated alkenes see: Waser J, Nambu H, Carreira EM. J. Am. Chem. Soc. 2005;127:8294. doi: 10.1021/ja052164r.Waser J, Gaspar B, Nambu H, Carreira EM. J. Am. Chem. Soc. 2006;128:11693. doi: 10.1021/ja062355+.Gaspar B, Carreira E. Angew. Chem., Int. Ed. 2007;46:4519. doi: 10.1002/anie.200700575.Gaspar B, Carreira EM. Angew. Chem., Int. Ed. 2008;47:5758. doi: 10.1002/anie.200801760.
- 15.Okada M, Nakamura Y, Horikawa H, Inoue T, Taguchi T. J. Fluorine Chem. 1997;82:157. [Google Scholar]
- 16.Stavber S, Jereb M, Zupan M. Synlett. 1999:1375. [Google Scholar]
- 17.Baumberger F, Vasella A. Helv. Chim. Acta. 1983;66:2210. [Google Scholar]
- 18.Reactions with styrenes were performed under both an inert atmosphere and under air. In either case, none to only trace amounts of the fluorinated product were observed, with the major byproducts being the benzylic alcohol and the dimer of the intermediate benzylic radical.
- 19.a Vincent SP, Burkart MD, Tsai C-Y, Zhang Z, Wong C-H. J. Org. Chem. 1999;64:5264. doi: 10.1021/jo990686h. [DOI] [PubMed] [Google Scholar]; b Differding E, Rüegg GM. Tetrahedron Lett. 1991;32:3815. [Google Scholar]; c Differding E, Wehrli M. Tetrahedron Lett. 1991;32:3810. [Google Scholar]
- 20.Akana JA, Bhattacharyya KX, Müller P, Sadighi JP. J. Am. Chem. Soc. 2007;129:7736. doi: 10.1021/ja0723784. [DOI] [PubMed] [Google Scholar]
- 21.a Michaudel Q, Thevenet D, Baran PS. J. Am. Chem. Soc. 2012;134:2547. doi: 10.1021/ja212020b. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Yin F, Wang Z, Li Z, Li C. J. Am. Chem. Soc. 2012;134:10401. doi: 10.1021/ja3048255. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.