Abstract
Upregulation of indoleamine 2,3-dioxygenase (IDO) by proinflammatory cytokines has been implicated as a biological mediator of inflammation-related mood disorders. Clinical reports on this neuro-immune interaction remain correlative, while mechanism-centered preclinical experiments have focused on a relatively narrow, and somewhat controversial, survey of depression-like behaviors that include the forced swim and tail suspension tests. Here, we sought to determine whether peripheral immune challenge with E. coli, lipopolysaccharides (LPS) precipitates the development of translationally relevant depression-like behaviors and to investigate the role of IDO in mediating these LPS-induced behaviors. Intraperitoneal injection of C57BL/6J mice with LPS resulted in a robust, but transient, reduction in exploratory locomotor activity (eLMA) that returned to near baseline levels by 24h. Sucrose preference, a preclinical correlate of anhedonia, was diminished by more than 20% in LPS-treated compared to saline-treated control mice, and LPS induced a significant increase in anxiety-like behavior at 24h that was independent eLMA. Pretreatment of mice with an IDO inhibitor, 1-methyltryptophan (1MT), ablated the anxiogenic effects of LPS, while having no impact on sickness associated changes in body weight or eLMA. Additionally, 1MT pretreatment attenuated the LPS-induced reduction in sucrose preference, which was also confirmed in IDO-1 null mice. Interestingly, acute systemic administration of L-kynurenine, the enzymatic product of IDO, precipitated an anhedonic and anxiogenic effect in naïve mice without effect on eLMA. In a preclinical model, these data implicate IDO as a pivotal mediator of LPS-induced depression- and anxiety-like behavior.
Keywords: Anxiety, Depression, Inflammation, Tryptophan, Kynurenine, Neuro-immune, Sucrose Preference, Behavior
Introduction
A wide spectrum of proinflammatory conditions, including infection, chronic disease and immunotherapy are recognized risk factors for the development of major depression (Dantzer, O'Connor et al. 2008). The striking incidence of major depression (30–50%) and depressive symptomology (>80%) that occurs in patients following initiation of interferon (IFN)-α immunotherapy provides compelling evidence of a causal role of proinflammatory cytokines in the subsequent depressive disorders (Capuron and Miller 2004). Nearly all of these patients initially develop neurovegetative symptoms reminiscent of influenza-like sickness and fatigue. Later, a significant proportion develop depressed mood and anhedonia, anxiety and irritability and cognitive disturbances (Capuron and Miller 2004). In an effort to identify neurobiological mechanisms linking inflammation to depression, vaccines or bacterial endotoxins (lipopolysaccharide or LPS) have been used to experimentally activate the innate immune system, in both healthy human volunteers and in rodents. Patients challenged with endotoxin develop a transient deterioration in subjective scores of mood (Reichenberg, Yirmiya et al. 2001). In mice, moderate intraperitoneal (ip) doses of LPS (10–25 µg per mouse) initiate behavioral sequelae that closely resemble the etiology of IFN-α induced depression (Frenois, Moreau et al. 2007; Godbout, Moreau et al. 2008; O'Connor, Lawson et al. 2009; Fu, Zunich et al. 2010). Mice exhibit a sickness response that includes a transient reduction in food intake, body weight and exploratory locomotor activity (eLMA). Importantly, when these ‘recovered’ mice are subsequently tested in the forced swim or tail suspension tests, the duration of immobility remains increased, suggesting a depression-like phenotype.
The metabolic enzyme indoleamine 2,3-dioxygenase (IDO, EC 1.13.11.52) appears to be a biological mediator of inflammation related depressive disorders (O'Connor, Lawson et al. 2009; O'Connor, Lawson et al. 2009; Raison, Dantzer et al. 2010; Dantzer, O'Connor et al. 2011). IDO is the initial and rate limiting extrahepatic enzyme of the kynurenine pathway, which metabolizes tryptophan to N-formylkynurenine and permits the subsequent generation of neurochemically active kynurenine metabolites. Kynurenine is further metabolized along two principle routes. Briefly, kynurenine aminotransferase enzymes yield kynurenic acid, an N-methyl-D-aspartate (NMDA) receptor and α7-nicotinic acetylcholine receptor antagonist, while kynurenine monooxygenase is the first enzyme of a multistep pathway that generates the NMDA receptor agonist, quinolinic acid. Several intermediate kynurenine metabolites in the latter pathway also generate free radicals causing oxidative damage. L-kynurenine readily crosses the blood brain barrier via large neutral amino acid transporters, and LPS potently upregulates IDO expression (Jung, Lee et al. 2007; Wang, Lawson et al. 2010). Thus, generation of kynurenine both in the periphery and within the brain has the capacity to modulate neural transmission with functional behavioral consequences.
The concentration of kynurenine in circulation and within the cerebrospinal fluid, as well as the kynurenine/tryptophan ratio, are significantly increased by IFN-α immunotherapy (Raison, Dantzer et al. 2010), and depression scores are positively correlated with IDO activity (Bonaccorso, Marino et al. 2002; Capuron, Neurauter et al. 2003). In mice, chronic inflammation induced by an attenuated strain of Mycobacterium bovis, BCG not only increased the kynurenine/tryptophan ratio in the plasma and within brain tissue, but also increased the duration of immobility in the forced swim and tail suspension tests (Moreau, Andre et al. 2008; O'Connor, Andre et al. 2009). Inhibition of IDO with 1-methyl-D,L-tryptophan (1MT) normalized the kynurenine/tryptophan ratios and attenuated depressive-like behavior without effect on the acute sickness behavior response. IDO deficient mice were protected from BCG induced depressive-like behavior (O'Connor, Lawson et al. 2009). Similarly, 1MT inhibited the LPS-induced increase in floating during the forced swim test and immobility in the tail suspension test, but IDO inhibition had no impact on the acute sickness behavior response of LPS-challenged mice (O'Connor, Andre et al. 2009). It remains to be determined whether IDO mediates other inflammation-induced depression-like behaviors. Here, we examined whether LPS precipitated the development of anhedonia and anxiety-like behavior independent of the acute sickness behavior response, as assessed by eLMA. We utilized well-accepted behavioral tests with good face and predictive validity within the temporal framework of our previously validated acute model of inflammation-induced depression-like behavior. Pharmacological and genetic approaches were employed to demonstrate, for the first time, that increased kynurenine production as a result of immune challenge drives the development of an anhedonic and anxiety-like behavioral phenotype in LPS challenged mice.
Material and Method
Mouse housing and treatments
All animal care and use was carried out in accord with the Guide for the Care and Use of Laboratory Animals, 8th edition (NRC) and approved by the Institutional Animal Care and Use Committee. Wild-type control C57BL/6J (Jax Strain # 000664) mice and Indoleamine 2,3 dioxygenase null (B6.129-Ido1tm1Alm/J, Jax Strain #005867) mice originated from The Jackson Laboratory (Bar Harbor, ME). All mice were individually housed in standard microisolator-topped shoebox cages within a temperature and humidity controlled facility with a modified 12:12 hr reverse light cycle (lights on 23:00–11:00) and were allowed ad libitum access to food and water. LPS isolated from E. coli (cat# L-3129, serotype 0127:B8) was purchased from Sigma (St. Louis, MO) and injections were prepared daily from 1 mg/ml stock solutions on the morning of injections. LPS was dissolved in sterile, endotoxin-free 0.09% saline and injected ip at a dose of 0.83 mg/kg. This dose of LPS acutely induces a transient sickness behavior response followed by the development of a distinct depressive-like behavioral phenotype in the forced swim and tail suspension tests (O'Connor, Lawson et al. 2009). 1-methyl-DL-tryptophan (1MT) (cat# 860646) and L-kynurenine (cat# K8265) were purchased from Sigma (St. Louis, MO) and prepared fresh daily on the morning of injections. 4X concentrated solutions were prepared in 0.1M HCl, neutralized with an equal volume of 0.1M NaOH, buffered with 2X phosphate buffered saline and filtered through a 0.2 µm syringe filter. In 1MT experiments, all mice received a three injection pretreatment regimen (48h, 24h and 30 minutes prior to LPS) of either 1MT (50 mg/kg) or vehicle, administered subcutaneously. For L-kynurenine dose-response experiments, mice received a single subcutaneous injection, of the indicated dose, 2h prior to behavioral testing. This interval is sufficient to allow for an increase in peripheral and central kynurenine metabolites, as previously reported (Chiarugi, Carpenedo et al. 1996).
Behavioral Testing
General-All mice were monitored daily by research staff beginning two weeks prior to the experiment. Mice were handled once daily for one week prior to the start of injections to acclimate them to gentle manipulation. Measures of general health and vigor (food intake, body weight, spontaneous home cage ambulatory activity and grooming) were observed or recorded daily, beginning one week prior to and continuing through the end of the experiment. All behavioral testing occurred during the first four hours of the dark photoperiod.
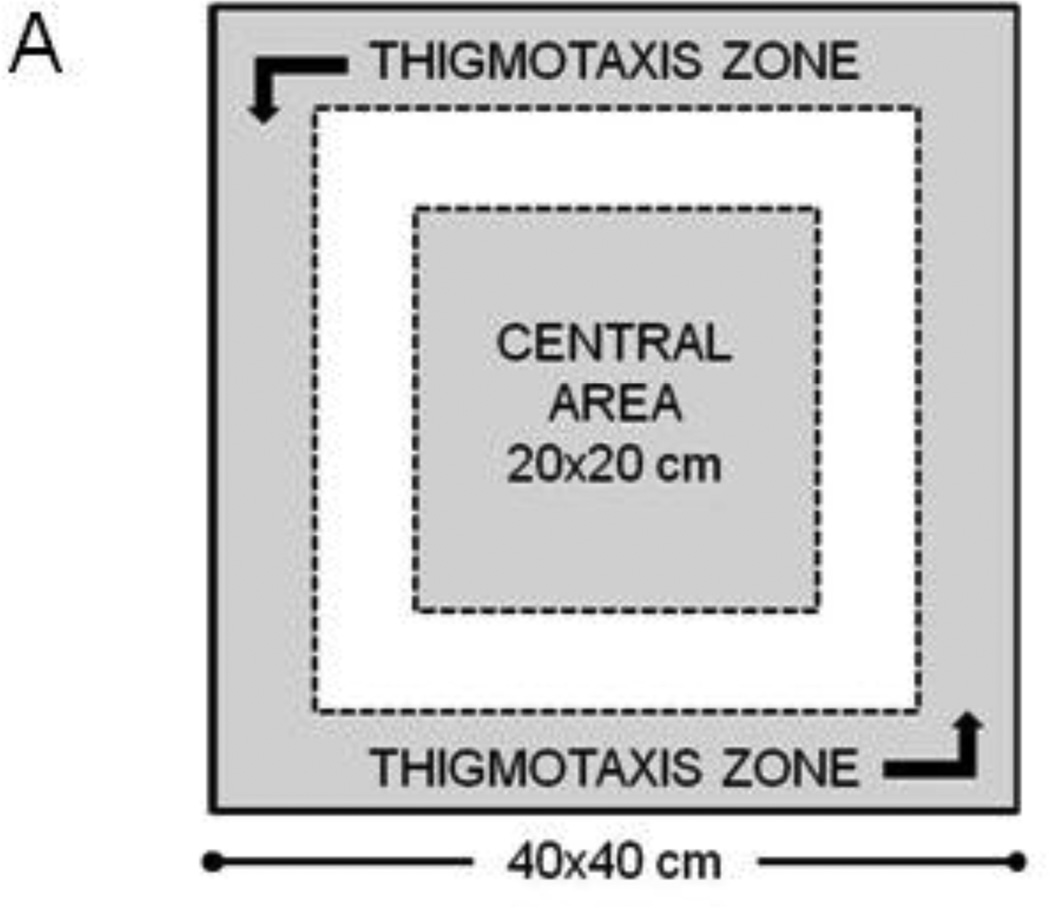
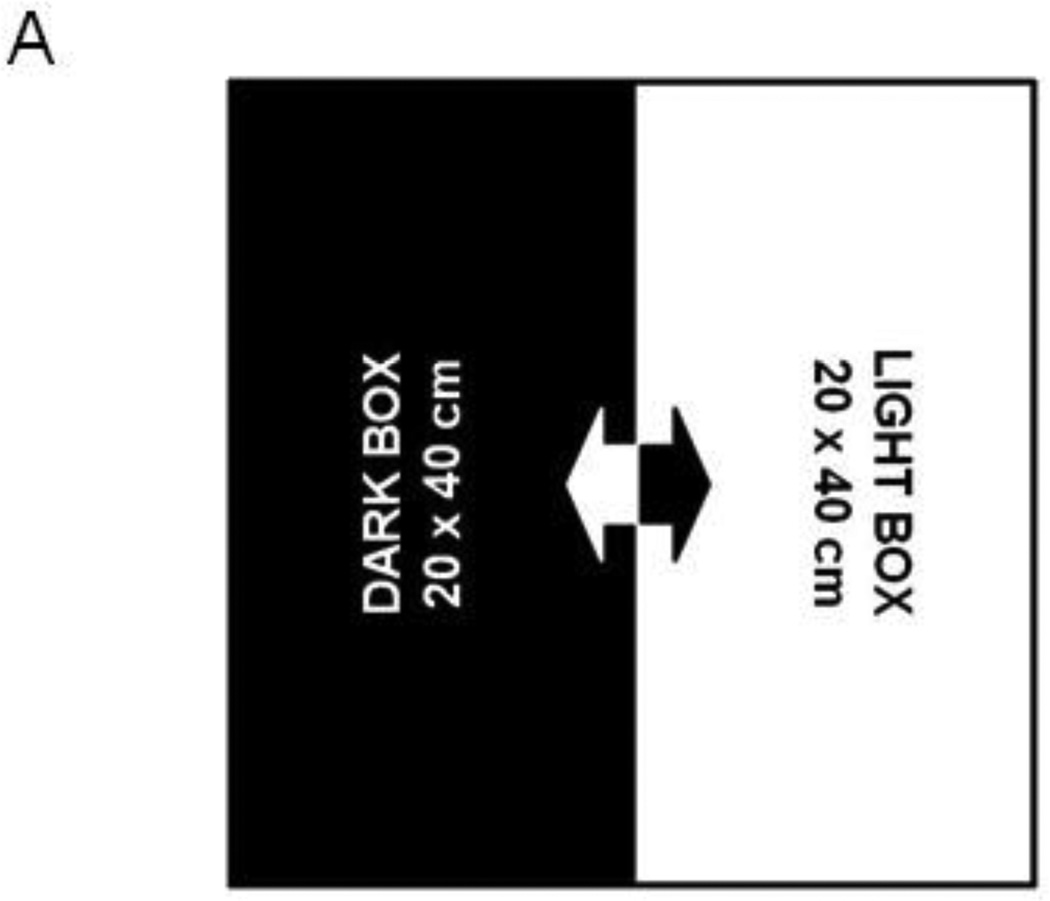
Open Field Test was utilized to assess exploratory locomotor activity (eLMA), measured as distance traveled by each mouse within a testing chamber. eLMA is a preferred behavioral marker of acute sickness behavior, as it reflects the mouse’s capacity and willingness to move freely and engage in exploratory behavior. Additionally, eLMA correlates tightly to LPS-induced fever responses (Kozak, Conn et al. 1994) and does not involve a compensatory phase, like recovery of lost body weight. The anxiety-like phenotype of an individual mouse’s behavior was assessed by determining the amount of time during the test that was spent exploring the central area of the chamber or hugging the perimeter chamber walls (thigmotaxis). The open field chamber consisted of a 40cm (W) × 40cm (L) × 30cm (H) acrylic box with opaque walls. The chamber floor was dimly illuminated from overhead (3–10 lux). A 5 minute testing session was video recorded, and the chamber was cleaned with 70% isopropanol between mice. Video files were analyzed using Ethovision XT 7 tracking software (Noldus, Leesburg, VA). The central area was defined as the 20 × 20 cm interior portion of the chamber, while thigmotaxis was scored when the test mouse was within 5 cm of the chamber wall (Fig. 1A).
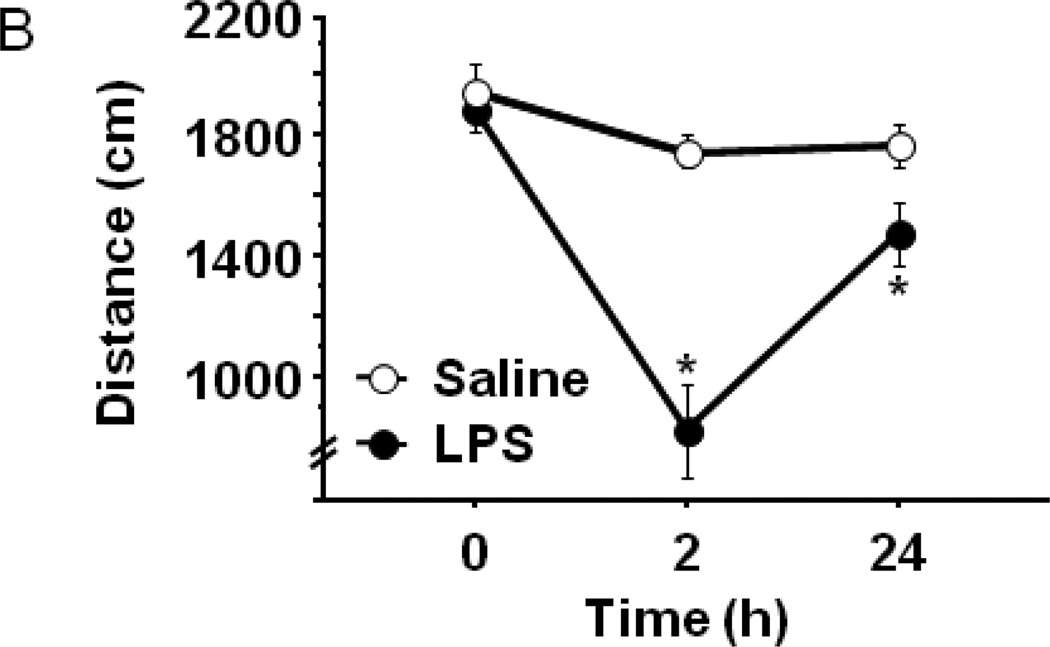
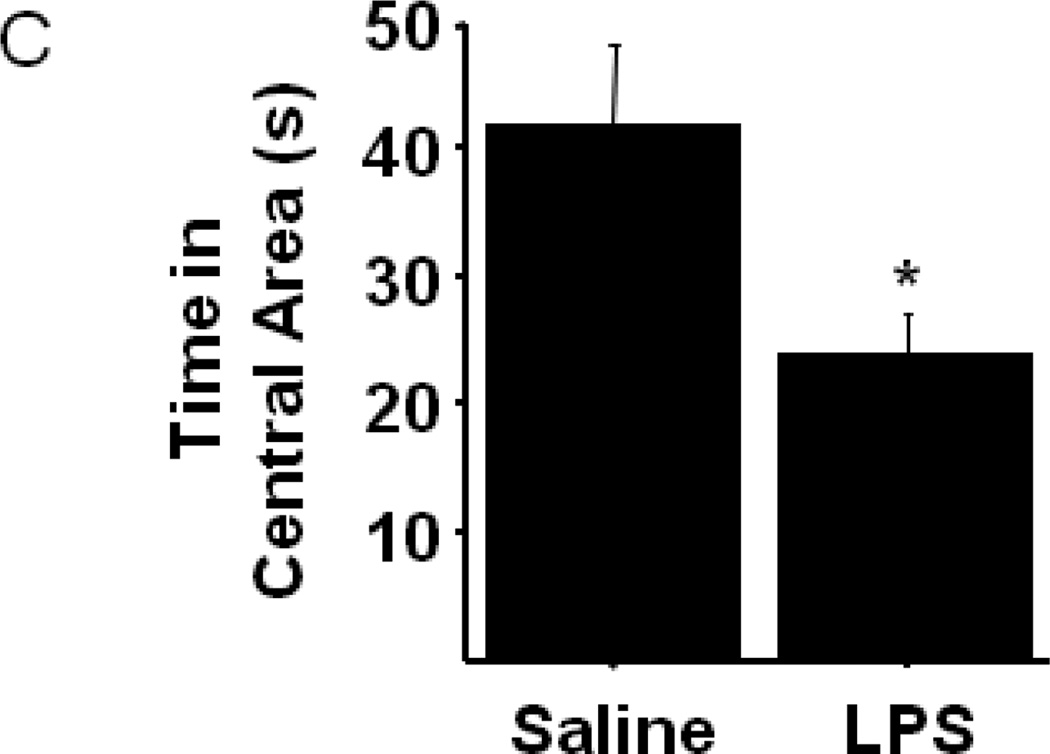
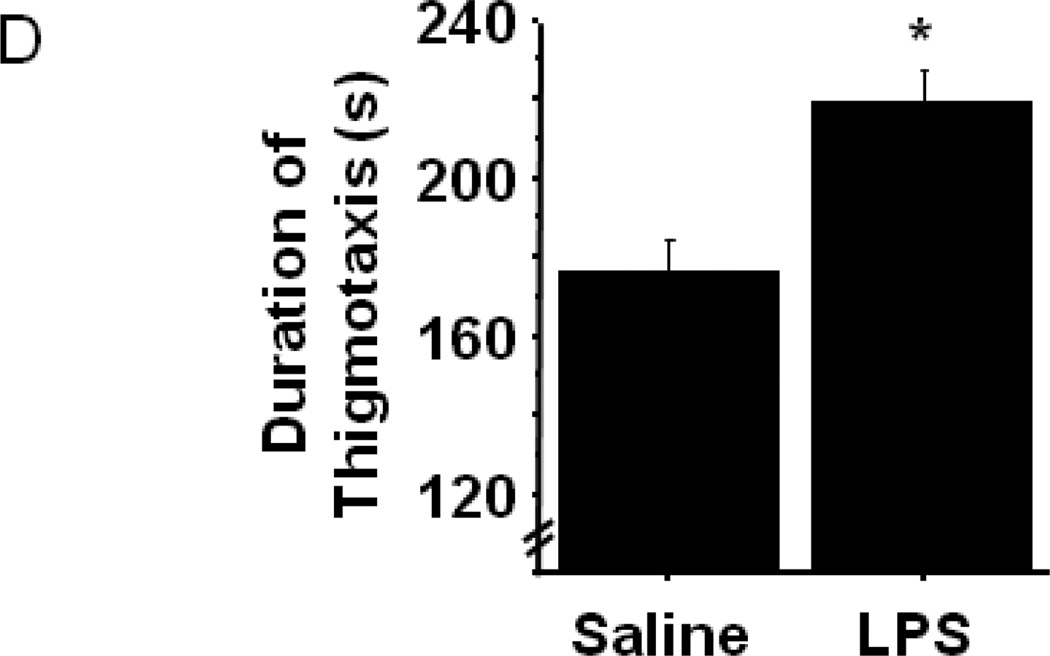
Figure 1.
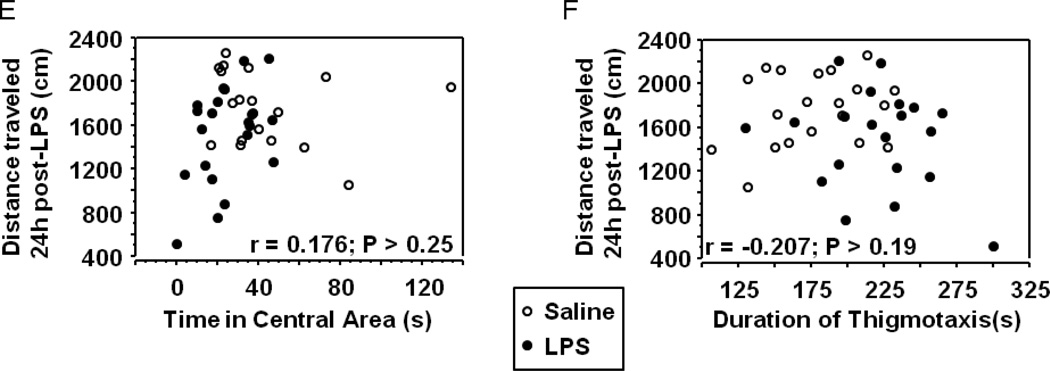
Peripheral LPS challenge precipitated anxiety-like behavior in the open field test. Mice were observed in the testing chambers during a 5 minute session at the indicated times. (A) General dimensions and scoring zones for the open field test. (B) Intraperitoneal LPS injection causes a transient reduction in exploratory locomotor activity, and LPS-challenged mice exhibit (C) a reduced time spent in the central area of the arena and an (D) increase in the amount of time spent in close proximity to the arena walls (thigmotaxis). Correlation analysis indicates that treatment-induced changes in (E) central area time or (F) thigmotaxis are not merely a consequence of fluctuations in 24h locomotor activity. Data represent group means ± SEM, n=19–20 mice per group in at least two independent experimental replications. *p<0.05 versus control.
Light-Dark Box Test was used as a second test of anxiety-like behavior. As figure 2A illustrates, the test chamber was evenly divided into two 20 × 40 cm chambers. The light chamber was brightly illuminated from overhead (450–500 lux) while the dark chamber consisted of an enclosed acrylic box. There was a 5 × 5 cm open passage between the two chambers. Mice were placed inside the dark chamber, with the passage closed, for 2 minutes prior to initiating the test. The passage was then opened, and the mouse was allowed to freely explore either chamber for 10 min. The test was video recorded, and the amount of time spent in the light chamber (all four paws) during the test was recorded.
Figure 2.
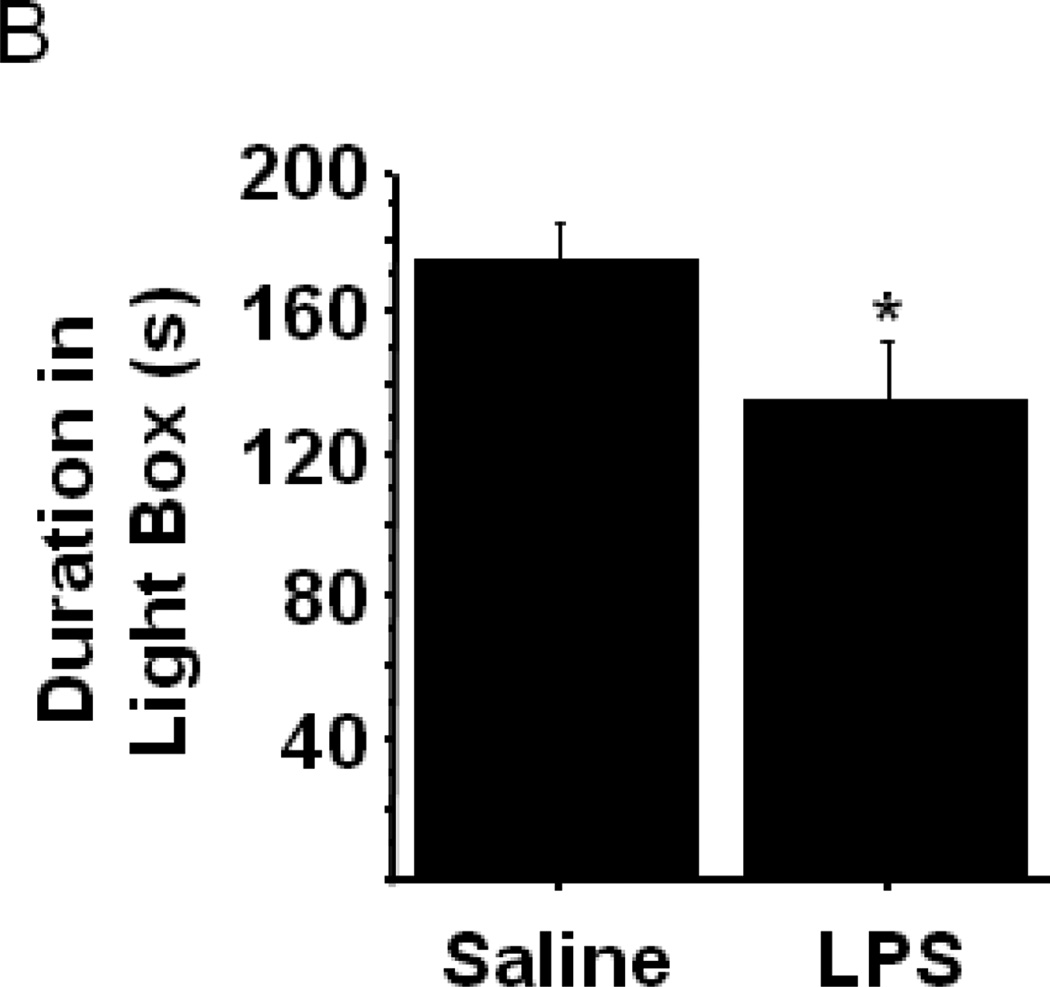
Anxiety-like behavior in the Light-Dark Box is increased by peripheral LPS challenge. (A) General dimensions of the light-dark box test chamber. (B) The duration of time spent exploring the illuminated side of the light-dark box is reduced by LPS. Data represent group means ± SEM, n=17–18 mice per group in at least two independent experimental replications. *p<0.05 versus control.
Sucrose Preference Test was performed after a two-day training period, during the 24 h interval following LPS administration. Mice were presented with two identical volumetric drinking tubes containing either water or a 1% sucrose solution. Bottles were weighed to measure fluid intake during each 24 h period. The position (right v. left) was alternated each period to eliminate potential bias from place preference. Sucrose preference was calculated using the following formula: [Sucrose intake/(Water intake + Sucrose intake)] × 100.
Statistical analyses
One factor (treatment) ANOVA, with repeated measures where appropriate, or two factor (pretreatment/genotype vs. treatment) ANOVA were performed using Statview 5.0.1 (SAS Institute, Inc. Cary, NC). Pearson product moment correlation coefficient (r) was calculated to compare the relationship between 24h eLMA and anxiety-like behavior in the open field test. When significant interactions were identified, post hoc testing was performed using Fisher’s protected least significant differences. Data were collected from at least two independent experiments repeated at different times.
Results and Discussion
Peripheral innate immune challenge induces anxiety-like behavior in mice that is independent of locomotor activity
We have previously demonstrated that chronic or acute peripheral immune challenge results in an increased duration of immobility in the forced swim and tail suspension tests independent from acute locomotor deficits and feeding behavior referred to as the sickness response (Frenois, Moreau et al. 2007; Moreau, Andre et al. 2008; O'Connor, Andre et al. 2009; O'Connor, Lawson et al. 2009; O'Connor, Lawson et al. 2009). We interpreted the increased immobility data from the forced swim and tail suspension tests to be reflective of a diminished motivational state or a depressive-like behavioral phenotype. This interpretation was based on the rationale that a reduction in the duration of immobility during these two tests is classically used to screen treatments for anti-depressant efficacy; thus the inverse behavioral response would be reflective of a depressive phenotype (Yan, Cao et al. 2010). Additionally, when placed in an inescapable aversive environment, mice employ either active or passive coping strategies in seeking an escape. Floating, as opposed to swimming or climbing, is interpreted as a passive coping strategy, which is the predominant method of coping exhibited by patients suffering from depression (Li, Cooper et al. 2011).
This interpretation has been met with a certain degree of skepticism because there is little face validity in translating these preclinical behaviors to a clinical interpretation of neuropsychiatric symptoms, as precisely what immobility during the test represents is not clear. Therefore, it was critical to determine whether LPS-induced depressive-like behavior could be identified across other symptom domains of depression. Anxiety and depression are highly comorbid with chronic inflammatory conditions (Evans, Charney et al. 2005; Roy-Byrne, Davidson et al. 2008), and anxiety-related symptoms are among the most frequently reported by patients undergoing IFN-α immunotherapy (Capuron and Miller 2004). Therefore, we subjected mice to two well-accepted tests of anxiety-like behavior, the open field test and the light-dark box test, using the same temporal paradigm as we had previously employed. After being injected ip with either LPS (0.83 mg/kg) or an equal volume of non-pyrogenic physiological saline, eLMA and anxiety-like behavior were measured in 8–12 week old male C57BL6/J mice (n=19–20 mice/group). Figure 1B indicates that baseline (0h) eLMA was no different between the two groups of mice (Sal-1945.9±96.1 vs. LPS-1892.6±77.8). Consistent with our previous findings, LPS induced an acute 52% decline in eLMA at 2h post LPS compared to control mice that had returned to 80% of baseline levels by 24h post-LPS (Fig. 1B; treatment × time- F1,74=10.28, p<0.001).
To determine whether LPS treated mice display anxiety-like behavior in the open field test, the nature of their movement within the chamber was more specifically analyzed. The open field test places a mouse in a situation of conflict where its innate curiosity and natural inclination to explore novel areas is pitted against its reluctance to enter open, unprotected spaces. Prior to LPS treatment, the amount of time spent exploring the central area as opposed to remaining in close proximity to the chamber walls was not different between groups (data not shown). However, figure 1C shows that the duration of time spent in the central area of the open field chamber was significantly reduced in LPS-treated mice 24h post-injection (Sal-42.3±6.2 vs. LPS-24.2±3.2; treatment- F1,38=6.64, p<0.02). Conversely, LPS-challenged mice spent 24% more time than saline controls in close proximity to the chamber walls during the testing session (Fig. 1D; treatment- F1,38=13.42, p<0.001). The present data are not the first to demonstrate an anxiogenic effect of LPS in the open field test, but to our knowledge, they are the first to demonstrate that LPS-induced anxiety-like behaviors are distinct from the acute reduction in eLMA that occurs during a sickness behavior response.
LPS-induced anxiety-like behavior in the open field test has been demonstrated in both mice (Swiergiel and Dunn 2007) and rats (Bassi, Kanashiro et al. 2011), but in both cases the open field test was administered only 2–4 hours after LPS challenge. At these times points, overall locomotor activity was substantially reduced, as we also observed (Fig. 1B), precluding a clear interpretation of the behaviors as anxiogenic. A recent clinical study did, in fact, demonstrate that low-dose endotoxin depressed mood and increased anxiety scores (Grigoleit, Kullmann et al. 2011), but rigorous interpretation of neuroimmunology experiments in rodents must consider locomotor activity when assessing behavioral data from tests, such as the open field. While their overt phenotype was indiscernible from control mice, 24h eLMA in LPS-treated mice remained modestly, but significantly, lower than saline control mice (Fig. 1B). To confirm that the modestly reduced activity levels 24h post-LPS were not a confound to interpreting anxiety-like behaviors in the open field, correlation analysis was performed. Figure 1E and 1F demonstrate that no significant relationship was apparent between 24h eLMA and either time in the central area (Fig. 1E) or thigmotaxis (Fig. 1F). Separate analysis of individual treatment groups did not reveal a significant correlation (data not shown), thus all data are included together. Together, these data indicate that peripheral immune challenge with LPS, precipitates the development of an anxiety-like phenotype in the open field test that was independent of the eLMA.
To confirm that the behavioral phenotype observed within the open field test in response to LPS was indeed reflective of an anxiety-like state, we utilized a second behavioral test, the light-dark box, in separate groups of mice that were challenged with LPS or an equal volume of non-pyrogenic saline (n=17–18 mice/group). The latency to emerge from the dark chamber, the number of nose pokes from dark to light and the duration of time spent entirely within the light chamber were recorded 24h post-treatment. Consistent with our analysis of the open field behavioral data, LPS-challenged mice spent significantly less time within the light chamber (Fig. 2B; saline-176.4 ± 9.9 vs. LPS-136.7 ± 16.2; treatment- F1,33=4.48, p<0.05). No significant differences in the latency to emerge from the dark box or the number of nose pokes from within the dark box were detected between treatment groups (data not shown). The present data demonstrate that LPS-challenge precipitates an anxiety-like phenotype, distinct from the acute sickness behavioral response, in mice.
Inflammation-induced anxiety-like behavior is mediated by indoleamine 2,3-dioxygenase
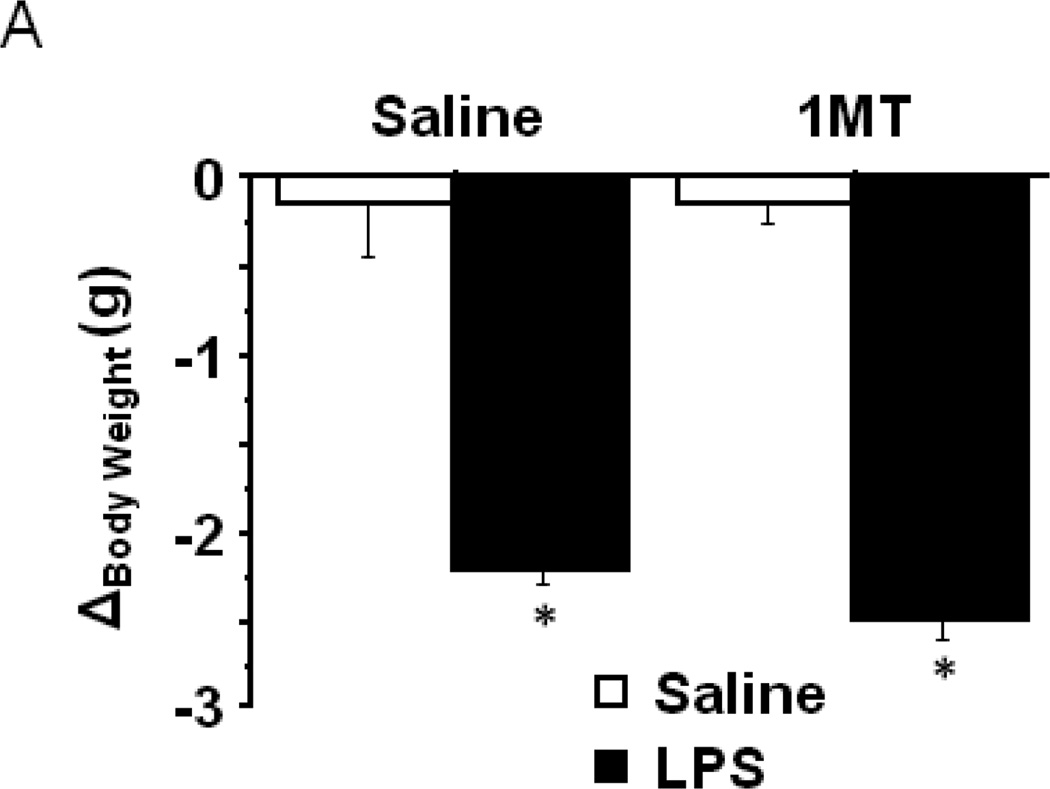
IDO and the kynurenine pathway have been consistently associated with depression and anxiety disorders in diverse clinical populations. For example, early studies identified positive correlations between anxiety scores and the plasma kynurenine/tryptophan ratio in depressed patients (Orlikov, Prakhye et al. 1994), in women during the puerperium (Maes, Verkerk et al. 2002) and in patients undergoing cytokine immunotherapy (Capuron, Neurauter et al. 2003). More recently, an increased concentration of kynurenine metabolites in blood and cerebrospinal fluid were positively correlated with depression scores (Raison, Dantzer et al. 2010), and on the genetic level, a single nucleotide polymorphism (rs9657182) in the promoter region of the IDO1 gene was found to be associated with severity of depressive symptoms in Caucasians undergoing IFN-α therapy (Smith, Simon et al. 2011). Mice lacking the stress and substrate inducible hepatic tryptophan 2,3-dioxygenase enzyme exhibit an anxiolytic phenotype (Kanai, Funakoshi et al. 2009), and we have demonstrated that IDO mediates LPS-induced depressive-like behavior in the forced swim or tail suspension tests (O'Connor, Lawson et al. 2009). Upregulation of peripheral IDO activity by chronic unpredictable mild stress was positively correlated to anxiety/depression-like behavioral symptoms (Laugeray, Launay et al. 2011). To determine the role of IDO in mediating LPS-induced anxiety-like behavior, mice were pretreated with 1MT, a competitive inhibitor of IDO, prior to LPS challenge (n=7–12 mice /group). As expected, LPS challenge precipitated a drop in body weight characteristic of an acute sickness response, and importantly pretreatment with 1MT had no impact (Fig. 3A) (treatment- F1,28=191.15, p<0.001). When eLMA was assessed in the same mice 24h post-LPS challenge, no significant differences between any of the experimental groups were apparent (Fig. 3B).
Figure 3.
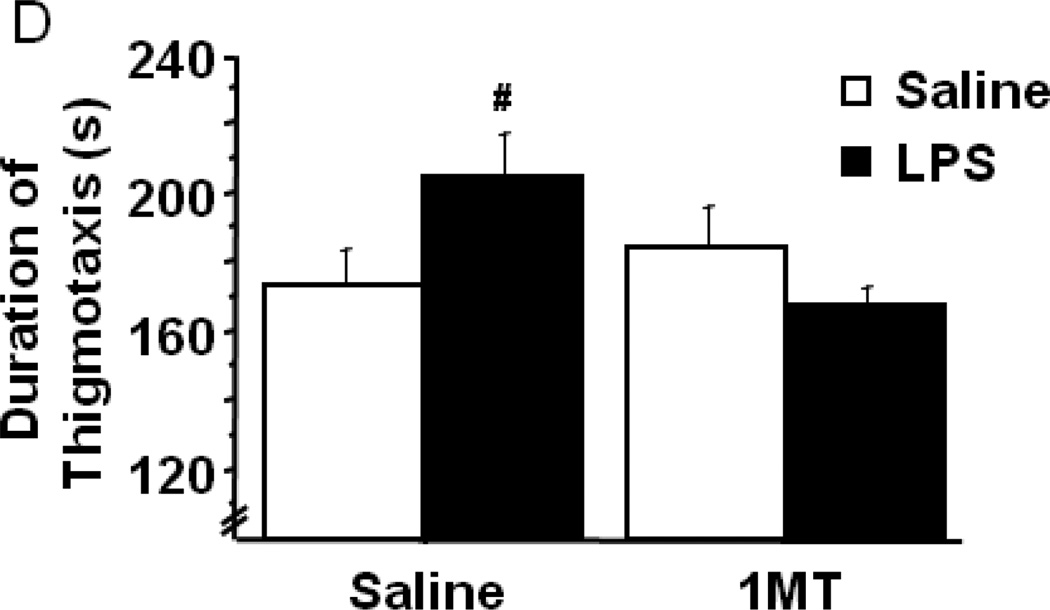
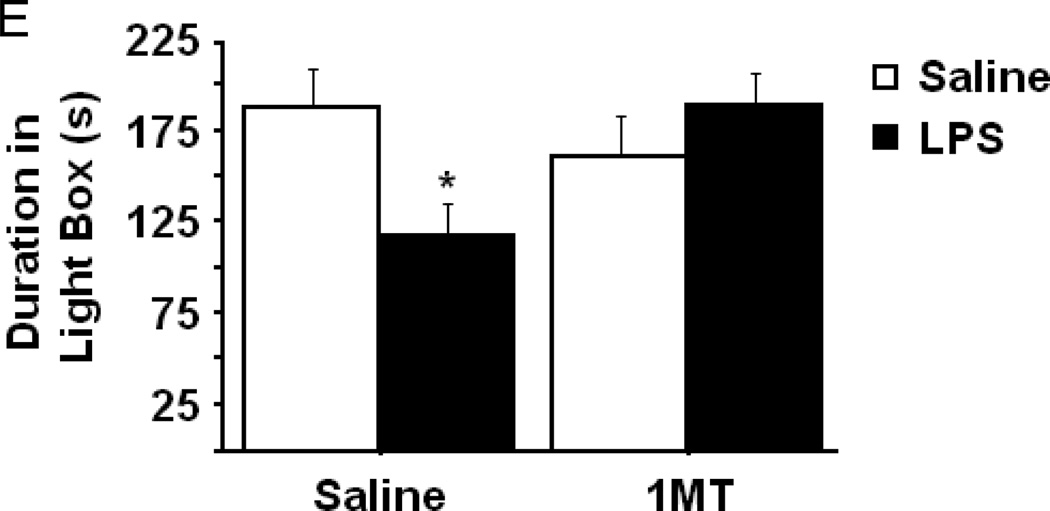
Indoleamine 2,3-dioxygenase mediates LPS-induced anxiety-like behavior in the open field test. After pretreatment with either vehicle or 1-methyltryptophan, the effects of LPS on (A) body weight and (B) locomotor activity over a 24h interval. (C) The duration of time spent within the central area or (D) in close proximity to the chamber walls was measured in the open field test at 24h post-LPS challenge. (F) The duration of time spent exploring the illuminated side of a light-dark box was also measured in different mice 24h post-LPS. Data represent group means ± SEM, n=7–12 mice per group in at least two independent experimental replications. *p<0.05 versus same pretreatment saline control, #p<0.05 versus 1MT/LPS.
After establishing that LPS-induced deficits in locomotor activity were no longer present, the nature of each mouse’s behavior within the open field was further examined. There was a significant interaction between 1MT pretreatment and LPS treatment in both the duration of time spent within the central area of the open field chamber and thigmotaxis (Figs. 3C and 3D). Post hoc testing revealed that time spent in the central area was significantly reduced by LPS (Fig. 3C, saline-36.3 ± 4.6 vs. LPS-21.3 ± 3.1), while pretreatment with 1MT completely ablated the LPS-induced reduction in central area time (pretreatment × treatment interaction- F1,28=4.25, p<0.05). As might be expected, the duration of thigmotactic behavior (movement in close proximity to the chamber wall) was significantly increased in response to LPS, and pretreatment with 1MT prevented the increase in thigmotaxis (Fig. 3D; pretreatment × treatment interaction- F1,28=5.02, p<0.05). Separate groups of mice were subjected to a second anxiety-like behavioral paradigm, the light-dark box, to confirm the apparent role of IDO in mediating LPS-induced anxiety-like behavior. A significant 1MT × LPS interaction was apparent in the duration of time spent within the brightly illuminated light chamber (pretreatment × treatment interaction- F1,32=4.59, p<0.05). Figure 3E shows that LPS challenge precipitated a decrease in the amount of time spent exploring within the light chamber (saline-31.4 ± LPS-3.7 vs. 19.7 ± 2.9), and inhibition of IDO prevented the LPS-induced anxiety-like response. These convergent data illustrate an important role for IDO in mediating the anxiety-like behavioral response to LPS.
Direct administration of L-kynurenine dose-dependently induces anxiety-like behavior in the open field test
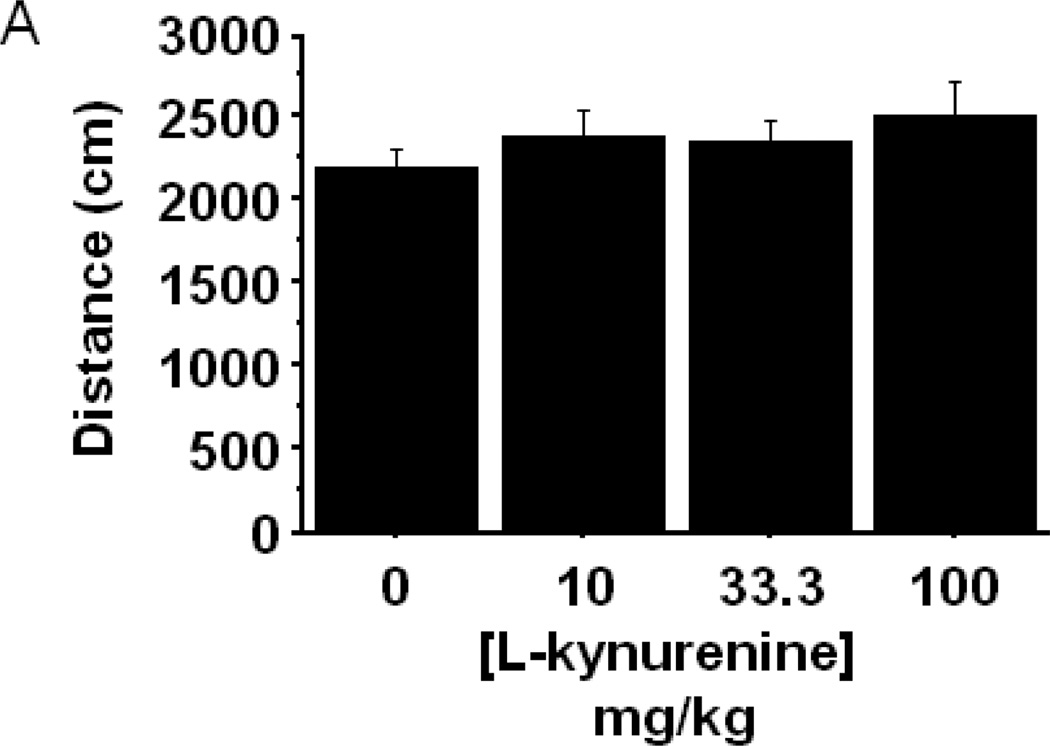
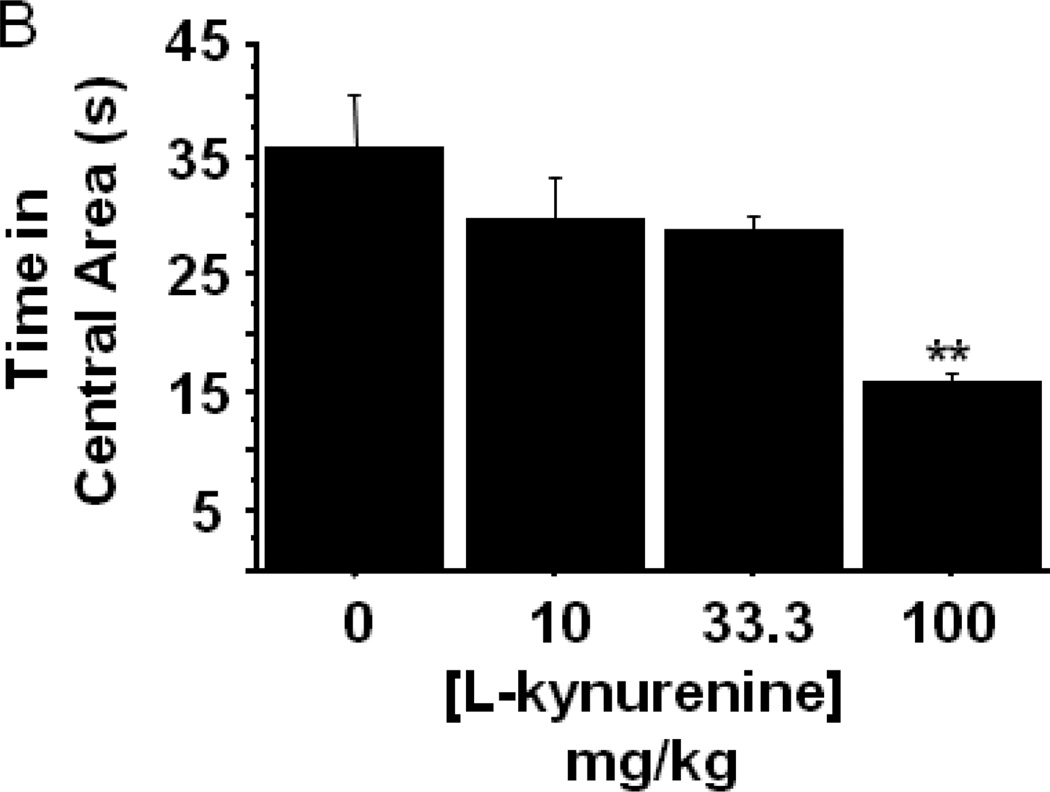
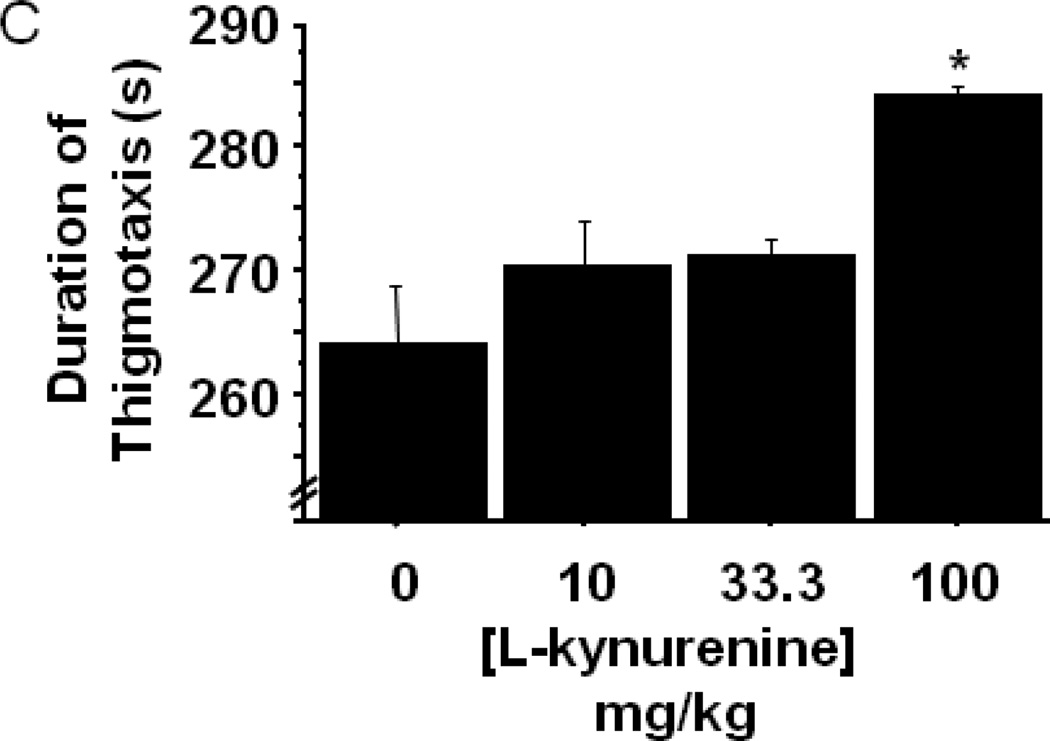
The enzymatic product of IDO, L-kynurenine, has been directly and indirectly implicated in driving behavioral and physiological responses. To determine whether systemic administration of L-kynurenine was sufficient to trigger anxiety-like behavior, mice were subcutaneously injected with increasing doses (0–100 mg/kg) of L-kynurenine or a vehicle control (n=6–7 mice/group). Two hours later, eLMA and anxiety-like behavior were measured in the open field test. Interestingly, L-kynurenine treatment elicited no change in general eLMA (Fig. 4A) or in average velocity during the test (data not shown). There was, however, a dose dependent reduction in the duration of time spent within the central area of the chamber (Fig. 4B; treatment- F3,22=7.59, p<0.001), with the 100 mg/kg L-kynurenine treated mice exhibiting greater than 50% reduction when compared to vehicle treated controls. With regard to thigmotaxis, mice receiving 100 mg/kg spent significantly more time in close proximity to the wall during the test (Fig. 4C; treatment- F3,22=7.61, p<0.01). It is interesting to note that, the effective anxiogenic dose of L-kynurenine (100 mg/kg) in the present experiments has been demonstrated to impair cognitive performance in rats (Chess, Landers et al. 2009; Alexander, Wu et al. 2011), but it is higher than was required to induce immobility in the forced swim and tail suspension tests in outbred CD-1 mice (O'Connor, Lawson et al. 2009). Together, these data implicate increasing kynurenine concentration as a potential precipitant of neuropsychiatric-like behaviors, but genetic and experimental factors are likely modulators of the neurobehavioral effects of kynurenine.
Figure 4.
Peripheral administration of L-kynurenine dose-dependently precipitates anxiety-like behavior in the open field test. Mice were injected subcutaneously with the indicated doses of L-kynurenine. Two hours later, (A) exploratory locomotor activity, (B) time spent in the central area and (C) time spent in close proximity to the chamber wall was measured in the open field test. Data represent group means ± SEM, n=6–7 mice per group in at least two independent experimental replications. *p<0.05 and **p<0.01 versus all other treatment groups.
Indoleamine 2,3-dioxygenase is an important mediator of LPS-induced anhedonia
Preclinically, anhedonia is measured using behavioral paradigms that demonstrate a preference for sweetened solutions or by intracranial self stimulation of the central reward centers. LPS is known to induce anhedonia in both of these paradigms, but understanding of the neurobiological mechanisms that mediate LPS-induced anhedonia have not been well characterized. Eisenberger et. al. recently demonstrated in healthy human volunteers that endotoxin-induced anhedonia was associated to changes in neuronal activity within the ventral striatum (Eisenberger, Berkman et al. 2010), and previous clinical studies associated elevated interleukin-6 levels (Harrison, Brydon et al. 2009) with poor mood. We have previously shown that peripheral LPS challenge upregulates neuronal activity in the hippocampus and hypothalamus (Frenois, Moreau et al. 2007), while proinflammatory cytokine and IDO mRNA are also upregulated in these brain regions (Andre, O'Connor et al. 2008). Our previous data demonstrated that IDO mediated LPS-induced floating in the forced swim test and immobility in the tail suspension test (O'Connor, Lawson et al. 2009), and Henry et. al. showed that minocycline, an anti-inflammatory antibiotic often used to inhibit microglial activation, attenuated LPS-induced reduction in sucrose preference while also reducing cytokine and IDO mRNA in the hippocampus (Henry, Huang et al. 2008).
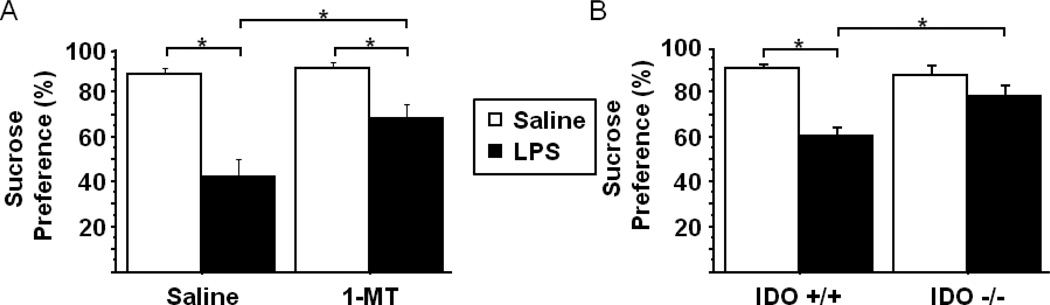
To determine whether IDO played an important mechanistic role or was merely a neurobiological marker of LPS-induced anhedonia, mice were pretreated with either saline or 1MT prior to LPS challenge (n=6 mice/group). Consistent with previous reports, LPS challenged mice exhibited a reduced preference for the sucrose solution (saline-90.1% ± 0.03 vs. LPS-42.5% ±0.08) during the 24h post-LPS interval (Fig. 5A). Importantly, pretreatment with 1MT attenuated the LPS-induced reduction in sucrose preference (pretreatment × treatment- F1,20=4.58, p<0.05) ,while 1MT alone had no significant impact. LPS induced a significant reduction within the 1MT pretreated group, whereas our previously reported LPS-induced depressive-like behavioral data in the forced swim and tail suspension test indicated a more complete inhibitory effect of 1MT. The partial inhibitory effect of 1MT could be due differences in dosing or the 1MT delivery method (continuous release vs. ip injection) or to the strain of mouse being used (CD-1 vs. C57BL6/J), all of which could be responsible for variability in the 1MT × LPS interaction between the two reports. To clarify the potential role of IDO in mediating LPS-induced anhedonia, wild-type (IDO +/+) or IDO deficient (IDO −/−) mice were injected ip with LPS or saline followed by determination of sucrose preference over the ensuing 24h period (n=8–11 mice/group). Figure 5B indicates that, while LPS precipitates a robust decline in sucrose preference in IDO +/+ mice (Saline-90.9% ± 1.5 vs. LPS-60.8% ± 3.2), IDO −/− mice do not exhibit a significant reduction in response to LPS (pretreatment × treatment- F1,37=10.77, p<0.01). Together, these data suggest that inhibition of IDO is sufficient to prevent the LPS-induced reduction in sucrose preference and implicate IDO in mediating the anhedonic effects of inflammation.
Figure 5.
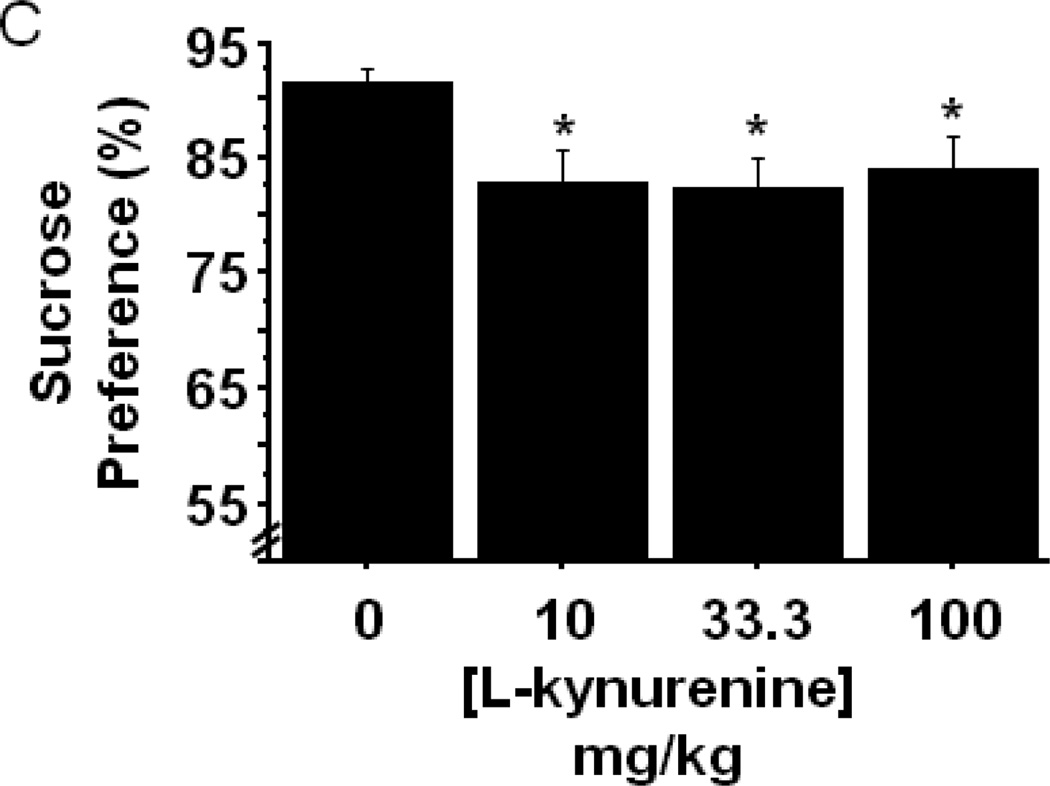
Indoleamine 2,3-dioxygenase mediates LPS-induced anhedonia. The effect of LPS challenge on sucrose preference was measured over the 24h post-LPS interval in (A) mice that had been pretreated with either vehicle or 1-methyltryptophan (n=6 mice per group) or (B) in WT or IDO null mice (n=8–11 mice per group). (C) Sucrose preference was determined in WT mice that were subcutaneously administered the indicated doses of L-kynurenine (n=11 mice per group). Data represent group means ± SEM, in at least two independent experimental replications. *p<0.05 versus the indicated groups in A and B, *p<0.05 versus control group in C.
Our previous reports have demonstrated that peripheral immune activation resulted in an increase in the kynurenine/tryptophan ratio, and inhibition of IDO, whether pharmacologically or genetically, normalized this index of IDO activity. Direct administration of L-kynurenine dose-dependently increased the duration of immobility in the forced swim/tail suspension tests and open field anxiety-like behavior (Figs. 4B and 4C), without altering locomotor activity. Manipulation of kynurenine metabolism elicits a number of cognitive effects. For example, 100mg/kg L-kynurenine impairs contextual, but not cue-dependent, fear conditioning (Chess, Landers et al. 2009), and the same dose, in a separate report, disrupted attentional set-shifting performance in rats (Alexander, Wu et al. 2011). To determine if direct administration of L-kynurenine, in the absence of inflammation, would produce an anhedonic behavioral phenotype, naïve mice were injected subcutaneously with increasing doses of L-kynurenine or an equal volume of vehicle buffered saline (n=11 mice/group). Figure 5C indicates that the single injection of L-kynurenine significantly reduced sucrose preference by 7–9% (treatment- F3,40=3.12, p<0.05) compared to vehicle controls at each dose tested. The magnitude of the reduction in sucrose preference is smaller than that observed following LPS, which is likely because a single injection elevates systemic kynurenine in a more transient manner than LPS, and immune activation is known to upregulate enzymes downstream of IDO that could differentially metabolize L-kynurenine. Even so, the dose of L-kynurenine necessary to elicit a reduction in sucrose preference is at least 10-fold lower than the dose required to induce anxiety-like behavior, while the anhedonia inducing doses are consistent with our previous data demonstrating that direct administration of L-kynurenine increases depressive-like behavior in the forced swim and tail suspension tests. Together, these data suggest that the various behaviors induced by peripheral LPS challenge are mediated through distinct neurobiological pathways or mechanisms, yet upregulation of IDO and kynurenine production appears to be an integral factor permitting the development of LPS-induced depressive-like and anxiety-like behaviors.
Conclusion
While it has been demonstrated that acute administration of L-kynurenine is sufficient to elevate kynurenine metabolites in the circulation and within the brain (Chiarugi, Carpenedo et al. 1996), a limitation of the present set of experiments is that kynurenine levels were not measured following injection. Here, the focus remained on the behavioral outcomes of treatment with LPS or L-kynurenine. IDO activity is positively associated with mortality caused by septic shock in patients, and inhibition of IDO protects rodents from LPS-induced endotoxic shock. Recent reports indicate that inhibition of kynurenine metabolism, downstream of IDO, may improve cognitive performance and reduce anxiety-like behavior (Potter, Elmer et al. 2010; Zwilling, Huang et al. 2011). However, the precise mechanisms, downstream of IDO activation and kynurenine production, that drive each particular inflammation-induced neuropsychiatric-like behavior will need to be investigated independently and are beyond the scope of the present report. It is important to note that an acute increase in L-kynurenine levels has the capacity to elicit both direct vaso-dilatory effects on vascular endothelial cells (Wang, Liu et al. 2010) and indirect effects on neurotransmitter systems via the generation of neuroactive metabolites. The vascular relaxing physiological effects of kynurenine have not been studied within the context of LPS-induced neuroimmuine responses, but it could be speculated that acute elevations in kynurenine that occur during immune challenge might alter the permeability of the blood brain barrier to inflammatory molecules and cells. The recent influx of new pharmacologic and genetic tools (enzyme inhibitors and novel transgenic mouse strains) will allow further investigation into the neuroimmunologic role of downstream kynurenine metabolites. Upregulation of IDO activity clearly appears to be a rate limiting point of regulation of diverse neurobiological and physiological responses to LPS. The present results not only extend evidence of IDO’s critical role in mediating translationally relevant inflammation-induced behaviors, but also they implicate the kynurenine pathway as a potential target of novel therapeutic treatments against inflammation-related anxiety and depression.
Highlights.
Peripheral immune challenge with LPS precipitates anhedonia and anxiety-like behavior in mice
Indoleamine 2,3-dioygenase mediates LPS-induced anhedonia and anxiety-like behavior
Direct administration of L-kynurenine causes anhedonia and anxiety-like behaviors in mice
These translationally relevant behaviors are distinct from the acute LPS-induced sickness response
Acknowledgements
This research was supported by grants R01-MH090127 and P30-MH089868 from the National Institute of Mental Health, and BLG received support from an ASPET-Pharmacology Summer Undergraduate Research Fellowship. The content is the sole the responsibility of the authors and does not necessarily represent the views of the National Institute of Mental Health or the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement: Jason O’Connor has received an honorarium from Lundbeck Research, USA.
References
- Alexander KS, Wu HQ, et al. Acute elevations of brain kynurenic acid impair cognitive flexibility: normalization by the alpha7 positive modulator galantamine. Psychopharmacology (Berl) 2011 doi: 10.1007/s00213-011-2539-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andre C, O'Connor JC, et al. Spatio-temporal differences in the profile of murine brain expression of proinflammatory cytokines and indoleamine 2,3-dioxygenase in response to peripheral lipopolysaccharide administration. J Neuroimmunol. 2008;200(1–2):90–99. doi: 10.1016/j.jneuroim.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassi GS, Kanashiro A, et al. Lipopolysaccharide-Induced Sickness Behaviour Evaluated in Different Models of Anxiety and Innate Fear in Rats. Basic Clin Pharmacol Toxicol. 2011 doi: 10.1111/j.1742-7843.2011.00824.x. [DOI] [PubMed] [Google Scholar]
- Bonaccorso S, Marino V, et al. Increased depressive ratings in patients with hepatitis C receiving interferon-alpha-based immunotherapy are related to interferon-alpha-induced changes in the serotonergic system. J Clin Psychopharmacol. 2002;22(1):86–90. doi: 10.1097/00004714-200202000-00014. [DOI] [PubMed] [Google Scholar]
- Capuron L, Miller AH. Cytokines and psychopathology: lessons from interferon-alpha. Biol Psychiatry. 2004;56(11):819–824. doi: 10.1016/j.biopsych.2004.02.009. [DOI] [PubMed] [Google Scholar]
- Capuron L, Neurauter G, et al. Interferon-alpha-induced changes in tryptophan metabolism. relationship to depression and paroxetine treatment. Biol Psychiatry. 2003;54(9):906–914. doi: 10.1016/s0006-3223(03)00173-2. [DOI] [PubMed] [Google Scholar]
- Chess AC, Landers AM, et al. L-kynurenine treatment alters contextual fear conditioning and context discrimination but not cue-specific fear conditioning. Behav Brain Res. 2009;201(2):325–331. doi: 10.1016/j.bbr.2009.03.013. [DOI] [PubMed] [Google Scholar]
- Chiarugi A, Carpenedo R, et al. Kynurenine disposition in blood and brain of mice: effects of selective inhibitors of kynurenine hydroxylase and of kynureninase. J Neurochem. 1996;67(2):692–698. doi: 10.1046/j.1471-4159.1996.67020692.x. [DOI] [PubMed] [Google Scholar]
- Dantzer R, O'Connor JC, et al. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9(1):46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, O'Connor JC, et al. Inflammation-associated depression: from serotonin to kynurenine. Psychoneuroendocrinology. 2011;36(3):426–436. doi: 10.1016/j.psyneuen.2010.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberger NI, Berkman ET, et al. Inflammation-induced anhedonia: endotoxin reduces ventral striatum responses to reward. Biol Psychiatry. 2010;68(8):748–754. doi: 10.1016/j.biopsych.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans DL, Charney DS, et al. Mood disorders in the medically ill: scientific review and recommendations. Biol Psychiatry. 2005;58(3):175–189. doi: 10.1016/j.biopsych.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Frenois F, Moreau M, et al. Lipopolysaccharide induces delayed FosB/DeltaFosB immunostaining within the mouse extended amygdala, hippocampus and hypothalamus, that parallel the expression of depressive-like behavior. Psychoneuroendocrinology. 2007;32(5):516–531. doi: 10.1016/j.psyneuen.2007.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu X, Zunich SM, et al. Central administration of lipopolysaccharide induces depressive-like behavior in vivo and activates brain indoleamine 2,3 dioxygenase in murine organotypic hippocampal slice cultures. J Neuroinflammation. 2010;7:43. doi: 10.1186/1742-2094-7-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godbout JP, Moreau M, et al. Aging exacerbates depressive-like behavior in mice in response to activation of the peripheral innate immune system. Neuropsychopharmacology. 2008;33(10):2341–2351. doi: 10.1038/sj.npp.1301649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigoleit JS, Kullmann JS, et al. Dose-dependent effects of endotoxin on neurobehavioral functions in humans. PLoS One. 2011;6(12):e28330. doi: 10.1371/journal.pone.0028330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison NA, Brydon L, et al. Inflammation causes mood changes through alterations in subgenual cingulate activity and mesolimbic connectivity. Biol Psychiatry. 2009;66(5):407–414. doi: 10.1016/j.biopsych.2009.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry CJ, Huang Y, et al. Minocycline attenuates lipopolysaccharide (LPS)-induced neuroinflammation, sickness behavior, and anhedonia. J Neuroinflammation. 2008;5:15. doi: 10.1186/1742-2094-5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung ID, Lee CM, et al. Differential regulation of indoleamine 2,3-dioxygenase by lipopolysaccharide and interferon gamma in murine bone marrow derived dendritic cells. FEBS Lett. 2007;581(7):1449–1456. doi: 10.1016/j.febslet.2007.02.073. [DOI] [PubMed] [Google Scholar]
- Kanai M, Funakoshi H, et al. Tryptophan 2,3-dioxygenase is a key modulator of physiological neurogenesis and anxiety-related behavior in mice. Mol Brain. 2009;2:8. doi: 10.1186/1756-6606-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak W, Conn CA, et al. Lipopolysaccharide induces fever and depresses locomotor activity in unrestrained mice. Am J Physiol. 1994;266(1 Pt 2):R125–R135. doi: 10.1152/ajpregu.1994.266.1.R125. [DOI] [PubMed] [Google Scholar]
- Laugeray A, Launay JM, et al. Evidence for a key role of the peripheral kynurenine pathway in the modulation of anxiety-and depression-like behaviours in mice: focus on individual differences. Pharmacol Biochem Behav. 2011;98(1):161–168. doi: 10.1016/j.pbb.2010.12.008. [DOI] [PubMed] [Google Scholar]
- Li R, Cooper C, et al. Coping strategies and psychological morbidity in family carers of people with dementia: A systematic review and meta-analysis. J Affect Disord. 2011 doi: 10.1016/j.jad.2011.05.055. [DOI] [PubMed] [Google Scholar]
- Maes M, Verkerk R, et al. Depressive and anxiety symptoms in the early puerperium are related to increased degradation of tryptophan into kynurenine, a phenomenon which is related to immune activation. Life Sci. 2002;71(16):1837–1848. doi: 10.1016/s0024-3205(02)01853-2. [DOI] [PubMed] [Google Scholar]
- Moreau M, Andre C, et al. Inoculation of Bacillus Calmette-Guerin to mice induces an acute episode of sickness behavior followed by chronic depressive-like behavior. Brain Behav Immun. 2008;22(7):1087–1095. doi: 10.1016/j.bbi.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor JC, Andre C, et al. Interferon-gamma and tumor necrosis factor-alpha mediate the upregulation of indoleamine 2,3-dioxygenase and the induction of depressive-like behavior in mice in response to bacillus Calmette-Guerin. J Neurosci. 2009;29(13):4200–4209. doi: 10.1523/JNEUROSCI.5032-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor JC, Lawson MA, et al. Induction of IDO by bacille Calmette-Guerin is responsible for development of murine depressive-like behavior. J Immunol. 2009;182(5):3202–3212. doi: 10.4049/jimmunol.0802722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor JC, Lawson MA, et al. Lipopolysaccharide-induced depressive-like behavior is mediated by indoleamine 2,3-dioxygenase activation in mice. Mol Psychiatry. 2009;14(5):511–522. doi: 10.1038/sj.mp.4002148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlikov AB, Prakhye IB, et al. Kynurenine in blood plasma and DST in patients with endogenous anxiety and endogenous depression. Biol Psychiatry. 1994;36(2):97–102. doi: 10.1016/0006-3223(94)91189-4. [DOI] [PubMed] [Google Scholar]
- Potter MC, Elmer GI, et al. Reduction of endogenous kynurenic acid formation enhances extracellular glutamate, hippocampal plasticity, and cognitive behavior. Neuropsychopharmacology. 2010;35(8):1734–1742. doi: 10.1038/npp.2010.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison CL, Dantzer R, et al. CSF concentrations of brain tryptophan and kynurenines during immune stimulation with IFN-alpha: relationship to CNS immune responses and depression. Mol Psychiatry. 2010;15(4):393–403. doi: 10.1038/mp.2009.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichenberg A, Yirmiya R, et al. Cytokine-associated emotional and cognitive disturbances in humans. Arch Gen Psychiatry. 2001;58(5):445–452. doi: 10.1001/archpsyc.58.5.445. [DOI] [PubMed] [Google Scholar]
- Roy-Byrne PP, Davidson KW, et al. Anxiety disorders and comorbid medical illness. Gen Hosp Psychiatry. 2008;30(3):208–225. doi: 10.1016/j.genhosppsych.2007.12.006. [DOI] [PubMed] [Google Scholar]
- Smith AK, Simon JS, et al. Association of a polymorphism in the indoleamine-2,3-ioxygenase gene and interferon-alpha-induced depression in patients with chronic hepatitis C. Mol Psychiatry. 2011 doi: 10.1038/mp.2011.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swiergiel AH, Dunn AJ. Effects of interleukin-1beta and lipopolysaccharide on behavior of mice in the elevated plus-maze and open field tests. Pharmacol Biochem Behav. 2007;86(4):651–659. doi: 10.1016/j.pbb.2007.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Lawson MA, et al. LPS-induced indoleamine 2,3-dioxygenase is regulated in an interferon-gamma-independent manner by a JNK signaling pathway in primary murine microglia. Brain Behav Immun. 2010;24(2):201–209. doi: 10.1016/j.bbi.2009.06.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Liu H, et al. Kynurenine is an endothelium-derived relaxing factor produced during inflammation. Nat Med. 2010;16(3):279–285. doi: 10.1038/nm.2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan HC, Cao X, et al. Behavioral animal models of depression. Neurosci Bull. 2010;26(4):327–337. doi: 10.1007/s12264-010-0323-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwilling D, Huang SY, et al. Kynurenine 3-monooxygenase inhibition in blood ameliorates neurodegeneration. Cell. 2011;145(6):863–874. doi: 10.1016/j.cell.2011.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]