Abstract
Background
Peripheral immunoregulation depends on T regulatory cell trafficking into the allograft in order to modulate the local alloresponse. Little is known about the relevance of trafficking receptors for Tregs after solid organ transplantation in humans. In this study expression of the peripheral chemokine receptors CXCR3 and CCR5 on CD4+FOXP3+ Treg cells was analyzed and correlated with allograft function in renal transplant recipients.
Methods
Flow cytometry analysis of peripheral blood mononuclear cells of 54 renal transplant recipients receiving a calcineurininhibitor based immunosuppression was performed for CD4, CD25, FOXP3, CXCR3 and CCR5 within the first 18 months posttransplantation. Correlation analysis of chemokine receptor expression and glomerular filtration rate as calculated by MDRD (eGFR) was performed.
Results
Expression of the peripheral homing receptors CXCR3 (r=0.44, p<0.05) and CCR5 (r=0.45, p<0.05) on FOXP3+ Tregs correlated with renal allograft function (eGFR) in patients receiving tacrolimus (n=28), but not CsA (n=26). CsA but not tracrolimus reduced surface expression of CXCR3 on FOXP3+ Tregs in renal transplant recipients as correlated to trough levels (r-0.42, p<0.05). In contrast to CD4+CXCR3+CD25lo T cells, flow sorted CD4+CXCR3+CD25hi Tregs isolated from healthy individuals did not produce IFNγ or IL-17 ex vivo and expressed high levels of GARP mRNA both at baseline as well as after TCR activation indicating functional regulatory activity.
Conclusion
Expression of the peripheral trafficking receptors CXCR3 and CCR5 on FOXP3+ Tregs is associated with renal allograft function. These results suggest that Treg trafficking may also depend on the interaction of CXCR3 or CCR5 and their respective ligands.
Keywords: Chemotaxis, Chemokine Receptors, Regulatory T cells, Renal transplantation
2. Introduction
Coordinate trafficking and retention of regulatory T cells is an indispensable mechanism of the immune system to mediate peripheral immunoregulation [1, 2]. Regulatory CD25hiFOXP3+ CD4+ T cells (Tregs) have been demonstrated to be essential for maintaining self-tolerance and mediating immune tolerance to allograft antigens [3, 4]. Tregs exert suppressive function to neutralize cytopathic effector cells by various mechanisms [3]. Their characteristic marker is the transcription factor FOXP3, which largely controls phenotype and function of Treg cells [5]. Studies undertaken in murine animal models demonstrated that Tregs modulate immune responses beyond secondary lymphoid organs through selective migration into the inflamed target tissue by expressing a combination of adhesion molecules [1] and chemokine receptors [2, 6–8]. Tolerance to alloantigens was shown to be only achieved if Tregs were allowed to recirculate in an appropriate pattern within secondary lymphoid organs and allografts [9, 10]. Whereas these murine studies have demonstrated that compartmentalization of Treg cells and their selective retention in the periphery is required to ensure peripheral tolerance [1, 9, 11–16], data from the human system are rare. Previous human studies suggest that the peripheral chemokine receptor CXCR3 which is classically associated with the recruitment of alloreactive effector T cells into rejecting allografts may also be involved in Treg trafficking [17, 18]. In these studies, expression and functional signaling of CXCR3 has been described in CD4+ T cells exhibiting a regulatory phenotype [17] and suppressive function [19]. In addition, IP-10 (CXCL-10), one of the chemokine ligands for CXCR3, was found to mediate migration of CXCR3hiCD25hiCD127lo CD4+ Tregs in vitro [19]., Recent findings from Oo and coworkers demonstrated that human CXCR3+ Treg cells isolated from chronically inflamed livers were found to function regulatory ex vivo [20]. Ukena et al. provided evidence that immune tolerant patients that underwent allogeneic stem cell transplantation exhibited a higher expression of CXCR3 both on RNA and protein level in contrast to those subjects with GvHD [21]. Elevated expression of peripheral chemokine receptors might therefore facilitate Treg migration into the allograft and thus may modulate and dampen the local allogeneic immune response resulting in improved graft function.
The aim of this study was to evaluate the relevance of the peripheral chemokine receptor CXCR3 expressed on circulating CD4+FOXP3+ T cells in 54 renal transplant recipients receiving standardized calcineurin inhibitor (CNI) based immunosuppression.
3. Material and methods
Patient samples
From June 2009 to June 2011, 70 Caucasian patients underwent renal transplantation receiving a calcineurininhibitor (CNI) in combination with mycophenolate mofetil (MPA) and steroids. Peripheral blood mononuclear cells (PBMCs) were analyzed by flow cytometry within two to 18 months posttransplantation. Active infections may stimulate or induce Tregs by inflammatory cytokines produced in response to the cognate pathogen. Therefore, only those patients were included that had a negative history of autoimmune disease (e.g. SLE, M. Wegener) or active infections with viral replication of EBV, CMV, Polyoma or Hepatitis A-C virus. 16 patients were excluded from analysis because of (1) acute rejection events or (2) other inflammatory events (CrP > 0.5 mg/dL) or (3) treatment with azathioprine or thymoglobuline. Renal allograft function was estimated as eGFR and calculated by MDRD. Clinical chemistry was done out of the same specimen that were used for FACS aquisition. Twelve healthy age matched individuals (male n=5) served as control group. The study protocol was approved by the Ethics Committee of the University Duisburg-Essen. Informed consent was obtained before study entry from each patient.
The study group of 54 patients consisted of 24 males, with a mean age of 52.0 ± 12 years, a mean time after transplantation of 5.7 ± 4.5 months, a mean eGFR of 46.3 ± 10.4 ml/min/1.73m2 (MDRD), mean amount of HLA mismatches of 2.6 ± 1.6, mean leukocyte count of 7.2 ± 2.5/nL and mean CrP of 0.13 ± 0.1 mg/dL. 26 patients received cyclosporine A (CsA) and 28 tacrolimus. Mean trough levels of CsA were 189 ± 64 ng/mL, of tacrolimus 7 ± 2.3 ng/mL and of MPA 3.5 ± 2.2 ng/mL. Subgroups of patients treated with CsA or tacrolimus did not differ with regard to age, months after Tx, MPA trough levels or eGFR (44.7±10.6 vs. 47.9±10.1 ml/min), respectively.
Reagents and Antibodies
Mouse anti-human CD4-PerCP (SK3), anti-human CXCR3-PE (1C6), anti-human CD25-FITC (M-A251), anti-human CD127-FITC (hIL-7R-M21), anti-human FOXP3-APC (clone 259D/C7) and murine monoclonal subclass-specific isotype controls were purchased from BD Biosciences (San Jose, CA, USA). Mouse anti-human CCR5-FITC (HEK/1/85a) was obtained from Biolegend (San Diego, CA, USA). For intracellular cytokine staining, mouse anti-human IFNγ-APC (clone 4S.B3), anti-human IL17a-APC (clone eBio 64DEC17) and their respective subclass-specific isotype controls were employed (all eBioscience, San Diego, USA).
For functional assays, mouse anti-human CD3 (HIT3a) and anti-human CD28 (CD28.2) were purchased from Biolegend (San Diego, USA). Recombinant IL-2 was obtained from Invitrogen (Darmstadt, Germany) and fetal bovine serum from Lonza (Cologne, Germany). Cyclosporine A (CsA) was purchased from Novartis Pharmaceuticals (Basel, CH) and Tacrolimus from BioXcell (Lebanon, NH, USA).
Flow cytometry
Freshly isolated PBMCs were stained for CD4, CD25 and CXCR3 with direct-conjugated fluorescent antibodies for 30 min at 4°C in the dark. After extensive washing, cells were fixed and permeabilized using the Fixation & Permeabilization Kit (BD Biosciences). Intracellular staining of FOXP3 was done according to the manufacturer’s recommendations. Phenotyping of T cell subsets was performed by gating on the lymphocyte subset and subsequently on the CD4+ T cell population. Aquisition was performed on a FACSCalibur (BD Biosciences) using CellQuest software (BD Biosciences). Data were analyzed using FlowJo Software, Version 8.7.3 (Tree Star, Inc., USA).
Cell Sorting
For cell sorting experiments, CD4+ cells were enriched from buffy coat specimen containing 6 to 8×108 PBMCs by autoMACS separation using the CD4+ T cell Isolation Kit II (Miltenyi, Bergisch-Gladbach, Germany) as recommended by the manufacturer, stained for anti-human CD25-FITC (M-A251) and CXCR3-PE (1C6), and isolated using a FACS Vantage (BD Biosciences) apparatus. Buffy coats were freshly prepared and provided by the Institute for Transfusion Medicine, University Hospital Essen.
Cell culture
Cell culture of PBMCs was performed at 37°C in 5% CO2 atmosphere in complete RPMI 1640 media (Cambrex, Charles City, IA) containing 10% fetal calf serum, 2mM L-glutamine, 100 U/ml penicillin/streptomycin (Gibco-Invitrogen, Carlsbad, CA), 1% sodium bicarbonate and 1% sodium pyruvate (Cambrex) in 96-well, round-bottom plates (Corning Life Sciences, Lowell, MA).
In vitro mitogen stimulation assays
CD4+ T cells were isolated from PBMCs using the CD4+ T cell Isolation Kit II (Miltenyi, Bergisch-Gladbach, Germany) according to the manufacturer’s instructions. Mitogen-dependent assays were performed in 96-well round bottom cell culture plates in triplicate wells (2×105 CD4+ cells/well) in a final volume of 200μl. T cells were stimulated with plate-bound anti-human CD3 (5μg/mL), soluble anti-human CD28 (2μg/mL) and IL-2 (100 U/mL) either in presence or absence of CsA (100 or 500 ng/mL) or tacrolimus (1 or 10 ng/mL) and analyzed by flow cytometry at the indicated time points.
Isolation of total RNA and Real-Time PCR analysis of GARP transcripts
For assessing mRNA expression of GARP, cell sorted CD4+CD25hiCXCR3+ and CD25lo/−CXCR3+ cells were stimulated in triplicates of 50000 cells/well with platebound anti-human CD3 (5μg/mL), soluble anti-human CD28 (2μg/mL) and IL-2 (100 U/mL). Cultured cells were harvested after two and six hours. Total RNA was prepared using the RNeasy® Mini Kit (Qiagen, Hilden, Germany) and cDNA was synthesized using the cloned AMV first-strand Synthesis Kit (Invitrogen, Carlsbad, CA). Quantitative PCR (qPCR) for each subset was performed on an ABI Prism 7500 Sequence Detection System (Applied Biosystems, Weiterstadt, Germany) using the TaqMan Universal Master Mix and validated gene-specific primer-probe sets for either human GARP (LRRC32) or the internal houskeeping gene RPL13A as control (Applied Biosystems). All cDNA samples were analyzed in triplicate and fold change in mRNA expression was calculated using standard comparative ΔCt-methodology. Expression of GARP in the distinct subsets was then normalized to its expression in unstimulated positively isolated CD4+ T cells.
Determination of intracellular cytokine production by flow cytometry
Positively isolated CD4+ T cells were mitogen stimulated using the cell stimulation cocktail (500X) and protein transport inhibitor (eBioscience, San Diego, USA) according to the manufacturer’s recommendations and incubated for 3 hours in 37°C and 5% CO2 atmosphere. After washing, cells were stained for surface molecules for 30 min. at 4°C in the dark followed by permeabilization and intracellular staining using the Cytofix/Cytoperm kit (BD Biosciences) according to the manufacturer’s recommendations.
Statistical analysis
Statistical analysis for flow cytometry assays was performed using GraphPad Prism V4.0. Correlation analysis was performed with Pearson’s test after proving normal distribution of the data. Treg chemokine receptor expression of healthy individuals and allograft recipients were compared with Mann-Whitney U test. A p value < 0.05 was considered as statistically significant.
4. Results
Human CXCR3+FOXP3+ CD4+ T cells exhibit a regulatory phenotype and highly express GARP mRNA transcripts
CD4+CXCR3+FOXP3+ T cells exhibit a T regulatory phenotype since they lack expression of CD127 compared to the CXCR3+FOXP3− subset (Figure 1a, upper histogram). Similar to the CXCR3+ cells, the CXCR3− CD4+FOXP3+ T cell subset shows low expression of CD127 (lower histogram). To examine whether CXCR3+ Tregs exhibit stable Treg cell line programming we evaluated mRNA expression of the proregulatory transcription factor GARP [22] in fluorescence cell sorted CD4+CD25hi expressing subsets. In contrast to CD4+CD25lo/− T cells, the CXCR3+CD25hi Tregs expressed high levels of GARP mRNA both at baseline as well as after TCR activation (Figure 1b) indicating stable Treg programming and thus suggesting functional regulatory activity Expression of GARP was comparable between TCR stimulated CXCR3− and CXCR3+ Tregs although there was a tendency to higher expression both at baseline and after stimulation in the CXCR3− subset (Figure 1b). In addition, CD4+CD25hi T cells that express CXCR3 neither express the inflammatory Th1 type cytokine IFNγ or the Th17 type cytokine IL-17 (Figure 1c). In contrast, half of the CD4+CD25lo cells that express CXCR3 produced high amounts of IFNγ reflecting their effector cell function (lower panel of Figure 1c).
Figure 1. Expression of CXCR3 on CD4+FOXP3+ Tregs.
The representative dot plot in (a) shows co-expression of CXCR3 and FOXP3 in CD4+ T cells in PBMCs of a healthy subject. The histograms (right) demonstrate that CXCR3+FOXP3+ Tregs lack CD127 expression in contrast to CXCR3+FOXP3− cells. (b) mRNA expression of GARP in flow sorted populations (as illustrated) of CD4+CD25hiCXCR3+ (white bars), CD4+CD25hiCXCR3− (black bars) or CD4+CD25lo/−CXCR3+ (grey bars) cells from PBMCs of a healthy subject. Expression was evaluated in unactivated cell subsets (baseline) or following 2 and 6 hrs treatment with anti-CD3/anti-CD28 and normalized to the total CD4+ T cell subset at baseline. Representative of n=3 experiments with similar results (triplicates, mean±SEM). (c) Representative dot blot of positively isolated CD4+ T cells that were stimulated with PMA/ionomycin for 4 hours. The histograms on the right of (c) show expression of IFNγ and IL-17 after gating on CD4+CD25hi or CD25lo subsets that are CXCR3 positive or CXCR3 negative.
Expression of CXCR3 and CCR5 positively correlates with allograft function in tacrolimus but not CsA treated patients
The percentages of CD4+FOXP3+ cells was 5.6±2.1% and of CD25hiFOXP3+ 2.6±1.2% in patients receiving CsA (n=26). Kidney transplant recipients treated with tacrolimus had 4.9±2.8% CD4+FOXP3+ and 2.4±1.6% CD25hiFOXP3+ CD4+ T cells (n=28). For both tacrolimus and CsA treated patient groups there was no difference in the percentages of CD4+FOXP3+ or CD4+CD25hiFOXP3+ Tregs compared to healthy subjects (6.0±2.3% and 2.2±1.0%, respectively). No correlation with eGFR was observed for both FOXP3+ and CD25hiFOXP3+ Treg subsets for neither of the immunosuppressive drugs (data not shown).
In contrast to tacrolimus treated subjects, CXCR3 expression on CD4+FOXP3+ Treg cells was significantly reduced in patients treated with CsA (38.5±8.4%; n=26) but not tacrolimus (41.8±6.0%; n=28) as compared to healthy subjects (44.5±7.4%; n=15; p<0.05). Comparison of CXCR3 expression in CsA (38.5±8.4%) vs. tacrolimus (41.8±6.0%) treated patients exhibited no significant difference. Similar to CXCR3, CCR5 expression on CD4+FOXP3+ Treg cells was significantly reduced in patients treated with CsA (21.4±8.6%; n=26) but not tacrolimus (25.5±8.0%; n=28) as compared to healthy subjects (44.5±7.4%; n=15; p<0.05). Comparison of CCR5 expression in CsA (21.4±8.5%; n=25) vs. tacrolimus (25.7±8.3%; n=27) treated patients exhibited no significant difference.
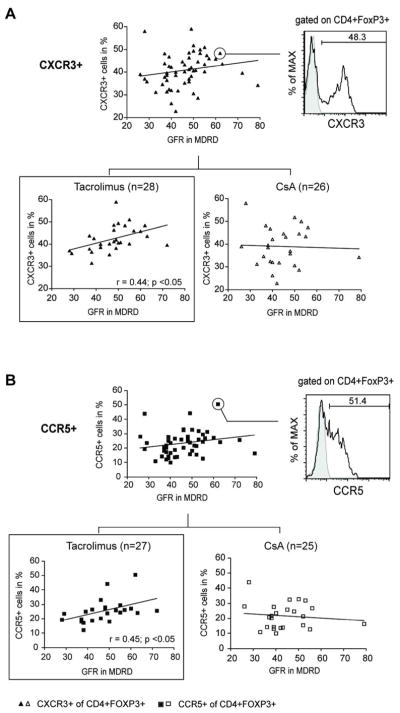
There was no correlation with eGFR (MDRD) between the percentages of CXCR3+ or CCR5+ cells among the CD4+CD25hiFOXP3+ subset (data not shown). However, gating on CD4+FOXP3+ Tregs revealed a positive correlation of CXCR3 expression with eGFR (Figure 2a). Moreover, subgroup analysis demonstrated that in tacrolimus but not CsA treated patients CXCR3+ (r=0.44, p<0.05) and CCR5+ (r=0.45, p<0.05) exhibited a significant correlation with kidney transplant function (Figures 2a,b).
Figure 2. Expression of the peripheral chemokine receptor CXCR3 and CCR5 on Tregs correlates with allograft function in patients receiving tacrolimus.
Scatter plot graphs show correlation analysis for CXCR3 and CCR5 after gating on CD4+FOXP3+ Tregs and MDRD in transplant recipients receiving CNIs (n=54; upper graphs) and after subdeviding into Tacrolimus (n=28) and CsA (n=26) treated patients (lower graphs). The representative histograms/dot blot on the right depicts CD4+FOXP3+ Tregs expressing CXCR3 and CCR5, respectively.
Correlation analysis of CXCR3 expression in the total CD4+ T cell subset detected no association with eGFR in CsA and tacrolimus treated patients.
Cyclosporine but not tacrolimus trough levels negatively correlate with the expression of CXCR3 on Treg cells in the peripheral blood of renal transplant recipients
To rule out that a decreased surface expression of CXCR3 and CCR5 as well as a decline in allograft function are caused by toxic side effects of calcineurin inhibition, we correlated drug trough levels with chemokine receptor expression and eGFR (MDRD). MDRD and trough levels displayed no correlation (not shown). However, CsA trough levels negatively correlated with the expression of CXCR3 on CD4+FOXP3+ Treg cells (Figure 3a, right panel) whereas treatment with tacrolimus did not affect surface expression of either CXCR3 nor CCR5 (Figure 3a,b).
Figure 3. Cyclosporine but not tacrolimus trough levels negatively correlate with the expression of CXCR3 on Treg cells in renal transplant recipients.
Scatter plot graphs demonstrate correlation analysis between the expression of CXCR3 (a) and CCR5 (b) on CD4+FOXP3+ Tregs and trough levels of transplant patients receiving tacrolimus (left panel) or CsA (right panel).
Surface CXCR3 expression in mitogen activated CD25+FOXP3+ T cells is suppressed after treatment with Cyclosporine and Tacrolimus in vitro
To examine the in vitro effect of CNIs on the expression of CXCR3 on Tregs, we stimulated purified CD4+ T cells with immobilized anti-human CD3, soluble anti-human CD28 and IL-2 (100 IU/mL) in absence or presence of tacrolimus (10 ng/mL) or cyclosporine (500 ng/mL) As expected, both tacrolimus and CsA inhibited Treg expansion after 5 days compared to untreated controls (n=4, Figure 4a). In addition, both drugs inhibited expression of CXCR3 on CD25+FOXP3+ cells (Figure 4b). In a second series of experiments with lower dosages of both drugs, again tacrolimus suppressed CXCR3 expression at 10 but not at 1 ng/mL (n=3; Figures 4c,d). This is in line with the results obtained in vivo (Figure 2). We interpret these observations to indicate that both proliferation of FOXP3+ T cells as well as expression of surface CXCR3 was inhibited in presence of CNI.
Figure 4. Calcineurin inhibitors suppress surface expression of CXCR3 on mitogen activated Treg cells in vitro.
CD4+ T cells were stimulated with platebound anti-human CD3 and soluble anti-human CD28 in the absence or presence of tacrolimus (Tac) or CsA as indicated, and were stained with fluorescent antibodies to CD25, FOXP3 and CXCR3 or isotype controls (shaded histograms). (a), dot blots of a representative experiment demonstrating the co-expression of CD25+ and FOXP3+ on CD4+ T cells on day 0, 2 and day 5 following mitogen activation and summarized data (right) illustrating the kinetics of co-expression of CD25+ and FOXP3+ over time (n=4; mean∓SEM). (b), representative experiment depicting the expression of CXCR3 (open histograms) on CD4+CD25hiFOXP3+ Tregs on day 0, 2 and day 5 following activation and summarized data (right) illustrating the expression kinetics of CXCR3 over time (n=4; mean±SEM). (c), representative expression of CXCR3 (open histograms) on CD4+CD25hiFOXP3+ T cells at days 0 and 5 and summarized for day 5 in (d) as box plots for CsA and tacrolimus (each n=3).
5. Discussion
The main finding of this study is that in stable renal transplant patients without active infections, surface expression of the peripheral homing receptors CXCR3 and CCR5 on peripherally circulating CD4+FOXP3+ T cells positively correlate with eGFR. Functionally active Tregs play a substantial role for the homeostasis of the immune system with respect to migration capacity, their clonal expansion and suppressive function. In acute rejection, the immune system insufficiently controls alloreactive effector T cells that are expanded in secondary lymphoid organs and recruited into allografts. Similar to effector T cells, regulatory T cells have been demonstrated to express both adhesion molecules and chemokine receptors [6, 17, 18]. In murine studies, trafficking of Tregs into secondary lymphoid organs as well as within the transplanted graft has been proposed to be important for alloimmune tolerance induction [9, 10], as well as for the prevention of chronic rejection [23]. This observation implies that homing receptor expression on human Tregs has similar potential to define effective migration patterns and peripheral immunoregulation in vivo. Indeed, humans Treg cells have been shown to accumulate in kidney allografts [24, 25]. Bestard and coworkers demonstrated that the FOXP3+ Treg/CD3+ T cell ratio of intragraft T cell infiltrates are positively correlated with graft function at 2 years after transplantation [25].
Whereas the peripheral leukocyte homing receptor CCR5 is well known to be of functional importance for both murine [9, 26] and human Tregs [17], CXCR3 signaling has classically been linked to T effector cell trafficking and recruitment of conventional alloreactive T cells into rejecting allografts [27–32]. However, recent studies demonstrated CXCR3 to be involved also in Treg trafficking [33–35]. In a CXCR3−/− murine model for experimental autoimmune encephalitis (EAE), CXCR3 signaling augmented Treg recruitment and effector T cell interaction, thus limiting autoimmune-mediated tissue damage [33]. In addition, Hasegawa et al. demonstrated that CXCR3-transfected Tregs migrate efficiently into and accumulate in GvHD affected organs, resulting in significant amelioration of GvHD in liver, lung and intestine [34]. In humans, the expression and functional signaling of CXCR3 has been recently described in CD4+ T cells exhibiting regulatory phenotype [17] and function [19]. That CXCR3+ Tregs indeed may play a role in vivo is supported by studies of Oo and coworkers in which human CXCR3+ Treg cells isolated from chronically inflamed livers were found to function regulatory ex vivo [20]. Very recently, Ukena et al. provided evidence that immune tolerant patients exhibited a higher expression of CXCR3 both on RNA and protein level in contrast to those subjects with GvHD after stem cell transplantation [21].
In this study, we demonstrate that CD4+CXCR3+FOXP3hi cells circulating in the periphery of renal transplant recipients exhibit a Treg phenotype since they show low/negative surface expression of the IL-7 receptor α-chain CD127. Furthermore, CXCR3+ Tregs isolated from healthy individuals highly express GARP, a proregulatory transcription factor [22] associated with suppressive Treg function that demonstrates stable Treg cell programming. Moreover, Wang et al. provided evidence that expression of GARP selectively identifies suppressive activated human FOXP3+ Tregs enabling discrimination from IL-17-secreting CD4+CD25+ T cells [36, 37]. Thus, CD4+FOXP3+ Tregs of patients with higher surface expression of CXCR3 might properly migrate to inflammatory sites such as allografts capable of controlling potentially alloreactive Teff clones. This may be supported by recent data from Lo et al. who demonstrated that the inflammatory chemokine ligands CXCL9-11 are elevated in renal transplants with stable function without any histologic signs of rejection [38].
It has been reported that both murine and human Tregs express multiple chemokine receptors [13, 14, 17–19]. In previous studies, we found that human Tregs co-express the lymph node homing receptors CD62L and CCR7 as well as the peripheral chemokine receptors CCR4, CCR5 and/or CXCR3 [19]. The combination of trafficking receptors are thought to allow Tregs to sequentially migrate into secondary lymphoid organs and into the periphery [6, 9]. Our results demonstrate that co-expression of CCR5 or CXCR3 on human CD4+FOXP3+ T cells is also associated with better renal transplant function suggesting an interplay or amplification of each single receptor signaling to optimize peripheral Treg trafficking. However, with respect to the multifaceted chemokine receptor equipment of a Treg cell it needs to be emphasized that the results provided in this study only represent parts of the whole picture of Treg migration.
We have also investigated the pharmacological effect of tacrolimus and CsA on the expression of the chemokine receptors CXCR3 and CCR5 on Treg cells. The results demonstrate that in contrast to CsA treatment with tacrolimus – at least in clinical applied dosages – does not alter surface expression of all investigated chemokine receptors in vivo. Potential confounding effects of mycophenolate mofetil acid (MPA) could be excluded since CsA and tacrolimus treated patients exhibited comparable MPA trough levels. In murine studies, it was recently shown that TCR-induced upregulation of CXCR3 is in part mediated by activation of PI3Kγ [39]. One of the major functions of CNIs is to prevent the activation of NFAT and thereby to suppress T cell activation. In addition, and this may serve as a possible explanation, it has been described that calcineurin inhibitors effectively inhibit the PI3K/AKT kinase pathway [40, 41]. However, whether this or other signalling mechanisms may be involved in the suppression of CXCR3 remains to be elucidated.
In summary, our observations demonstrate that CD4+FOXP3+ Treg cells that co-express CXCR3 and CCR5 correlate with renal allograft function in patients receiving a tacrolimus based immunosuppressive regimen. CXCR3 and CCR5 expressing Treg subsets that display the capacity to enter inflamed sites may have a role in the control of established immune reactions such as an allogeneic solid organ transplant. Further studies involving higher patient numbers including protocol biopsies are needed to further elucidate this finding.
Acknowledgments
This work was supported by an IFORES Research fellowship from the University Duisburg-Essen Medical School to AH.
The authors wish to thank Dr. Klaus Lennartz (University Duisburg-Essen, Institute for Cell Biology) for excellent technical assistance in cell sorting and Professor Peter Horn (University Duisburg-Essen, Institute for Transfusion Medicine) for providing blood donor buffy coat specimen for CD4+ T cell subset isolation.
Abbreviations Used
- FOXP3
Forkhead Box P3
- CNI
Calcineurin Inhibitor
- GvHD
Graft versus Host Disease
- PBMC
peripheral blood mononuclear cells
- Treg
Regulatory T cell
- Tx
transplantation
References
- 1.Huehn J, Siegmund K, Hamann A. Migration rules: functional properties of naive and effector/memory-like regulatory T cell subsets. Curr Top Microbiol Immunol. 2005;293:89–114. doi: 10.1007/3-540-27702-1_5. [DOI] [PubMed] [Google Scholar]
- 2.Piccirillo CA. Regulatory T cells in health and disease. Cytokine. 2008;43:395–401. doi: 10.1016/j.cyto.2008.07.469. [DOI] [PubMed] [Google Scholar]
- 3.Sakaguchi S, Miyara M, Costantino CM, et al. FOXP3+ regulatory T cells in the human immune system. Nat Rev Immunol. 2010;10:490–500. doi: 10.1038/nri2785. [DOI] [PubMed] [Google Scholar]
- 4.Li XC, Turka LA. An update on regulatory T cells in transplant tolerance and rejection. Nat Rev Nephrol. 2010;6:577–583. doi: 10.1038/nrneph.2010.101. [DOI] [PubMed] [Google Scholar]
- 5.Campbell DJ, Ziegler SF. FOXP3 modifies the phenotypic and functional properties of regulatory T cells. Nat Rev Immunol. 2007;7:305–310. doi: 10.1038/nri2061. [DOI] [PubMed] [Google Scholar]
- 6.Huehn J, Hamann A. Homing to suppress: address codes for Treg migration. Trends Immunol. 2005;26:632–636. doi: 10.1016/j.it.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 7.Sakaguchi S, Ono M, Setoguchi R, et al. Foxp3+ CD25+ CD4+ natural regulatory T cells in dominant self-tolerance and autoimmune disease. Immunol Rev. 2006;212:8–27. doi: 10.1111/j.0105-2896.2006.00427.x. [DOI] [PubMed] [Google Scholar]
- 8.Sather BD, Treuting P, Perdue N, et al. Altering the distribution of Foxp3(+) regulatory T cells results in tissue-specific inflammatory disease. J Exp Med. 2007;204:1335–1347. doi: 10.1084/jem.20070081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang N, Schroppel B, Lal G, et al. Regulatory T cells sequentially migrate from inflamed tissues to draining lymph nodes to suppress the alloimmune response. Immunity. 2009;30:458–469. doi: 10.1016/j.immuni.2008.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Long ET, Baker S, Oliveira V, et al. Alpha-1,2-mannosidase and hence N-glycosylation are required for regulatory T cell migration and allograft tolerance in mice. PLoS One. 5:e8894. doi: 10.1371/journal.pone.0008894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huehn J, Siegmund K, Lehmann JC, et al. Developmental stage, phenotype, and migration distinguish naive- and effector/memory-like CD4+ regulatory T cells. J Exp Med. 2004;199:303–313. doi: 10.1084/jem.20031562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boehmer v. Mechanisms of suppression by suppressor T cells. Nature Immunology. 2005;6:338–344. doi: 10.1038/ni1180. [DOI] [PubMed] [Google Scholar]
- 13.Wysocki CA, Jiang Q, Panoskaltsis-Mortari A, et al. Critical role for CCR5 in the function of donor CD4+CD25+ regulatory T cells during acute graft-versus-host disease. Blood. 2005;106:3300–3307. doi: 10.1182/blood-2005-04-1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee I, Wang L, Wells AD, et al. Recruitment of Foxp3+ T regulatory cells mediating allograft tolerance depends on the CCR4 chemokine receptor. J Exp Med. 2005;201:1037–1044. doi: 10.1084/jem.20041709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Siegmund K, Feuerer M, Siewert C, et al. Migration matters: regulatory T-cell compartmentalization determines suppressive activity in vivo. Blood. 2005;106:3097–3104. doi: 10.1182/blood-2005-05-1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roncarolo MG, Battaglia M. Regulatory T-cell immunotherapy for tolerance to self antigens and alloantigens in humans. Nat Rev Immunol. 2007;7:585–598. doi: 10.1038/nri2138. [DOI] [PubMed] [Google Scholar]
- 17.Lim HW, Broxmeyer HE, Kim CH. Regulation of trafficking receptor expression in human forkhead box P3+ regulatory T cells. J Immunol. 2006;177:840–851. doi: 10.4049/jimmunol.177.2.840. [DOI] [PubMed] [Google Scholar]
- 18.Hirahara K, Liu L, Clark RA, et al. The majority of human peripheral blood CD4+CD25highFoxp3+ regulatory T cells bear functional skin-homing receptors. J Immunol. 2006;177:4488–4494. doi: 10.4049/jimmunol.177.7.4488. [DOI] [PubMed] [Google Scholar]
- 19.Hoerning A, Koss K, Datta D, et al. Subsets of human CD4(+) T regulatory cells express the peripheral homing receptor CXCR3. Eur J Immunol. 2011;41(8):2291–302. doi: 10.1002/eji.201041095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oo YH, Weston CJ, Lalor PF, et al. Distinct roles for CCR4 and CXCR3 in the recruitment and positioning of regulatory T cells in the inflamed human liver. J Immunol. 2010;184:2886–2898. doi: 10.4049/jimmunol.0901216. [DOI] [PubMed] [Google Scholar]
- 21.Ukena SN, Velaga S, Geffers R, et al. Human regulatory T cells in allogeneic stem cell transplantation. Blood. 2011;118:e82–92. doi: 10.1182/blood-2011-05-352708. [DOI] [PubMed] [Google Scholar]
- 22.Probst-Kepper M, Geffers R, Kroger A, et al. GARP. a key receptor controlling FOXP3 in human regulatory T cells. J Cell Mol Med. 2009;13:3343–3357. doi: 10.1111/j.1582-4934.2009.00782.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nadig SN, Wieckiewicz J, Wu DC, et al. In vivo prevention of transplant arteriosclerosis by ex vivo-expanded human regulatory T cells. Nat Med. 2010;16:809–813. doi: 10.1038/nm.2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Muthukumar T, Dadhania D, Ding R, et al. Messenger RNA for FOXP3 in the urine of renal-allograft recipients. N Engl J Med. 2005;353:2342–2351. doi: 10.1056/NEJMoa051907. [DOI] [PubMed] [Google Scholar]
- 25.Bestard O, Cruzado JM, Rama I, et al. Presence of FoxP3+ regulatory T Cells predicts outcome of subclinical rejection of renal allografts. J Am Soc Nephrol. 2008;19:2020–2026. doi: 10.1681/ASN.2007111174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nozaki T, Rosenblum JM, Schenk AD, et al. CCR5 is required for regulation of alloreactive T-cell responses to single class II MHC-mismatched murine cardiac grafts. Am J Transplant. 2009;9:2251–2261. doi: 10.1111/j.1600-6143.2009.02786.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hancock WW, Lu B, Gao W, et al. Requirement of the chemokine receptor CXCR3 for acute allograft rejection. J Exp Med. 2000;192:1515–1520. doi: 10.1084/jem.192.10.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Melter M, Exeni A, Reinders ME, et al. Expression of the chemokine receptor CXCR3 and its ligand IP-10 during human cardiac allograft rejection. Circulation. 2001;104:2558–2564. doi: 10.1161/hc4601.098010. [DOI] [PubMed] [Google Scholar]
- 29.Hancock WW, Gao W, Csizmadia V, et al. Donor-derived IP-10 initiates development of acute allograft rejection. J Exp Med. 2001;193:975–980. doi: 10.1084/jem.193.8.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hancock WW. Chemokine receptor-dependent alloresponses. Immunol Rev. 2003;196:37–50. doi: 10.1046/j.1600-065x.2003.00084.x. [DOI] [PubMed] [Google Scholar]
- 31.Panzer U, Reinking RR, Steinmetz OM, et al. CXCR3 and CCR5 positive T-cell recruitment in acute human renal allograft rejection. Transplantation. 2004;78:1341–1350. doi: 10.1097/01.tp.0000140483.59664.64. [DOI] [PubMed] [Google Scholar]
- 32.Schnickel GT, Hsieh GR, Garcia C, et al. Role of CXCR3 and CCR5 in Allograft Rejection. Transplant Proc. 2006;38:3221–3224. doi: 10.1016/j.transproceed.2006.10.164. [DOI] [PubMed] [Google Scholar]
- 33.Muller M, Carter SL, Hofer MJ, et al. CXCR3 signaling reduces the severity of experimental autoimmune encephalomyelitis by controlling the parenchymal distribution of effector and regulatory T cells in the central nervous system. J Immunol. 2007;179:2774–2786. doi: 10.4049/jimmunol.179.5.2774. [DOI] [PubMed] [Google Scholar]
- 34.Hasegawa H, Inoue A, Kohno M, et al. Therapeutic effect of CXCR3-expressing regulatory T cells on liver, lung and intestinal damages in a murine acute GVHD model. Gene Ther. 2008;15:171–182. doi: 10.1038/sj.gt.3303051. [DOI] [PubMed] [Google Scholar]
- 35.Yamada Y, Okubo Y, Shimada A, et al. Acceleration of diabetes development in CXC chemokine receptor 3 (CXCR3)-deficient NOD mice. Diabetologia. 2012 Apr; doi: 10.1007/s00125-012-2547-8. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 36.Wang R, Kozhaya L, Mercer F, et al. Expression of GARP selectively identifies activated human FOXP3+ regulatory T cells. Proc Natl Acad Sci U S A. 2009;106:13439–13444. doi: 10.1073/pnas.0901965106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tran DQ, Andersson J, Wang R, et al. GARP (LRRC32) is essential for the surface expression of latent TGF-beta on platelets and activated FOXP3+ regulatory T cells. Proc Natl Acad Sci U S A. 2009;106:13445–13450. doi: 10.1073/pnas.0901944106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lo DJ, Weaver TA, Kleiner DE, et al. Chemokines and Their Receptors in Human Renal Allotransplantation. Transplantation. 2011;91:70–77. doi: 10.1097/TP.0b013e3181fe12fc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barbi J, Cummings HE, Lu B, et al. PI3Kgamma (PI3Kgamma) is essential for efficient induction of CXCR3 on activated T cells. Blood. 2008;112:3048–3051. doi: 10.1182/blood-2008-02-135715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cho ML, Ju JH, Kim KW, et al. Cyclosporine A inhibits IL-15-induced IL-17 production in CD4+ T cells via down-regulation of PI3K/Akt and NF-kappaB. Immunol Lett. 2007;108:88–96. doi: 10.1016/j.imlet.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 41.Datta D, Flaxenburg JA, Laxmanan S, et al. Ras-induced Modulation of CXCL10 and Its Receptor Splice Variant CXCR3-B in MDA-MB-435 and MCF-7 Cells: Relevance for the Development of Human Breast Cancer. Cancer Res. 2006;66:9509–9518. doi: 10.1158/0008-5472.CAN-05-4345. [DOI] [PubMed] [Google Scholar]