Abstract
All-trans-retinoic acid (RA), the major active metabolite of vitamin A, is a regulator of gene expression with many roles in cell differentiation. In the present study, we investigated RA in the regulation of MafB, a basic leucine-zipper transcription factor with broad roles in embryonic development, hematopoiesis and monocyte-macrophage differentiation. In RA-treated THP-1 human monocytic cells, MafB mRNA and protein levels were up-regulated by RA dose and time-dependently, while, additionally, RA and tumor necrosis factor (TNF)α, also known to induce monocyte to macrophage differentiation, increased MafB expression synergistically. Screening of potential targets containing Maf recognition elements (MARE motifs) in their promoter regions identified SPOCK1, Blimp1 and CCL2 as potential targets; these genes are related to cell communication, recruitment and differentiation, respectively. Across cell treatments, SPOCK1, Blimp1 and CCL2 mRNA levels were highly correlated (P<0.001) with MafB. ChIP assays demonstrated increased MafB protein binding to MARE elements in the promoter regions of SPOCK1, Blimp1 and CCL2 in RA and TNFα-treated cells, as well as acetylation of histone-H4 in MARE-containing regions, indicative of chromatin activation. Conversely, reducing MafB protein by microRNA silencing significantly decreased the expression of SPOCK1, Blimp1 and CCL2 (P<0.01). Moreover, the reduction in MafB expression and these downstream targets correlated with decreased cell differentiation as determined by cell-surface CD11b expression and phagocytic activity. We conclude that MafB may be a key factor in mediating the ability of RA and TNFα to regulate monocytic cell communication, recruitment and differentiation through regulation of MafB target genes including SPOCK1, CCL2 and Blimp1.
Keywords: retinoic acid, MafB, MafB target genes, MafB silencing, monocytic cell differentiation
Introduction
Retinoids (vitamin A and its metabolites) are required for normal innate and adaptive immune responses to agents of infectious diseases [1–3]. All-trans-retinoic acid (RA)3 exerts pleiotropic effects on many cellular processes, including cell growth, differentiation and apoptosis [4], which mainly result from RA-induced changes in gene expression. The binding of RA to nuclear retinoic acid receptors (RARs) can directly alter the transcriptional activity of target genes [5], while other genes are also regulated physiologically by RA through either indirect or undefined mechanisms [6]. In studies conducted in human THP-1 cells, a useful model of monocytic cell differentiation [7], we have previously have shown that RA regulates cell cycle progression [8–9], while increasing the expression of CD11b, a cell surface marker of macrophage differentiation, and increasing phagocytic activity [8]. However, the pathways through which RA acts to regulate cellular processes in monocytic cells are incompletely understood and could include many molecular factors.
MafB is a transcription factor, belonging to the Maf family [10]. Similar to other so-called large Maf proteins, MafB contains a basic leucine zipper structure at the carboxy-terminal portion, which mediates DNA binding and subunit dimerization, and is involved in the regulation of gene expression. Maf proteins bind to a specific DNA sequence, called Maf recognition elements (MAREs). MafB is best known as a regulator of early embryonic development [11–13], but it has also been reported to be involved in islet beta-cell differentiation [14–17] and to be expressed in monocytes and macrophages where it is thought to be an important regulator of macrophage differentiation [18–20]. Overexpression of MafB was shown to limit myeloid progenitor proliferation while promoting functional macrophage differentiation [18, 21]. Moreover, a deficiency of MafB inhibited monocyte/macrophage differentiation in avian myeloid progenitors [18]. Together, these results strongly suggest a critical function of MafB in monocytic differentiation. However, relatively little is known of the factors that regulate MafB gene expression.
The regulation of MafB by RA was first suggested to us by the results of a microarray analysis from RA-treated THP-1 cells, in which MafB was one of the more strongly retinoid-responsive genes. In the present study, based on confirmation of these results we hypothesized that MafB may be a significant mediator of the effects of RA on the regulation of downstream genes that potentially regulate several aspects of monocytic cell functions, such as cell differentiation and response to inflammatory stimuli. Additionally, we found that TNFα, one of the key mediators of inflammation that induces monocyte maturation and activation [22], acts synergistically with RA to upregulate MafB expression. Thus, the present studies have focused on the regulation of MafB by RA and TNFα, and on identification of putative downstream genes that may mediate their effects on monocytic cell differentiation.
Materials and Methods
Cell and culture conditions
THP-1 cells (ATCC, Rockville, MD) were maintained in RPMI 1640 medium with 10% fetal bovine serum (FBS), 500 mM β-mercaptoethanol, and 100 units/ml penicillin and streptomycin [8], and used within 15 passages [23]. For each experiment, the cells were grown in the same medium containing 3% FBS; this change was made to reduce the concentration of albumin for better uptake of RA. All-trans RA (Sigma-Aldrich, St. Louis, MO) was added at the desired final concentrations and recombinant human TNFα (R&D Systems, Minneapolis, MN) was added either alone or with RA. Ethanol was used as a vehicle control and did not exceed 0.01%. After completion of the various treatments, the cells were harvested and subjected to RNA or protein analysis. Cell viability was monitored by addition of 0.4% Trypan Blue dye (Sigma-Aldrich) and was consistently > 95%.
Microarray analysis
For microarray analysis, THP-1 cells were pre-treated with 20 nM RA or vehicle at t=0 h and after 16 h cells were treated with a second dose of RA and/or with TNFα (5 ng/ml). At 40 h, the cells were harvested. Total RNA was prepared and sent to the Microarray Facility of the National Cancer Institute, Frederick MD, for analysis, as described earlier [24]. These data have been deposited under GEO accession GSE28995. We sorted genes from the full database (54,675 targets) that were upregulated by treatment with both RA and TNFα and that showed significant regulation as detected by one-way ANOVA using the criterion of a P value of <0.001. Putative promoter sequences (2000 bps upstream of the transcription starting site for each gene) of these RA- and TNFα-upregulated genes were downloaded from the UCSC genome browser website (http://genome.ucsc.edu). A Python script (http://www.python.org) was used to scan these sequences for the presence of MARE sequences (TGCTGACTCAGCA and TGCTGACGTCAGCA) and for 5′-AT-rich MARE half-sites, on both the sense and anti-sense strands of the promoter sequences.
Quantitative real-time PCR (q-PCR)
Total RNA was extracted from cells using RNeasy Mini Kit (Qiagen, Valencia, CA). For reverse transcription, as described before [25], 1 μg of total RNA was subjected to reverse transcription using Moloney murine leukemia virus reverse transcriptase (Promega, Madison, WI). The diluted reaction product (1/20th of the reaction products) was applied for quantitative real-time PCR analysis using iQ SYBR Green Supermix from BioRad (Emory, CA) in a final volume of 20 μl. 18S RNA was amplified at the same time as an internal control. Primers for MafB were: 5′-CCGCTGGCCATGGAGTATGT-3′ (forward), 5′-GGCGACGCTTGGTGAT-3′ (reverse). Primers for SPOCK1 were 5′-GACCGGGACAAGTACTGGAA-3′ (forward), 5-‘TCCTTTCTGCCTTGTGCTTT-3′ (reverse); for Blimp1: 5′-TGGACATGGA GGACGCGGATATG-3′ (forward), 5′-GGTTGGCAGGGATAGGCTTAATAG-3′ (reverse); and for CCL2: 5′-CCCAGTCACCTGCTGGTTAT-3′ (forward), 5′-TGGAATCCTGAACCCACTTC-3′ (reverse).
Immunoblotting
Whole cell lysates were prepared [8] and 50 μg of protein per sample was denatured, separated by SDS-polyacrylamide gel electrophoresis, and transferred to nitrocellulose membranes, which were sequentially incubated in primary antibody, anti-MafB, and horseradish peroxidase (HRP)–conjugated secondary antibody (both from Santa Cruz Biotechnology, Santa Cruz, CA). The HRP conjugate was detected using an ECL system (Pierce Biotechnology, Rockford, IL). Loading controls utilized an anti-β -actin antibody (Santa Cruz Biotechnology)
Chromatin Immunoprecipitation (ChIP) Assay
Cell nuclei were subjected to ChIP assays similar to as described [25]. Antibodies specific to MafB and acetylated histone H4 were used for immunoprecipitation. Anti-acetylated histone H4 (Millipore, Billerica, MA) was used as chromatin activation indicator, and nonspecific IgG (Santa Cruz Biotechnology) as a negative control. The immunoprecipitated DNA (~500bps) was subjected to q-PCR with oligonucleotide primer pairs designed for the MARE region on the promoters of SPOCK1, Blimp1 and CCL2, using methods described above for MafB. Primers for the MARE-containing regions in the promoter were: for SPOCK1, 5′-GGATGGGCAGTGCAGATATT-3′ (forward), 5′-CACCTGGGTGGAGAGAGAAG-3′ (reverse); for Blimp1, 5′-CAGCAGTTGCATGATGGTGT-3′ (forward), 5′-AGAGAAATCCAGCCTGCTCA-3′ (reverse); and for CCL2, 5′-CGCTGGAAAGTATGTCAGCA-3′ (forward), 5′-AAATGCATGGGGTT TCTTGA-3′ (reverse).
Micro RNA (miRNA) silencing
MiRNAs were introduced into THP-1 cells using a lentiviral Pol II miR RNAi expression system (Invitrogen Corp., Madison, WI) according to the manufacturer’s protocol. Four miRNAs targeting different areas of the MafB mRNA were tested, and position 2 (cctgctcaagttcgacgtgaa) which caused the greatest reduction in MafB protein expression in RA plus TNFα-treated cells was selected for further experiments. As a negative control we used a miRNA (Invitrogen Corp., Madison, WI) predicted not to target any known vertebrate gene. After transduction, the cells were cultured in complete medium with 5 ng/ml blasticidin for 10 days to eliminate untransfected cells, then the cells were treated with vehicle or with the combination of RA and TNFα as described above for 24 h.
Flow cytometry and phagocytosis assay
For determination of CD11b expression, THP-1 cells were treated with vehicle or with RA and TNFα for 24 h. Then cells were stained with phycoerythrin (PE)-conjugated anti-CD11b antibody (BD Biosciences, San Jose, CA) fixed, and subjected to flow cytometry [8]. To assess phagocytosis, at the end of the treatment time, FITC-labeled E. coli bioparticles (Molecular Probes) were added (E. coli to cell ratio of 10:1) for 2 h at 37°C [8]. The cells were then pelleted, resuspended in 0.25% Trypan Blue dye for 1 min to quench the surface-bound fluorescence, washed twice with PBS, and stained with PE-labeled anti-CD11b, followed by two-color flow cytometric analysis.
Statistical analysis
Results are shown as the mean ± SE of at least 3 experiments each. ANOVA tests were conducted using Prism 5 software (GraphPad Software, La Jolla, CA). A P value less than 0.05 was considered statistically significant.
Results
MafB expression is upregulated synergistically by RA and TNFα
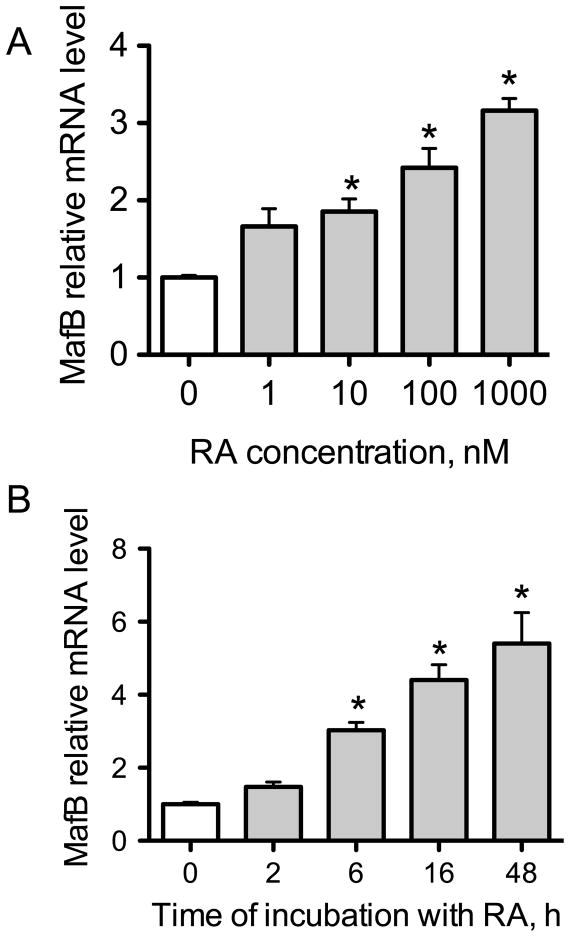
We first tested the effect of RA on MafB expression. After a 6-h incubation, MafB mRNA was increased by RA dose-dependently, with as little as 10 nM RA increasing expression significantly (Fig. 1A). Based on this dose response, we chose 20 nM RA for later treatments. MafB mRNA expression continued to increase with time of incubation with RA, reaching a 6-fold increase after 48 h (Fig. 1B).
Fig. 1. MafB mRNA is upregulated by RA time- and dose-dependently.
(A) Q-PCR analysis of MafB mRNA in THP-1 cells. Cells were treated with different concentrations of RA from 0 to 1000 nM for 6 h. (B) Q-PCR analysis of MafB mRNA. Cells were treated with 20 nM RA for 2, 6, 16, or 48 h. * P < 0.05 compared with vehicle control (0).
TNFα also is known to induce THP-1 cell differentiation [8], and thus we also tested TNFα (5 ng/ml) alone and in combination with RA (20 nM) on MafB mRNA and protein levels. Whereas either agent alone increased MafB mRNA time-dependently after 6 and 24 h, the combination of RA and TNFα was much stronger, resulting in a synergistic increase in MafB expression that was significant by 3 h (Fig. 2A). For MafB protein (Fig. 2B), RA increased the expression moderately while TNFα or RA and TNFα combined resulted in levels much greater than in control cells. Furthermore, lipopolysaccharide (LPS), which is known to induce the production and secretion of TNFα from monocytes [26], also induced MafB expression, and RA also enhanced the LPS-induced expression of MafB, similar to that observed with RA and TNFα (Fig. 2C).
Fig. 2. RA and TNFα synergize in inducing MafB mRNA and protein.
(A) Q-PCR analysis of MafB mRNA in THP-1 cells. Cells were treated with 20 nM RA, or 5 ng/ml TNFα, or both for 0 (control), 1, 3, 6, or 24 h. * P < 0.05 compared with vehicle control; # P < 0.05 compared with RA treatment and TNFα treatment. (B) Immunoblot analysis of MafB protein. Cells were treated with 20 nM RA, or 5 ng/ml TNFα, or both for 6 h. (C) MafB mRNA was induced by RA and LPS. Cells were treated with 20 nM RA or 100 ng/ml of LPS, or both, for 24 h and analyzed as for panel A. * P < 0.05 compared with vehicle control; # P<0.05 compared with either RA or LPS alone.
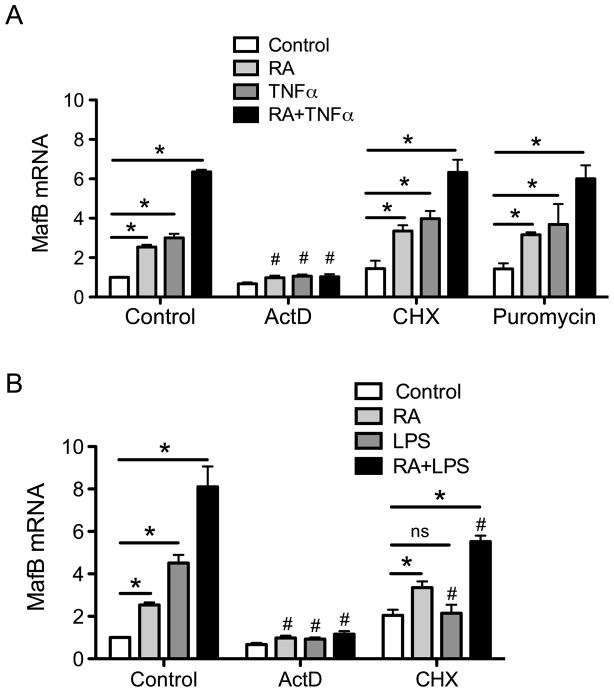
To determine whether RA and TNFα regulate MafB at the level of transcription, THP-1 cells were treated with actinomycin D, an inhibitor of RNA synthesis, or with cycloheximide, an inhibitor of protein synthesis, prior to treatment with RA and TNFα. Actinomycin D completely blocked the increase in MafB mRNA expression, as expected (Fig. 3A). However when the cells were pretreated with cycloheximide, RA and TNFα still increased MafB expression similar to cells without cycloheximide (Fig. 3A). Results from similar cells treated with puromycin instead of cycloheximide supported the results shown for cycloheximide in Fig. 3A. These results indicate that the regulation of MafB is likely by a direct increase in MafB gene expression that does not require new protein factors. It is interesting that, in contrast, the induction of MafB expression by LPS was abolished or attenuated by both actinomycin D and cycloheximide (Fig. 3B); this result further supports the importance of mediators, such as TNFα, in the LPS-induced increase in MafB expression.
Fig. 3. Treatment wth actinomycin D (ActD), cycloheximide (CHX) or puromycin differentially affect the induction of MafB mRNA by RA and TNFα (A) or RA and LPS (B).
In A, THP-1 cells were pretreated with 5 μg/ml of Act or 5 μg/ml of CHX or 5 μg/ml of puromycin for 30 min and then with either vehicle, 20 nM RA, 5 ng/ml TNFα or both for 6 h. In B, the same pretreatments were followed by 20 nM of RA, 100 ng/ml of LPS or both for 6 h. *, P<0.05 compared to control for same pretreatment; #, P<0.05 compared to vehicle-treated cells (comparison among the same color bars).
SPOCK1, CLL2 and Blimp1 are predicted as MafB target genes
To identify MafB target genes, we first sorted genes from our microarray data (see Materials and Methods) that were significantly upregulated by both RA and TNFα, using a value of P < 0.001 as our screening criterion. Then, sequences upstream of the respective transcription start sites of the identified genes were screened for potential MafB binding sites, using the MARE sequences TGCTGACTCAGCA and TGCTGACGTCAGCA described previously [10], and a 5′-AT-rich MARE half-site [27]. Among the 637 genes that were responsive to treatment with RA and TNFα in THP-1 cells, 64 genes were predicted to contain potential binding sites in their promoter regions (Table 1); these genes included SPOCK1, CCL2 and Blimp. We selected these 3 genes for further study because of their potential involvement in several different aspects of monocytic cell differentiation, including cell communication and extracellular matrix functions, SPOCK1, a secreted factor; cell differentiation, Blimp1, a regulatory transcription factor; and response to inflammation and recruitment, CCL2, a well-known chemokine.
Table 1.
RA and TNFα-upregulated genes containing putative MARE in their promoter regions
| REFSEQ | Gene Annotation |
|---|---|
| NM_001561 | Homo sapiens tumor necrosis factor receptor superfamily, member 9 (TNFRSF9) |
| NM_006142 | Homo sapiens stratifin (SFN) |
| NM_020365 | Homo sapiens eukaryotic translation initiation factor 2B, subunit 3 gamma,(EIF2B3) |
| NM_002053 | Homo sapiens guanylate binding protein 1, interferon-inducible, 67kDa (GBP1) |
| NM_004833 | Homo sapiens absent in melanoma 2 (AIM2) |
| NM_012394 | Homo sapiens prefoldin subunit 2 (PFDN2) |
| NM_032174 | Homo sapiens translocase of outer mitochondrial membrane 40 homolog (yeast)-like (TOMM40L), nuclear gene encoding mitochondrial protein, |
| NM_003101 | Homo sapiens sterol O-acyltransferase (acyl-Coenzyme A: cholesterol acyltransferase) 1 (SOAT1) |
| NM_002928 | Homo sapiens regulator of G-protein signalling 16 (RGS16) |
| NM_030769 | Homo sapiens N-acetylneuraminate pyruvate lyase (dihydrodipicolinate synthase) (NPL) |
| NM_003338 | Homo sapiens ubiquitin-conjugating enzyme E2D 1 (UBC4/5 homolog, yeast) (UBE2D1) |
| NM_144590 | Homo sapiens ubiquitin-conjugating enzyme E2D 1 (UBC4/5 homolog, yeast) (UBE2D1) |
| NM_004811 | Homo sapiens leupaxin (LPXN) |
| NM_016582 | Homo sapiens solute carrier family 15, member 3 (SLC15A3) |
| NM_153611 | Homo sapiens cytochrome b, ascorbate dependent 3 (CYBASC3) |
| NM_022003 | Homo sapiens FXYD domain containing ion transport regulator 6 (FXYD6) |
| NM_002223 | Homo sapiens inositol 1,4,5-triphosphate receptor, type 2 (ITPR2) |
| NM_018169 | Homo sapiens chromosome 12 open reading frame 35 (C12orf35) |
| NM_005475 | Homo sapiens SH2B adaptor protein 3 (SH2B3) |
| NM_024738 | Homo sapiens chromosome 12 open reading frame 49 (C12orf49) |
| NM_153218 | Homo sapiens chromosome 13 open reading frame 31 (C13orf31) |
| NM_016150 | Homo sapiens ankyrin repeat and SOCS box-containing 2 (ASB2) |
| NM_018602 | Homo sapiens DnaJ (Hsp40) homolog, subfamily A, member 4 (DNAJA4) |
| NM_004530 | Homo sapiens matrix metallopeptidase 2 (gelatinase A, 72kDa gelatinase, 72kDa type IV collagenase) (MMP2) |
| NM_014732 | Homo sapiens KIAA0513 (KIAA0513) |
| NM_000676 | Homo sapiens adenosine A2b receptor (ADORA2B) |
| NM_018404 | Homo sapiens centaurin, alpha 2 (CENTA2) |
| NM_002982 | Homo sapiens chemokine (C-C motif) ligand 2 (CCL2) |
| NM_005623 | Homo sapiens chemokine (C-C motif) ligand 8 (CCL8) |
| NM_173626 | Homo sapiens solute carrier family 26, member 11 (SLC26A11) |
| NM_006033 | Homo sapiens lipase, endothelial (LIPG) |
| NM_021155 | Homo sapiens CD209 molecule (CD209) |
| NM_145256 | Homo sapiens leucine rich repeat containing 25 (LRRC25), |
| NM_002936 | Homo sapiens ribonuclease H1 (RNASEH1) |
| NM_012249 | Homo sapiens ras homolog gene family, member Q (RHOQ) |
| NM_002664 | Homo sapiens pleckstrin (PLEK) |
| NM_025076 | Homo sapiens UDP-glucuronate decarboxylase 1 (UXS1) |
| NM_020548 | Homo sapiens diazepam binding inhibitor (GABA receptor modulator, acyl-CoA binding protein) (DBI) |
| NM_005168 | Homo sapiens Rho family GTPase 3 (RND3) |
| NM_007115 | Homo sapiens tumor necrosis factor, alpha-induced protein 6 (TNFAIP6) |
| NM_004288 | Homo sapiens cytohesin 1 interacting protein (CYTIP) |
| NM_004591 | Homo sapiens chemokine (C-C motif) ligand 20 (CCL20) |
| NM_005301 | Homo sapiens G protein-coupled receptor 35 (GPR35) |
| NM_001188 | Homo sapiens BCL2-antagonist/killer 1 (BAK1) |
| NM_004994 | Homo sapiens matrix metallopeptidase 9 (gelatinase B, 92kDa gelatinase, 92kDa type IV collagenase) (MMP9) |
| NM_001336 | Homo sapiens cathepsin Z (CTSZ) |
| NM_006395 | Homo sapiens ATG7 autophagy related 7 homolog (S. cerevisiae) (ATG7), transcript variant 1 |
| NM_023067 | Homo sapiens forkhead box L2 (FOXL2) |
| NM_004087 | Homo sapiens discs, large homolog 1 (Drosophila) (DLG1) |
| NM_019027 | Homo sapiens RNA binding motif protein 47 (RBM47) |
| NM_000857 | Homo sapiens guanylate cyclase 1, soluble, beta 3 (GUCY1B3) |
| NM_002185 | Homo sapiens interleukin 7 receptor (IL7R) |
| NM_004598 | Homo sapiens sparc/osteonectin, cwcv and kazal-like domains proteoglycan (testican) 1 (SPOCK1) |
| NM_030939 | Homo sapiens chromosome 6 open reading frame 62 (C6orf62) |
| NM_004556 | Homo sapiens nuclear factor of kappa light polypeptide gene enhancer in B- cells inhibitor, epsilon (NFKBIE) |
| NM_138441 | Homo sapiens chromosome 6 open reading frame 150 (C6orf150) |
| NM_018384 | Homo sapiens GTPase, IMAP family member 5 (GIMAP5) |
| NM_005384 | Homo sapiens nuclear factor, interleukin 3 regulated (NFIL3) |
| NM_145177 | Homo sapiens dehydrogenase/reductase (SDR family) X-linked (DHRSX) |
| NM_004729 | Homo sapiens zinc finger, BED-type containing 1 (ZBED1) |
| NM_002621 | Homo sapiens complement factor properdin (CFP) |
| NM_145177 | Homo sapiens dehydrogenase/reductase (SDR family) X-linked (DHRSX) |
| NM_004729 | Homo sapiens zinc finger, BED-type containing 1 (ZBED1) |
| NM_001198 | Homo sapiens PR domain containing 1, with ZNF domain (PRDM1) |
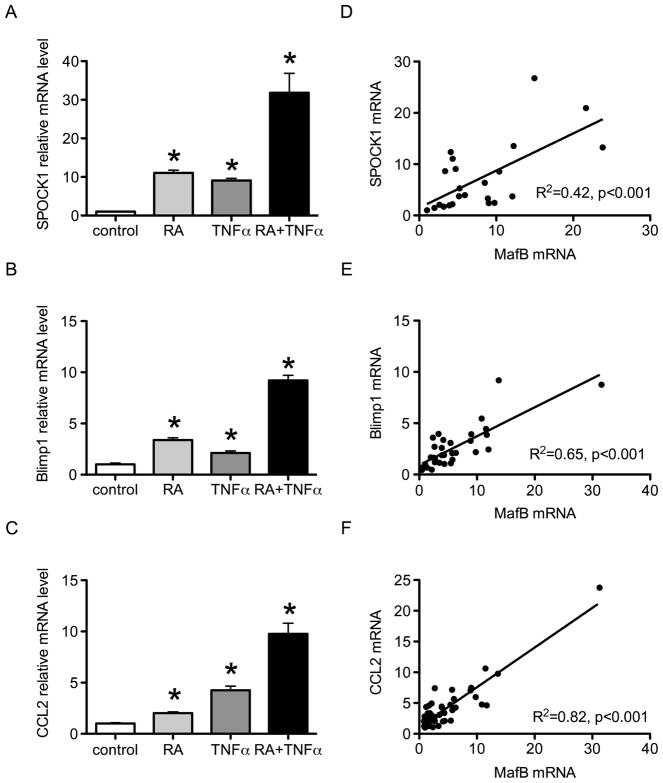
Q-PCR results were first obtained to validate the microarray data for SPOCK1, Blimp1 and CCL2 mRNA. Each of them was significantly increased by RA and TNFα alone (Fig. 4A, B & C), and the effect of the combined treatment was more than additive. Moreover, correlation analysis based on data pooled from multiple experiments showed that SPOCK1, Blimp1 and CCL2 were each highly correlated with MafB mRNA (all P < 0.001, Fig. 4D, E & F, respectively).
Fig. 4. Expression of SPOCK1, Blimp1 and CCL2 are correlated with MafB.
(A–C) Q-PCR analysis of SPOCK1 (A), Blimp1 (B) and CCL2 (C) in THP-1 cells treated with 20 nM RA, or 5 ng/ml TNFα, or both combined for 24 h. * P < 0.05 compared with vehicle control. (D–F) Correlation analysis of SPOCK1 mRNA (D), Blimp1 mRNA (E), and CCL2 mRNA (F) with MafB mRNA. Data were derived over several independent experiments shown in Fig 4A–C and additional analyses.
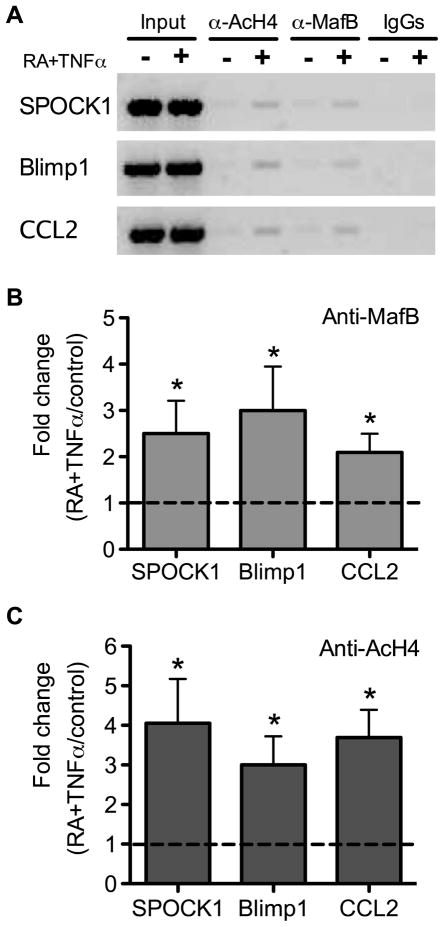
To test directly whether MafB protein occupies the promoter region of these genes, ChIP assays were conducted. The results of these assays showed that the binding of MafB protein to each promoter was detectable and was increased 3–4 times after THP-1 cells were treated with RA plus TNFα, as compared to control cells (value set at 1.00) (Fig. 5A,B), Furthermore, the acetylation of histone H4, which we measured as an indictor of chromatin activation, was also increased 3–4 fold in the MARE-containing regions in THP-1 cells treated with RA plus TNFα (Fig. 5C).
Fig. 5. MafB binding events to the promoter regions of SPOCK1, Blimp1 and CCL2 are enhanced by RA and TNFα.
(A) PCR analysis of DNA after chromatin immunoprecipitation with antibodies to MafB, acetylated histone H4, and IgG as control. Results are shown for SPOCK1, Blimp1, and CLL2 promoter regions after precipitation with anti-MafB antibody (B) and anti-acetylated histone 4 (Anti-AcH4) antibody (C) in ChIP assays. MARE-containing regions were amplified by q-PCR using primers given in Materials and Methods. The Y-axis in B and C represents the fold changes of MafB binding events to MARE-containing regions for cells treated with 20 nM RA plus 5 ng/ml TNFα compared with vehicle-treated control cells (defined as 1.00). Results represented four independent experiments. * P < 0.05 compared to vehicle-treated control cells.
Reduction in MafB down-regulates the expression of the MARE-containing target genes SPOCK1, Blimp1 and CCL2
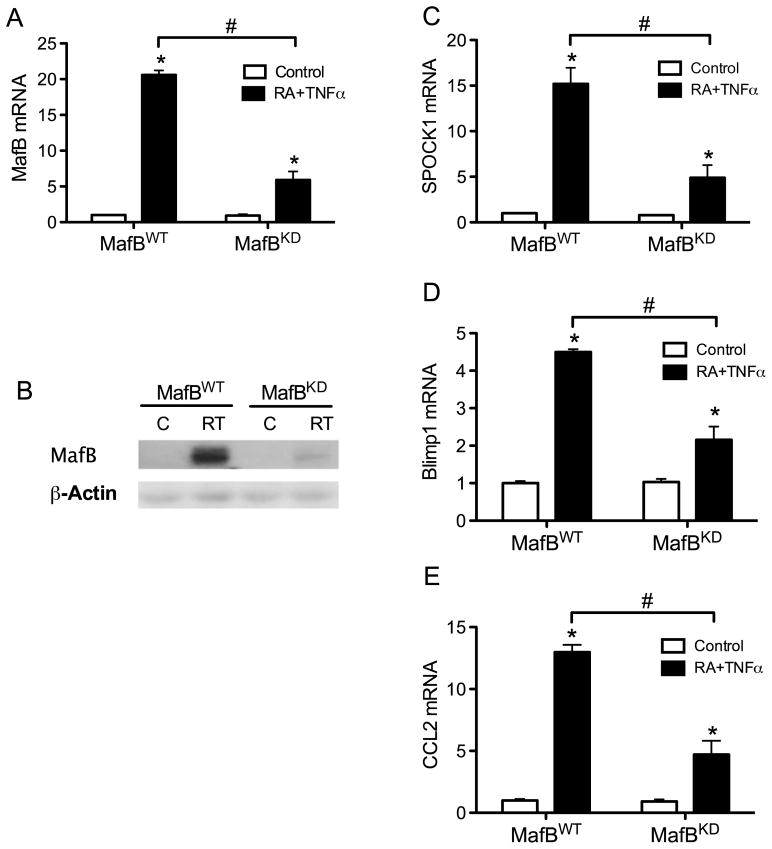
To further determine the requirement of MafB in the expression of these putative target genes, we established a MafB knockdown model by introducing micro RNA (miRNA) into THP-1 cells. A vector-based miRNA construct was packed into lentivirus, and then transduced into the cells. MafB knockdown was verified by testing the levels of MafB mRNA (Fig. 6A) and protein (Fig. 6B). In MafB knockdown (MafBKD) cells, the responsiveness of SPOCK1, Blimp1 and CCL2 to RA and TNFα were all significantly decreased compared with wild-type (MafBWT) cells (Fig. 6C–E), further supporting a regulatory role for MafB in the expression of these factors, which themselves are important in monocytic cell regulation.
Fig. 6. Expression of MafB, SPOCK1, Blimp1 and CCL2 is reduced in MafBKD cells.
MafB knock down (KD) cells were prepared by introducing a specific miRNA targeting MafB mRNA (see Materials and Methods). As a negative control, designated MafBWT, a miRNA that is predicted not to target any known vertebrate gene was used. The MafB knock down was first verified at the mRNA (A) and protein (B) levels. Cells were treated with vehicle or 20 nM RA plus 5 ng/ml TNFα for 24 h. Then SPOCK1 (C), Blimp1 (D), and CCL2 (E) in MafBWT and MafBKD cells were analysis by q-PCR. * P < 0.01 compared with control treatment within each genotype; # P < 0.01 compared between genotypes.
Effect of MafB on RA-induced monocytic differentiation
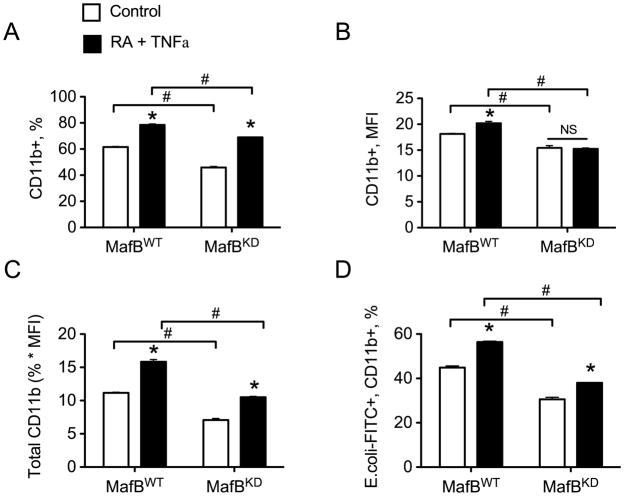
Next we examined THP-1 cell differentiation and functional activity in the MafBKD cells because RA and TNF-α had previously been shown [8] to induce monocyte-macrophage differentiation, determined as increased expression of CD11b and macrophage phagocytic function. To determine the effect of reducing MafB expression on these functions, MafBKD or MafBWT cells were treated with 20 nM RA and 5 ng/ml TNFα for 24 h and then subjected to flow cytometry to determine the CD11b expression level (Fig. 7). The percentage of CD11b+ cells was moderately but significantly reduced in MafBKD cells compared with MafBWT cells; nevertheless both cells responded to RA plus TNFα treatment with an increase in CD11b, observed by the % of positively-staining cells (Fig. 7A). Additionally, the mean fluorescence intensity (MFI) of MafBKD cells was significantly lower than in MafBWT cells when treated with RA plus TNFα (Fig. 7B), indicating fewer CD11b molecules per cell in the presence of RA and TNFα treatment. For this parameter, however, MafBKD cells showed no increase whereas MafBWT cells expressed on average slightly higher amounts of CD11b per cell after treatment (Fig. 7B). Overall, the total CD11b expression (combination of percentage and MFI) in MafBKD cells was reduced approximately 40% compared to control cells (Fig. 7C).
Fig. 7. Differentiation and function are decreased in MafBKD cells.
(A) MafBWT and MafBKD cells treated with vehicle or 20 nM RA plus 5 ng/ml TNFα for 24 h were subjected to flow cytometry to determine the percentage of CD11b positive cells. (B) Mean fluorescence intensity (MFI) of CD11b positive cells. (C) The total CD11b expression was calculated by multiplying the percentage of CD11b-PE positive cells by the MFI. (D) After treatment with RA plus TNFα, MafBWT and MafBKD cells were incubated with FITC-tagged E. coli bioparticles, and then subjected to flow cytometry to analyze cells positive for both CD11b and FITC. * P < 0.01 compared with control treatment within each genotype; # P < 0.01 compared between genotype.
Finally, we assessed phagocytosis, a function which is associated with monocyte maturation, using fluorescein-tagged E. coli bioparticles to detect the uptake of opsonized particles by CD11b-positive cells [8]. The percentage of CD11b+ cells that were also FITC+ positive after incubation was reduced in MafBKD cells compared to MafBWT cells (Fig. 7D); however both cells showed a significant response to RA and TNFα which was about equal in proportion to the basal level in vehicle controls in both MafBWT and MafBKD cells (Fig. 7D). These data suggest that differentiated MafBKD cells are also moderately less phagocytic, although they are still capable of increasing their phagocytic activity in response to stimulation with RA and TNFα.
Discussion
The present study has identified RA and TNFα as regulators of the expression of the transcription factor MafB, and has identified new targets of MafB which may be involved in a variety of monocyte-related functions. The MafB gene appears to be a direct transcriptional target of RA based on its rapid induction by a physiological concentration of RA, which occurred independently of new protein synthesis. TNFα in particular was a strong regulator of MafB protein level, suggesting that MafB expression is regulated both transcriptionally and post-transcriptionally. The same conditions that produced a much higher level of the MafB gene products also led to an increased expression of numerous other genes, suggesting that the response of MafB is part of a program of cell differentiation that can be induced by RA and promoted under inflammatory conditions by TNFα.
No previous studies have identified these agents as regulators of MafB, and thus it was of interest to further explore downstream targets of MafB activity. We therefore first examined the promoter regions of these putative target genes for the presence of MARE sequences, the consensus binding motifs that have been described for large Maf proteins [10, 27]. From our initial microarray data, 64 genes, which were initially identified as being significantly upregulated by RA and TNFα at the P < 0.001 level, were identified to contain potential MafB binding sites in their promoter regions. We therefore narrowed our investigation to focus further on three of them, which we selected due to their putative or known involvement in monocyte-macrophage cell differentiation and/or function -- SPOCK1, Blimp1 and CCL2. SPOCK1 has been reported to have calcium binding affinity and to be involved in cell adhesion [28–30], and RA and TNFα-treated THP-1 cells are noticeably more attached and spread in appearance compared to untreated cells, which grow in suspension. Thus, adhesion appears to be an important part of the differentiation program initiated by RA and TNFα, and SPOCK1 could potentially participate in this process. Blimp1, a transcriptional repressor, has been shown as a trigger for monocytic differentiation [31]. Blimp1 was originally identified as a master regulator of B cell plasmacytic differentiation [32], but it also is involved in several other cell differentiation processes. Over-expression of Blimp1 was shown to drive macrophage differentiation of U937 cells [31]. CCL2/monocyte chemotactic protein-1 (MCP1) is well known as a chemoattractant for monocytes, with roles in cell differentiation and cell trafficking [33]. Furthermore, increased expression of CCL2 in monocytes during their differentiation, associated with its receptors, provides a feedback mechanism in the control of macrophage recruitment and activation [34–35]. In the present study, we showed that, for each of these genes, SPOCK1, Blimp1 and CCL2, expression was increased after treatment of cells with RA and TNFα. Binding events of MafB to each promoter were enhanced by the treatment of RA plus TNFα, and shown by ChIP assays. Moreover, lower responses of THP-1 cells to treatment with RA plus TNFα were observed in MafBKD cells. Together, these results strongly suggest that MafB modulates cell behavior through targeting SPOCK1, Blimp1 and CCL2. Other pathways may also be involved, as suggested by the presence of putative MARE sequences in other genes regulated by RA and TNFα in THP-1 cells (Table 1), which we have not yet explored. Thus MafB and RA- and TNFα-induced MafB levels may have more widespread downstream effects on monocyte biology than has been revealed in our present study.
The mechanism by which RA regulates the MafB gene is still unknown. Our study has provided evidence for direct transcriptional regulation but did not identify its mechanism. RA is a ligand for nuclear receptors RARα, β, and γ, which dimerize with retinoid X receptors (RXRα, β, γ) and bind to specific DNA sites, known as retinoic acid response elements (RARE) [5, 36]. A computational analysis of the MafB promoter did not locate a classical direct repeat DR2 or DR5 motif, however, we note that there are two hexameric motifs separated by 7 nucleotides, which might be RAR/RXR binding sites, since RAR/RXR hererodimers are able to bind to motifs with spacing nucleotides other than 2 or 5 [37]. We also detected a potential NF-KB binding site in the 5′-untranslated region in the MafB gene, which may explain the rapid increase of MafB expression by TNFα. Additionally, both RA and TNFα could potentially influence gene expression and protein production in many ways besides through nuclear receptors. For example, RA and TNFα have been indicated to rapidly induce the MAPK phosphorylation cascade [38–40], increase acetylation of histone H4, and increase the recruitment of polymerase II [25, 41]. The p300 and CBP [cAMP-response-element-binding protein (CREB)-binding protein)] are transcriptional co-activators, and both have intrinsic histone-acetyltransferase activity, and may act directly on chromatin-associated histones to facilitate transcription [42]. Recruitment of CBP/p300 was enhanced by RA and TNFα [41, 43]. Moreover, CBP/p300 protein levels were induced by RA [43]. Thus, the synergistic effect of RA and TNFα identified in this study could potentially be exerted through recruitment of CBP/p300. It seems likely that RA and TNFα may potentially coordinate in several pathways to enhance MafB expression and further studies on this interesting interaction appear warranted.
It is also worthy of note that we tested c-maf, another large Maf family member also involved in monocytic differentiation [44], in THP-1 cells and found that c-maf mRNA was increased 2-fold by RA and TNFα (data not shown), which was much less than for MafB (~20 fold). In our studies using MafBKD cells and MafBWT THP-1 cells, the knockdown of MafB did not affect c-maf expression (data not shown). This suggests that c-maf could act together with but independently of MafB in the process of RA-induced monocytic differentiation and function. Although the reduction in MafB was quite extensive, we did not observe major changes in THP-1 cell differentiation. The reasons for this are not known but could be related to a possible redundancy at the level of function of c-maf, which as noted above was not changed in cells with reduced MafB expression, with MafB. It could also indicate that differentiation requires less MafB than is required for other functions such as maintenance of the genes CCL2, SPOCK1, and Blimp-1 that we investigated in this study. Some of the other genes listed in table 1, such as MMP2 and MMP9, could be involved in cell mobilization by cleaving extracellular matrix. The diversity of functions associated with the genes in this list, including transcription factors, adapter molecules, transporters and enzymes, suggests that MafB could play a broad role in maintaining cellular functions.
To date, the MafB gene has not been deleted in vivo, but an X-ray mutagenesis induced mutation, known as the kreisler mutation, affects MafB expression in rhombomeres 5 and 6 and results in defects in oto-neurogenic development in kr/kr mutant mice [20, 45]. Only relatively recently has MafB been implicated in broader functions including hematopoiesis [18–20] and islet beta-cell maturation [14–15]. Additionally, gene association studies have implicated MafB as potentially involved in other development defects, such as cleft lip [46](--) and in diseases such as tuberculosis [47–48]. MafB has also been described as a type I interferon rheostat [49], through modulation of the transcription activity of IRF-3 [50], which may further link MafB expression to immune responses to viruses and/or other pathogens.
Conclusion
Our studies using RA at a physiological level, similar to that in plasma, and TNFα at a relatively low dose, provide evidence that these agents, individually and especially in combination, positively regulate the levels and activity of MafB, which can then mediate effects through MARE sites on downstream target genes. Thus RA- and TNFα-regulated changes in MafB expression could potentially be important in regulating the morphology, migration characteristics and other functions of monocytic cells. These results add to what is known about MafB in monocytic cells by showing that MafB expression is, itself, highly regulated by the cell’s external environment, and by identifying several new potential targets that can be regulated at least in part by MafB. Thus MafB may play a wider role in monocyte biology than has been previously understood.
Acknowledgments
We thank Dr. Michael Borland for advice on the ChIP assay, the NIH Microarray facility, Frederick MD, and mAdb Support, NIH/CIT, Bethesda, MD, for their helpful assistance. This work was supported by NIH DK41479, an ARRA supplement 3R01DK41479-20S1, CA90214, and a Bioactive Nutrient Gene Expression Omnibus (BANGEO) supplement, CA-90214S.
Footnotes
The abbreviations used are: atRA, all-trans-retinoic acid; miRNA, microRNA; MafBKD, cells with reduced (knocked down) MafB expression; MafBWT, cells with wildtype (control level) MafB expression; ANOVA, analysis of variance; NS, not significantly different.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Semba RD. Vitamin A and immunity to viral, bacterial and protozoan infections. Proc Nutr Soc. 1999;58:719–727. doi: 10.1017/s0029665199000944. [DOI] [PubMed] [Google Scholar]
- 2.Montrone M, Martorelli D, Rosato A, Dolcetti R. Retinoids as critical modulators of immune functions: new therapeutic perspectives for old compounds. Endocr Metab Immune Disord Drug Targets. 2009;9:113–131. doi: 10.2174/187153009788452435. [DOI] [PubMed] [Google Scholar]
- 3.Manicassamy S, Pulendran B. Retinoic acid-dependent regulation of immune responses by dendritic cells and macrophages. Semin Immunol. 2009;21:22–27. doi: 10.1016/j.smim.2008.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fields AL, Soprano DR, Soprano KJ. Retinoids in biological control and cancer. J Cell Biochem. 2007;102:886–898. doi: 10.1002/jcb.21530. [DOI] [PubMed] [Google Scholar]
- 5.Bastien J, Rochette-Egly C. Nuclear retinoid receptors and the transcription of retinoid-target genes. Gene. 2004;328:1–16. doi: 10.1016/j.gene.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 6.Balmer JE, Blomhoff R. Gene expression regulation by retinoic acid. J Lipid Res. 2002;43:1773–1808. doi: 10.1194/jlr.r100015-jlr200. [DOI] [PubMed] [Google Scholar]
- 7.Tsuchiya S, Yamabe M, Yamaguchi Y, Kobayashi Y, Konno T, Tada K. Establishment and characterization of a human acute monocytic leukemia cell line (THP-1) Int J Cancer. 1980;26:171–176. doi: 10.1002/ijc.2910260208. [DOI] [PubMed] [Google Scholar]
- 8.Chen Q, Ross AC. Retinoic acid regulates cell cycle progression and cell differentiation in human monocytic THP-1 cells. Exp Cell Res. 2004;297:68–81. doi: 10.1016/j.yexcr.2004.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kronenberg HM. Developmental regulation of the growth plate. Nature. 2003;423:332–336. doi: 10.1038/nature01657. [DOI] [PubMed] [Google Scholar]
- 10.Motohashi H, Shavit JA, Igarashi K, Yamamoto M, Engel JD. The world according to Maf. Nucleic Acids Res. 1997;25:2953–2959. doi: 10.1093/nar/25.15.2953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maves L, Kimmel CB. Dynamic and sequential patterning of the zebrafish posterior hindbrain by retinoic acid. Dev Biol. 2005;285:593–605. doi: 10.1016/j.ydbio.2005.07.015. [DOI] [PubMed] [Google Scholar]
- 12.Hernandez RE, Rikhof HA, Bachmann R, Moens CB. vhnf1 integrates global RA patterning and local FGF signals to direct posterior hindbrain development in zebrafish. Development. 2004;131:4511–4520. doi: 10.1242/dev.01297. [DOI] [PubMed] [Google Scholar]
- 13.Grapin-Botton A, Bonnin MA, Sieweke M, Le Douarin NM. Defined concentrations of a posteriorizing signal are critical for MafB/Kreisler segmental expression in the hindbrain. Development. 1998;125:1173–1181. doi: 10.1242/dev.125.7.1173. [DOI] [PubMed] [Google Scholar]
- 14.Kataoka K, Shioda S, Ando K, Sakagami K, Handa H, Yasuda K. Differentially expressed Maf family transcription factors, c-Maf and MafA, activate glucagon and insulin gene expression in pancreatic islet alpha- and beta-cells. J Mol Endocrinol. 2004;32:9–20. doi: 10.1677/jme.0.0320009. [DOI] [PubMed] [Google Scholar]
- 15.Matsuoka TA, Zhao L, Artner I, Jarrett HW, Friedman D, Means A, Stein R. Members of the large Maf transcription family regulate insulin gene transcription in islet beta cells. Mol Cell Biol. 2003;23:6049–6062. doi: 10.1128/MCB.23.17.6049-6062.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Artner I, Blanchi B, Raum JC, Guo M, Kaneko T, Cordes S, Sieweke M, Stein R. MafB is required for islet beta cell maturation. Proc Natl Acad Sci U S A. 2007;104:3853–3858. doi: 10.1073/pnas.0700013104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Artner I, Blanchi B, Raum JC, Guo M, Kaneko T, Cordes S, Sieweke M, Stein R. MafB is required for islet beta cell maturation. Proc Natl Acad Sci U S A. 2007;104:3853–3858. doi: 10.1073/pnas.0700013104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kelly LM, Englmeier U, Lafon I, Sieweke MH, Graf T. MafB is an inducer of monocytic differentiation. Embo J. 2000;19:1987–1997. doi: 10.1093/emboj/19.9.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bakri Y, Sarrazin S, Mayer UP, Tillmanns S, Nerlov C, Boned A, Sieweke MH. Balance of MafB and PU.1 specifies alternative macrophage or dendritic cell fate. Blood. 2005;105:2707–2716. doi: 10.1182/blood-2004-04-1448. [DOI] [PubMed] [Google Scholar]
- 20.Eichmann A, Grapin-Botton A, Kelly L, Graf T, Le Douarin NM, Sieweke M. The expression pattern of the mafB/kr gene in birds and mice reveals that the kreisler phenotype does not represent a null mutant. Mech Dev. 1997;65:111–122. doi: 10.1016/s0925-4773(97)00063-4. [DOI] [PubMed] [Google Scholar]
- 21.Gemelli C, Montanari M, Tenedini E, Zanocco Marani T, Vignudelli T, Siena M, Zini R, Salati S, Tagliafico E, Manfredini R, Grande A, Ferrari S. Virally mediated MafB transduction induces the monocyte commitment of human CD34+ hematopoietic stem/progenitor cells. Cell Death Differ. 2006;13:1686–1696. doi: 10.1038/sj.cdd.4401860. [DOI] [PubMed] [Google Scholar]
- 22.Winston BW, Krein PM, Mowat C, Huang Y. Cytokine-induced macrophage differentiation: a tale of 2 genes. Clin Invest Med. 1999;22:236–255. [PubMed] [Google Scholar]
- 23.Kremlev SG, Phelps DS. Effect of SP-A and surfactant lipids on expression of cell surface markers in the THP-1 monocytic cell line. Am J Physiol. 1997;272:L1070–1077. doi: 10.1152/ajplung.1997.272.6.L1070. [DOI] [PubMed] [Google Scholar]
- 24.Pai T, Chen Q, Zhang Y, Zolfaghari R, Ross AC. Galactomutarotase and other galactose-related genes are rapidly induced by retinoic acid in human myeloid cells. Biochemistry. 2007;46:15198–15207. doi: 10.1021/bi701891t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang Y, Zolfaghari R, Ross AC. Multiple retinoic acid response elements cooperate to enhance the inducibility of CYP26A1 gene expression in liver. Gene. 2010;464:32–43. doi: 10.1016/j.gene.2010.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.White B, Schmidt M, Murphy C, Livingstone W, O’Toole D, Lawler M, O’Neill L, Kelleher D, Schwarz HP, Smith OP. Activated protein C inhibits lipopolysaccharide-induced nuclear translocation of nuclear factor kappaB (NF-kappaB) and tumour necrosis factor alpha (TNF-alpha) production in the THP-1 monocytic cell line. Br J Haematol. 2000;110:130–134. doi: 10.1046/j.1365-2141.2000.02128.x. [DOI] [PubMed] [Google Scholar]
- 27.Yoshida T, Ohkumo T, Ishibashi S, Yasuda K. The 5′-AT-rich half-site of Maf recognition element: a functional target for bZIP transcription factor Maf. Nucleic Acids Res. 2005;33:3465–3478. doi: 10.1093/nar/gki653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bocock JP, Edgell CJ, Marr HS, Erickson AH. Human proteoglycan testican-1 inhibits the lysosomal cysteine protease cathepsin L. Eur J Biochem. 2003;270:4008–4015. doi: 10.1046/j.1432-1033.2003.03789.x. [DOI] [PubMed] [Google Scholar]
- 29.Hausser HJ, Decking R, Brenner RE. Testican-1, an inhibitor of pro-MMP-2 activation, is expressed in cartilage. Osteoarthritis Cartilage. 2004;12:870–877. doi: 10.1016/j.joca.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 30.Kohfeldt E, Maurer P, Vannahme C, Timpl R. Properties of the extracellular calcium binding module of the proteoglycan testican. FEBS Lett. 1997;414:557–561. doi: 10.1016/s0014-5793(97)01070-3. [DOI] [PubMed] [Google Scholar]
- 31.Chang DH, Angelin-Duclos C, Calame K. BLIMP-1: trigger for differentiation of myeloid lineage. Nat Immunol. 2000;1:169–176. doi: 10.1038/77861. [DOI] [PubMed] [Google Scholar]
- 32.Turner CA, Jr, Mack DH, Davis MM. Blimp-1, a novel zinc finger-containing protein that can drive the maturation of B lymphocytes into immunoglobulin-secreting cells. Cell. 1994;77:297–306. doi: 10.1016/0092-8674(94)90321-2. [DOI] [PubMed] [Google Scholar]
- 33.Serbina NV, Jia T, Hohl TM, Pamer EG. Monocyte-mediated defense against microbial pathogens. Annu Rev Immunol. 2008;26:421–452. doi: 10.1146/annurev.immunol.26.021607.090326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gruss HJ, Brach MA, Schumann RR, Herrmann F. Regulation of MCP-1/JE gene expression during monocytic differentiation. J Immunol. 1994;153:4907–4914. [PubMed] [Google Scholar]
- 35.Fantuzzi L, Borghi P, Ciolli V, Pavlakis G, Belardelli F, Gessani S. Loss of CCR2 expression and functional response to monocyte chemotactic protein (MCP-1) during the differentiation of human monocytes: role of secreted MCP-1 in the regulation of the chemotactic response. Blood. 1999;94:875–883. [PubMed] [Google Scholar]
- 36.Wei LN. Retinoid receptors and their coregulators. Annu Rev Pharmacol Toxicol. 2003;43:47–72. doi: 10.1146/annurev.pharmtox.43.100901.140301. [DOI] [PubMed] [Google Scholar]
- 37.Wang L, Tankersley LR, Tang M, Potter JJ, Mezey E. Regulation of the murine alpha(2)(I) collagen promoter by retinoic acid and retinoid X receptors. Arch Biochem Biophys. 2002;401:262–270. doi: 10.1016/S0003-9861(02)00058-9. [DOI] [PubMed] [Google Scholar]
- 38.Bruck N, Vitoux D, Ferry C, Duong V, Bauer A, de The H, Rochette-Egly C. A coordinated phosphorylation cascade initiated by p38MAPK/MSK1 directs RARalpha to target promoters. Embo J. 2009;28:34–47. doi: 10.1038/emboj.2008.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gianni M, Parrella E, Raska I, Jr, Gaillard E, Nigro EA, Gaudon C, Garattini E, Rochette-Egly C. P38MAPK-dependent phosphorylation and degradation of SRC-3/AIB1 and RARalpha-mediated transcription. Embo J. 2006;25:739–751. doi: 10.1038/sj.emboj.7600981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aggarwal BB. Signalling pathways of the TNF superfamily: a double-edged sword. Nat Rev Immunol. 2003;3:745–756. doi: 10.1038/nri1184. [DOI] [PubMed] [Google Scholar]
- 41.Clarke DL, Clifford RL, Jindarat S, Proud D, Pang L, Belvisi M, Knox AJ. TNFalpha and IFNgamma synergistically enhance transcriptional activation of CXCL10 in human airway smooth muscle cells via STAT-1, NF-kappaB, and the transcriptional coactivator CREB-binding protein. J Biol Chem. 2010;285:29101–29110. doi: 10.1074/jbc.M109.0999952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chan HM, La Thangue NB. p300/CBP proteins: HATs for transcriptional bridges and scaffolds. J Cell Sci. 2001;114:2363–2373. doi: 10.1242/jcs.114.13.2363. [DOI] [PubMed] [Google Scholar]
- 43.Dietze EC, Troch MM, Bowie ML, Yee L, Bean GR, Seewaldt VL. CBP/p300 induction is required for retinoic acid sensitivity in human mammary cells. Biochem Biophys Res Commun. 2003;302:841–848. doi: 10.1016/s0006-291x(03)00266-3. [DOI] [PubMed] [Google Scholar]
- 44.Hegde SP, Zhao J, Ashmun RA, Shapiro LH. c-Maf induces monocytic differentiation and apoptosis in bipotent myeloid progenitors. Blood. 1999;94:1578–1589. [PubMed] [Google Scholar]
- 45.Vázquez-Echeverría C, Dominguez-Frutos E, Charnay P, Schimmang T, Pujades C. Analysis of mouse kreisler mutants reveals new roles of hindbrain-derived signals inthe establishment of the otic neurogenic domain. Develop Biol. 2008;322:167–176. doi: 10.1016/j.ydbio.2008.07.025. [DOI] [PubMed] [Google Scholar]
- 46.Beaty TH, Murray JC, Marazita ML, Munger RG, Ruczinski I, Hetmanski JB, Liang KY, Wu T, Murray T, Fallin MD, Redett RA, Raymond G, Schwender H, Jin SC, Cooper ME, Dunnwald M, Mansilla MA, Leslie E, Bullard S, Lidral AC, Moreno LM, Menezes R, Vieira AR, Petrin A, Wilcox AJ, Lie RT, Jabs EW, Wu-Chou YH, Chen PK, Wang H, Ye X, Huang S, Yeow V, Chong SS, Jee SH, Shi B, Christensen K, Melbye M, Doheny KF, Pugh EW, Ling H, Castilla EE, Czeizel AE, Ma L, Field LL, Brody L, Pangilinan F, Mills JL, Molloy AM, Kirke PN, Scott JM, Arcos-Burgos M, Scott AF. A genome-wide association study of cleft lip with and without cleft palate identifies risk variants near MAFB and ABCA4. Nat Genet. 2010;42:525–529. doi: 10.1038/ng.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mahasirimongkol S, Yanai H, Mushiroda T, Promphittayarat W, Wattanapokayakit S, Phromjai J, Yuliwulandari R, Wichukchinda N, Yowang A, Yamada N, Kantipong P, Takahashi A, Kubo M, Sawanpanyalert P, Kamatani N, Nakamura Y, Tokunaga K. Genome-wide association studies of tuberculosis in Asians identify distinct at-risk locus for young tuberculosis. J Hum Genet. 2012 May 3; doi: 10.1038/jhg.2012.35. [DOI] [PubMed] [Google Scholar]
- 48.Berry MP, Graham CM, McNab FW, Xu Z, Bloch SA, Oni T, Wilkinson KA, Banchereau R, Skinner J, Wilkinson RJ, Quinn C, Blankenship D, Dhawan R, Cush JJ, Mejias A, Ramilo O, Kon OM, Pascual V, Banchereau J, Chaussabel D, O’Garra A. An interferon-inducible neutrophil-driven blood transcriptional signature in human tuberculosis. Nature. 2010;466:973–977. doi: 10.1038/nature09247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Motohashi H, Igarashi K. MafB as a type I interferon rheostat. Nat Immunol. 2010;11:695–696. doi: 10.1038/ni0810-695. [DOI] [PubMed] [Google Scholar]
- 50.Kim H, Seed B. The transcription factor MafB antagonizes antiviral responses by blocking recruitment of coactivators to the transcription factor IRF3. Nat Immunol. 2010;11:743–750. doi: 10.1038/ni.1897. [DOI] [PMC free article] [PubMed] [Google Scholar]