Abstract
Zebrafish form shoals in nature and in the laboratory. The sight of conspecifics has been found reinforcing in zebrafish learning tasks. However, the mechanisms of shoaling, and those of its reinforcing properties, are not known. The dopaminergic system has been implicated in reward among other functions and it is also engaged by drugs of abuse as shown in a variety of vertebrates including zebrafish. The ontogenetic changes in dopamine levels and, to a lesser degree, in serotonin levels, have been found to accompany the maturation of shoaling in zebrafish. Thus, we hypothesized that the dopaminergic system may contribute to shoaling in zebrafish. To test this we employed a D1-receptor antagonist and quantified behavioral responses of our subjects using a social preference (shoaling) paradigm. We found significant reduction of social preference induced by the D1-R antagonist, SCH23390, in the AB strain of zebrafish, an alteration that was not accompanied by changes in motor function or vision. We also detected D1-R antagonist induced changes in the level of dopamine, DOPAC, serotonin and 5HIAA, respectively, in the brain of AB zebrafish as quantified by HPLC with electrochemical detection. We found the antagonist induced behavioral changes to be absent and the levels of these neurochemicals to be lower in another zebrafish population, SF, demonstrating naturally occurring genetic variability in these traits. We conclude that this variability may be utilized to unravel the mechanisms of social behavior in zebrafish, a line of research that may be extended to other vertebrates including our own species.
Keywords: dopamine, serotonin, shoaling, social behavior, strain differences, zebrafish
INTRODUCTION
There are several human brain disorders associated with abnormal social behavior including the autism spectrum disorders (e.g. Schreibman, 1988; Tager-Flusberg, 2010) and schizophrenia (e.g. Bromley & Brekke, 2010; Kalkstein et al., 2010) in which the dopaminergic system, e.g. the role of the dopamine D1 subtype receptor (D1-R), has been implicated (Hettinger et al., 2008; Verhoeff, 1999; Emilien et al., 1999). However, due to limited understanding of the mechanisms of social behavior and of these diseases, efficient treatment has not been developed. Animal models may facilitate the analysis of these mechanisms and thus, ultimately, may lead to betterment of human health.
The zebrafish, successfully employed in embryology and genetics (Grunwald and Eisen, 2002), has been gaining popularity in behavioral neuroscience (Sison et al., 2006; Gerlai, 2010). It represents an optimal compromise between system complexity and practical simplicity. It is a small and prolific vertebrate with a brain whose anatomical layout, physiology, and neurochemistry are similar to those of other vertebrates (Tropepe and Sive, 2003; Alsop and Vijayan, 2008; Chatterjee and Gerlai, 2009). The nucleotide sequence of its genes and the amino acid sequence of its proteins are homologous (reaching 80–90% sequence homology in some cases) to those of mammalian counterparts (e.g. Reimers et al., 2004; Renier et al., 2007).
Zebrafish is a social species. In nature as well as in the laboratory zebrafish form groups in which individual members swim close to each other, a behavior termed shoaling. The distance individual shoal members keep between each other has been thoroughly investigated and notable temporal changes in this measure of shoaling have been identified. On a short time scale Miller and Gerlai (2008) revealed dynamic oscillation of shoal cohesion with a cycle length of 5–10 sec. On a long time scale Buske and Gerlai (2011a) found a quasi linear strengthening of shoal cohesion from 7 days post fertilization to adulthood. Although the neurobiological mechanisms subserving shoaling, and social behavior in general, have not been identified in zebrafish, the analysis of these mechanisms has been started. For example, the levels of neurochemicals dopamine and DOPAC have been shown to increase during ontogenesis of zebrafish (Buske and Gerlai, 2011b). Serotonin and its metabolite, 5HIAA, have also been found to exhibit agedependent increase in their levels, albeit unlike for dopamine and DOPAC, the trajectory has been found to be step-wise, which correlated less well with the ontogenesis of shoaling.
Notably, the sight of conspecifics has been found rewarding for zebrafish (Al-Imari and Gerlai, 2008; Sison and Gerlai, 2011). The dopaminergic system has been known to subserve reward, among other functions (Wise and Rompre, 1989; Bromberg-Martin et al., 2010) and thus may also be involved in shoaling. Also importantly, alcohol (Gerlai et al., 2009) and cocaine (López-Patiño et al., 2008) have both been shown to engage the dopaminergic system in zebrafish and have also been shown to alter shoaling responses (e.g. Gerlai et al., 2009). Shoaling in zebrafish, and social behavior in general, is likely to be dependent upon a large number of mechanisms. Nevertheless, the above findings implicate the involvement of the dopaminergic system in social behavior in zebrafish, a hypothesis that we aim to test in the current paper.
To investigate the role of the dopaminergic system in social behavior of zebrafish we employed a D1 dopamine receptor antagonist (SCH23390) and analyzed its effects on behavior as quantified using a social preference paradigm (e.g. Gerlai et al., 2009). This drug was chosen because D1-R, the target of SCH23390, is one of the most abundantly expressed dopamine receptor subtypes in the zebrafish brain (Li et al., 2007). Furthermore, SCH23390 has been thoroughly investigated for its psychopharmacological properties in mammals (e.g. Cameron & Williams, 1993; Darbin et al., 2010; Steketee, 1998; Kurata & Shibata, 1991). Last, this drug is water soluble and thus it could be administered to zebrafish by simple immersion of the subject in the drug solution.
The sight of conspecifics, including that of a synthetic shoal (animated images of zebrafish), has previously been found sufficiently rewarding to support associative learning and to elicit attraction and approach in zebrafish (Al-Imari and Gerlai, 2008; Fernandes and Gerlai, 2009). To investigate if this attraction and approach response is modified by D1-R antagonism, we presented our test subjects with animated (moving) images of conspecific fish on a computer monitor and measured the response of the subject.
Dopamine D1 receptors (D1-R’s) are known to mediate a major component of dopaminergic neurotransmission in mammals and are involved in reward and reinforcement, among other functions (Wise and Rompre, 1989; Bromberg-Martin et al., 2010). D1-R’s, highly homologous to the mammalian counterparts, have been identified in zebrafish (Li et al., 2007). Given the high nucleotide sequence homology between the mammalian and the zebrafish genes encoding D1-R, we expect the amino-acid sequence and thus the structural and functional characteristics of these mammalian and zebrafish receptors to be similar too. Consequently, we also expect D1-R specific antagonists developed using mammalian systems to also work in the zebrafish. This expectation is supported by a study in which the D1-R selective antagonist, SCH23390 has been successfully employed in gold fish (Mora-Ferrer & Gangluff, 2002), a cyprinid closely related to zebrafish. In summary, we employed this D1-R antagonist and hypothesized that it would diminish social behavior in zebrafish.
As a follow up analysis after the examination of the behavioral effects of the administration of the D1-R antagonist, we also quantified the levels of dopamine, its metabolite DOPAC, as well as serotonin and its metabolite 5HIAA from whole brain extracts of zebrafish treated with the antagonist. Neurochemical and psychopharmacological analysis of the adult zebrafish is relatively new (e.g. Gerlai e al., 2009; López-Patiño et al., 2008 and references therein). Furthermore, D1-R has been shown to exhibit a complex subcellular localization (has been found pre-, extra- as well as post-synaptically) and region specific expression in the mammalian brain (Cameron & Williams, 1993; Yung et al., 1995; Caillé et al., 1996). Therefore, the effects of the pharmacological treatment on neurochemical responses in zebrafish are difficult to predict. For example, antagonism of pre-synaptic or extra-synaptic D1-R autoreceptors (Cameron & Williams, 1993) may lead to increased dopamine production and release, whereas antagonism of a post-synaptically localized D1-R (Yung et al., 1995) may result in the opposite, i.e. decreased downstream signaling leading to reduced neurotransmitter production and release. Although both of these outcomes are possible, antagonism of D1-R using the compound we employed here, i.e. SCH23390, has been shown to reduce dopamine (Steketee, 1998; Kurata & Shibata, 1991) as well as serotonin release (Darbin et al., 2010) in mammals. It is also important to note that the level of neurotransmitter production has been shown to correlate with the level of their metabolites (Diop et al., 1988). Although the latter four studies were conducted with mammals, based on their results one may suggest that SCH23390 could act similarly in zebrafish, i.e. could reduce levels of dopamine, DOPAC (one metabolite of dopamine), serotonin and 5HIAA (a metabolite of serotonin), respectively.
Previously, behavioral and neurochemical differences (in dopamine, DOPAC, serotonin and 5HIAA) have been demonstrated between two genetically distinct zebrafish populations, AB and SF, in response to alcohol treatment (Gerlai et al., 2009). Our second goal with the current study was to investigate whether such differences exist in response to the D1-R antagonist in social behavioral and neurochemical responses in these populations of zebrafish. Our hope is that discovery of such population differences will lay the foundation of research and will lead to better understanding of the mechanisms of social behavior of vertebrates.
METHODS
Animals and Housing
The details of methods of zebrafish breeding and maintenance have been published elsewhere (Sison and Gerlai, 2011). Briefly, males and females were allowed to spawn naturally in breeding tanks. After spawning the parents were removed and their eggs were kept in the tank. Five day-post-fertilization (dpf) old larvae were transferred to a zebrafish nursery where they received artificial plankton at least three times a day (100 µm particle size, ZeiglerBros, Inc., Gardners, PA, USA). From the age of 10 dpf, the developing fish were fed nauplii of brine shrimp (Artemia salina), and from the age of 15 dpf they also received a 50–50 % mixture of Tetramin Tropical fish flake food (Tetra Co, Melle, Germany) and spirulina (Jehmco Inc., Lambertville, NJ, USA). After reaching the age of 15 dpf, fish were moved to their permanent holding tanks (10–15 fish per 3 liter system rack tank), which were connected to a recirculating filtration system. This system had a mechanical, biological, and activated carbon filter and a UV sterilizing unit (system rack by Aquaneering Inc., San Diego, Ca, USA). The system water (maintained at 27°C) was reverse osmosis purified and was supplemented with 60mg/l Instant Ocean Sea Salt. Light cycle was 12h light/12h dark with lights on at 7:00 h.
Zebrafish of the AB and short-fin wild-type (SF) populations were tested at their age of 4–6 months (sexually mature young adults) in all experiments. We refer to these populations as “strains” although SF, a heterogeneous stock, has not been genetically characterized. The SF population originates from founders we obtained at a local pet store (Big Al’s Aquarium Warehouse Inc., Mississauga, Ontario, Canada) one year before the start of the experiments (i.e. 2 generations of laboratory breeding). The AB strain was established about four decades ago (approximately 60–70 generations of laboratory breeding). Individuals of the two strains look identical and exhibit a wild type phenotype in their appearance. However, SF fish are expected to possess high degree of heterozygosity at the individual level and substantial genetic variance at the population level. SF fish may show species-typical features as they originate from breeding facilities in Singapore where the effective population size is large (Big Al’s Aquarium Warehouse Inc., Mississauga, ON, Canada, personal communication). On the other hand, AB, due to the decades of laboratory breeding, is expected to have experienced genetic drift, i.e. random fixation of alleles. Briefly, AB fish may have peculiar, strain-specific features. All experimental fish were bred, raised and maintained in the same fish room under the identical conditions as described before (Sison and Gerlai, 2011).
Experimental design and drug exposure procedure
We employed a 2 × 3 factorial between-subject experimental design with which we investigated the effect of strain origin (2 levels: AB or SF) and the effect of the D1-receptor antagonist SCH23390 (R-(+)-8-chloro-2,3,4,5-tetrahydro-3-methyl-5-phenyl-1H-3-benzazepine-7- ol; Cat # D054; Sigma-Aldrich, Oakville, ON, Canada; the 3 doses employed were 0.0, 0.1 and 1.0 mg/liter external bath concentration).
Fish were randomly assigned to their respective dose group. The sex ratio in both strains were found to be 50:50% and thus equal number of males and females were used throughout this study. Sex differences were found non-significant. Therefore, the data for sexes were pooled. Fish were placed in drug exposure beakers (300 ml in volume). The beakers were equipped with air-stones connected via rubber tubing to air compressors, which facilitated proper oxygenation of water and homogeneous distribution of the drug. Fish remained in the respective D1-Receptor antagonist solution for 30 min. The order of the fish was randomized according to strain origin and drug concentration. SCH23390 is a water soluble substance and fish absorb it through the vasculature of their gills and skin. Behavioral recording commenced 1h after the exposure ended. Receptor occupancy, drug absorption, distribution, metabolism and excretion (ADME) studies have not been carried out in zebrafish for this particular drug, but based upon the mammalian literature (e.g. Cameron & Williams, 1993; Darbin et al., 2010; Steketee, 1998; Kurata & Shibata, 1991 and references therein) as well as on our own pilot behavioral studies, we found the 30 min exposure period to be sufficiently long for the drug to reach the zebrafish brain and exert behavioral effects. We also found the one hour long acclimation period to be sufficient for the fish to recover from any potential negative (fear inducing) consequences of the dosing procedure but not too long for the drug to wash out. After the acclimation period, zebrafish were behaviorally tested. The order of behavioral testing followed the random order of the drug dosing.
Behavioral test procedure
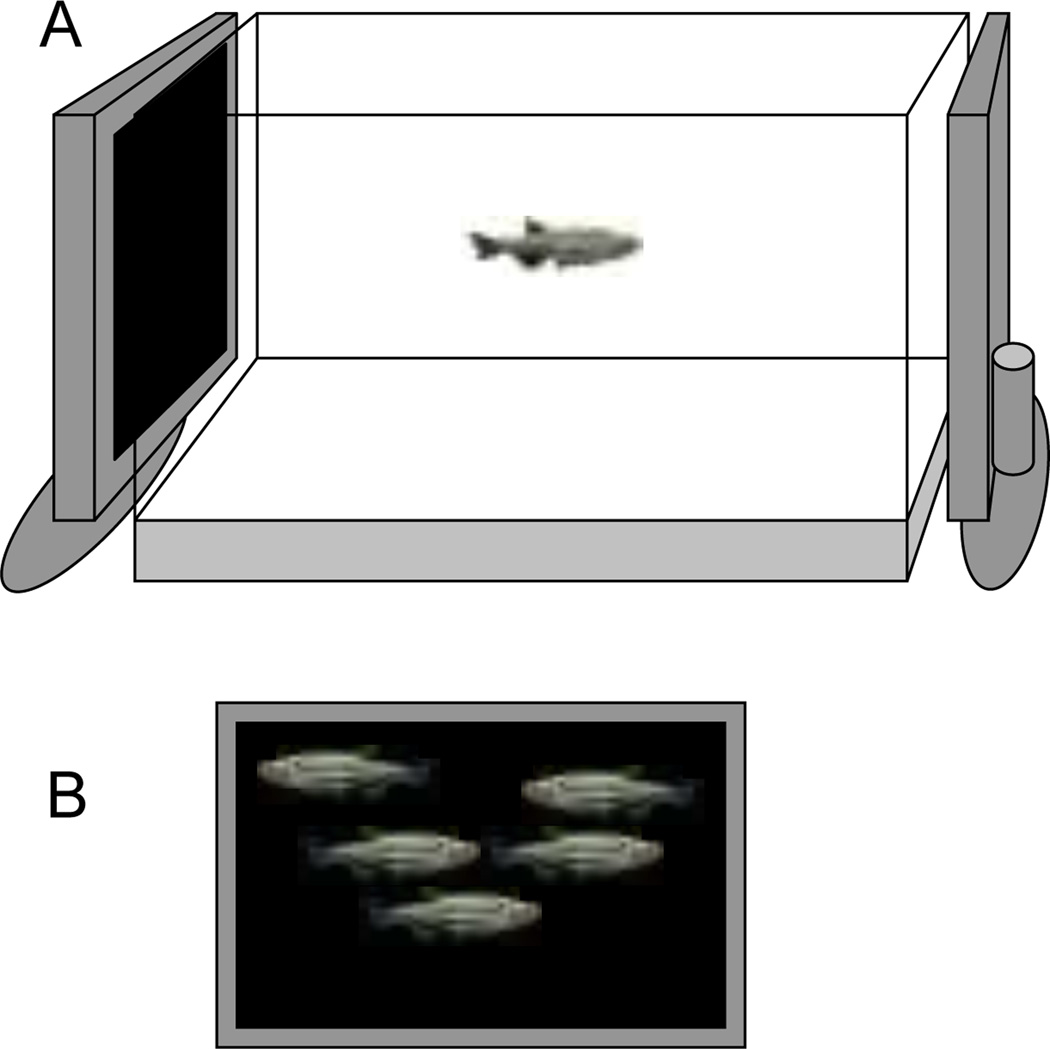
The experimental paradigm was based upon methods described previously (Fernandes and Gerlai, 2009). Briefly, the experimental fish were placed singly into a 37 liter test tank (50cm × 25 cm × 30cm, length × width × height) which had a flat LCD computer monitor (17 inch Samsung SyncMaster 732N monitor) on its left and right side (Fig. 1A). Computer animated images could be displayed on these screens (Saverino and Gerlai, 2008; Fernandes and Gerlai, 2009; also see Fig. 1B). The behavior of experimental fish was recorded onto the hard drive of a camcorder (JVC GZ -MG37u and GZ-MG50) viewing the tank from the front. The digital recordings were later replayed and analyzed using the Ethovision Color Pro Videotracking software application (10 video-frames per second sampling rate, Version 3, Noldus Info Tech, Wageningen, The Netherlands).
Figure 1.
The test apparatus consisted of a 37 liter tank with an LCD computer monitor on each of the two sides (A). The monitor could present animated (moving) images of conspecifics (zebrafish) of identical size and appearance to the experimental subject (B).
We presented animated images of female zebrafish, a “synthetic shoal”, on a black background on the LCD monitor. The rationale for choosing females for this purpose was that females are uniformly preferred by both male and female zebrafish whereas males are only preferred by females (a point discussed in more detail by Fernandes and Gerlai, 2009). A custom software application (first employed by Saverino and Gerlai, 2008) allowed us to set the speed (1–4 cm/sec), location (entire screen area), and the number of moving fish images presented (5 in this case). The sequence of stimulus presentation was as follows: 8 min no stimulus (acclimation period) and 8 min zebrafish shoal image presentation. The stimulus was presented only on one side for each experimental fish but the side of presentation changed randomly across experimental subjects.
The dopaminergic system has been implicated in motivation/reward, motor function and vision as well (Young & Penney, 1984; Bromberg-Martin et al., 2010; Witkovsky, 2004). D1-R antagonism may affect any or all of these functions. We chose behavioral parameters that may be sensitive to these functions. The following three swim path parameters were quantified: 1, distance to stimulus screen (expressed in cm), whose value is expected to decrease when socially attractive stimuli are being shown; 2, distance swum (expressed in cm), a measure of activity which has also been found to decrease in response to the shoal image (Fernandes & Gerlai, 2009); 3, approach (the distance weighted horizontal component of position change, from one video-frame to the next, of the subject relative to the stimulus screen expressed as cm/frame (Noldus Information Technology, EthoVision Reference Manual Version 3, 2004; also see Spruijt et al., 1992). This latter parameter essentially weighs the movement towards the target with the distance of the subject from the target. When the subject is close to the target and is moving towards it, the response is weighed more than when the subject makes the same movement but farther away from the target. The value of this measure is expected to increase in response to social stimuli in zebrafish similarly to what has been shown in rodents (Spruijt et al., 1992).
Quantification of neurochemicals using HPLC
We quantified the level of dopamine, its metabolite DOPAC (3,4-Dihydroxyphenylacetic acid), serotonin and its metabolite 5HIAA (5-Hydroxyindoleacetic acid) using HPLC (high precision liquid chromatography) with electrochemical detection in a single run, i.e. from the same tissue samples as described before (Chatterjee and Gerlai, 2009). The fish used for neurochemical analysis were of the same age, size, and strain origin as those tested behaviorally. The methods of neurochemicals analysis have been adopted and modified to zebrafish as described before (Chatterjee and Gerlai, 2009). Fish were decapitated and their brains were dissected on dry ice. The brains were suspended in artificial cerebrospinal fluid and were sonicated. The sonicate was analyzed for protein content by BioRad protein assay reagent (BioRad, Hercules, CA, United States). It was centrifuged and the supernatant was collected for HPLC analysis. A BAS 460 Microbore HPLC system with electrochemical detection (Bioanalytical Systems Inc., West Lafayette, IN, USA) was employed with a Uniget C-18 reverse phase microbore column as the stationary phase (BASi, Cat no. 8949). The mobile phase consisted of buffer (0.1 M monochloro acetic acid, 0.5 mM Na-EDTA, 0.15 g/L Na-octylsulfonate and 10 nM sodium chloride, pH 3.1 [all chemicals were from Sigma]), acetonitrile and tetrahydrofuran (all solvents from Fisher Scientific) at a ratio 94:3.5:0.7. The flow rate was set to 1.0 ml/min and the working electrode (Uniget 3 mm glassy carbon, BAS P/N MF-1003) was set at 550 mV vs. Ag/Ag/Cl reference electrode. Detection gain was 1.0 nA, filter was 0.2 Hz and detection limit was set at 20 nA. Five µl of the sample supernatant was directly injected into the HPLC system for analysis. Standard neurochemicals (all from Sigma) were employed to quantify and identify the peaks on the chromatographs.
Data analysis
Data were analyzed using SPSS (version 14.1) for the PC. The behavioral data were calculated for the two intervals (habituation and stimulus presentation intervals) separately. First the baseline (habituation interval) data were analyzed using Variance Analysis (ANOVA) with two factors: strain (two levels) and D1-R antagonist dose (3 levels). This analysis, in principle, is supposed to be able to reveal both main effects and the interaction between the main effects, the strain and D1-R antagonist dose. However, ANOVA has been shown to be insensitive to detect genotype × environment interaction (Wahlsten, 1990) and indeed, despite the occasionally apparent (see figures) interaction, we did not find the interaction terms to reach significance for any of the behaviors quantified. Therefore, we do not report the two factorial ANOVA results. Instead, following Wahlsten’s (1990) arguments, we conducted uni-factorial ANOVAs separately for each strain to investigate the effect of the D1-R antagonist and we only report the results of these analyses. These analyses indeed turned out to be more powerful and detected strain specific D1-R antagonist effects. In case of significant D1-R antagonist effect, we conducted the post hoc multiple comparison Tukey Honestly Significant Difference (HSD) test. In addition to analyzing the baseline (habituation interval) behavioral responses, we also analyzed the effect of stimulus presentation (the sight of conspecific shoal). We subtracted the value for the corresponding behavior obtained for the habituation period from the one obtained for the stimulus presentation period and performed statistical analyses on this difference score as described above. In addition, we also conducted one-sample t-tests with Bonferroni correction for multiple comparisons and compared the values of certain behavioral measures to 0 (no change in response to the shoal stimulus) as explained in the results section. The effect of D1-R antagonist on neurochemical measures was also analyzed separately for the two strains as described above. The null hypothesis of no D1-R effect was rejected when its probability (p) was found to be below 0.05.
RESULTS
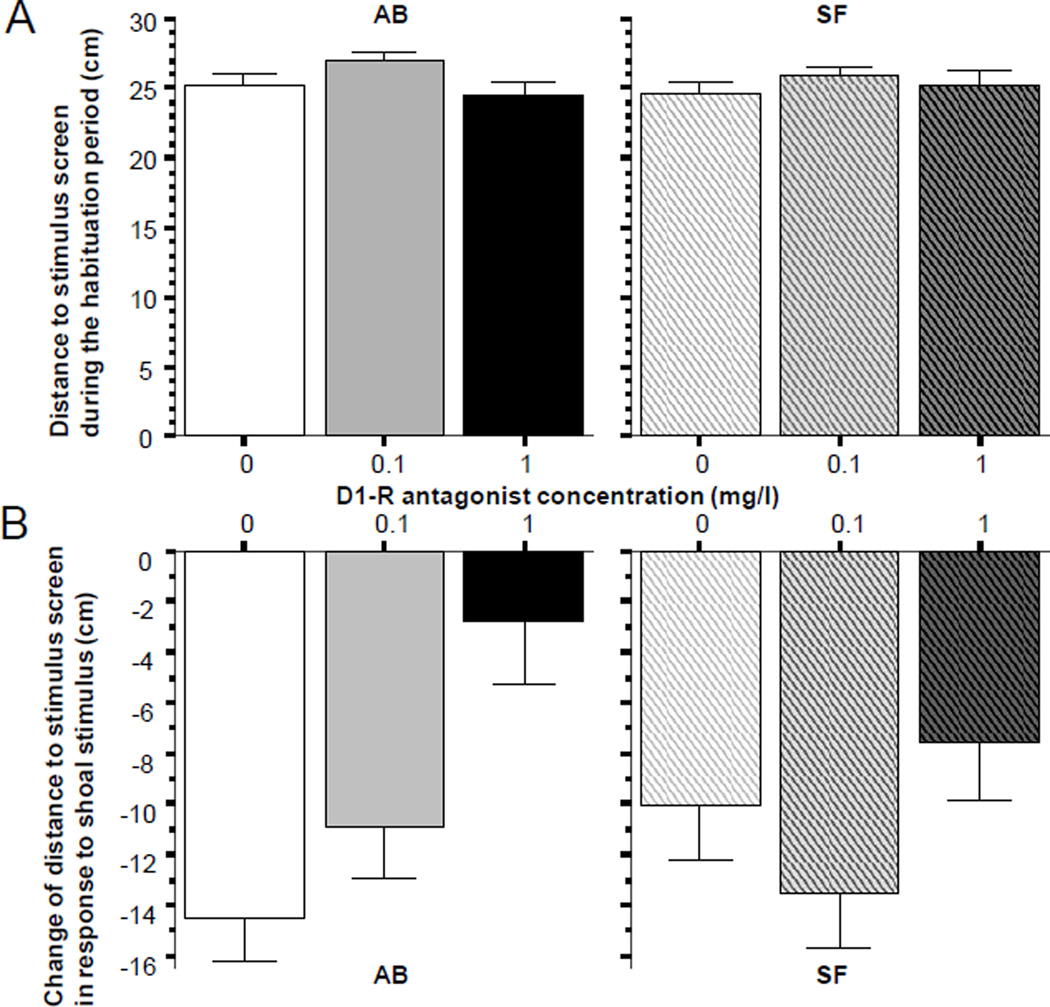
During the habituation session fish did not appear to prefer any sides of the experimental tank. This observation was confirmed by the results shown on Fig. 1 (panel A), which demonstrates that on average fish were about 25 cm away from the stimulus screen (the tank was 50 cm long). Furthermore, ANOVA revealed no significant D1-R antagonist effect for either strain (AB, F(2, 57) = 2.353, p > 010; SF F(2, 57) = 0.527, p > 0.55). Presentation of the stimulus, however, significantly altered the swim pattern of fish. As expected, the distance to the stimulus screen dramatically decreased (Fig 1, panel B). For example, control fish of the AB strain (0 mg/l SCH23390) swam 14 cm closer to the screen when the stimulus was turned on compared to when it was off. Importantly, this response was robustly diminished by SCH23390 (ANOVA F(2, 57) = 8.436, p < 0.001), a finding that demonstrates a significant drug effect. Post hoc Tukey HSD revealed that fish immersed in the 0 mg/l solution significantly (p < 0.05) differed from fish immersed in the high concentration (1mg/l) but not from the fish immersed in the low concentration (0.1 mg/l) SCH23390 solution. This analysis also found fish immersed in the low concentration to significantly (p < 0.05) differ from fish immersed in the high concentration. Briefly, the high concentration SCH23390 compound impaired the shoaling response. In fact this dose abolished the shoaling response as shown by a t-test that demonstrated the change in response to the shoaling image to be not significantly different from zero (t = 1.121, df = 19, p > 0.25). Interestingly, the D1-R antagonist effect seen in the AB strain zebrafish could not be found in SF zebrafish (Fig 2, panel B right bar graph). This observation was confirmed by ANOVA, which showed no significant D1-R antagonist effect (F(2, 57) = 1.834), p > 0.15)
Figure 2.
Distance to stimulus screen, a measure of social attraction (AB strain solid bars, SF strain striped bars, also indicated by letters above and below the graphs). Panel A shows the baseline response, i.e. the distance to the stimulus screen during the habituation period when no shoal stimulus was presented. Panel B shows the change of distance to stimulus screen (stimulus period distance minus habituation period distance) elicited by the stimulus (moving images of 5 zebrafish, the “synthetic shoal”), which was presented during the 8 minutes that immediately followed the habituation period. Note that the negative values mean reduction of distance compared to baseline. Also note that the reduction of distance was significantly attenuated by the D1-R antagonist but only in AB fish (lower left graph). Mean ± S.E.M. are shown. Sample sizes (n) equal 20 for all concentration groups in each strain. The concentration of the drug indicated represents the external bath concentrations.
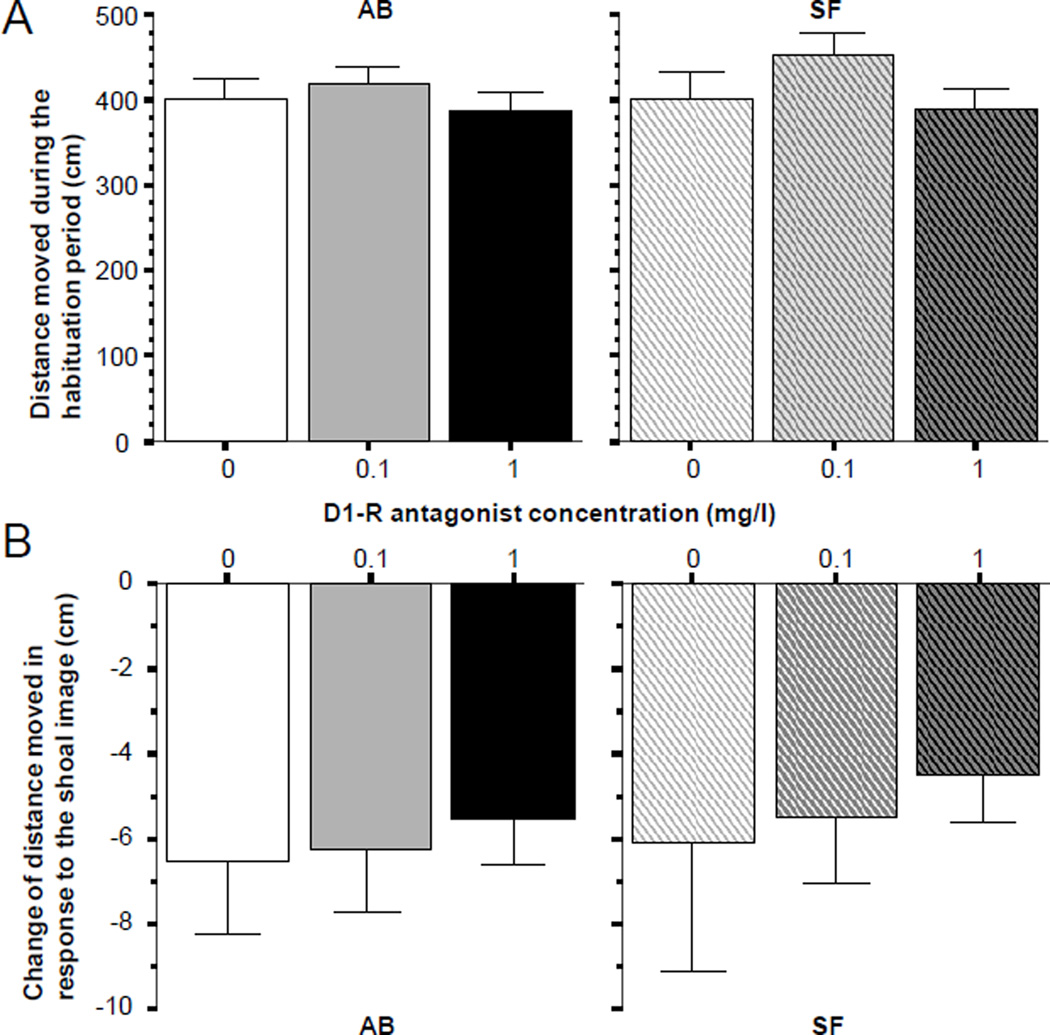
Figure 3 shows the results obtained for the parameter, total distance moved. Panel A demonstrates that fish of both strains were active during the habituation period. Furthermore, it appears that fish of both strains moved about the same distance irrespective of whether they were exposed to the high or low concentration of the D1-R antagonist or no antagonist at all. This conclusion is supported by ANOVA, which showed no significant D1-R antagonist effect for AB (F(2, 57) = 0.460, p > 0.60) or SF zebrafish (F(2, 57) = 1.543, p > 0.20). Panel B of figure 3 also suggests that irrespective of strain origin, and also independently of D1-R antagonist dose, all fish reduced their activity in response to the shoaling stimulus. The lack of significant D1-R antagonist effect was confirmed by ANOVA for both strains (AB, F(2, 57) = 0.349, p > 0.70; SF F(2, 57) = 0.320, p > 0.70) and the significant reduction of activity was confirmed for all groups by one sample t-tests with Bonferroni correction (t > 5.199, df = 19, p < 0.01).
Figure 3.
Distance moved, a measure of locomotory activity (AB strain solid bars, SF strain striped bars, also indicated by letters above and below the graphs). Panel A shows the baseline response, i.e. the distance moved during the habituation period when no shoal stimulus was presented. Panel B shows the change of distance moved (stimulus period distance minus habituation period distance) elicited by the stimulus (moving images of 5 zebrafish, the “synthetic shoal”), which was presented during the 8 minutes that immediately followed the habituation period. Note that the negative values mean reduction of distance compared to baseline. Also note that in response to the presentation of the shoal image, fish all dose groups and in both strains responded with a similar reduction of activity. Mean ± S.E.M. are shown. Sample sizes (n) are 20 for all concentration groups in each strain. The concentration of the drug indicated represents the external bath concentrations.
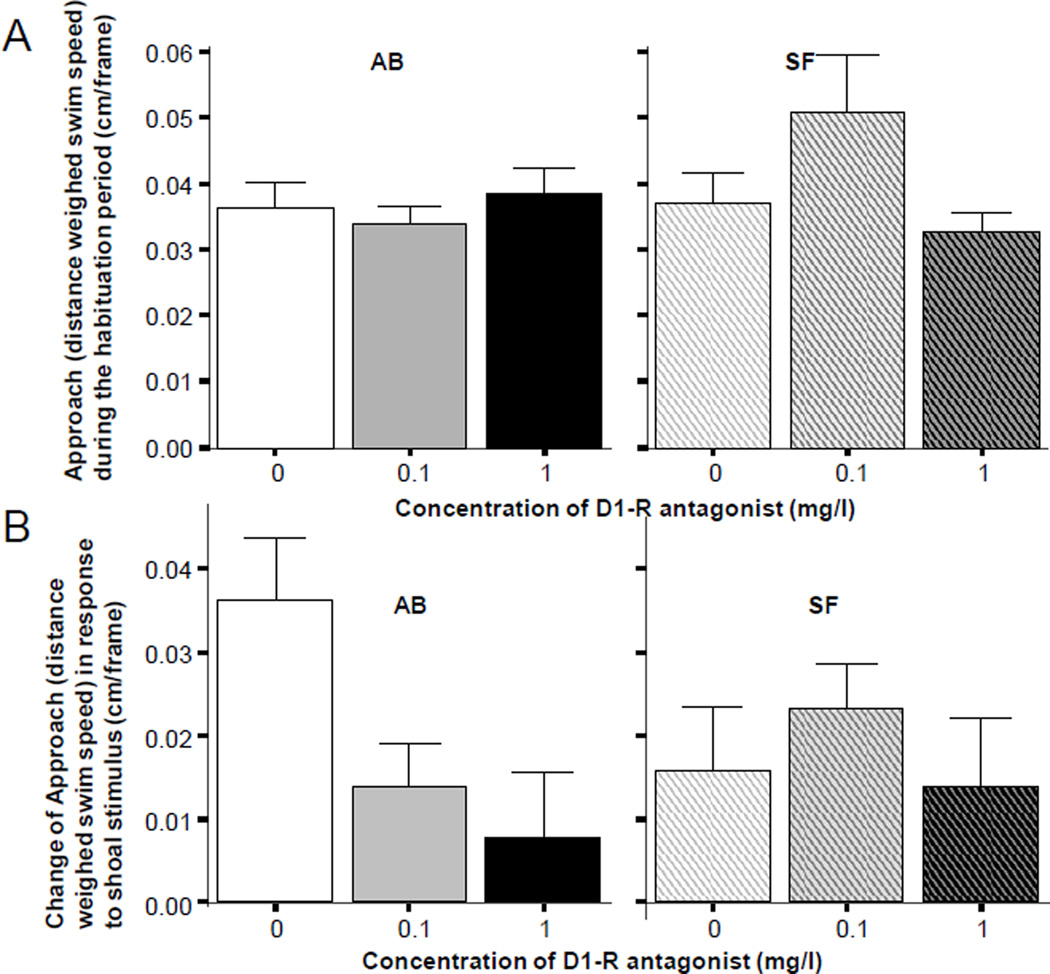
The results obtained for Approach are shown on Figure 4. They are highly similar to those obtained for the behavioral measure, distance to the stimulus screen. The results shown on panel A of figure 4 suggest that zebrafish of both strains and of all three drug treatment groups behaved similarly during the habituation period. All fish showed a similar approach behavior to the stimulus screen when the screen had no shoaling stimuli. ANOVA found the effect of the D1-R antagonist non-significant for both strains (AB F(2, 57) = 0.481, p > 0.60; SF F(2, 57) = 2.615, p > 0.05). However, when the shoaling stimulus was turned on, zebrafish of the AB strain performed in a D1-R antagonist dose dependent manner (figure 4, panel B). AB fish not treated with the drug (control) increased their approach compared to their habituation period baseline level. However, AB fish exposed to the D1-R antagonist appeared to show only a blunted increase (Figure 4, panel B left graph). These observations were confirmed by ANOVA, which showed a significant D1-R antagonist effect (F(2, 57) = 4.569, p < 0.05), and Tukey HSD post hoc multiple comparison test, which revealed that the highest dose group (immersion in 1mg/l SCH23390 solution) significantly (p < 0.05) differed from control (0 mg/l SCH23390 solution) while other group differences were non-significant. Importantly, these drug treatment effects were not found in SF fish (Figure 4 panel B right graph; ANOVA (F(2, 57) = 0.468, p > 0.60).
Figure 4.
Distance weighed horizontal component of position change, a measure approach (AB strain solid bars, SF strain striped bars, also indicated by letters above the graphs). Panel A shows the baseline response, i.e. approach of the stimulus screen during the habituation period when no shoal stimulus was presented. Panel B shows the change of approach of the stimulus screen (stimulus period approach minus habituation period approach) elicited by the stimulus (moving images of 5 zebrafish, the “synthetic shoal”), which was presented during the 8 minutes that immediately followed the habituation period. Note that the positive values mean increase of approach of the stimulus screen during the stimulus presentation period compared to baseline. Also note that the increase of approach was significantly attenuated by the D1-R antagonist but only in AB fish (lower left graph). Mean ± S.E.M. are shown. Sample sizes (n) equal 20 for all concentration groups in each strain. The concentration of the drug indicated represents the external bath concentrations.
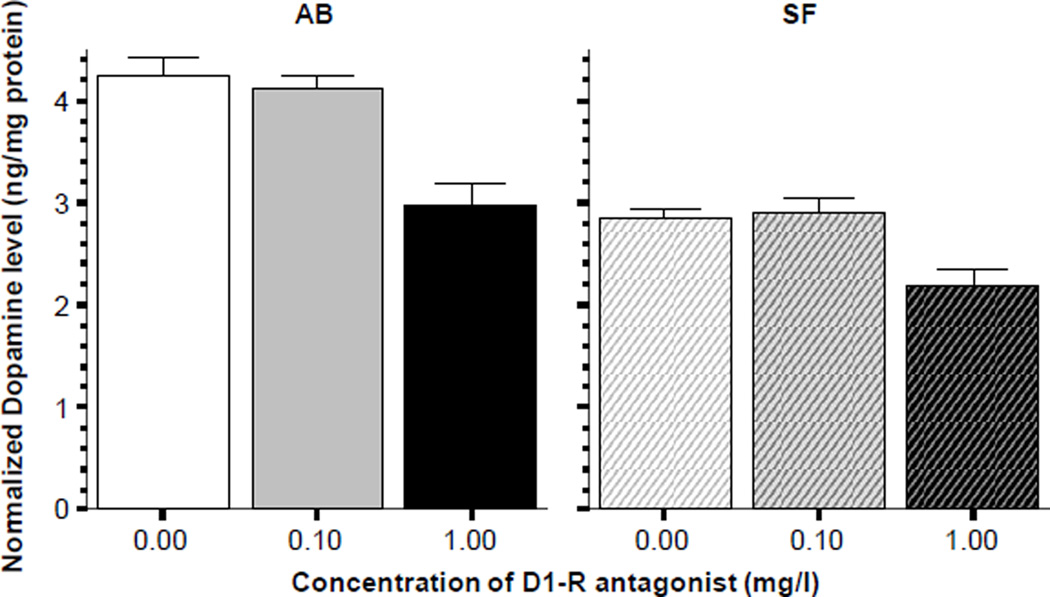
In addition to the behavioral effects, the D1-R antagonist also significantly altered levels of neurochemicals in the brain of the exposed zebrafish. Figure 5 suggests that dopamine levels were reduced by the highest concentration of the D1-R antagonist in both the AB and SF fish (Figure 5). This observation was confirmed by ANOVA, which revealed a significant D1-R antagonist effect for AB (F(2, 27) = 17.85, p < 0.001) and also for SF (F(2, 27) = 8.401, p < 0.01). Tukey HSD post hoc test showed that for both strains the highest dose group (immersion in 1 mg/l SCH23390) significantly (p < 0.05) differed from the other two groups. It is also notable that ANOVA also revealed that the SF fish had significantly less dopamine in their brain compared to the AB fish across all treatment groups (F1, 54) = 85.535, p < 0.001).
Figure 5.
Normalized Dopamine levels in the brain of zebrafish. Note that the D1-receptor antagonist SCH23390 significantly reduced the amount of dopamine in the brain of zebrafish in both strains (AB solid bars SF striped bars, also indicated above the graphs). Mean ± S.E.M. are shown. Sample sizes (n) are 10 for all concentration groups in each strain. The concentration of the drug indicated below the graphs represents the external bath concentration. Note that zebrafish of the AB strain were found to have significantly higher level of dopamine in their brain as compared to SF, a strain effect that was found independent of the drug effect.
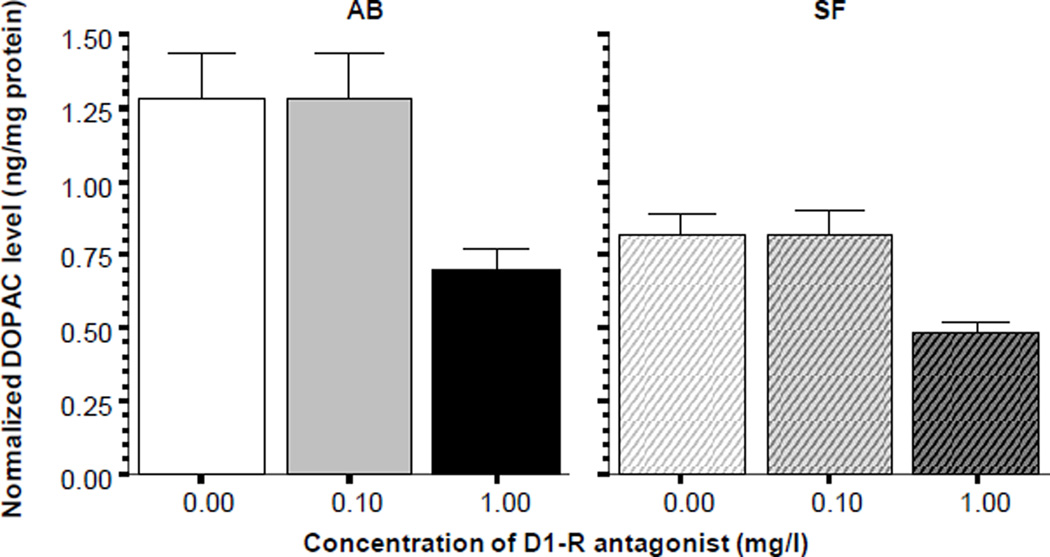
Analysis of DOPAC levels (figure 6) showed a similar picture. ANOVA found the D1-R antagonist treatment to exert a significant effect in both AB (F(2, 27) = 6.474, p < 0.01) and SF fish (F(2, 27) = 7.317, p < 0.01) and post hoc Tukey HSD showed again that the highest dose group differed significantly (p < 0.05) from the other two treatment groups in both strains. Also notably, ANOVA demonstrated a significant reduction of DOPAC levels independent of the drug treatment in SF fish compared to AB (F1, 54) = 2.231, p < 0.001).
Figure 6.
Normalized DOPAC levels in the brain of zebrafish. Note that the D1-receptor antagonist SCH23390 significantly reduced the amount of DOPAC in the brain of zebrafish in both strains (AB solid bars SF striped bars, also indicated above the graphs). Mean ± S.E.M. are shown. Sample sizes (n) are 10 for all concentration groups in each strain. The concentration of the drug indicated below the graphs represents the external bath concentration. Note that zebrafish of the AB strain were found to have significantly higher level of dopamine in their brain as compared to SF, a strain effect that was found independent of the drug effect.
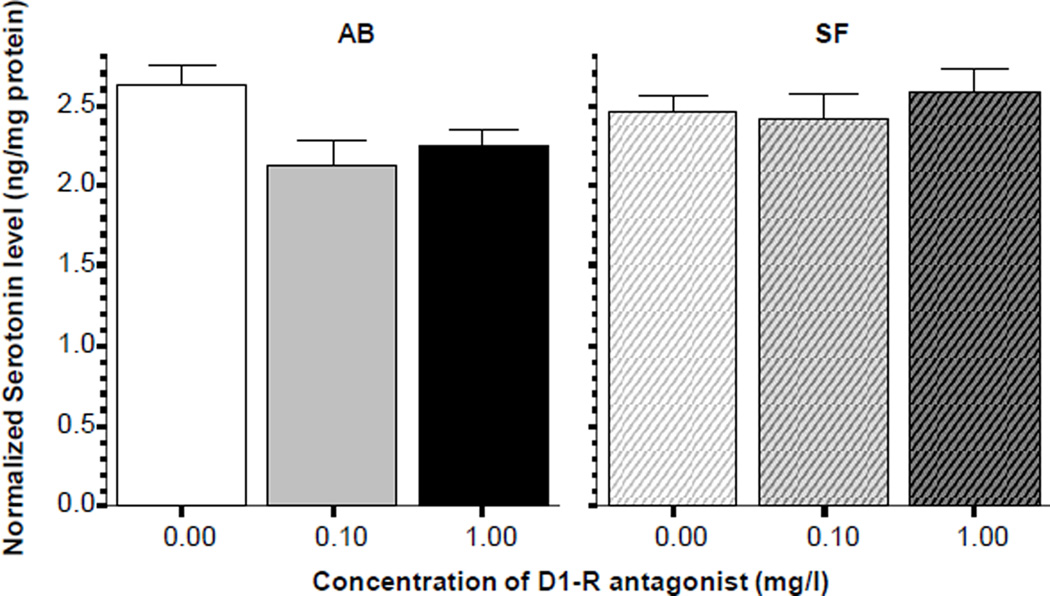
Analysis of the levels of serotonin showed a different pattern of results (figure 7). While the differences among dose groups of AB fish did not appear large, ANOVA did reveal a significant D1-R antagonist effect (F(2, 27) = 4.022, p < 0.05) and Tukey HSD showed that the control fish had significantly (p < 0.05) higher serotonin levels compared to the low D1-R antagonist dose treated fish, while other group differences were non-significant. SF fish exhibited no such drug effect (ANOVA F(2, 27) = 0.442, p > 0.60).
Figure 7.
Normalized serotonin levels in the brain of zebrafish. Note that the D1-receptor antagonist SCH23390 was found to significantly reduce serotonin levels only in the AB strain (solid bars) but not in the SF strain (striped bars). Mean ± S.E.M. are shown. Sample sizes (n) are 10 for all concentration groups in each strain. The concentration of the drug indicated below the graphs represents the external bath concentration.
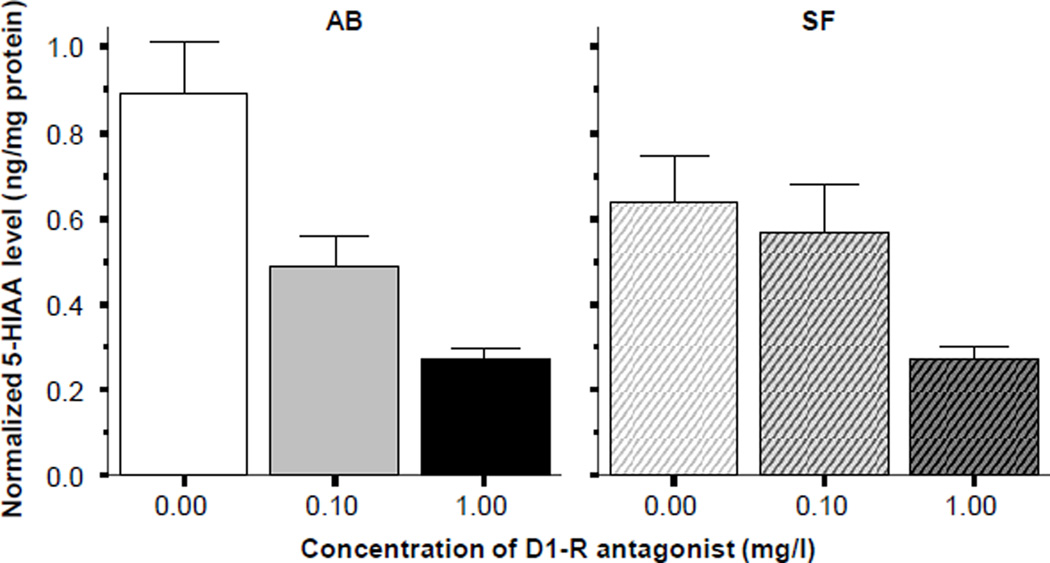
Unlike serotonin, 5HIAA did appear to show robust changes in response to the D1-R antagonist treatment (figure 8). ANOVA confirmed this observation and showed a significant drug treatment effect in AB (F(2, 27) = 14.848, p < 0.001) and Tukey HSD revealed that control fish differed significantly (p < 0.05) from both the low and the high D1-R antagonist dose treated fish, while these latter two groups did not significantly differ from each other. Analysis of the results obtained for SF fish also showed a significant D1-R antagonist effect (ANOVA F(2, 27) = 4.470, p < 0.05) and Tukey HSD found the control fish and the fish of the highest D1-R antagonist dose group to differ significantly (p < 0.05). Strain differences were not found significant for serotonin or 5HIAA.
Figure 8.
Normalized 5HIAA levels in the brain of zebrafish. Note that the D1-receptor antagonist SCH23390 significantly reduced the amount of 5-HIAA in the brain of zebrafish in both strains (AB solid bars SF striped bars, also indicated above the graphs). Mean ± S.E.M. are shown. Sample sizes (n) are 10 for all concentration groups in each strain. The concentration of the drug indicated below the graphs represents the external bath concentration.
DISCUSSION
Laboratory animal models have been proposed to facilitate the discovery of the mechanisms of human brain and behavior functions as well as the mechanisms of pathological alterations of the brain. Among other species, the zebrafish has been proposed as a translational research tool for the modeling of human biology and pathology (e.g. Ingham, 2009; Mathur and Guo, 2010, Dooley and Zon, 2000). The zebrafish is one of the most social laboratory species and thus may be particularly appropriate for the analysis of social behavior and its mechanisms and abnormalities (Saverino and Gerlai, 2008, Miller and Gerlai, 2011, Buske and Gerlai, 2011a, 2011b).
In this paper, we demonstrate a significant D1-R antagonism induced dose dependent disruption of preference of zebrafish to move towards and stay close to social stimuli, a shoal image shown on the computer monitor. Importantly, the preference to stay close to the shoal stimulus was specific to this stimulus as experimental fish did not show such preference towards the monitor itself nor did they prefer any side of the test tank when the stimulus was not presented.
The dopaminergic system is involved in a number of brain functions, one of which is motor function. Thus D1-R antagonism could potentially lead to altered shoaling responses as a result of abnormal locomotory activity or impaired motor patterns. However, the treated fish did not exhibit abnormal motor or posture patterns and their activity level was also unaltered. For example, analysis of total distance the fish swam showed no differences among any experimental groups during the habituation period when no stimulus was shown.
It is also interesting to note that in response to the presentation of the shoal image, all fish reduced their activity. A similar reduction of activity induced by the shoaling image was reported before (Fernandes & Gerlai, 2009). Importantly, in response to the shoal image the same level of reduction of the total distance swum was seen in all drug treatment groups as well as in the control group. This finding is noteworthy for two reasons. One, it confirms unaltered motor function. Two, it also suggests that all fish were able to perceive the stimulus presented. The latter point is important because D1-R receptors have been found in the retina of mammals (Nguyen-Legros et al., 2004) and of fish (Mora-Ferrer & Gangluff, 2002), and thus the D1-R antagonist treatment employed here could, in principle, have exerted its effects via impairing vision of the experimental zebrafish. However, given that all fish responded to the shoal image by reducing their locomotory activity to the same level and given that the only modality they could use to perceive the stimulus was their vision (the stimulus was presented on a computer screen that was outside of the fish tank and thus heat, vibrations or auditory signals could not guide the experimental fish), we conclude that the impaired response to the social stimulus resulting from the D1-R antagonist treatment could not be due to altered vision. Because motor function was also found unaltered in the drug treated fish, we conclude that the reduced preference for the social stimulus is likely to be mediated by central mechanisms unrelated to perception and motor function. Furthermore, given the involvement of the dopaminergic system in reward, our results are compatible with the suggestion that the D1-R induced reduction of social response to the shoal image may be due to impaired motivation and/or reward mechanisms.
Another notable finding of the current study is that the D1-R induced changes appeared specific to one zebrafish strain, AB, and could not be detected in the other strain, SF, studied. We stress that these two genetically distinct groups of zebrafish were bred, raised and maintained in the same vivarium room under identical conditions and they were tested in a fully randomized and blind manner. Therefore, we conclude that the observed differences between the two strains in their response to the D1-R antagonist can only be the result of the genetic differences between these strains. Previously, we documented differences between the AB and SF zebrafish strains in their response to alcohol (Gerlai et al., 2009). Briefly, SF fish were found to be less vulnerable to the effects of acute alcohol administration and also developed less robust tolerance to alcohol after chronic administration of this substance (Gerlai et al., 2009). The current study now extends these findings in a new direction and shows that SF fish are also less affected by D1-R antagonism as compared to AB fish.
The AB zebrafish strain has been bred in the laboratory for four decades and is homozygous at an estimated 80% of its loci (Guryev et al., 2006). Briefly, the AB strain must have experienced genetic drift due to loss of alleles at the majority of its loci and thus is likely to have developed idiosyncratic features. On the other hand, SF individuals are likely to have numerous loci in a heterozygous form because this strain was established only a year before the experiments were conducted and because the founding individuals were from a large genetically heterogeneous stock. It is notable that reduced vulnerability to, i.e. better buffering against, environmental insults have been found in genetically heterogeneous stocks before (see e.g. Crusio, 2006 and references therein) and such buffering may explain the reduced D1-R effects in SF fish we are reporting here. Nevertheless, at this point the exact mechanisms underlying the observed differences between the AB and SF strains are not known. Also, the question as to how the D1-R antagonist treatment led to the observed social behavior deficits remains unanswered. Last, we do not know whether a mechanistic link may exist between the blunted alcohol and D1-R antagonist effects seen in SF relative to AB fish or, alternatively, whether these characteristics only represent spurious associations. These mechanistic questions will be investigated in the future.
In the current paper we have started the analysis of the potential mechanisms underlying zebrafish social behavior. We showed that the dopaminergic system was likely to be involved. We showed that in addition to alterations in behavior, the D1-R antagonist also changed the level of neurochemicals in the zebrafish brain. However, we have to emphasize that the behavioral and neurochemical findings did not correlate well and definitely do not represent causation. For example, while the D1-R antagonist impaired the social behavioral response of AB fish and also reduced the level of the measured neurochemicals, this correlation was not observed in SF fish, which although also showed the neurochemical responses, did not alter their behavior as a result of the drug treatment.
Briefly, in response to the D1-R antagonist treatment dopamine, DOPAC and 5HIAA responded with a significant reduction in both strains. The significantly reduced dopamine levels imply reduced dopamine production and/or increased dopamine degradation in response to the acutely employed D1-R antagonist (e.g. Diop et al., 1988 and references therein). Whereas the reduced DOPAC and 5HIAA levels may be interpreted as impaired dopaminergic and serotoninergic neurotransmission (dopamine is metabolized after release from the presynaptic terminal and one metabolic product of this process is DOPAC, and similarly 5HIAA is a metabolite of serotonin and is produced after this neurotransmitter is released from synaptic vesicles). It is also notable that, although the drug induced reduction was found significant in both strains, the absolute level of reduction of these neurochemicals induced by the D1-R antagonist was about half as much in SF compared to AB. It is possible that the behavioral differences observed between these two strains in response to the D1-R antagonist are, at least partially, due to these differential neurochemical characteristics.
The physiological role for the dopamine D1 receptor has been difficult to define even in mammals because of the complex subcellular localization of this receptor. For example, in the mammalian midbrain D1 receptors were found to be localized presynaptically on the terminals of afferent GABA neurons (Cameron & Williams, 1993). Others have shown D1-R to be localized to the post-synaptic terminals of neurons of the mammalian basal ganglia (Yung et al., 1995). Yet others have shown expression of D1-R at extra-synaptic sites, for example, on striatonigral neurons (Caillé et al., 1996). Clearly, depending on subcellular localization of D1-R, the employed antagonist may exert highly different effects that may lead to enhanced or reduced neurochemical levels. For example, blockade of pre-synaptic or extra-synaptic autoreceptors are expected to increase neurotransmitter production whereas blockade of post-synaptic neurotransmitter receptors may impair signaling downstream and thus reduce neurotransmitter release. Given the systemic application of the D1-R antagonist, we could not distinguish brain region and subcellular localization specific effects. Nevertheless, the very same D1-R antagonist we employed here, SCH23390, has been shown to reduce dopamine (Steketee, 1998; Kurata & Shibata, 1991) as well as serotonin release (Darbin et al., 2010) in mammals, results that are compatible with our finding of reduced DOPAC and 5HIAA levels in zebrafish. Furthermore, the level of neurotransmitter production has been shown to correlate with the level of their metabolites in mammals (Diop et al., 1988), which again is in line with our finding of reduced dopamine levels in response to the systemic D1-R antagonist treatment in zebrafish.
One may expect a strong cross talk between different neurotransmitter systems and thus using a dopamine receptor antagonist, even if it is highly selective for a particular dopamine receptor (as is likely the case with SCH23390 employed here), does not guarantee that only the dopaminergic system will be affected. Teasing out how neurotransmitter systems interact with each other and how their interaction leads to particular behavioral responses is a complex task, and our work is only a first step in unraveling this complexity in zebrafish. Clearly, there may be numerous neurochemicals, other than dopamine and serotonin and their metabolites, whose levels may be altered by the D1-R antagonist. At this point, we do not know which of these, if any, may underlie the observed strain differences and the observed D1-R antagonist induced behavioral alterations.
Furthermore, there may be several other neurobiological mechanisms that may be revealed at other levels of analyses. For example, DNA microarrays may allow one to identify changes in the transcriptome in response to D1-R antagonist treatment and may also illuminate the mechanisms underlying strain dependent drug responses. The importance of analysis of strain differences is underscored by a recent gene expression analysis (Pan et al., 2011b) in which the AB and SF zebrafish strains were compared. This analysis found that out of the ten genes studied, three exhibited significant expression level differences between the strains: the gene encoding the D1 dopamine receptor (but not the genes encoding D2-R or D4-R) was found overexpressed, while the genes for GABAA-R1 and for solute carrier family protein 6 (SLC6) were underexpressed in the brain of SF fish as compared to AB. These results are notable for two reasons. First, they demonstrate that there may be potentially large number of genetic differences between the strains (hence the need for comprehensive analyses). Second, they also show that transcription of genes implicated in drug abuse as well as reward mechanisms may differ between AB and SF. For example, both the dopaminergic and the GABAergic systems have been implicated in drug abuse and/or reward (Fibiger and Phillips, 1988; Wise, 1987; Volicer, 1981; Buckett, 1981) and solute carrier family proteins (e.g. SLC6) have been recently implicated in mediating chronic alcohol induced changes in the brain (Pan et al., 2011a).
Although we cannot, at this point, pinpoint the exact mechanisms underlying the D1-R antagonist induced behavioral impairment or those underlying the differences in these responses between the strains, one may regard the results reported here as an important start. For example, our results underscore the need to characterize the available zebrafish strains. Knowing such characteristics will aid us in selecting the appropriate host strain for mutagenesis and the mapping strain for linkage analysis crosses. Similarly, knowing that a strain is resistant or responsive to certain drugs is also crucial when choosing a zebrafish strain for drug screening. Furthermore, the specific differences between the AB and SF strains reported here may be utilized in quantitative trait locus (QTL) mapping, which may lead one to identify the loci and eventually the genes underlying social behavior and its rewarding properties.
Fish are considered the most primitive vertebrates. Nevertheless, the zebrafish has been successfully utilized to model and study numerous human diseases (Dooley and Zon, 2010). Analysis of zebrafish behavior and brain function may also have promise in translational research (Mathur and Guo, 2010). It is our hope that the findings reported here will facilitate research on the mechanisms of vertebrate social behavior, which may ultimately lead to better understanding of human brain disorders associated with social behavior abnormalities.
ACKNOWLEDGEMENTS
This study was supported by an NIH/NIAAA R01 (USA) grant awarded to Robert Gerlai, and an NSERC (CANADA) summer undergraduate research scholarship awarded to Tanya Scerbina.
REFERENCES
- Al-Imari L, Gerlai R. Conspecifics as reward in associative learning tasks for zebrafish (Danio rerio) Behav. Brain Res. 2008;189:216–219. doi: 10.1016/j.bbr.2007.12.007. [DOI] [PubMed] [Google Scholar]
- Alsop D, Vijayan MM. Development of the corticosteroid stress axis and receptor expression in zebrafish. Am J Physiol Regul Integr Comp Physiol. 2008;294:R711–R719. doi: 10.1152/ajpregu.00671.2007. [DOI] [PubMed] [Google Scholar]
- Bromberg-Martin ES, Matsumoto M, Hikosaka O. Dopamine in motivational control: rewarding, aversive, and alerting. Neuron. 2010;68:815–834. doi: 10.1016/j.neuron.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromley E, Brekke JS. Assessing function and functional outcome in schizophrenia. Curr Top Behav Neurosci. 2010;4:3–21. doi: 10.1007/7854_2010_40. [DOI] [PubMed] [Google Scholar]
- Buckett WR. The role of GABA in analgesia and drug dependence. Rev Pure Appl Pharmacol Sci. 1981;2:115–141. [PubMed] [Google Scholar]
- Buske C, Gerlai R. Shoaling develops with age in zebrafish (Danio rerio) Prog Neuro-Psychopharm Biol Psych. 2011a;35:1409–1415. doi: 10.1016/j.pnpbp.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buske C, Gerlai R. Maturation of shoaling behavior is accompanied by changes in the dopaminergic and serotoninergic systems in zebrafish. Dev Psychobiol. 2011b doi: 10.1002/dev.20571. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caillé I, Dumartin B, Bloch B. Ultrastructural localization of D1 dopamine receptor immunoreactivity in rat striatonigral neurons and its relation with dopaminergic innervation. Brain Res. 1996;730:17–31. doi: 10.1016/0006-8993(96)00424-6. [DOI] [PubMed] [Google Scholar]
- Cameron DL, Williams JT. Dopamine D1 receptors facilitate transmitter release. Nature. 1993;366:344–347. doi: 10.1038/366344a0. [DOI] [PubMed] [Google Scholar]
- Chatterjee D, Gerlai R. High precision liquid chromatography analysis of dopaminergic and serotoninergic responses to acute alcohol exposure in zebrafish. Behav. Brain Res. 2009;200:208–213. doi: 10.1016/j.bbr.2009.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crusio WE. Inheritance of behavioral and neuroanatomical phenotypical variance: hybrid mice are not always more stable than inbreds. Behav Genet. 2006;36:723–731. doi: 10.1007/s10519-005-9039-2. [DOI] [PubMed] [Google Scholar]
- Dankova J, Bédard P, Langelier P, Poirier LJ. Dopaminergic agents and circling behaviour. Gen Pharmacol. 1978;9:295–302. doi: 10.1016/0306-3623(78)90064-2. [DOI] [PubMed] [Google Scholar]
- Darbin O, Risso JJ, Rostain JC. Dopaminergic control of striatal 5-HT level at normobaric condition and at pressure. Undersea Hyperb Med. 2010;37:159–166. [PubMed] [Google Scholar]
- Diop L, Gottberg E, Brière R, Grondin L, Reader TA. Distribution of dopamine D1 receptors in rat cortical areas, neostriatum, olfactory bulb and hippocampus in relation to endogenous dopamine contents. Synapse. 1988;2:395–405. doi: 10.1002/syn.890020406. [DOI] [PubMed] [Google Scholar]
- Dooley K, Zon LI. Zebrafish: a model system for the study of human disease. Curr Opin Genet Dev. 2000;10:252–256. doi: 10.1016/s0959-437x(00)00074-5. [DOI] [PubMed] [Google Scholar]
- Emilien G, Maloteaux JM, Geurts M, Hoogenberg K, Cragg S. Dopamine receptors--physiological understanding to therapeutic intervention potential. Pharmacol Ther. 1999;84:133–156. doi: 10.1016/s0163-7258(99)00029-7. [DOI] [PubMed] [Google Scholar]
- Fernandes Y, Gerlai R. Long-term behavioral changes in response to early developmental exposure to ethanol in zebrafish. Alcohol Clin Exp Res. 2009;33:601–609. doi: 10.1111/j.1530-0277.2008.00874.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fibiger HC, Phillips AG. Mesocorticolimbic dopamine systems and reward. Ann N Y Acad Sci. 1988;537:206–215. doi: 10.1111/j.1749-6632.1988.tb42107.x. [DOI] [PubMed] [Google Scholar]
- Gerlai R, Chatterjee D, Pereira T, Sawashima T, Krishnannair R. Acute and chronic alcohol dose: Population differences in behavior and neurochemistry of zebrafish. Genes Brain Behav. 2009;8:586–599. doi: 10.1111/j.1601-183X.2009.00488.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlai R. High-throughput behavioral screens: the first step towards finding genes involved in vertebrate brain function using zebrafish. Molecules. 2010;15:2609–2622. doi: 10.3390/molecules15042609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunwald DJ, Eisen JS. Timeline: Headwaters of the zebrafish—emergence of a new model vertebrate. Nat Rev Genet. 2002;3:717–724. doi: 10.1038/nrg892. [DOI] [PubMed] [Google Scholar]
- Guryev V, Koudijs MJ, Berezikov E, Johnson SL, Plasterk RH, van Eeden FJ, Cuppen E. Genetic variation in the zebrafish. Genome Res. 2006;16:491–497. doi: 10.1101/gr.4791006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hettinger JA, Liu X, Schwartz CE, Michaelis RC, Holden JJ. A DRD1 haplotype is associated with risk for autism spectrum disorders in male-only affected sib-pair families. Am J Med Genet B Neuropsychiatr Genet. 2008;147B:628–636. doi: 10.1002/ajmg.b.30655. [DOI] [PubMed] [Google Scholar]
- Ingham PW. The power of the zebrafish for disease analysis. Hum Mol Genet. 2009;18:R107–R112. doi: 10.1093/hmg/ddp091. [DOI] [PubMed] [Google Scholar]
- Kalkstein S, Hurford I, Gur RC. Neurocognition in schizophrenia. Curr Top Behav Neurosci. 2010;4:373–390. doi: 10.1007/7854_2010_42. [DOI] [PubMed] [Google Scholar]
- Kurata K, Shibata R. Effects of D1 and D2 antagonists on the transient increase of dopamine release by dopamine agonists by means of brain dialysis. Neurosci Lett. 1991;25:133, 77–80. doi: 10.1016/0304-3940(91)90061-w. [DOI] [PubMed] [Google Scholar]
- Li P, Shah S, Huang L, Carr AL, Gao Y, Thisse C, Thisse B, Li L. Cloning and spatial and temporal expression of the zebrafish dopamine D1 receptor. Dev Dyn. 2007;236:1339–1346. doi: 10.1002/dvdy.21130. [DOI] [PubMed] [Google Scholar]
- López-Patiño MA, Yu L, Cabral H, Zhdanova IV. Anxiogenic effects of cocaine withdrawal in zebrafish. Physiol Behav. 2008;93:160–171. doi: 10.1016/j.physbeh.2007.08.013. [DOI] [PubMed] [Google Scholar]
- Mathur P, Guo S. Use of zebrafish as a model to understand mechanisms of addiction and complex neurobehavioral phenotypes. Neurobiol Dis. 2010;40:66–72. doi: 10.1016/j.nbd.2010.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miklósi A, Andrew RJ. Right eye use associated with decision to bite in zebrafish. Behav. Brain Res. 1999;105:199–205. doi: 10.1016/s0166-4328(99)00071-6. [DOI] [PubMed] [Google Scholar]
- Miller N, Gerlai R. Oscillations in shoal cohesion in zebrafish (Danio rerio) Behav. Brain Res. 2008;193:148–151. doi: 10.1016/j.bbr.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller N, Gerlai R. Shoaling in zebrafish: What we don’t know. Rev. Neurosci. 2011;22:17–25. doi: 10.1515/RNS.2011.004. [DOI] [PubMed] [Google Scholar]
- Mora-Ferrer C, Gangluff V. D2-dopamine receptor blockade modulates temporal resolution in goldfish. Vis Neurosci. 2002;19:807–815. doi: 10.1017/s0952523802196106. [DOI] [PubMed] [Google Scholar]
- Nguyen-Legros J, Simon A, Caillé I, Bloch B. Immunocytochemical localization of dopamine D1 receptors in the retina of mammals. Vis Neurosci. 1997;14:545–551. doi: 10.1017/s0952523800012207. [DOI] [PubMed] [Google Scholar]
- Noldus Information Technology bv. EthoVision Reference Manual Version 3. Wageningen: The Netherlands; 2004. p. 327. [Google Scholar]
- Pan Y, Mo K, Razak Z, Westwood JT, Gerlai R. Chronic alcohol exposure induced gene expression changes in the zebrafish brain. Behav. Brain Res. 2011a;216:66–76. doi: 10.1016/j.bbr.2010.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Y, Chaterjee D, Gerlai R. Zebrafish strains differ in gene expression and in levels of neurochemicals in their brain: A semiquantitative RT-PCR and HPLC analysis. Behav. Genet. 2011b;216:66–76. [Google Scholar]
- Reimers MJ, Hahn ME, Tanguay RL. Two zebrafish alcohol dehydrogenases share common ancestry with mammalian class I, II, IV, and V alcohol dehydrogenase genes but have distinct functional characteristics. J Biol Chem. 2004;279:38303–38312. doi: 10.1074/jbc.M401165200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renier C, Faraco JH, Bourgin P, Motley T, Bonaventure P, Rosa F, Mignot E. Genomic and functional conservation of sedative-hypnotic targets in the zebrafish. Pharmacogen Genomics. 2007;17:237–253. doi: 10.1097/FPC.0b013e3280119d62. [DOI] [PubMed] [Google Scholar]
- Saverino C, Gerlai R. The social zebrafish: Behavioral responses to conspecific, heterospecific, and computer animated fish. Behav. Brain Res. 2008;191:77–87. doi: 10.1016/j.bbr.2008.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreibman L. Diagnostic features of autism. J Child Neurol. 1988;3(Suppl):S57–S64. doi: 10.1177/0883073888003001s11. [DOI] [PubMed] [Google Scholar]
- Sison M, Cawker J, Buske C, Gerlai R. Fishing for genes of vertebrate behavior: Zebra fish as an upcoming model system. Lab Anim. 2006;35:33–39. doi: 10.1038/laban0506-33. [DOI] [PubMed] [Google Scholar]
- Sison M, Gerlai R. Associative learning performance is impaired in zebrafish (Danio rerio) by the NMDA-R antagonist MK-801. Neurobiol Learn Mem. 2011;96:230–237. doi: 10.1016/j.nlm.2011.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spruijt BM, Hol T, Rousseau J. Approach, avoidance, and contact behavior of individually recognized animals automatically quantified with an imaging technique. Physiol Behav. 1992;51:747–752. doi: 10.1016/0031-9384(92)90111-e. [DOI] [PubMed] [Google Scholar]
- Steketee JD. Injection of SCH 23390 into the ventral tegmental area blocks the development of neurochemical but not behavioral sensitization to cocaine. Behav Pharmacol. 1998;9:69–76. [PubMed] [Google Scholar]
- Tager-Flusberg H. The origins of social impairments in autism spectrum disorder: studies of infants at risk. Neural Netw. 2010;23:1072–1076. doi: 10.1016/j.neunet.2010.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tropepe V, Sive HL. Can zebrafish be used as a model to study the neurodevelopmental causes of autism? Genes Brain Behav. 2003;2:268–281. doi: 10.1034/j.1601-183x.2003.00038.x. [DOI] [PubMed] [Google Scholar]
- Verhoeff NP. Radiotracer imaging of dopaminergic transmission in neuropsychiatric disorders. Psychopharmacol. 1999;147:217–249. doi: 10.1007/s002130051163. [DOI] [PubMed] [Google Scholar]
- Volicer L. GABA receptors in alcoholism. Ann Neurol. 1981;10:401–402. doi: 10.1002/ana.410100425. [DOI] [PubMed] [Google Scholar]
- Wahlsten D. Insensitivity of the analysis of variance to heredity-environment interaction. Behav Brain Sci. 1990;13:109–116. [Google Scholar]
- Wise RA. The role of reward pathways in the development of drug dependence. Pharmacol Ther. 1987;35:227–263. doi: 10.1016/0163-7258(87)90108-2. [DOI] [PubMed] [Google Scholar]
- Wise RA, Rompre PP. Brain dopamine and reward. Annu Rev Psychol. 1989;40:191–225. doi: 10.1146/annurev.ps.40.020189.001203. [DOI] [PubMed] [Google Scholar]
- Witkovsky P. Dopamine and retinal function. Doc Ophthalmol. 2004;108:17–40. doi: 10.1023/b:doop.0000019487.88486.0a. [DOI] [PubMed] [Google Scholar]
- Young AB, Penney JB. Neurochemical anatomy of movement disorders. Neurol Clin. 1984;2:417–433. [PubMed] [Google Scholar]
- Yung KK, Bolam JP, Smith AD, Hersch SM, Ciliax BJ, Levey AI. Immunocytochemical localization of D1 and D2 dopamine receptors in the basal ganglia of the rat: light and electron microscopy. Neurosci. 1995;65:709–730. doi: 10.1016/0306-4522(94)00536-e. [DOI] [PubMed] [Google Scholar]