Abstract
Complications in treatment of large bone defects using bone grafting still remain. Our understanding of the endogenous bone regeneration cascade has inspired the exploration of a wide variety of growth factors (GFs) in an effort to mimic the natural signaling that controls bone healing. Biomaterial-based delivery of single exogenous GFs has shown therapeutic efficacy, and this likely relates to its ability to recruit and promote replication of cells involved in tissue development and the healing process. However, as the natural bone healing cascade involves the action of multiple factors, each acting in a specific spatiotemporal pattern, strategies aiming to mimic the critical aspects of this process will likely benefit from the usage of multiple therapeutic agents. This article reviews the current status of approaches to deliver single GFs, as well as ongoing efforts to develop sophisticated delivery platforms to deliver multiple lineage-directing morphogens (multiple GFs) during bone healing.
Keywords: Growth Factor Delivery, Bone Regeneration, Biomaterials, Tissue Engineering, Spatial regulation, Temporal regulation, Bio-inspired
1. Relevance of the topic
Bone transplantation is one of the most common clinical procedures worldwide. Bone loss from trauma injury, tissue resection of tumors, revision surgery, osteonecrosis, spinal deformities, trabecular void, and infection can lead to significant bone loss with poor healing capacities or non-unions that pose a major clinical and socioeconomic problem. It is estimated that there are approximately 6.3 million fractures each year in the United States, with roughly 1 million who have skeletal defects [1] and over 500,000 requiring bone grafting procedures, costing approximately $ 2.5 billion [2]. These costs are estimated on fractures that heal on a first time successful surgical outcome. However, there is only an 85% success rate on a first procedure using bone grafting, and costs associated with complications and revision surgeries are estimated at 3 times this cost and more. A recent forecast of population development from the Federal Statistics Office predicts that in the year 2050, half of the population in Germany will be older than 48 and one third will be at least 60 years old. The proportion of people over the age of 80 will triple [3]. The aging population will lead to an increase in the demand for surgery for joint replacement and fracture treatment. The need for effective and scalable osteosynthesis strategies will take center stage in the coming decade.
1.1 Classical treatment approaches for large bone defects and their limitations
Although bone repair in fracture healing is usually an efficient process, there is a subset of cases that have been reported as complications, most often during the treatment of large bone defects and resulting non-unions. While there is no universally accepted definition of non-union resulting from segmental or large bone defects, it can be considered the failure of a bony union within 6–8 months [4] without any signs of further progression toward healing. The diagnosis for such cases in humans is based on a combination of clinical symptoms and physical findings, including radiographic evidence of a failure in osseous union. For non-union or critical-sized defect scenarios, a temporary material must be used to fill the bone defect. Historically, management of posttraumatic large bone defects and the resultant poor outcomes was done by amputation. In the past decades, massive cancellous bone autografts, free vascularized grafts, bone transport with distraction osteogenesis, often in combination with metal implants have been the alternatives to amputation for large bone defects, but still often involve delayed union, prolonged treatment time, and revision surgeries.
The current gold standard treatment of critical-sized bone defects is autogenous bone grafting. In this treatment, host bone is removed from another site (typically from the pelvis or iliac crest) and used to fill the bone defect [5, 6]. However, the complication rate in autogenous bone grafting is high and may include donor site morbidity, pain, paresthesia, prolonged hospitalization and rehabilitation, increased risk of deep infection, inflammation, and restricted availability. Another option for patients and surgeons is the use of bone allografts (typically derived from human cadavers). Many orthopedic allograft procedures have been FDA-approved and utilized for years. Success in both autograft and allograft procedures is attributed to the physical and biological similarity in donor (site or patient) and host tissue. However, orthopedic allografts carry risks of donor to recipient infection and disease transmission, and host immune responses. As a last resort, patients requiring bone repair or replacement may also consider a xenograft, or tissue grafting from a non-human. Early success in xeno-transplantation of a variety of cells, tissues, and organs created optimism towards this approach. However, after decades of research investigation as well as global clinical trials, bone xenografts are now considered to be unsuitable for transplantation due to risk of disease or virus transmission, infection, toxicity associated with sterilization, immunogenicity, and finally host rejection [7]. Synthetic bone graft substitutes have evolved, as a result of the limitations of bone grafts, and continue to be developed, but not yet reached clinical efficacy for the treatment of posttraumatic segmental bone defects. The limited bone regeneration potential of allograft, xenograft and synthetic replacements has prompted the pursuit of tissue engineering approaches for the regeneration of functional bone tissue [8–10].
1.2. New strategies in the treatment of segmental bone defects
Although, the classical approaches have not been entirely successful in restoration of functional bone tissue, they still serve as motivation and a gold standard for newer tissue engineering approaches. The ability of bone grafts, or devitalized, de-mineralized bone to release osteo-inductive signals and induce a response in adult stem-like cells, and osteogenic cells has unveiled promising strategies in bone tissue engineering, based on delivery of cells, matrix and bioactive molecules. Numerous growth factor (GF) proteins with an important role in this auto-inductive process have been investigated for their therapeutic potential in regeneration of segmental bone defects, including bone morphogenetic proteins (BMPs), transforming growth factor-beta (TGF-b), fibroblast growth factor (FGF), insulin-like growth factor (IGF), vascular growth factor (VEGF), platelet derived growth factor (PDGF), and stromal derived growth factor (SDF1) [11, 12]. However, these strategies still remain inferior, costlier and more complicated compared to the gold standard treatment of bone grafting. These inferior results may be due to the mode and timing of their delivery, poor control over the subsequent distribution of the factors locally and systemically, rapid degradation of the factors, undesirable systemic effects and toxicity, and an insufficient local concentration for the required time frame during regeneration. Consequently, the need to orchestrate the spatiotemporal delivery of single or multiple cues (simultaneously or sequentially) capable of instructing both cells resident in the tissue and transplanted cells will likely be key to the ultimate success of the regenerative process during bone healing [13]. The aim of this article is to review recent progress in delivering lineage- directing morphogens in the treatment of bone healing, with a specific focus in segmental or large bone defects. The article will cover:
-
(i)
An overview of biological mechanisms in bone healing,
-
(ii)
A brief review of current tissue engineering and regeneration strategies for bone,
-
(ii)
Growth factors and delivery methods in treatment of bone defects,
-
(iii)
Approaches that could provide greater control over bone regeneration driven by delivered GFs,
-
(iv)
Future outlook in bone tissue regeneration strategies.
This review does not attempt systematic comparisons of different morphogens and their outcomes in bone healing, or cover recent advances in small molecule therapies, treatment of bone healing in disease conditions, or extensively review cell and viral based gene therapies in the treatment of bone healing. There have been several excellent recent reviews on these other topics [14–23].
2. Endogenous mechanisms in secondary bone healing
A fracture occurs when the upper limit of bone strain is exceeded. Fracture repair, which aims at regaining the functional competence of a bone, is a complex and multi-factorial process, which is assumed to recapitulate bone development [24]. In optimal conditions and in contrast to most other tissues, injured bone can be reconstituted almost identically to its original shape without a scar. Depending on the fixation stability a primary (direct) or a secondary (indirect) healing pattern proceeds [25]. Primary fracture healing can be defined as primary cortical healing which involves a direct attempt by the cortex to re-establish itself. This type of healing occurs after anatomical reduction with minimal inter-fragmentary movement of the fracture fragments, followed by discrete remodeling units known as ‘cutting cones’ that cross the fracture site[26]. Under such conditions endochondral ossification is absent and little or no callus formation occurs. This repair method is nowadays less frequently aimed for and only seen with rigid compression fixation, and in small bone cracks (e.g. following stress fractures) [27].Fractures in the diaphyseal region affect cortical bone. Repair of these fractures, specifically those with motion between bone fragments or inter-fragmentary gaps, occur by secondary bone healing. This review deals with secondary bone healing in the diaphyseal region of the long bone, with the femur taken as an example.
2.1 Background of secondary bone healing
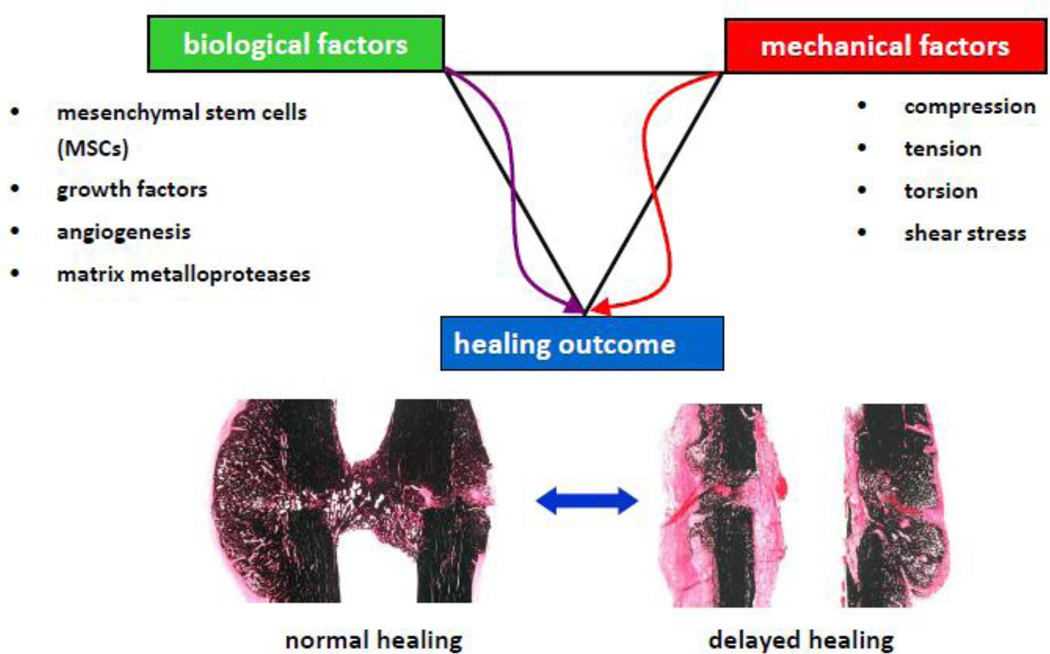
Secondary bone defect healing is a more frequent process of bone repair, and has been studied in greater detail in animal fracture models, and will be the focus of this article. The key tissues in secondary diaphyseal bone repair are the bone marrow and the periosteum, as these contain committed osteoprogenitor cells and uncommitted undifferentiated mesenchymal cells; both cell populations contribute to the bone healing process. Secondary healing of bones involves the classical stages of fracture healing. Healing begins with (i) inflammation that is followed by (ii) the formation of a periosteal callus through intra-membranous ossification, (iii) bridging of the bone defect by endochondral ossification, and finally (iv) the callus is resorbed during remodeling. The process of bone healing is highly sensitive to biological and mechanical stimuli, both influencing the path and the outcome (Figure 1). Tremendous efforts have been exerted in past decades to understand the separate influences of mechanics, and biology on bone healing outcome. In reality, these processes do not function independently. This has led to a new field termed “mechanobiology” in bone healing [28, 29]. Understanding the mechanisms of the endogenous bone healing process, and especially (in comparison) the biology of impaired/delayed bone healing, will generate treatment possibilities that provide better control of regenerating bone tissue.
Figure 1.
Biological and mechanical stimuli both influence the endogenous regeneration pathway, and control the outcome of bone defect healing. The optimal mechanical and biological stimulus would result in fast and uncomplicated healing outcome; an inappropriate stimulus leads to impaired/ delayed healing.
2.1.1 Normal healing
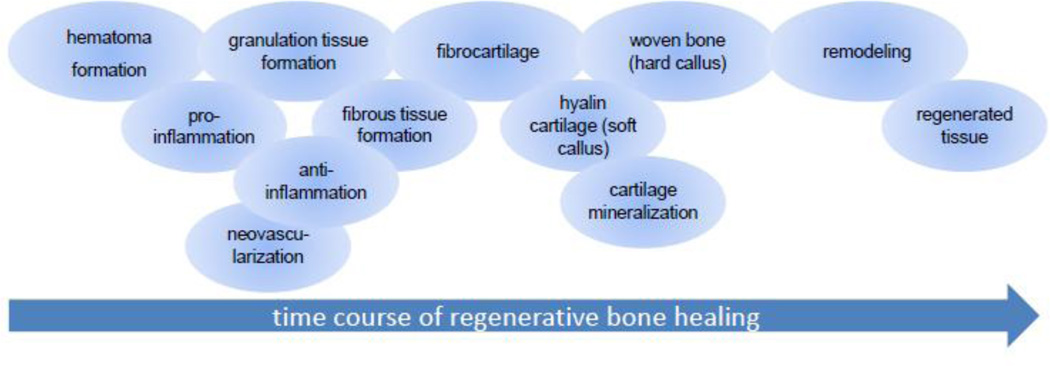
Normal bone healing proceeds through the following stages: hematoma formation, inflammation, revascularization, fibrocartilage, hyaline cartilage, cartilage mineralization, woven bone formation, and remodeling (Figure 2)[30]. When a bone fracture occurs, the ruptured blood vessels provide blood flow that fills the fracture site with a blood clot, and this also leads to a localized hypoxia and acidosis. The bioactive blood clot activates platelets and their release of alpha granules, transforming the clot into a fibrin-rich extracellular matrix (ECM)-hematoma. A combination of inflammatory cells, neutrophils, monocytes, and lymphocytes appear consecutively from ruptured blood vessels, and the bone marrow cavity via migration into the extracellular matrix of the hematoma. The hypoxic conditions in this healing phase induce the angiogenic HIF1α (hypoxia inducible factor 1 alpha) pathway [31–;34]. Therefore the revascularization process in the injured region starts in the hematoma, which has been reported to be inherently angiogenic [35]. Mesenchymal progenitors in the periosteal and endosteal envelopes, as well as from the bone marrow are activated by means of signaling molecules such as Interleukin 6 (IL6), Tumor necrosis factor-alpha (TNF alpha), Granulocyte colony-stimulating factor (GCSF), Stromal cell-derived factor-1 (SDF 1), Macrophage colony-stimulating factor (MCSF), and matrix proteases, and are recruited to the fracture site [36]. Once mesenchymal progenitors are present in the fracture gap, mechanical loading leads to matrix metalloproteinase (MMP) secretion and further enhance angiogenesis [37–39]. The recruited cells can participate in immune modulation [40], and differentiate into osteoblasts or chondrogenic cells.
Figure 2.
The consecutive overlapping phases of the regenerative bone healing process.
Osteoblasts are of mesenchymal origin, deriving from the non-hematopoietic part of bone marrow which contains the bone marrow mesenchymal stem cells (BMSCs). Osteoblast differentiation is under control of variety of growth factors, hormones and transcription factors. The TGF-beta super family includes the bone morphogenetic proteins (BMPs). These factors have either a direct influence on osteoblast activity, or an effect on other bone growth regulators. The BMPs’ major effects include enhancing osteoprogenitor cell proliferation and differentiation, while other members of the TGF-beta family control differentiated cell functions such as proliferation and matrix synthesis[41].
Chondrocytes of the soft callus proliferate and become hypertrophic while the extracellular matrix becomes calcified. Concurrently, blood vessels penetrate the chondrogenic tissue, bringing chondroclast and mesenchymal progenitors that initiate cartilage replacement with woven and lamellar bone. TGF-β2 (transforming growth factor beta 2), TGF-β3 and GDF5 (growth and differentiation factor 5) are involved in chondrogenesis [42]. VEGF (vascular endothelial growth factor) expression in hypertrophic cartilage is a key regulator in the transformation of the cartilaginous matrix into a vascularized osseous tissue [43]. This osseous matrix, the hard callus, is gradually replaced with woven bone coincident with expression of collagen 1, osteocalcin and alkaline phosphatase [27]. Woven bone is then remodeled into lamellar bone, leading to highly organized structures that lead to mechanical and biological restoration of bone function. The regenerative bone healing process is concluded with the remodeling stage, which gives rise to bone “restitution ad integrum”. The entire healing cascade is orchestrated under tight control, both spatially and temporally, by biological cues produced by cells and ECM at the injury site. However, in unstable fractures and large bone defects, blood vessel formation is either impaired or limited, mechanical stimulus is altered, there is an absence of endochondral bone formation, and mesenchymal progenitor cells remain in too low of numbers to induce bone formation. The spatiotemporal expression of the biological and mechanical cues is perturbed, resulting in a delayed healing outcome[44].
2.1.1.1 Spatiotemporal presentation of regenerative cues in bone healing
Multiple factors regulate the bone healing cascade by affecting different aspects of osteoblast and chondroblast biology, including their migration, proliferation, chemotaxis, differentiation, inhibition, and extracellular protein synthesis. Signaling molecules that influence bone healing can be summarized by the processes they influence: the initial pro and anti-inflammatory response, cellular growth, differentiation and tissue morphogenesis, angiogenesis, feedback through inhibitory molecules, extracellular matrix cues (Table 1).
Table 1.
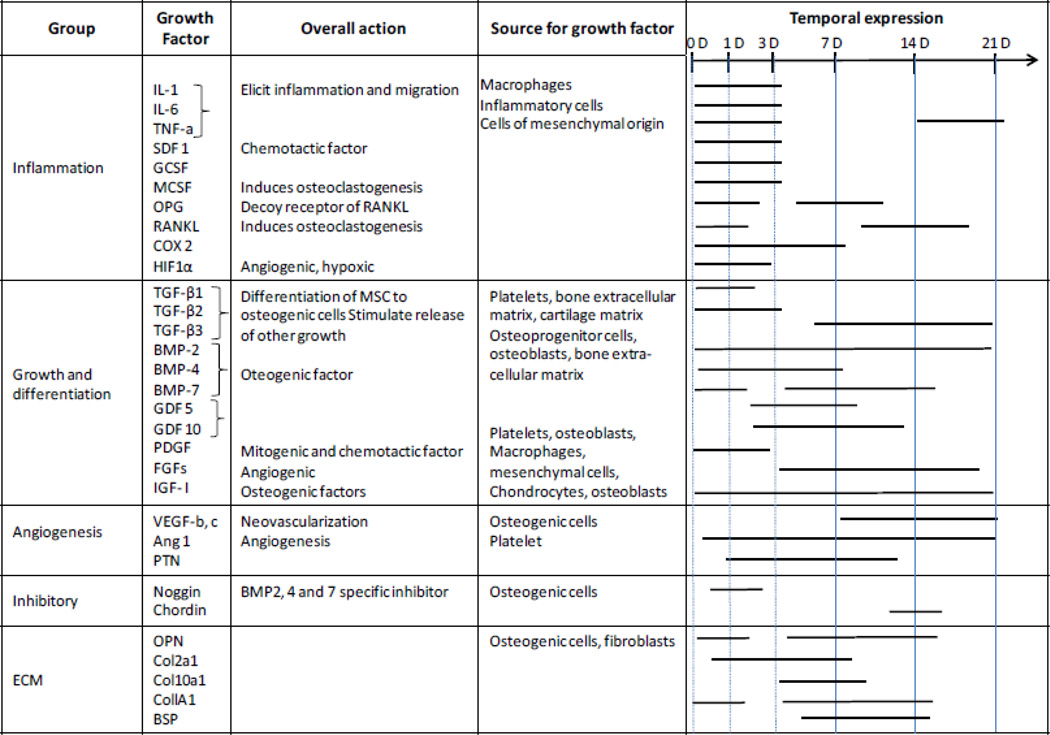 |
Vascularization is key for bone healing, as supported by a failure in bone healing when revascularization is disturbed, even at an early stage [45]. An initial pro-inflammatory response involves secretion of TNFα (tumor necrosis factor alpha), IL-1 (interleukin 1), IL-6, IL-11 and IL-18 [24]. These factors promote angiogenesis, and cell mobilization [46]. Without TNFα the fracture healing becomes impaired, emphasizing its importance in the tightly regulated healing process [47, 48].
The cartilaginous step of the bone healing process is influenced by the fracture stability [49–51]. Limited movement, in a 3 mm osteotomy gap not more than 1 mm compression or 1 mm shear, promotes cartilage formation and is beneficial for the healing outcome; [49]. However, larger movements, even though resulting in larger callus formation, hinder the bony bridging and consolidation of the fracture callus [52, 53]. Tissue formation is mechanoregulated, as cells sense tissue strain and hydrostatic pressure. Direct intramembranous bone formation occurs at very small strains (<5%) and hydrostatic pressures (<−0.15MPa), endochondral ossification occurs at larger values (<15% and >−0.15MPa) whereas high values of strain (>15%) lead to fibrocartilage and connective tissue formation and thus prevent bone healing [54–56].
Transformation of the soft callus to a mineralized callus and woven bone rely on the key players of bone homeostasis RANKL (receptor activator of nuclear factor kappa-β ligand), RANK (receptor activator of nuclear factor kappa-β), and OPG (osteoprotegerin) [57, 58]; these have been harnessed for clinical applications [59, 60].
Remodeling reinstates a stabilized mineral callus that adapts to the loads occurring in each individual overtime, so as to restore normal bone anatomy, structure and functional properties. However, remodeling can result in weak bone, as seen with altered loading in hip implant patients [61]. In the worst case this could result in consecutive fractured bone.
Although sources for stem and progenitor cells are similar (periosteum, vasculature, bone marrow and adjacent muscle, vascular networks)[62], different fracture locations probably recruit cells from these sources in a distinct manner. For example, metaphyseal fractures have a good opportunity to recruit stem cells from the cell-rich bone marrow, while muscle coverage in this area of the bone is scarce. Diaphyseal fractures, on the other hand, have good muscle coverage, while the bone marrow develops with age to a cell-poor fatty marrow [63]. As both fracture types can heal, cell recruitment is often adequate, even though different cell sources are likely exploited. Utilization of these cell depots by clinical techniques such as micro-fracturing or bone marrow cavity reaming, depending on the site of injury, enables mobilization of these cells, and their subsequent participation in the bone repair process. The loss of these cell sources for example due to a segmental bone defect where bone marrow volume is compromised, or during complicated fracture trauma where muscle volume is compromised, leads to impaired healing outcome. As a consequence, there is a need for tissue engineering strategies to supplement these cell sources, and aid in recruitment of cells into the injury site during such clinical cases. There is growing evidence that the vascular system and the MSC niche in the muscles provide a source of progenitor cells for bone fracture healing [64, 65]. Consequently, this may have an impact on the mechanism of healing in fracture vs. segmental defects, as the extent of injury and dislocation of muscle differs in both cases [66]. Future studies are needed to clarify the varying contribution of different cell sources towards bone healing in various scenarios.
2.2 Failure in bone healing
Despite advances in the treatment of fractures, failure in bone healing still occurs. Some of these failures are due to mechanically challenging conditions at the fracture and once these are corrected, healing takes place. Other failures are due to biological limitations, and in most of these cases we lack an understanding of the involved molecular mechanisms that lead to delayed healing and non-unions. This is likely the reason that single growth factor therapies of fixed dose and limited duration exhibit limited therapeutic success: bone heals instead with a complex hierarchy of processes that involve complex pathways and dynamic dosing of multiple factors in close coordination. Clinically relevant small and large animal models of delayed bone healing are being utilized in an effort to better understand the cascades that lead to a healing delay or to non-unions [67–70]. Data from studies utilizing these animal models have indicated that all bone healing stages still occur during delayed bone healing, albeit with a difference in temporal onset and spatial distribution of callus tissue compared to normal healing subjects [71]. It has been observed that the hematoma that forms in subjects with a delayed healing has an altered composition, and remains for a longer duration through the healing phase [72]. There is also a delay in expression of angiogenic factors [73], down regulation of the endothelial cell marker vWF occurs, and the expression of extracellular matrix and markers of chondrogenesis and osteogenesis are changed [74]. Eventually, if the window of opportunity is missed due to a late onset in normal healing patterns, this can lead to either a hypertrophic- or atrophic non-union.
During the formation of non-unions there is a soft tissue prolapse into the defect, lack of cartilage formation, and negligible mechanical properties of the callus [44, 68]. Other than the obvious lack of mechanical stimuli in the defect and insufficient biological control and resources to fill the defect in a timely manner, in-depth knowledge of the biological mechanisms during the development of non-unions still remain unclear.
Although bones have an intrinsic ability to regenerate themselves, beyond a certain defect size, bone is not capable of restoring its architecture and function when only mechanically stabilized. The defect healing capacity is limited to a few millimeters in humans, and healing of a gap of 3 mm is significantly delayed. In critical sized defects, healing does not occur at all. In such cases autologous cancellous bone grafts may be used. The success of autologous cancellous bone grafts [75–78] demonstrates at least three characteristics essential for bone tissue engineering, namely the ability of a material to be osteo-conductive, osteo-inductive, and exhibit osteogenic potential. Osteo-conductive scaffolds promote the attachment of osteoblasts and migration of osteo-progenitor cells at the injury site, osteo-inductivity then furthers the differentiation of osteo-progenitor cells to form bone. Osteogenic potential refers to the cells that are vital and can differentiate and proliferate to build new bone.
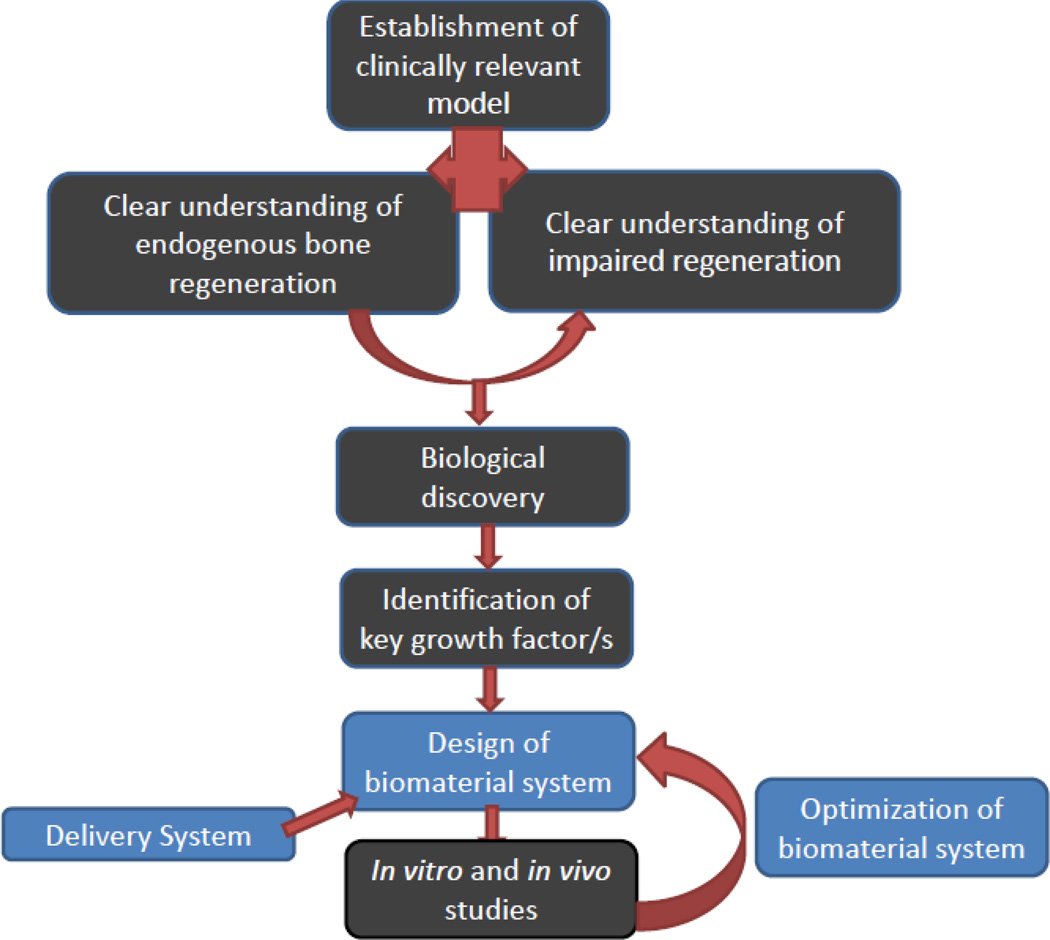
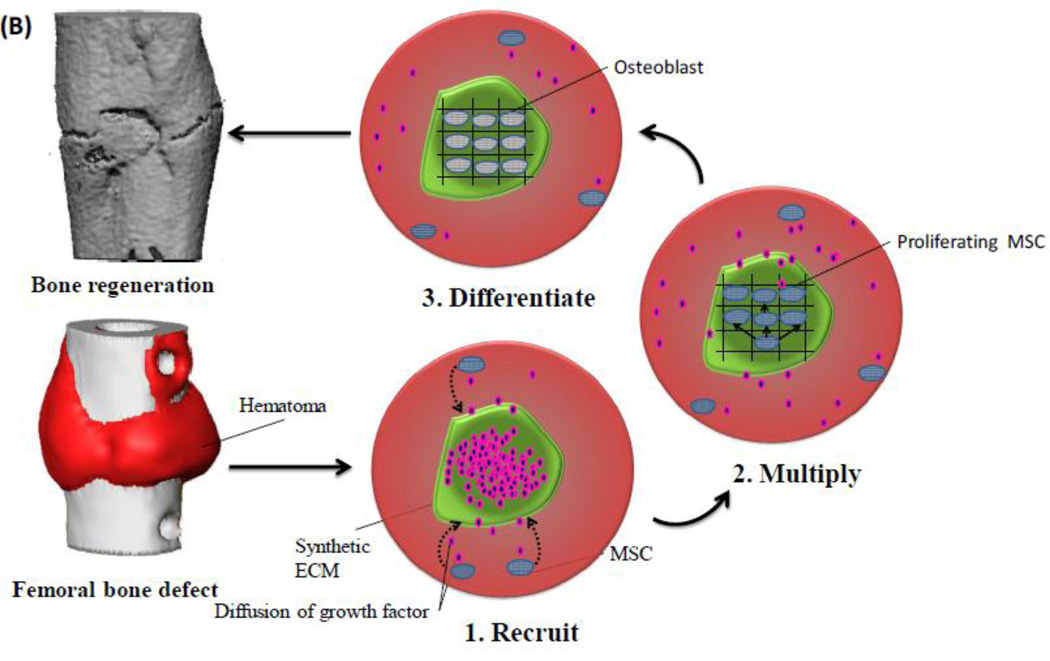
The current understanding of endogenous bone regeneration and clinical success with autografts has inspired a number of regenerative medicine strategies. To mimic endogenous repair strategies, a wide variety of growth factors (GFs) have been utilized using bio-inspired strategies (Figure 4) to create mineralized tissue either in vitro or in vivo. However, mimicking key aspects of the natural biological process is challenging, and may require provision of multiple therapeutic agents at an optimized ratio, physiological doses, and in a specific spatiotemporal pattern.
Figure 4.
Schematic illustration of a bio-inspired engineering approach to develop a suitable delivery system, based on identification of endogenous regeneration processes and failures [85]. The aim of the resultant system is to mimic critical aspects of natural healing processes, and provide suitable regenerative cues that control the participating cells.
Bone tissue engineering concepts that are in the clinic today include the use of growth factors, BMP2 and BMP7 [79–81], and platelet-derived growth factor (PDGF) [11]. Platelet rich plasma [82–84] has also been widely explored. However, these either have to be used in supraphysiological dosages or show no clinical evidence of a real benefit. A major demand on regenerative medicine strategies to bone healing is the often complex situation, as one must take into account the extrinsic mechanical environment, extracellular matrix properties, inflammation, cell migration and differentiation, angiogenic processes, soluble factor transport/ nutrition; these must be balanced to allow for tissue regeneration. Research directed to influence the inflammatory phase of the bone healing process [85], to enhance angiogenesis [86–88], to mimic growth factor release [89–95], and improve cell recruitment [96–99] underpin efforts of the tissue engineering field to further improve bone regeneration.
An alternative to growth factor strategies is the delivery of cells either capable of directly forming bone tissue, or capable of secreting factors that induce regeneration by host cells. The former concept is based on the rationale that single or repeated boluses of exogenous stem cells or progenitor cells could enhance the regenerative potential in the bone defect by directly increasing the local cell population [100]. The tissue engineering approach using ex vivo-cultivated stem cells typically involves the harvest of suitable autologous donor tissues to recover resident stem cells, typically mesenchymal stem cells (MSCs), followed by their expansion in culture to a large population. These cells are either transplanted back into the patient by injection (systemic or local), or they are seeded into a prefabricated three- dimensional scaffold and then implanted back into the patient. Despite advances in stem cell research, cell transplantation remains problematic due to concerns associated with cell sources and numbers, and the use of ex vivo-manipulated cells may have as yet undetermined, unwanted, or poorly controlled consequences in addition to the practical barriers and translational limitations [101]. Tissue regeneration by stem cell transplantation is also hindered by many other hurdles to clinical translation, including potential immune rejection, pathogen transmission, potential tumorigenesis, labor-intensive procedures, high cost, and difficulties in regulatory approval. Added to this are issues that will take considerable time to resolve, such as the optimization of existing scaffold materials for cell delivery, vascularization, cell integration with the host tissue, and the capacity of delivered cells to form specific structures in vivo. All of these motivate the conceptually simpler approach of delivering signaling cues (e.g., growth factors) that stimulate endogenous cells to migrate to the defect and initiate repair.
3. Growth factor delivery for bone tissue engineering
While mimicking the endogenous regeneration cascade for bone healing seems like a reasonable approach, it is important to distinguish between the endogenous GFs that are naturally presented for bone formation and remodeling, and the utilization of exogenous GFs. Endogenous bone regeneration involves local production of minute quantities of the proteins continually. On the other hand, delivery of exogenous GFs relies typically on the one-time application of a supra-physiological concentration. The original reported applications of growth factors as therapeutic agents involved intravenous injection, but this delivery method is not localized to the target tissue and is also ineffective because of the growth factors’ short half-lives [102, 103]. Consequently, no further pharmacological activity is achieved once protein levels are reduced below a threshold concentration. The chemical identity, concentration, duration, and context of growth factors also contain information that controls cell fate in various ways (Table 2). Coordinated interactions with other GFs, cells, and extracellular matrices (ECM) define a local cellular microenvironment that cells sense and that performs complex and dynamic regulation of cellular processes [104, 105]. The cellular effects can be controlled by variables as listed in Table 3, and as reviewed previously [102]. For example, the endogenous GF activities are regulated by binding to extracellular matrices, and presentation to cells that provide a tightly regulated feedback, along with inhibitory signals. The critical drug delivery challenge for exogenous factors is to identify a biomaterial carrier that maintains a sufficient concentration of the GF at the application site in a manner that mimics the normal physiologic control required for bone regeneration. Altogether, these challenges have motivated the development of controlled delivery systems that allow the sustained, dynamic and localized delivery of small amounts of these factors to the target cell population and tissue site.
Table 2.
Manifestations of growth factors on cellular effects are multifaceted.
| Action of growth factors on cells | |
|---|---|
| - | an onset of vectorial migration (chemotaxis effect) |
| - | an onset of random migration (chemokinesis effect) |
| - | a stimulation of cell division (mitogenic effect) |
| - | an induction of cell differentiation (control cell fate) |
| - | patterning of cells (morphogenesis) |
| - | an initiation of programmed cell death (apoptotic effect) |
| - | an inhibition of cellular activity |
| - | a modulation of metabolic activity |
| - | a combination of above |
Table 3.
Variables that need to be considered during the design of a biomaterial system that enables tight control over cell response.
| Design variables that provide control over cell response | |
|---|---|
| - | Concentration of GF |
| - | Gradient formation |
| - | Microenvironment |
| - matrix composition | |
| - mechanical cues | |
| - matrix architecture | |
| - presence of other cells | |
| - presence of other growth factors/cytokines/chemokines | |
Bolus delivery of GFs is technically simple, but the subsequent distribution of the factors throughout the body and their rapid degradation may lead to undesirable systemic effects and toxicity, and an insufficient local concentration for the required time frame, respectively. The use of polymeric vehicles to locally deliver the factors in various formats provides a method of controlled, localized delivery for the desired time frame. Growth factors can be incorporated into the polymer delivery systems in a variety of different ways [106, 107]. Depending on the method of incorporation, the release rate of the growth factor may be controlled by processes including diffusion of factor, charge interactions, erosion or degradation of the polymer, swelling of the polymer, osmosis wetting phenomenon or dissolution. Challenges to polymer delivery include the denaturing and deactivation of proteins resulting from various encapsulation processes utilizing harsh solvents, cross-linking agents and high temperatures. A variety of processing techniques have been developed to bypass these issues including soaking the scaffold in a solution of growth factor after processing, use of hydrogel delivery systems where growth factor incorporation can be achieved at low temperatures [103], and supercritical carbon dioxide processing [108]. Chemical/ genetic modifications of growth factors to improve stability and bioactivity have also shown success. For example, acidic Fibroblast growth factor (aFGF) was conjugated with heparin sulfate proteoglycan to protect it from proteolytic degradation [109]. Additionally, peptide mimics of growth factors provide an alternative system to preserve bioactivity. For example, a BMP-2 mimic covalently coupled to alginate hydrogel induced ectopic bone formation in rats [110]. Peptide mimics often allow for harsher processing treatments to incorporate into a carrier system than would be possible with growth factor encapsulation.
A wide variety of growth factors have been identified and isolated for analysis in in vitro and in vivo osteoinduction (Table 4). The general principle of growth factor presentation follows a similar path; a key subset of growth factors (BMPs) is reviewed here as an example for illustration of the strategy in bone healing. The delivery of growth factors in biomaterials for tissue engineering has been pursued by: (i) physical encapsulation of growth factors in the delivery system and, (ii) chemical immobilization of the growth factor into or onto the matrix. The former approach is achieved by the encapsulation of the factor, followed by the diffusion and pre-programmed release of growth factor from the substrate into the surrounding tissue. The latter approach typically involves chemical binding or affinity interaction between the growth factor and polymer substrate. Both general concepts and recent advances in these two strategies will be discussed in this section. In addition, the delivery of multiple factors and growth factor release on demand provide another level of control, and will be discussed.
Table 4.
Single GF release systems in bone regeneration.
| Single growth factor system |
Carrier | Species | Model | Ref. |
|---|---|---|---|---|
| BMP-2 | Heparin/apatite-coated Titanium implant |
Rat | Tibial defect | [231] |
| Autogenic graft | Human | Tibia non-union | [232] | |
| Collagen | Goat | Closed tibia fracture | [233] | |
| PLA coating | Sheep | Spine fusion | [234] | |
| Collagen | Rat | Femoral defect | [235] | |
| Alginate | Rat | Femoral defect | [236] | |
| BMP-7 | Collagen | Baboon | Calvarial defect | [237] |
| BMP-3 | Hydroxyapatite/TCP | Rat | Segmental femoral defect | [238] |
| BMP-13 | Collagen | Rat | Intramuscular tendon defect | [239] |
| TGF-β1 | Demineralized bone matrix/ carboxymethyl cellulose |
Rabbit | Calvarial defect | [240] |
| TCP+bone marrow | Rabbit | Radius defect | [241] | |
| TGF-β2 | Bolous/BSA solution | Rabbit | Tibia fracture | [242] |
| TGF-β3 | TCP | Baboon | Spine defect | [243] |
| IGF-I | osmotic pump device | Rat | Calvarial defect | [244] |
| PLGA microparticles | Sheep | Tibia defect, diaphyseal defect | [245] | |
| PLA coating | Minipig | Tibia defect | [246] | |
| IGF-II | Collagen | Rat | Facial defect | [247] |
| Basic-FGF | Injectable composite | Rabbit | Segmental defect | [248] |
| Hyaluronan gel | Baboon | Fibula defect | [249] | |
| Gelatin hydrogel | Dog | Maxillary furcation defect | [250] | |
| Acidic-FGF | Agarose | Rat | Calvarial defect | [251] |
| PDGF BB | Collagen | Rabbit | Tibia defect | [252] |
| Chitosan/TCP | Rat | Calvarial defect | [253] | |
| GDF-5 | Collagen | Rat | Femoral defect | [235] |
| VEGF | PLGA scaffolds | Rat | Calvarial defect | [254] |
3.1 Physical entrapment of growth factor within matrices
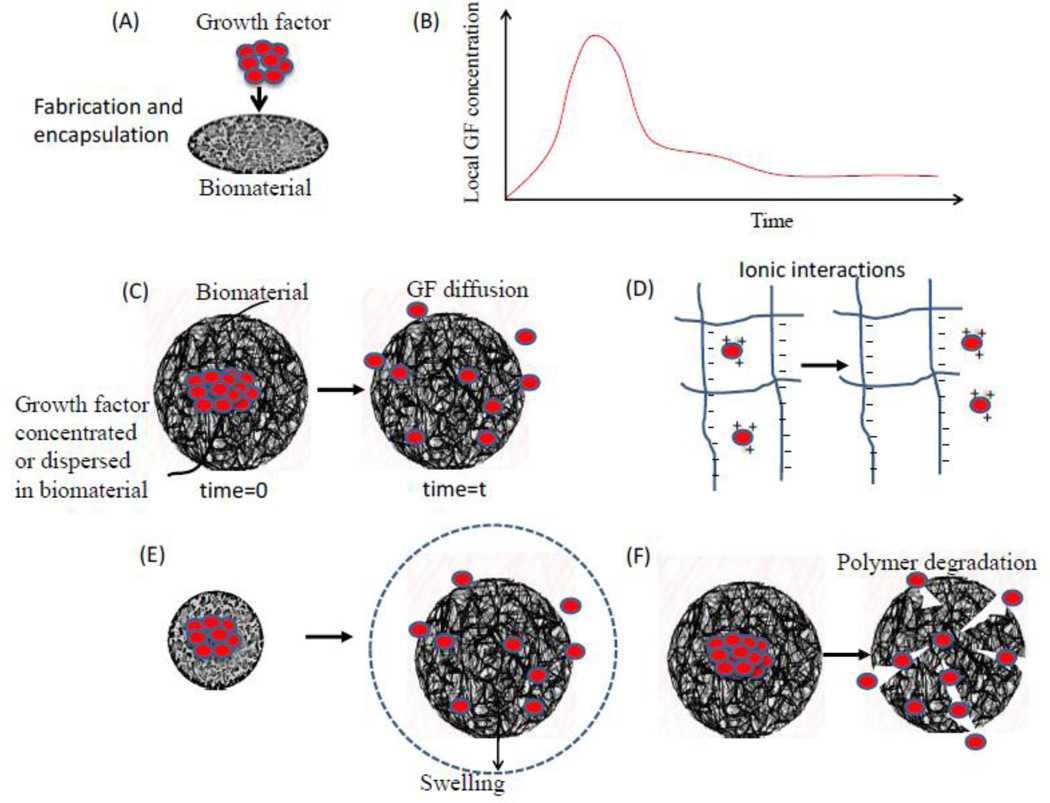
The simplest method for encapsulating growth factors into a polymer matrix is by mixing the factor with the polymer before its gelation or solidification (Figure 5). Subsequent protein release is often characterized by an initial burst release of a large amount of the factor (surface associated factor) followed by a phase of slower release from the carrier system due to diffusion through the polymer. More generally, diffusion often controls release of growth factors from biomaterials, especially in hydrogel systems [111]. The mesh size and environmental conditions control solute diffusion in ionic gels [112], and hydrogels can be tailored to exhibit zero-order or near zero-order release. With special modifications to the carrier or protein, the release mechanisms can be further manipulated. For example, chemical modification of hydrophilic alginate with long alkyl chains rendered them hydrophobic, which significantly altered release profiles [113]. Modifications of the protein itself also allows for additional changes in carrier affinity, bioactivity, stability and bioavailability. To modulate changes in carrier affinity, succinylated [114], acetylated [115] or biotinylated [116] BMP derivatives have been demonstrated to modulate protein–carrier interactions. Plasmin cleavage [117] is another approach to change the affinity to a carrier material. Truncation of BMP-2 by plasmin cleavage removed a positively charged fraction of the N-terminus, yielding a protein with a lowered pI-value and, therefore, reduced electrostatic interactions with the negatively charged proteoglycans of the ECM [116]. It should also be noted that hydrophobic matrices that degrade by surface erosion can exhibit zero-order release kinetics without a burst release phase, if surface erosion occurs more rapidly than drug diffusion through the polymer.
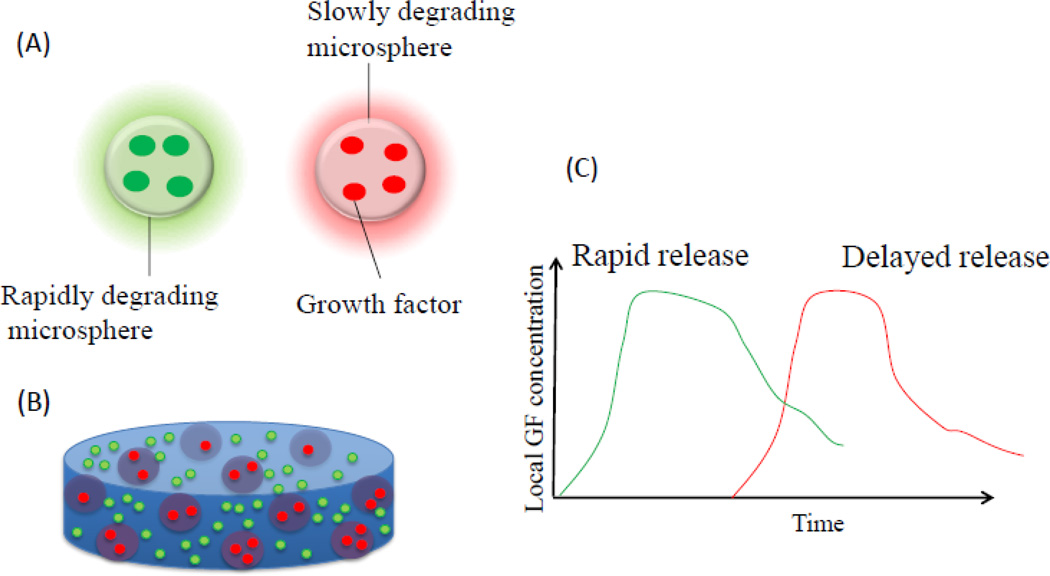
Figure 5.
GFs are frequently incorporated into a scaffold by physical encapsulation, using direct mixing before solidification of the biomaterial (image A). This typically leads to a burst release of a significant quantity of the GF, followed by a lower rate of release over an extended time period (image B). Examples of such single growth factor release systems are illustrated in C-F. Image C demonstrates a simple system wherein a growth factor is physically encapsulated into a biomaterial and is allowed to diffuse out of the material overtime. Image D, demonstrates a system wherein the growth factor release is controlled by charge interactions between the factor and the biomaterial or by addition of other intermediates such as heparin. Image E, demonstrates a system wherein the growth factor release is based on the physical expansion of the biomaterial system. Image F, demonstrates a system wherein an encapsulated growth factor is released based on the degradation of the biomaterial.
Bone morphogenetic proteins (BMPs) are a well-characterized family of growth factors, and have been investigated for their capacity to improve bone healing [118, 119]. Among BMPs, BMP-2 is an osteogenic growth factor, FDA approved, and routinely used in orthotropic sites for bone generation. Delivery of BMP-2 has been the focus of a variety of preclinical and clinical models of bone regeneration. Delivery of BMP-2 protein from an absorbable collagen sponge induces bone formation and heals bony defects [120]. In relation to intra-oral defects, the repair of large critical-sized defects in the canine mandible can be accomplished with BMP-2 protein delivery from a collagen scaffold [121]. BMP-2 can also be delivered from synthetic materials, such as poly (ethylene glycol) hydrogels, to promote the repair of bone defects [122]. US Food and Drug Administration (FDA)-approved BMP products for bone regeneration are now available. However, the transition into clinical studies has had only moderate success, and relies on large doses of BMPs for bone formation. One such product is the InFUSE™ bone graft (Medtronic SofamorDanek, Memphis, TN). The Infuse bone graft consists of recombinant human BMP2 within a collagen substrate that is housed in a spinal cage. While effective, it has also shown deleterious effects [123–126]. Endogenously, BMPs are released naturally by cells, and mere nanogram quantities of the proteins per gram of bone matrix are enough to trigger the bone repair cascade. However, microgram quantities of BMP per gram of matrix material are needed to produce the same effect with the current recombinant human BMP products [127]. The side-effects found with InFuse product may result from its inability to provide a controlled release and effective localization of the BMP- 2, leading to ectopic bone formation[128]. Another example illustrating similar problems is the use of VEGF [129]. Variable reports in the literature regarding VEGF delivery suggest that determining the optimal release rate and local dose for therapeutic effect will be critical [130]. OP-1™ (Stryker, Kalamazoo, MI) was granted a humanitarian device exemption by the FDA for the treatment of established non-unions. In addition to BMP, sustained release of other osteo-inductive factors, such as fibroblast growth factor-2 and insulin-like growth factor-I, can also accelerate bone formation, as shown in calvarial defect and tibial fracture models [92, 131]. However, clinical applications of these factors in bone regeneration have yet to be achieved. While considerable success, and promise, has been shown with these approaches, the poor control over factor release and localization with biomaterials used to date in the clinic motivates the development of more sophisticated delivery systems.
As bone formation is an extended process, it is necessary to maintain protein bioactivity within the material for sustained release. Delivery of osteoinductive factors via microparticles or nanoparticles, alone or contained in a scaffold, may enable more continuous release to induce tissue formation [132–137]. The factors are still physically encapsulated, but their release can be regulated by the polymer degradation; tailoring the degradation rates of the particles makes it possible to change the release profiles of the growth factors. Synthetic polymers, such as gelatin and PLG, have demonstrated the capacity for prolonged delivery of TGF-β and recombinant human BMP-2 (rhBMP-2), along with sustained bioactivity over long periods of time to promote bone healing [138, 139]. Furthermore, particles containing different factors can be embedded and combined into bulk scaffolds, enabling sequential and tighter control of release profiles (Figure 6) [140].
Figure 6.
Encapsulation of growth factors into polymer microspheres that degrade at various rates (A), for example by altering the extend of cross-linking, enable control over timing of release (C). Similarly, in scaffold delivery systems (B), factors can be encapsulated into the more rapidly degrading bulk for rapid release, or within more slowly degrading microspheres embedded in the bulk polymer for a delayed release.
3.2 Chemically immobilizing growth factors in a delivery vehicle
Immobilization strategies of bioactive molecules onto matrices come in two flavors: (i) conditional/temporary, and (ii) permanent (Figure 7). In the conditional immobilization strategies, factors that are chemically immobilized in a delivery vehicle can be released with well controlled kinetics mimicking the ability of natural ECMs to locally bind, store and release bioactive growth factors to direct them to the right place at the right time [141, 142]. Polymer scaffolds can be modified (with or without linkers) to interact with GF molecules, thereby hindering their diffusion out of the scaffold and prolonging their release [143]. Immobilization of GFs can be made to occur through reversible association with the scaffold (i.e. binding/de-binding interactions), or irreversible binding to the polymer. GFs can also be released upon degradation of the matrix itself [144]. The number of binding sites, the affinity of the factor for these sites, and the degradation rate of the scaffold can be optimized such that the amount of bound signal, as well as the release profile can be accurately controlled. Using hydrogels it is possible to control growth factor release kinetic by ECM binding interactions. For example, functionalized alginate hydrogels provided sustained release of BMPs, VEGF, or other proteins over several days to weeks by locally binding to the alginate matrix, and promoted segmental bone repair [90]. A more recent study highlights spatiotemporal control of the regenerative process. This study utilized a hybrid protein delivery system comprised of two parts: (i) a perforated cylindrical polycaprolactone nanofibrous mesh that spatially confines the defect region and, (ii) a functionalized alginate hydrogel that provides temporal BMP growth factor release. BMP-mediated functional restoration of challenging 8 mm femoral defects in a rat model was compared to current clinical standard of collagen delivery [145, 146]. The nanofiber mesh tube was essential to promote maximal mineralized matrix synthesis, prevent extra-anatomical mineralization, and guide an integrated pattern of bone formation, while, the growth factor reversibly associated with the alginate hydrogel, slowing the release.
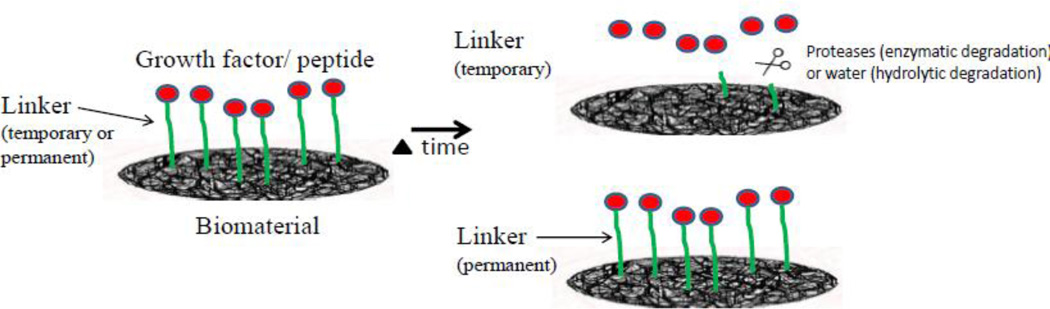
Figure 7.
Growth factors chemically cross-linked to a biomaterial via a bifunctional linker can be released via degradation of the bond, or remain permanently bound for a continuous presentation to interacting cells.
Permanent immobilization of biomolecules leads to long-term presentation of bioactive molecules to cells from the surface. This general approach to factor presentation has been demonstrated with a number of different molecules [147]. Covalently tethered EGF leads to an enhanced response of MSCs, as compared to soluble EGF [148]. Similarly, tethering BMP-2 [149] via self assembly, or directly on silk fibroin films induced enhanced osteogenic behavior of cells, compared to cells cultured in the presence of soluble osteogenic stimulants [150]. A smaller amount of immobilized growth factor may also be required as a result of multivalency of presentation and high local concentrations [151–153]. Multivalent ligands enable clustering of cell surface receptors, more effectively activating signaling pathways [154]. Furthermore, immobilization of biomolecules may inhibit down-regulation of signaling molecules that results from internalization of the signal-receptor complex, and as a result the signal duration may be increased [155]. In place of entire molecules, mimics of BMP 2 have been covalently tethered to alginate gels, and shown to accelerate bone healing in rats [156, 157]. Further, by immobilizing specific matrix proteins [158, 159] and adhesive peptides [160, 161] onto a material it is possible to more closely mimic the extracellular matrix (ECM) environment, and provide a multifunctional cell-adhesive surface [162] [146, 152, 163–165]. For example, modification of alginate with an RGD-containing peptide promoted osteoblast differentiation, whereas minimal cell adhesion was observed on unmodified hydrogels. Controlling the adhesion ligand density, valency, and nanoscale spacing between adhesive regions can alter cell proliferation [152, 166]. Cross-talk between growth factor and integrin receptors may allow one to selectively gain control over the behavior of cells that come in contact with the biomaterial.
3.3 Delivery of multiple signals (combinations or sequences)
Tissue regeneration may be enhanced by delivery of combinations or sequences of factors, as single growth factor delivery has a number of limitations (Table 5). Significant efforts have been made in recent years to develop schemes for combinatorial or sequential delivery of multiple growth factors [145, 167, 168]. Challenges with this approach include the selection of proper GF cocktails, understanding their synergies, and rigorously controlling their concentrations, gradients, and timing (Figure 8). Each growth factor has a specific physiological mechanism of action, and this drives the selection of a specific release profile. Often, though, the most effective dosage and release profile is not known, and must be empirically explored.
Table 5.
Limitations of single GF systems.
| Drawbacks | Implications | |||
|---|---|---|---|---|
| - | Control of cellular behavior |
- | Inadequate control of : migration, proliferation, differentiation, morphogenesis, apoptosis |
|
| Single growth factor |
- | Inability to mimic dynamics of physiological cell signal controls |
||
| - | Co-activation/ priming |
- | Reduced bioactivity of cells due to insufficient activation and crosstalk |
|
| - | Receptor dimerization | |||
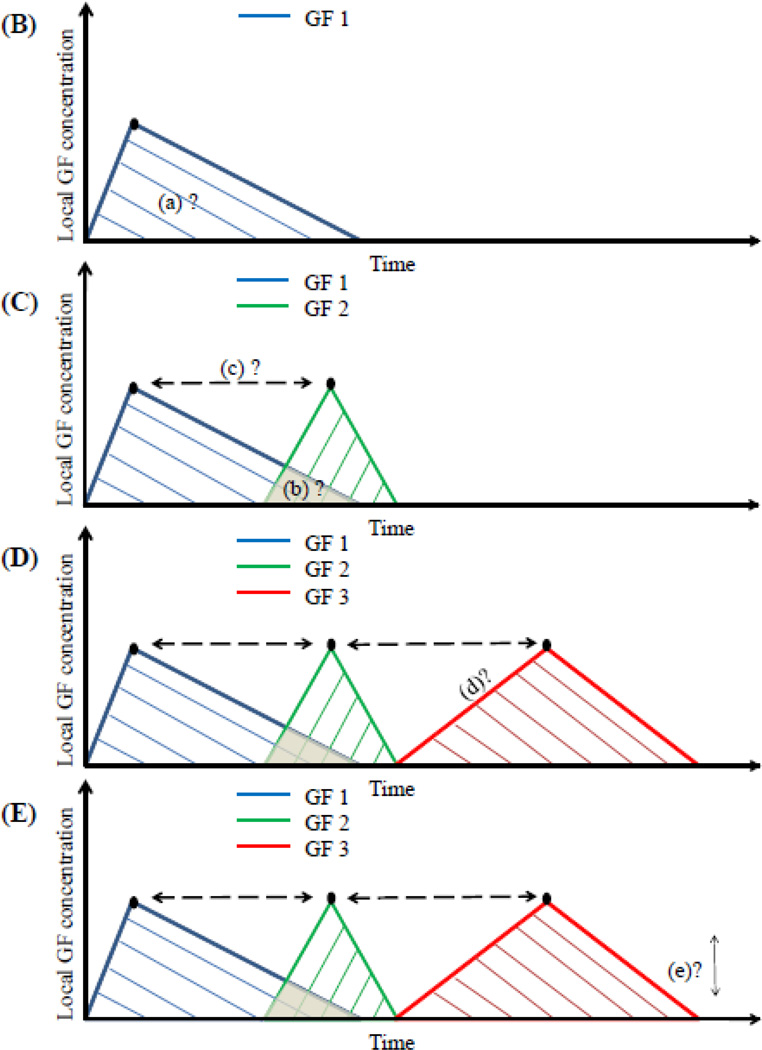
Figure 8.
Bio-inspired approach to mimic a template for desired cellular response during the stages of fracture healing (A). Multiple morphogens can be delivered in sequence or in combination to gain control of the different phases in healing. The time interval between each growth factor delivery, the total amount and concentrations, and synergies between factors need to be considered for the design of these delivery systems. (B) the release of a single growth factor- GF 1. The area under the curve (a), the peak concentration, and duration of exposure can be used to control cell behavior. (C) release of two growth factors (GF1 and GF2). The factors can be delivered with some overlap (region b) or purely in sequence, and the timing of their peak concentration may also be regulated (c). The choice of overlap depends on their synergistic or inhibitory effects on osteogenic behavior. Multiple growth factors can also be released using controlled delivery from biomaterials (GF 3). (D) rate of release (parameter d) can be defined based on in vitro experiments that demonstrate cellular response to a desired concentration and time of exposure for a certain behavioral response. (E) the effective dosage that elicits a maximum cellular response may have a lower and upper limit defined by a range (parameter e). A range outside this may cause a lowered or a different response in cellular behavior. All these parameters may be similar or different for varying GF’s (1, 2, 3, or more).
The benefit of sequential GF delivery from a single structural polymer scaffold was first demonstrated in the context of angiogenesis [169]. Subsequently, simultaneous delivery of low doses of two growth factors, BMP-2 and TGF-beta 3, was shown to increase bone formation as compared to single factor delivery [90, 167]. A number of systems have now been developed to allow simultaneous or sequential delivery of morphogens for bone regeneration (Table 6). With the goal of mimicking endogenous healing patterns, a sequential presentation of an angiogenic factor (VEGF) was followed by an osteogenic factor (BMP-4), and this led to greater control of MSC-driven bone repair [170]. Titanium wire [171] has been coated with multiple layers of poly (D,L-lactide) (PDLLA) to allow sequential release of gentamicin (antibiotic), BMP-2, and IGF-I, and this increased bone formation. To provide even greater temporal resolution, four-layer mesh structures were developed to allow tight regulation over the timing of factor release [172]. Hydrogels are also being explored for their utility in sequential release [173–175]. Presentation of RGD ligands along with soluble BMP 2 has been shown to increase co-activation of receptors and osteogenic properties of progenitor cells [176]. These findings are not surprising, as during tissue morphogenesis and repair, cellular processes typically require a complex network of several signaling pathways, and are usually induced by more than one growth factor.
Table 6.
Multiple GF release systems in bone regeneration.
| Multiple growth factor system |
Carrier | Species | Model | Testing temporal regulation |
Ref. |
|---|---|---|---|---|---|
| PDGF/IGF-I | Titanium implant | Dog | Jaw bone | No | [107] |
| IGF-I/TGF-beta 1 | poly(D,L-lactide)- coated titanium K-wires |
Rat | Tibia | No | [178] |
| BMP 2/ FGF | Collagen sponge | Rat | Femur | No | [179] |
| BMP-2/ TGF-β3 | Alginate scaffold | Rat | Femoral defect | No | [255] |
| BMP-2/ TGF-β3 | Composite scaffold | Mice | Muscle | No | [256] |
| BMP-4/ VEGF | PLGA scaffold | Mice | Subcutaneous | No | [170] |
| BMP 7/ TGF-β1 | Collagen | Baboon | Extraskeletal sites | No | [257] |
| Bone Protein/ PDGF/IGF | Collagen/PLG | Rat | Skull caps | No | [177] |
| BMP2/ VEGF | Composite scaffold | Rat | Cranial | Yes | [258] |
| BMP2/ VEGF | Composite scaffold | Rat | Subcutaneous and femoral defect |
Yes | [259] |
If not appropriately chosen, the delivery of a combination of growth factors could lead to inhibitory, as well as stimulatory responses in bone formation [177]. For example, in a rabbit tibia fracture model, the combined application of IGF-I and TGF-β1 showed a synergistic effect on fracture healing [92, 178], whereas the combination of BMP-2 and bFGF resulted in decreased bone formation [179]. Therefore, even though there is a desire to present cells with multiple growth factors to gain control over their behavior, it is also important to choose and screen these growth factors, often using in vitro experiments that probe the osteogenic behavior of cells in combination and sequence [89, 180, 181]. One recent study exploring these issues utilized a two-layered, crosslinked gelatin gel system for sequential delivery of two bone growth factors, BMP-2 and IGF-I. Alkaline phosphatase activity in cultured C3H10T1/2 (C3H) cells was greatest with the addition of BMP-2 followed by either IGF-I or a combination of BMP-2+IGF-I. However, simultaneous presentation of BMP-2+IGF-I at all times was not as effective. Further, prolonged high affinity binding of growth factors to receptors can not only result in negative feedback and downregulation of signaling pathways, but also pathological effects such as cellular transformation [182].
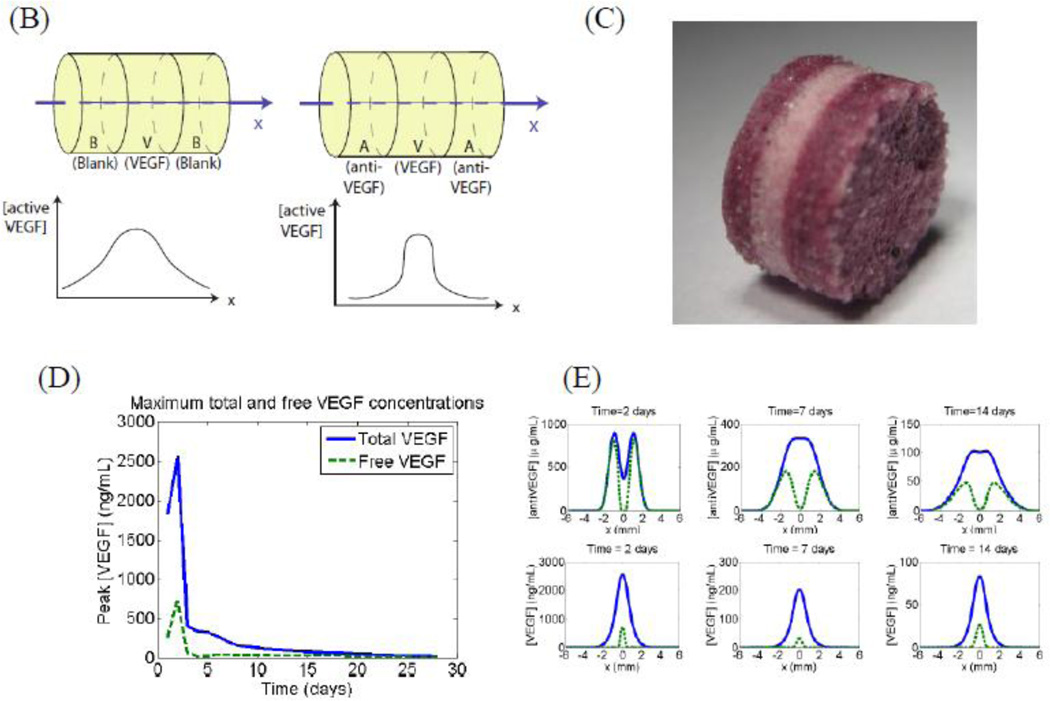
Nature frequently utilizes opposing factors to create a stable activator gradient to robustly control pattern formation, and this concept has inspired recent work with growth factor delivery. A biomimicry approach was developed to deliver both pro- and anti-growth factors from spatially restricted zones of a synthetic polymer (Figure 9 A). This resulted in a temporally stable and spatially restricted regenerative zone [183], and was exploited to spatially control angiogenesis (Figure 9 B). Delivery of only the stimulatory molecule led to angiogenesis in a broad tissue zone, while release of the two spatially separated agents led to narrow and tightly defined angiogenic region. The resulting spatially restrictive and temporally sustained profiles of active signaling allowed the creation of a spatially heterogeneous and functional vasculature. Such an approach would be beneficial in situations where spatial control of the regeneration of bony tissue is desired, for example in regeneration of spine defects wherein ectopic bone formation is undesirable and can be deleterious, or in filling osteochondral, spinal, or growth plate defects where juxtaposed bone and cartilage is required (Figure 9 C).
Figure 9.
Spatial control of growth factor and inhibitor release to provide tight spatial control over regeneration. The location of factor release and orientation of the scaffold is chosen to create a zone where the regeneration of the tissue is desired. The promoter and inhibitor partially cancel each other’s effects, leading to a sharp cut-off in the spatial location where regeneration is promoted (A). This approach has been shown to be effective in creating an angiogenic zone with high spatio-temporal resolution. Layered scaffolds were made delivering VEGF and Anti-VEGF (B-C). Simulation of concentration profiles of unbound VEGF and total VEGF in the surrounding tissues over time shows initial bursts in both VEGF and antibody, they react quickly and the resulting concentration of active VEGF is significantly lower than the total VEGF in the first two days (D). (E) simulation plots of concentration profiles of anti-VEGF and VEGF at 3, 7, 14, and 21 d in which VEGF and anti-VEGF are regulated to partially cancel each other to enable spatial resolution. During implantation, the orientation of the scaffold was chosen to create an angiogenic zone directly over the section of the femoral artery that was ligated—with a goal of creating new blood vessels that would bypass the ligated vessel. The study showed that using this design, it was possible to create an angiogenic zone of 1mm wide that lasts for 3 weeks. In contrast, no incorporation of the antibody in the side layers resulted in an angiogenic zone that starts out very broadly and shrinks over time. The approach of delivering antagonists may be used in other morphogenic processes where spatial control is important, for example juxtaposed cartilage and bone in spine and limbs (F-G).
3.4 Local and external on-demand release
Polymers traditionally used in tissue engineering have limited sensitivity to external stimuli. This has spurred the development of bioresponsive materials in which the release of factors is regulated locally or externally. Bioresponsive materials can be designed to undergo structural, often reversible, changes with alterations in environmental factors (e.g. temperature, pH, electric field, solute concentrations, light), allowing one to build in functionality (phase transition, alteration in shape or release of encapsulated growth factors or cells [62] and [63]) on-demand. For example, biomaterials can be engineered to mimic the ability of natural extracellular matrix to undergo cell-triggered proteolysis and matrix remodeling (e.g. with a combination of matrix metalloproteinase substrates and cell adhesion sites) [184]. A proteolytically cleavable material system was shown to lead to bone formation following cell mediated matrix remodeling and subsequent release of BMP -2 [144]. This approach mimics the mechanism by which factors are often released in vivo from stores in the natural ECM by invading cells. Similarly, as tissues in the body are subjected to mechanical stimuli, this signaling can be exploited to allow on-demand release of growth factors. The release of growth factors reversibly bound to a vehicle was demonstrated to dramatically increase with strain, enhancing tissue formation [185].
On-demand" growth factor release systems (ie enzymes secreted by infiltrating cells regulate release) represent a potentially important concept, and are based on the perspective that “natureknows-better" in their design. These systems allow the wound healing host responses to decide when factors are released. It will be complex, however, to adapt this approach to multiple-GF loaded devices. It may be possible, though, to combine these systems with a more traditional growth factor release system that is preprogrammed to release a factor(s) at a pre-defined time, in order to appropriately sequence and combine factor availability.
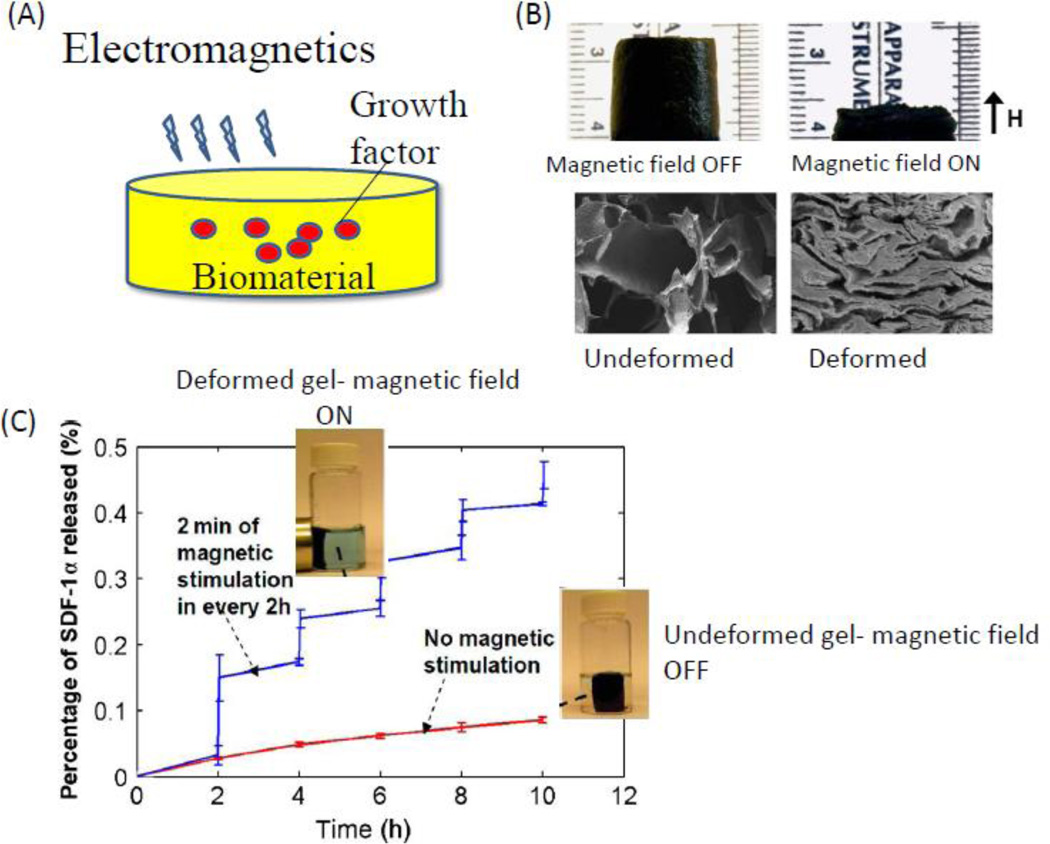
A logical extension of this concept is to trigger factor release externally. This may be useful in situations where one wishes inflammation to diminish prior to factor release, or to release distinct factors as specific stages in regeneration are reached. A variety of strategies are being pursued with this goal [186, 187]. For example, magnetic field gradients and ultrasound are being utilized for the on-demand release of growth factors and cells, in vitro and in vivo [188, 189] (Figure 10). The release of biological agents from conventional porous scaffolds is typically governed by molecular diffusion, material degradation, and cell migration, which do not allow for dynamic external regulation. Figure 10 illustrates a new active porous scaffold that can be remotely controlled by a magnetic field to deliver various biological agents on demand. The deformation and volume variation allows a new mechanism to trigger and enhance the release of various drugs including mitoxantrone, plasmid DNA, and a chemokine from the scaffold. The porous scaffold can also act as a depot of various cells, whose release can be controlled by external magnetic fields. The use of such techniques can be used as a method to activate on demand a cell population at the bone injury site with controlled release of a GF that is intended as a short burst, for example IFN or TNF alpha, which has been shown to upregulate CXCR4 receptors in MSC to a migratory phenotype [46], but the inflammatory cytokines are not favorable in long-term.
Figure 10.
On-demand release of GFs using external stimuli provided in a non-invasive manner (e.g., electromagnetic stimulus) (A). (B) magnetic fields can be used to mechanically deform a macroporous ferrogel. (C) the magnetically-triggered deformation system can be used to release drugs on demand. Graph demonstrates a step increase in cumulative release of SDF-1α following each 2 min of magnetic stimulation (repeated every 2 hour) vs. no magnetic stimulation. Images adopted from Zhao et.al [188].
3.5 Plasmid DNA delivery from Polymer scaffolds
An approach that aims to bypass limitations of direct protein delivery from polymer scaffolds is the use of local gene delivery with delivery of plasmid DNA encoding the protein. Gene delivery using both viral and non-viral vectors provides a versatile approach to promote local, sustained expression of tissue-inductive factors. While viral approaches today provide the most efficient means of gene transfer, they have associated safety concerns that hinder their translation into the clinic. Non-viral vectors employing plasmid DNA are believed to be safer, and have a clearer path to clinical translation in tissue regeneration [190]. However, bolus delivery of plasmid DNA in vivo has not been shown to be effective, and is typically associated with low levels of gene transfer and cellular expression, perhaps due to a limited exposure of cells to the plasmid [16]. Delaying clearance of the plasmid DNA from the tissue, protecting against its degradation, and providing repeated opportunities for cellular internalization are key to maintaining effective concentrations for long times [6–9] and making this approach feasible [10]. Polymeric delivery represents an approach that can increase residence time within the tissue and protect against degradation [191]. A pioneering study employing naturally-derived matrices demonstrated significant bone regeneration [191, 192]. Subsequently, sustained delivery of plasmid DNA from synthetic polymers allowed tight control over the time-frame of plasmid availability, and resulted in transfection of a large numbers of cells at a localized site [193]. The delivery of condensed plasmids enabled more efficient gene expression and enhanced bone regeneration [194]. Other studies have demonstrated non-viral vectors delivered from natural scaffolds promote tissue formation in a variety of injury models, such as bone, and the key role that microenvironmental cues play in regulating the extent of plasmid uptake and expression [190–192, 195, 196]. Design parameters of the delivery vehicle (e.g., porosity, loading) [197] may be exploited to regulate the quantity and duration of protein production, and the number and location of cells expressing the transgene [198]. Finally, in vivo transfection efficiencies and controlled delivery from polymeric carriers can also be improved through application of external stimulus using electroporation [199, 200], nucleofection [201], and sonoporation [202].
4. Conclusions and future outlook
Strategies for growth factor delivery from biomaterials have successfully enhanced tissue formation and healing in many animal models and patients. Materials for tissue engineering have significantly progressed over the years, from being initially viewed as biologically inert structural supports, to platforms capable of providing signals to cells and tissues and orchestrating regeneration. Studies, to date, have mainly investigated the delivery of a single growth factor for tissue regeneration [203, 204], but the failure to provide more complex combinations of signals present in the normal physiologic environment may limit their success in certain settings. Endogenous bone regeneration relies on several growth factors and cells that work in concert to form functional tissue. To successfully regenerate bone in many clinical scenarios, appropriate combinations of inductive factors in a specific spatial and temporal sequence may be required. More sophisticated release mechanisms, often responding to local or externally-imposed environmental conditions are likely to provide greater control over the various stages of bone regeneration.
The delivery of multiple factors, each potentially with distinct spatial and temporal presentation, creates a very large variable space that must be explored. The delivery of multiple growth factors should mimic the endogenous profile of growth factor production during bone repair. Therefore, a greater understanding of the required therapeutic doses and durations will be important to obtain the benefits resulting from the association of growth factors and scaffolds. Although this concept seems logical and appealing, the intrinsic properties of growth factors limit the number of materials and preparation methods that can be used to prepare growth factor releasing scaffolds. This likely will require changes in the current approach to design factor delivery vehicles. In addition, delivery materials in the future are also likely to program bone regeneration by directing multiple stages of this process. These concepts are briefly discussed in the following sections.
4.1 Approaches to screening the large variable space
Optimization of temporal and spatial release using modeling
In the past, successful drug delivery systems were typically developed as a result of an empirical, trial and error selection of constituents and configurations. However, the large variable space created by the delivery of multiple factors with distinct spatiotemporal presentation will make this approach extremely cumbersome, slow, and expensive. An increased utilization of mathematical modeling would allow one to much more rapidly and easily design vehicles capable of the desired factor(s) presentation in tissues. Modeling can facilitate vehicle design by identifying key parameters and molecule release mechanisms [173]. Computer aided mathematical modeling promises to provide high-throughput screening, thereby reducing the number of experiments needed to design a new vehicle [205]. Simple reaction/diffusion models have already been utilized to design systems releasing two factors to achieve distinct spatial compartmentalization [183]. Models varying from simple empirical models to complex mechanistic models that consider diffusion, swelling, and erosion processes simultaneously have been reported [206]. Combining mathematical models with 3D cell culture models (see below) can provide a particularly powerful approach to designing delivery systems [207].
Organ of on a chip testing
The application of microfluidic 3D cell culture devices is another rapidly developing field that would aid in the screening of growth factors and their effects on cellular behavior, and design of delivery vehicles [208]. These systems can provide an intermediate between traditional cell culture and in vivo testing, (e.g., explant-based microfluidics) [209]. For example, culturing tissue biopsies in a microfluidic device may provide a more holistic model for detecting cellular response to growth factors. Compared to traditional cell culture models, biopsy tissue retains the complex cell–cell and cell–matrix interactions. Typically, 3D cell culture models mimic in vivo tissue physiology much more accurately than traditional 2D cell culture models [210, 211], and the integration of 3D tissue mimics with microfluidics [212] allows one to create large numbers of tissue mimics that could be utilized in the development of growth factor delivery vehicles. Importantly, these types of organs on a chip allow for time lapse monitoring of cellular responses that may not be easily followed in vivo.
High-throughput screening
Biomaterials for bone tissue regeneration have traditionally been individually developed and tested for their effect on cells, but emerging technologies that enable high-throughput screening may dramatically impact this development process. Alongside in situ computer simulations, microscale technologies can facilitate high-throughput experimentation by miniaturization of assays, thereby providing a powerful tool for screening combinations of growth factors, small molecules, matrix materials and cellular secretome and kinetic analysis and responses [213, 214]. These systems may be especially useful for investigating synergistic and inhibitory effects of combinations of biomolecules. Recent developments in laboratory automation, such as robotic spotters capable of dispensing and immobilizing nanoliters of material, can be used in optimization of cell interactions using combinatorial matrixes in a high-throughput manner. For example, synthetic biomaterial arrays including thousands of combinations of polymeric materials have been assayed for their effects on differentiation of human embryonic stem cells and human mesenchymal stem cells [215, 216]. While there exists a large array of endogenously expressed growth factors (Table 1), so far only a small set of growth factors (Table 4 and 6) have been tested in vitro. Using high throughput methods, many other growth factors could potentially be rapidly screened.
In vivo molecular imaging and cellular imaging
Imaging is one of the few technologies that can generate longitudinal data sets in intact host environments [217] and so may provide key information regarding temporal effects of factors and combinations on bone regeneration[218]. A thorough review of the different imaging technologies is described elsewhere [219]. These technologies may meet a critical need for information describing local drug delivery and GF-release [220], cell responses [90, 221], and functional outcomes [222, 223], during bone repair. In vivo multimodal imaging methods are already proving, in experimental animals and human subjects, helpful in the design smart biomaterials for controlled release of GFs [222, 223].
4.2 In situ programming
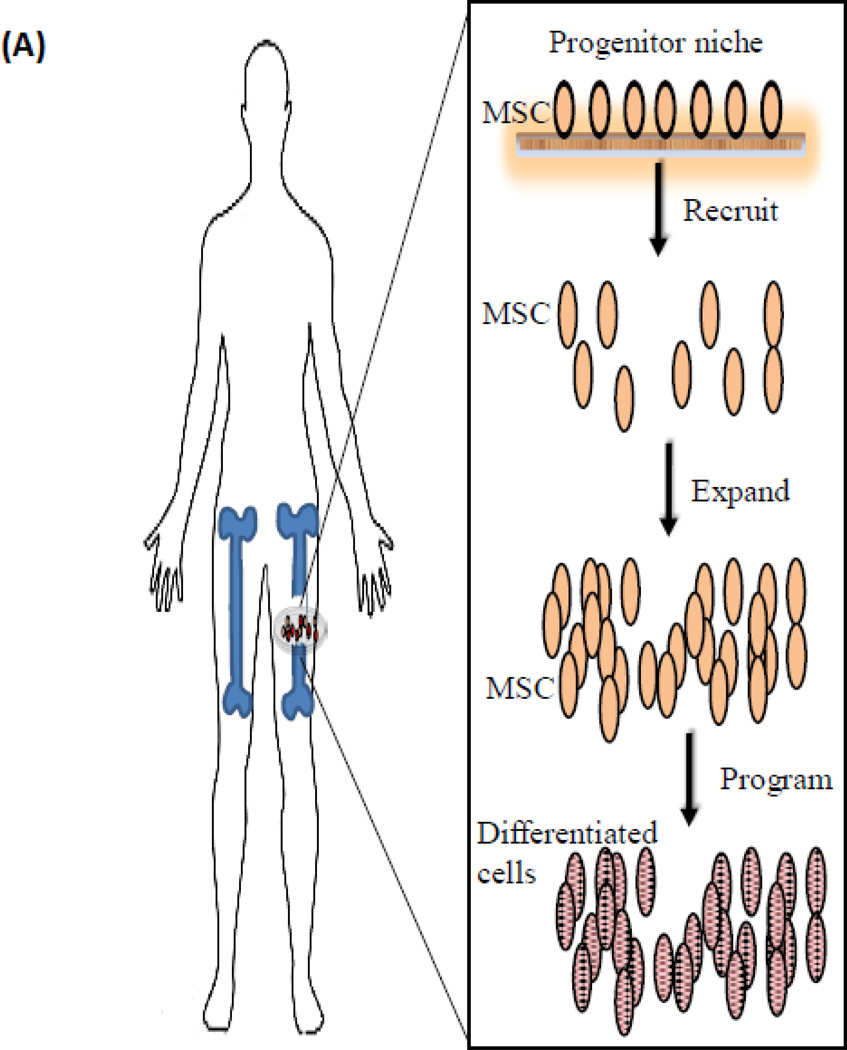
As one looks ahead, growth factor delivery systems may transition to in situ cell programming strategies that use biomaterials to recruit stem cells from the surrounding tissue, expand the cells, and differentiate them to promote functional tissue regeneration (Figure 11 A). Currently, many tissue engineering approaches focus on a rigid bone substitute with a high load bearing capacity - targeting the end-point of regeneration. Such rigid materials may block the self-adapting capacity of living bone tissue and control of cell fate [28]. Adult bone marrow derived mesenchymal stem cells represent an important source of cells for bone tissue regeneration. A large pool of MSC’s in the bone marrow exists (BMSCs). The ability to recruit and program in situ the host BMSCs to aid regeneration of bone after severe trauma is very promising. Cell fate can be programmed using biological cues [104, 105, 224], however, this concept has not been extensively implemented using controlled GF delivery from biomaterials. In the future, one may specifically recruit, expand and differentiate host BMSCs in a critical size bone defect, and promote functional bone restoration. The in situ approach would involve identification of (i) chemoattractant(s) that recruits the target cell population (BMSCs), (ii) identification of a mitogenic agent that promotes proliferation of the BMSCs, and (iii) identification of a osteogenic agent that promotes differentiation of the BMSCs. Using biomaterials it possible to deliver these growth factors in a sequential manner to provide tight control of the BMSC behavior to promote bone regeneration (Figure ll B). This approach could avoid ex vivo manipulation of autologous cells, while maintaining many of the advantages of cell therapies. To drive successful regeneration via endogenous stem cell homing, it will be key to direct stem cells to find their way to the defect site, and to provide the recruited stem cells with a local environment – both mechanically and biologically - where they can proliferate and differentiate efficiently.
Figure 11.
Future approach to bone regeneration. (A) Illustration of the concept for in situ programming: recruiting cells (e.g., MSCs) from a progenitor niche, expanding and differentiating the MSCs at the site of injury. (B) Schematic of an implantable biomaterial system that mimics the microenvironment of a bone injury, allowing the delivery of a (i) chemoattractant that recruits the target cell population (MSCs), (ii) subsequent delivery of mitogenic agent that promotes proliferation of the MSCs, and (iii) presentation of an osteogenic agent that promotes differentiation of the MSCs.
While biomaterials in the past have mainly been used as space fillers, current tissue engineering concepts use biomaterials to guide local cell behavior. Next generation biomaterial development will take form of a more life-like multi-functional materials that are able to simultaneously provide complex biological signals (chemical, structural and mechanical), replace mechanical function and respond to environmental stimuli. In the future, biomaterials may be designed to manipulate specific cell populations that reside in the host at a significant distance from the implant site. The immune system, which is often overlooked, has been shown to play a role in bone tissue repair [225], and future endeavors in material design will potentially expand beyond stem cell recruitment to include immune-modulation. Recent demonstrations of biomaterials as regulators of the immune system [226] illustrate the concept of recruiting and manipulating immune cells locally, in order to generate a systemic response. Combinations of systemic and local manipulations may open new possibilities for treating bone healing problems in diseased patients.
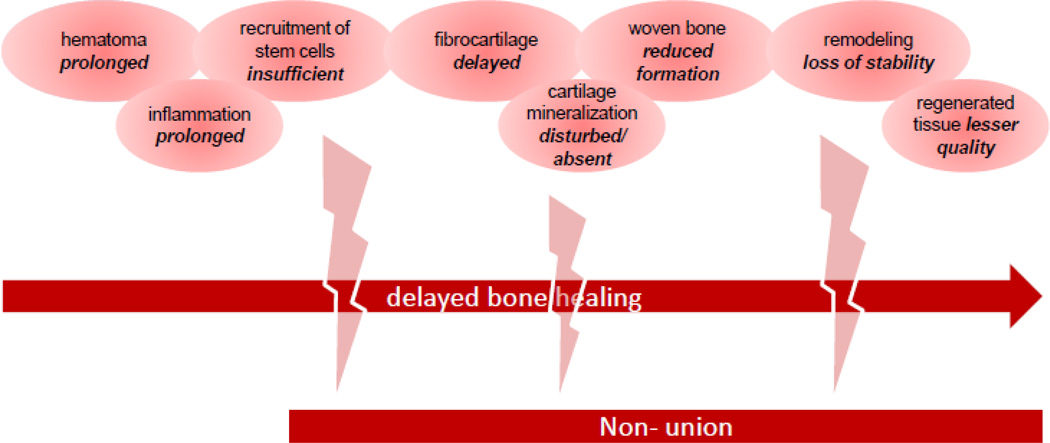
Figure 3.
The bone healing sequel can be interrupted or disturbed I n each phase of the regenerative healing process, leading to non-union. This challenges the current treatments, and is leading to the new approaches in regenerative medicine that allow a timely and effective restoration of bone defects.
Acknowledgments
The authors gratefully acknowledge the award of an Einstein Visiting Fellowship by the Einstein Foundation Berlin through the Charité – Universitätsmedizin Berlin, Berlin-Brandenburg School for Regenerative Therapies GSC 203 (DM), and financial support from the Nachwuchsakademie Medizintechnik (DFG- ME 4083/1-1), and NIH (R37 DE013033).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Langer R, Vacanti J. Tissue engineering. Science. 1993;260:920–926. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- 2.Laurencin C, Khan Y, El-Amin SF. Bone graft substitutes. Expert review of medical devices. 2006;3:49–57. doi: 10.1586/17434440.3.1.49. [DOI] [PubMed] [Google Scholar]
- 3.Bundesamt S. Bevölkerung Deutschlands bis 2050. 2003 [Google Scholar]
- 4.Rodriguez-Merchan EC, Forriol F. Nonunion: General principles and experimental data. Clin Orthop Relat R. 2004:4–12. [PubMed] [Google Scholar]
- 5.Muscolo DL, Ayerza MA, Aponte-Tinao LA. Massive allograft use in orthopedic oncology. Orthop Clin N Am. 2006;37:65-+. doi: 10.1016/j.ocl.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 6.DeCoster TA, Gehlert R, Mikola EA, Pirela-Cruz MA. Management of posttraumatic segmental bone defects. J Am Acad Orthop Sur. 2004;12:28–38. doi: 10.5435/00124635-200401000-00005. [DOI] [PubMed] [Google Scholar]
- 7.Parikh SN. Bone graft substitutes: past, present, future. J Postgrad Med. 2002;48:142–148. [PubMed] [Google Scholar]
- 8.Eppley BL, Pietrzak WS, Blanton MW. Allograft and alloplastic bone substitutes: a review of science and technology for the craniomaxillofacial surgeon. Journal of craniofacial surgery. 2005;16:981–989. doi: 10.1097/01.scs.0000179662.38172.dd. [DOI] [PubMed] [Google Scholar]
- 9.Peter SJ, Miller MJ, Yasko AW, Yaszemski MJ, Mikos AG. Polymer concepts in tissue engineering. J Biomed Mater Res. 1998;43:422–427. doi: 10.1002/(sici)1097-4636(199824)43:4<422::aid-jbm9>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 10.Rose FRAJ, Oreffo ROC. Bone tissue engineering: Hope vs hype. Biochemical and biophysical research communications. 2002;292:1–7. doi: 10.1006/bbrc.2002.6519. [DOI] [PubMed] [Google Scholar]
- 11.Lieberman JR, Daluiski A, Einhorn TA. The role of growth factors in the repair of bone. Biology and clinical applications. The Journal of bone and joint surgery. American volume. 2002;84-A:1032–1044. doi: 10.2106/00004623-200206000-00022. [DOI] [PubMed] [Google Scholar]
- 12.Kitaori T, Ito H, Schwarz EM, Tsutsumi R, Yoshitomi H, Oishi S, Nakano M, Fujii N, Nagasawa T, Nakamura T. Stromal cell–derived factor 1/CXCR4 signaling is critical for the recruitment of mesenchymal stem cells to the fracture site during skeletal repair in a mouse model. Arthritis & Rheumatism. 2009;60:813–823. doi: 10.1002/art.24330. [DOI] [PubMed] [Google Scholar]
- 13.Guldberg RE. Spatiotemporal Delivery Strategies for Promoting Musculoskeletal Tissue Regeneration. Journal of Bone and Mineral Research. 2009;24:1507–1511. doi: 10.1359/jbmr.090801. [DOI] [PubMed] [Google Scholar]
- 14.Tseng SS, Lee MA, Reddi AH. Nonunions and Potential of Stem Cells in Fracture-Healing. Bone. 2010:92–98. doi: 10.2106/JBJS.G.01192. [DOI] [PubMed] [Google Scholar]
- 15.Kimelman N, Pelled G, Gazit Z, Gazit D. Applications of gene therapy and adult stem cells in bone bioengineering. Regen Med. 2006;1:549–561. doi: 10.2217/17460751.1.4.549. [DOI] [PubMed] [Google Scholar]
- 16.Caplan AI. New era of cell-based orthopedic therapies. Tissue engineering. Part B, Reviews. 2009;15:195–200. doi: 10.1089/ten.teb.2008.0515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Biondi M, Ungaro F, Quaglia F, Netti PA. Controlled drug delivery in tissue engineering. Adv Drug Deliv Rev. 2008;60:229–242. doi: 10.1016/j.addr.2007.08.038. [DOI] [PubMed] [Google Scholar]
- 18.Phillips JE, Gersbach CA, Garcia AJ. Virus-based gene therapy strategies for bone regeneration. Biomaterials. 2007;28:211–229. doi: 10.1016/j.biomaterials.2006.07.032. [DOI] [PubMed] [Google Scholar]
- 19.Santos JL, Pandita D, Rodrigues J, Pego AP, Granja PL, Tomas H. Non-viral gene delivery to mesenchymal stem cells: methods, strategies and application in bone tissue engineering and regeneration. Current gene therapy. 2011;11:46–57. doi: 10.2174/156652311794520102. [DOI] [PubMed] [Google Scholar]
- 20.Kofron MD, Laurencin CT. Bone tissue engineering by gene delivery. Adv Drug Deliv Rev. 2006;58:555–576. doi: 10.1016/j.addr.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 21.Partridge KA, Oreffo RO. Gene delivery in bone tissue engineering: progress and prospects using viral and nonviral strategies. Tissue Eng. 2004;10:295–307. doi: 10.1089/107632704322791934. [DOI] [PubMed] [Google Scholar]
- 22.Schugar RC, Robbins PD, Deasy BM. Small molecules in stem cell self-renewal and differentiation. Gene therapy. 2008;15:126–135. doi: 10.1038/sj.gt.3303062. [DOI] [PubMed] [Google Scholar]
- 23.Bonadio J. Tissue engineering via local gene delivery: update and future prospects for enhancing the technology. Adv Drug Deliv Rev. 2000;44:185–194. doi: 10.1016/s0169-409x(00)00094-6. [DOI] [PubMed] [Google Scholar]
- 24.Gerstenfeld LC, Cullinane DM, Barnes GL, Graves DT, Einhorn TA. Fracture healing as a post-natal developmental process: molecular, spatial, and temporal aspects of its regulation. J Cell Biochem. 2003;88:873–884. doi: 10.1002/jcb.10435. [DOI] [PubMed] [Google Scholar]
- 25.Marsell R, Einhorn TA. The biology of fracture healing. Injury. 2011;42:551–555. doi: 10.1016/j.injury.2011.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Willenegger H, Perren SM, Schenk R. Primary and secondary healing of bone fractures. Chirurg. 1971:241–252. [PubMed] [Google Scholar]
- 27.Einhorn TA. The cell and molecular biology of fracture healing. Clin Orthop Relat Res. 1998:S7–S21. doi: 10.1097/00003086-199810001-00003. [DOI] [PubMed] [Google Scholar]
- 28.Willie BM, Petersen A, Schmidt-Bleek K, Cipitria A, Mehta M, Strube P, Lienau J, Wildemann B, Fratzl P, Duda G. Designing biomimetic scaffolds for bone regeneration: why aim for a copy of mature tissue properties if nature uses a different approach? Soft Matter. 2010;6:4976–4987. [Google Scholar]
- 29.Epari DR, Duda GN, Thompson MS. Mechanobiology of bone healing and regeneration: in vivo models. P I Mech Eng H. 2010;224:1543–1553. doi: 10.1243/09544119JEIM808. [DOI] [PubMed] [Google Scholar]
- 30.Kolar P, Schmidt-Bleek K, Schell H, Gaber T, Toben D, Schmidmaier G, Perka C, Buttgereit F, Duda GN. The early fracture hematoma and its potential role in fracture healing. Tissue Eng Part B Rev. 2010;16:427–434. doi: 10.1089/ten.TEB.2009.0687. [DOI] [PubMed] [Google Scholar]
- 31.Gaber T, Dziurla R, Tripmacher R, Burmester GR, Buttgereit F. Hypoxia inducible factor (HIF) in rheumatology: low O2! See what HIF can do! Ann Rheum Dis. 2005;64:971–980. doi: 10.1136/ard.2004.031641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wan C, Gilbert SR, Wang Y, Cao XM, Shen X, Ramaswamy G, Jacobsen KA, Alaql ZS, Gerstenfeld LC, Einhorn TA, Eberhardt AW, Deng L, Guldberg RE, Clemens TL. Role of hypoxia inducible factor-1alpha pathway in bone regeneration. J Musculoskelet Neuronal Interact. 2008;8:323–324. [PubMed] [Google Scholar]
- 33.Wang Y, Wan C, Deng L, Liu X, Cao X, Gilbert SR, Bouxsein ML, Faugere MC, Guldberg RE, Gerstenfeld LC, Haase VH, Johnson RS, Schipani E, Clemens TL. The hypoxia-inducible factor alpha pathway couples angiogenesis to osteogenesis during skeletal development. J Clin Invest. 2007;117:1616–1626. doi: 10.1172/JCI31581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wilson CJ, Kasper G, Schutz MA, Duda GN. Cyclic strain disrupts endothelial network formation on Matrigel. Microvasc Res. 2009;78:358–363. doi: 10.1016/j.mvr.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 35.Street J, Winter D, Wang JH, Wakai A, McGuinness A, Redmond HP. Is human fracture hematoma inherently angiogenic? Clin Orthop Relat Res. 2000:224–237. doi: 10.1097/00003086-200009000-00033. [DOI] [PubMed] [Google Scholar]
- 36.Raheja LF, Genetos DC, Wong A, Yellowley CE. Hypoxic regulation of mesenchymal stem cell migration: the role of RhoA and HIF-1alpha. Cell Biol Int. 2011 doi: 10.1042/CBI20100733. [DOI] [PubMed] [Google Scholar]
- 37.Kasper G, Ode A, Groothuis A, Glaeser J, Gaber T, Wilson CJ, Geissler S, Duda GN. Validation of beta-actin used as endogenous control for gene expression analysis in mechanobiology studies: amendments. Stem cells. 2010;28:633–634. doi: 10.1002/stem.305. [DOI] [PubMed] [Google Scholar]
- 38.Kasper G, Mao L, Geissler S, Draycheva A, Trippens J, Kuhnisch J, Tschirschmann M, Kaspar K, Perka C, Duda GN, Klose J. Insights into mesenchymal stem cell aging: involvement of antioxidant defense and actin cytoskeleton. Stem cells. 2009;27:1288–1297. doi: 10.1002/stem.49. [DOI] [PubMed] [Google Scholar]
- 39.Groothuis A, Duda GN, Wilson CJ, Thompson MS, Hunter MR, Simon P, Bail HJ, van Scherpenzeel KM, Kasper G. Mechanical stimulation of the pro-angiogenic capacity of human fracture haematoma: involvement of VEGF mechano-regulation. Bone. 2010;47:438–444. doi: 10.1016/j.bone.2010.05.026. [DOI] [PubMed] [Google Scholar]
- 40.Nauta AJ, Fibbe WE. Immunomodulatory properties of mesenchymal stromal cells. Blood. 2007;110:3499–3506. doi: 10.1182/blood-2007-02-069716. [DOI] [PubMed] [Google Scholar]
- 41.Pneumaticos SG, Triantafyllopoulos GK, Basdra EK, Papavassiliou AG. Segmental bone defects: from cellular and molecular pathways to the development of novel biological treatments. Journal of cellular and molecular medicine. 2010;14:2561–2569. doi: 10.1111/j.1582-4934.2010.01062.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cho TJ, Gerstenfeld LC, Einhorn TA. Differential temporal expression of members of the transforming growth factor beta superfamily during murine fracture healing. J Bone Miner Res. 2002;17:513–520. doi: 10.1359/jbmr.2002.17.3.513. [DOI] [PubMed] [Google Scholar]
- 43.Keramaris NC, Calori GM, Nikolaou VS, Schemitsch EH, Giannoudis PV. Fracture vascularity and bone healing: a systematic review of the role of VEGF. Injury. 2008;39(Suppl 2):S45–S57. doi: 10.1016/S0020-1383(08)70015-9. [DOI] [PubMed] [Google Scholar]
- 44.Marsh D. Concepts of fracture union, delayed union, and nonunion. Clin Orthop Relat R. 1998:S22–S30. doi: 10.1097/00003086-199810001-00004. [DOI] [PubMed] [Google Scholar]
- 45.Pelissier P, Villars F, Mathoulin-Pelissier S, Bareille R, Lafage-Proust MH, Vilamitjana-Amedee J. Influences of vascularization and osteogenic cells on heterotopic bone formation within a madreporic ceramic in rats. Plast Reconstr Surg. 2003;111:1932–1941. doi: 10.1097/01.PRS.0000055044.14093.EA. [DOI] [PubMed] [Google Scholar]
- 46.Ponte AL, Marais E, Gallay N, Langonne A, Delorme B, Herault O, Charbord P, Domenech J. The in vitro migration capacity of human bone marrow mesenchymal stem cells: comparison of chemokine and growth factor chemotactic activities. Stem cells. 2007;25:1737–1745. doi: 10.1634/stemcells.2007-0054. [DOI] [PubMed] [Google Scholar]
- 47.Gerstenfeld LC, Cho TJ, Kon T, Aizawa T, Tsay A, Fitch J, Barnes GL, Graves DT, Einhorn TA. Impaired fracture healing in the absence of TNF-alpha signaling: the role of TNF-alpha in endochondral cartilage resorption. J Bone Miner Res. 2003;18:1584–1592. doi: 10.1359/jbmr.2003.18.9.1584. [DOI] [PubMed] [Google Scholar]
- 48.Gerstenfeld LC, Cho TJ, Kon T, Aizawa T, Cruceta J, Graves BD, Einhorn TA. Impaired intramembranous bone formation during bone repair in the absence of tumor necrosis factor-alpha signaling. Cells Tissues Organs. 2001;169:285–294. doi: 10.1159/000047893. [DOI] [PubMed] [Google Scholar]
- 49.Epari DR, Kassi JP, Schell H, Duda GN. Timely fracture-healing requires optimization of axial fixation stability. J Bone Joint Surg Am. 2007;89:1575–1585. doi: 10.2106/JBJS.F.00247. [DOI] [PubMed] [Google Scholar]
- 50.Epari DR, Schell H, Bail HJ, Duda GN. Instability prolongs the chondral phase during bone healing in sheep. Bone. 2006;38:864–870. doi: 10.1016/j.bone.2005.10.023. [DOI] [PubMed] [Google Scholar]
- 51.Trepczik B, Lienau J, Schell H, Epari DR, Thompson MS, Hoffmann JE, Kadow-Romacker A, Mundlos S, Duda GN. Endochondral ossification in vitro is influenced by mechanical bending. Bone. 2007;40:597–603. doi: 10.1016/j.bone.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 52.Claes L, Wolf S, Augat P. [Mechanical modification of callus healing] Chirurg. 2000;71:989–994. doi: 10.1007/s001040051172. [DOI] [PubMed] [Google Scholar]
- 53.Claes L, Blakytny R, Gockelmann M, Schoen M, Ignatius A, Willie B. Early dynamization by reduced fixation stiffness does not improve fracture healing in a rat femoral osteotomy model. J Orthop Res. 2009;27:22–27. doi: 10.1002/jor.20712. [DOI] [PubMed] [Google Scholar]
- 54.Carter DR, Beaupre GS, Giori NJ, Helms JA. Mechanobiology of skeletal regeneration. Clin Orthop Relat Res. 1998:S41–S55. doi: 10.1097/00003086-199810001-00006. [DOI] [PubMed] [Google Scholar]
- 55.Claes LE, Heigele CA. Magnitudes of local stress and strain along bony surfaces predict the course and type of fracture healing. J Biomech. 1999;32:255–266. doi: 10.1016/s0021-9290(98)00153-5. [DOI] [PubMed] [Google Scholar]
- 56.Prendergast PJ, Huiskes R, Søballe K. Biophysical stimuli on cells during tissue differentiation at implant interfaces. Journal of biomechanics. 1997;30:539–548. doi: 10.1016/s0021-9290(96)00140-6. [DOI] [PubMed] [Google Scholar]
- 57.Kearns AE, Khosla S, Kostenuik PJ. Receptor activator of nuclear factor kappaB ligand and osteoprotegerin regulation of bone remodeling in health and disease. Endocr Rev. 2008;29:155–192. doi: 10.1210/er.2007-0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Boyce BF, Xing L. Functions of RANKL/RANK/OPG in bone modeling and remodeling. Arch Biochem Biophys. 2008;473:139–146. doi: 10.1016/j.abb.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kostenuik PJ, Shalhoub V. Osteoprotegerin: a physiological and pharmacological inhibitor of bone resorption. Curr Pharm Des. 2001;7:613–635. doi: 10.2174/1381612013397807. [DOI] [PubMed] [Google Scholar]
- 60.Hofbauer LC, Schoppet M. Clinical implications of the osteoprotegerin/RANKL/RANK system for bone and vascular diseases. JAMA. 2004;292:490–495. doi: 10.1001/jama.292.4.490. [DOI] [PubMed] [Google Scholar]
- 61.Perka C, Heller M, Wilke K, Taylor WR, Haas NP, Zippel H, Duda GN. Surgical approach influences periprosthetic femoral bone density. Clin Orthop Relat Res. 2005:153–159. doi: 10.1097/01.blo.0000149814.40480.8e. [DOI] [PubMed] [Google Scholar]
- 62.Bosch P, Musgrave D, Lee J, Cummins J, Shuler T, Ghivizzani T, Evans T, Robbins T, Huard J. Osteoprogenitor cells within skeletal muscle. Journal of Orthopaedic Research. 2000;18:933–944. doi: 10.1002/jor.1100180613. [DOI] [PubMed] [Google Scholar]
- 63.Rosen CJ, Ackert-Bicknell C, Rodriguez JP, Pino AM. Marrow Fat and the Bone Microenvironment: Developmental, Functional, and Pathological Implications. Crit Rev Eukar Gene. 2009;19:109–124. doi: 10.1615/critreveukargeneexpr.v19.i2.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Harry L, Sandison A, Pearse M, Paleolog E, Nanchahal J. Comparision of the vascularity of fasciocutaneous tissue and muscle for coverage of open tibial fractures. Plastic & Reconstructive Surgery. 2009;124:1211–1219. doi: 10.1097/PRS.0b013e3181b5a308. [DOI] [PubMed] [Google Scholar]
- 65.Kumar S, Wan C, Ramaswamy G, Clemens TL, Ponnazhagan S. Mesenchymal Stem Cells Expressing Osteogenic and Angiogenic Factors Synergistically Enhance Bone Formation in a Mouse Model of Segmental Bone Defect. Molecular Therapy. 2010;18:1026–1034. doi: 10.1038/mt.2009.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liu R, Birke O, Morse A, Peacock L, Mikulec K, Little D, Schindeler A. Myogenic progenitors contribute to open but not closed fracture repair. BMC Musculoskeletal Disorders. 2011;12:288. doi: 10.1186/1471-2474-12-288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schell H, Thompson MS, Bail HJ, Hoffmann JE, Schill A, Duda GN, Lienau J. Mechanical induction of critically delayed bone healing in sheep: radiological and biomechanical results. J Biomech. 2008;41:3066–3072. doi: 10.1016/j.jbiomech.2008.06.038. [DOI] [PubMed] [Google Scholar]
- 68.Mehta M, Schell H, Schwarz C, Peters A, Schmidt-Bleek K, Ellinghaus A, Bail HJ, Duda GN, Lienau J. A 5-mm femoral defect in female but not in male rats leads to a reproducible atrophic non-union. Arch Orthop Trauma Surg. 2011;131:121–129. doi: 10.1007/s00402-010-1155-7. [DOI] [PubMed] [Google Scholar]
- 69.Willie B, Adkins K, Zheng X, Simon U, Claes L. Mechanical characterization of external fixator stiffness for a rat femoral fracture model. J Orthop Res. 2009;27:687–693. doi: 10.1002/jor.20792. [DOI] [PubMed] [Google Scholar]
- 70.Kratzel C, Bergmann C, Duda G, Greiner S, Schmidmaier G, Wildemann B. Characterization of a rat osteotomy model with impaired healing. BMC Musculoskelet Disord. 2008;9:135. doi: 10.1186/1471-2474-9-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Peters A, Schell H, Bail HJ, Hannemann M, Schumann T, Duda GN, Lienau J. Standard bone healing stages occur during delayed bone healing, albeit with a different temporal onset and spatial distribution of callus tissues. Histol Histopathol. 2010;25:1149–1162. doi: 10.14670/HH-25.1149. [DOI] [PubMed] [Google Scholar]
- 72.Schmidt-Bleek K, Schell H, Schulz N, Hoff P, Perka C, Buttgereit F, Volk HD, Lienau J, Duda GN. Inflammatory phase of bone healing initiates the regenerative healing cascade. Cell Tissue Res. 2011 doi: 10.1007/s00441-011-1205-7. [DOI] [PubMed] [Google Scholar]
- 73.Lienau J, Schmidt-Bleek K, Peters A, Haschke F, Duda GN, Perka C, Bail HJ, Schutze N, Jakob F, Schell H. Differential regulation of blood vessel formation between standard and delayed bone healing. J Orthop Res. 2009;27:1133–1140. doi: 10.1002/jor.20870. [DOI] [PubMed] [Google Scholar]
- 74.Lienau J, Schmidt-Bleek K, Peters A, Weber H, Bail HJ, Duda GN, Perka C, Schell H. Insight into the molecular pathophysiology of delayed bone healing in a sheep model. Tissue Eng Part A. 2010;16:191–199. doi: 10.1089/ten.TEA.2009.0187. [DOI] [PubMed] [Google Scholar]
- 75.Einhorn TA, Lane JM, Burstein AH, Kopman CR, Vigorita VJ. The healing of segmental bone defects induced by demineralized bone matrix. A radiographic and biomechanical study. J Bone Joint Surg Am. 1984;66:274–279. [PubMed] [Google Scholar]
- 76.Theos C, Koulouvaris P, Kottakis S, Demertzis N. Reconstruction of tibia defects by ipsilateral vascularized fibula transposition. Arch Orthop Trauma Surg. 2008;128:179–184. doi: 10.1007/s00402-007-0301-3. [DOI] [PubMed] [Google Scholar]
- 77.Perka C, Schultz O, Spitzer RS, Lindenhayn K, Burmester GR, Sittinger M. Segmental bone repair by tissue-engineered periosteal cell transplants with bioresorbable fleece and fibrin scaffolds in rabbits. Biomaterials. 2000;21:1145–1153. doi: 10.1016/s0142-9612(99)00280-x. [DOI] [PubMed] [Google Scholar]
- 78.Lane JM, Tomin E, Bostrom MP. Biosynthetic bone grafting. Clin Orthop Relat Res. 1999:S107–S117. doi: 10.1097/00003086-199910001-00011. [DOI] [PubMed] [Google Scholar]
- 79.Han D, Liu W, Ao Q, Wang G. Optimal delivery systems for bone morphogenetic proteins in orthopedic applications should model initial tissue repair structures by using a heparin-incorporated fibrin-fibronectin matrix. Med Hypotheses. 2008;71:374–378. doi: 10.1016/j.mehy.2008.01.035. [DOI] [PubMed] [Google Scholar]
- 80.Haidar ZS, Hamdy RC, Tabrizian M. Delivery of recombinant bone morphogenetic proteins for bone regeneration and repair. Part A: Current challenges in BMP delivery. Biotechnol Lett. 2009;31:1817–1824. doi: 10.1007/s10529-009-0099-x. [DOI] [PubMed] [Google Scholar]
- 81.Frost HM. A determinant of bone architecture. The minimum effective strain. Clin Orthop Relat Res. 1983:286–292. [PubMed] [Google Scholar]
- 82.Griffin XL, Smith CM, Costa ML. The clinical use of platelet-rich plasma in the promotion of bone healing: a systematic review. Injury. 2009;40:158–162. doi: 10.1016/j.injury.2008.06.025. [DOI] [PubMed] [Google Scholar]
- 83.Calori GM, Tagliabue L, Gala L, d'Imporzano M, Peretti G, Albisetti W. Application of rhBMP-7 and platelet-rich plasma in the treatment of long bone non-unions: a prospective randomised clinical study on 120 patients. Injury. 2008;39:1391–1402. doi: 10.1016/j.injury.2008.08.011. [DOI] [PubMed] [Google Scholar]
- 84.Sarkar MR, Augat P, Shefelbine SJ, Schorlemmer S, Huber-Lang M, Claes L, Kinzl L, Ignatius A. Bone formation in a long bone defect model using a platelet-rich plasma-loaded collagen scaffold. Biomaterials. 2006;27:1817–1823. doi: 10.1016/j.biomaterials.2005.10.039. [DOI] [PubMed] [Google Scholar]
- 85.Mountziaris PM, Spicer PP, Kasper FK, Mikos AG. Harnessing and Modulating Inflammation in Strategies for Bone Regeneration. Tissue Eng Part B Rev. 2011 doi: 10.1089/ten.teb.2011.0182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Peng H, Wright V, Usas A, Gearhart B, Shen HC, Cummins J, Huard J. Synergistic enhancement of bone formation and healing by stem cell-expressed VEGF and bone morphogenetic protein-4. J Clin Invest. 2002;110:751–759. doi: 10.1172/JCI15153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Geiger F, Bertram H, Berger I, Lorenz H, Wall O, Eckhardt C, Simank HG, Richter W. Vascular endothelial growth factor gene-activated matrix (VEGF165-GAM) enhances osteogenesis and angiogenesis in large segmental bone defects. J Bone Miner Res. 2005;20:2028–2035. doi: 10.1359/JBMR.050701. [DOI] [PubMed] [Google Scholar]
- 88.Takeshita S, Zheng LP, Brogi E, Kearney M, Pu LQ, Bunting S, Ferrara N, Symes JF, Isner JM. Therapeutic angiogenesis. A single intraarterial bolus of vascular endothelial growth factor augments revascularization in a rabbit ischemic hind limb model. J Clin Invest. 1994;93:662–670. doi: 10.1172/JCI117018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Basmanav FB, Kose GT, Hasirci V. Sequential growth factor delivery from complexed microspheres for bone tissue engineering. Biomaterials. 2008;29:4195–4204. doi: 10.1016/j.biomaterials.2008.07.017. [DOI] [PubMed] [Google Scholar]
- 90.Oest ME, Dupont KM, Kong HJ, Mooney DJ, Guldberg RE. Quantitative assessment of scaffold and growth factor-mediated repair of critically sized bone defects. J Orthop Res. 2007;25:941–950. doi: 10.1002/jor.20372. [DOI] [PubMed] [Google Scholar]
- 91.Schmidmaier G, Wildemann B, Cromme F, Kandziora F, Haas NP, Raschke M. Bone morphogenetic protein-2 coating of titanium implants increases biomechanical strength and accelerates bone remodeling in fracture treatment: a biomechanical and histological study in rats. Bone. 2002;30:816–822. doi: 10.1016/s8756-3282(02)00740-8. [DOI] [PubMed] [Google Scholar]
- 92.Schmidmaier G, Wildemann B, Ostapowicz D, Kandziora F, Stange R, Haas NP, Raschke M. Long-term effects of local growth factor (IGF-I and TGF-beta 1) treatment on fracture healing - A safety study for using growth factors. Journal of Orthopaedic Research. 2004;22:514–519. doi: 10.1016/j.orthres.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 93.Dupoirieux L, Pohl J, Hanke M, Pourquier D. A preliminary report on the effect of dimeric rhGDF-5 and its monomeric form rhGDF-5C465A on bone healing of rat cranial defects. J Craniomaxillofac Surg. 2009;37:30–35. doi: 10.1016/j.jcms.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 94.Yoshimoto T, Yamamoto M, Kadomatsu H, Sakoda K, Yonamine Y, Izumi Y. Recombinant human growth/differentiation factor-5 (rhGDF-5) induced bone formation in murine calvariae. J Periodontal Res. 2006;41:140–147. doi: 10.1111/j.1600-0765.2005.00847.x. [DOI] [PubMed] [Google Scholar]
- 95.Yonamine Y, Matsuyama T, Sonomura T, Takeuchi H, Furuichi Y, Uemura M, Izumi Y, Noguchi K. Effectable application of vascular endothelial growth factor to critical sized rat calvaria defects. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;109:225–231. doi: 10.1016/j.tripleo.2009.09.010. [DOI] [PubMed] [Google Scholar]
- 96.Quarto R, Mastrogiacomo M, Cancedda R, Kutepov SM, Mukhachev V, Lavroukov A, Kon E, Marcacci M. Repair of large bone defects with the use of autologous bone marrow stromal cells. N Engl J Med. 2001;344:385–386. doi: 10.1056/NEJM200102013440516. [DOI] [PubMed] [Google Scholar]
- 97.Peters A, Toben D, Lienau J, Schell H, Bail HJ, Matziolis G, Duda GN, Kaspar K. Locally applied osteogenic predifferentiated progenitor cells are more effective than undifferentiated mesenchymal stem cells in the treatment of delayed bone healing. Tissue Eng Part A. 2009;15:2947–2954. doi: 10.1089/ten.TEA.2009.0058. [DOI] [PubMed] [Google Scholar]
- 98.Zhang X, Awad HA, O'Keefe RJ, Guldberg RE, Schwarz EM. A perspective: engineering periosteum for structural bone graft healing. Clin Orthop Relat Res. 2008;466:1777–1787. doi: 10.1007/s11999-008-0312-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Stangenberg L, Schaefer DJ, Buettner O, Ohnolz J, Mobest D, Horch RE, Stark GB, Kneser U. Differentiation of osteoblasts in three-dimensional culture in processed cancellous bone matrix: quantitative analysis of gene expression based on real-time reverse transcription-polymerase chain reaction. Tissue Eng. 2005;11:855–864. doi: 10.1089/ten.2005.11.855. [DOI] [PubMed] [Google Scholar]
- 100.Kadiyala S, Jaiswal N, Bruder SP. Culture-expanded, bone marrow-derived mesenchymal stem cells can regenerate a critical-sized segmental bone defect. Tissue Engineering. 1997;3:173–185. [Google Scholar]
- 101.Mooney DJ, Vandenburgh H. Cell delivery mechanisms for tissue repair. Cell stem cell. 2008;2:205–213. doi: 10.1016/j.stem.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 102.Tayalia P, Mooney DJ. Controlled growth factor delivery for tissue engineering. Advanced materials (Deerfield Beach, Fla.) 2009;21:3269–3285. doi: 10.1002/adma.200900241. [DOI] [PubMed] [Google Scholar]
- 103.Chen RR, Mooney DJ. Polymeric growth factor delivery strategies for tissue engineering. Pharm Res. 2003;20:1103–1112. doi: 10.1023/a:1025034925152. [DOI] [PubMed] [Google Scholar]
- 104.Discher DE, Mooney DJ, Zandstra PW. Growth factors, matrices, and forces combine and control stem cells. Science (New York, N.Y.) 2009;324:1673–1677. doi: 10.1126/science.1171643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sands RW, Mooney DJ. Polymers to direct cell fate by controlling the microenvironment. Curr Opin Biotechnol. 2007;18:448–453. doi: 10.1016/j.copbio.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Langer RS, Peppas NA. Present and Future Applications of Biomaterials in Controlled Drug Delivery Systems. Biomaterials. 1981;2:201–214. doi: 10.1016/0142-9612(81)90059-4. [DOI] [PubMed] [Google Scholar]
- 107.Stefani CM, Machado MA, Sallum EA, Sallum AW, Toledo S, Nociti FH., Jr Platelet-derived growth factor/insulin-like growth factor-1 combination and bone regeneration around implants placed into extraction sockets: a histometric study in dogs. Implant Dent. 2000;9:126–131. doi: 10.1097/00008505-200009020-00004. [DOI] [PubMed] [Google Scholar]
- 108.Mooney DJ, Baldwin DF, Suh NP, Vacanti LP, Langer R. Novel approach to fabricate porous sponges of poly(D,L-lactic-co-glycolic acid) without the use of organic solvents. Biomaterials. 1996;17:1417–1422. doi: 10.1016/0142-9612(96)87284-x. [DOI] [PubMed] [Google Scholar]
- 109.Yoneda A, Asada M, Oda Y, Suzuki M, Imamura T. Engineering of an FGF-proteoglycan fusion protein with heparin-independent, mitogenic activity. Nat Biotechnol. 2000;18:641–644. doi: 10.1038/76487. [DOI] [PubMed] [Google Scholar]
- 110.Suzuki Y, Tanihara M, Suzuki K, Saitou A, Sufan W, Nishimura Y. Alginate hydrogel linked with synthetic oligopeptide derived from BMP-2 allows ectopic osteoinduction in vivo. J Biomed Mater Res. 2000;50:405–409. doi: 10.1002/(sici)1097-4636(20000605)50:3<405::aid-jbm15>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 111.Slaughter BV, Khurshid SS, Fisher OZ, Khademhosseini A, Peppas NA. Hydrogels in Regenerative Medicine. Advanced Materials. 2009;21:3307–3329. doi: 10.1002/adma.200802106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Amende MT, Hariharan D, Peppas NA. Factors Influencing Drug and Protein-Transport and Release from Ionic Hydrogels. React Polym. 1995;25:127–137. [Google Scholar]
- 113.Pillay V, Danckwerts MP, Muhidinov Z, Fassihi R. Novel modulation of drug delivery using binary zinc-alginate-pectinate polyspheres for zero-order kinetics over several days: Experimental design strategy to elucidate the crosslinking mechanism. Drug Dev Ind Pharm. 2005;31:191–207. doi: 10.1081/ddc-200047806. [DOI] [PubMed] [Google Scholar]
- 114.Winn SR, Uludag H, Hollinger JO. Sustained release emphasizing recombinant human bone morphogenetic protein-2. Adv Drug Deliv Rev. 1998;31:303–318. doi: 10.1016/s0169-409x(97)00126-9. [DOI] [PubMed] [Google Scholar]
- 115.Uludag H, D'Augusta D, Golden J, Li J, Timony G, Riedel R, Wozney JM. Implantation of recombinant human bone morphogenetic proteins with biomaterial carriers: A correlation between protein pharmacokinetics and osteoinduction in the rat ectopic model. J Biomed Mater Res. 2000;50:227–238. doi: 10.1002/(sici)1097-4636(200005)50:2<227::aid-jbm18>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 116.Ruppert R, Hoffmann E, Sebald W. Human bone morphogenetic protein 2 contains a heparin-binding site which modifies its biological activity. Eur J Biochem. 1996;237:295–302. doi: 10.1111/j.1432-1033.1996.0295n.x. [DOI] [PubMed] [Google Scholar]
- 117.Winn SR, Uludag H, Hollinger JO. Carrier systems for bone morphogenetic proteins. Clin Orthop Relat R. 1999:S95–S106. doi: 10.1097/00003086-199910001-00010. [DOI] [PubMed] [Google Scholar]
- 118.Riley EH, Lane JM, Urist MR, Lyons KM, Lieberman JR. Bone morphogenetic protein-2: biology and applications. Clin Orthop Relat R. 1996:39–46. [PubMed] [Google Scholar]
- 119.Kirker-Head CA. Potential applications and delivery strategies for bone morphogenetic proteins. Adv Drug Deliv Rev. 2000;43:65–92. doi: 10.1016/s0169-409x(00)00078-8. [DOI] [PubMed] [Google Scholar]
- 120.Li G, Bouxsein ML, Luppen C, Li XJ, Wood M, Seeherman HJ, Wozney JM, Simpson H. Bone consolidation is enhanced by rhBMP-2 in a rabbit model of distraction osteogenesis. Journal of Orthopaedic Research. 2002;20:779–788. doi: 10.1016/S0736-0266(01)00166-8. [DOI] [PubMed] [Google Scholar]
- 121.Wozney JM, Rosen V. Bone morphogenetic protein and bone morphogenetic protein gene family in bone formation and repair. Clin Orthop Relat R. 1998:26–37. [PubMed] [Google Scholar]
- 122.Hubbell JA, Lutolf MR, Weber FE, Schmoekel HG, Schense JC, Kohler T, Muller R. Repair of bone defects using synthetic mimetics of collagenous extracellular matrices. Nat Biotechnol. 2003;21:513–518. doi: 10.1038/nbt818. [DOI] [PubMed] [Google Scholar]
- 123.Carragee EJ, Hurwitz EL, Weiner BK. A critical review of recombinant human bone morphogenetic protein-2 trials in spinal surgery: emerging safety concerns and lessons learned. The spine journal : official journal of the North American Spine Society. 2011;11:471–491. doi: 10.1016/j.spinee.2011.04.023. [DOI] [PubMed] [Google Scholar]
- 124.Buchowski JM, Riew KD, Nussenbaum B. In reference to Acute airway obstruction in cervical spinal procedures with bone morphogenetic proteins. The Laryngoscope. 2011;121:2501. doi: 10.1002/lary.21784. author reply 2502–2503. [DOI] [PubMed] [Google Scholar]
- 125.Yaremchuk KL, Toma MS, Somers ML, Peterson E. Acute airway obstruction in cervical spinal procedures with bone morphogenetic proteins. The Laryngoscope. 2010;120:1954–1957. doi: 10.1002/lary.21096. [DOI] [PubMed] [Google Scholar]
- 126.Epstein NE. Pros, cons, and costs of INFUSE in spinal surgery. Surgical neurology international. 2011;2:10. doi: 10.4103/2152-7806.76147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Service RF. Tissue engineers build new bone. Science. 2000;289:1498–1500. doi: 10.1126/science.289.5484.1498. [DOI] [PubMed] [Google Scholar]
- 128.Shields LB, Raque GH, Glassman SD, Campbell M, Vitaz T, Harpring J, Shields CB. Adverse effects associated with high-dose recombinant human bone morphogenetic protein-2 use in anterior cervical spine fusion. Spine. 2006;31:542–547. doi: 10.1097/01.brs.0000201424.27509.72. [DOI] [PubMed] [Google Scholar]
- 129.Li R, Stewart DJ, von Schroeder HP, Mackinnon ES, Schemitsch EH. Effect of cell-based VEGF gene therapy on healing of a segmental bone defect. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2009;27:8–14. doi: 10.1002/jor.20658. [DOI] [PubMed] [Google Scholar]
- 130.Street J, Bao M, deGuzman L, Bunting S, Peale FV, Jr, Ferrara N, Steinmetz H, Hoeffel J, Cleland JL, Daugherty A, van Bruggen N, Redmond HP, Carano RA, Filvaroff EH. Vascular endothelial growth factor stimulates bone repair by promoting angiogenesis and bone turnover. Proc Natl Acad Sci U S A. 2002;99:9656–9661. doi: 10.1073/pnas.152324099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kimoto T, Hosokawa R, Kubo T, Maeda M, Sano A, Akagawa Y. Continuous administration of basic fibroblast growth factor (FGF-2) accelerates bone induction on rat calvaria--an application of a new drug delivery system. J Dent Res. 1998;77:1965–1969. doi: 10.1177/00220345980770120301. [DOI] [PubMed] [Google Scholar]
- 132.Ennett AB, Kaigler D, Mooney DJ. Temporally regulated delivery of VEGF in vitro and in vivo. J Biomed Mater Res A. 2006;79:176–184. doi: 10.1002/jbm.a.30771. [DOI] [PubMed] [Google Scholar]
- 133.Santo VE, Frias AM, Carida M, Cancedda R, Gomes ME, Mano JF, Reis RL. Carrageenan-Based Hydrogels for the Controlled Delivery of PDGF-BB in Bone Tissue Engineering Applications. Biomacromolecules. 2009;10:1392–1401. doi: 10.1021/bm8014973. [DOI] [PubMed] [Google Scholar]
- 134.Chen FM, Chen R, Wang XJ, Sun HH, Wu ZF. In vitro cellular responses to scaffolds containing two microencapulated growth factors. Biomaterials. 2009;30:5215–5224. doi: 10.1016/j.biomaterials.2009.06.009. [DOI] [PubMed] [Google Scholar]
- 135.Patel ZS, Young S, Tabata Y, Jansen JA, Wong ME, Mikos AG. Dual delivery of an angiogenic and an osteogenic growth factor for bone regeneration in a critical size defect model. Bone. 2008;43:931–940. doi: 10.1016/j.bone.2008.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Hasirci V, Yilgor P, Hasirci N. Sequential BMP-2/BMP-7 delivery from polyester nanocapsules. Journal of Biomedical Materials Research Part A. 2010;93A:528–536. doi: 10.1002/jbm.a.32520. [DOI] [PubMed] [Google Scholar]
- 137.Oldham JB, Lu L, Zhu X, Porter BD, Hefferan TE, Larson DR, Currier BL, Mikos AG, Yaszemski MJ. Biological activity of rhBMP-2 released from PLGA microspheres. J Biomech Eng-T Asme. 2000;122:289–292. doi: 10.1115/1.429662. [DOI] [PubMed] [Google Scholar]
- 138.Hong L, Tabata Y, Miyamoto S, Yamada K, Aoyama I, Tamura M, Hashimoto N, Ikada Y. Promoted bone healing at a rabbit skull gap between autologous bone fragment and the surrounding intact bone with biodegradable microspheres containing transforming growth factor-beta 1. Tissue Engineering. 2000;6:331–340. doi: 10.1089/107632700418056. [DOI] [PubMed] [Google Scholar]
- 139.Lu LC, Yaszemski MJ, Mikos AG. TGF-beta 1 release from biodegradable polymer microparticles: Its effects on marrow stromal osteoblast function. Journal of Bone and Joint Surgery-American Volume. 2001;83A:S82–S91. [PubMed] [Google Scholar]
- 140.Hasirci V, Yilgor P, Tuzlakoglu K, Reis RL, Hasirci N. Incorporation of a sequential BMP-2/BMP-7 delivery system into chitosan-based scaffolds for bone tissue engineering. Biomaterials. 2009;30:3551–3559. doi: 10.1016/j.biomaterials.2009.03.024. [DOI] [PubMed] [Google Scholar]
- 141.Lutolf MP, Hubbell JA. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat Biotechnol. 2005;23:47–55. doi: 10.1038/nbt1055. [DOI] [PubMed] [Google Scholar]
- 142.Jeon O, Song SJ, Kang SW, Putnam AJ, Kim BS. Enhancement of ectopic bone formation by bone morphogenetic protein-2 released from a heparin-conjugated poly(L-lactic-co-glycolic acid) scaffold. Biomaterials. 2007;28:2763–2771. doi: 10.1016/j.biomaterials.2007.02.023. [DOI] [PubMed] [Google Scholar]
- 143.van de Wetering P, Metters AT, Schoenmakers RG, Hubbell JA. Poly(ethylene glycol) hydrogels formed by conjugate addition with controllable swelling, degradation, and release of pharmaceutically active proteins. J Control Release. 2005;102:619–627. doi: 10.1016/j.jconrel.2004.10.029. [DOI] [PubMed] [Google Scholar]
- 144.Lutolf MP, Weber FE, Schmoekel HG, Schense JC, Kohler T, Muller R, Hubbell JA. Repair of bone defects using synthetic mimetics of collagenous extracellular matrices. Nat Biotech. 2003;21:513–518. doi: 10.1038/nbt818. [DOI] [PubMed] [Google Scholar]
- 145.Kolambkar YM, Boerckel JD, Dupont KM, Bajin M, Huebsch N, Mooney DJ, Hutmacher DW, Guldberg RE. Spatiotemporal delivery of bone morphogenetic protein enhances functional repair of segmental bone defects. Bone. 2011;49:485–492. doi: 10.1016/j.bone.2011.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Guldberg RE, Oest ME, Dupont KM, Kong HJ, Mooney DJ. Quantitative assessment of scaffold and growth factor-mediated repair of critically sized bone defects. Journal of Orthopaedic Research. 2007;25:941–950. doi: 10.1002/jor.20372. [DOI] [PubMed] [Google Scholar]
- 147.Ito Y. Covalently immobilized biosignal molecule materials for tissue engineering. Soft Matter. 2008;4:46–56. doi: 10.1039/b708359a. [DOI] [PubMed] [Google Scholar]
- 148.Fan VH, Au A, Tamama K, Littrell R, Richardson LB, Wright JW, Wells A, Griffith LG. Tethered Epidermal Growth Factor Provides a Survival Advantage to Mesenchymal Stem Cells. Stem cells. 2007;25:1241–1251. doi: 10.1634/stemcells.2006-0320. [DOI] [PubMed] [Google Scholar]
- 149.Pohl TLM, Boergermann JH, Schwaerzer GK, Knaus P, Cavalcanti-Adam EA. Surface immobilization of bone morphogenetic protein 2 via a self-assembled monolayer formation induces cell differentiation. Acta biomaterialia. 2012;8:772–780. doi: 10.1016/j.actbio.2011.10.019. [DOI] [PubMed] [Google Scholar]
- 150.Karageorgiou V, Meinel L, Hofmann S, Malhotra A, Volloch V, Kaplan D. Bone morphogenetic protein-2 decorated silk fibroin films induce osteogenic differentiation of human bone marrow stromal cells. Journal of Biomedical Materials Research Part A. 2004;71A:528–537. doi: 10.1002/jbm.a.30186. [DOI] [PubMed] [Google Scholar]
- 151.Weissleder R, Kelly K, Sun EY, Shtatland T, Josephson L. Cell-specific targeting of nanoparticles by multivalent attachment of small molecules. Nat Biotechnol. 2005;23:1418–1423. doi: 10.1038/nbt1159. [DOI] [PubMed] [Google Scholar]
- 152.Alsberg E, Anderson KW, Albeiruti A, Franceschi RT, Mooney DJ. Cell-interactive alginate hydrogels for bone tissue engineering. J Dent Res. 2001;80:2025–2029. doi: 10.1177/00220345010800111501. [DOI] [PubMed] [Google Scholar]
- 153.Gong J, Ito Y. Peptide immobilized on gold particles enhances cell growth. Cytotechnology. 2008;58:141–144. doi: 10.1007/s10616-008-9179-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Kiessling LL, Gestwicki JE, Strong LE. Synthetic multivalent ligands as probes of signal transduction. Angew Chem Int Edit. 2006;45:2348–2368. doi: 10.1002/anie.200502794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Ito Y, Zheng J, Imanishi Y, Yonezawa K, Kasuga M. Protein-free cell culture on an artificial substrate with covalently immobilized insulin. Proc Natl Acad Sci U S A. 1996;93:3598–3601. doi: 10.1073/pnas.93.8.3598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Tanihara M, Saito A, Suzuki Y, Ogata S, Ohtsuki C. Accelerated bone repair with the use of a synthetic BMP-2-derived peptide and bone-marrow stromal cells. Journal of Biomedical Materials Research Part A. 2005;72A:77–82. doi: 10.1002/jbm.a.30208. [DOI] [PubMed] [Google Scholar]
- 157.Aono A, Hazama M, Notoya K, Taketomi S, Yamasaki H, Tsukuda R, Sasaki S, Fujisawa Y. Potent ectopic bone-inducing activity of bone morphogenetic protein-4/7 heterodimer. Biochemical and biophysical research communications. 1995;210:670–677. doi: 10.1006/bbrc.1995.1712. [DOI] [PubMed] [Google Scholar]
- 158.Bauer S, Park J, Pittrof A, Song YY, von der Mark K, Schmuki P. Covalent functionalization of TiO(2) nanotube arrays with EGF and BMP-2 for modified behavior towards mesenchymal stem cells. Integr Biol (Camb) 2011;3:927–936. doi: 10.1039/c0ib00155d. [DOI] [PubMed] [Google Scholar]
- 159.Feito MJ, Lozano RM, Alcaide M, Ramirez-Santillan C, Arcos D, Vallet-Regi M, Portoles MT. Immobilization and bioactivity evaluation of FGF-1 and FGF-2 on powdered silicon-doped hydroxyapatite and their scaffolds for bone tissue engineering. J Mater Sci Mater Med. 2011;22:405–416. doi: 10.1007/s10856-010-4193-3. [DOI] [PubMed] [Google Scholar]
- 160.Evangelista MB, Hsiong SX, Fernandes R, Sampaio P, Kong HJ, Barrias CC, Salema R, Barbosa MA, Mooney DJ, Granja PL. Upregulation of bone cell differentiation through immobilization within a synthetic extracellular matrix. Biomaterials. 2007;28:3644–3655. doi: 10.1016/j.biomaterials.2007.04.028. [DOI] [PubMed] [Google Scholar]
- 161.Ceylan H, Kocabey S, Tekinay AB, Guler MO. Surface-adhesive and osteogenic self-assembled peptide nanofibers for bioinspired functionalization of titanium surfaces. Soft Matter. 2012;8:3929–3937. [Google Scholar]
- 162.Ratner BD. The engineering of biomaterials exhibiting recognition and specificity. J Mol Recognit. 1996;9:617–625. doi: 10.1002/(sici)1099-1352(199634/12)9:5/6<617::aid-jmr310>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 163.Alsberg E, Kong HJ, Hirano Y, Smith MK, Albeiruti A, Mooney DJ. Regulating bone formation via controlled scaffold degradation. J Dent Res. 2003;82:903–908. doi: 10.1177/154405910308201111. [DOI] [PubMed] [Google Scholar]
- 164.Comisar WA, Kazmers NH, Mooney DJ, Linderman JJ. Engineering RGD nanopatterned hydrogels to control preosteoblast behavior: a combined computational and experimental approach. Biomaterials. 2007;28:4409–4417. doi: 10.1016/j.biomaterials.2007.06.018. [DOI] [PubMed] [Google Scholar]
- 165.Mooney DJ, Rowley JA, Madlambayan G. Alginate hydrogels as synthetic extracellular matrix materials. Biomaterials. 1999;20:45–53. doi: 10.1016/s0142-9612(98)00107-0. [DOI] [PubMed] [Google Scholar]
- 166.Kim J, Cao L, Shvartsman D, Silva EA, Mooney DJ. Targeted Delivery of Nanoparticles to Ischemic Muscle for Imaging and Therapeutic Angiogenesis. Nano Lett. 2011;11:694–700. doi: 10.1021/nl103812a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Simmons CA, Alsberg E, Hsiong S, Kim WJ, Mooney DJ. Dual growth factor delivery and controlled scaffold degradation enhance in vivo bone formation by transplanted bone marrow stromal cells. Bone. 2004;35:562–569. doi: 10.1016/j.bone.2004.02.027. [DOI] [PubMed] [Google Scholar]
- 168.Shah NJ, Macdonald ML, Beben YM, Padera RF, Samuel RE, Hammond PT. Tunable dual growth factor delivery from polyelectrolyte multilayer films. Biomaterials. 2011;32:6183–6193. doi: 10.1016/j.biomaterials.2011.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Richardson TP, Peters MC, Ennett AB, Mooney DJ. Polymeric system for dual growth factor delivery. Nat Biotechnol. 2001;19:1029–1034. doi: 10.1038/nbt1101-1029. [DOI] [PubMed] [Google Scholar]
- 170.Huang Y-C, Kaigler D, Rice KG, Krebsbach PH, Mooney DJ. Combined Angiogenic and Osteogenic Factor Delivery Enhances Bone Marrow Stromal Cell-Driven Bone Regeneration. Journal of Bone and Mineral Research. 2005;20:848–857. doi: 10.1359/JBMR.041226. [DOI] [PubMed] [Google Scholar]
- 171.Strobel C, Bormann N, Kadow-Romacker A, Schmidmaier G, Wildemann B. Sequential release kinetics of two (gentamicin and BMP-2) or three (gentamicin, IGF-I and BMP-2) substances from a onecomponent polymeric coating on implants. J Control Release. 2011;156:37–45. doi: 10.1016/j.jconrel.2011.07.006. [DOI] [PubMed] [Google Scholar]
- 172.Okuda T, Tominaga K, Kidoaki S. Time-programmed dual release formulation by multilayered drug-loaded nanofiber meshes. J Control Release. 2010;143:258–264. doi: 10.1016/j.jconrel.2009.12.029. [DOI] [PubMed] [Google Scholar]
- 173.Lin CC, Metters AT. Hydrogels in controlled release formulations: network design and mathematical modeling. Adv Drug Deliv Rev. 2006;58:1379–1408. doi: 10.1016/j.addr.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 174.Lee KY, Mooney DJ. Alginate: Properties and biomedical applications. Prog Polym Sci. 2012;37:106–126. doi: 10.1016/j.progpolymsci.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Hao X, Silva EA, Månsson-Broberg A, Grinnemo K-H, Siddiqui AJ, Dellgren G, Wärdell E, Brodin LÅ, Mooney DJ, Sylvén C. Angiogenic effects of sequential release of VEGF-A165 and PDGF-BB with alginate hydrogels after myocardial infarction. Cardiovascular Research. 2007;75:178–185. doi: 10.1016/j.cardiores.2007.03.028. [DOI] [PubMed] [Google Scholar]
- 176.Moore NM, Lin NJ, Gallant ND, Becker ML. Synergistic enhancement of human bone marrow stromal cell proliferation and osteogenic differentiation on BMP-2-derived and RGD peptide concentration gradients. Acta biomaterialia. 2011;7:2091–2100. doi: 10.1016/j.actbio.2011.01.019. [DOI] [PubMed] [Google Scholar]
- 177.Strayhorn CL, Garrett JS, Dunn RL, Benedict JJ, Somerman MJ. Growth factors regulate expression of osteoblast-associated genes. J Periodontol. 1999;70:1345–1354. doi: 10.1902/jop.1999.70.11.1345. [DOI] [PubMed] [Google Scholar]
- 178.Schmidmaier G, Wildemann B, Gabelein T, Heeger J, Kandziora F, Haas NP, Raschke M. Synergistic effect of IGF-I and TGF-beta1 on fracture healing in rats: single versus combined application of IGF-I and TGF-beta1. Acta orthopaedica Scandinavica. 2003;74:604–610. doi: 10.1080/00016470310018036. [DOI] [PubMed] [Google Scholar]
- 179.Vonau RL, Bostrom MP, Aspenberg P, Sams AE. Combination of growth factors inhibits bone ingrowth in the bone harvest chamber. Clin Orthop Relat Res. 2001:243–251. doi: 10.1097/00003086-200105000-00032. [DOI] [PubMed] [Google Scholar]
- 180.Raiche AT, Puleo DA. In vitro effects of combined and sequential delivery of two bone growth factors. Biomaterials. 2004;25:677–685. doi: 10.1016/s0142-9612(03)00564-7. [DOI] [PubMed] [Google Scholar]
- 181.Huang Z, Ren PG, Ma T, Smith RL, Goodman SB. Modulating osteogenesis of mesenchymal stem cells by modifying growth factor availability. Cytokine. 2010;51:305–310. doi: 10.1016/j.cyto.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 182.Wells A, Welsh JB, Lazar CS, Wiley HS, Gill GN, Rosenfeld MG. Ligand-Induced Transformation by a Noninternalizing Epidermal Growth-Factor Receptor. Science. 1990;247:962–964. doi: 10.1126/science.2305263. [DOI] [PubMed] [Google Scholar]
- 183.Yuen WW, Du NR, Chan CH, Silva EA, Mooney DJ. Mimicking nature by codelivery of stimulant and inhibitor to create temporally stable and spatially restricted angiogenic zones. Proc Natl Acad Sci U S A. 2010;107:17933–17938. doi: 10.1073/pnas.1001192107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Ulijn RV, Bibi N, Jayawarna V, Thornton PD, Todd SJ, Mart RJ, Smith AM, Gough JE. Bioresponsive hydrogels. Mater Today. 2007;10:40–48. [Google Scholar]
- 185.Lee KY, Peters MC, Anderson KW, Mooney DJ. Controlled growth factor release from synthetic extracellular matrices. Nature. 2000;408:998–1000. doi: 10.1038/35050141. [DOI] [PubMed] [Google Scholar]
- 186.Kost J, Langer R. Responsive polymeric delivery systems. Adv Drug Deliv Rev. 2001;46:125–148. doi: 10.1016/s0169-409x(00)00136-8. [DOI] [PubMed] [Google Scholar]
- 187.Stubbe BG, De Smedt SC, Demeester J. "Programmed polymeric devices" for pulsed drug delivery. Pharm Res. 2004;21:1732–1740. doi: 10.1023/b:pham.0000045223.45400.01. [DOI] [PubMed] [Google Scholar]
- 188.Zhao X, Kim J, Cezar CA, Huebsch N, Lee K, Bouhadir K, Mooney DJ. Active scaffolds for on-demand drug and cell delivery. Proc Natl Acad Sci U S A. 2011;108:67–72. doi: 10.1073/pnas.1007862108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Paliwal S, Mitragotri S. Ultrasound-induced cavitation: applications in drug and gene delivery. Expert Opinion on Drug Delivery. 2006;3:713–726. doi: 10.1517/17425247.3.6.713. [DOI] [PubMed] [Google Scholar]
- 190.Goldstein SA. In vivo nonviral delivery factors to enhance bone repair. Clin Orthop Relat Res. 2000:S113–S119. doi: 10.1097/00003086-200010001-00015. [DOI] [PubMed] [Google Scholar]
- 191.Fang J, Zhu YY, Smiley E, Bonadio J, Rouleau JP, Goldstein SA, McCauley LK, Davidson BL, Roessler BJ. Stimulation of new bone formation by direct transfer of osteogenic plasmid genes. Proc Natl Acad Sci U S A. 1996;93:5753–5758. doi: 10.1073/pnas.93.12.5753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Bonadio J, Smiley E, Patil P, Goldstein S. Localized, direct plasmid gene delivery in vivo: prolonged therapy results in reproducible tissue regeneration. Nature medicine. 1999;5:753–759. doi: 10.1038/10473. [DOI] [PubMed] [Google Scholar]
- 193.Shea LD, Smiley E, Bonadio J, Mooney DJ. DNA delivery from polymer matrices for tissue engineering. Nat Biotechnol. 1999;17:551–554. doi: 10.1038/9853. [DOI] [PubMed] [Google Scholar]
- 194.Huang YC, Simmons C, Kaigler D, Rice KG, Mooney DJ. Bone regeneration in a rat cranial defect with delivery of PEI-condensed plasmid DNA encoding for bone morphogenetic protein-4 (BMP-4) Gene therapy. 2005;12:418–426. doi: 10.1038/sj.gt.3302439. [DOI] [PubMed] [Google Scholar]
- 195.Endo M, Kuroda S, Kondo H, Maruoka Y, Ohya K, Kasugai S. Bone regeneration by modified gene-activated matrix: effectiveness in segmental tibial defects in rats. Tissue Eng. 2006;12:489–497. doi: 10.1089/ten.2006.12.489. [DOI] [PubMed] [Google Scholar]
- 196.Jang JH, Houchin TL, Shea LD. Gene delivery from polymer scaffolds for tissue engineering. Expert Rev Med Devices. 2004;1:127–138. doi: 10.1586/17434440.1.1.127. [DOI] [PubMed] [Google Scholar]
- 197.Kong HJ, Liu J, Riddle K, Matsumoto T, Leach K, Mooney DJ. Non-viral gene delivery regulated by stiffness of cell adhesion substrates. Nat Mater. 2005;4:460–464. doi: 10.1038/nmat1392. [DOI] [PubMed] [Google Scholar]
- 198.Jang JH, Rives CB, Shea LD. Plasmid delivery in vivo from porous tissue-engineering scaffolds: transgene expression and cellular transfection. Molecular therapy : the journal of the American Society of Gene Therapy. 2005;12:475–483. doi: 10.1016/j.ymthe.2005.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Kawai M, Maruyama H, Bessho K, Yamamoto H, Miyazaki J, Yamamoto T. Simple strategy for bone regeneration with a BMP-2/7 gene expression cassette vector. Biochemical and biophysical research communications. 2009;390:1012–1017. doi: 10.1016/j.bbrc.2009.10.099. [DOI] [PubMed] [Google Scholar]
- 200.Wells DJ. Gene therapy progress and prospects: Electroporation and other physical methods. Gene therapy. 2004;11:1363–1369. doi: 10.1038/sj.gt.3302337. [DOI] [PubMed] [Google Scholar]
- 201.Aslan H, Zilberman Y, Arbeli V, Sheyn D, Matan Y, Liebergall M, Li JZ, Helm GA, Gazit D, Gazit Z. Nucleofection-based ex vivo nonviral gene delivery to human stem cells as a platform for tissue regeneration. Tissue Engineering. 2006;12:877–889. doi: 10.1089/ten.2006.12.877. [DOI] [PubMed] [Google Scholar]
- 202.Sheyn D, Kimelman-Bleich N, Pelled G, Zilberman Y, Gazit D, Gazit Z. Ultrasound-based nonviral gene delivery induces bone formation in vivo. Gene therapy. 2008;15:257–266. doi: 10.1038/sj.gt.3303070. [DOI] [PubMed] [Google Scholar]
- 203.Burdick JA, Mason MN, Hinman AD, Thorne K, Anseth KS. Delivery of osteoinductive growth factors from degradable PEG hydrogels influences osteoblast differentiation and mineralization. J Control Release. 2002;83:53–63. doi: 10.1016/s0168-3659(02)00181-5. [DOI] [PubMed] [Google Scholar]
- 204.Bessho K, Carnes DL, Cavin R, Ong JL. Experimental studies on bone induction using low-molecular-weight poly (DL-lactide-co-glycolide) as a carrier for recombinant human bone morphogenetic protein-2. J Biomed Mater Res. 2002;61:61–65. doi: 10.1002/jbm.10169. [DOI] [PubMed] [Google Scholar]
- 205.Tzafriri AR, Lerner EI, Flashner-Barak M, Hinchcliffe M, Ratner E, Parnas H. Mathematical modeling and optimization of drug delivery from intratumorally injected microspheres. Clinical cancer research : an official journal of the American Association for Cancer Research. 2005;11:826–834. [PubMed] [Google Scholar]
- 206.Kanjickal DG, Lopina ST. Modeling of drug release from polymeric delivery systems--a review. Critical reviews in therapeutic drug carrier systems. 2004;21:345–386. doi: 10.1615/critrevtherdrugcarriersyst.v21.i5.10. [DOI] [PubMed] [Google Scholar]
- 207.Chen RR, Silva EA, Yuen WW, Brock AA, Fischbach C, Lin AS, Guldberg RE, Mooney DJ. Integrated approach to designing growth factor delivery systems. Faseb J. 2007;21:3896–3903. doi: 10.1096/fj.06-7873com. [DOI] [PubMed] [Google Scholar]
- 208.Baker M. Tissue models: a living system on a chip. Nature. 2011;471:661–665. doi: 10.1038/471661a. [DOI] [PubMed] [Google Scholar]
- 209.Webster A, Dyer CE, Haswell SJ, Greenman J. A microfluidic device for tissue biopsy culture and interrogation. Anal Methods-Uk. 2010;2:1005–1007. [Google Scholar]
- 210.Zahir N, Weaver VM. Death in the third dimension: apoptosis regulation and tissue architecture. Curr Opin Genet Dev. 2004;14:71–80. doi: 10.1016/j.gde.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 211.Cukierman E, Pankov R, Stevens DR, Yamada KM. Taking cell-matrix adhesions to the third dimension. Science. 2001;294:1708–1712. doi: 10.1126/science.1064829. [DOI] [PubMed] [Google Scholar]
- 212.van Midwoud PM, Merema MT, Verpoorte E, Groothuis GMM. Microfluidics Enables Small-Scale Tissue-Based Drug Metabolism Studies With Scarce Human Tissue. Jala-J Lab Autom. 2011;16:468–476. doi: 10.1016/j.jala.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 213.Yliperttula M, Chung BG, Navaladi A, Manbachi A, Urtti A. High-throughput screening of cell responses to biomaterials. European journal of pharmaceutical sciences : official journal of the European Federation for Pharmaceutical Sciences. 2008;35:151–160. doi: 10.1016/j.ejps.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 214.Pernagallo S, Diaz-Mochon JJ. Polymer microarrays for cellular high-content screening. Methods Mol Biol. 2011;706:171–180. doi: 10.1007/978-1-61737-970-3_14. [DOI] [PubMed] [Google Scholar]
- 215.Anderson DG, Levenberg S, Langer R. Nanoliter-scale synthesis of arrayed biomaterials and application to human embryonic stem cells. Nat Biotechnol. 2004;22:863–866. doi: 10.1038/nbt981. [DOI] [PubMed] [Google Scholar]
- 216.Anderson DG, Putnam D, Lavik EB, Mahmood TA, Langer R. Biomaterial microarrays: rapid, microscale screening of polymer-cell interaction. Biomaterials. 2005;26:4892–4897. doi: 10.1016/j.biomaterials.2004.11.052. [DOI] [PubMed] [Google Scholar]
- 217.Snoeks TJ, Khmelinskii A, Lelieveldt BP, Kaijzel EL, Lowik CW. Optical advances in skeletal imaging applied to bone metastases. Bone. 2011;48:106–114. doi: 10.1016/j.bone.2010.07.027. [DOI] [PubMed] [Google Scholar]
- 218.Kempen DH, Yaszemski MJ, Heijink A, Hefferan TE, Creemers LB, Britson J, Maran A, Classic KL, Dhert WJ, Lu L. Non-invasive monitoring of BMP-2 retention and bone formation in composites for bone tissue engineering using SPECT/CT and scintillation probes. J Control Release. 2009;134:169–176. doi: 10.1016/j.jconrel.2008.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Weissleder R, Pittet MJ. Imaging in the era of molecular oncology. Nature. 2008;452:580–589. doi: 10.1038/nature06917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Lo Celso C, Fleming HE, Wu JWW, Zhao CX, Miake-Lye S, Fujisaki J, Cote D, Rowe DW, Lin CP, Scadden DT. Live-animal tracking of individual haematopoietic stem/progenitor cells in their niche. Nature. 2009;457:U92–U96. doi: 10.1038/nature07434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Guldberg RE, Ballock RT, Boyan BD, Duvall CL, Lin ASP, Nagaraja S, Oest M, Phillips J, Porter BD, Robertson G, Taylor WR. Analyzing bone, blood vessels, and biomaterials with microcomputed tomography. Ieee Eng Med Biol. 2003;22:77–83. doi: 10.1109/memb.2003.1256276. [DOI] [PubMed] [Google Scholar]
- 222.Kozloff KM, Volakis LI, Marini JC, Caird MS. Near-infrared fluorescent probe traces bisphosphonate delivery and retention in vivo. J Bone Miner Res. 2010;25:1748–1758. doi: 10.1002/jbmr.66. [DOI] [PubMed] [Google Scholar]
- 223.Borselli C, Cezar CA, Shvartsman D, Vandenburgh HH, Mooney DJ. The role of multifunctional delivery scaffold in the ability of cultured myoblasts to promote muscle regeneration. Biomaterials. 2011;32:8905–8914. doi: 10.1016/j.biomaterials.2011.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Chan G, Mooney DJ. New materials for tissue engineering: towards greater control over the biological response. Trends Biotechnol. 2008;26:382–392. doi: 10.1016/j.tibtech.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 225.Toben D, Schroeder I, El Khassawna T, Mehta M, Hoffmann JE, Frisch JT, Schell H, Lienau J, Serra A, Radbruch A, Duda GN. Fracture healing is accelerated in the absence of the adaptive immune system. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2011;26:113–124. doi: 10.1002/jbmr.185. [DOI] [PubMed] [Google Scholar]
- 226.Ali OA, Huebsch N, Cao L, Dranoff G, Mooney DJ. Infection-mimicking materials to program dendritic cells in situ. Nat Mater. 2009;8:151–158. doi: 10.1038/nmat2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Devescovi V, Leonardi E, Ciapetti G, Cenni E. Growth factors in bone repair. La Chirurgia degli organi di movimento. 2008;92:161–168. doi: 10.1007/s12306-008-0064-1. [DOI] [PubMed] [Google Scholar]
- 228.Kempen DHR, Creemers LB, Alblas J, Lu L, Verbout AJ, Yaszemski MJ, Dhert WJa. Growth factor interactions in bone regeneration. Tissue engineering. Part B, Reviews. 2010;16:551–566. doi: 10.1089/ten.teb.2010.0176. [DOI] [PubMed] [Google Scholar]
- 229.Deschaseaux F, Sensébé L, Heymann D. Mechanisms of bone repair and regeneration. Trends in molecular medicine. 2009;15:417–429. doi: 10.1016/j.molmed.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 230.Huang Z, Nelson ER, Smith RL, Goodman SB. The sequential expression profiles of growth factors from osteoprogenitors [correction of osteroprogenitors] to osteoblasts in vitro. Tissue Eng. 2007;13:2311–2320. doi: 10.1089/ten.2006.0423. [DOI] [PubMed] [Google Scholar]
- 231.Ishibe T, Goto T, Kodama T, Miyazaki T, Kobayashi S, Takahashi T. Bone formation on apatite-coated titanium with incorporated BMP-2/heparin in vivo. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;108:867–875. doi: 10.1016/j.tripleo.2009.06.039. [DOI] [PubMed] [Google Scholar]
- 232.Johnson EE, Urist MR, Finerman GAM. Repair of Segmental Defects of the Tibia with Cancellous Bone-Grafts Augmented with Human-Bone Morphogenetic Protein - a Preliminary-Report. Clin Orthop Relat R. 1988:249–257. [PubMed] [Google Scholar]
- 233.Welch RD, Jones AL, Bucholz RW, Reinert CM, Tjia JS, Pierce WA, Wozney JM, Li XJ. Effect of recombinant human bone morphogenetic protein-2 on fracture healing in a goat tibial fracture model. J Bone Miner Res. 1998;13:1483–1490. doi: 10.1359/jbmr.1998.13.9.1483. [DOI] [PubMed] [Google Scholar]
- 234.Kandziora F, Bail H, Schmidmaier G, Schollmeier G, Scholz M, Knispel C, Hiller T, Pflugmacher R, Mittlmeier T, Raschke M, Haas NP. Bone morphogenetic protein-2 application by a poly(D,L-lactide)-coated interbody cage: in vivo results of a new carrier for growth factors. J Neurosurg. 2002;97:40–48. doi: 10.3171/spi.2002.97.1.0040. [DOI] [PubMed] [Google Scholar]
- 235.Wulsten D, Glatt V, Ellinghaus A, Schmidt-Bleek K, Petersen A, Schell H, Lienau J, Sebald W, Ploger F, Seemann P, Duda GN. Time Kinetics of Bone Defect Healing in Response to Bmp-2 and Gdf-5 Characterised by in Vivo Biomechanics. Eur Cells Mater. 2011;21:177–192. doi: 10.22203/ecm.v021a14. [DOI] [PubMed] [Google Scholar]
- 236.Kolambkar YM, Dupont KM, Boerckel JD, Huebsch N, Mooney DJ, Hutmacher DW, Guldberg RE. An alginate-based hybrid system for growth factor delivery in the functional repair of large bone defects. Biomaterials. 2011;32:65–74. doi: 10.1016/j.biomaterials.2010.08.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Ripamonti U, VandenHeever B, Sampath TK, Tucker MM, Rueger DC, Reddi AH. Complete regeneration of bone in the baboon by recombinant human osteogenic protein-1 (hOP-1, bone morphogenetic protein-7) Growth Factors. 1996;13:273-&. doi: 10.3109/08977199609003228. [DOI] [PubMed] [Google Scholar]
- 238.Stevenson S, Cunningham N, Toth J, Davy D, Reddi AH. The Effect of Osteogenin (a Bone Morphogenetic Protein) on the Formation of Bone in Orthotopic Segmental Defects in Rats. Journal of Bone and Joint Surgery-American Volume. 1994;76A:1676–1687. doi: 10.2106/00004623-199411000-00011. [DOI] [PubMed] [Google Scholar]
- 239.Forslund C, Aspenberg P. CDMP-2 induces bone or tendon-like tissue depending on mechanical stimulation. Journal of Orthopaedic Research. 2002;20:1170–1174. doi: 10.1016/S0736-0266(02)00078-5. [DOI] [PubMed] [Google Scholar]
- 240.McKinney L, Hollinger JO. A bone regeneration study: Transforming growth factor-beta(1) and its delivery. Journal of Craniofacial Surgery. 1996;7:36–45. [PubMed] [Google Scholar]
- 241.Beck LS, Wong RL, DeGuzman L, Lee WP, Ongpipattanakul B, Nguyen TH. Combination of bone marrow and TGF-beta1 augment the healing of critical-sized bone defects. Journal of pharmaceutical sciences. 1998;87:1379–1386. doi: 10.1021/js9800883. [DOI] [PubMed] [Google Scholar]
- 242.Critchlow MA, Bland YS, Ashhurst DE. The effect of exogenous transforming growth factor-beta-2 on healing fractures in the rabbit. Bone. 1995;16:521–527. doi: 10.1016/8756-3282(95)00085-r. [DOI] [PubMed] [Google Scholar]
- 243.Steffen T, Stoll T, Arvinte T, Schenk RK. Porous tricalcium phosphate and transforming growth factor used for anterior spine surgery. Eur Spine J. 2001;10:S132–S140. doi: 10.1007/s005860100325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Thaller SR, Dart A, Tesluk H. The effects of insulin-like growth factor-1 on critical-size calvarial defects in Sprague-Dawley rats. Annals of plastic surgery. 1993;31:429–433. doi: 10.1097/00000637-199311000-00007. [DOI] [PubMed] [Google Scholar]
- 245.Meinel L, Zoidis E, Zapf J, Hassa P, Hottiger MO, Auer JA, Schneider R, Gander B, Luginbuehl V, Bettschart-Wolfisberger R, Illi OE, Merkle HP, von Rechenberg B. Localized insulin-like growth factor I delivery to enhance new bone formation. Bone. 2003;33:660–672. doi: 10.1016/s8756-3282(03)00207-2. [DOI] [PubMed] [Google Scholar]
- 246.Raschke M, Wildemann B, Inden P, Bail H, Flyvbjerg A, Hoffmann J, Haas NP, Schmidmaier G. Insulin-like growth factor-1 and transforming growth factor-beta1 accelerates osteotomy healing using polylactide-coated implants as a delivery system: a biomechanical and histological study in minipigs. Bone. 2002;30:144–151. doi: 10.1016/s8756-3282(01)00640-8. [DOI] [PubMed] [Google Scholar]
- 247.Toung JS, Ogle RC, Morgan RF, Lindsey WH. Insulinlike growth factor 1- and 2-augmented collagen gel repair of facial osseous defects. Arch Otolaryngol Head Neck Surg. 1999;125:451–455. doi: 10.1001/archotol.125.4.451. [DOI] [PubMed] [Google Scholar]
- 248.Komaki H, Tanaka T, Chazono M, Kikuchi T. Repair of segmental bone defects in rabbit tibiae using a complex of beta-tricalcium phosphate, type I collagen, and fibroblast growth factor-2. Biomaterials. 2006;27:5118–5126. doi: 10.1016/j.biomaterials.2006.05.031. [DOI] [PubMed] [Google Scholar]
- 249.Radomsky ML, Aufdemorte TB, Swain LD, Fox WC, Spiro RC, Poser JW. Novel formulation of fibroblast growth factor-2 in a hyaluronan gel accelerates fracture healing in nonhuman primates. Journal of Orthopaedic Research. 1999;17:607–614. doi: 10.1002/jor.1100170422. [DOI] [PubMed] [Google Scholar]
- 250.Murakami S, Takayama S, Kitamura M, Shimabukuro Y, Yanagi K, Ikezawa K, Saho T, Nozaki T, Okada H. Recombinant human basic fibroblast growth factor (bFGF) stimulates periodontal regeneration in class II furcation defects created in beagle dogs. Journal of Periodontal Research. 2003;38:97–103. doi: 10.1034/j.1600-0765.2003.00640.x. [DOI] [PubMed] [Google Scholar]
- 251.Cuevas P, dePaz V, Cuevas B, MarinMartinez J, PiconMolina M, FernandezPereira A, GimenezGallego G. Osteopromotion for cranioplasty: An experimental study in rats using acidic fibroblast growth factor. Surg Neurol. 1997;47:242–246. doi: 10.1016/s0090-3019(96)00438-7. [DOI] [PubMed] [Google Scholar]
- 252.Nash TJ, Howlett CR, Martin C, Steele J, Johnson KA, Hicklin DJ. Effect of Platelet-Derived Growth-Factor on Tibial Osteotomies in Rabbits. Bone. 1994;15:203–208. doi: 10.1016/8756-3282(94)90709-9. [DOI] [PubMed] [Google Scholar]
- 253.Lee YM, Park YJ, Lee SJ, Ku Y, Han SB, Klokkevold PR, Chung CP. The bone regenerative effect of platelet-derived growth factor-BB delivered with a chitosan/tricalcium phosphate sponge carrier. J Periodontol. 2000;71:418–424. doi: 10.1902/jop.2000.71.3.418. [DOI] [PubMed] [Google Scholar]
- 254.Kaigler D, Wang Z, Horger K, Mooney DJ, Krebsbach PH. VEGF scaffolds enhance angiogenesis and bone regeneration in irradiated osseous defects. Journal of Bone and Mineral Research. 2006;21:735–744. doi: 10.1359/jbmr.060120. [DOI] [PubMed] [Google Scholar]
- 255.Oest ME, Dupont KM, Kong HJ, Mooney DJ, Guldberg RE. Quantitative assessment of scaffold and growth factor-mediated repair of critically sized bone defects. Journal of Orthopaedic Research. 2007;25:941–950. doi: 10.1002/jor.20372. [DOI] [PubMed] [Google Scholar]
- 256.Si X, Jln Y, Yang L. Induction of new bone by ceramic bovine bone with recombinant human bone morphogenetic protein 2 and transforming growth factor β. International journal of oral and maxillofacial surgery. 1998;27:310–314. doi: 10.1016/s0901-5027(05)80622-8. [DOI] [PubMed] [Google Scholar]
- 257.Ripamonti U, Duneas N, VandenHeever B, Bosch C, Crooks J. Recombinant transforming growth factor-beta 1 induces endochondral bone in the baboon and synergizes with recombinant osteogenic protein-1 (bone morphogenetic protein-7) to initiate rapid bone formation. Journal of Bone and Mineral Research. 1997;12:1584–1595. doi: 10.1359/jbmr.1997.12.10.1584. [DOI] [PubMed] [Google Scholar]
- 258.Young S, Patel ZS, Kretlow JD, Murphy MB, Mountziaris PM, Baggett LS, Ueda H, Tabata Y, Jansen JA, Wong M, Mikos AG. Dose Effect of Dual Delivery of Vascular Endothelial Growth Factor and Bone Morphogenetic Protein-2 on Bone Regeneration in a Rat Critical-Size Defect Model. Tissue Eng Pt A. 2009;15:2347–2362. doi: 10.1089/ten.tea.2008.0510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Kempen DHR, Lu LC, Heijink A, Hefferan TE, Creemers LB, Maran A, Yaszemski MJ, Dhert WJA. Effect of local sequential VEGF and BMP-2 delivery on ectopic and orthotopic bone regeneration. Biomaterials. 2009;30:2816–2825. doi: 10.1016/j.biomaterials.2009.01.031. [DOI] [PubMed] [Google Scholar]