Abstract
Peroxiredoxin 2 has immune regulatory functions, but its expression in human peripheral blood lymphocytes and levels in extracellular fluid in healthy subjects and rheumatoid arthritis patients are poorly described. In the present study, the median intracellular peroxiredoxin 2 protein content of lymphocytes from rheumatoid arthritis patients was more than two-fold higher compared with healthy subjects’ lymphocytes. Intracellular peroxiredoxin 3 levels were similar in healthy and rheumatoid arthritis lymphocytes. Flow cytometry detected peroxiredoxin 2 on the surface of ca. 8% of T cells and ca. 56% of B cells (median % values) of all subjects analyzed. Exofacial thioredoxin-1 was also observed. In the total lymphocyte population from rheumatoid arthritis patients, few cells (median, 6%) displayed surface peroxiredoxin 2. In contrast, a significantly increased proportion of interleukin-17+ve lymphocytes were exofacially peroxiredoxin 2+ve (median, 39%). Prdx2 was also detected in human extracellular fluids. We suggest that crucial inflammatory cell subsets, i.e. interleukin-17+ve T cells, exhibit increased exofacial redox-regulating enzymes and that peroxiredoxin 2 may be involved in the persistence of pro-inflammatory cells in chronic inflammation.
Abbreviations: DAPI, 4’,6-diamidino-2-phenylindole; ECL, enhanced chemiluminescence; HS, healthy subjects; EDTA, ethylenediaminetetraacetic acid; TLR4, toll-like receptor 4; OA, osteoarthritis; PBLs, peripheral blood lymphocytes; PBMCs, peripheral blood mononuclear cells; PE, phycoerythrin; Prdx, peroxiredoxin; Q-RT-PCR, quantitative real-time polymerase chain reaction; RA, rheumatoid arthritis; PBS, phosphate buffered saline; ROS, reactive oxygen species; RT-PCR, reverse transcription polymerase chain reaction; Srx, sulfiredoxin; Trx, thioredoxin; TrxR, thioredoxin reductase
Keywords: Inflammation, Interleukin 17, Cell surface, Autoimmune disease, Peroxiredoxin, Thioredoxin
1. Introduction
Peroxiredoxins (Prdxs) are important antioxidant enzymes. They function as thioredoxin peroxidases, with six mammalian isozymes (Prdxs 1–6). Typical 2-Cys Prdxs (Prdxs 1–4) use a redox-active cysteine residue, termed the peroxidatic cysteine, to reduce hydrogen peroxide (H2O2) (Wood et al., 2003b). This residue is subsequently reduced to a thiol (Cys-SH) when a resolving cysteine (Cys-SH) residue attacks the peroxidatic cysteine (Cys-SOH), forming an inter-subunit disulfide bond which is then reduced by thioredoxin (Trx) (Wood et al., 2003b). Trx is maintained in a reduced state by virtue of the activity of thioredoxin reductase (TrxR) (Arner and Holmgren, 2000). Besides acting as antioxidants, Prdxs have cell signaling roles (Kang et al., 2005), partly due to their susceptibility to “overoxidation” whereby the sulfenic acid reaction intermediate is oxidized to sulfinic acid by H2O2, allowing a local, transient, increase in H2O2 concentration (Wood et al., 2003a). Prdxs 1–4 use sulfiredoxin (Srx) or sestrins as electron donors for the reduction of their sulfinic acid form to sulfenic acid (Biteau et al., 2003; Budanov et al., 2004). Prdx2 is a modulator of inflammatory and immune responses, probably through its antioxidant activity (Szabó et al., 2009).
Rheumatoid arthritis (RA) is an autoimmune disorder which affects about 1–2% of the population worldwide (Hakim et al., 2006). Although RA primarily affects synovial joints, it is a systemic disease, and Th1 and Th17 (IL-17+ve) lymphocytes have been implicated in RA pathogenesis (Annunziato et al., 2009). Key features of the disease are chronic inflammation, oxidative stress and dysfunctional apoptosis. The overproduction of reactive oxygen species (ROS) leads to the exhaustion of some antioxidants (Gilston et al., 1998; Pattison and Winyard, 2008). Oxidative damage within the peripheral blood lymphocytes (PBLs) in RA patients is demonstrated by an increase in the levels of the mutagenic 8-hydroxydeoxyguanosine in lymphocyte DNA compared with healthy control subjects (Bashir et al., 1993). Chronic exposure of lymphocytes to oxidative stress is associated with resistance to apoptosis (Brown and Bicknell, 2001), and defective apoptosis is a feature of a number of inflammatory autoimmune diseases (Eggleton et al., 2010; Tarr et al., 2010). The lack of apoptotic clearance of inflammatory immune cells has been implicated in the chronicity of inflammation in RA (Liu and Pope, 2003). Both Prdx2 and Prdx3 are inhibitors of apoptosis (Zhang et al., 1997), and therefore these Prdxs may regulate the removal of inflammatory cells.
Despite the above indirect evidence that Prdxs might be involved in RA pathogenesis, direct evidence is limited, although a small number of studies have examined the role of Trx1. Serum and synovial fluid Trx1 levels were elevated in RA patients compared with osteoarthritis (OA) patients and healthy subjects (HS), and plasma and synovial fluid Trx1 concentrations correlated with disease activity and progression (Jikimoto et al., 2001; Maurice et al., 1999). Furthermore, treatment of human synovial fibroblast cultures with Trx1 augmented IL-1 and IL-6 production upon TNF-α stimulation (Yoshida et al., 1999). These results support the idea that Trx1 is pro-inflammatory. However, information on the role in RA of Prdxs – as redox partners of Trx1 – remains scant. The aim of the present study was to investigate the potential role of intracellular and exofacial Prdx2 – along with selected other members of the Prdx-based system – in peripheral blood lymphocytes from RA and HS.
2. Materials and methods
2.1. Materials
Recombinant human Prdx2 and Prdx3 were obtained from AbFrontier (Seoul, South Korea), and Trx1 was a gift of Dr Kamal Hamidi (University of Exeter). Mouse monoclonal and rabbit polyclonal anti-human antibodies to Prdx2 (1E8, 9A1), Prdx3 (4G10), overoxidized Prdx (10A1) and Trx1 (8A1) were from AbFrontier. Horseradish peroxidase (HRP)-conjugated goat anti-rabbit antibody was from Abcam (Cambridge, UK). The HRP-conjugated goat anti-mouse antibody and the monoclonal anti-human β-tubulin (TUB2.1) antibody were from Sigma (Dorset, UK). Fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse or anti-rabbit IgG secondary antibodies were from Beckman-Coulter (High Wycombe, UK). FITC-conjugated goat anti-mouse Fab2 antibody was from Jackson ImmunoResearch Laboratories (Suffolk, UK).
Phycoerythrin (PE)-conjugated anti-human CD3 and PE-conjugated anti-human CD19 antibodies were from Biolegend (San Diego, USA). FITC-conjugated mouse and rabbit IgG1 isotype control antibodies were from Santa Cruz Biotechnology (Santa Cruz, USA) and a FITC-conjugated mouse IgG1 κ isotype control antibody (MOPC21) was from BD Pharmingen (Pecs, Hungary). ProLong® Gold Antifade Reagent with 4′,6-diamidino-2-phenylindole (DAPI) was from Invitrogen (Budapest, Hungary). Ficoll-Paque Plus was from GE Healthcare (Amersham, UK) and Histopaque-1077 was from Sigma (Budapest, Hungary). Cell culture reagents such as RPMI culture medium, fetal bovine serum and penicillin-streptomycin were from Lonza (Wokingham, UK). NP40 lysis buffer and Magic Marker molecular mass markers were purchased from Invitrogen (Paisley, UK). The Bradford reagent was from Sigma. Commercially prepared 4–20% gradient SDS-PAGE gels were obtained from Thermo Fisher Scientific (Northumberland, UK). PVDF membranes and 30% acrylamide were purchased from Bio-Rad (Hemel Hempstead, UK). The Quantichrom Hemoglobin Assay was from BioAssay Systems (Hayward, USA). CytoStim T cell stimulant and the IL-17 secretion assay were supplied by Miltenyi Biotec (Bisley, UK).
2.2. RA and healthy subjects
RA patients attending the outpatient clinics of the Royal Devon and Exeter Foundation Trust Hospital (Exeter, UK) were asked to take part in the study. All patients and HS gave written consent and the study was authorized by the North and East Devon Research Ethics Committee. In total, 45 patients with diagnosed RA based on the 1987 American College of Rheumatology criteria for RA (Arnett et al., 1988) were recruited for various aspects of the study. EDTA anti-coagulated blood samples (up to 40 ml) were obtained from all subjects. Plasma was obtained by centrifugation of EDTA anticoagulated blood samples at 1611 × g for 10 min at RT. Synovial fluid samples from RA patients were obtained during routine knee aspirations. Due to the relatively large volumes of blood needed to perform all the assays in replicate, it was not possible to use the same patients for all of the analyses used in this study. For full details of the volunteers, see Supplementary Data 1.
2.3. Peripheral blood lymphocyte isolation
Peripheral blood mononuclear cells (PBMCs) were isolated from EDTA anticoagulated whole blood of RA patients and HS using Ficoll-Paque Plus™ or Histopaque-1077, according to the manufacturer's instructions. Any residual red blood cell contamination was removed by hypotonic lysis. In order to eliminate platelets and monocytes and obtain a pure fraction of PBLs, PBMCs were cultured overnight at 37 °C in 5% CO2/air in RPMI culture medium supplemented with 10% (v/v) fetal bovine serum, 100 U/ml penicillin and 100 μg/ml streptomycin.
2.4. Western blotting
Cell lysis was performed using NP40 lysis buffer according to the manufacturer's instructions. Briefly, lysis buffer was added to the cell pellets, followed by 30 min incubation on ice with intermittent vortexing, and finally by centrifugation at 10,000 g for 10 min. Protein concentrations of the samples were measured by the Bradford assay. Protein lysates (30 μg), molecular mass markers and purified recombinant Prdx2, Prdx3 and Trx1 were separated on 4–20% reducing or non-reducing SDS-PAGE gels, transferred onto PVDF membranes, incubated with 1:2000 (0.5 μg/ml) primary mouse monoclonal anti-human antibodies to Prdx2, Prdx3, overoxidized Prdx, or Trx1, followed by incubation with HRP-conjugated goat anti-mouse secondary antibody at 1:2000 dilution and detection by enhanced chemiluminescence (ECL) using a Chemidoc XRS imager system (Bio-Rad). Tubulin bands resolved on separate reducing blots were used as a loading control, and bands showing unequal loading were excluded from the analysis. Densitometry analysis was performed using Quantity One 1-D analysis software (Bio-Rad).
2.5. Reverse transcription PCR (RT-PCR) and quantitative real-time PCR (Q-RT-PCR)
mRNA was extracted from RA (n = 8) and HS (n = 8) lymphocytes and RT-PCR of Prdxs 1–6 was performed as described (Lehtonen et al., 2004a). mRNA was extracted from additional RA (n = 19) and HS (n = 13) lymphocytes, from which cDNA was synthesized for subsequent Q-RT-PCR analysis. The expression of selected genes (Prdxs 2, 3, 5, Srx, Trx1 and TR) was quantified using Q-RT-PCR using our previously published methods (Eggleton et al., 2010; Tarr et al., 2010).
2.6. Flow cytometry analysis of exofacial Prdx2 and Trx1
PBLs (isolated as described above) were dispensed at a density of 0.2 × 106 cells per 100 μl cold buffer (PBS supplemented with 0.5% (w/v) bovine serum albumin and 2 mM EDTA) in sterile Eppendorf tubes. The cells were incubated with 5 μg/ml mouse monoclonal or rabbit polyclonal primary antibody to Prdx2 or Trx1 for 15 min on ice. Subsequently, the cells were washed twice in PBS and incubated in 100 μl cold buffer containing 0.25 μl FITC-conjugated goat anti-mouse or anti-rabbit IgG secondary antibody. Following 15 min incubation on ice and two washing steps, the cells were incubated for 30 min with 5 μl PE-conjugated anti-human CD3 or PE-conjugated anti-human CD19 – T and B lymphocyte markers, respectively. The following controls were also used: unstained cells, cells stained with FITC-conjugated secondary antibody only, cells treated with PE-conjugated anti-CD3 and, finally, cells treated with a FITC-conjugated mouse or rabbit IgG1 as an isotype control. Samples were analyzed on a Cell Lab Quanta SC flow cytometer (Beckman Coulter, High Wycombe, UK).
2.7. Fluorescence microscopy of intracellular and exofacial Prdx2 and Trx1 in peripheral blood lymphocytes
PBLs were isolated as described above and resuspended in blocking buffer (PBS containing 0.5% (w/v) bovine serum albumin and 2 mM EDTA) at 2 × 106 cells/ml density. Samples for intracellular staining were subsequently pelleted and permeabilized by incubation in methanol for 20 min at −20 °C, followed by washing and resuspension in blocking buffer at the indicated density. Aliquots (100 μl) of the cells were then incubated for 15 min on ice with 0.5 μl mouse monoclonal antibody to Prdx 2, Trx 1 or 2 μl FITC-conjugated mouse IgG1 κ isotype control antibody. This was followed by washing and incubation for 15 min on ice with 0.75 μl of a FITC-labeled goat anti-mouse Fab2 antibody. The cells were subsequently washed and mounted on microscopy slides as described below.
Cells for exofacial staining were used live, at the indicated density. Aliquots of the live cells (100 μl, containing 0.2 × 106 cells) were incubated for 30 min on ice with 2.5 μl mouse monoclonal antibody to Prdx 2 or Trx 1. This was followed by washing and incubation on ice for 15 min with 0.25 μl of a FITC-labeled goat anti-mouse Fab2 antibody. An aliquot stained with the secondary antibody only was used as a negative control. After washing, the cells were fixed using 4% paraformaldehyde.
The stained and fixed cells were subjected to cytospin, washed using PBS, then mounted on the slide by adding a drop of ProLong Gold Antifade Reagent with DAPI and covering the drop with a coverslip. Images were taken using a Nikon H600L fluorescence microscope equipped with a SpotFlex FX1520 digital camera.
2.8. T cell stimulation and assay of IL-17 secretion
After isolation from peripheral blood, PBMCs were maintained overnight, at 1.5 × 106 cells/well in 96 well plates in RPMI medium supplemented with 5% (v/v) autologous serum, in 5% CO2/air at 37 °C. A sensitive secretion assay was used to detect IL-17-secreting cells, according to the instructions of the manufacturer (Miltenyi Biotec). In this assay, T cells were stimulated by incubation for 4 h with CytoStim, a superantigen T cell stimulant (used at 20 μl/ml medium). The IL-17-secreting cells were labeled according to the manufacturer's instructions, and this step was followed by labeling with an anti-Trx1 or anti-Prdx2 rabbit polyclonal antibody and a FITC-conjugated anti-rabbit secondary antibody. Unstimulated cells were collected, to serve as negative controls. Stimulated and unstimulated cells from RA patients (n = 3) were also used for RNA extraction and subsequent Q-RT-PCR analysis of Prdx2 levels as described above.
2.9. Statistical analysis
Pair-wise differences between mRNA, intracellular and cell surface protein levels between groups were tested using a Mann–Whitney U-test. Correlations between mRNA/protein levels and clinical parameters were tested using Spearman's rank correlation coefficient (rs).
3. Results
3.1. Intracellular protein levels of Prdx2, Prdx3 and Trx1 in RA subjects compared to healthy subjects
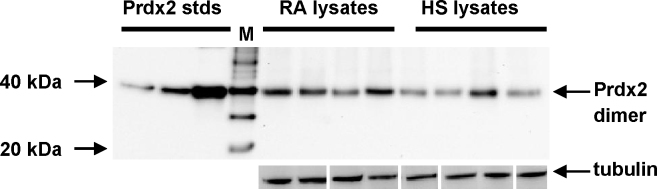
Lymphocyte cell lysates from RA patients (n = 10) and healthy subjects (n = 9) were analyzed by non-reducing gradient SDS-PAGE and immunoblotted for Prdx2 (Fig. 1) and Prdx3 (Fig. 2). Non-reducing blots were used in order to reveal if the Prdxs were present in the overoxidized form in these samples: upon cell lysis, reduced Prdxs oxidize and form dimers, whereas overoxidized Prdxs are unable to dimerize and resolve as monomers on non-reducing blots (Peskin et al., 2007). On non-reducing gels, Prdx2 in all samples exhibited an oligomeric mobility corresponding to a dimer. The monomeric band (22 kDa) was not detectable in most cases (Fig. 1), indicating that the majority of Prdx2 was not overoxidized in these lysates. Prdx2 protein content was elevated in the RA samples compared with HS (Mann–Whitney U-test, p < 0.05) (see Supplementary Data 2, Supplementary Fig. 1).
Fig. 1.
A representative Western blot of the intracellular expression of Prdx2 in blood lymphocyte lysates from RA patients compared with healthy subjects. Lysates of lymphocytes from RA patients and healthy subjects (HS) were loaded (30 μg total protein/lane) onto 4–20% gradient SDS-PAGE gels under non-reducing conditions and analyzed by Western blotting with anti-Prdx2. Molecular mass markers are denoted by ‘M’. Samples of purified human Prdx2 (10, 25 and 100 ng/lane) were loaded as standards on each blot. Equal loading of total protein was confirmed by the Bradford assay and aliquots of the lysates were resolved on SDS-PAGE under reducing conditions, blotted and probed with anti-tubulin. The bands in this tubulin blot image originate from two blots and have been moved around to match the order of samples in the Prdx2 blot.
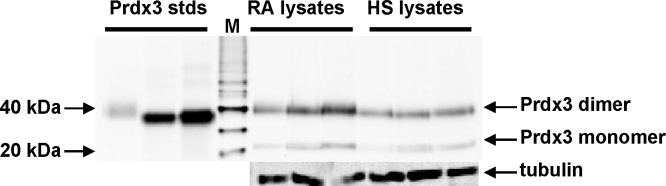
Fig. 2.
A representative Western blot of the intracellular expression of Prdx3 in blood lymphocyte lysates from RA patients compared with healthy subjects. Lysates of lymphocytes from RA patients and healthy subjects (HS) were loaded (30 μg total protein/lane) onto 4–20% gradient SDS-PAGE gels under non-reducing conditions and analyzed by Western blotting with anti-Prdx3. Molecular mass markers are denoted by ‘M’. Samples of purified human Prdx3 (10, 50 and 100 ng/lane) were loaded as standards on each blot. Equal loading of total protein was confirmed by the Bradford assay and aliquots of the lysates were resolved on SDS-PAGE under reducing conditions, blotted and probed with anti-tubulin.
Prdx3 protein was also expressed in all lymphocyte lysate samples (Fig. 2). Prdx3 was resolved as a dimer on non-reducing blots (RA: n = 6, healthy: n = 6), indicating that the majority of Prdx3 was in the reduced state in the cells. Slight monomeric bands were also visible in most samples, potentially showing some overoxidized Prdx3. Prdx3 content was very similar in the RA and healthy samples (see Supplementary Data 2).
Having obtained initial evidence from the above non-reducing blots of Prdx2 and Prdx3, that these Prdxs were not extensively overoxidized to cysteine sulfinic acid, we wished to confirm this finding by probing blots with a monoclonal antibody that recognizes the overoxidized forms of Prdxs 1–4. Non-reducing blots of lymphocyte lysate samples (RA: n = 6 and healthy: n = 6) were probed. Lysates of Jurkat cells treated for 10 min with H2O2 (200 μM, 2 mM) were used as positive controls in the experiment (Baty et al., 2005). Although the positive controls displayed a single band (between 20 and 30 kDa, where overoxidized forms of Prdx 1–4 are expected to appear), the RA and healthy lymphocyte lysates gave weak bands, or no bands at all (data not shown), indicating that Prdx overoxidation did not occur to a large extent in untreated human peripheral blood lymphocytes, or that recycling of overoxidized Prdxs was very efficient in these cells. Overoxidation of Prdxs in intact human lymphocytes was nevertheless inducible using 200 μM H2O2 for 10 min (data not shown).
Western blots of reducing SDS-PAGE were prepared, in order to detect total Trx1 protein expression in lymphocytes from RA patients (n = 6) and healthy subjects (n = 7). Trx1 was expressed in all analyzed subjects (data not shown), at similar levels in the RA (n = 6) and healthy groups (n = 7).
3.2. Relative mRNA levels of the Prdx-based gene family in isolated lymphocytes from healthy individuals and RA patients
Having observed differences in Prdx 2 but not Prx3 at the protein level in lymphocytes from RA patients compared to healthy subjects, we investigated whether there were differences in the Prdx-based gene family at the mRNA level. RT-PCR analysis (RA: n = 8, HS: n = 8) showed that all six Prdxs were expressed in PBLs, both in RA patients and healthy subjects. PBLs from additional RA (n = 19) and healthy (n = 13) subjects were used for Q-RT-PCR analysis of Prdx2, Prdx3, Prdx5, Srx, Trx1 and TR 1. These members of the Prdx-based family were selected for the following reasons: Srx, Trx1 and TR 1 are reducing partners of Prdx2 (Szabó et al., 2009). Prdx3 is a mitochondrial Prdx and has anti-apoptotic functions (Cox et al., 2008), and could therefore be relevant in RA where a failure of apoptosis of inflammatory cells contributes to disease pathology (see Section 1). Finally, Prdx5 over-expression has been shown in OA (Wang et al., 2002). Employing Q-RT-PCR, we found that all of the mentioned six genes were expressed in all subjects. However, there were no significant differences in gene expression between individual genes in RA and HS subjects (data not shown). A strong positive correlation was present between Trx1 protein and Trx1 mRNA levels (rs = 0.94; p < 0.0001; n = 13). In contrast, we did not observe significant correlations between protein and mRNA levels of Prdx2 and Prdx3 in lymphocytes.
Disease duration showed a positive correlation with Prdx2 mRNA expression levels (rs = 0.68, p = 0.01, n = 13). In the RA group, there was a negative correlation between age, and (a) the level of Srx mRNA (rs = −0.55, p = 0.02, n = 19) and (b) the level of TR mRNA (rs = −0.50, p = 0.03, n = 19). No significant correlations were observed between age and the relative mRNA expression of Prdx2, Prdx3, Prdx5, Srx, Trx1, or TR1 in the healthy group.
3.3. Prdx2 and Trx1 are both present on the surface of human lymphocytes and are differentially displayed in lymphocyte subpopulations
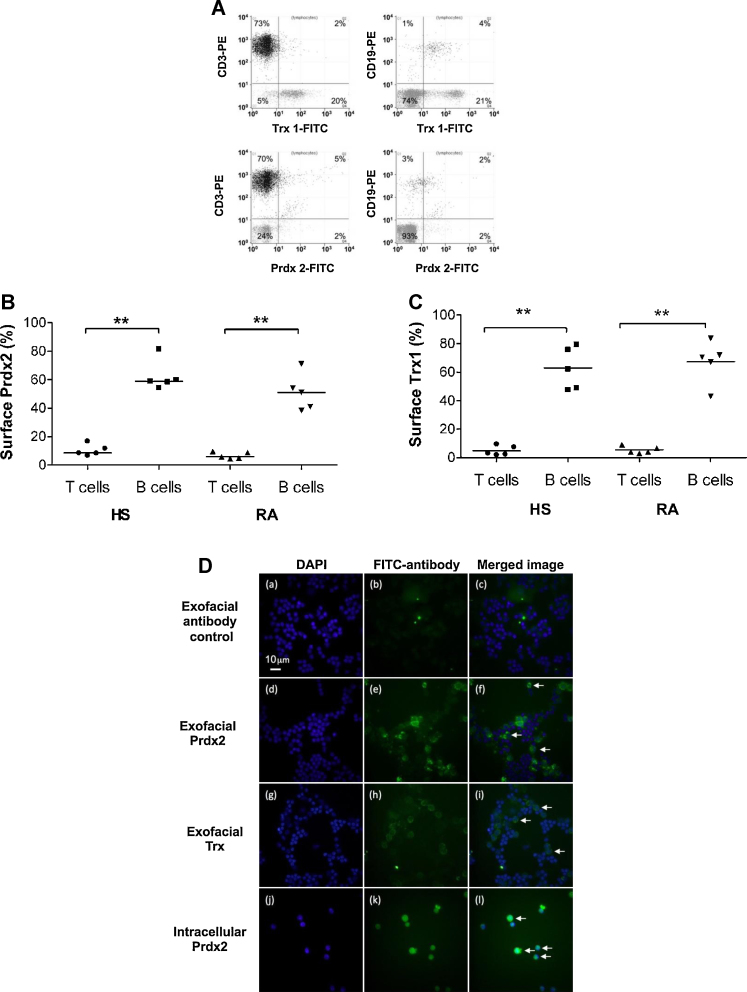
Exofacial Prdx2 and Trx1 were both present on human B and T lymphocytes, as detected by flow cytometry. CD3 was used as a marker of T cells and CD19 was used as a marker of B cells. Prdx2 was displayed on both CD3+ve and CD19+ve cells (Fig. 3A), and the proportion of CD19+ve cells displaying Prdx2 was significantly higher compared with CD3+ve cells, in both the healthy and the RA group (Fig. 3B). In the healthy group (n = 5), 59% [median %] (range 54–81%) of CD19+ve cells were positive for Prdx2 staining, whereas only 8% [median %] (range 7–17%) of CD3+ve cells were positive for Prdx2 (p < 0.01). In the RA group (n = 5), 51% (range 38–71%) of CD19+ve cells exhibited positive staining for Prdx2, whereas only 6% (range 5–10%) of CD3+ve cells were positive for Prdx2 staining (p < 0.01).
Fig. 3.
Presence of exofacial Prdx2 and Trx1 on lymphocytes. (A) Representative flow cytometry dot plots of the presence of exofacial Prdx2 and Trx1 on T and B lymphocytes. The Y-axes show the proportion of lymphocytes with a T cell (CD3-PE) or B cell (CD19-PE) phenotype. The X-axes show the proportion of lymphocytes that stain for cell surface Trx1 (Trx1-FITC indirect staining) or Prdx2 (Prdx2-FITC indirect staining). Thus the upper right-hand quadrant of each graph shows the proportion of T or B cells that stain for Trx1 or Prdx2. The numbers in each graph represent the percentage of cells contained in each quadrant. (B) The % of T and B lymphocytes in RA and healthy (HS) lymphocytes with exofacial Prdx2 is shown. Horizontal lines indicate median values. Two asterisks indicate a statistically significant difference at the p < 0.01 level (Mann–Whitney U-test). (C) The % of T and B lymphocytes in RA and healthy (HS) lymphocytes with exofacial Trx1 is shown. Horizontal lines indicate median values. Two asterisks indicate a statistically significant difference at the p < 0.01 level (Mann–Whitney U-test). (D) Immunohistochemical detection of exofacial Prdx2 and Trx1, and intracellular Prdx2 in peripheral blood lymphocytes from a healthy subject. PBLs were isolated from peripheral blood. Exofacial Prdx2 (panel e) and Trx1 (panel h) was detected on live cells using mouse monoclonal antibodies to human Prdx2 and Trx1, respectively, in conjunction with a FITC-conjugated goat anti-mouse Fab2 fragment of IgG. Cells labeled with the secondary antibody only were used as controls (panels a–c). After labeling, the cells were fixed with 4% paraformaldehyde and mounted on slides with antifade fluid supplemented with DAPI for nuclear staining (panels a, d, g). Cells permeabilized and fixed with methanol were used to label intracellular Prdx2 (panel k). The permeabilized and labeled cells were mounted on slides with antifade fluid supplemented with DAPI for nuclear staining (panel j). The arrows in panels f, i, and l depict DAPI-stained cells that were positive for exofacial Prdx2 (panel f), exofacial Trx1 (panel i), or intracellular Prdx2 (panel l), respectively.
The median expression of Trx1 on CD3+ve lymphocytes (T cells) was 3% in the healthy (n = 5, range: 2–9%) and 4% in the RA group (n = 5, range: 3–9%; Fig. 3C). The proportion of CD19+ve cells (B lymphocytes) displaying Trx1 was significantly higher (p < 0.01) than the proportion of CD3+ve T cells displaying Trx1: Trx1 was present on 62% and 71% (median values) of all CD19+ve cells in the healthy group (n = 5, range: 48–79%) and RA group (n = 5, range: 43–83%), respectively (Fig. 3C). To confirm the exofacial presence of Prdx2 and Trx1, immunocytochemistry was performed on non-permeabilized PBLs (Fig. 3D; panels a–i), using a FITC-conjugated goat anti-mouse Fab2 antibody as a negative control (panels a–c) and antibodies against Prdx2 (panels d–f) and Trx1 (panels g–i) in conjunction with a FITC-conjugated goat anti-mouse Fab2 antibody. We also examined permeabilized lymphocytes for intracellular Prdx2, using anti-Prdx2 (panels j–l) in conjunction with a FITC-conjugated goat anti-mouse Fab2 antibody. Exofacial localization of both Trx1 and Prdx2 was observed (shown by arrows in panels i and f, respectively). Intracellular Prdx2 appeared more abundant within lymphocytes (shown by arrows in panel l). There was no detection of non-specific binding to cells of the FITC-conjugated goat anti-mouse Fab2 (panel c, merged image).
3.4. The proportion of IL-17-secreting RA lymphocytes displaying exofacial Prdx2 is increased compared with the total RA lymphocyte population
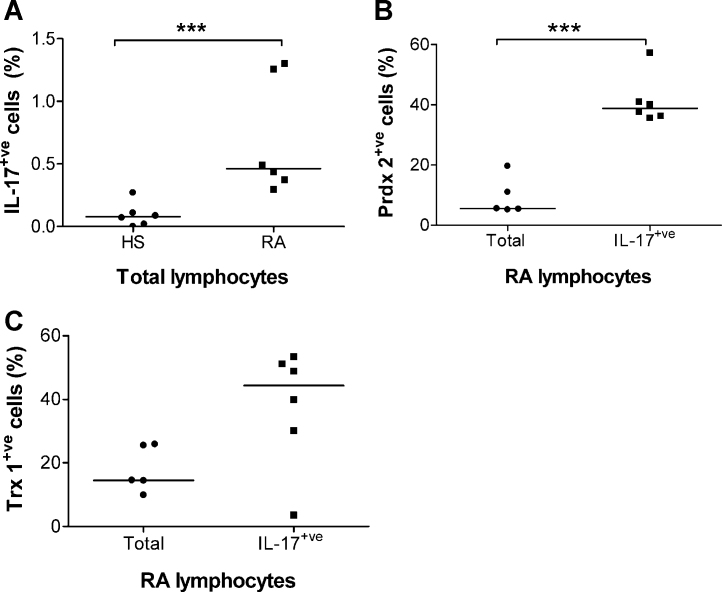
IL-17-secreting T cells were detected using a flow cytometry IL-17 capture assay in the blood lymphocyte population from RA (n = 6) and healthy (n = 6) subjects. As expected, the percentage of IL-17-secreting cells within the total lymphocyte population was significantly higher (Mann–Whitney U-test, p < 0.005) in the RA group (median: 0.46%, range: 0.3–1.3%) than in the healthy group (median: 0.08%, range: 0–0.27%; Fig. 4A). A subset of the general lymphocyte population displayed Prdx2 (Fig. 4B) and Trx1 (Fig. 4C) on their surface. Due to the extremely small number of IL-17-secreting PBLs in the healthy samples, only the RA samples were used to estimate the percentage of Prdx2+ve IL17+ve and Trx1+ve IL17+ve cells. Prdx2 was displayed on 36–57% (median: 39%) of IL-17-secreting PBLs (Fig. 4B), while Trx1 was displayed on 3–53% (median: 44%) of IL-17-secreting PBLs in the RA patients screened (Fig. 4C). The median percentage of Prdx2+ve cells in the IL-17-secreting population, was significantly greater (p < 0.005) than the median percentage of Prdx2+ve cells in the total lymphocyte population (Fig. 4B). There was no statistically significant difference in the median percentage of Trx1+ve cells in the IL-17-secreting population compared with the total PBL population (Fig. 4C).
Fig. 4.
Percentages of lymphocytes from healthy subjects (HS) and RA patients (RA) that were positive for IL-17 secretion and exofacial Prdx2 and Trx1, as determined by flow cytometry. See “Section 2” for details. (A) Percentages of IL-17-secreting cells within the total lymphocyte population in healthy subjects and in RA patients. (B) Comparison of the percentages of Prdx2+ve cells within the IL-17-secreting and the total lymphocyte population in RA patients. (C) Comparison of the percentages of Trx1+ve cells within the IL-17-secreting and the total lymphocyte population in RA patients. In Panels (A) and (B), three asterisks indicate a statistically significant difference at the p < 0.005 level (Mann–Whitney U-test). Horizontal lines in A–C indicate median values.
Remarkably, the median proportion of all lymphocytes (i.e. the total lymphocyte population-not the IL-17+ve cells only) exhibiting Prdx2 on their surface increased significantly after stimulation with CytoStim for 4 h in RA patients (p < 0.005, Mann–Whitney U-test) (Supplementary Fig. 2B). The mRNA level corresponding to Prdx2 was measured by Q-RT-PCR in RA PBLs (n = 3) before and after stimulation, but no evidence was found of an increase in Prdx2 production at the mRNA level following stimulation with CytoStim for 4 h (data not shown).
4. Discussion
The present study showed that Prdx2, Prdx3 and Trx1 were all present, at the protein level, in peripheral blood lymphocytes from both healthy human subjects and RA patients. Semi-quantitative analysis of western blots suggested that the lymphocyte content of Prdx2 – but not two related proteins, Prdx3 and Trx1 – was elevated in RA patients. Not only was Prdx2 protein content elevated in RA lymphocytes in the peripheral circulation but intracellular Prdx2 levels were in positive correlation with the serum concentrations of CRP, an inflammatory marker. This suggests the possibility that Prdx2 may be elevated as part of the inflammatory response in RA lymphocytes, and is reminiscent of the well-known induction of plasma Trx1 during the inflammatory response in RA (Jikimoto et al., 2001). Although Prdx2 in RA blood lymphocytes, plasma and synovial fluid has not been studied before, previous studies (Bo et al., 2009; Kim et al., 2006) showed that Prdx2 protein was elevated in the synovial tissue of RA patients compared with the synovial tissue from OA patients, and Prdx2 was also elevated in cultured RA and OA synovial fibroblasts compared with healthy control fibroblasts.
Prdx2 is a redox enzyme with extensive roles in immune regulation: Prdx2 knockout mice suffer from the expansion of certain immune cell populations (Moon et al., 2006) and intracellular Prdx2 is a negative regulator of the pro-inflammatory toll-like receptor 4 (TLR4) signaling pathway (Yang et al., 2007). At the same time, by consuming H2O2, Prdx2 contributes to the sustenance of the mitogen activated protein kinase kinase/extracellular signal-regulated kinase pathway of T cell receptor signaling (Kwon et al., 2003). Moreover, Prdx2 is an inhibitor of apoptosis (Zhang et al., 1997). Therefore, it is unclear whether the observed increase in the intracellular Prdx2 content of the peripheral blood lymphocytes from RA patients plays a pathological or protective role. However, a reducing environment intracellularly in lymphocytes is necessary to maintain levels of extracellular thiols, which in turn are necessary for the functioning of T helper cells (Griffiths et al., 2011). Therefore, it is possible that by maintaining a reducing environment, the Prdx system contributes to T cell activation in RA.
In the current study, immunoblots of non-reducing gels of lysates from healthy and RA lymphocytes showed the presence of Prdx2 and Prdx3 dimers, with only weak monomeric bands, indicating that the majority of the Prdx2 and Prdx3 molecules were not overoxidized (Figs. 1 and 2). Moreover, when probed with a specific antibody to the overoxidized conserved site cysteine residue of peroxiredoxins, blots revealed no detectable staining for the overoxidized forms of Prdx2 or Prdx3. The lack of large-scale overoxidation of Prdxs may indicate that the enzymes were redox active in these samples.
The expression of mRNA for Prdxs 1–3 in different mammalian tissues appears ubiquitous (Chae et al., 1999). Nevertheless, few systematic studies have been performed describing their expression, particularly in a clinical setting. To our knowledge, this is the first study to demonstrate that all six Prdxs are expressed in human lymphocytes, at least at the mRNA level. Previously, it has been observed that the cellular mRNA and protein levels of the Prdxs do not necessarily correlate with each other (Lehtonen et al., 2004b; Seo et al., 2000) indicating that post-transcriptional mechanisms may play important roles in modulating Prdx protein levels. In the present study, we found no relationship between the amount of Prdx2/3 mRNA and the corresponding protein levels. Thus there was no difference in lymphocyte Prdx2 mRNA levels between RA patients and healthy subjects, despite the elevated Prdx2 protein content of RA lymphocytes.
The presence of exofacial Trx1 is well known on a wide range of human cells (Wollman et al., 1997; Martin and Dean, 1991; Sahaf et al., 1997), and has been suggested to play a role in cell adhesion during the inflammatory response (Hara et al., 2007). To date, the presence of exofacial Prdx2 has not been described in higher eukaryotes. However, in the protozoan, Entamoeba histolytica, a 29 kDa Prdx, assumed to protect the pathogen from oxidant attack by the host, is the major thiol-containing surface antigen (Choi et al., 2005). Moreover, Prdx1 was detected on the surface of a 2B4 mouse T cell hybridoma line (Metcalfe et al., 2011). In the current study, the presence of Prdx2 on the cell surface of human lymphocytes was demonstrated for the first time. We also showed that the redox-regulating enzymes Prdx2 and Trx1 were differentially displayed on the surface of human peripheral blood lymphocyte subsets. These results are schematically summarized in Fig. 5. While a high proportion of unstimulated B cells displayed exofacial Trx1 and Prdx2, few T cells displayed these two enzymes on the surface under resting conditions. These observations are in line with previous studies (Lawrence et al., 1996; Pedersen-Lane et al., 2007), in which differences were detected between human lymphocyte subsets with regard to their surface thiol levels: CD19+ve cells (i.e. B cells) had the highest surface thiol levels, followed by CD8+ve T cells, then by CD4+ve T cells.
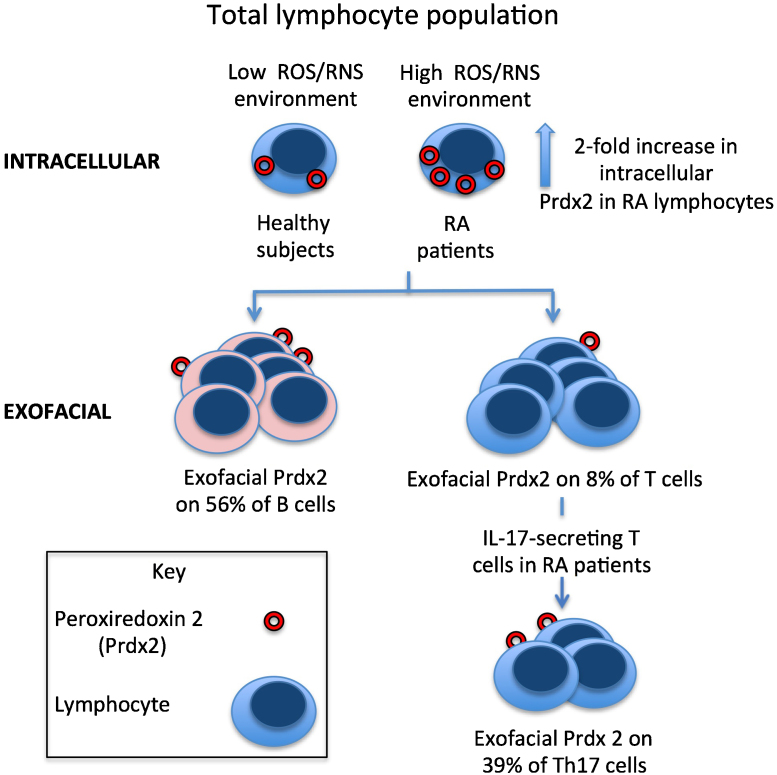
Fig. 5.
A basic summary of some of the major lymphocyte subsets in relation to the levels of intracellular and exofacial Prdx2. The diagram summarizes the key results found in the present study, comparing healthy subjects with RA patients. For simplicity, a number of subtypes of T cell have been omitted from the figure. Prdx2 is schematically shown as a red ring, representing the crystal structure of the decameric human red blood cell Prdx 2 (Schröder et al., 2000). It is unknown if the exofacial Prdx2 is in the decameric, or a different oligomeric form. Intracellular Prdx2 protein content was elevated in RA lymphocytes compared with lymphocytes from healthy subjects. The median proportion of B cells displaying exofacial Prdx2 and Trx1 was higher compared with Prdx2+ve and Trx1+ve T cells in both the RA and healthy subjects. The indicated values of 56% Prdx2+ve B cells and 8% Prdx2+ve T cells are median values of percentages obtained from all the analysed PBL preparations (n = 10; made up of the pooled values from the 5 RA patients and 5 healthy subjects shown in Fig 3B). The medain proportion of Th17 cells displaying exofacial Prdx2 was higher compared with the medain proportion of Prdx2+ve cells in the total lymphocyte population in RA patients. (Th: T helper cell).
Surface thiols are extremely important in the regulation and activity of lymphocytes. Although there is some controversy in the literature, elevated lymphocyte surface thiol levels have been shown to increase susceptibility to RA (Gelderman et al., 2006), suggesting a key role for the redox regulation of exofacial thiol-containing proteins in autoimmunity. Moreover, T cells from RA patients display decreased basal ROS levels and lower ROS production upon stimulation compared with healthy controls (Griffiths et al., 2011). Although the redox regulation of immune responses is complex and cell population-specific, the current view is that effector T lymphocytes need a reducing extracellular microenvironment for their activation (Angelini et al., 2002; Yan and Banerjee, 2010). On the contrary, regulatory T cells (Tregs) have a different capacity for redox control in comparison with effector lymphocytes: e.g. Tregs have a lowered sensitivity to oxidative stress-induced cell death (Mougiakakos et al., 2009) which is due to an increased production of Trx1 in these cells (Mougiakakos et al., 2011). In the present study, there was no difference between the RA and healthy subject groups in relation to the percentage of unstimulated lymphocytes displaying exofacial Prdx2 and Trx1. However, upon antigenic stimulation, the percentage of Prdx2+ve lymphocytes increased in the RA group, but not in the healthy group. Mitogenic activation also increased surface thiol levels of T lymphocytes in another study (Lawrence et al., 1996). As expected, we observed that the percentage of IL-17-secreting cells (Th17 cells) was higher in RA blood lymphocytes compared with lymphocytes from healthy subjects. The pro-inflammatory Th17 cells, have been suggested to provoke the pathology observed in RA (Bennett, 2008). In our study, the percentage of PBLs displaying exofacial Prdx2 (but not Trx1) was higher among the IL-17-secreting cells than in the general lymphocyte population in RA patients (summarized in Fig. 5). These results suggest that Prdx2 may sustain redox-mediated immunoregulatory events at the surface of specific pro-inflammatory T cell subsets, such as Th17 cells. However, such a role for Prdx2 would clearly be dependent on the maintenance of the activity of the enzyme, when present in the extracellular (exofacial) environment, as well as an available supply of reducing substrates for Prdx2.
We detected extracellular Prdx2 in the plasma of RA and healthy subjects and in the synovial fluid of RA patients, and this extracellular Prdx2 did not appear to be an artefact (see Supplementary Data 4).
The effects of different drug treatments on the expression of the Prdx-based system were analyzed by Spearman's rank correlation where drug doses were available, or – where drug doses were not available – by dividing the patients into subgroups based on the presence or absence of a certain drug treatment and comparing the means of analyzed parameters between the two groups. Using these approaches, we found no evidence for any effects of different drug treatments on the analyzed parameters.
The clinical relevance of our findings is two-fold, as they point to the possibility that Prdx2 may be used as a marker of inflammation and as a drug target. The potential of Prdx2 as a marker of inflammation is supported by: (a) the observed increase in the levels of Prdx2 protein in RA lymphocytes compared with healthy subject lymphocytes, (b) the correlation of intracellular levels of Prdx2 in RA lymphocytes with serum concentrations of CRP, and (c) the higher percentage of Prdx2+ve IL17+ve lymphocytes compared with the Prdx2+ve total lymphocyte population in RA patients.
This study has not addressed the functional role of Prdx2 as an inhibitor of apoptosis of inflammatory cells – a research subject actively pursued with regard to malignant cells (Wang et al., 2005). As Prdx2 protein concentrations may be elevated intracellularly and on the surface of inflammatory cells in RA, the role of Prdx2 should be addressed in these cell populations in future studies, preferably in a leukocyte subpopulation-specific manner.
In summary, the present study showed that the intracellular levels of Prdx2 protein in lymphocytes were increased in RA patients compared with healthy subjects and that the Prdx2 levels correlated with the degree of inflammation as indicated by the serum CRP concentration. We also detected exofacial Prdx2 in isolated human lymphocytes. In RA, the proportion of cells that displayed exofacial Prdx2 was enhanced in the Th17 population compared with the total lymphocyte population, suggesting the possibility that Prdx2 might contribute to the activation of these pro-inflammatory cells.
Acknowledgements
The authors would like to thank to Drs Kirsty Line, Adrian Todd, Robert Morse, Claire Bennett, Janet Holley, Tamás G. Szabó, Lorna Harries and Giada Alberigo for useful discussions and their help in the laboratory. We are grateful to Miriam Haas of the NIHR Clinical Research Facility for providing details of the clinical variables corresponding to the RA patient samples. We are greatly indebted to all volunteers who donated samples for the purposes of this study. This study was supported by the Northcott Devon Medical Foundation, the Peninsula Medical School, a grant from the European Union FP7 Marie Curie ITN program (no. 215009), and an equipment grant for flow cytometry (no. 17231) from Arthritis Research UK.
Footnotes
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.biocel.2012.04.016.
Appendix A. Supplementary data
The following are the supplementary data to this article:
References
- Angelini G., Gardella S., Ardy M., Ciriolo M.R., Filomeni G., Di Trapani G. Antigen-presenting dendritic cells provide the reducing extracellular microenvironment required for T lymphocyte activation. Proceedings of the National Academy of Sciences. 2002;99:1491–1496. doi: 10.1073/pnas.022630299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annunziato F., Cosmi L., Liotta F., Maggi E., Romagnani S. Type 17T helper cells – origins, features and possible roles in rheumatic disease. Nature Reviews Rheumatology. 2009;5:325–331. doi: 10.1038/nrrheum.2009.80. [DOI] [PubMed] [Google Scholar]
- Arner E.S.J., Holmgren A. Physiological functions of thioredoxin and thioredoxin reductase. European Journal of Biochemistry. 2000;267:6102–6109. doi: 10.1046/j.1432-1327.2000.01701.x. [DOI] [PubMed] [Google Scholar]
- Arnett F.C., Edworthy S.M., Bloch D.A., McShane D.J., Fries J.F., Cooper N.S. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis and Rheumatism. 1988;31:315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- Bashir S., Harris G., Denman M.A., Blake D.R., Winyard P.G. Oxidative DNA damage and cellular sensitivity to oxidative stress in human autoimmune diseases. Annals of the Rheumatic Diseases. 1993;52:659–666. doi: 10.1136/ard.52.9.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baty J.W., Hampton M.B., Winterbourn C.C. Proteomic detection of hydrogen peroxide-sensitive thiol proteins in Jurkat cells. Biochemical Journal. 2005;389:785–795. doi: 10.1042/BJ20050337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett J.C. The role of T lymphocytes in rheumatoid arthritis and other autoimmune diseases. Arthritis & Rheumatism. 2008;58:S53–S57. doi: 10.1002/art.23045. [DOI] [PubMed] [Google Scholar]
- Biteau B., Labarre J., Toledano M.B. ATP-dependent reduction of cysteine-sulphinic acid by S. cerevisiae sulphiredoxin. Nature. 2003;425:980–984. doi: 10.1038/nature02075. [DOI] [PubMed] [Google Scholar]
- Bo G.P., Zhou L.N., He W.F., Luo G.X., Jia X.F., Gan C.J. Analyses of differential proteome of human synovial fibroblasts obtained from arthritis. Clinical Rheumatology. 2009;28:191–199. doi: 10.1007/s10067-008-1013-y. [DOI] [PubMed] [Google Scholar]
- Brown N.S., Bicknell R. Hypoxia and oxidative stress in breast cancer: oxidative stress – its effects on the growth, metastatic potential and response to therapy of breast cancer. Breast Cancer Research. 2001;3:323–327. doi: 10.1186/bcr315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budanov A.V., Sablina A.A., Feinstein E., Koonin A.V., Chumakov P.M. Regeneration of peroxiredoxins by p53-regulated sestrins, homologs of bacterial AhpD. Science. 2004;304:596–600. doi: 10.1126/science.1095569. [DOI] [PubMed] [Google Scholar]
- Chae H.Z., Kim H.J., Kang S.W., Rhee S.G. Characterization of three isoforms of mammalian peroxiredoxin that reduce peroxides in the presence of thioredoxin. Diabetes Research and Clinical Practice. 1999;45:101–112. doi: 10.1016/s0168-8227(99)00037-6. [DOI] [PubMed] [Google Scholar]
- Choi M-H., Sajed D., Poole L., Hirata K., Herdman S., Torian B.E. An unusual surface peroxiredoxin protects invasive Entamoeba histolytica from oxidant attack. Molecular and Biochemical Parasitology. 2005;143:80. doi: 10.1016/j.molbiopara.2005.04.014. [DOI] [PubMed] [Google Scholar]
- Cox A.G., Pullar J.M., Hughes G., Ledgerwood E.C., Hampton M.B. Oxidation of mitochondrial peroxiredoxin 3 during the initiation of receptor-mediated apoptosis. Free Radical Biology and Medicine. 2008;44:1001–1009. doi: 10.1016/j.freeradbiomed.2007.11.017. [DOI] [PubMed] [Google Scholar]
- Eggleton P., Harries L.W., Alberigo G., Wordsworth P., Viner N., Haigh R. Changes in apoptotic gene expression in lymphocytes from rheumatoid arthritis and systemic lupus erythematosus patients compared with healthy lymphocytes. Journal of Clinical Immunology. 2010;30:649–658. doi: 10.1007/s10875-010-9429-y. [DOI] [PubMed] [Google Scholar]
- Gelderman K.A., Hultqvist M., Holmberg J., Olofsson P., Holmdahl R. T cell surface redox levels determine T cell reactivity and arthritis susceptibility. Proceedings of the National Academy of Sciences. 2006;103:12831–12836. doi: 10.1073/pnas.0604571103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilston V., Blake D.R., Winyard P.G. The rheumatoid joint: redox paradox? In: Montagnier L., Olivier R., Pasquier C., editors. Oxidative stress in cancer, AIDS, and neurodegenerative diseases. Marcel Dekker Inc.; New York: 1998. pp. 517–535. [Google Scholar]
- Griffiths H.R., Dunston C.R., Bennett S.J., Grant M.M., Phillips D.C., Kitas G.D. Free radicals and redox signalling in T-cells during chronic inflammation and ageing. Biochemical Society Transactions. 2011;39:1273–1278. doi: 10.1042/BST0391273. [DOI] [PubMed] [Google Scholar]
- Hakim A.J., Clunie G.P.R., Haq I. Oxford University Press; Oxford: 2006. Oxford handbook of rheumatology. [Google Scholar]
- Hara T., Kondo N., Nakamura H., Okuyama H., Mitsui A., Hoshino Y. Cell-surface thioredoxin-1: possible involvement in thiol-mediated leukocyte-endothelial cell interaction through lipid rafts. Antioxidants and Redox Signalling. 2007;9:1427–1438. doi: 10.1089/ars.2007.1661. [DOI] [PubMed] [Google Scholar]
- Jikimoto T., Nishikubo Y., Koshiba M., Kanagawa S., Morinobu S., Morinobu A. Thioredoxin as a biomarker for oxidative stress in patients with rheumatoid arthritis. Molecular Immunology. 2001;38:765–772. doi: 10.1016/s0161-5890(01)00113-4. [DOI] [PubMed] [Google Scholar]
- Kang S.W., Rhee S.G., Chang T.S., Jeong W., Choi M.H. 2-Cys peroxiredoxin function in intracellular signal transduction: therapeutic implications. Trends in Molecular Medicine. 2005;11:571–578. doi: 10.1016/j.molmed.2005.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim C.W., Cho E.H., Lee Y.J., Kim Y.H., Hah Y., Kim D.R. Disease specific proteins from rheumatoid arthritis patients. Journal of Korean Medical Science. 2006;21:478–484. doi: 10.3346/jkms.2006.21.3.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon J., Devadas S., Williams M.S. T cell receptor-stimulated generation of hydrogen peroxide inhibits MEK-ERK activation and lck serine phosphorylation. Free Radical Biology and Medicine. 2003;35:406–417. doi: 10.1016/s0891-5849(03)00318-6. [DOI] [PubMed] [Google Scholar]
- Lawrence D.A., Song R., Weber P. Surface thiols of human lymphocytes and their changes after in vitro and in vivo activation. Journal of Leukocyte Biology. 1996;60:611–618. doi: 10.1002/jlb.60.5.611. [DOI] [PubMed] [Google Scholar]
- Lehtonen S.T., Svensk A.M., Soini Y., Pääkkö P., Hirvikoski P., Kang S.W. Peroxiredoxins a novel protein family in lung cancer. International Journal of Cancer. 2004;111:514–521. doi: 10.1002/ijc.20294. [DOI] [PubMed] [Google Scholar]
- Lehtonen S.T., Svensk A.M., Soini Y., Pääkkö P., Hirvikoski P., Kang S.W. A novel protein family in lung cancer. International Journal of Cancer. 2004;111:514–521. doi: 10.1002/ijc.20294. [DOI] [PubMed] [Google Scholar]
- Liu H., Pope R.M. The role of apoptosis in rheumatoid arthritis. Current Opinion in Pharmacology. 2003;3:317–322. doi: 10.1016/s1471-4892(03)00037-7. [DOI] [PubMed] [Google Scholar]
- Martin H., Dean M. Identification of a thioredoxin-related protein associated with plasma membranes. Biochemical and Biophysical Research Communications. 1991;175:123–128. doi: 10.1016/s0006-291x(05)81209-4. [DOI] [PubMed] [Google Scholar]
- Maurice M.M., Nakamura H., Gringhuis S., Okamoto T., Yoshida S., Kullmann F. Expression of the thioredoxin–thioredoxin reductase system in the inflamed joints of patients with rheumatoid arthritis. Arthritis & Rheumatism. 1999;42:2430–2439. doi: 10.1002/1529-0131(199911)42:11<2430::AID-ANR22>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Metcalfe C., Cresswell P., Ciaccia L., Thomas B., Barclay A.N. Labile disulfide bonds are common at the leucocyte cell surface. Open Biology. 2011;1:1–24. doi: 10.1098/rsob.110010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon E.Y., Noh Y.W., Han Y.H., Kim S.U., Kim J.M., Yu D.Y. T lymphocytes and dendritic cells are activated by the deletion of peroxiredoxin II (Prx II) gene. Immunology Letters. 2006;102:184–190. doi: 10.1016/j.imlet.2005.09.003. [DOI] [PubMed] [Google Scholar]
- Mougiakakos D., Johansson C.C., Jitschin R., Böttcher M., Kiessling R. Increased thioredoxin-1 production in human naturally occurring regulatory T cells confers enhanced tolerance to oxidative stress. Blood. 2011;117:857–861. doi: 10.1182/blood-2010-09-307041. [DOI] [PubMed] [Google Scholar]
- Mougiakakos D., Johansson C.C., Kiessling R. Naturally occurring regulatory T cells show reduced sensitivity toward oxidative stress-induced cell death. Blood. 2009;113:3542–3545. doi: 10.1182/blood-2008-09-181040. [DOI] [PubMed] [Google Scholar]
- Pattison D.J., Winyard P.G. Dietary antioxidants in inflammatory arthritis: do they have any role in etiology or therapy. Nature Clinical Practice Rheumatology. 2008;4:590–596. doi: 10.1038/ncprheum0920. [DOI] [PubMed] [Google Scholar]
- Pedersen-Lane J.H., Zurier R.B., Lawrence D.A. Analysis of the thiol status of peripheral blood leukocytes in rheumatoid arthritis patients. Journal of Leukocyte Biology. 2007;81:934–941. doi: 10.1189/jlb.0806533. [DOI] [PubMed] [Google Scholar]
- Peskin A.V., Low F.M., Paton L.N., Maghzal G.J., Hampton M.B., Winterbourn C.C. The high reactivity of peroxiredoxin 2 with H2O2 is not reflected in its reaction with other oxidants ad thiol reagents. Journal of Biological Chemistry. 2007;282:11885–11892. doi: 10.1074/jbc.M700339200. [DOI] [PubMed] [Google Scholar]
- Sahaf B., Söderberg A., Spyrou G., Barral A.M., Pekkari K., Holmgren A. Thioredoxin expression and localization in human cell lines: detection of full-length and truncated species. Experimental Cell Research. 1997;236:181–192. doi: 10.1006/excr.1997.3699. [DOI] [PubMed] [Google Scholar]
- Schröder E., Littlechild J.A., Lebedev A.A., Errington N., Vagin A.A., Isupov M.N. Crystal structure of decameric 2-Cys peroxiredoxin from human erythrocytes at 1.7 Å resolution. Structure. 2000;8:605–615. doi: 10.1016/s0969-2126(00)00147-7. [DOI] [PubMed] [Google Scholar]
- Seo M.S., Kang S.W., Kim K., Baines I.C., Lee T.H., Rhee S.G. Identification of a new type of mammalian peroxiredoxin that forms an intramolecular disulfide as a reaction intermediate. Journal of Biological Chemistry. 2000;275:20346–20354. doi: 10.1074/jbc.M001943200. [DOI] [PubMed] [Google Scholar]
- Szabó KÉ, Line K., Eggleton P., Littlechild J.A., Winyard P.G. Structure and function of the human peroxiredoxin-based antioxidant system: the interplay between peroxiredoxins, thioredoxins, thioredoxin reductases, sulfiredoxins and sestrins. In: Winyard P.G., Jacob C., editors. Redox signalling and regulation in biology and medicine. Wiley-VCH; Weinheim: 2009. pp. 143–179. [Google Scholar]
- Tarr J.M., Winyard P.G., Ryan B., Harries L.W., Haigh R., Viner N. Extracellular calreticulin is present in the joints of rheumatoid arthritis patients and inhibits FasL (CD95L) mediated apoptosis of T cells. Arthritis & Rheumatism. 2010;62:2919–2929. doi: 10.1002/art.27602. [DOI] [PubMed] [Google Scholar]
- Wang M.X., Wei A., Yuan J., Trickett A., Knoops B., Murrell G.A.C. Expression and regulation of peroxiredoxin 5 in human osteoarthritis. FEBS Letters. 2002;531:359. doi: 10.1016/s0014-5793(02)03511-1. [DOI] [PubMed] [Google Scholar]
- Wang T., Tamae D., LeBon T., Shively J.E., Yen Y., Li J.J. The role of peroxiredoxin II in radiation-resistant MCF-7 breast cancer cells. Cancer Research. 2005;65:10338–10346. doi: 10.1158/0008-5472.CAN-04-4614. [DOI] [PubMed] [Google Scholar]
- Wollman E.E., Kahan A., Fradelizi D. Detection of membrane associated thioredoxin on human cell lines. Biochemical and Biophysical Research Communications. 1997;230:602–606. doi: 10.1006/bbrc.1996.6015. [DOI] [PubMed] [Google Scholar]
- Wood Z.A., Poole L.B., Karplus P.A. Peroxiredoxin evolution and the regulation of hydrogen peroxide signaling. Science. 2003;300:650–653. doi: 10.1126/science.1080405. [DOI] [PubMed] [Google Scholar]
- Wood Z.A., Schröder E., Harris J.R., Poole L.B. Structure, mechanism and regulation of peroxiredoxins. Trends in Biochemical Sciences. 2003;28:32–40. doi: 10.1016/s0968-0004(02)00003-8. [DOI] [PubMed] [Google Scholar]
- Yan Z., Banerjee R. Redox remodeling as an immunoregulatory strategy. Biochemistry. 2010;49:1059–1066. doi: 10.1021/bi902022n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C.S., Lee D.S., Song C.H., An S.J., Li S., Kim J.M. Roles of peroxiredoxin II in the regulation of proinflammatory responses to LPS and protection against endotoxin-induced lethal shock. Journal of Experimental Medicine. 2007;204:583–594. doi: 10.1084/jem.20061849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida S., Katoh T., Tetsuka T., Uno K., Matsui N., Okamoto T. Involvement of thioredoxin in rheumatoid arthritis: its costimulatory roles in the TNF-α-induced production of IL-6 and IL-8 from cultured synovial fibroblasts. Journal of Immunology. 1999;163:351–358. [PubMed] [Google Scholar]
- Zhang P., Liu B., Kang S., Seo M., Rhee S., Obeid L. Thioredoxin peroxidase is a novel inhibitor of apoptosis with a mechanism distinct from that of Bcl-2. Journal of Biological Chemistry. 1997;272:30615–30618. doi: 10.1074/jbc.272.49.30615. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.