Abstract
Black-pearl (Blp) is a highly conserved, essential inner-mitochondrial membrane protein. The yeast Blp homologue, Magmas/Pam16, is required for mitochondrial protein transport, growth, and survival. Our purpose was to determine the role of Drosophila Blp in mitochondrial function, cell survival, and proliferation. To this end, we performed mitotic recombination in Drosophila melanogaster, RNAi-mediated knockdown, MitoTracker staining, measurement of reactive oxygen species (ROS), flow cytometry, electron transport chain complex assays, and hemocyte isolation from Drosophila larvae. Proliferation-defective, Blp-deficient Drosophila Schneider cells exhibited mitochondrial membrane depolarization, a 60% decrease in ATP levels, increased amounts of ROS (3.5-fold), cell cycle arrest, and activation of autophagy that were associated with a selective 65% reduction of cytochrome c oxidase activity. N-acetyl cysteine (NAC) rescued Blp-RNAi-treated cells from cell cycle arrest, indicating that increased production of ROS is the primary cause of the proliferation and survival defects in Blp-depleted cells. blp hypomorph larvae had a 35% decreased number of plasmatocytes with a 45% reduced active mitochondrial staining and their viability was increased 2-fold by administration of NAC, which blocked melanotic lesions. Loss of Blp decreases cytochrome c oxidase activity and uncouples oxidative phosphorylation, causing ROS production, which selectively affects mitochondria-rich plasmatocyte survival and function, leading to melanotic lesions in Blp-deficient flies.—Roy, S., Short, M. K., Stanley, E. R., Jubinsky, P. T. Essential role of Drosophila black-pearl is mediated by its effects on mitochondrial respiration.
Keywords: reactive oxygen species, electron transport chain, melanotic inclusions, Magmas, Pam 16
The mouse homologue of black-pearl (Blp), mitochondria-associated granulocyte macrophage colony-stimulating factor (CSF) signaling (Magmas), was identified by its expression in response to granulocyte-macrophage CSF (GM-CSF) (1) and is subcellularly localized in mitochondria (1, 2). Increased levels of Magmas mRNA in high-energy-requiring tissues, such as heart, skeletal muscles, and testes (1, 3), together with its mitochondrial location, suggest that Magmas plays a role in mitochondrial ATP generation and cell metabolism.
Magmas is a highly conserved, essential gene in multiple species. A functional genomic screen in Caenorhabditis elegans has shown that the Magmas homologue, tim16, is essential for cell division and development (4); similarly, deletion of the yeast ortholog, PAM16, is lethal in Saccharomyces cerevisiae (5). Homozygous germ-line clones of blp mutants are embryonically lethal, with segmentation defects (6). However, in a recessive semilethal P-element insertion line, blpp, almost 5% of the homozygotes are escapers, developing pigment-encapsulated cell clusters in the abdomen (7). Their presence is indicative of an aberrant innate immune system, resulting either from an autoimmune response to disrupted basement membrane or from overactivation of hemocytes (8, 9).
In Drosophila, hemocytes include plasmatocytes, lamellocytes, and crystal cells. Plasmatocytes make up the vast majority (90–95%) of hemocytes and are responsible for the phagocytosis of bacteria and other small foreign particles. Lamellocytes are larger than plasmatocytes and encapsulate larger pathogens. Infection by larger pathogens results in the attachment of plasmatocytes to the pathogen, and this binding stimulates the proliferation and differentiation of prohemocytes into lamellocytes. Crystal cells rupture to release cytoplasmic crystalline prophenoloxidase (proPO) into the hemolymph (reviewed in ref. 10). ProPO is cleaved by proPO-activating enzyme and oxidizes tyrosine and phenols to form melanin (11), killing the pathogen by oxidative stress from free radicals (quinones and semiquinones), generated as reaction intermediates (reviewed in refs. 10–12). The appearance of melanotic inclusions in blpp flies may result from inadequate plasmatocyte-mediated clearance of pathogens and an increased proliferation and differentiation of prohemocytes into lamellocytes or, alternatively, from an autoimmune-type response involving activated lamellocytes and/or crystal cells.
In the present study, we show that Blp-depletion impairs normal plasmatocyte function through its effects on mitochondrial activity. Mosaic analysis in Drosophila eye discs and studies of Drosophila Schneider (S2) cell line of embryonic hemocyte origin demonstrated that Blp-depletion led to severe proliferation defects. Further studies in S2 cells showed that reduced Blp expression decreased ATP levels and increased reactive oxygen species (ROS), leading to cell cycle arrest and autophagy. Decreased cellular ATP resulted from a specific loss of cytochrome c oxidase (complex IV) activity in Blp-depleted cells. The homozygous blpp larvae had fewer plasmatocytes with reduced numbers of active mitochondria per cell, consistent with the differential sensitivity of these mitochondria-rich cells that are specialized to generate large amounts of ROS in the immune response.
MATERIALS AND METHODS
Mitotic clone generation in Drosophila eye imaginal discs
Homozygous blp-null mutant clones (blp locus mapping to 89A8 on chromosome 3R) were obtained by the FLP-FRT-mediated mitotic recombination technique (13). The yw1118; FRT82B blpΔ3/TM3,GFP flies carrying the blp-null allele were crossed with yweyF1.1; FRT82B arm-lacZ/TM3,sb1 or ywhsF; FRT82B arm-lacZ/TM3,sb1 flies. In the case of hsF, larvae were subjected to heat shock (1 h, 37°C) 60 ± 12 h after egg laying and dissected 72 h later, and the eye imaginal discs were stained with mouse anti-β-galactosidase (mAb40-1a; Developmental Studies Hybridoma Bank, University of Iowa, Iowa City, IA, USA) antibody alone, or with mouse anti-CM1 (anti-active human caspase-3; Cell Signaling Technology, Danvers, Massachusetts, USA) antibody, and examined by confocal microscopy.
RNAi preparation and treatment
The forward and reverse primers for Blp cDNA amplification, as the template for RNA preparation, were designed to include a 5′ T7 RNA polymerase binding site (underlined), as follows: 5′-TAATACGACTCACTATAGGGCCCCAAGAAGCGTGATCTTA-3′ and 5′-TAATACGACTCACTATAGGGAAAACGTGGACGCTATCACC-3′. S2 cells (0.5×106 cells/ml) were cultured in Schneider's S2 cell medium (Invitrogen, Carlsbad, CA, USA), containing 10% fetal bovine serum (FBS; without penicillin-streptomycin; Gemini Bio-Products, West Sacramento, CA, USA) in 35-mm tissue culture dishes. After 24 h, 5 μg of Blp-RNAi was added, and the cells were cultured for 3, 4, 5, 6, and 9 d.
MitoTracker staining
The S2 cells (cultured in 35-mm tissue culture dishes, 2 ml medium) were treated with Blp- or LIM- and SH3 domain protein (LASP, control)-RNAi (5 μg) and, at indicated time points, incubated in serum-free S2 medium containing 50 nM MitoTracker (MitoTracker Red CMXRos; Invitrogen) and Hoechst (33342; Invitrogen) for 30 min, then fixed and imaged as described previously (14).
Luciferin-luciferase based ATP assay
RNAi-treated S2 cells (2×104) were resuspended in 100 μl nucleotide releasing buffer and 50 μl enzyme mix (ATP assay kit; Calbiochem, San Diego, CA, USA), and the emitted luminescence was recorded.
Fluorescence-activated cell scanning (FACS) for cell cycle analysis—propidium iodide and 5-bromo-2′-deoxyuridine (BrdU) staining
RNAi-treated S2 cells were fixed, resuspended in PBS containing 0.25 mg/ml RNase (Sigma-Aldrich, St. Louis, Missouri, USA), and 40 μg/ml propidium iodide (Sigma-Aldrich) and incubated for 30 min (15).
For BrdU labeling, the RNAi-treated S2 cells were incubated with 15 μM BrdU (Sigma-Aldrich) for 30 min at 22°C. The cells were fixed, incubated in 2 M HCl for 30 min, and washed with PBS containing 0.1% BSA and 0.2% Tween-20, prior to staining with mouse monoclonal anti-BrdU antibody (2 μg/ml; Roche Applied Science, Indianapolis, Indiana, USA) and propidium iodide.
5-(and-6)-Chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate acetyl ester (DCFDA) assay for ROS
RNAi-treated S2 cells were resuspended in S2 medium containing 5% FBS and DCFDA (final concentration, 100 μM; Invitrogen) and incubated for 30 min at 22°C. The emitted fluorescence from 1 × 105 cells was determined using a plate reader (excitation wavelength: 485 nm; emission wavelength: 520 nm; ref. 16) and expressed as fold increase of fluorescence units with respect to LASP-RNAi-treated cells.
N-acetyl cysteine (NAC) and Mito-Tempo treatment
RNAi-treated S2 cells were incubated with NAC (0.5 mg/ml; Sigma-Aldrich) for 30 min at 22°C. The cells were then treated with DCFDA, as above, to measure ROS levels. For cell cycle experiments, NAC (0.5 mg/ml) was added 36 h prior to propidium iodide staining and FACS analysis. For fly treatment, blpp/TM3,GFP flies were grown on food containing NAC at 0, 1, 5, and 10 mg/ml. For Mito-Tempo treatment, RNAi-treated S2 cells were preincubated with Mito-Tempo (2 μM, 4 h; Enzo Life Sciences, Farmingdale, NY, USA; ref. 17) before staining with MitoTracker.
Mitochondrial membrane potential (MMP) assay
RNAi- or Blp-inhibitor-treated S2 cells were homogenized, and mitochondria were isolated as described previously (18). MMP was determined by incubating the isolated mitochondria with JC-1 (2 μM, 20 min, 25°C, in dark; Invitrogen). The fluorescence intensity was measured using a microplate reader, and the MMP was expressed as the ratio of emission at 590 nm (red) to 529 nm (green).
Scanning electron microscopy
RNAi-treated S2 cells were fixed with 2.5% glutaraldehyde in 0.1 M cacodylate buffer and postfixed with 1% osmium tetraoxide, followed by 1% uranyl acetate. The samples were then dehydrated and embedded in LX112 resin (Ladd Research Industries, Williston, VT, USA). Ultrathin sections were stained with uranyl acetate, followed by lead citrate.
Drosophila larval hemocyte isolation and MitoTracker staining
The non-GFP larvae from the blpp/TM3, GFP progeny were selected, the hemolymph was collected, and the cells were allowed to settle on the coverslips (4 h, 18°C). Following incubation in 50 nM MitoTracker, the cells were fixed and imaged (19).
Western blot analysis
RNAi-treated S2 cells were lysed and centrifuged (16,000 g, 15 min, 4°C) to pellet nuclei and cell debris (20). Primary antibodies: rabbit anti-yeast Magmas, rabbit anti-cyclin B and anti-cyclin E (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA); mouse anti-AMPK and anti-tubulin (Sigma-Aldrich); rabbit anti-GAPDH (Imgenex Corp., San Diego, CA, USA); rabbit anti-LASP (gift from Dr. Andreas Jenny, Albert Einstein College of Medicine), mouse anti-p53 (Santa Cruz Biotechnology, Inc.), rabbit anti-LC3 (Cell Signaling), and mouse anti-Hsp60 (Millipore, Billerica, MA, USA).
In vitro assay of electron transport chain (ETC) complex activity
Complex I activity assay
RNAi-treated S2 cell lysates were added to 1 ml assay buffer (10 mM potassium phosphate buffer, pH 8.0, containing 0.25 mM potassium EDTA, 1 mM potassium cyanide, 10 μM decylubiquinone, and 20 mM phosphatidylcholine) and allowed to equilibrate (22°C, 2 min). The reaction was started by addition of 50 μl of 1 mM NADH (final concentration, 50 μM), and the absorbance was recorded for 2 min at 340 nm (ε=6.81 mM/cm) (21–23).
Complex II activity assay
Cell lysates were added to 1 ml assay buffer [50 mM potassium phosphate buffer, pH 7.4, containing 20 mM succinate, pH 7.4, 50 μM dichlorophenolindophenol (DCPIP), 2 μg/ml of rotenone (stock solution: 400 μg/ml in 100% ethanol), 2 μg/ml of antimycin A, and 2 mM potassium cyanide] and allowed to equilibrate (22°C, 2 min). The reaction was initiated by addition of 5 μl of 10 mM decylubiquinone (final concentration, 50 μM), and the absorbance was recorded for 2 min at 640 nm (ε=19.1 mM/cm) (23).
Complex III activity assay
Cell lysates were added to 1 ml assay buffer (50 mM Tris-HCl, pH 7.4, containing 250 mM sucrose, 1 mM EDTA, 2 mM KCN, and 50 μM cytochrome c) and allowed to equilibrate (22°C, 2 min). The reaction was initiated by addition of 5 μl of reduced decylubiquinone (DBH2, final concentration, 50 μM), and the absorbance was recorded for 2 min at 550 nm (ε=19.0 mM/cm) (23).
Complex IV activity assay
Cell lysates were added to 1 ml assay buffer (10 mM Tris-HCl, pH 7.0, containing 250 mM sucrose) and allowed to equilibrate (22°C for 2 min). The reaction was initiated with 50 μl of 0.22 mM reduced cytochrome c, and absorbance was measured at 550 nm (ε=19.0 mM/cm; CytoCOX1; Sigma; ref. 23). The cytochrome c was reduced with 0.1 M dithiothreitol (DTT). DTT was removed by size exclusion using a Sephadex G25 column (1×3 cm). The absorbance ratio (550 nm/565 nm>10) was checked to ensure its reduced state, and the solution was maintained under N2 to prevent oxidation.
Calculation of enzyme activity
All assays were carried out using 50 μl of lysate (100–150 μg protein). Enzyme activity per milligram protein was calculated from the initial rates using the formula:
where ΔA/min is the change in absorbance per minute (initial rates); DF is dilution factor; 1 cm is the spectrophotometer cell path length, and ε is molar absorptivity (in mM/cm).
Mitochondrial ETC complex isolation and separation in 1-D nondenaturing gel
S2 cells were homogenized in 2 ml of cold 1× hypotone buffer (83 mM sucrose, 10 mM MOPS, pH 7.2). Hypotone buffer (3×, 2 ml) was added and centrifuged (1000 g, 3 min, 4°C) to remove nuclei and unbroken cells, and the supernatant was centrifuged again (8000 g, 15 min, 4°C) to pellet the mitochondria. The mitochondrial pellet was resuspended in 300 μl of extraction buffer (750 mM 6-aminocaproic acid, 50 mM BisTris, 1% TritonX-100, 1 mM benzamidine, 10 μg/ml leupeptin, and 5 μg/ml aprotinin, pH 7.0), then subjected to ultracentrifugation (100,000 g, 30 min, 4°C), and the supernatant was collected and loaded onto a nondenaturing polyacrylamide gel (24, 25). The protein bands were visualized by silver staining.
RESULTS
Blp depletion inhibits cell survival and proliferation
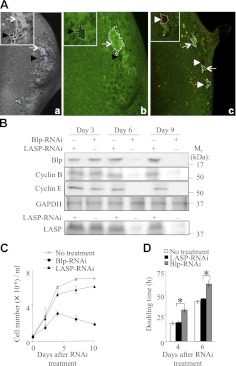
To study the role of Blp in cell survival and proliferation in vivo, we generated blpΔ3 clones in the Drosophila eye imaginal disc by FLP-FRT-mediated mitotic recombination using either the constitutive eyeless-Flp (eyF) or the inducible hsp70-Flp (hsF). In this mosaic system, homozygous blpΔ3 clones and their respective twin spots were generated simultaneously, adjacent to each other. Only very small or, often, single-cell clones of blpΔ3 (Fig. 1Aa, b, unstained cell clusters; black arrowheads) were obtained in the posterior region of the eye disc compared to their larger twin spots (Fig. 1Aa, b, bright green spots; arrows), suggesting that the blpΔ3 clones displayed impaired proliferation. All the observed blpΔ3 clones were posterior to the morphogenetic furrow, where they had an increased chance of survival in the absence of cell competition. Furthermore, the presence of active caspase-3 staining indicated that blpΔ3 clone cells were undergoing apoptosis (Fig. 1Ac, white arrowheads). We were unable to obtain larger blp-null clones by using different blp-null lines (blpΔ1and blpΔ2) or different promoters for Flp expression, or by generating clones in the minute background to provide a growth advantage for weaker cells (data not shown). These results demonstrate that Blp is required for survival and proliferation in vivo.
Figure 1.
Blp is required for cell survival and proliferation. A) Reduced survival and proliferation of cells in blpΔ3 clones in the Drosophila eye imaginal disc. a, b) Black blpΔ3 clones (black arrowheads) are marked by the absence of β-galactosidase expression, whereas the bright green twin spots (arrows) stain strongly with anti-β-galactosidase staining. blpΔ3 clones were generated using ey-Flp (a) and hs-Flp (b). (c) Costaining of active caspase-3 using CM1 antibody (red) with anti-β-galactosidase antibody (green) shows apoptotic cells in blpΔ3 clones (white arrowheads), generated by ey-Flp. Clones of each type are outlined. B) Western blot showing reduced levels of Blp, cyclin B, and cyclin E from d 6 in Blp-RNAi-treated S2 cells compared to GAPDH (loading control). LASP-RNAi treatment showed effective knockdown of LASP levels at d 6 after RNAi treatment. C) Growth curves showing reduced cell numbers from d 4 in Blp-RNAi-treated cells (means±sd). D) Doubling time of S2 cells at d 4 and 6, with no RNAi, LASP-RNAi, or Blp-RNAi. Blp-RNAi inhibits proliferation. Values are means ± sd; n = 3. *P < 0.001; Student's t test.
To independently confirm whether the loss of Blp decreases cell survival and proliferation, we examined the effect of RNAi-mediated Blp knockdown in S2 cells. Following a 5-d treatment with 5 μg of Blp-RNAi, significantly lower levels of Blp were detected by Western blotting compared to control LASP-RNAi treatment (data not shown). This low level persisted for ≥9 d after treatment (Fig. 1B). In addition, S2 cells cultured in the presence of 5 μg of Blp-RNAi displayed reduced proliferation (Fig. 1C) and significantly increased doubling times (Fig. 1D) starting at 4 d posttreatment, compared to cells treated with the control LASP-RNAi. Using trypan blue exclusion to determine cell viability, there was no significant increase in cell death up to d 8 after Blp- or LASP-RNAi treatment, compared to untreated cells (Supplemental Fig. S1D). Blp-RNAi treatment resulted in reduced cyclin B and E protein levels at 6 and 9 d (Fig. 1B), indicating a Blp-dependent proliferation defect, as we observed in the blpΔ3 clones. Taken together, our genetic and knockdown approaches indicate that Blp is required for cell proliferation and survival.
Blp-knockdown cells have dysfunctional mitochondria and reduced cellular ATP levels
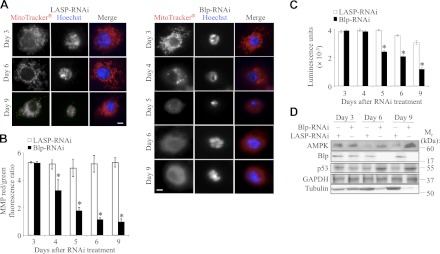
Previous studies in mammalian cells (1) and in yeast (2, 26, 27) have demonstrated that the Blp homologs are localized to the matrix side of the inner mitochondrial membrane. Since Blp-RNAi impairs cell proliferation, we examined the possibility that alterations in mitochondrial function cause the cell proliferation defects in these cells. To determine whether Blp regulates mitochondrial activity, S2 cells were treated with Blp-RNAi or LASP-RNAi (control) and incubated with MitoTracker Red CMXRos, which accumulates in active mitochondria. Control cells showed an intense, punctuate, red fluorescent signal, indicative of healthy mitochondria with active membrane potential (Fig. 2A). In contrast, viable Blp-RNAi-treated S2 cells exhibited slight reductions in MitoTracker intensity at 4 d and severe reduction (diffuse cell staining due to loss of MitoTracker accumulation in active mitochondria) from 5 d after treatment (Fig. 2A), indicating a loss of mitochondrial membrane polarity. RNAi-treated S2 cells were also incubated with JC-1, a lipophilic cationic dye, which emits red fluorescence at high MMPs and green fluorescence at low MMPs (18). The ratio of the emitted red fluorescence to green fluorescence provides a measure of MMP. The Blp-RNAi-treated cells showed significant reduction in the MMP at 4 d after RNAi treatment compared to the control cells (Fig. 2B), confirming our MitoTracker results and indicating that Blp-depletion impairs mitochondrial function.
Figure 2.
Blp deficiency in S2 cells leads to loss of MMP, reduced cellular ATP levels, and elevation of AMPK. A) Immunofluorescence images of LASP-RNAi and Blp-RNAi-treated cells stained with MitoTracker Red CMXRos to visualize active mitochondria and Hoechst stain for nuclei at different times after RNAi treatment. A heterogenous population was observed, showing slight reduction at d 4 and drastic reduction from d 5 in MitoTracker Red CMXRos staining after Blp-RNAi treatment. B) Ratio of red:green fluorescence after JC-1 staining of RNAi-treated S2 cells, showing loss of MMP from d 4 of Blp-RNAi treatment. C) Cellular ATP levels measured by luciferin-luciferase assay, showing significant reduction of ATP-levels in Blp-RNAi-treated cells from d 5 compared to LASP-RNAi-treated control cells. Each data point is normalized to DAPI staining (DNA). D) Western blot of cell lysates showing a Blp-RNAi-induced increase in AMPK and p53 expression and a decrease in tubulin levels compared with GAPDH (loading control) at d 6 and 9. Scale bars = 20 μm. Values are means ± sd; n = 3. *P < 0.001; Student's t test.
Based on these observations, we hypothesized that the mitochondrial membrane depolarization in Blp-knockdown cells would affect ATP production. Therefore, we measured the ATP levels in the Blp-RNAi-treated S2 cells using a luciferin-luciferase-based assay and observed that within 5 d of RNAi treatment, cellular ATP levels were reduced by 40% (Fig. 2C) and by 75% at 9 d after Blp-RNAi treatment. An elevation in AMPK levels and a p53-mediated suppression of cell cycling by down-regulation of cyclin E are normal cellular survival responses to reduced ATP levels (28). Consistent with the 75% reduction in cellular ATP levels in Blp-deficient cells (Fig. 2C), we observed elevated AMPK and p53 expression (Fig. 2D) and reduced cyclin E (Fig. 1B), in agreement with the known role of p53 as a suppressor of cell cycle and activator of autophagy, especially during genotoxic stress. Genotoxic stress activates and stabilizes p53, inducing autophagy through transcription-dependent activation of AMPK (reviewed in ref. 29).
Impaired proliferation due to Blp deficiency is associated with G1- and S-phase arrest
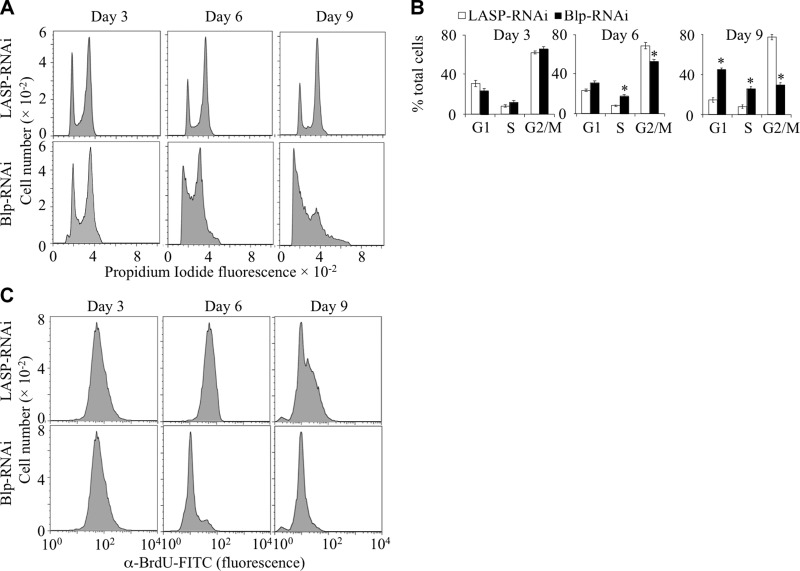
The tenured-null allele (tend) of the Drosophila cytochrome oxidase subunit, Va, causes G1-phase cell cycle arrest in cells of the Drosophila eye imaginal disc and a drop in cellular ATP levels (28), similar to the effects observed in Blp-RNAi-treated S2 cells. To compare the cell cycle effects of Blp-depletion to those of tend, Blp-RNAi-treated S2 cells were incubated with propidium iodide, and the cell cycle profile was analyzed by flow cytometry (cell gating shown in Supplemental Fig. S3A). In contrast to the accumulation of tend cells in G1 phase (28), Blp-RNAi-treated S2 cells accumulated in both G1 and S phases of the cell cycle at 6 and 9 d after treatment (Fig. 3A, bottom panel; B). A concomitant reduction of cells in G2/M phases indicated decreased DNA synthesis. LASP-RNAi treatment did not affect the cell cycle profile (Fig. 3A, top panel). S phase is highly energy dependent and sensitive to cellular ATP levels. To confirm the reduction of DNA synthesis, implied by the observed accumulation of cells in S phase (Fig. 3A), the treated S2 cells were analyzed for BrdU incorporation (cell gating shown in Supplemental Fig. S3C). At d 6 and 9, flow cytometric analysis showed that significantly reduced numbers of Blp-RNAi-treated cells were incorporating BrdU and with reduced BrdU staining intensity during the 30-min labeling period, demonstrating a reduced rate of DNA synthesis that resulted in slower S-phase progression (Fig. 3C). Thus, despite the higher proportion of Blp-deficient cells in S phase compared with control cells at d 6 and 9 (Fig. 3A), the Blp-deficient cells had reduced rate of BrdU incorporation. These results show that Blp-depletion results in a partial S-phase arrest and restriction of cells to G2/M-phases.
Figure 3.
Cell cycle arrest and reduced DNA synthetic rate of Blp-deficient S2 cells. A, B) Flow cytometric analysis of cells after Blp- or LASP-RNAi treatment stained with propidium iodide (A), and quantitation of the percentage of cells in different phases of the cell cycle (B), showing significant accumulation in both G1 and S phases of the cell cycle at d 6 and 9 and a reduction of cells in G2/M phases. LASP-RNAi treatment did not affect the cell cycle profile. Values are means ± sd; n = 3. *P < 0.001; Student's t test. C) Cellular BrdU incorporation rate (30 min labeling with BrdU) after Blp-RNAi treatment, showing significantly reduced numbers of BrdU+ cells at d 6 and 9, indicating fewer cells undergoing active DNA synthesis during the labeling period, leading to a reduced number of cells incorporating BrdU. Only BrdU+ cells were gated for analysis.
Blp depletion increases ROS levels and induces autophagy
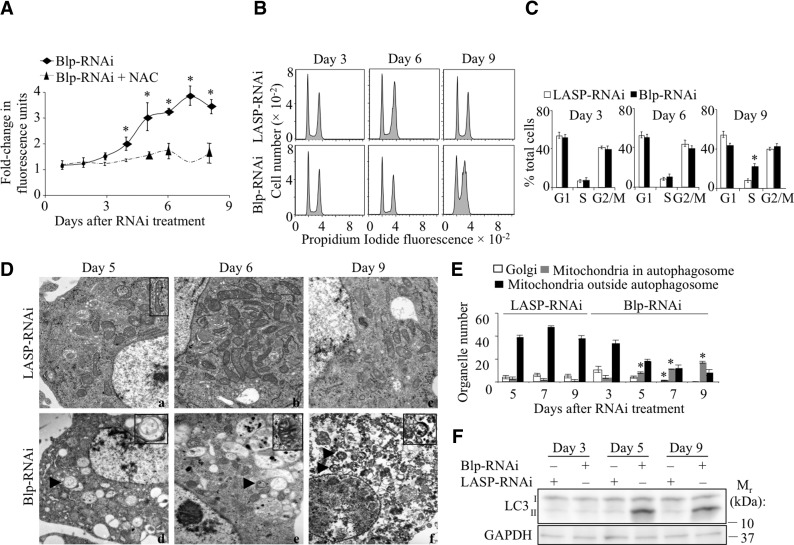
The mitochondrial ETC complexes, present in the inner membrane, are critical for mitochondrial ATP production and for mitochondrial membrane polarity (reviewed in ref. 30). During oxidative phosphorylation, a small percentage of electrons “leak” to oxygen, forming ROS (30, 31). The increased ROS production that occurs with impaired oxidative phosphorylation can cause DNA damage and result in a block in DNA synthesis and cell cycle arrest as we observed with decreased Blp (Fig. 3A, C). We therefore measured ROS levels in Blp-RNAi-treated S2 cells using the membrane permeable dye, DCFDA, which fluoresces on reaction with ROS (32). An ∼2-fold increase in ROS levels was detected at 4 d after Blp-RNAi treatment (Fig. 4A), and ROS levels rose to 3.5 times the control levels at d 8. When the Blp-depleted cells were pretreated with a thiol antioxidant (NAC) for 30 min prior to the addition of DCFDA and ROS measurement, the fluorescence signal was reduced to background levels, confirming that the observed fluorescence was due to ROS (Fig. 4A).
Figure 4.
Increased cellular ROS and autophagy in Blp-deficient S2 cells and effect of NAC treatment. A) Cellular ROS levels in Blp-RNAi-treated cells, data expressed as fold increase of fluorescence units with respect to LASP-RNAi-treated cells (mean±sd fluorescence; n=3), preincubated with or without NAC. Blp depletion results in an increase in ROS levels at d 4, which is reduced with NAC treatment. B) Flow cytometric analysis of propidium iodide-stained S2 cells treated with NAC and Blp-RNAi or LASP-RNAi. Results show reversal of cell cycle arrest at d 6 and partial reversal at d 9 (compared to Fig. 3A). C) Quantitation of the percentage of cells in different phases of the cell cycle; n = 3. D) Electron micrographs of Blp-RNAi-treated (a–c) and LASP-RNAi-treated (d–f) S2 cells at d 5 (a, d), d 6 (b, e), and d 9 (c, f). Arrowheads indicate autophagic vesicles. Insets: normal mitochondrion (a) and autophagic vesicles containing single mitochondria (d, f) and a swollen mitochondrion (e). E) Morphometric analysis showing increased mitophagy. Data represent organelle number. F) Western blot analysis showing increased levels of the LC3-II isoform at d 5 and 9 with Blp-RNAi treatment compared to LASP-RNAi control, indicating the activation of autophagy. Values are means ± sd. *P < 0.001; Student's t test.
Increased cellular ROS causes oxidation of cellular components. Several protective mechanisms, such as up-regulation of antioxidants and removal of damaged proteins and organelles by autophagy, have evolved to prevent ROS-induced damage (reviewed in refs. 33, 34). We observed a gradual reduction of cell size following Blp-RNAi treatment (data not shown) that is characteristic of cells undergoing autophagy, by flow cytometry and reduced tubulin levels (Fig. 2D). We also detected elevated AMPK levels, which have been shown to be required for autophagy (35, 36). Finally, S2 cells survived for up to 12 d after RNAi addition despite the block in cell cycle progression and severe mitochondrial dysfunction, consistent with the activation of autophagy and possibly other rescue pathways.
We then examined Blp-knockdown S2 cells by transmission electron microscopy (Fig. 4D) for the morphological changes that occur during autophagy. Compared to control cells, S2 cells treated for 5 d with Blp-RNAi showed globular-shaped mitochondria with prominent structural deformations and swelling of their internal cristae. Most mitochondria were found encapsulated in double-membrane vesicles (Fig. 4D, bottom panels). At d 9 of Blp-RNAi treatment, the cells were at late-stage autophagy, with excess vacuoles and autolysosomes present. Morphometric analysis (Fig. 4E) showed significantly increased numbers of mitochondria in autophagosomes and loss of rough ER and the Golgi apparatus 5 d after Blp-RNAi treatment. These results and the high levels of LC3-II isoform present (Fig. 4F) demonstrate that Blp-deficiency results in autophagy.
Antioxidant treatment rescues the cell cycle in Blp-deficient cells
Since ROS induces DNA damage (37) that leads to cell cycle arrest, we tested whether NAC could rescue the cell cycle defect in Blp-deficient S2 cells. Treatment with NAC for 36 h completely released the block in cell cycle at d 6 and partially released it at d 9 of Blp-RNAi treatment (Fig. 4B, C; cf. Fig. 3A, B), suggesting the involvement of ROS.
Blp deficiency specifically reduces complex IV activity without affecting the activity of complexes I-III
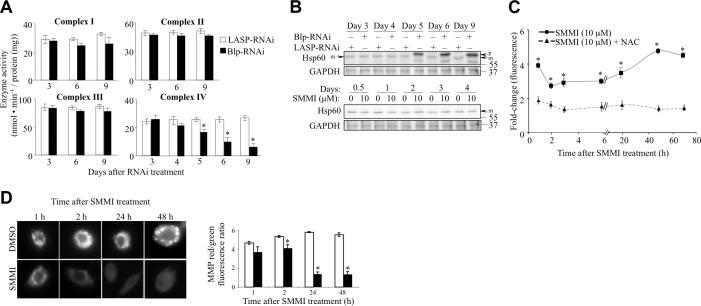
Mass spectrometric analysis of immunoaffinity-purified Magmas complexes, as well as mitochondrial complex isolation and analysis, have demonstrated an association of Magmas with complexes of the ETC (unpublished results and ref. 38). In addition, isolation and separation of ETC complexes from S2 cells in nondenaturing polyacrylamide gel, followed by staining and Western blotting, showed that Blp is associated with complexes I and IV (Supplemental Fig. S2A). These data suggest a direct role for Blp in the ETC.
To determine whether the increased ROS following the disruption of Blp function resulted from the uncoupling of oxidative phosphorylation, we measured the activity of complexes I–IV in Blp-deficient cells at d 3, 6, and 9 following RNAi treatment (Fig. 5A). Blp-RNAi treatment did not affect the activity of complex I. Compared to control cells, complex II and complex III displayed a slight reduction of activity at d 6 and 9 of Blp-RNAi treatment. In contrast, complex IV (cytochrome c oxidase) activity was markedly impaired by d 5 of treatment. These data indicate a selective role for Blp in complex IV function. Similar results were obtained in human cells with antisense to Magmas (unpublished results).
Figure 5.
Blp-RNAi-mediated knockdown differentially reduces complex IV (cytochrome c oxidase) activity. A) Activities of oxidative phosphorylation complexes I–IV in S2 cells at d following RNAi treatment. Blp-RNAi treatment showed selective reduction of complex IV activity at d 5, 6, and 9, compared to LASP-RNAi-treated control cells. B) Top panels: Western blot showing accumulation of the precursor form (p) of Hsp60 (indicative of nonfunctional mitochondrial protein import machinery) at d 5, 6, and 9 after Blp-RNAi treatment. Bottom panels: failure of Blp-inhibitor treatment to cause the accumulation of the precursor form of Hsp60 up to 4 d posttreatment. Differences in protein import between Blp-RNAi and Blp-inhibitor (SMMI) treatments are possibly due to the difference in their mechanism of action. RNAi treatment leads to a reduction in the Blp protein levels, whereas the SMMI causes the inactivation of Blp without causing Blp depletion. C) Changes in ROS levels were measured with DCFDA dye in SMMI-treated cells, preincubated with or without NAC and expressed as fold increase in fluorescence units with respect to DMSO treatment. Blp inactivation leads to a significant increase in ROS levels by 1 h of treatment, compared to the levels in control NAC-treated cells. D) Immunofluorescence microscopy images of MitoTracker-stained S2 cells after SMMI or DMSO treatment at the indicated times, showing a decrease in membrane polarity. E) MMP (ratio of red:green JC-1 fluorescence) in S2 cells, after SMMI treatment at the indicated times. Values are means ± sd; n = 3. *P < 0.001; Student's t test.
Complex IV, the terminal enzyme in ETC, receives electrons from cytochrome c and transfers them to oxygen to form water. The reduced complex IV activity at d 5 coincides with the loss of mitochondrial membrane polarity, the drop in cellular ATP levels, and the elevation of ROS levels. As complexes I and III are responsible for generation of ROS, the inhibition of the downstream complex IV by Blp deficiency is expected to cause elevation of ROS (30), which we observed (Fig. 4A).
Blp deficiency/inhibition reduces MMP prior to the impairment of mitochondrial protein import
The yeast Blp ortholog, Pam16, has been shown to have an essential role in the translocation of nuclear-encoded proteins into the mitochondria (2, 27, 39). Disruption of the interaction between Pam16 and its heterodimerization partner, Pam18, leads to the inhibition of mitochondrial protein import, which can be detected by the accumulation of the nonprocessed precursor form (p) of the mitochondrial-targeted protein Hsp60 (2, 40). During the normal function of the mitochondrial protein import apparatus, the precursor form of Hsp60 is converted into the matured form (m) to be imported into mitochondria. When the import complex is impaired, the unprocessed high-molecular-weight form of Hsp60 (containing the mitochondria targeting sequence) is observed by SDS-PAGE. Western blot analysis of RNAi-treated cell lysates detected the accumulation of the p form of Hsp60 at 5 d post-Blp-RNAi treatment (Fig. 5B), whereas the loss of MMP (Fig. 2A) and increased ROS levels (Fig. 4A) occurred at 4 d, indicating that the effect of Blp depletion on oxidative phosphorylation is more sensitive and occurs earlier than the effect of Blp depletion on mitochondrial protein import. To confirm this, we used a small molecule that rapidly inhibits Magmas activity, small molecule Magmas inhibitor (SMMI; ref. 1, 41) to determine whether the effect of reduced Blp activity on oxidative phosphorylation preceded or followed its effect on mitochondrial protein import. Treatment of S2 cells with SMMI resulted in reduced cell proliferation (Supplemental Fig. S2B), increased ROS levels at 1 h (Fig. 5C), loss of mitochondrial membrane polarity within 2 h (Fig. 5D), reduced ATP levels, cell cycle arrest, induction of autophagy (data not shown), and reduced complex IV activity by 3 d (Supplemental Fig. S2C), all changes observed more rapidly than in response to Blp-RNAi treatment. However, accumulation of the precursor form of Hsp60 (Fig. 5B, bottom panel) was not observed even 4 d after a significant difference in the MMP was observed at 2 h (Fig. 5D). Together, the Blp-RNAi and SMMI results indicate that the uncoupling of oxidative phosphorylation is among the initial effects of depletion or inactivation of Blp and suggest that the inhibition of protein import may be secondary, as it is ATP dependent.
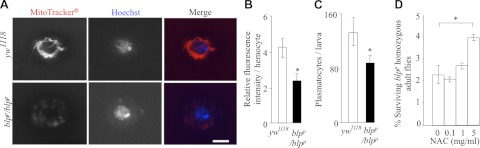
Plasmatocytes from blpp homozygous larvae are reduced in number and possess fewer active mitochondria
Our results with S2 cells suggest that the impaired proliferation and survival of cells in blpΔ3 clones (Fig. 1A) and the cause of the early larval lethality of blpΔ3 flies are primarily a consequence of defective oxidative phosphorylation. It is likely that mitochondria-rich, metabolically-active plasmatocytes would be significantly affected by the loss of Blp activity. Previous studies have shown that blpp escapers exhibit melanotic inclusions (7). This could be the consequence of decreased clearance of pathogens or cell debris by plasmatocytes, followed by lamellocyte proliferation and crystal cell-mediated melanization. Defective oxidative phosphorylation would severely impair plasmatocyte-mediated phagocytosis. To assess mitochondrial function in Blp-deficient plasmatocytes, third-instar larval plasmatocytes from blpp homozygote and control yw1118 larvae were stained with MitoTracker. Compared to the control plasmatocytes (Fig. 6A, B), blpp homozygote larval plasmatocytes exhibited a 45% reduction in the intensity of MitoTracker staining. Furthermore, there was a 35% reduction in the number of recovered blpp homozygote plasmatocytes, compared with control plasmatocytes (Fig. 6C). These results indicate defective mitochondrial function and reduced survival of Blp-deficient plasmatocytes.
Figure 6.
Reduced number and mitochondrial activity of blpp homozygous larval plasmatocytes and increased survival of blpp homozygotes to adulthood with NAC treatment. A) Immunofluorescence microscopy of third-instar larval plasmatocytes from blpp homozygous and yw1118 (control) larvae stained with MitoTracker and Hoechst stain. Results show decreased MitoTracker fluorescence in blpp homozygous cells, indicating reduced mitochondrial activity in blpp homozygous larvae. Scale bar = 20 μm. B) Quantitation of MitoTracker fluorescence, showing a significant reduction in the fluorescence intensity of plasmatocytes from blpp homozygous larvae (n=300). *P < 0.001; Student's t test. C) Number of plasmatocytes isolated from third-instar larvae. There are reduced numbers of surviving plasmatocytes in blpp homozygous larvae, compared to yw1118 (control) larvae (n=30). *P < 0.001; Student's t test. D) Effect of NAC on the survival of blpp homozygous adult flies (non-GFP) from blpp/TM3,GFP crosses. Surviving blpp/ blpp adult flies were scored and expressed as percentage of total surviving flies. At 5 mg/ml NAC, a statistically significant improvement in survival was observed (n≥4500) *P < 0.05; Student's t test. NAC had no effect on blpp/TM3,GFP flies. This suggests that Blp depletion inhibits cell growth through ROS.
Survival of blpp homozygotes is improved by NAC treatment
If, similar to Blp-deficient S2 cells, the reduction in the plasmatocyte numbers in blpp homozygotes results from increased cellular ROS levels, their deleterious effects should be counteracted by addition of antioxidants. Therefore, blpp/TM3, GFP flies were grown on food containing varying concentrations of the antioxidant NAC, and the number of escapers was scored by their failure to fluoresce under UV microscopy. NAC (5 mg/ml) addition resulted in an ∼2-fold (Fig. 6D) increase in the survival to adulthood of the blpp homozygous flies, but not the survival of control blpp/TM3,GFP flies. In addition, compared to untreated flies, we failed to observe melanotic inclusions in the surviving NAC-treated flies. These studies demonstrate that ROS contribute to the poor survival of blpp homozygotes to adulthood by mechanisms similar to those observed in RNAi- and SMMI-treated S2 cells.
DISCUSSION
Previous investigators have shown that Blp is required for the survival of Drosophila embryos (6). Our experiments demonstrate that Blp supports cell survival and proliferation and show that it has a critical role in the oxidative phosphorylation. Blp-RNAi-treated cells exhibited loss of MMP that was associated with elevated levels of ROS, reduced cellular ATP levels, cell cycle arrest, and activation of autophagy. Analysis of ETC complex activity in these cells indicated that, at the time of onset of these characteristics, the activity of complex IV, but not of complexes I–III, was affected. The data suggest that uncoupling of oxidative phosphorylation due to complex IV inactivation is a key event. The electrons generated from the oxidation of NADH and FADH2 by ETC complexes are transferred via cytochrome c to complex IV. This flow of electrons through the ETC complexes creates an electrochemical proton gradient across the inner mitochondrial membrane, leading to the formation of MMP. Complex IV utilizes the bulk of the electrons generated by complexes I and III to produce water. However, 1–2% of the electrons generated by the complexes I and III leak to O2 to form superoxides and other ROS (30, 31).
The selective effect of Blp depletion on complex IV inactivation results in a disruption in the flow of electrons from complexes I–III to complex IV. This leads to an uncoupling of oxidative phosphorylation and loss of mitochondrial membrane polarity, as demonstrated by reduced MitoTracker fluorescent intensity. The accumulation of excess electrons at complexes I and III results in an overproduction of ROS, which enters the cytoplasm, causing cellular damage and cell cycle arrest. Treatment of Blp-deficient cells with NAC or the mitochondrially targeted antioxidant, Mito-Tempo, failed to restore MitoTracker staining (Supplemental Fig. S1A, B), although it reduced total cellular ROS levels (Supplemental Fig. S1C), indicating that the loss of mitochondrial membrane polarity in Blp-deficient cells is primarily due to the uncoupling of oxidative phosphorylation. In contrast, NAC treatment rescued the cell cycle defect (Fig. 4B), indicating that reduced cell proliferation phenotype is due to the overproduction of ROS. Finally, the addition of NAC increased the survival of blpp homozygotes, suggesting that the survival defect in blp mutants is also ROS dependent (Fig. 6D).
Melanotic lesions are characteristic of a dysfunctional innate immune response in Drosophila. Decreased plasmatocyte function impairs pathogen clearance and causes lamellocyte proliferation and crystal cell-mediated melanization. Plasmatocytes are expected to be more susceptible to elevated ROS than other adult cell lineages due to their higher metabolic requirements. We have shown that plasmatocytes isolated from blpp homozygous third-instar larvae are reduced in number and that the surviving plasmatocytes contain fewer active mitochondria. Our failure to detect melanotic lesions in NAC-treated blpp homozygous flies suggests that the plasmatocytes are the cells that are predominantly affected.
The yeast Blp ortholog, Pam16, has been shown to be essential for the import of nuclear-encoded proteins into the mitochondria (2, 27). Mitochondrial protein import is an energy-dependent process (42–44), requiring the MMP (Δψ) and ATP for the translocation of the mitochondrial matrix- and inner membrane-targeted precursor proteins, which contain a positively charged cleavable presequence (45, 46). For matrix-targeted proteins, the translocase of the inner membrane 23 (TIM23) complex, lacking Tim21, associates with the ATP-dependent PAM complex (including Tim44, Mge1, Pam16, Pam17, Pam18, and mitochondrial heat shock protein-70; reviewed in ref. 46), located on the matrix side of the inner membrane, to form the TIM23MOTOR, which completes the transfer of the precursor protein into the matrix (46, 47). In yeast, Pam16 has been shown to associate with the PAM complex through direct interaction with Pam18, and mutations in Pam16 disrupt this interaction and inhibit protein import (2, 39). The J-like domain of Pam16 binds to the J domain of Pam18, which is required for the recruitment of Pam18 to the TIM23 complex (26) and thus, Pam16 regulates the mitochondrial Hsp70-stimulatory ability of Pam18 (40, 48). The sequestration of the J domain of Pam18 by Pam16, acting as a negative regulator of protein import (49), is proposed to act as an on/off switch, regulating the binding of Pam18 at the translocon. Others have suggested that the interaction between the J domains of Pam16 and Pam18 is required most importantly for the stabilization of Pam18 at the translocon (50). Hence the mechanism of Pam16 action in the protein import machinery is currently unclear.
The Pam16-Pam18 complex is not restricted to the TIM23MOTOR and has been shown to be part of the ETC supercomplexes, containing complex III and IV, that are associated with TIM23SORT (38). Thus, it is also possible that the Pam16 has a protein import complex-independent role in the ETC, or that it is acting as a regulatory module to synchronize the ETC with the protein import machinery. If so, perturbation of the Pam16/Magmas/Blp levels could immediately affect both the respiratory chain and the protein import. Our demonstration that Blp deficiency uncouples oxidative phosphorylation by selectively inhibiting complex IV activity, leading to decreased ATP levels and loss of Δψ, suggests that the primary effect of the deficiency could occur at the level of the ETC. In contrast, if the primary effect of Blp deficiency were at the level of protein transport, we would not expect that the inhibition would be at such a specific point in the ETC. Our Blp-RNAi experiments suggest that the uncoupling of oxidative phosphorylation precedes the inhibition of protein import in response to depletion of Blp. However, while the Blp inhibitor, SMMI, inhibits the MMP within 2 h, we fail to see inhibition of protein import at 4 d, suggesting that inhibition of protein import can be independent of the uncoupling of oxidative phosphorylation. At present, the reason why the Blp inhibitor apparently fails to affect protein import is not clear. One possibility is that Blp interacts with proteins within complex IV that are disrupted by the inhibitor, but those proteins are not present in the import complex, so function there is preserved.
Interestingly, a truncation mutation of DnaJC19, the mammalian homologue of Pam18, that prevents Magmas/DnaJC19 heterodimerization, is associated with dilated cardiomyopathy with ataxia syndrome (2). Thus, similar to hemocytes, cells from human tissues that are enriched in mitochondria, such as cardiac and skeletal myocytes and neuronal cells, are particularly sensitive to loss of Magmas (Pam16) function. Finally, Magmas has been reported to be highly overexpressed in several human malignancies, including prostate cancer (3) and human pituitary adenomas (51), suggesting that Magmas could be a potential therapeutic target for treating human cancer.
Supplementary Material
Acknowledgments
The authors thank Dr. Nicholas Baker (Albert Einstein College of Medicine) for reagents and advice with the mosaic analysis and hemocyte isolation, Dr. Erich Buchner (Biozentrum der Universität Würzburg, Am Hubland, Würzburg, Germany) for the Drosophila blp lines, the Einstein Analytical Imaging and Flow Cytometry Core Facilities, and Donna Alex, Pete Walker, and Mary and Robert Cancilla for their support.
This work was supported by U.S. National Institutes of Health grant CA26504 (E.R.S.), the Albert Einstein College of Medicine Cancer Center grant 5P30-CA13330, the James B. Ax Family Foundation, The SAS Foundation for Cancer Research, and the Magmas Research Fund (P.T.J.).
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
- Blp
- black-pearl
- BrdU
- 5-bromo-2′-deoxyuridine
- DCFDA
- 5-(and-6)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate acetyl ester
- ETC
- electron transport chain
- eyF
- eyeless-Flp
- FACS
- fluorescence-activated cell scanning
- FBS
- fetal bovine serum
- hsF
- hsp70-Flp
- Magmas
- mitochondria-associated granulocyte macrophage colony-stimulating factor signaling
- MMP
- mitochondrial membrane potential
- NAC
- N-acetyl cysteine
- ROS
- reactive oxygen species
- SMMI
- small molecule Magmas inhibitor
- TIM
- translocase of the inner membrane
REFERENCES
- 1. Jubinsky P. T., Messer A., Bender J., Morris R. E., Ciraolo G. M., Witte D. P., Hawley R. G., Short M. K. (2001) Identification and characterization of Magmas, a novel mitochondria-associated protein involved in granulocyte-macrophage colony-stimulating factor signal transduction. Exp. Hematol. 29, 1392–1402 [DOI] [PubMed] [Google Scholar]
- 2. Sinha D., Joshi N., Chittoor B., Samji P., D'Silva P. (2010) Role of Magmas in protein transport and human mitochondria biogenesis. Hum. Mol. Genet. 19, 1248–1262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jubinsky P. T., Short M. K., Mutema G., Morris R. E., Ciraolo G. M., Li M. (2005) Magmas expression in neoplastic human prostate. J. Mol. Histol. 36, 69–75 [DOI] [PubMed] [Google Scholar]
- 4. Gonczy P., Echeverri C., Oegema K., Coulson A., Jones S. J., Copley R. R., Duperon J., Oegema J., Brehm M., Cassin E., Hannak E., Kirkham M., Pichler S., Flohrs K., Goessen A., Leidel S., Alleaume A. M., Martin C., Ozlü N., Bork P., Hyman A. A. (2000) Functional genomic analysis of cell division in C. elegans using RNAi of genes on chromosome III. Nature 408, 331–336 [DOI] [PubMed] [Google Scholar]
- 5. Winzeler E. A., Shoemaker D. D., Astromoff A., Liang H., Anderson K., Andre B., Bangham R., Benito R., Boeke J. D., Bussey H., Chu A. M., Connelly C., Davis K., Dietrich F., Dow S. W., El Bakkoury M., Foury F., Friend S. H., Gentalen E., Giaever G., Hegemann J. H., Jones T., Laub M., Liao H., Liebundguth N., Lockhart D. J., Lucau-Danila A., Lussier M., M'Rabet N., Menard P., Mittmann M., Pai C., Rebischung C., Revuelta J. L., Riles L., Roberts C. J., Ross-MacDonald P., Scherens B., Snyder M., Sookhai-Mahadeo S., Storms R. K., Véronneau S., Voet M., Volckaert G., Ward T. R, Wysocki R., Yen G. S., Yu K., Zimmermann K., Philippsen P., Johnston M., Davis R. W. (1999) Functional characterization of the S. cerevisiae genome by gene deletion and parallel analysis. Science 285, 901–906 [DOI] [PubMed] [Google Scholar]
- 6. Perrimon N., Lanjuin A., Arnold C., Noll E. (1996) Zygotic lethal mutations with maternal effect phenotypes in Drosophila melanogaster. II. Loci on the second and third chromosomes identified by P-element-induced mutations. Genetics 144, 1681–1692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Becker S., Gehrsitz A., Bork P., Buchner S., Buchner E. (2001) The black-pearl gene of Drosophila defines a novel conserved protein family and is required for larval growth and survival. Gene 262, 15–22 [DOI] [PubMed] [Google Scholar]
- 8. Hanratty W. P., Dearolf C. R. (1993) The Drosophila tumorous-lethal hematopoietic oncogene is a dominant mutation in the hopscotch locus. Mol. Gen. Genet. 238, 33–37 [DOI] [PubMed] [Google Scholar]
- 9. Watson K. L., Johnson T. K., Denell R. E. (1991) Lethal(1) aberrant immune response mutations leading to melanotic tumor formation in Drosophila melanogaster. Dev. Genet. 12, 173–187 [DOI] [PubMed] [Google Scholar]
- 10. Lemaitre B., Hoffmann J. (2007) The host defense of Drosophila melanogaster. Annu. Rev. Immunol. 25, 697–743 [DOI] [PubMed] [Google Scholar]
- 11. Hultmark D. (2003) Drosophila immunity: paths and patterns. Curr. Opin. Immunol. 15, 12–19 [DOI] [PubMed] [Google Scholar]
- 12. Meister M. (2004) Blood cells of Drosophila: cell lineages and role in host defence. Curr. Opin. Immunol. 16, 10–15 [DOI] [PubMed] [Google Scholar]
- 13. Xu T., Rubin G. M. (1993) Analysis of genetic mosaics in developing and adult Drosophila tissues. Development 117, 1223–1237 [DOI] [PubMed] [Google Scholar]
- 14. Pile L. A., Spellman P. T., Katzenberger R. J., Wassarman D. A. (2003) The SIN3 deacetylase complex represses genes encoding mitochondrial proteins: implications for the regulation of energy metabolism. J. Biol. Chem. 278, 37840–37848 [DOI] [PubMed] [Google Scholar]
- 15. Bjorklund M., Taipale M., Varjosalo M., Saharinen J., Lahdenpera J., Taipale J. (2006) Identification of pathways regulating cell size and cell-cycle progression by RNAi. Nature 439, 1009–1013 [DOI] [PubMed] [Google Scholar]
- 16. Nemoto S., Takeda K., Yu Z. X., Ferrans V. J., Finkel T. (2000) Role for mitochondrial oxidants as regulators of cellular metabolism. Mol. Cell. Biol. 20, 7311–7318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Liang H. L., Sedlic F., Bosnjak Z., Nilakantan V. (2010) SOD1 and MitoTEMPO partially prevent mitochondrial permeability transition pore opening, necrosis, and mitochondrial apoptosis after ATP depletion recovery. Free Radic. Biol. Med. 49, 1550–1560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wang Y. C., Lee C. M., Lee L. C., Tung L. C., Hsieh-Li H. M., Lee-Chen G. J., Su M. T. (2011) Mitochondrial dysfunction and oxidative stress contribute to the pathogenesis of spinocerebellar ataxia type 12 (SCA12). J. Biol. Chem. 286, 21742–21754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sriram V., Krishnan K. S., Mayor S. (2003) deep-orange and carnation define distinct stages in late endosomal biogenesis in Drosophila melanogaster. J. Cell Biol. 161, 593–607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yu W., Chen J., Xiong Y., Pixley F. J., Dai X. M., Yeung Y. G., Stanley E. R. (2008) CSF-1 receptor structure/function in MacCsf1r-/- macrophages: regulation of proliferation, differentiation, and morphology. J. Leukoc. Biol. 84, 852–863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ladha J. S., Tripathy M. K., Mitra D. (2005) Mitochondrial complex I activity is impaired during HIV-1-induced T-cell apoptosis. Cell Death Differ. 12, 1417–1428 [DOI] [PubMed] [Google Scholar]
- 22. Schwarze S. R., Weindruch R., Aiken J. M. (1998) Oxidative stress and aging reduce COX I RNA and cytochrome oxidase activity in Drosophila. Free Radic. Biol. Med. 25, 740–747 [DOI] [PubMed] [Google Scholar]
- 23. Trounce I. A., Kim Y. L., Jun A. S., Wallace D. C. (1996) Assessment of mitochondrial oxidative phosphorylation in patient muscle biopsies, lymphoblasts, and transmitochondrial cell lines. Methods Enzymol. 264, 484–509 [DOI] [PubMed] [Google Scholar]
- 24. Schagger H., von Jagow G. (1991) Blue native electrophoresis for isolation of membrane protein complexes in enzymatically active form. Anal. Biochem. 199, 223–231 [DOI] [PubMed] [Google Scholar]
- 25. Van Coster R., Smet J., George E., De Meirleir L., Seneca S., Van Hove J., Sebire G., Verhelst H., De Bleecker J., Van Vlem B., Verloo P., Leroy J. (2001) Blue native polyacrylamide gel electrophoresis: a powerful tool in diagnosis of oxidative phosphorylation defects. Pediatr. Res. 50, 658–665 [DOI] [PubMed] [Google Scholar]
- 26. D'Silva P. R., Schilke B., Hayashi M., Craig E. A. (2008) Interaction of the J-protein heterodimer Pam18/Pam16 of the mitochondrial import motor with the translocon of the inner membrane. Mol. Biol. Cell 19, 424–432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Frazier A. E., Dudek J., Guiard B., Voos W., Li Y., Lind M., Meisinger C., Geissler A., Sickmann A., Meyer H. E., Bilanchone V., Cumsky M. G., Truscott K. N., Pfanner N., Rehling P. (2004) Pam16 has an essential role in the mitochondrial protein import motor. Nat. Struct. Mol. Biol. 11, 226–233 [DOI] [PubMed] [Google Scholar]
- 28. Mandal S., Guptan P., Owusu-Ansah E., Banerjee U. (2005) Mitochondrial regulation of cell cycle progression during development as revealed by the tenured mutation in Drosophila. Dev. Cell. 9, 843–854 [DOI] [PubMed] [Google Scholar]
- 29. Levine B., Abrams J. (2008) p53: the Janus of autophagy? Nat. Cell Biol. 10, 637–639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Poyton R. O., Ball K. A., Castello P. R. (2009) Mitochondrial generation of free radicals and hypoxic signaling. Trends Endocrinol. Metab. 20, 332–340 [DOI] [PubMed] [Google Scholar]
- 31. Cadenas E., Boveris A., Ragan C. I., Stoppani A. O. (1977) Production of superoxide radicals and hydrogen peroxide by NADH-ubiquinone reductase and ubiquinol-cytochrome c reductase from beef-heart mitochondria. Arch. Biochem. Biophys. 180, 248–257 [DOI] [PubMed] [Google Scholar]
- 32. Vanden Hoek T. L., Li C., Shao Z., Schumacker P. T., Becker L. B. (1997) Significant levels of oxidants are generated by isolated cardiomyocytes during ischemia prior to reperfusion. J. Mol. Cell. Cardiol. 29, 2571–2583 [DOI] [PubMed] [Google Scholar]
- 33. Lemasters J. J. (2005) Selective mitochondrial autophagy, or mitophagy, as a targeted defense against oxidative stress, mitochondrial dysfunction, and aging. Rejuvenation Res. 8, 3–5 [DOI] [PubMed] [Google Scholar]
- 34. Scherz-Shouval R., Elazar Z. (2007) ROS, mitochondria and the regulation of autophagy. Trends Cell Biol. 17, 422–427 [DOI] [PubMed] [Google Scholar]
- 35. Meley D., Bauvy C., Houben-Weerts J. H., Dubbelhuis P. F., Helmond M. T., Codogno P., Meijer A. J. (2006) AMP-activated protein kinase and the regulation of autophagic proteolysis. J. Biol. Chem. 281, 34870–34879 [DOI] [PubMed] [Google Scholar]
- 36. Wang Z., Wilson W. A., Fujino M. A., Roach P. J. (2001) Antagonistic controls of autophagy and glycogen accumulation by Snf1p, the yeast homolog of AMP-activated protein kinase, and the cyclin-dependent kinase Pho85p. Mol. Cell. Biol. 21, 5742–5752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Vafa O., Wade M., Kern S., Beeche M., Pandita T. K., Hampton G. M., Wahl G. M. (2002) c-Myc can induce DNA damage, increase reactive oxygen species, and mitigate p53 function: a mechanism for oncogene-induced genetic instability. Mol. Cell. 9, 1031–1044 [DOI] [PubMed] [Google Scholar]
- 38. Wiedemann N., van der Laan M., Hutu D. P., Rehling P., Pfanner N. (2007) Sorting switch of mitochondrial presequence translocase involves coupling of motor module to respiratory chain. J. Cell Biol. 179, 1115–1122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. D'Silva P. R., Schilke B., Walter W., Craig E. A. (2005) Role of Pam16's degenerate J domain in protein import across the mitochondrial inner membrane. Proc. Natl. Acad. Sci. U. S. A. 102, 12419–12424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. D'Silva P. D., Schilke B., Walter W., Andrew A., Craig E. A. (2003) J protein cochaperone of the mitochondrial inner membrane required for protein import into the mitochondrial matrix. Proc. Natl. Acad. Sci. U. S. A. 100, 13839–13844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Jubinsky P. T., Short M. K., Ghanem M., Das B. C. (2011) Design, synthesis, and biological activity of novel Magmas inhibitors. Bioorg. Med. Chem. Lett. 21, 3479–3482 [DOI] [PubMed] [Google Scholar]
- 42. D'Silva P., Liu Q., Walter W., Craig E. A. (2004) Regulated interactions of mtHsp70 with Tim44 at the translocon in the mitochondrial inner membrane. Nat. Struct. Mol. Biol. 11, 1084–1091 [DOI] [PubMed] [Google Scholar]
- 43. Liu Q., D'Silva P., Walter W., Marszalek J., Craig E. A. (2003) Regulated cycling of mitochondrial Hsp70 at the protein import channel. Science 300, 139–141 [DOI] [PubMed] [Google Scholar]
- 44. Schneider H. C., Berthold J., Bauer M. F., Dietmeier K., Guiard B., Brunner M., Neupert W. (1994) Mitochondrial Hsp70/MIM44 complex facilitates protein import. Nature 371, 768–774 [DOI] [PubMed] [Google Scholar]
- 45. Sirrenberg C., Bauer M. F., Guiard B., Neupert W., Brunner M. (1996) Import of carrier proteins into the mitochondrial inner membrane mediated by Tim22. Nature 384, 582–585 [DOI] [PubMed] [Google Scholar]
- 46. Van der Laan M., Hutu D. P., Rehling P. (2010) On the mechanism of preprotein import by the mitochondrial presequence translocase. Biochim. Biophys. Acta 1803, 732–739 [DOI] [PubMed] [Google Scholar]
- 47. Chacinska A., Lind M., Frazier A. E., Dudek J., Meisinger C., Geissler A., Sickmann A., Meyer H. E., Truscott K. N., Guiard B., Pfanner N., Rehling P. (2005) Mitochondrial presequence translocase: switching between TOM tethering and motor recruitment involves Tim21 and Tim17. Cell 120, 817–829 [DOI] [PubMed] [Google Scholar]
- 48. Truscott K. N., Voos W., Frazier A. E., Lind M., Li Y., Geissler A., Dudek J., Müller H., Sickmann A., Meyer H. E., Meisinger C., Guiard B., Rehling P., Pfanner N. (2003) A J-protein is an essential subunit of the presequence translocase-associated protein import motor of mitochondria. J. Cell Biol. 163, 707–713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Li Y., Dudek J., Guiard B., Pfanner N., Rehling P., Voos W. (2004) The presequence translocase-associated protein import motor of mitochondria. Pam16 functions in an antagonistic manner to Pam18. J. Biol. Chem. 279, 38047–38054 [DOI] [PubMed] [Google Scholar]
- 50. Pais J. E., Schilke B., Craig E. A. (2011) Reevaluation of the role of the Pam18: Pam16 interaction in translocation of proteins by the mitochondrial Hsp70-based import motor. Mol. Biol. Cell 22, 4740–4749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Tagliati F., Gentilin E., Buratto M., Mole D., degli Uberti E. C., Zatelli M. C. (2010) Magmas, a gene newly identified as overexpressed in human and mouse ACTH-secreting pituitary adenomas, protects pituitary cells from apoptotic stimuli. Endocrinology 151, 4635–4642 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.