Abstract
In the past decade, it has become increasingly evident that the circadian clock system plays an important role in many physiological processes. The circadian clock can be divided into 2 parts: the central clock, residing in the suprachiasmatic nucleus of the hypothalamus, which receives light cues, and the peripheral clocks that reside in various tissues throughout the body. The peripheral clocks play an integral and unique role in each of their respective tissues, driving the circadian expression of specific genes involved in a variety of physiological functions. The goal of this review is to provide an introduction to and overview of the peripheral clocks, including potential mechanisms, targets, and implications for disease states. The peripheral clocks include the cardiovascular, metabolic, endocrine, immune, and reproductive systems.— Richards, J., Gumz, M. L. Advances in understanding the peripheral circadian clocks.
Keywords: cardiovascular, metabolism, immune, endocrine, renal
Over the past decade, the field of chronobiology has rapidly expanded as researchers have begun unraveling the complex and integral role the circadian clock plays in most physiological processes. The field of chronobiology is not new, however. Since the 18th century, scientists have observed that many physiological processes in plants and mammals exhibited apparent 24-h rhythms. In the 1970s, researchers began experimenting with the circadian clock in Drosophila melanogaster with crucial work done by Konopka and Benzer (1) that demonstrated the phenomenon of period length and rhythmicity, enabling them to identify a gene locus important for these processes. Over the course of the past 40 yr, the circadian clock has been further studied in multiple genetic systems; notably, the mammalian homologs (human and mice) of per were identified prior to the 21st century (2), which opened the door for extensive research into the role of the circadian clock in physiology and disease states.
CENTRAL CLOCK VS. PERIPHERAL CLOCK
On a basic level, the circadian clock can be divided into 2 parts: the central clock, residing in the suprachiasmatic nucleus (SCN) of the brain, and the peripheral clocks that are present in nearly every tissue and organ system tested (3, 4). Light enters through the retina of the eye, causing electrical signals to pass through the retinal hypothalamic tract, which are converted to chemical signals in the SCN. Light signals and other physiological factors, such as feeding cues, entrain the central circadian clock. There has been much debate among chronobiologists about the relationship between the central clock and the peripheral clock with 2 major theories emerging. The first is the “master-slave” model (discussed in ref. 4), which gives complete synchronization power to the central clock. In this model, the peripheral clocks are synchronized solely by the central clock and are not otherwise affected by external or internal stimuli. The second model was developed by Albrecht and colleagues (4) and is referred to as the “orchestra” model. In this model, the central clock behaves as a conductor, with each peripheral clock as an orchestra member. Each member has the ability to play its own “instrument,” but the conductor's direction allows for efficient and guided direction of the melody, which would include physiological output rhythms. Thus, each peripheral clock can adapt to its own external and internal stimuli, such as feeding cues for the liver, kidney, and pancreas, but is “conducted” by the light-dark cues sensed by the central clock. This model implies a more balanced relationship between the central and peripheral clocks. Recent studies provide increasing evidence for the independence of the peripheral clocks. The importance of understanding the role of the peripheral clocks in each tissue, and their relationship to one another is paramount in order to better comprehend the role of the peripheral clocks in circadian physiology. Hence, the goals of this review are to consider the role of the circadian clock in the various peripheral clocks and their unique and physiologically important targets, provide further insight into debate about the divisions of power in the 2 pieces of the clock, and to highlight recent and important advances in peripheral clock research. This review will start with a brief introduction of the canonical clock mechanism, then overview the peripheral clocks starting with the cardiovascular system including the kidney and the muscle and the circadian clock in metabolism, and end with the endocrine, immune, and reproductive systems.
TRANSCRIPTION TRANSLATION OSCILLATING (TTO) LOOP MODEL
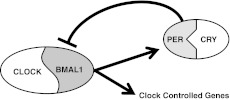
At the start of the new millennium, the consensus among circadian researchers was that on the molecular level, the circadian clock could be modeled by a loop involving transcription and translation of specific core clock proteins. These core clock proteins affect transcription of themselves as well as clock-controlled genes. This gene regulation then resulted in translation of the circadian proteins and their targets in a rhythmic manner. This model became known as the TTO loop model (Fig. 1). The circadian protein circadian locomotor output cycles kaput protein (Clock) and brain and muscle Arnt-like protein-1 (Bmal1) heterodimerize and interact with E-boxes in the promoters of clock-controlled genes, which drives the positive transcription of the TTO loop (5). These 2 proteins promote the transcription of clock-controlled genes and the 2 circadian proteins Period (Per; homologs: 1, 2, and 3) and cryptochrome-like protein (Cry; homologs: 1 and 2). These 2 proteins also interact, translocate into the nucleus, and inhibit the activity of Clock and Bmal1, which promotes the canonical transcriptional repression arm of the TTO loop (4). Other accessory proteins include the D-box binding protein (DBP) and the nuclear orphan receptors retinoid-related orphan receptor (ROR) and nuclear receptor subfamily 1, group D, member 1 (NR1D1; REV-ERB), which modulate the activity of the loop. As research into the mechanism has progressed, more accessory proteins have been identified that modulate the activity of the clock (reviewed in ref. 6). Understanding the mechanism and function of the circadian clock is an increasingly active area of investigation.
Figure 1.
TTO loop model. Clock and Bmal1 heterodimerize and transcriptionally activate Per and Cry and other clock-controlled genes. Per and Cry interact to inhibit Clock and Bmal1 action.
CIRCADIAN CLOCK MECHANISM
Even though the circadian clock is not fully understood, much of the work done in the past 2 decades contributed to formation of the TTO loop model. The canonical understanding is that the 4 core circadian proteins Clock, Bmal1, Cry, and Per interact with each other and that they are the major driving force behind the rhythmic expression of clock-controlled genes. Though the focus of this review is on the peripheral clocks and the canonical mechanism is well understood, the reader is directed to a review by Zhang and Kay (6) for further inquiry on the complexities of the core clock mechanism.
POST-TRANSLATIONAL MODIFICATIONS
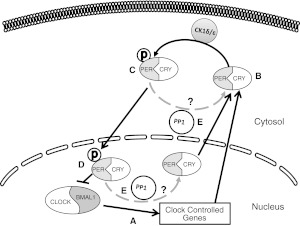
Aside from the transcriptional and translational regulation of the clock described in the TTO loop model, investigation into post-translational modifications of the clock proteins is increasing. Regulation of nuclear entry and export of the Per proteins is integral for overall circadian control (7). Casein kinases 1 and 2 (CK1 and CK2) are involved in the phosphorylation of the Period proteins. The mechanism of this role in regulation is still being studied. It is well established that CK1 inhibition results in circadian phase delays (8). There is evidence that CK1ε phosphorylation conceals the nuclear localization signal of Period homologue 1 (Per1), which results in cytoplasmic localization in HEK293 cells (9). There is also evidence that CK1δ/ε phosphorylation results in Per1 import rather than export (10). Per1 was predominantly cytoplasmic in fibroblasts from CK1δ/ε-knockout (KO) animals and following pharmacological inhibition of CK1δ/ε in mpkCCD14 cells (a model of the mouse principal cells of the cortical collecting duct of the kidney; unpublished results). However, the role of CK1δ/ε might be tissue specific and thus needs to be studied in each peripheral clock. Protein phosphatase 1 (PP1) interacts with Per1 and likely dephosphorylates the protein, resulting in cytoplasmic localization of Per1 (10). It is thought that this balance between CK1δ/ε phosphorylation and dephosphorylation by PP1 may be a key regulator of the post-translational regulation of the Period proteins (Fig. 2).
Figure 2.
Post-translational modifications in the circadian clock. The current understanding for post-translational regulation of the circadian clock is modeled. A) Clock and Bmal1 dimerize and transcriptionally activate clock-controlled genes, including Per and Cry. B) Per and Cry interact in the cytosol. C) Per is phosphorylated by CK1δ/ε, causing it to translocate into the nucleus. D) Per and Cry repress Clock and Bmal1 activity. E) Phosphorylated Per1 is potentially dephosphorylated by protein phosphatase 1 (PP1), either in the cytosol or in the nucleus (causing nuclear export). P, phosphorylation. Dotted lines indicate hypothetical mechanisms.
CARDIOVASCULAR SYSTEM, KIDNEY, AND MUSCLE
Blood pressure
It has been known since the middle of the 20th century that blood pressure drops at night and rises during the day (11, 12). Individuals who do not experience this nighttime “dip” in blood pressure are known as “nondippers” and have an increased risk for adverse cardiovascular events (13, 14). It remains to be determined whether this nondipper phenotype is associated with specific circadian genetic mutations. Increasing evidence suggests that the circadian clock plays an integral role in the regulation of blood pressure.
Vascular clock
Since the late 1960s, it has been known that blood flow in humans exhibits a circadian rhythm (15). Sympathetic and vascular tones, response to adrenergic receptor agonists, human forearm vascular resistance, and flow-mediated dilation of the brachial artery exhibit circadian variations (16, 17). On a molecular level, mRNA levels of various clock components (Per, Clock, Cry, Bmal1) oscillate in vascular smooth muscle cells, cultured veins and arteries, endothelial cells, and vascular fibroblasts. Thrombomodulin and plasminogen activator inhibitor 1 (PAI-1), both of which function in thrombus formation, were identified as clock-controlled genes (refs. 18, 19; reviewed in ref. 20).
With implications for the vasculature, work has been done on studying the role of peroxisome proliferator-activated receptor γ (PPARγ) in endothelial cells. Interestingly, endothelial cell-specific PPARγ-KO mice have reduced levels of Bmal1, which has been linked to the reduction in mean arterial pressure in the active phase of these animals (21). When the same deletion was made in smooth muscle cells it caused an increase in mean arterial blood pressure during their resting phase. Whether this apparent contradiction reflects tissue-specific functions for Bmal1 is not clear; however, it has been shown that Bmal1 siRNA in mouse hepatoma cells significantly reduces PPARα mRNA (22), and it has been shown that Per3 inhibits PPARγ activity (23). Clearly, more work elucidating the mechanism is needed in order to fully understand the connection between PPARγ, the circadian clock, and mean arterial blood pressure.
Ambulatory Blood Pressure Monitoring for Prediction of Cardiovascular Events (MAPEC) study
The MAPEC trial was a hallmark study with critical implications for the use of chronotherapy in cardiovascular disease (CVD). Chronotherapy is the timed administration of medication that takes into account inherent circadian rhythms. The MAPEC study goal was to evaluate the effect of timed administration of antihypertensive drugs and the effect on CVD risk. The study looked at the difference between taking antihypertensive medication at bedtime vs. the more common administration on waking in the morning (24). Of those enrolled, 2156 people saw the study to completion, with 1084 subjects randomized to morning administration and 1072 to bedtime administration of just one antihypertensive drug. Strikingly, those who took their medication before sleeping experienced on average a 39% decrease in CVD events compared to individuals taking all of their antihypertensive medications on waking. Also intriguing is that those who experienced a decrease in sleeping blood pressure, resulting in a normalized dipping pattern, exhibited reduced risk for adverse cardiovascular events. These data support a role for chronotherapy in the treatment of CVD.
Kidney
Multiple renal functions exhibit circadian variation, including glomerular filtration rate, renal blood flow, potassium excretion, and sodium excretion (reviewed in ref. 25). Multiple circadian mutant mouse models display specific renal- and aldosterone-related phenotypes. Clock-KO mice exhibit kidney-related defects, including hypotension, partial diabetes insipidus, and disregulated sodium excretion (26). It has also been shown that Clock-KO mice have decreased levels of 20-hydroxyeicosatetraenoic acid (20-HETE; a vasoconstrictor of renal preglomerular arteries, which results in increased blood pressure), which may contribute to the hypotensive phenotype of these mice (27). It was shown that Na+/H+ exchanger 3 (NHE3) mRNA exhibits circadian oscillations in the mouse renal medulla (28). E-cadherin and claudin-4, both important for the regulation of cell-cell junctions, were shown to oscillate in a circadian manner in rat kidneys and may play a role in sodium excretion (28). Cry1/Cry2 double-KO mice display salt-sensitive hypertension, which results from increased synthesis of aldosterone (29). Increased aldosterone levels resulted from increased production of 3-β-dehydrogenase-isomerase, an adrenal gland-specific enzyme in the aldosterone synthesis pathway. We have shown that Per1 is involved in the basal and aldosterone-induced regulation of the α subunit of the epithelial sodium channel (ENaC), in the renal collecting duct (30, 31). ENaC is crucial for sodium transport in the collecting duct and contributes to the fine-tuning of blood volume control. Notably, Per1-KO mice exhibit reduced blood pressure and increased renal ET-1 levels, which may contribute to the blood pressure phenotype in these animals (32).
Angiotensin
Angiotensin is a peptide hormone involved in blood pressure regulation in the renin-angiotensin system (RAS). PAI-1, a clock-controlled gene, is increased in a dose-dependent manner by angiotensin II (33). Cry1/2 double-KO mice exhibit complete ablation of the rhythmic expression of PAI-1, while angiotensin type 1 (AT1a) receptor-KO mice had disruption of rhythmic expression of PAI-1 in the kidney, but not in the lung and liver, providing evidence for tissue-specific circadian regulation of PAI-1 by AT1a-mediated signaling (34).
Recently there has been research that has provided evidence that the angiotensin II receptor blocker (ARB) olmesartan can restore the rhythmic pattern of blood pressure in human patients, i.e., converting a nondipper into a dipper. ARB treatment enhanced sodium excretion in the urine during the day and lowered blood pressure at night, resulting in a dipping phenotype. This restoration of the dipping phenotype of patients was accredited to suppression of tubular sodium reabsorption (35). This effect was also seen by another group using the ARB irbesartan (36). Aside from ARBs, treatment with the diuretic hydrocholorothiazide also restored dipping effects (37). These studies provide evidence for the importance of renal sodium handling in the control of the nighttime dipping of blood pressure and the importance of understanding the circadian mechanism of action in the regulation of renal sodium transport.
Cardiomyocyte
Much of the current work on the circadian clock in the heart, specifically the cardiomyocyte, and cardiovascular system has been done by Young and colleagues. Recent major advances in the field are summarized below, and the reader is directed to one of his excellent reviews for a more in-depth analysis (38). These advances include the generation of the cardiomyocyte-specific clock protein mutant, heart rate, growth, and repair.
Cardiomyocyte-specific clock mutant (CCM)
One of the first major advances was the generation of the CCM, a cardiomyocyte cell-specific transgenic mutant that expresses a dominant negative mutant of the Clock protein (39). This model was used to help identify cardiomyocyte clock-controlled genes involved in fatty acid metabolism [including adiponutrin (ADPN), peroxisome proliferator-activated receptor, coactivator 1α (PGC1α), and PPARα], helping establish a connection between the circadian clock in the heart and its responsiveness to fatty acids, triglyceride metabolism, and increased rate of fatty acid oxidation (reviewed in ref. 38).
Cardiac function
Like blood pressure, heart rate exhibits circadian variation (reviewed in ref. 38). As discussed above, endothelial cell-specific PPARγ-KO animals, in addition to having reduced mean arterial pressure during active phase, exhibit a reduced heart rate (21). It was also shown that CCM mice exhibit reduced heart rate, specifically during the active phase. Whether these two phenomena share a similar mechanism is unknown. However, several intracellular calcium channels, important for heart rate regulation, are clock controlled (40).
It also has recently been shown that repolarization is circadian controlled, specifically transient outward potassium current by Krüppel-like factor 15 (Klf15; ref. 41). Klf15 modulated the transcription of KCHIP2, a critical modulator of the outward rectifier potassium channel. Klf15-null mice or mice overexpressing Klf15 have loss of rhythmic QT variation, abnormal repolarization, and increased occurrence of ventricular arrhythmias. It will be interesting to see whether the observations regarding cardiac function in the CCM mice or PPARγ-KO animals are associated with the actions of Klf15.
Circadian clock and hypertrophy
CCM mice were shown to have physiological hypertrophy, also known as “athlete's heart,” displaying the phenotype of increased heart muscle mass, decreased heart rate, and larger ejection fraction (38). This same phenotype was evident in mice lacking 3 circadian clock-regulated transcription factors, DBP, hepatic leukemia factor (HLF), and thyrotroph embryonic factor (TEF) (these 3 transcription factors are involved in the modulation of circadian rhythms); these animals also exhibit lower blood pressure (42). Phosphoinositide 3-kinase (PI3K), an upstream kinase involved in multiple pathways, plays a role in hypertrophy by activating v-akt murine thymoma viral oncogene homologue 1 (Akt), resulting in the subsequent phosphorylation of glycogen synthase kinase (GSK)-3 β, inactivating the latter. This releases its repression of several translation initiation factors, allowing for increased protein synthesis, a hallmark of hypertrophic growth. The regulatory subunit of PI3K (P85) is a clock-controlled gene and was repressed in CCM mouse hearts. Thus, PI3K would be unregulated and in this case constitutively active, which results in the observed elevation of the active state of Akt and inactive state of GSK-3β seen in these animals (reviewed in ref. 38).
Cardiovascular disease
It is becoming increasingly clear that the circadian clock plays an integral role in the physiology and pathophysiology of the cardiovascular system. Two areas of pathological cardiovascular circadian research have come to the forefront in recent years: the circadian clock in ischemia and infarction and loss of synchronization and cardiovascular disease.
Clock in ischemia
Ischemia, specifically in the heart, is caused by the blocking of arteries to part or all of the heart, resulting in oxygen deprivation to the tissues, leading to necrosis. The resulting lesions are called infarcts. It has been shown that acute myocardial infarctions (commonly called heart attacks) occur with a circadian pattern, with incidence peaking in the early morning. The reasoning behind this was attributed to the rise of systolic blood pressure and heart rate in the morning, which results in increased oxygen demand of the heart. Interestingly, PAI-1, which was discussed above, integral for fibrinolysis, also peaks in the morning and down-regulates tissue-plasminogen activator (t-PA), which is involved in the opening of occluded vessels (reviewed in ref. 43).
Young and colleagues (44) tested the effect of ischemic injury based on time of day intervention using ischemia/reperfusion (I/R) on wild-type (WT) and CCM mice, using 2 time points: zeitgeiber time (ZT) 0 (moment when lights turn on in a 12-h light-dark cycle) and ZT 12 (lights off). WT mice experiencing I/R at ZT 12 had significantly larger infarct size and more severe fibrosis compared to mice who were reperfused at ZT 0. This difference was absent in CCM mice, providing evidence for a circadian clock-controlled mechanism. Notably, in humans, infarct size and myocardial injury also exhibit circadian fluctuations, with the highest injury peaking in the early morning hours (1:00 AM–5:00 AM; ref. 45). Likewise, Fournier and colleagues (46) showed an independent correlation between infarction size and the time of day when symptoms occurred. In a convincing study, it was recently shown that stabilization of Per2 by adenosine receptor A2b (Adora2b) results in increased stability of hypoxia inducible factor 1α (HIF1α) (47). HIF1α enhances the glycolytic capacity of the heart, which is integral for ischemic adaptation. This study provides mechanistic evidence for the previously described observations regarding circadian rhythmicity of infarctions.
Loss of synchronization in cardiovascular disease
Pathologically, loss of synchronization of the clock can lead to increased risk of cardiovascular disease. For example, a recent study examined the association of Japanese male rotational shift workers and adverse cardiac events. (“Rotational shift” workers do not have set schedules and can work night/day or whole-day schedules at random intervals). The rotational shift workers had a 2.3-fold higher risk of death of ischemia of the heart compared to fixed-schedule workers (48). Another report demonstrated that African American women who did >2 yr of rotational night-shift work over a 12-mo period in the last 2 yr of the study had an increase in hazard ratio of cardiovascular events, while Caucasian women had no change (49). These two studies bring up an interesting concept. The circadian clock is entrained by light cues, and thus night-shift workers in most cases are in lit environments and sleep with the lights off; therefore, they experience a light-dark cycle, but opposite that of day-shift workers. Rotational shift workers experienced constant disruption of their light-dark cycles due to rotational shift work. Because of this phenomenon, chronobiologists have begun contemplating various ways to address and perhaps assist in alleviating the disruption of rotational shift workers' circadian rhythms (reviewed in ref. 50). These results also suggest the potential for research in the field of pharmacogenomics. Identification of mutations in circadian genes could help determine disease risk for shift workers and potential chronotherapy opportunities, though much work is needed in order to determine the potential benefit of such pursuits.
Gastrointestinal (GI) tract, the gut, and skeletal muscle
The circadian clocks in skeletal muscle and the GI tract have a very close relationship to the vascular clock previously mentioned. Work on the GI tract clock has mostly been done in smooth muscle and thus is relatable to the work discussed above on the vascular smooth muscle.
GI tract and the gut
Circadian clock genes were identified and shown to oscillate in smooth muscle cells as discussed in the vascular clock section. The focus in this section will be on the unique GI tract aspects that are affected by the circadian clock. Many gut-related phenomena, such as motility of the GI tract, acid secretion, maintenance of the mucosa, and digestive enzyme production, oscillate in a circadian rhythm; consequently, pathologies related to these processes, including acid reflux disease and ulcer flare-ups, have also been shown to oscillate (reviewed in ref. 51). Interestingly, and consistent with its regulation in the kidney, ENaC activity oscillates in the colon (42).
Ghrelin, a peptide hormone produced in the stomach that is responsible for the stimulation of hunger, exhibited rhythmic oscillation under constant feeding (52). Ghrelin and motilin are two of the hormones responsible for the generation of migrating motor complexes, which are the peristaltic waves in the GI tract that provide the locomotion of substances through the tract. Ghrelin is also involved in the generation of the segmental contractions in the small intestines and the colon. Whether the circadian clock is involved in these phenomena remains unclear. The study of these phenomena in circadian mutant mice should shed insight on the involvement of the circadian clock in these tissues.
Skeletal muscle
Circadian clock research in skeletal muscle is still in its infancy. Myoblast determination protein 1 homolog (MyoD) is a transcription factor involved in muscle differentiation and has recently been identified as a clock-controlled gene (53). A study in zebrafish implicated myogenic factor 6 (a regulator of MyoD) as a clock-controlled protein (54). Interestingly, microRNA miR-206 appears to be involved in the regulation of Bmal1, Clock, and MyoD in skeletal muscle. Using a systems modeling approach, it was shown that miR-206 deletion resulted in a slight decrease of Clock and Bmal1 amplitude of expression but resulted in significant changes in MyoD rhythmic expression (55). Further experimentation is required in order to fully understand the role of microRNA regulation of the circadian clock in skeletal muscle, although increasing evidence is being shown for the involvement of microRNAs in the post-transcriptional regulation of the circadian clock (discussed in ref. 56).
METABOLISM
The circadian clock in metabolism is one of the most studied areas in the field, outside of the central clock. Many of the general mechanistic understandings of the clock came from studying metabolic processes. It has also been known for some time that feeding cues synchronize several of the peripheral clocks, such as those in the liver, pancreas, kidney, and heart (57, 58). In most cases, the feeding cues are in synch with light-dark cycles; however, inversion of feeding times in rodents uncouples the feeding cues from the light-dark cycle (59). This effectively creates two separate rhythms. This phenomenon brought forth the concept of “two clocks,” the food clock and the light clock. Evidence from transcriptome analysis revealed that many rate-limiting enzymes in nearly all metabolic pathways are rhythmically expressed (60). A small-scale human study showed that ∼15% of the metabolites from both blood and saliva showed circadian oscillations (61). In mice, 34% of liver metabolites displayed circadian variation (62). Interestingly, recent work has highlighted the importance of the circadian clock in nitrogen metabolism. Klf15, which contributes to the circadian regulation of cardiac repolarization, as discussed above, also appears to be critical for circadian regulation of amino acid, urea, and ammonia metabolism (63). The rhythms for these metabolic processes were ablated in Klf15-KO mice. It was also demonstrated that serum levels of several amino acids, urea, and ornithine each displayed rhythmic oscillation in humans. It will be interesting to see whether Klf15 is involved in the circadian control of other physiological processes, as well. For the sake of brevity and because of the well-characterized role for the circadian clock in metabolism, the reader is directed to 2 reviews for further information (4, 64). The following section highlights the most recent advances in the field, specifically silent mating type information regulation 2 homologue 1 (SIRT1), the “NAD clock” and circadian disruptions and metabolic disorders.
SIRT1 and the NAD clock
The topics of SIRT1 and the role of NAD in the circadian metabolic clock are of particular interest. As with many of the peripheral clocks, unique mechanisms of circadian rhythmicity and control are not fully understood. However, in recent years research into the protein SIRT1 and the metabolite NAD have begun to shed additional light on the metabolic circadian clock.
SIRT1: a circadian regulator
SIRT1, also known as NAD-dependent deacetylase, plays an important role in nutrient availability, liver gluconeogenesis, fat breakdown (lipolysis), and insulin secretion (65). A recent review highlighted the connection between SIRT1 and the circadian clock (66). SIRT1 deacetylation activity was shown to oscillate, and SIRT1 was shown to physically interact with Clock and Bmal1, presumably suppressing their activity. SIRT1 ablation resulted in an overall increase in DBP and Per2 mRNA (60). The evidence for the physical interaction of SIRT1 with the circadian clock is striking; as mentioned previously, SIRT1 is involved in a plethora of metabolic processes and could be a viable “master regulator” of circadian metabolism. Additional investigation is needed in order to test this hypothesis.
NAD clock
As previously mentioned, feeding cycles can alter the metabolic circadian clock, but work has shown that NAD levels also affect the circadian clock. Clock-Bmal1 DNA binding activity is modulated by the ratio of NAD+/NADH (67). The rate-limiting enzyme in NAD biosynthesis is nicotinamide phosphoribosyltransferase (NAMPT); increased NAMPT results in increased synthesis of NAD, which then increases the activity of SIRT1. Notably, both NAMPT and NAD levels have been shown to oscillate in a circadian manner; this effect is lost in Clock- and Bmal1-mutant mice. Pharmacological inhibition of NAMPT resulted in increased Clock and Bmal1 levels, presumably through a SIRT1-dependent mechanism (68). This phenomenon of NAD-controlled SIRT1-mediated suppression of Clock and Bmal1 brings forth an interesting concept known as the NAD clock, where NAD levels modulate the levels of Clock and Bmal1. Whether this is a crucial element of circadian metabolism or contributes to how feeding cues are processed remains to be seen, but the evidence for its existence is compelling.
Circadian disruptions and diabetes
Chronobiologists have known for some time that circadian disturbances can lead to metabolic diseases, such as diabetes; however, not until the past decade have we begun to fully understand the link between the circadian clock and metabolic disorders, specifically diabetes. Other metabolic disorders, such as obesity, have been well characterized and discussed above. In this section, the focus will be on the circadian clock and its involvement in diabetes.
Interestingly, as researchers began to unravel the various phenotypes of each circadian protein mutant, it became clear that many of the circadian mutants displayed a variety of diabetic phenotypes. Bmal1- and Clock-KO mice have reduced insulin in the blood and are diabetic (69). Another study showed that HIP rats (rats that overexpress human islet amyloid polypeptide, leading to pancreatic β-cell failure) had accelerated diabetes onset on circadian disruption (constant light or shifting light/dark) (70). Another study showed that overexpression of Cry1 resulted in lower blood glucose concentrations and insulin sensitivity in db/db (mouse model for diabetes) mice (71), and consequently, mice expressing a mutant form of Cry (C414A mutation) have a diabetic phenotype, and mice with a double KO of Cry1/2 are hyperglycemic (72). Bmal1-KO mice have impaired glucose-stimulated insulin secretion (GSIS), due to an increase in mitochondrial uncoupling, caused by an up-regulation of mitochondrial uncoupling protein 2 (Ucp2; ref. 73). Transcriptome analysis of islets obtained from healthy and type 2 diabetic patients showed that Per2, Per3, and Cry2 were down-regulated in patients with type 2 diabetes (74). In mouse islet cells, Reverb-α knockdown resulted in impaired GSIS, decreased fatty acid synthase, and decreased sterol regulatory element-binding protein 1-c (SREBP-1c) (both critical enzymes/regulators in fat metabolism; ref. 75). It will be interesting to determine whether the varying circadian diabetic phenotypes are caused by similar mechanisms, such as GSIS with Bmal1 and Reverb-α, or whether other mechanisms are responsible.
ENDOCRINE, IMMUNE, AND REPRODUCTIVE SYSTEM
Endocrine system
Because the central clock is thought to synchronize the peripheral clocks in part through humoral factors, many researchers propose the endocrine system as a major regulatory component of the circadian clock. Thus, the endocrine system and its role in the secretion of hormones are crucial elements of circadian rhythms in the central and peripheral clocks. Melatonin and glucocorticoids have both been linked to peripheral clock regulation and are discussed below.
Melatonin
Melatonin is secreted from the pineal gland and other tissues during darkness, and levels are not affected by activity cycles (it is secreted in darkness for both diurnal and nocturnal animals). It is believed to be partially responsible for synchronization of the peripheral clocks by the central clock and behaves as a feedback mechanism for the SCN by inhibiting SCN firing (ref. 76; reviewed in ref. 77). Melatonin acts via binding to its 2 receptors, melatonin receptor 1 and 2. It has been known for decades that melatonin can entrain the clock (ref. 78; reviewed in ref. 79). Of particular note regarding melatonin and the peripheral clocks in a nonsynchronizing role is its involvement in inhibition of insulin secretion, specifically GSIS. Melatonin inhibits insulin-stimulated secretion through the secondary messenger cAMP (reviewed in ref. 80). As mentioned previously, Bmal1 and Reverb-α have been shown to increase GSIS (as their knockdown results in impairment). It would be interesting to see whether melatonin-, MT1-, or MT2-KO mice have a diabetes protective phenotype; however, rats with their pineal glands removed have a diabetic phenotype that was partially restored on melatonin supplementation (81). Whether this discrepancy is due to actions from the circadian clock genes remains to be seen, and further research is needed in order to further our understanding of the role of melatonin and the core clock genes in diabetes. Another study showed that melatonin treatment of human prostate cancer cell lines resulted in increased expression of Per2 and Clock and reduction of Bmal1; Per2 and DBP were found to be dysregulated in these cells. The physiological implications of these effects remain to be seen (82), but melatonin may have anticarcinogenic effects (reviewed in ref. 83).
Glucocorticoids
Glucocorticoids are a group of steroid hormones synthesized in the adrenal cortex that are involved in multiple functions, including inflammation, metabolism, and cardiovascular and neurological function (reviewed in ref. 84). These hormones have also been implicated in the synchronization of the central clock and may act in the peripheral clocks (85, 86). Much of the recent research concerning this class of steroid hormones has been conducted on the glucocorticoid receptor (GR). Of note, a study published in the middle of the last decade showed that Per1-KO mice exhibited increased levels of the glucorcorticoid corticosterone (87). It has been shown that GR interacts with and is repressed by Cry 1 and 2 (88). Cry1 and Cry2 are also involved in the modulation of dexamethasone-controlled genes, most likely due to their repressive effects on GR. Another study looked at the effect of dexamethasone on circadian gene expression in human osteoblast cells (89). Dexamethasone treatment induced Per1-3 and Bmal1 expression. Dexamethasone treatment in neutrophils and lymphocytes resulted in decreased expression of Clock, Cry1, and reverb-α and increased Per1 in neutrophils (90). In lymphocytes, expression of Per1 and Per2 was slightly increased on treatment. A convincing study showed that expression of the core circadian genes and their targets in the liver was synchronized following dexamethasone treatment; these effects were shown to be mediated by the nuclear receptor hepatocyte nuclear factor 4α (HNF4α; ref. 91). It would be of great interest to determine whether the glucocorticoid synchronization in other peripheral tissues is also HNF4 α mediated as this receptor is expressed in the kidney, heart, and other organs.
Immune system
As one of the more recently discovered peripheral clocks, research into the circadian clock in the immune system is rapidly expanding. Both glucocorticoids (92) and melatonin (93) have roles in the immune system and, as mentioned above, are critical players in the circadian clock. The core circadian clock genes were shown to oscillate in a circadian manner in mice splenic macrophages, dendritic cells, and B cells (94). Human lymphocyte population levels also oscillate (reviewed in ref. 95).
The most recent work has delved into the role of the circadian clock in the mounting of the immune response and the development of immunities. A convincing study demonstrated that Toll-like receptor 9 (TLR9) expression was rhythmic and was dysregulated in Per2-mutant animals in an immune tissue-specific manner (up-regulated in whole spleen, but down-regulated in splenic macrophages). The TLRs are responsible for identifying molecules from foreign microbes or pathogens; TLR9 recognizes unmethylated CpGs (found most abundantly in bacteria and viruses). TLR9 expression was reduced by Clock siRNA treatment in peritoneal macrophages. Mice treated at the peak of TLR9 expression with a TLR9 agonist exhibited a greater innate immune response. In a model of sepsis (extreme inflammatory state resulting in shock) mice had increased sepsis severity during the peak of TLR9 expression (96). It would be very interesting to look at other circadian mutant mouse models in order to determine whether any of the other TLRs are circadian controlled. Such investigation would help determine to what extent the innate immune response is controlled by the circadian clock. Another important study demonstrated circadian control of the T cell antigen response (97). It was shown that T-cell antigen response is limited in Clock-KO mice and that the downstream kinase ZAP70 is rhythmically expressed. Mice immunized during the day had a stronger T-cell response than mice immunized at night. E4 promoter-binding protein 4 (E4BP4), a circadian protein involved in negative regulation of DBP-controlled genes, positively regulates natural killer cell development, B-cell class switch to IgE, and T-cell response (reviewed in ref. 98). Rats who had their circadian clocks disrupted by shifted light-dark cycles displayed decreased natural killer cell cytolytic activity (99). Taken together, these studies provide substantial evidence for the involvement of the circadian clock in the immune response.
Reproductive system
The reproductive system is the most recently discovered peripheral clock and is perhaps the least understood. However, recent work has been done on both the male and female reproductive clocks and provides a foundation for our understanding of the ovarian and testicular clocks.
Ovarian clock
The various clock genes were shown to oscillate in the ovaries of rats, mice, and bovines (reviewed in ref. 100). A study published in 2006 showed the circadian variation of Per1 and Per2 expression in rat ovaries (101). Immunofluorescence studies demonstrated that intracellular localization of Per1 and Per2 was time dependent. Per1 was almost completely nuclear from ZT 0 to ZT 10 but was ubiquitously expressed at ZT 16. In contrast, Per2 was observed in the nucleus only after ZT 10 and remained mostly cytosolic until then. This study is one of the first to investigate time-dependent localization of the clock proteins in any tissue. It will be interesting to see whether the time-dependent intracellular localization of these clock proteins varies among the peripheral clocks. Although it has been shown that the circadian clock is present in the ovaries, the physiological implications are not understood, though there is growing evidence for the involvement of the circadian clocks in ovulation as SCN lesions result in disruption of luteinizing hormone secretion, ovulation, and estrous cycling in rats (reviewed in ref. 100). One study showed that Bmal1-KO male and female mice are infertile due to decreased sex steroid hormone production (102); however, others have shown that Bmal1-KO mice are fertile but have delayed estrous cycles and implantation defects (103). Further work is needed in order to sort out these conflicting results and to further elucidate the mechanism of the circadian clock in the ovary.
Testicular clock
Ironically, there is evidence that the testis is one of the few tissues that does not contain a circadian clock (104); however, this study only examined Per1 and Bmal1 transcripts. The clock could act through another mechanism involving other clock genes. Another report showed that in the testicular clock in hamsters, Bmal1 levels were constant, but Per1 expression oscillated (105). They also concluded that testicular function, specifically spermatogenesis, is not circadian. Clearly, more work is needed in order to fully test the presence of a testicular clock.
CONCLUSIONS
The peripheral clocks are an integral and important part of physiology. It is common knowledge that the various systems of the body can communicate with one another, whether through hormonal cascades, metabolites, or other circulating factors. However, one of the interesting debates and questions in the circadian field regards the mechanism of cross-communication between the peripheral clocks and the central clock, and the balance of control between them. The peripheral clocks appear to share the same core clock machinery but have unique and tissue specific gene targets. Mechanisms such as SIRT1 and the NAD clock have been identified in the liver and heart, specifically in the metabolic clocks; it will be interesting to see whether this is a shared mechanism between the peripheral clocks. It remains to be seen whether the NAD clock is the mechanism by which feeding cues are recognized.
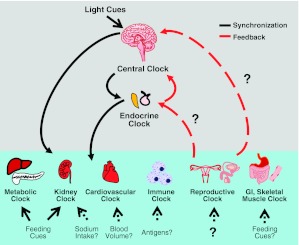
A synchronization/feedback loop almost certainly exists between the endocrine clock and the central clock, but is that also true for the central clock and the other peripheral clocks? Perhaps the peripheral clocks feed back to the endocrine clock, which then behaves as a messenger and gatekeeper of the circadian clock. In support of this concept, it has become apparent that the endocrine clock, via glucocorticoids, plays a substantial role in the synchronization of the peripheral clocks. There is evidence that the peripheral clocks cannot autonomously keep synchronization in hamster livers (106) but can autonomously oscillate in fibroblasts (107), and that cells require hormonal signaling or SCN input in order to oscillate (reviewed in ref. 4). However, as mentioned previously, there is evidence for synchronization aside from light (mediated by the SCN) with feeding cycles for the metabolic clock. It is tempting to speculate that there are other cues that could synchronize the peripheral clocks (Fig. 3). Perhaps each peripheral clock requires a tissue-specific input, such as salt intake for the kidney or antigen exposure for the immune clock. However, it should be noted that salt intake occurs during active cycles, making it difficult to separate peripheral clock inputs from the entrainment of the central clock. In years to come, researchers will continue to examine the interplay between the central and peripheral clocks and their roles in physiological processes. Such investigation should shed significant light on the role of the circadian clock in disease states.
Figure 3.
Connection between the central and peripheral clocks. Model for communication between the central clock, the endocrine clock, and the other peripheral clocks. The central clock processes light cues and synchronizes the peripheral clocks. The endocrine clock, through neurohormonal action, provides feedback to the central clock. The endocrine clock also synchronizes the peripheral clocks through the actions of glucocorticoids and melatonin. Potential feedback loops for the peripheral clocks are shown as dotted red lines. Feeding cues synchronize the metabolic and kidney clocks. Dotted arrows represent hypothetical synchronizers for the other peripheral clocks.
Acknowledgments
This work was supported by U.S. National Institutes of Health (NIH) grant DK085193 and University of Florida, Division of Nephrology, funds to M.L.G., and NIH grant T32 DK-07518 to J.R.
Footnotes
- AKT
- v-akt urine thymoma viral oncogene homolog 1
- ARB
- angiotensin II receptor blocker
- AT1a
- angiotensin type 1a
- BMAL1
- brain and muscle Arnt-like protein-1
- CCM
- cardiomyocyte-specific circadian clock mutant
- CK
- casein kinase
- CK1δ/ε
- casein kinase 1 δ/ε
- CLOCK
- circadian locomotor output cycles kaput
- Cry
- cryptochrome
- DBP
- D-box binding protein
- CVD
- cardiovascular disease
- ENaC
- epithelial sodium channel
- GI
- gastrointestinal
- GR
- glucocorticoid receptor
- GSIS
- glucose-stimulated insulin secretion
- GSK
- glycogen synthase kinase
- HIF1α
- hypoxia inducible factor 1α
- HNF4α
- hepatocyte nuclear factor 4α
- I/R
- ischemia/reperfusion
- Klf15
- Krüppel-like factor 15
- KO
- knockout
- MAPEC
- Ambulatory Blood Pressure Monitoring for Prediction of Cardiovascular Events
- MyoD
- myoblast determination protein 1 homolog
- NAMPT
- nicotinamide phosphoribosyltransferase
- PAI-1
- plasminogen activator inhibitor 1
- Per
- period
- PI3K
- phosphoinositide 3-kinase
- PPARα
- peroxisome proliferator-activated receptor α
- PPARγ
- peroxisome proliferator-activated receptor γ
- REV-ERB
- nuclear receptor subfamily 1, group D, member 1 (NR1D1)
- ROR
- retinoid-related orphan receptor
- SCN
- suprachiasmatic nucleus
- SIRT1
- silent mating type information regulation 2 homolog 1
- TLR9
- toll-like receptor 9
- TTO
- transcription translation oscillating
- WT
- wild type
- ZT
- zeitgeiber time
REFERENCES
- 1. Konopka R. J., Benzer S. (1971) Clock mutants of Drosophila melanogaster. Proc. Natl. Acad. Sci. U. S. A. 68, 2112–2116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tei H., Okamura H., Shigeyoshi Y., Fukuhara C., Ozawa R., Hirose M., Sakaki Y. (1997) Circadian oscillation of a mammalian homologue of the Drosophila period gene. Nature 389, 512–516 [DOI] [PubMed] [Google Scholar]
- 3. Albrecht U. (2004) The mammalian circadian clock: a network of gene expression. Front. Biosci. 9, 48–55 [DOI] [PubMed] [Google Scholar]
- 4. Dibner C., Schibler U., Albrecht U. (2010) The mammalian circadian timing system: organization and coordination of central and peripheral clocks. Annu. Rev. Physiol. 72, 517–549 [DOI] [PubMed] [Google Scholar]
- 5. Gekakis N., Staknis D., Nguyen H. B., Davis F. C., Wilsbacher L. D., King D. P., Takahashi J. S., Weitz C. J. (1998) Role of the CLOCK protein in the mammalian circadian mechanism. Science 280, 1564–1569 [DOI] [PubMed] [Google Scholar]
- 6. Zhang E. E., Kay S. A. (2010) Clocks not winding down: unravelling circadian networks. Nat. Rev. Mol. Cell Biol. 11, 764–776 [DOI] [PubMed] [Google Scholar]
- 7. Virshup D. M., Eide E. J., Forger D. B., Gallego M., Harnish E. V. (2007) Reversible protein phosphorylation regulates circadian rhythms. Cold Spring Harbor Symp. Quant. Biol. 72, 413–420 [DOI] [PubMed] [Google Scholar]
- 8. Sprouse J., Reynolds L., Kleiman R., Tate B., Swanson T. A., Pickard G. E. (2010) Chronic treatment with a selective inhibitor of casein kinase I delta/epsilon yields cumulative phase delays in circadian rhythms. Psychopharmacology (Berl.) 210, 569–576 [DOI] [PubMed] [Google Scholar]
- 9. Vielhaber E., Eide E., Rivers A., Gao Z. H., Virshup D. M. (2000) Nuclear entry of the circadian regulator mPER1 is controlled by mammalian casein kinase I epsilon. Mol. Cell. Biol. 20, 4888–4899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lee H. M., Chen R., Kim H., Etchegaray J. P., Weaver D. R., Lee C. (2011) The period of the circadian oscillator is primarily determined by the balance between casein kinase 1 and protein phosphatase 1. Proc. Natl. Acad. Sci. U. S. A. 108, 16451–16456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Agarwal R. (2010) Regulation of circadian blood pressure: from mice to astronauts. Curr. Opin. Nephrol. Hypertension 19, 51–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Agarwal R., Light R. P. (2010) The effect of measuring ambulatory blood pressure on nighttime sleep and daytime activity—implications for dipping. Clin. J. Am. Soc. Nephrol. 5, 281–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Larochelle P. (2002) Circadian variation in blood pressure: dipper or nondipper. J. Clin. Hypertens. (Greenwich) 4, 3–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Despotovic N. (2002) [Circadian rhythm of blood pressure and obesity–blood pressure variation and obesity]. Med. Pregl. 55, 419–421 [DOI] [PubMed] [Google Scholar]
- 15. Kaneko M., Zechman F. W., Smith R. E. (1968) Circadian variation in human peripheral blood flow levels and exercise responses. J. Appl. Physiol. 25, 109–114 [DOI] [PubMed] [Google Scholar]
- 16. Panza J. A., Epstein S. E., Quyyumi A. A. (1991) Circadian variation in vascular tone and its relation to alpha-sympathetic vasoconstrictor activity. New Engl. J. Med. 325, 986–990 [DOI] [PubMed] [Google Scholar]
- 17. Hossmann V., Fitzgerald G. A., Dollery C. T. (1980) Circadian rhythm of baroreflex reactivity and adrenergic vascular response. Cardiovasc. Res. 14, 125–129 [DOI] [PubMed] [Google Scholar]
- 18. Takeda N., Maemura K., Horie S., Oishi K., Imai Y., Harada T., Saito T., Shiga T., Amiya E., Manabe I., Ishida N., Nagai R. (2007) Thrombomodulin is a clock-controlled gene in vascular endothelial cells. J. Biol. Chem. 282, 32561–32567 [DOI] [PubMed] [Google Scholar]
- 19. Schoenhard J. A., Smith L. H., Painter C. A., Eren M., Johnson C. H., Vaughan D. E. (2003) Regulation of the PAI-1 promoter by circadian clock components: differential activation by BMAL1 and BMAL2. J. Mol. Cell. Cardiol. 35, 473–481 [DOI] [PubMed] [Google Scholar]
- 20. Paschos G. K., FitzGerald G. A. (2010) Circadian clocks and vascular function. Circ. Res. 106, 833–841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wang N., Yang G., Jia Z., Zhang H., Aoyagi T., Soodvilai S., Symons J. D., Schnermann J. B., Gonzalez F. J., Litwin S. E., Yang T. (2008) Vascular PPARgamma controls circadian variation in blood pressure and heart rate through Bmal1. Cell Metab. 8, 482–491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wang C., Xu C. X., Krager S. L., Bottum K. M., Liao D. F., Tischkau S. A. (2011) Aryl hydrocarbon receptor deficiency enhances insulin sensitivity and reduces PPAR-alpha pathway activity in mice. Environ. Health Perspect. 119, 1739–1744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Costa M. J., So A. Y., Kaasik K., Krueger K. C., Pillsbury M. L., Fu Y. H., Ptacek L. J., Yamamoto K. R., Feldman B. J. (2011) Circadian rhythm gene period 3 is an inhibitor of the adipocyte cell fate. J. Biol. Chem. 286, 9063–9070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hermida R. C., Ayala D. E., Mojon A., Fernandez J. R. (2010) Influence of circadian time of hypertension treatment on cardiovascular risk: results of the MAPEC study. Chronobiol. Int. 27, 1629–1651 [DOI] [PubMed] [Google Scholar]
- 25. Stow L. R., Gumz M. L. (2011) The circadian clock in the kidney. J. Am. Soc. Nephrol. 22, 598–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zuber A. M., Centeno G., Pradervand S., Nikolaeva S., Maquelin L., Cardinaux L., Bonny O., Firsov D. (2009) Molecular clock is involved in predictive circadian adjustment of renal function. Proc. Natl. Acad. Sci. U. S. A. 106, 16523–16528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nikolaeva S., Pradervand S., Centeno G., Zavadova V., Tokonami N., Maillard M., Bonny O., Firsov D. (2012) The circadian clock modulates renal sodium handling. [E-pub ahead of print] J. Am. Soc. Nephrol. doi: 10.1681/ASN.2011080842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nishinaga H., Komatsu R., Doi M., Fustin J. M., Yamada H., Okura R., Yamaguchi Y., Matsuo M., Emoto N., Okamura H. (2009) Circadian expression of the Na+/H+ exchanger NHE3 in the mouse renal medulla. Biomed. Res. 30, 87–93 [DOI] [PubMed] [Google Scholar]
- 29. Doi M., Takahashi Y., Komatsu R., Yamazaki F., Yamada H., Haraguchi S., Emoto N., Okuno Y., Tsujimoto G., Kanematsu A., Ogawa O., Todo T., Tsutsui K., van der Horst G. T., Okamura H. (2010) Salt-sensitive hypertension in circadian clock-deficient Cry-null mice involves dysregulated adrenal Hsd3b6. Nat. Med. 16, 67–74 [DOI] [PubMed] [Google Scholar]
- 30. Gumz M. L., Stow L. R., Lynch I. J., Greenlee M. M., Rudin A., Cain B. D., Weaver D. R., Wingo C. S. (2009) The circadian clock protein Period 1 regulates expression of the renal epithelial sodium channel in mice. J. Clin. Invest. 119, 2423–2434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gumz M. L., Cheng K. Y., Lynch I. J., Stow L. R., Greenlee M. M., Cain B. D., Wingo C. S. (2010) Regulation of alphaENaC expression by the circadian clock protein Period 1 in mpkCCD(c14) cells. Biochim. Biophys. Acta 1799, 622–629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Stow L. R., Richards J., Cheng K. Y., Lynch I. J., Jeffers L. A., Greenlee M. M., Cain B. D., Wingo C. S., Gumz M. L. (2012) The circadian protein period 1 contributes to blood pressure control and coordinately regulates renal sodium transport genes. Hypertension 59, 1151–1156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Feener E. P., Northrup J. M., Aiello L. P., King G. L. (1995) Angiotensin II induces plasminogen activator inhibitor-1 and -2 expression in vascular endothelial and smooth muscle cells. J. Clin. Invest. 95, 1353–1362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Masuda Y., Emoto N., Nonaka H., Yagita K., Todo T., Okamura H., Yokoyama M., Hirata K. (2009) Role of angiotensin and the clock system in the circadian regulation of plasminogen activator inhibitor-1. Kobe J. Med. Sci. 54, E264–271 [PubMed] [Google Scholar]
- 35. Fukuda M., Wakamatsu-Yamanaka T., Mizuno M., Miura T., Tomonari T., Kato Y., Ichikawa T., Miyagi S., Shirasawa Y., Ito A., Yoshida A., Kimura G. (2011) Angiotensin receptor blockers shift the circadian rhythm of blood pressure by suppressing tubular sodium reabsorption. Am. J. Physiol. 301, F953–957 [DOI] [PubMed] [Google Scholar]
- 36. Polonia J., Diogo D., Caupers P., Damasceno A. (2003) Influence of two doses of irbesartan on non-dipper circadian blood pressure rhythm in salt-sensitive black hypertensives under high salt diet. J. Cardiovasc. Pharmacol. 42, 98–104 [DOI] [PubMed] [Google Scholar]
- 37. Uzu T., Kimura G. (1999) Diuretics shift circadian rhythm of blood pressure from nondipper to dipper in essential hypertension. Circulation 100, 1635–1638 [DOI] [PubMed] [Google Scholar]
- 38. Durgan D. J., Young M. E. (2010) The cardiomyocyte circadian clock: emerging roles in health and disease. Circ. Res. 106, 647–658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Durgan D. J., Trexler N. A., Egbejimi O., McElfresh T. A., Suk H. Y., Petterson L. E., Shaw C. A., Hardin P. E., Bray M. S., Chandler M. P., Chow C. W., Young M. E. (2006) The circadian clock within the cardiomyocyte is essential for responsiveness of the heart to fatty acids. J. Biol. Chem. 281, 24254–24269 [DOI] [PubMed] [Google Scholar]
- 40. Ko G. Y., Shi L., Ko M. L. (2009) Circadian regulation of ion channels and their functions. J. Neurochem. 110, 1150–1169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Jeyaraj D., Haldar S. M., Wan X., McCauley M. D., Ripperger J. A., Hu K., Lu Y., Eapen B. L., Sharma N., Ficker E., Cutler M. J., Gulick J., Sanbe A., Robbins J., Demolombe S., Kondratov R. V., Shea S. A., Albrecht U., Wehrens X. H., Rosenbaum D. S., Jain M. K. (2012) Circadian rhythms govern cardiac repolarization and arrhythmogenesis. Nature 483, 96–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wang Q., Maillard M., Schibler U., Burnier M., Gachon F. (2010) Cardiac hypertrophy, low blood pressure, and low aldosterone levels in mice devoid of the three circadian PAR bZip transcription factors DBP, HLF, and TEF. Am. J. Physiol. 299, R1013–1019 [DOI] [PubMed] [Google Scholar]
- 43. Takeda N., Maemura K. (2011) Circadian clock and cardiovascular disease. J. Cardiol. 57, 249–256 [DOI] [PubMed] [Google Scholar]
- 44. Durgan D. J., Pulinilkunnil T., Villegas-Montoya C., Garvey M. E., Frangogiannis N. G., Michael L. H., Chow C. W., Dyck J. R., Young M. E. (2010) Short communication: ischemia/reperfusion tolerance is time-of-day-dependent: mediation by the cardiomyocyte circadian clock. Circ. Res. 106, 546–550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Reiter R., Swingen C., Moore L., Henry T. D., Traverse J. H. (2012) Circadian dependence of infarct size and left ventricular function after ST elevation myocardial infarction. Circ. Res. 110, 105–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Fournier S., Eeckhout E., Mangiacapra F., Trana C., Lauriers N., Beggah A. T., Monney P., Cook S., Bardy D., Vogt P., Muller O. (2012) Circadian variations of ischemic burden among patients with myocardial infarction undergoing primary percutaneous coronary intervention. Am. Heart J. 163, 208–213 [DOI] [PubMed] [Google Scholar]
- 47. Eckle T., Hartmann K., Bonney S., Reithel S., Mittelbronn M., Walker L. A., Lowes B. D., Han J., Borchers C. H., Buttrick P. M., Kominsky D. J., Colgan S. P., Eltzschig H. K. (2012) Adora2b-elicited Per2 stabilization promotes a HIF-dependent metabolic switch crucial for myocardial adaptation to ischemia. Nat. Med. 18, 774–782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Fujino Y., Iso H., Tamakoshi A., Inaba Y., Koizumi A., Kubo T., Yoshimura T. (2006) A prospective cohort study of shift work and risk of ischemic heart disease in Japanese male workers. Am. J. Epidemiol. 164, 128–135 [DOI] [PubMed] [Google Scholar]
- 49. Lieu S. J., Curhan G. C., Schernhammer E. S., Forman J. P. (2012) Rotating night shift work and disparate hypertension risk in African-Americans. J. Hypertens. 30, 61–66 [DOI] [PubMed] [Google Scholar]
- 50. Monk T. H. (2000) What can the chronobiologist do to help the shift worker? J. Biol. Rhythms 15, 86–94 [DOI] [PubMed] [Google Scholar]
- 51. Konturek P. C., Brzozowski T., Konturek S. J. (2011) Gut clock: implication of circadian rhythms in the gastrointestinal tract. J. Physiol. Pharmacol. 62, 139–150 [PubMed] [Google Scholar]
- 52. LeSauter J., Hoque N., Weintraub M., Pfaff D. W., Silver R. (2009) Stomach ghrelin-secreting cells as food-entrainable circadian clocks. Proc. Natl. Acad. Sci. U. S. A. 106, 13582–13587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Zhang X., Patel S. P., McCarthy J. J., Rabchevsky A. G., Goldhamer D. J., Esser K. A. (2012) A non-canonical E-box within the MyoD core enhancer is necessary for circadian expression in skeletal muscle. Nucleic Acids Res. 40, 3419–3430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Amaral I. P., Johnston I. A. (2012) Circadian expression of clock and putative clock-controlled genes in skeletal muscle of the zebrafish. Am. J. Physiol. 302, R193–206 [DOI] [PubMed] [Google Scholar]
- 55. Zhou W., Li Y., Wang X., Wu L., Wang Y. (2011) MiR-206-mediated dynamic mechanism of the mammalian circadian clock. BMC Syst. Biol. 5, 141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kojima S., Shingle D. L., Green C. B. (2011) Post-transcriptional control of circadian rhythms. J. Cell Sci. 124, 311–320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Damiola F., Le Minh N., Preitner N., Kornmann B., Fleury-Olela F., Schibler U. (2000) Restricted feeding uncouples circadian oscillators in peripheral tissues from the central pacemaker in the suprachiasmatic nucleus. Genes Dev. 14, 2950–2961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Wu T., Ni Y., Dong Y., Xu J., Song X., Kato H., Fu Z. (2010) Regulation of circadian gene expression in the kidney by light and food cues in rats. Am. J. Physiol. 298, R635–R641 [DOI] [PubMed] [Google Scholar]
- 59. Stokkan K. A., Yamazaki S., Tei H., Sakaki Y., Menaker M. (2001) Entrainment of the circadian clock in the liver by feeding. Science 291, 490–493 [DOI] [PubMed] [Google Scholar]
- 60. Storch K. F., Lipan O., Leykin I., Viswanathan N., Davis F. C., Wong W. H., Weitz C. J. (2002) Extensive and divergent circadian gene expression in liver and heart. Nature 417, 78–83 [DOI] [PubMed] [Google Scholar]
- 61. Dallmann R., Viola A. U., Tarokh L., Cajochen C., Brown S. A. (2012) The human circadian metabolome. Proc. Natl. Acad. Sci. U. S. A. 109, 2625–2629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Eckel-Mahan K. L., Patel V. R., Mohney R. P., Vignola K. S., Baldi P., Sassone-Corsi P. (2012) Coordination of the transcriptome and metabolome by the circadian clock. Proc. Natl. Acad. Sci. U. S. A. 109, 5541–5546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Jeyaraj D., Scheer F. A., Ripperger J. A., Haldar S. M., Lu Y., Prosdocimo D. A., Eapen S. J., Eapen B. L., Cui Y., Mahabeleshwar G. H., Lee H. G., Smith M. A., Casadesus G., Mintz E. M., Sun H., Wang Y., Ramsey K. M., Bass J., Shea S. A., Albrecht U., Jain M. K. (2012) Klf15 orchestrates circadian nitrogen homeostasis. Cell Metab. 15, 311–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Bass J., Takahashi J. S. (2010) Circadian integration of metabolism and energetics. Science 330, 1349–1354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Schug T. T., Li X. (2011) Sirtuin 1 in lipid metabolism and obesity. Ann. Med. 43, 198–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Imai S. (2010) “Clocks” in the NAD World: NAD as a metabolic oscillator for the regulation of metabolism and aging. Biochim. Biophys. Acta 1804, 1584–1590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Rutter J., Reick M., Wu L. C., McKnight S. L. (2001) Regulation of clock and NPAS2 DNA binding by the redox state of NAD cofactors. Science 293, 510–514 [DOI] [PubMed] [Google Scholar]
- 68. Ramsey K. M., Yoshino J., Brace C. S., Abrassart D., Kobayashi Y., Marcheva B., Hong H. K., Chong J. L., Buhr E. D., Lee C., Takahashi J. S., Imai S., Bass J. (2009) Circadian clock feedback cycle through NAMPT-mediated NAD+ biosynthesis. Science 324, 651–654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Marcheva B., Ramsey K. M., Buhr E. D., Kobayashi Y., Su H., Ko C. H., Ivanova G., Omura C., Mo S., Vitaterna M. H., Lopez J. P., Philipson L. H., Bradfield C. A., Crosby S. D., JeBailey L., Wang X., Takahashi J. S., Bass J. (2010) Disruption of the clock components CLOCK and BMAL1 leads to hypoinsulinaemia and diabetes. Nature 466, 627–631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Gale J. E., Cox H. I., Qian J., Block G. D., Colwell C. S., Matveyenko A. V. (2011) Disruption of circadian rhythms accelerates development of diabetes through pancreatic beta-cell loss and dysfunction. J. Biol. Rhythms 26, 423–433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Zhang E. E., Liu Y., Dentin R., Pongsawakul P. Y., Liu A. C., Hirota T., Nusinow D. A., Sun X., Landais S., Kodama Y., Brenner D. A., Montminy M., Kay S. A. (2010) Cryptochrome mediates circadian regulation of cAMP signaling and hepatic gluconeogenesis. Nat. Med. 16, 1152–1156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Tanida M., Yamatodani A., Niijima A., Shen J., Todo T., Nagai K. (2007) Autonomic and cardiovascular responses to scent stimulation are altered in cry KO mice. Neurosci. Lett. 413, 177–182 [DOI] [PubMed] [Google Scholar]
- 73. Lee J., Kim M. S., Li R., Liu V. Y., Fu L., Moore D. D., Ma K., Yechoor V. K. (2011) Loss of Bmal1 leads to uncoupling and impaired glucose-stimulated insulin secretion in beta-cells. Islets 3, 381–388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Stamenkovic J. A., Olsson A. H., Nagorny C. L., Malmgren S., Dekker-Nitert M., Ling C., Mulder H. (2012) Regulation of core clock genes in human islets. [E-pub ahead of print] Metabolism doi: 10.1016/j.metabol.2011.11.013 [DOI] [PubMed] [Google Scholar]
- 75. Vieira E., Marroqui L., Batista T. M., Caballero-Garrido E., Carneiro E. M., Boschero A. C., Nadal A., Quesada I. (2012) The clock gene Rev-erbalpha regulates pancreatic beta-cell function: modulation by leptin and high-fat diet. Endocrinology 153, 592–601 [DOI] [PubMed] [Google Scholar]
- 76. Liu C., Weaver D. R., Jin X., Shearman L. P., Pieschl R. L., Gribkoff V. K., Reppert S. M. (1997) Molecular dissection of two distinct actions of melatonin on the suprachiasmatic circadian clock. Neuron 19, 91–102 [DOI] [PubMed] [Google Scholar]
- 77. Prasai M. J., Pernicova I., Grant P. J., Scott E. M. (2011) An endocrinologist's guide to the clock. J. Clin. Endocrinol. Metab. 96, 913–922 [DOI] [PubMed] [Google Scholar]
- 78. Redman J., Armstrong S., Ng K. T. (1983) Free-running activity rhythms in the rat: entrainment by melatonin. Science 219, 1089–1091 [DOI] [PubMed] [Google Scholar]
- 79. Challet E. (2007) Minireview: Entrainment of the suprachiasmatic clockwork in diurnal and nocturnal mammals. Endocrinology 148, 5648–5655 [DOI] [PubMed] [Google Scholar]
- 80. Peschke E., Muhlbauer E. (2010) New evidence for a role of melatonin in glucose regulation. Best Pract. Res. Clin. Endocrinol. Metab. 24, 829–841 [DOI] [PubMed] [Google Scholar]
- 81. La Fleur S. E., Kalsbeek A., Wortel J., van der Vliet J., Buijs R. M. (2001) Role for the pineal and melatonin in glucose homeostasis: pinealectomy increases night-time glucose concentrations. J. Neuroendocrinol. 13, 1025–1032 [DOI] [PubMed] [Google Scholar]
- 82. Jung-Hynes B., Huang W., Reiter R. J., Ahmad N. (2010) Melatonin resynchronizes dysregulated circadian rhythm circuitry in human prostate cancer cells. J. Pineal. Res. 49, 60–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Bukowska A. (2011) [Anticarcinogenic role of melatonin—potential mechanisms]. Med. Pr. 62, 425–434 [PubMed] [Google Scholar]
- 84. Chung S., Son G. H., Kim K. (2011) Circadian rhythm of adrenal glucocorticoid: its regulation and clinical implications. Biochim. Biophys. Acta 1812, 581–591 [DOI] [PubMed] [Google Scholar]
- 85. Balsalobre A., Brown S. A., Marcacci L., Tronche F., Kellendonk C., Reichardt H. M., Schutz G., Schibler U. (2000) Resetting of circadian time in peripheral tissues by glucocorticoid signaling. Science 289, 2344–2347 [DOI] [PubMed] [Google Scholar]
- 86. Son G. H., Chung S., Kim K. (2011) The adrenal peripheral clock: glucocorticoid and the circadian timing system. Front. Neuroendocrinol. 32, 451–465 [DOI] [PubMed] [Google Scholar]
- 87. Dallmann R., Touma C., Palme R., Albrecht U., Steinlechner S. (2006) Impaired daily glucocorticoid rhythm in Per1 (Brd) mice. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 192, 769–775 [DOI] [PubMed] [Google Scholar]
- 88. Lamia K. A., Papp S. J., Yu R. T., Barish G. D., Uhlenhaut N. H., Jonker J. W., Downes M., Evans R. M. (2011) Cryptochromes mediate rhythmic repression of the glucocorticoid receptor. Nature 480, 552–556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Komoto S., Kondo H., Fukuta O., Togari A. (2012) Comparison of beta-adrenergic and glucocorticoid signaling on clock gene and osteoblast-related gene expressions in human osteoblast. Chronobiol. Int. 29, 66–74 [DOI] [PubMed] [Google Scholar]
- 90. Nebzydoski S. J., Pozzo S., Nemec L., Rankin M. K., Gressley T. F. (2010) The effect of dexamethasone on clock gene mRNA levels in bovine neutrophils and lymphocytes. Vet. Immunol. Immunopathol. 138, 183–192 [DOI] [PubMed] [Google Scholar]
- 91. Reddy A. B., Maywood E. S., Karp N. A., King V. M., Inoue Y., Gonzalez F. J., Lilley K. S., Kyriacou C. P., Hastings M. H. (2007) Glucocorticoid signaling synchronizes the liver circadian transcriptome. Hepatology 45, 1478–1488 [DOI] [PubMed] [Google Scholar]
- 92. Franchimont D. (2004) Overview of the actions of glucocorticoids on the immune response: a good model to characterize new pathways of immunosuppression for new treatment strategies. Ann. N. Y. Acad. Sci. 1024, 124–137 [DOI] [PubMed] [Google Scholar]
- 93. Radogna F., Diederich M., Ghibelli L. (2010) Melatonin: a pleiotropic molecule regulating inflammation. Biochem. Pharmacol. 80, 1844–1852 [DOI] [PubMed] [Google Scholar]
- 94. Silver A. C., Arjona A., Hughes M. E., Nitabach M. N., Fikrig E. (2011) Circadian expression of clock genes in mouse macrophages, dendritic cells, and B cells. Brain Behav. Immun. 26, 407–413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Mazzoccoli G., Southern R. B., De Cata A., Giuliani F., Fontana A., Copetti M., Pellegrini F., Tarquini R. (2011) A timetable of 24-hour patterns for human lymphocyte subpopulations. J. Biol. Regul. Homeost. Agents 25, 387–395 [PubMed] [Google Scholar]
- 96. Silver A. C., Arjona A., Walker W. E., Fikrig E. (2012) The circadian clock controls toll-like receptor 9-mediated innate and adaptive immunity. Immunity 36, 251–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Fortier E. E., Rooney J., Dardente H., Hardy M. P., Labrecque N., Cermakian N. (2011) Circadian variation of the response of t cells to antigen. J. Immunol. 187, 6291–6399 [DOI] [PubMed] [Google Scholar]
- 98. Male V., Nisoli I., Gascoyne D. M., Brady H. J. (2011) E4BP4: an unexpected player in the immune response. Trends Immunol. 33, 98–102 [DOI] [PubMed] [Google Scholar]
- 99. Logan R. W., Zhang C., Murugan S., O'Connell S., Levitt D., Rosenwasser A. M., Sarkar D. K. (2012) Chronic shift-lag alters the circadian clock of NK cells and promotes lung cancer growth in rats. J. Immunol. 188, 2583–2591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Sellix M. T., Menaker M. (2010) Circadian clocks in the ovary. Trends Endocrinol. Metab. 21, 628–636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Fahrenkrug J., Georg B., Hannibal J., Hindersson P., Gras S. (2006) Diurnal rhythmicity of the clock genes Per1 and Per2 in the rat ovary. Endocrinology 147, 3769–3776 [DOI] [PubMed] [Google Scholar]
- 102. Alvarez J. D., Hansen A., Ord T., Bebas P., Chappell P. E., Giebultowicz J. M., Williams C., Moss S., Sehgal A. (2008) The circadian clock protein BMAL1 is necessary for fertility and proper testosterone production in mice. J. Biol. Rhythms 23, 26–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Ratajczak C. K., Boehle K. L., Muglia L. J. (2009) Impaired steroidogenesis and implantation failure in Bmal1−/− mice. Endocrinology 150, 1879–1885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Morse D., Cermakian N., Brancorsini S., Parvinen M., Sassone-Corsi P. (2003) No circadian rhythms in testis: Period1 expression is clock independent and developmentally regulated in the mouse. Mol. Endocrinol. 17, 141–151 [DOI] [PubMed] [Google Scholar]
- 105. Klose M., Grote K., Lerchl A. (2011) Temporal control of spermatogenesis is independent of the central circadian pacemaker in Djungarian hamsters (Phodopus sungorus). Biol. Reprod. 84, 124–129 [DOI] [PubMed] [Google Scholar]
- 106. Guo H., Brewer J. M., Lehman M. N., Bittman E. L. (2006) Suprachiasmatic regulation of circadian rhythms of gene expression in hamster peripheral organs: effects of transplanting the pacemaker. J. Neurosci. 26, 6406–6412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Balsalobre A., Damiola F., Schibler U. (1998) A serum shock induces circadian gene expression in mammalian tissue culture cells. Cell 93, 929–937 [DOI] [PubMed] [Google Scholar]