Abstract
A-kinase anchoring proteins (AKAPs) have emerged as important regulatory molecules that can compartmentalize cAMP signaling transduced by β2-adrenergic receptors (β2ARs); such compartmentalization ensures speed and fidelity of cAMP signaling and effects on cell function. This study aimed to assess the role of AKAPs in regulating global and compartmentalized β2AR signaling in human airway smooth muscle (ASM). Transcriptome and proteomic analyses were used to characterize AKAP expression in ASM. Stable expression or injection of peptides AKAP-IS or Ht31 was used to disrupt AKAP-PKA interactions, and global and compartmentalized cAMP accumulation stimulated by β-agonist was assessed by radioimmunoassay and membrane-delineated flow through cyclic nucleotide-gated channels, respectively. ASM expresses multiple AKAP family members, with gravin and ezrin among the most readily detected. AKAP-PKA disruption had minimal effects on whole-cell cAMP accumulation stimulated by β-agonist (EC50 and Bmax) concentrations, but significantly increased the duration of plasma membrane-delineated cAMP (τ=251±51 s for scrambled peptide control vs. 399±79 s for Ht31). Direct PKA inhibition eliminated decay of membrane-delineated cAMP levels. AKAPs coordinate compartmentalized cAMP signaling in ASM cells by regulating multiple elements of β2AR-mediated cAMP accumulation, thereby representing a novel target for manipulating β2AR signaling and function in ASM.—Horvat, S. J., Deshpande, D. A., Yan, H., Panettieri, R. A., Codina, J., DuBose Jr., T. D., Xin, W., Rich, T. C., Penn, R. B. A-kinase anchoring proteins regulate compartmentalized cAMP signaling in airway smooth muscle.
Keywords: asthma, β2-adrenergic receptor, phosphodiesterase, gravin
In many cell types, β-agonist stimulation of the β2-adrenergic receptor (β2AR) Gs-adenylyl cyclase transmembrane signaling cascade generates the second messenger cAMP. cAMP, in turn, activates the cAMP-dependent protein kinase A (PKA), as well as the exchange protein activated by cAMP (Epac), which regulate numerous cell functions. In airway smooth muscle (ASM), these functions include contraction, cell proliferation, and cytokine production. Via their capacity to prevent or reverse ASM contraction in vivo, β-agonists are the drug of choice for treatment of acute bronchospasm in asthmatic subjects (1).
How β2ARs and other Gs-coupled receptors function and are regulated has been an area of intense investigation for more than 30 years. More recently, studies have demonstrated that cAMP generation as well as its downstream signaling can be highly compartmentalized within the cell. Although several cellular mechanisms underlie cAMP compartmentalization, A-kinase anchoring proteins (AKAPs) appear to play a central role (2–4). AKAP-mediated localization of receptors, adenylyl cyclase (AC), phosphodiesterases (PDEs), PKA, and phosphatases ensures both speed and fidelity within the cAMP signaling pathway while limiting the spatial spread of cAMP signals. Thus, AKAPs represent a potential therapeutic target, given their important role in regulating Gs-coupled receptor signaling and function.
Previous studies of AKAPs have been conducted in a variety of cell and tissue types, including heart (5, 6), brain (7), and T cells (8). In addition to information on individual AKAP family members (of which currently >50 are known; ref. 9), these studies all support the conclusion that specific AKAPs are important in specific cell types. In the current study, we identify those AKAPs expressed in human ASM (HASM) and assess their role in β2AR-mediated cAMP accumulation.
MATERIALS AND METHODS
Materials
Purified RII and catalytic PKA subunits were provided by Susan Taylor (University of California, San Diego, CA, USA). Constructs encoding AKAP in silico (AKAP-IS) and Ht31 were provided by Carmen Dessauer (University of Texas, Houston, TX, USA) and Meredith Bond (University of Maryland, Baltimore, MD, USA), respectively. The antibody for gravin was provided by Irwin Gelman (Roswell Park Cancer Institute, Buffalo, NY, USA). The antibody for AKAP3 was provided by Sarah Fielder (Portland Veterans Affairs Medical Center, Portland, OR, USA), and the AKAP9 antibody was provided by Carmen Dessauer. Antibodies against MAP2B and AKAP79 were purchased from BD Biosciences (San Jose, CA, USA). The AKAP2 antibody was purchased from Novus Biologicals (Littleton, CO, USA). The antibody for ezrin was purchased from Neo Markers (Fremont, CA, USA). IRDye 680 or 800 secondary antibodies were from Rockland (Gilbertsville, PA, USA). All other materials were obtained from Sigma (St. Louis, MO, USA) or from previously identified sources.
Cell culture
Human ASM cultures were established from human tracheae as described previously (10). Third- to fifth-passage cells, naive or stably selected after retroviral infection as described below, were plated at a density of 104 cells/cm2 in either 24-well (cAMP radioimmunoassay) or 6-well plates (immunoblots) and maintained in Ham's F-12 medium supplemented with 10% fetal bovine serum (FBS). At 24 h prior to stimulation, cells were arrested in Ham's F-12 medium supplemented with 0.1% bovine serum albumin (BSA).
Assessment of AKAP mRNA
Microarray data previously generated (11) were analyzed for relative expression of various AKAPs. In addition, AKAP expression in HASM cells was analyzed by real-time PCR. Briefly, HASM cells were grown to near confluence in 60-mm dishes and washed before isolation of RNA using TRIzol (Invitrogen, Grand Island, NY, USA), followed by chloroform addition. RNA was resuspended in nuclease-free water and converted to cDNA, and real-time RT-PCR was performed via the TaqMan system (Applied Biosystems, Carlsbad, CA, USA). The cycle threshold (Ct) was calculated with Applied Biosystems 7300 software.
RII-overlay assay
Radiolabeling of purified bovine brain RII-β subunits was achieved by incubation of 1 μg RII-β with 1 μg C subunit in 25 μl (final volume) of 50 mM MOPS (pH 6.8), containing 5 μl [γ-32P]ATP (3000 Ci/mmol), 20 mM NaCl, 2 mM MgCl2, and 1 mM dithiothreitol. After 15 min incubation at 37°C, the reaction was stopped by placing the tube on ice and separating the free ATP from the bound ATP using a prepacked disposable PD-10 desalting column (GE Heathcare, Piscataway, NJ, USA). The 32P-labeled RII-β was labeled to a specific activity of ∼1 mol 32P incorporated to each mol of RII-β. The 32P-labeled RII preparation contained a single radiolabeled polypeptide with an apparent molecular weight of 55,000.
Proteins in cell lysates were separated by SDS-PAGE, transferred to nitrocellulose, and blocked overnight at 4°C. The filters were then incubated with 32P-labeled RII-β (0.3×106 cpm/ml) in fresh Blotto and PBS for 4–6 h at room temperature. Next, the filters were washed 3 times with Blotto and BSA for 15 min. Binding of 32P-labeled RII-β to proteins was visualized by autoradiography using Kodak XAR-5 film (Eastman Kodak, Rochester, NY, USA).
Immunoblotting
Tissue lysates were prepared by grinding whole-tissue samples in a glass homogenizer cooled with liquid N2. RIPA buffer was added, and the tissue was ground again to homogenize further. Samples were sonicated thoroughly, and a portion of this lysate was removed as the whole-tissue lysate. The remainder was centrifugated at 5000 rpm for 5 min at 4°C. The supernatant was removed and stored separately from the pellet. Pellets were resuspended in RIPA buffer prior to electrophoresis and immunoblotting as below.
Cultured HASM cells were grown to near confluence in 6-well plates, and growth was arrested for 24 h in serum-free Ham's F-12 and 0.1% BSA as described above. Cells were lysed as reported previously (12), sonicated briefly, then electrophoresed on 10% SDS-polyacrylamide gels (4–15% gels for analysis of AKAP9), transferred to nitrocellulose membranes, and subsequently probed with the indicated primary antibodies and secondary antibodies conjugated with infrared fluorophores as reported previously (13).
Retroviral expression of AKAP-disrupting peptides
Stable expression of a yellow fluorescent protein (YFP) chimera of AKAP-IS (14), Ht31 (15), or a scrambled (SCR) peptide control was achieved by retroviral infection, as described previously (10). Briefly, constructs encoding YFP chimeras of SCR peptide, AKAP-IS, or Ht31 were subcloned into the retroviral vector pLNCX2. Retrovirus was produced by cotransfecting GP2–293 cells with pVSV-G vector (encoding the pantropic VSV-G envelope protein) and either pLNCX2-SCR-YFP, pLNCX2-AKAP-IS-YFP, or pLNCX2-Ht31-YFP. At 48 h after transfection, supernatants were harvested and used to infect human ASM cultures, with effective virus concentrations established by immunoblot analysis. Cultures were selected to homogeneity (typically >95% YFP positive, as demonstrated in ref. 12) with 250 μg/ml G418. Stable lines expressing YFP alone exhibited properties similar to those of uninfected naive cells with respect to mitogen-stimulated DNA synthesis and cell proliferation, as reported previously (16).
Cellular cAMP accumulation
Human ASM cells stably expressing SCR-YFP, AKAP-IS-YFP, or Ht31-YFP were grown to near confluence in 24-well plates as above and were stimulated in PBS containing vehicle, 1 mM RO-20-1724, or 1 mM isobutylmethylxanthine (IBMX); and vehicle, 50 nM and 1 μM isoproterenol (ISO), or 100 μM forskolin (FSK) for 10 min. Stimulation was terminated by aspiration of medium and quenching with 100% cold ethanol, after which cAMP was isolated and quantified by radioimmunoassay as reported previously (17).
Adenoviral expression of CNG channels
Expression of cyclic nucleotide-gated (CNG) channels was achieved using adenoviral constructs as described previously (18). HASM cultures were infected with adenovirus expressing either green fluorescent protein (AdGFP) or a mutant CNG channel containing the double mutation C460W/E583M (AdCNG). This double mutation enhances both the selectivity and sensitivity of the channel to cAMP (19). Viral titer was determined on all stocks using the Adeno-X kit (BD Biosciences). HASM cells were infected at matched multiplicities of infection of 20 for patch-clamp recordings. Experiments were performed 2–3 d after infection.
CNG channel measurement of compartmentalized cAMP
Near-membrane cAMP signals were measured as described previously (3, 4, 18). Briefly, measurements were made using an Heka EPC10 patch-clamp amplifier (Heka Elektronik, Lambrecth/Pfalz, Germany) in whole-cell configuration. To ensure adequate voltage control, pipette resistance was limited to 4 MΩ and averaged 2.5 ± 0.1 MΩ, and access resistance was 5.7 ± 0.3 MΩ (n=31). Voltage offsets were zeroed with the pipette in the bath solution; no additional corrections were made for the liquid junction potential difference. After achieving whole-cell configuration, the preparation was allowed to equilibrate >10 min. Current records were sampled at 10 kHz and filtered at 2 kHz, and recorded during 400-ms steps to a membrane potential of +30 mV from a holding potential of 0 mV. Cells in which the baseline current was above 200 pA were not included in the analysis. Baseline current was most likely due to both leak current and current through CNG channels induced by basal cAMP levels near the plasma membrane. The pipette solution contained 140 mM KCl, 0.5 mM MgCl2, 10 mM HEPES, 5 mM Na2ATP, and 0.5 mM Na2GTP (pH 7.4). In indicated experiments, either 20 nM PKI or 10 μM stearated Ht31 (st-Ht31) was added to the pipette solution. This approach allowed us to precisely control the intracellular concentrations of these peptides (not possible with overexpression or treatment with membrane permeant peptides). The bath was continually perfused with solution containing 140 mM NaCl, 4 mM KCl, 10 mM d-glucose, 10 mM HEPES, and either 0.1 or 10 mM MgCl2 (pH 7.4), with an exchange time of ∼30 s. Extracellular solutions containing ISO or a PDE inhibitor cocktail [PDE-I, containing 100 μM (final) 8MM-IBMX (relatively selective for PDE1), 10 μM cilostamide (PDE3), and 10 μM rolipram (PDE4)] were applied using the SF-77B fast-step solution switcher (Warner Instruments, Hamden, CT, USA), with an exchange time between 0.06 and 0.1 s. Electrophysiological data presented are representative of ≥4 experiments. Data were converted to formats compatible with MATLAB software using a custom script provided by Bruxton Corp. (Seattle, WA, USA).
Equations describing the β2 adrenergic signaling pathway in ASM cells
To better understand the effects of localization of PKA, and thus localized PKA activity, we have modeled β2AR-mediated signaling in ASM cells. We used a model framework developed previously (3, 4) with two important extensions: the model presented here incorporates both low- and high-affinity PDEs and incorporates PKA-mediated inhibition of cAMP production. The latter may be due to either PKA-mediated receptor desensitization or inhibition of AC activity; however, for parsimony we have modeled inhibition of AC activity, because at the high (saturating) agonist concentrations used in this study, G-protein-coupled receptor kinase (GRK)-mediated receptor desensitization is more prevalent (20). These modifications resulted in the following changes to the system of equations:
| (1) |
| (2) |
| (3) |
| (4) |
| (5) |
| (6) |
| (7) |
where AC0 represents basal AC activity, AC0p represents phosphorylated basal AC activity, kPKA is the rate constant of PKA-mediated phosphorylation, kppAC is the rate constant of AC dephosphorylation, [PKI] is the concentration of PKA inhibitory peptide, KI-PKI is the inhibition constant for PKI, ACsyn represents the rate of ISO-mediated AC activity, ACsynp represents phosphorylated AC activity, [C] is the concentration of free (activated) PKA catalytic subunit, [GsGTP] is the concentration of activated G protein, KGsAC is the equilibrium constant between activated G protein and AC, EAC is the cAMP synthesis rate, EPDE is the total cAMP hydrolysis rate, [PDEha] and [PDEla] are the concentrations of high and low affinity PDEs, Kmha and Kmla are the Michaelis constants for the high and low affinity PDEs, KI is the inhibition constant for both PDEs, kPDEha and kPDEla are the hydrolysis rate constants for unphosphorylated high and low affinity PDEs, and kPDEhap and kPDElap are the hydrolysis rate constants for phosphorylated high- and low-affinity PDEs.
The full set of equations describes GRK-mediated desensitization of β2ARs, as well as receptor turnover. The equations also describe PKA-mediated stimulation of both high- and low-affinity PDE4 activities and PKA-mediated inhibition of AC activity. The effects of AKAP disruptors (Ht31s) on PKA-mediated regulation of AC and PDE activities were described by lowering the local PKA concentrations (analogous to dislodging R subunits from AKAPs). Initial conditions were obtained by setting the basal adenylyl cyclase activity and letting the system run to equilibrium (without stimulating receptor activity). Equations were coded and simulations conducted in the MATLAB programming environment using Runge Kutta solvers.
Statistical analysis
Data are presented as mean ± se values from n experiments, in which each experiment was performed using a different culture derived from a unique donor. Individual data points from a single cAMP radioimmunoassay experiment were calculated as the mean value from replicate observations. Statistically significant differences among groups were assessed either by ANOVA with Fisher's post hoc analysis, t test, or by t test for paired samples (as appropriate) with P < 0.05 sufficient to reject the null hypothesis.
RESULTS
AKAP expression in HASM was first assessed utilizing microarray data previously generated from 3 different HASM cultures (21). AKAP1, AKAP10–AKAP13, AKAP2, and ezrin all generated consistent present calls; the strongest signals were observed for AKAP1, AKAP12, AKAP2, MAP2B, and ezrin (Supplemental Fig. S1). AKAP3, AKAP4, and AKAP79 were consistently absent. Those AKAPs with positive array signals in HASM were investigated further using real-time PCR. Each of the AKAPs examined was present in HASM cultures to some extent, with the majority (AKAP2, AKAP10, AKAP12, AKAP13, and ezrin) exhibiting values of ΔCt < 7 (Table 1). Gravin (AKAP12) and ezrin were the most readily detected based on these data.
TABLE 1.
Investigation of AKAP isoform expression by real-time PCR
| Isoform | Ct | Ct GAPDH | ΔCt | n |
|---|---|---|---|---|
| AKAP1 | 26.40 ± 1.10 | 18.18 ± 1.24 | 8.22 ± 1.66 | 10 |
| AKAP2 | 25.00 ± 1.14 | 18.18 ± 1.24 | 6.82 ± 1.68 | 10 |
| AKAP10 | 24.09 ± 0.56 | 17.37 ± 0.84 | 6.72 ± 1.01 | 6 |
| AKAP11 | 25.53 ± 1.14 | 18.18 ± 1.24 | 7.35 ± 1.68 | 10 |
| AKAP12 | 20.80 ± 1.29 | 18.18 ± 1.24 | 2.62 ± 1.79 | 10 |
| AKAP13 | 24.34 ± 1.34 | 18.18 ± 1.24 | 6.16 ± 1.83 | 10 |
| Ezrin | 23.06 ± 1.44 | 18.18 ± 1.24 | 4.88 ± 1.90 | 10 |
| MAP2B | 25.16 ± 1.02 | 17.37 ± 0.84 | 7.79 ± 1.32 | 6 |
Multiple HASM cultures were analyzed for mRNA levels of the indicated AKAPs. Data are presented as mean ± se values.
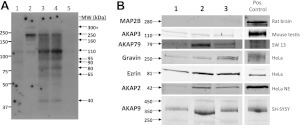
As a first means of assessing AKAP protein expression in human ASM, RII-overlay assays were performed using tissue and cell lysates derived from 3 different HASM samples. Tissue lysates were prepared and run as whole-tissue lysates, supernatant, or pellet; corresponding cultures of cells derived from the tissue were also run. A representative overlay (Fig. 1A) suggested ≥9 different AKAPs present in HASM. The molecular masses of the various overlay bands suggested the presence of AKAP9 (350 kDa), AKAP12/gravin (250 kDa), AKAP3 (110 kDa), AKAP2 (42 kDa), ezrin (81 kDa), and AKAP79 (79 kDa). Based on this information, combined with the array and PCR data, expression of selected AKAPs was investigated via immunoblotting of HASM cell lysates derived from 3 separate cultures (Fig. 1B). Gravin, ezrin, AKAP2, AKAP3, AKAP79, and AKAP9 (each of the 250, 350, and 450 kDa isoforms) were readily identified. MAP2B was also present in at least one of the lysates, though at very low levels.
Figure 1.
AKAP identification in HASM by RII-overlay assay and immunoblotting. A) Identification of AKAPs in HASM by RII-overlay assay of primary cultures (lanes 1 and 2; passages 3 and 4, respectively), whole-tissue lysate (lane 3), tissue supernatant (lane 4), and tissue pellet (lane 5) fractions. Bands that were likely to be AKAPs and their approximate molecular weight are indicated. B) Identification of various AKAPs by Western blot analyses in cell lysates prepared from 3 different HASM lines. Positive controls used for each AKAP are indicated.
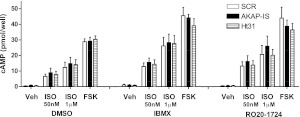
The signaling consequences of AKAP disruption were investigated utilizing HASM cultures stably expressing the pan-AKAP inhibitor peptides AKAP-IS or Ht31. AKAP-IS was designed using computer-aided optimization of the binding helix based on the PKA-binding regions of several AKAPs (14). This peptide binds preferentially to PKA-RII and thus prevents PKA docking on various AKAP scaffolds. Ht31 is a short peptide derived from the PKA-binding amphipathic helix of AKAP-Lbc (15) and inhibits PKA docking to AKAPs similarly to AKAP-IS. Whole-cell cAMP accumulation was assessed in response to FSK (an activator of AC independent of receptor activation) and approximate EC50 (50 nM) and maximal (1 μM) concentrations of ISO, each in the presence of either vehicle, the broad PDE inhibitor IBMX, or the PDE4-specific RO-20-1724 (Fig. 2). No significant effect of AKAP-IS or Ht31 expression on vehicle-, ISO-, or FSK-stimulated cAMP accumulation was observed under any conditions. The most prominent effect was observed between cells expressing SCR peptide and those expressing AKAP-IS or Ht31 occurred under the conditions of 50 nM and 1μM ISO stimulation without addition of the PDE inhibitor, in which AKAP-disrupting peptides increased cAMP accumulation by ∼20%. The variance in these data combined with the small experimental effect contributed to the lack of statistical significance.
Figure 2.
Agonist-induced global cAMP accumulation and effects of AKAP disruption. Multiple HASM lines were infected with retrovirus enabling expression of scramble peptide (SCR) or the AKAP disrupting peptides AKAP-IS or Ht31. Global cAMP accumulation was measured after 10 min stimulation with ISO (50 nM or 1 μM), or FSK (100 μM). Experiments were done in the absence or presence of the broad PDE inhibitor IMBX (1 mM) or the selective PDE4 inhibitor RO 20-1724 (1 mM). Data are presented as mean ± se values, n = 5.
Recent studies have provided data indicating that AKAP-mediated localization of PKA is critical for the proposed regulation of near-membrane cAMP signals in human embryonic kidney 293 (HEK293) cells (2–4, 22). To investigate the roles of AKAP-PKA interactions in regulation of near-membrane cAMP signals in HASM cells, we utilized adenovirus-mediated expression of C460W/E583M CNG channels as described previously (19, 23). cAMP binding triggers a conformational change that leads to an increase in CNG channel activity, which was monitored using the whole-cell patch-clamp technique. This approach allows detection of cAMP signals with minimal effect on the cAMP signals being measured. Specifically, near-membrane cAMP levels are readily detected by 100–2000 ion channels. At these low expression levels, CNG channels have minimal cAMP buffering capacity, and therefore do not substantively alter free cAMP levels (24). Also, in the experimental conditions used in this study, little or no Ca2+ influx occurs through CNG channels (18, 23, 25). In addition, CNG channels do not appear to be localized by protein–protein interactions. Rather, they appear to preferentially sequester into lipid rafts via lipid interactions (26). Thus, it is unlikely that overexpression of CNG channels substantively altered the composition of localized signaling complexes in ASM cells.
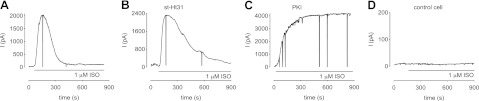
We observed that in ASM cells stimulation with 1 μM ISO triggered a transient increase in CNG channel activity (width at half height of 251±51 s, n=10; Fig. 3A). This increase in CNG channel activity reflects an underlying transient increase in near-membrane cAMP levels. To investigate the roles of localized PKA in regulating cAMP signals, we included either 10 μM st-Ht31 or 20 nM PKI in the patch pipette. We observed that ISO-induced cAMP signals remained transient with the inclusion of st-Ht31 in the patch pipette (Fig. 3B). However, the durations of transient signals (width at half height of 399±79 s, P < 0.05, n=6) were significantly longer than those observed in control cells. In contrast, direct inhibition of PKA with 20 nM PKI (a saturating concentration included in the patch pipette) completely ablated the decay of cAMP signals over the observed timeframe (Fig. 3C). Little or no ISO-induced current was observed in control cells (Fig. 3D). In a subset of experiments, we included the control peptide st-Ht31p in the patch pipette solution. st-Ht31p is a peptide similar to st-Ht31, except that it contains an isoleucine to proline substitution that disrupts the amphipathic helix and binding to PKA-RII (27). Under these conditions, the responses were similar (data not shown) to those from experiments with standard solution in the patch pipette (e.g., Fig. 3A). These data suggest that PKA activity contributes to the decline in transient, ISO-induced cAMP signals, and that this decline is at least in part regulated by an AKAP-localized pool of PKA.
Figure 3.
Effect of altering localized PKA activity of ISO-induced near-membrane cAMP signals in HASM cells. Localized cAMP signals were measured using C460W/E583M CNG channels as described in the text. A) ISO (1 μM) triggered transient activation of CNG channels, indicating a transient increase in near-membrane cAMP signals. Width at half height of this experiment is 167 s. B) Inclusion of 10 μM st-Ht31 in the patch pipette slowed the decay of transient cAMP signals. Width at half height of this experiment is 292 s. C) Inclusion of 20 nM PKI effectively prevented decays in cAMP signals. D) Response of a cell transfected with adenovirus encoding GFP to ISO. Little or no current was observed. Trace is representative of 7 experiments.
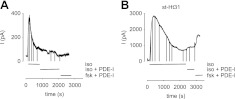
Previous studies from our group (12, 28, 29) suggest that PKA-mediated inhibition of G-protein-coupled receptor and AC activities regulates the rate of cAMP production in HASM cells. Thus, we sought to determine whether localization of PKA activity contributed to the rate of near-membrane cAMP accumulation using the following experimental protocol: C460W/E583M channel-expressing HASM cells were exposed to 1 μM ISO. Following ISO-triggered transient cAMP responses, cells were exposed to both ISO and the PDE inhibitor cocktail, PDE-I (Fig. 4). Following the initial ISO-induced transient cAMP response, exposure of cells to these inhibitors had little or no effect on CNG channel activity or the underlying cAMP signal (Fig. 4A). Similarly, subsequent exposure to 10 μM FSK and the PDE inhibitor cocktail triggered little or no increase in CNG channel activity. However, when 10 μM st-Ht31 was included in the pipette solution, exposure of cells to the PDE inhibitor cocktail triggered an increase in CNG channel activity. Subsequent exposure to 10 μM FSK further increased CNG channel activity. PDE inhibitors have no direct effect on CNG channel activity (19). Thus, the observed increase in CNG channel activity was likely due to an increase in near-membrane cAMP levels. To assess the data, we compared the ratio of the peak current to both ISO and FSK and the PDE inhibitor cocktails to the peak current in the presence of ISO alone. When st-Ht31 was included in the pipette solution, the ratio of peak currents was 0.16 ± 0.04 and 0.39 ± 0.09 for ISO and FSK, respectively; yet when st-Ht31 was absent from the pipette solution, the ratio of peak currents was significantly smaller (0.003±0.002 and 0.03±0.02 for ISO and FSK, P<0.05). Little or no response was observed in control cells (cells expressing GFP rather than CNG channels) in response to ISO or FSK in the presence of PDE inhibitors (data not shown). These data indicate that the PDE inhibitor cocktail revealed a sustained AC activity observable when PKA-AKAP interactions are disrupted. As such, these data indicate that β2AR activity, AC activity, or both are inhibited by a localized pool of PKA.
Figure 4.
Effect of disruption of AKAP-PKA interactions and PDE inhibitors on ISO-triggered near-membrane cAMP signals in HASM cells. Near-membrane cAMP signals were monitored using C460W/E583M CNG channels, as described in Materials and Methods. A) Rapid exposure of a single cell to ISO triggered a transient cAMP signal. Subsequent application of a PDE inhibitor cocktail [PDE-I: 100 μM 8-methoxy methyl IBMX (8MM-IBMX), 10 μM cilostamide, and 10 μM rolipram] triggered little or no increase in cAMP levels. Similarly, exposure to both 10 μM FSK and PDE-I triggered a further increase in CNG channel activity. B) ISO-triggered response of a single cell with 10 μM st-Ht31 in the whole-cell patch pipette solution. Subsequent exposure to ISO and PDE-I caused an increase in cAMP levels that reached a steady plateau. Exposure to 10 μM FSK and PDE-I caused an additional increase in CNG channel activity.
To examine potential contributions of localized PKA activity on cAMP synthesis and hydrolysis in ASM cells, we adapted computational models developed to describe prostaglandin- and β2AR-mediated cAMP signals (3, 4). These models considered the roles of PKA-mediated stimulation of PDE4 activity and β2AR desensitization in providing negative feedback in the cAMP pathway. The model presented here has been expanded to include PKA-mediated inhibition of cAMP production and hydrolysis by both high- and low-affinity PDEs. A schematic of the model is depicted in Fig. 5A. In this model, stimulation of β2ARs with 1 μM ISO triggers activation of Gαs and subsequent activation of AC. AC produces cAMP, and increasing cAMP concentrations activate localized PKA activity. PKA stimulates both low- and high-affinity PDE activities (Fig. 5A, solid green arrows) and inhibits AC activity (Fig. 5A, solid red arrows). GRK-mediated phosphorylation triggers β2AR desensitization (Fig. 5A, red arrow). PKA may also inhibit β2ARs by direct phosphorylation (Fig. 5A, dashed red arrow) or by stimulating GRK/arrestin-mediated receptor desensitization (Fig. 5A, dashed green arrow). The latter reactions are not considered in this model, because their potential contributions to the time course of cAMP signals cannot be differentiated from PKA-mediated inhibition of AC activity and GRK-mediated β2AR desensitization.
Figure 5.
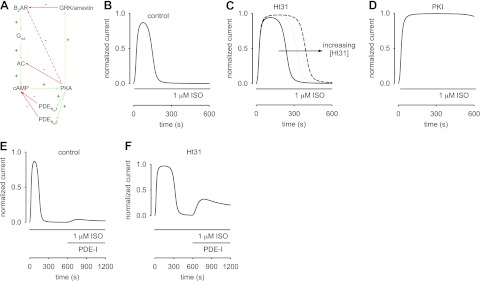
Mathematical modeling of β2AR-mediated cAMP signaling in ASM cells. A) Schematic of β2AR signaling pathway. Ligand binding to β2ARs triggers activation of Gαs, subsequent activation of AC, and increased cAMP production. Increased cAMP levels activate PKA, which in turn phosphorylates AC and both low- and high-affinity PDEs. Phosphorylation triggers a reduction in AC activity and an increase in both PDE activities. GRK-mediated desensitization lowers β2AR activity. It is likely that PKA activity triggers β2AR desensitization, and it may also trigger increased GRK activity (dashed arrows). The latter reactions are not described in the models, because their effects are not readily distinguished from PKA-mediated inhibition of AC activity. Red lines indicate inhibitory reactions; green lines indicate stimulatory reactions. B–F) Simulations of cAMP-mediated CNG channel activity. B) Simulated response of an ASM cell to 1 μM ISO. CNG channel response is similar to the response depicted in Fig. 3A. C) Simulated response to 1 μM ISO with 10 μM Ht31 in the patch pipette. Solid, dotted, and dashed lines represent simulations in which there is a 2-fold, 3-fold, and 5-fold reduction in local PKA activity, respectively. Based on these simulations, we estimate that 10 μM Ht31 causes a 2- to 3-fold reduction in local PKA activity. D) Simulated response to 1 μM ISO with 20 nM PKI in the patch pipette. The presence of 20 nM PKI causes a marked increase in cAMP accumulation and saturation of the CNG channel response. E, F) ISO was present throughout the simulation, and PDE inhibitors (PDE-I) were at concentrations 10-fold KI. E) In the control condition, PDE-I induced small currents through CNG channels. F) However, when the local PKA activity was reduced 3-fold (analogous to including Ht31 in the patch pipette), PDE-I caused a substantial cAMP-mediated increase in currents through CNG channels. In this model, the peak of the PDE-I induced currents is primarily dictated by the level of GRK activity. These simulations describe the experimental data presented in Fig. 4. Parameter values used to describe near-membrane cAMP signals: [βtot], total β2AR concentration, 1.4 μM; ACsyn, stimulated cAMP synthesis rate, 15 nM/s; Vmax1-PDE and Vmax2-PDE, maximal hydrolysis rate of unphosphorylated high- and low-affinity PDEs, 50 nM/s, Vmax1-PDEp and Vmax2-PDEp, maximal hydrolysis rate of phosphorylated high- and low-affinity PDEs, 150 nM/s, Km1 and Km2, Michaelis constants for high- and low-affinity PDEs, 0.2 and 2.0 μM; kPKA, rate constant of PKA-mediated phosphorylation, 0.01 μM−1 · s−1; kppAC, rate constant of AC dephosphorylation, 0.0005 s−1; kppPDE, rate constant of PDE dephosphorylation, 0.005 s−1. Parameters were manually fit to data. Other model parameters are as described in refs. 3 4 and the references therein.
Model simulations (Fig. 5B–D) well describe the transient time course of ISO-induced cAMP signals reflected in data from Figs. 3 and 4. The decay in the transient cAMP response is due to a decrease in the rate of cAMP synthesis (receptor desensitization and PKA-mediated inhibition of AC activity) as well as an increase in the rate of hydrolysis (a PKA-mediated increase in the Vmax of both high- and low-affinity PDE activities). To better understand how localization of PKA activity potentiates inhibition of cAMP synthesis and stimulation of cAMP hydrolysis, we modeled the effects of the AKAP disrupter Ht31 by decreasing local concentrations of PKA. Simulations indicate that reductions in local PKA (due to increasing Ht31 concentrations) cause a broadening of the cAMP response. This broadening is due to reduced PKA-mediated feedback onto both AC and PDE activities, and is consistent with the increase in the width at half height observed in Ht31-treated ASM cells (Fig. 3B). Interestingly, model simulations indicate that 3-fold reduction in the local concentration of PKA is required to describe the observed increase in the width at half height of cAMP signals in the presence of Ht31. This indicates that in ASM cells AKAPs cause a 3-fold increase in the local PKA concentration. This estimated increase in local PKA concentration is similar to the experimentally estimated 4-fold increase in local PKC concentration caused by binding to AKAP79 (30). Simulations also indicate that PKI-mediated inhibition of PKA activity allows ISO-induced cAMP levels to accumulate to levels that saturate CNG channels, similar to the data presented in Fig. 3C.
We next used the model to examine effects of PDE inhibitors on ISO-induced cAMP signals. Simulations indicate that following transient, ISO-induced cAMP signals, application of PDE inhibitors at intracellular concentrations of 10*KI caused a minimal increase in cAMP induced current through CNG channels, indicating that little cAMP synthesis remained following a ten minute exposure to 1 μM ISO (Fig. 5E). This condition was primarily due to GRK-mediated receptor desensitization as well as PKA-mediated inhibition of cAMP production and residual PDE activity (competitive PDE inhibitors were used). In contrast, when the local concentration of PKA was reduced (analogous to treatment with Ht31), treatment with PDE inhibitors caused a substantial increase in cAMP-mediated CNG channel activity (Fig. 5F). In the presence of Ht31, the peak of the PDE inhibitor-induced signal was lower than the initial ISO-induced peak, primarily due to GRK-mediated receptor desensitization. These simulation results are similar to the experimental results presented in Fig. 4.
While model simulations faithfully describe most experimental observations, they underestimate the slowing of decay in transient cAMP signals in the presence of increasing Ht31 (Fig. 5C). Specifically, Ht31 causes a parallel shift in the decay rather than a shift and a slowing of the time course. In the simulations, the parallel shifts in the decay in cAMP signals due to Ht31 are primarily due to the level of GRK-mediated receptor desensitization. This level of desensitization is required to blunt residual AC activity in response to ISO and PDE inhibitors (Fig. 5E) and shifts the balance of AC and PDE activity such that cAMP signals rapidly decline to baseline. Other feedback mechanisms not considered in the models presented here are also likely to be involved in the regulation of cAMP signals, including feedback from Gi signaling and Ca2+ signaling pathways. Thus, a careful balance between multiple feedback mechanisms is required to modulate near membrane cAMP signaling in ASM cells.
DISCUSSION
The current study demonstrates that multiple AKAPs are expressed in human ASM and disruption of AKAP binding with the regulatory subunit of PKA affects membrane-delineated cAMP accumulation mediated by β-agonist. This disruption translates into a reduction in compartmentalized PKA activity that in turn regulates multiple elements of β2AR-mediated cAMP accumulation, including the β2AR, adenylyl cyclase, and PDE activities. Accordingly, AKAPs represent a novel pharmacological target for manipulating β2AR signaling and function in ASM.
Using a combination of overlay assays, mRNA analyses, and immunoblotting, several AKAPs were observed to be expressed in HASM, including AKAP2, AKAP3, AKAP79, AKAP9, gravin, ezrin, and MAP2B. The high expression of gravin (AKAP12) and ezrin is of particular interest due to the potential roles of these AKAPs in β2AR internalization and recycling. Association of gravin with the β2AR is dynamic and is induced on agonist binding of the receptor; gravin is then internalized with receptor and dissociates on recycling of the receptor (31). Knockdown of gravin in HEK293 and A431 cells abolishes recycling of the β2AR and largely blunts recovery of the cAMP response to ISO following agonist washout (32). Ezrin is a known substrate for GRK2, and this phosphorylated, active form of ezrin colocalizes with internalized β2AR (33). Ezrin is also required for agonist-induced endocytosis of the receptor (33). Thus, gravin and ezrin possess multiple qualities that can regulate β2AR signaling, recycling, and resensitization in ASM. Disruption of AKAP-RII association by peptides did not significantly increase β-agonist-stimulated total (whole-cell) cAMP accumulation. However, analyses of membrane-delineated cAMP accumulation demonstrated that AKAP disruption caused a prolonged β-agonist induced cAMP signal within this compartment. A more profound effect was observed with direct inhibition of PKA, which resulted in a sustained level of membrane-delineated cAMP with no decay observed over the examined time frame.
The modest effect of AKAP disruption on global cAMP accumulation may be explained by the compartmentalized nature of AKAP-related signaling. Although not statistically significant, differences in β-agonist-stimulated whole cell cAMP accumulation between control and AKAP-IS or Ht31-expressing cells where greatest under conditions of no phosphodiesterase inhibition. This is potentially attributable to the loss of PKA-mediated PDE activation in AKAP-disrupted cells, as PKA is known to phosphorylate and activate certain PDE isoforms, including PDE4, in other cell types (34, 35). Gravin specifically has been implicated in the activation of subplasma membrane PDE in HEK293 cells (2). Although AKAPs may serve to localize PDE to reduce cAMP accumulation in specific subcellular domains, cAMP that diffuses away or escapes the spatial constraint is not necessarily subject to degradation. Thus, the net effect of AKAP disruption on total cellular cAMP accumulation may be rather modest, as suggested by our data.
Thus, we examined the effects of AKAP disruption on local cAMP flux, specifically constrained near the plasma membrane. Evidence of AKAP-mediated inhibition of cAMP synthesis in HASM cells was supported by the differences in near-membrane cAMP levels in the presence or absence of st-HT31 monitored with CNG channels. The effects of disrupting AKAP/PKA interactions on the rate of cAMP synthesis were particularly evident when cells were exposed to the PDE inhibitor cocktail following the ISO-induced transient cAMP responses. Increased cAMP synthesis was likely due to decreased β2AR desensitization and decreased negative feedback on AC activity (12). Disruption of AKAP/PKA interactions is also likely to have reduced PKA-mediated stimulation of PDE4 activity. It is likely that both an increase in cAMP production and a decrease in cAMP hydrolysis contribute to the prolonged cAMP signals observed when st-Ht31 was included in the pipette solution. Future studies are required to demonstrate regulation of PDE4 by AKAP-localized PKA activity and subsequent effects on localized cAMP signals in HASM.
Previous studies by our group and others (12, 29, 36–40) have demonstrated that β-agonist-stimulated cAMP accumulation in ASM cells is subject to multiple modes of regulation. GRKs (29, 38, 40) and arrestins (36, 37) effect agonist-specific desensitization of the β2AR in ASM, whereas PKA appears capable of attenuating β-agonist-stimulated cAMP accumulation by targeting both the β2AR and adenylyl cyclase (12). Of note, the PKA-sensitive adenylyl cyclase type 6 appears to be the most abundantly expressed adenylyl cyclase isoform in ASM (41). Data in the present study suggest that AKAPs are critical in the localization of PKA to the near-membrane space in order to provide the negative feedback required to reduce (primarily) membrane-delineated cAMP accumulation. The mathematical simulations presented here further strengthen this conclusion. The simulations clearly indicate that both inhibition of AC activity and stimulation of PDE activity are required to describe the data depicted in Figs. 3 and 4. Quantification of the extent to which they do this by localizing β2AR or AC proteins, facilitating PKA phosphorylation and desensitization of β2AR, inhibiting AC, or activating PDEs is a subject of future consideration.
The results presented here are markedly different than findings of previous studies using HEK293 cells (2–4). In previous studies, little or no agonist-induced reduction in the rate of cAMP production was observed in 10 min following stimulation of EP receptors; primarily GRK-mediated inhibition of β2AR activity, and the subsequent rate of cAMP production, was observed following stimulation of β2ARs. Here, we conclude that both GRK-mediated desensitization and PKA-mediated feedback onto AC and possibly β2AR activity contribute to the observed time course of cAMP signals. Similar to the previous studies, localization of components of the signaling pathway via AKAPs is critical for ensuring signaling fidelity.
Means of improving PKA activation in ASM cells have physiological relevance in airway biology and the clinical management of airway disease. Although inhaled β-agonists have proven useful as bronchoprotective and bronchodilatory drugs in the treatment of asthma and chronic obstructive pulmonary disease, their chronic use can be associated with tachyphylaxis and possibly increased mortality (reviewed in refs. 1, 42). Inhibition of GRK/arrestin-mediated β2AR desensitization improves β-agonist-stimulated cAMP accumulation and bronchorelaxation in ASM cell- and tissue-based models and in vivo (36, 38), although β-agonist regulation of more chronic ASM functions, such as proliferation, are unaffected by GRK/arrestin inhibition. Presumably, other mechanisms serving to attenuate cAMP accumulation or PKA activity in ASM are invoked to limit the functional consequences of GRK/arrestin inhibition. Because PKA is both a critical effector and feedback regulator of β2AR signaling, global targeting of PKA activity is not a feasible approach to improving β2AR function. Alternatively, selective targeting of PKA functions appears to be required. Accordingly, manipulation of AKAP function to selectively augment β2AR-mediated cAMP accumulation, while preserving those localized PKA activities important in mediating bronchomotor control, cell proliferation, and other ASM synthetic functions represents an intriguing therapeutic approach.
Supplementary Material
Acknowledgments
This study reflects equal contributions from the R.B.P. and T.C.R. laboratories and was funded in part by independent investigator grants from GlaxoSmithKline to R.B.P and T.C.R, P01 H066299 (T.C.R.), and HL58506 (R.B.P.).
S.J.H. is supported by U.S. National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) grant T32 AR07592.
The authors thank Anna Misior for preliminary transcriptome and proteome analyses. The authors declare no other conflicts of interest.
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
- β2AR
- β2-adrenergic receptor
- AC
- adenylyl cyclase
- AKAP
- A-kinase anchoring protein
- AKAP-IS
- A-kinase anchoring protein in silico
- ASM
- airway smooth muscle
- BSA
- bovine serum albumin
- CNG
- cyclic nucleotide-gated
- Epac
- exchange protein activated by cAMP
- FSK
- forskolin
- GFP
- green fluorescent protein
- GRK
- G-protein-coupled receptor kinase
- HASM
- human airway smooth muscle
- HEK
- human embryonic kidney
- IBMX
- isobutylmethylxanthine
- ISO
- isoproterenol
- PDE
- phosphodiesterase
- PKA
- protein kinase A
- SCR
- scrambled
- st-Ht31
- stearated Ht31
- YFP
- yellow fluorescent protein
REFERENCES
- 1. Billington C. K., Penn R. B. (2003) Signaling and regulation of G protein-coupled receptors in airway smooth muscle. Respir. Res. 4, 2. [PMC free article] [PubMed] [Google Scholar]
- 2. Willoughby D., Wong W., Schaack J., Scott J. D., Cooper D. M. (2006) An anchored PKA and PDE4 complex regulates subplasmalemmal cAMP dynamics. EMBO J. 25, 2051–2061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rich T. C., Xin W., Mehats C., Hassell K. A., Piggott L. A., Le X., Karpen J. W., Conti M. (2007) Cellular mechanisms underlying prostaglandin-induced transient cAMP signals near the plasma membrane of HEK-293 cells. Am. J. Physiol. Cell Physiol. 292, C319–C331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Xin W., Tran T. M., Richter W., Clark R. B., Rich T. C. (2008) Roles of GRK and PDE4 activities in the regulation of beta2 adrenergic signaling. J. Gen. Physiol. 131, 349–364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Stangherlin A., Stangherlin A., Zaccolo M. (2011) Local termination of cAMP signals: the role of AKAP-anchored phosphodiesterases. J. Cardiovasc. Pharmacol. 58, 345–353 [DOI] [PubMed] [Google Scholar]
- 6. Mauban J. R. H., O'Donnell M., Warrier S., Manni S., Bond M. (2009) AKAP-scaffolding proteins and regulation of cardiac physiology. Physiology 24, 78–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sanderson J. L., Dell'acqua M. L. (2011) AKAP signaling complexes in regulation of excitatory synaptic plasticity. Neuroscientist 17, 321–336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tasken K., Ruppelt A. (2006) Negative regulation of T-cell receptor activation by the cAMP-PKA-Csk signalling pathway in T-cell lipid rafts. Front. Biosci. 11, 2929–2939 [DOI] [PubMed] [Google Scholar]
- 9. Beene D. L., Scott J. D. (2007) A-kinase anchoring proteins take shape. Curr. Opin. Cell Biol. 19, 192–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Panettieri R. A., Murray R. K., DePalo L. R., Yadvish P. A., Kotlikoff M. I. (1989) A human smooth muscle cell line that retains physiological responsiveness. Am. J. Physiol. Cell Physiol. 256, C329–C335 [DOI] [PubMed] [Google Scholar]
- 11. Misior A. M., Deshpande D. A., Loza M. J., Pascual R. M., Hipp J. D., Penn R. B. (2009) Glucocorticoid- and protein kinase A-dependent transcriptome regulation in airway smooth muscle. Am. J. Respir. Cell Mol. Biol. 41, 24–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Guo M., Pascual R. M., Wang S., Fontana M. F., Valancius C. A., Panettieri R. A., Jr., Tilley S. L., Penn R. B. (2005) Cytokines regulate beta-2-adrenergic receptor responsiveness in airway smooth muscle via multiple PKA- and EP2 receptor-dependent mechanisms. Biochemistry 44, 13771–13782 [DOI] [PubMed] [Google Scholar]
- 13. Billington C. K., Kong K. C., Bhattacharyya R., Wedegaertner P. B., Panettieri R. A., Chan T. O., Penn R. B. (2005) Cooperative regulation of p70S6 kinase by receptor tyrosine kinases and G protein-coupled receptors augments airway smooth muscle growth. Biochemistry 44, 14595–14605 [DOI] [PubMed] [Google Scholar]
- 14. Alto N. M., Soderling S. H., Hoshi N., Langeberg L. K., Fayos R., Jennings P. A., Scott J. D. (2003) Bioinformatic design of A-kinase anchoring protein-in silico: a potent and selective peptide antagonist of type II protein kinase A anchoring. Proc. Natl. Acad. Sci. U. S. A. 100, 4445–4450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Carr D. W., Stofko-Hahn R. E., Fraser I. D. C., Bishop S. M., Acott T. S., Brennan R. G., Scott J. D. (1991) Interaction of the regulatory subunit (RII) of cAMP-dependent protein kinase with RII-anchoring proteins occurs through an amphipathic helix binding motif. J. Biol. Chem. 266, 14188–14192 [PubMed] [Google Scholar]
- 16. Misior A. M., Yan H., Pascual R. M., Deshpande D. A., Panettieri R. A., Jr., Penn R. B. (2008) Mitogenic effects of cytokines on smooth muscle are critically dependent on protein kinase A and are unmasked by steroids and cyclooxygenase inhibitors. Mol. Pharmacol. 73, 566–574 [DOI] [PubMed] [Google Scholar]
- 17. Penn R. B., Parent J. L., Pronin A. N., Panettieri R. A., Jr., Benovic J. L. (1999) Pharmacological Inhibition of protein kinases in intact cells: antagonism of beta adrenergic receptor ligand binding by H-89 reveals limitations of usefulness. J. Pharmacol. Exp. Ther. 288, 428–437 [PubMed] [Google Scholar]
- 18. Rich T. C., Fagan K. A., Nakata H., Schaack J., Cooper D. M., Karpen J. W. (2000) Cyclic nucleotide-gated channels colocalize with adenylyl cyclase in regions of restricted cAMP diffusion. J. Gen. Physiol. 116, 147–161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rich T. C., Tse T. E., Rohan J. G., Schaack J., Karpen J. W. (2001) In vivo assessment of local phosphodiesterase activity using tailored cyclic nucleotide-gated channels as cAMP sensors. J. Gen. Physiol. 118, 63–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tran T. M., Friedman J., Qunaibi E., Baameur F., Moore R. H., Clark R. B. (2004) Characterization of agonist stimulation of cAMP-dependent protein kinase and G protein-coupled receptor kinase phosphorylation of the beta2-adrenergic receptor using phosphoserine-specific antibodies. Mol. Pharmacol. 65, 196–206 [DOI] [PubMed] [Google Scholar]
- 21. Misior A. M., Deshpande D. A., Loza M. J., Pascual R. M., Hipp J. D., Penn R. B. (2009) Glucocorticoid- and protein kinase A-dependent transcriptome regulation in airway smooth muscle. Am. J. Respir. Cell Mol. Biol. 41, 24–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Terrin A., Di B. G., Pertegato V., Cheung Y. F., Baillie G., Lynch M. J., Elvassore N., Prinz A., Herberg F. W., Houslay M. D., Zaccolo M. (2006) PGE(1) stimulation of HEK293 cells generates multiple contiguous domains with different [cAMP]: role of compartmentalized phosphodiesterases. J. Cell Biol. 175, 441–451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rich T. C., Fagan K. A., Tse T. E., Schaack J., Cooper D. M., Karpen J. W. (2001) A uniform extracellular stimulus triggers distinct cAMP signals in different compartments of a simple cell. Proc. Natl. Acad. Sci. U. S. A. 98, 13049–13054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rich T. C., Karpen J. W. (2002) Review article: cyclic AMP sensors in living cells: what signals can they actually measure? Ann. Biomed. Eng. 30, 1088–1099 [DOI] [PubMed] [Google Scholar]
- 25. Piggott L. A., Hassell K. A., Berkova Z., Morris A. P., Silberbach M., Rich T. C. (2006) Natriuretic peptides and nitric oxide stimulate cGMP synthesis in different cellular compartments. J. Gen. Physiol. 128, 3–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Brady J. D., Rich T. C., Le X., Stafford K., Fowler C. J., Lynch L., Karpen J. W., Brown R. L., Martens J. R. (2004) Functional role of lipid raft microdomains in cyclic nucleotide-gated channel activation. Mol. Pharmacol. 65, 503–511 [DOI] [PubMed] [Google Scholar]
- 27. Vijayaraghavan S., Goueli S. A., Davey M. P., Carr D. W. (1997) Protein kinase A-anchoring inhibitor peptides arrest mammalian sperm motility. J. Biol. Chem. 272, 4747–4752 [DOI] [PubMed] [Google Scholar]
- 28. Pascual R. M., Billington C. K., Hall I. P., Panettieri R. A., Jr., Fish J. E., Peters S. P., Penn R. B. (2001) Mechanisms of cytokine effects on G protein-coupled receptor-mediated signaling in airway smooth muscle. Am. J. Physiol. Lung Cell. Mol. Physiol. 281, 1425–1435 [DOI] [PubMed] [Google Scholar]
- 29. Penn R. B., Panettieri R. A., Jr., Benovic J. L. (1998) Mechanisms of acute desensitization of the β2AR-adenylyl cyclase pathway in human airway smooth muscle. Am. J. Respir. Cell Mol. Biol. 19, 338–348 [DOI] [PubMed] [Google Scholar]
- 30. Tavalin S. J. (2008) AKAP79 selectively enhances protein kinase C regulation of GluR1 at a Ca2+-calmodulin-dependent protein kinase II/protein kinase C site. J. Biol. Chem. 283, 11445–11452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chen M. H., Malbon C. C. (2009) G-protein-coupled receptor-associated A-kinase anchoring proteins AKAP5 and AKAP12: Differential trafficking and distribution. Cell. Signal. 21, 136–142 [DOI] [PubMed] [Google Scholar]
- 32. Tao J., Malbon C. C. (2008) G-protein-coupled receptor-associated A-kinase anchoring proteins AKAP5 and AKAP12: differential signaling to MAPK and GPCR recycling. J. Mol. Signal. 3, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cant S. H., Pitcher J. A. (2005) G protein-coupled receptor kinase 2-mediated phosphorylation of ezrin is required for G protein-coupled receptor-dependent reorganization of the actin cytoskeleton. Mol. Biol. Cell 16, 3088–3099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sette C., Iona S., Conti M. (1994) The short-term activation of a roliproam-sensitive, cAMP-sepcific phosphodiesterase by thyroid-stimulating hormone in thyroid FRTL-5 cells is mediated by a cAMP-dependent phosphorylation. J. Biol. Chem. 269, 9245–9252 [PubMed] [Google Scholar]
- 35. Sette C., Conti M. (1996) Phosphorylation and activation of a cAMP-specific phosphodiesterase by the cAMP-dependent protein kinase. Involvement of serine 54 in the enzyme activation. J. Biol. Chem. 271, 16526–16534 [DOI] [PubMed] [Google Scholar]
- 36. Deshpande D. A., Theriot B. S., Penn R. B., Walker J. K. (2008) β-Arrestins specifically constrain β2-adrenergic receptor signaling and function in airway smooth muscle. FASEB J. 22, 2134–2141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Penn R. B., Pascual R. M., Kim Y.-M., Mundell S. J., Krymskaya V. P., Panettieri R. A., Jr., Benovic J. L. (2001) Arrestin specificity for G protein-coupled receptors in human airway smooth muscle. J. Biol. Chem. 276, 32648–32656 [DOI] [PubMed] [Google Scholar]
- 38. Kong K. C., Gandhi U., Martin T. J., Anz C. B., Yan H., Misior A. M., Pascual R. M., Deshpande D. A., Penn R. B. (2008) Endogenous Gs-coupled receptors in smooth muscle exhibit differential susceptibility to GRK2/3-mediated desensitization. Biochemistry 47, 9279–9288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hewitt M., Estell K., Davis I. C., Schwiebert L. M. (2010) Repeated bouts of moderate-intensity aerobic exercise reduce airway reactivity in a murine asthma model. Am. J. Respir. Cell Mol. Biol. 42, 243–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wang W. C. H., Mihlbachler K. A., Brunnett A. C., Liggett S. B. (2009) Targeted transgenesis reveals discrete attenuator functions of GRK and PKA in airway + 2-adrenergic receptor physiologic signaling. Proc. Nat. Acad. Sci. 106, 15007–15012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Billington C. K., Hall I. P., Mundell S. M., Parent J.-L., Panettieri R. A., Benovic J. L., Penn R. B. (1999) Inflammatory and contractile agents sensitize specific adenylyl cyclase isoforms in human airway smooth muscle. Am. J. Respir. Cell Mol. Biol. 21, 597–606 [DOI] [PubMed] [Google Scholar]
- 42. Walker J. K. L., Penn R. B., Hanania N. A., Dickey B. F., Bond R. A. (2011) New perspectives regarding β2-adrenoceptor ligands in the treatment of asthma. Br. J. Pharmacol. 163, 18–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.