Abstract
Cytochrome c oxidase (COX) is the terminal enzyme of the mitochondrial electron transport chain. The purpose of this study was to analyze the function of lung-specific cytochrome c oxidase subunit 4 isoform 2 (COX4i2) in vitro and in COX4i2-knockout mice in vivo. COX was isolated from cow lung and liver as control and functionally analyzed. COX4i2-knockout mice were generated and the effect of the gene knockout was determined, including COX activity, tissue energy levels, noninvasive and invasive lung function, and lung pathology. These studies were complemented by a comprehensive functional screen performed at the German Mouse Clinic (Neuherberg, Germany). We show that isolated cow lung COX containing COX4i2 is about twice as active (88 and 102% increased activity in the presence of allosteric activator ADP and inhibitor ATP, respectively) as liver COX, which lacks COX4i2. In COX4i2-knockout mice, lung COX activity and cellular ATP levels were significantly reduced (−50 and −29%, respectively). Knockout mice showed decreased airway responsiveness (60% reduced Penh and 58% reduced airway resistance upon challenge with 25 and 100 mg methacholine, respectively), and they developed a lung pathology deteriorating with age that included the appearance of Charcot-Leyden crystals. In addition, there was an interesting sex-specific phenotype, in which the knockout females showed reduced lean mass (−12%), reduced total oxygen consumption rate (−8%), improved glucose tolerance, and reduced grip force (−14%) compared to wild-type females. Our data suggest that high activity lung COX is a central determinant of airway function and is required for maximal airway responsiveness and healthy lung function. Since airway constriction requires energy, we propose a model in which reduced tissue ATP levels explain protection from airway hyperresponsiveness, i.e., absence of COX4i2 leads to reduced lung COX activity and ATP levels, which results in impaired airway constriction and thus reduced airway responsiveness; long-term lung pathology develops in the knockout mice due to impairment of energy-costly lung maintenance processes; and therefore, we propose mitochondrial oxidative phosphorylation as a novel target for the treatment of respiratory diseases, such as asthma.—Hüttemann, M., Lee, I., Gao, X., Pecina, P., Pecinova, A., Liu, J., Aras, S., Sommer, N., Sanderson, T. H., Tost, M., Neff, F., Aguilar-Pimentel, J. A., Becker, L., Naton, B., Rathkolb, B., Rozman, J., Favor, J., Hans, W., Prehn, C., Puk, O., Schrewe, A., Sun, M., Höfler, H., Adamski, J., Bekeredjian, R., Graw, J., Adler, T., Busch, D. H., Klingenspor, M., Klopstock, T., Ollert, M., Wolf, E., Fuchs, H., Gailus-Durner, V., Hrabě de Angelis, M., Weissmann, N., Doan, J. W., Bassett, D. J. P., Grossman, L. I. Cytochrome c oxidase subunit 4 isoform 2-knockout mice show reduced enzyme activity, airway hyporeactivity, and lung pathology.
Keywords: asthma, CCO4-2, COX4-2, COX4i2, inflammation, oxidative phosphorylation
The mitochondrial respiratory chain together with ATP synthase constitutes the oxidative phosphorylation (OxPhos) machinery, which produces >15 times more energy equivalents in the form of ATP than does the glycolytic pathway. Cytochrome c oxidase (COX; EC 1.9.3.1) is the terminal enzyme of the respiratory chain and consumes >90% of cellular oxygen. COX is located in the inner mitochondrial membrane and transfers electrons from cytochrome c (Cytc) to oxygen, a process coupled to the pumping of protons across the membrane that generates the mitochondrial membrane potential ΔΨm, which is utilized by ATP synthase to generate ATP. Mammalian COX has been crystallized as a dimer composed of 13 subunits per monomer (1).
In mammals, the rate and efficiency of the electron transport complexes have to be regulated according to physiological energy demand. The reaction catalyzed by COX is the suggested rate-limiting step of the electron transport chain (ETC) in intact cells in mammals under physiological conditions (2–7). This conversion of oxygen to water via COX has a free energy of ΔG° = −100 kJ/mol, which is about twice as high as the reactions catalyzed by complexes I and III (8). Consequently, this essentially irreversible step of the ETC is fine-tuned through multiple levels of regulation: phosphorylation, isoform expression, and allosteric control (reviewed in ref. 9). COX contains 3 known heart-type and liver-type isoforms of subunits VIa, VIIa, and VIII (10). In addition, a testes isoform of subunit VIb and a third isoform of subunit VIII have been reported (11, 12), but their functions have not been studied. In contrast, ubiquitously expressed COX subunit IV isoform 1 (COX4i1) has been studied in some detail. It plays a key regulatory role because it allosterically regulates COX by binding ATP (allosteric inhibitor) and ADP (allosteric activator), thereby adjusting energy production to energy demand (13–15).
We have recently identified a lung/trachea-specific isoform gene of this regulatory subunit, COX subunit 4 isoform 2 (COX4i2; ref. 16). In the lung, COX4i1 and COX4i2 are expressed at similar levels. COX4i2 is expressed in all cells of the lung, with highest levels found in the smooth muscle and the lining epithelium (16). Since the role of COX4i2 in lung function was previously unknown, this current study aimed to analyze COX4i2 in vitro as well as in vivo in a newly generated mouse line lacking COX4i2.
Lung tissue requires energy generated by OxPhos for the many processes performed by this organ, including secretory activity and mucus production, ciliary escalator action to fight airborne infection, and airway constriction, the latter of which occurs during asthmatic reactions. In addition, during episodes of hypoxia, lungs have a unique response mechanism called hypoxic pulmonary vasoconstriction (HPV). HPV leads to a constriction of the pulmonary arterial vessels, causing diversion of blood flow to better ventilated areas of the lung to improve arterial oxygenation. The possible physiological role of COX4i2 in any of these processes is not clear.
We here demonstrate that the knockout mice show reduced COX activity and decreased energy levels in the lung. COX4i2-knockout mice revealed decreased airway resistance to bronchoconstrictor challenge, and we propose a model linking mitochondria to airway responsiveness, since pulmonary airway constriction requires ATP provided by OxPhos. Our study suggests mitochondrial OxPhos as a novel functional target for lung pathologies with increased airway reactivity, such as asthma.
MATERIALS AND METHODS
Isolation of cow COX and Western blot analysis
Chemicals were purchased from Sigma-Aldrich (St. Louis, MO, USA) unless stated otherwise. Mitochondria and subsequently COX were isolated from cow lung and liver as control under standard conditions (17). SDS-PAGE in the presence of urea (Kadenbach gel) of isolated COX was performed as described previously (17).
Purified cow lung, liver, and heart COX (10 μg) were denatured in SDS-PAGE sample buffer for 40 min at room temperature and separated in a 10% acrylamide Tris/Tricine/SDS gel. Proteins were transferred to a PVDF membrane (0.2 μm; Bio-Rad, Hercules, CA, USA) with transfer buffer (25 mM Tris base, 192 mM glycine, and 20% methanol) for 60 min at 25 V. The membrane was blocked with blocking reagent (5% nonfat dry milk/PBS-0.1% Tween 20) for 1 h at room temperature. Antibodies were diluted in blocking reagent, and the blot was incubated with anti-COX4i2 (1:2000 dilution; H00084701-M01; Abnova, Boulder, CO, USA) overnight at 4°C, washed in PBS-T (PBS-0.1% Tween 20) 7 times for 5 min, and incubated with anti-mouse IgG-HRP (1:4,000 dilution; GE Healthcare, Piscataway, NJ, USA) for 2 h. For mouse tissues, both primary and secondary antibody concentrations were doubled, and secondary antibody incubation time was increased to 4 h. The membrane was washed 8 times in PBS-T and 1 time in PBS for 5 min, and signals were detected by the chemiluminescence method (ECL+ kit; GE Healthcare). The membrane was reprobed with anti-COX4i1 (1:8000 dilution; MS408; Mitosciences, Eugene, OR, USA) for 1 h, washed 3 times in PBS-T for 10 min, and incubated with anti-mouse IgG-HRP (1:10,000 dilution) for 1 h. Signals were detected as described above.
COX activity measurements of isolated COX and solubilized tissue
Isolated COX was dialyzed in the presence of ATP and cardiolipin to remove cholate and to replace damaged cardiolipin, as previously described in detail (17). Activity of isolated COX was determined using the polarographic method in a closed 200-μl chamber equipped with a micro-Clark-type oxygen electrode (Oxygraph system; Hansatech, Norfolk, UK) in measuring buffer (10 mM K-HEPES, pH 7.4; 40 mM KCl; 1% Tween 20; 2 μM oligomycin; 1 mM PMSF; 10 mM KF; and 2 mM EGTA) as described previously (18). COX activity was determined in the presence of 5 mM ADP, an allosteric activator of COX, or 5 mM ATP, an allosteric inhibitor of COX, after incubation with an ATP regenerating system, as described previously (18). Tissue samples were first sonicated, and measurements were performed at 25°C in the presence of 20 mM ascorbate and increasing amounts of cow heart Cytc from 0 to 45 μM. Enzymatic activity is defined as micromolar oxygen consumed per minute per micromolar COX.
COX-specific activity in lung tissue homogenates was determined after solubilization in measuring buffer after sonication as described (17). Specific activity is defined as nanomoles oxygen consumed per minute per milligram protein. Protein concentration was determined with the Dc protein assay kit (Bio-Rad).
Generation of COX4i2-knockout mice
All experiments conformed to Wayne State University Animal Investigation Committee guidelines on the ethical treatment of animals and to the U.S. Government Principles for the Utilization and Care of Vertebrate Animals Used in Testing, Research, and Training (approved protocol A04-21-08). Animal numbers were kept to a minimum consistent with statistical significance.
The COX4i2 gene is located on mouse chromosome 2 [see U.S. National Center for Biotechnology Information (NCBI) sequence NC_000068.6; 152579909-152590773]. The gene contains 5 exons (for mRNA sequence, see NCBI sequence AF271382). Exons II and III were replaced by homologous recombination with the Neo cassette as part of the pPNT vector (19). Upstream (5′) and downstream (3′) genomic DNA regions of the COX4i2 gene were amplified with the Expand Long Template PCR System (Roche, Indianapolis, IN, USA) using buffer system 3 according to the manufacturer's instructions (all PCRs were 50-μl reactions with nucleotide, primer, and template DNA concentrations used as recommended). Using total mouse DNA as template, the 5′ and 3′ genomic sequences were amplified as an outer PCR followed by a nested PCR (see Fig. 2A for primer locations). Outer primers to amplify the upstream flanking region were P1outer forward 5′-GAGATACATGAGGTAGGAACCAACCT-3′, P2outer reverse 5′-TGAGTCCTGTCCTCATTACCAGAC-3′ and nested primers containing restriction sites (italics; mutations introduced to generate restriction sites are underscored) were P3inner forward EcoRI 5-GAAAACTGGAATTCAGAGACATTAGGCCAC-3′, P4inner reverse BamHI 5-CATCTGCAGGATCCGAGACACATCTTGTTCAGC-3′. Using 300 ng of total mouse genomic DNA as template, an outer PCR was performed with primers P1 and P2, 2 min initial denaturation at 93°C; and 30 cycles: 30 s, 93°C; 30 s, 60°C; 2 min, 69°C. Nested PCR was performed with 1 μl of a 1:50 dilution of the outer PCR with primers P3 and P4 as a touch-up PCR, with 2 min initial denaturation at 93°C; 5 cycles: 30 s, 93°C; 30 s, 50°C; 2 min, 69°C; 25 cycles: 30 s, 93°C; 30 s, 60°C; 2 min, 69°C to allow efficient annealing of the restriction site-containing primers. The amplified DNA was purified, and restriction digestion was performed with EcoRI and BamHI. The resultant 1572-bp fragment was purified and cloned into the pPNT plasmid that was digested as above. To generate the downstream arm for homologous recombination an inner and outer PCR were performed as above, using primers P5outer forward 5′-GCGAGGAGCAGCGGGCCCTGAAGGAG-3′, P6outer reverse 5′-GTTGCTCTTCATGTCCAGGAGGCGCTGGAG-3′, P7inner forward XhoI 5′-GGGGGACTCGAGGAGAGTCACCTGGACTG-3′, and P8inner reverse NotI 5′-GGGTCTCCGCGGCCGCTGGGACTCTGAGCCTG-3′, with the following PCR parameters: outer PCR, 2 min initial denaturation at 93°C; 30 cycles: 30 s, 93°C; 30 s, 60°C; 10 min, 69°C with primers P5 and P6, followed by a nested touch-up PCR, 2 min initial denaturation at 93°C; 5 cycles: 30 s, 93°C; 30 s, 52°C; 10 min, 69°C; 25 cycles: 30 s, 93°C; 30 s, 65°C; 10 min, 69°C. The product in the expected size range was obtained as judged by agarose gel electrophoresis, purified, and digested with XhoI and NotI, and the resultant 7459-bp fragment was cloned into the corresponding restriction sites of the pPNT vector. Orientation and sequence were confirmed by sequencing. Finally, plasmid DNA containing the upstream and downstream sequences of the COX4i2 gene flanking the Neo cassette in the pPNT vector was linearized with NotI, resulting in a 16379-bp fragment. Electroporation of R1 mouse embryonic stem cells was performed as a paid service at the Transgenic Animal Model Core (TAMC), University of Michigan (Ann Arbor, MI, USA). Recombinant clones that had undergone homologous recombination in the correct locus were identified using a genotyping protocol (see next section). Positively identified clones were microinjected into C57BL/6J blastocysts at the TAMC, and chimeric animals with a high contribution from the ES cell clone and low contribution from the host embryo as judged by coat color contribution (embryonic stem cells derived from 129 mice produce agouti fur) were selected. Animals were genotyped (see below) and breeding was continued for >8 generations using C57BL/6J mice.
Figure 2.
Gene knockout of COX4i2. A) Schematic representation of the gene-knockout strategy. PCR-based amplification of the 5′ and 3′ COX4i2 regions (o-PCR, outer PCR; arrows) was followed by an inner PCR (i-PCR) with primers containing the indicated restriction sites (sphere-tailed arrows). Two fragments are generated, the promoter region to the end of intron I (1572-bp fragment), and the 6459-bp region spanning from the beginning of intron III to the end of exon IV. These fragments were cloned into the respective sites of the pPNT vector (pPNT4-2), leading to a replacement of exons II and III by the neo cassette in the recombinant. B) Northern blot analysis shows absence of COX4i2 transcripts in the knockouts. Total RNA (40 μg) derived from mouse lung tissues from knockout (KO), heterozygous (HT), and wild-type (WT) mice were loaded per lane. The blots were probed with the COX4i1 (left panel) and COX4i2 (right panel) digoxigenin-labeled antisense RNA. The size of the resulting signal was calculated to be ∼950 bases for both isoforms with the ribosomal bands consistent with the cloned cDNAs, allowing a poly(A) tail of 200–300 bases. KO and HT mice show absence and reduced COX4i2 transcript levels, respectively. C) Western blot analysis using lung and liver tissue from WT and KO mice indicates absence of COX4i2 protein in lung tissue of the KO mice.
COX4i2 genotyping
Genomic DNA was isolated from punched ear tissues with the Wizard Genomic DNA Purification Kit (Promega, Madison, WI, USA). PCR genotyping was performed using the Expand Long Template PCR System (Roche, Indianapolis, IN, USA) in conjunction with buffer 3 according to the manufacturer's instructions; all PCRs were 25-μl reactions with 100 ng template DNA and recommended nucleotide and primer concentrations. Two separate PCRs were performed to identify the wild-type and the recombinant alleles using primer pairs P9/P10 and P9/P11, respectively: forward primer P9 located upstream of the 5′ arm used for homologous recombination (5′-CACCGCCTATGAAGTGAGATACATGAGGTAG-3′), wild-type reverse primer P9 located in COX4i2 exon II/intron II junction (5′-CAGAATCTGAAGACTTACCAGCATCTCCTG-3′), recombinant-specific reverse primer P10 located in the Neo gene (5′-ACGGTATCGCCGCTCCCGATTCGCAG-3′). PCR conditions were 2 min initial denaturation at 93°C; 13 cycles: 18 s, 93°C; 40 s, 54°C; 2 min, 69°C; 22 cycles: 18 s, 93°C; 35 s, 55°C; 2 min, 69°C, plus 4 s per each new cycle. PCR products were loaded and separated on a 1% agarose gel containing ethidium bromide, and wild-type and recombinant PCR products were observed at ∼1.8 and 2.3 kb, respectively.
RNA isolation and Northern blot analysis
Total RNA was isolated from mouse lungs of all three genotypes by the phenol/chloroform/isoamyl extraction method (20). Digoxigenin-labeled antisense probe generation for COX4i1 and COX4i2 and Northern blot analysis was performed as described previously (16). The probes used were based on the corresponding rat COX4i1 and COX4i2 sequences that share >90% identity across the coding region between the mouse and rat orthologs, whereas homology between the COX4i1 and COX4i2 paralogs is <50%, allowing specific annealing to the respective isoform mRNAs.
Mitochondrial respiration measurements
Preparation of mouse lung homogenates for mitochondrial respiration measurements was performed as described previously (21, 22). Briefly, excised lungs were weighed, chopped into small pieces with scissors, and Polytron-homogenized in 5-fold volume ratio to tissue of cold buffer [225 mM mannitol, 75 mM sucrose, 5 mM 3-(N-morpholino)-propanesulfonate (MOPS), 20 mM ethylene glycol-bis-(B-aminoethyl ether)-N,N,N′,N′–tetraacetic acid (EGTA), and 2% (w/v) fatty acid free bovine serum albumin]. Homogenate samples were used for measuring pyruvate/malate, succinate, and glycerol 3-phosphate-dependent respiration using a micro-Clark-type oxygen electrode (see above) at 30°C; the respiration is defined as picomoles oxygen consumed per second per milligram wet tissue.
ATP assay
Lung ATP levels of flash-frozen lung samples were determined with the bioluminescent method in conjunction with the boiling method for inactivating enzymes, as previously reported (18, 23).
Lung physiology
For determination of the pulmonary arterial pressure (PAP) in isolated ventilated and perfused lungs, mouse lungs were removed from the chest under deep anesthesia and were artificially ventilated and perfused as described previously (24, 25). Briefly, mice were anesthetized with intraperitoneal injection of 80 mg/kg pentobarbital and anticoagulated with 1000 U/kg heparin. Catheters were inserted into the pulmonary artery and left atrium, and buffer perfusion via the pulmonary artery was started at a flow of 0.2 ml/min. In parallel, ventilation was changed from room air to a premixed gas (21% O2, 5% CO2, balanced with N2), left atrial pressure was set to 2.0 mmHg, and buffer flow was slowly increased from 0.2 to 2 ml/min. Positive pressure ventilation was performed with a tidal volume of 250 μl, 90 breaths/min, and a positive end-expiratory pressure of 2 cmH2O. For perfusion, Krebs-Henseleit buffer containing 120 mM NaCl, 4.3 mM KCl, 1.1 mM KH2PO4, 2.4 mM CaCl2, 1.3 mM MgCl2, 20 mM NaHCO3, and 13.32 mM glucose as well as 5% (w/v) hydroxyethylamylopectin (molecular weight 200,000) was employed. The perfusion medium was preequilibrated by gassing with the ventilation gas mixture, resulting in a pH of 7.37–7.40. PAP was measured after an initial steady-state period of 15 min at 2 ml/min (24, 25).
Noninvasive pulmonary function was assessed by placing unanesthetized mice within a whole-body plethysmograph equipped with a bias flow air supply and pneumotachograph and pressure transducer for determining changes in flow (Buxco Electronics Inc., Wilmington, DE, USA). Changes in pneumotach flow were used to assess alterations in breathing pattern brought about by exposure to increasing aerosol concentrations of the bronchoconstrictor methacholine and used to compute enhanced pauses in breathing (Penh), as previously investigated and evaluated (26–30). Airway reactivity to methacholine was also assessed on separate cohorts of anesthetized mice (urethane, 1.5 g/kg body wt i.p.) using an invasive method based on direct measurements of changes in airway resistance through measurements of whole-body plethysmograph flow and transpulmonary pressure using a saline-filled esophageal cannula (Buxco Electronics) as described previously (31). Both these methods required a 30 min acclimatization within plethysmographs prior to exposures of 2 min to increasing concentrations of methacholine aerosols (0 to 100 mg/ml) that corresponded to 0 to 6.25 g methacholine/m3. A 5-min recovery time was allowed between each methacholine application, during which time mean Penh or airway resistance changes were determined using a pulmonary function computer (Buxco Electronics). Data obtained for each mouse using both of these methods were subjected to polynomial regression analyses, as described previously (28, 32), demonstrating coefficients of >0.985 in all cases. The resulting quadratic equations were then used to establish single numbers for comparing the 3 mouse genotypes examined at single levels of methacholine aerosol challenge (30).
Inflammatory state determination
Whole lung tissue accumulation of inflammatory cells was assessed by incubating sliced perfused parenchymal tissue in Dulbecco's modified Eagle's medium containing collagenase (75 U/ml; Worthington Biochemical Corp., Lakewood, NJ, USA), fetal calf serum (10% v/v; Life Technologies, Inc., Grand Island, NY, USA), and 50 U/ml DNase, as described previously (32–34). The resulting cell populations were subjected to total cell counting and differential analyses following staining with modified Wright's stain for identification of tissue-recovered lymphocytes, neutrophils, and eosinophils (32, 34). A nonspecific esterase staining procedure confirmed the identification of the combined monocyte and macrophage population recovered from the tissue collagenase digests (35). Results were reported as total tissue-recovered cells per lung. Statistical analyses of data from pulmonary function cell analyses were performed by 1-way analysis of variance with comparison of the means using Dunnett's multiple range test for comparison with wild-type. Values of P <0.05 were considered significant, and data are presented as means ± se.
Phenotypic characterization at the German Mouse Clinic (GMC)
A comprehensive phenotypic characterization of the COX4i2-knockout mice was conducted at the GMC (Neuherberg, Germany). The following screens were performed: dysmorphology, cardiovascular, energy metabolism, clinical chemistry, eye, molecular phenotyping, behavior, neurology, nociception, immunology and allergy, steroid metabolism, and pathology as described in detail (36, 37). All procedures for animal handling and experiments were performed in accordance with protocols approved by the Regierung von Oberbayern (District Government of Upper Bavaria, Germany). Data sets showing significant phenotypic differences are presented in the text and in Supplemental Figs. S1–S3.
RESULTS
COX4i2 constitutes a subunit of isolated lung COX, a high-activity isozyme
Among the tissues previously tested, lung and liver contain the highest and lowest expressed levels of COX4i2, respectively (16). To characterize lung COX in comparison to liver COX, which lacks COX4i2, we isolated both isozymes. Since larger amounts of tissue are required for the procedure, we used 1 kg of cow lung, and as a control liver tissue, for the isolation of mitochondria and subsequently COX. Lung tissue contains a significant amount of connective tissue, which made mitochondria isolation cumbersome. Although we employed some modifications to our purification protocol, which is optimized for liver and heart COX purification, isolated lung COX contained more impurities compared to liver COX (Fig. 1A).
Figure 1.
Isolated lung COX is a high-turnover isozyme. A) SDS-PAGE of COX from cow lung and liver. COX from both organs was isolated side by side, and subunits were separated by SDS-PAGE. Subunits as indicated. Lung (left lane) and liver COX (right lane) were 37% ammonium sulfate-precipitated fractions. B) Western blot analysis of isolated cow lung, liver, and heart with COX4i2 (top panel) and COX4i1 (lower panel). COX4i2 was detected in isolated lung COX. COX isolated from heart, the organ with second-highest COX4i2 transcription levels among tissues previously tested (16), gave a weak signal, whereas no signal was detected in liver COX. COX4i1 and COX4i2 run at the same size of ∼18 kDa. M, molecular marker (kDa). C) Respiration kinetics of solubilized cow lung (triangles) and liver COX (squares) in the presence of allosteric activator ADP (solid symbols), or inhibitor ATP (open symbols) and the presence of an ATP-regenerating system. COX activity (TN, turnover) is defined as consumed micromolar O2 per second per micromolar COX. Both lung and liver COX show allosteric regulation by ADP and ATP. Lung COX is ∼2-fold more active at maximal turnover compared to liver COX in the presence of ADP or ATP. Data are representative of 3 independent experiments (sd<5% at maximal turnover).
To show that COX4i2 is a component of COX, we performed Western blot analysis with cow lung, using liver and heart as controls and using a commercial antibody (H00084701-M01, Abnova). Only lung tissue produced a strong signal, whereas no detectable signal was obtained with liver COX (Fig. 1B). Cow heart COX produced a weak signal after overexposure of the blot, in agreement with our previous finding that heart tissue has the second highest expression level of COX4i2 after lung among tissues tested (16). Another commercial COX4i2 antibody (sc-86386; Santa Cruz Biotechnology, Santa Cruz, CA, USA) could not distinguish between both isoforms and should therefore not considered to be COX4i2 specific (not shown).
We next assessed the kinetic properties of lung COX in comparison to liver COX. COX activity was determined in the presence of allosteric effectors ATP (inhibitor) and ADP (activator), which bind to subunit 4 (13). Both enzymes showed hyperbolic kinetics in the presence of adenine nucleotides. Allosteric regulation was also present in the lung enzyme, which suggests that the presence of COX4i2 does not abolish or otherwise interfere with allosteric regulation (Fig. 1C, compare open and closed symbols). Lung COX activity was about 2-fold increased compared to liver COX at maximal turnover (88% and 102% increased activity in the presence of allosteric activator ADP and inhibitor ATP, respectively), assigning an activating role to COX4i2.
Generation of COX4i2-knockout mice
To generate chimeric mice, our linearized recombinant construct was electroporated into mouse R1 embryonic stem cells (Fig. 2A). Exon II, which contains the start ATG, and exon III were replaced with the neo cassette. Northern blot analysis showed that COX4i2 transcripts were reduced in the heterozygotes and absent in the knockout mice (Fig. 2B). Western blot analysis using a COX4i2-specific antibody indicated absence of subunit 4i2 in the lungs of the knockout mice. (Fig. 2C).
COX activity and ATP levels are reduced in COX4i2-knockout mice
Since lung COX showed increased COX activity compared to the liver enzyme, which lacks COX4i2, we expected reduced COX activity in the lungs of COX4i2-knockout mice. Since isolation of COX from mouse lungs is not practical due to limitations in the amount of available tissue, we directly determined COX activity in lung tissue homogenates. Consistent with results from isolated cow lung (Fig. 1C), our findings indicated decreased activity of COX in the knockout mice. In the presence of allosteric inhibitor ATP or activator ADP, COX activity was 2-fold higher in the wild-type mice compared to the knockout mice (Fig. 3A). In both wild-type and knockout mice, the allosteric effect was present, resulting in ∼29 and 44% enzyme activation in the presence of ADP compared to ATP in the wild-type and knockout mice, respectively (Fig. 3A). Since COX is the proposed rate-limiting enzyme of the ETC in intact cells, changes in COX activity should result in differences in cellular energy levels. Analysis of ATP levels in lung tissue revealed a significant 29% decrease in the knockout mice compared to the wild-type mice (Fig. 3B). Other tissues tested; i.e., heart and liver, did not show significant differences in COX activity (Supplemental Fig. S4A) or ATP levels between wild-type and knockout mice (data not shown).
Figure 3.
COX activity and ATP levels are decreased in the COX4i2-knockout mice. A) COX activity was determined in total lung tissue homogenates in the presence of allosteric COX regulators ADP (activator, solid symbols) and ATP (inhibitor, open symbols) and an ATP-regenerating system using the polarographic method by increasing the amount of substrate Cytc. COX activity is defined as consumed nanomoles O2 per minute per milligram protein. Data are representative measurements (n=4 each; sd<5% at maximal turnover). B) ATP concentrations of lung median cross-sections were determined using the bioluminescence method. ATP levels were 12 and 29% decreased in the heterozygote (HT) and COX4i2-knockout (KO) mice in comparison to the wild-type (WT) mice, respectively (n=4 animals/group, measured in triplicates each).*P = 0.013.
We next examined mitochondrial respiration rates of knockout, heterozygote, and wild-type mice. Lung homogenates instead of isolated mitochondria were used for two reasons. Due to the high lipid content and extensive extracellular matrix elements in the lung, earlier studies to isolate mitochondria demonstrated that relatively low yields of intact coupled mitochondria could be obtained only if fatty acid free albumin and high levels of the calcium chelator EGTA were included during the homogenization steps (21). In addition, a recent study with rat heart, liver, brain, and kidney tissues indicated that mitochondrial studies using various substrates produced equivalent results for isolated mitochondria and tissue extracts (38). Using three different substrates, pyruvate/malate, succinate, and glycerol 3-phosphate (Table 1), we obtained lower respiration rates in lung compared to those reported in the aforementioned study on four other tissues, likely due to overall decreased mitochondria mass in the lung. Differences were mostly insignificant among the three genotypes. This finding may be surprising at first. However, preparation of tissue homogenates had to be performed in the absence of unspecific phosphatase inhibitors fluoride and vanadate, because they interfere with the performed mitochondrial experiments, but are required to maintain COX phosphorylation (17). Under such conditions, COX may not be rate-limiting, likely due to dephosphorylation, as we have discussed in detail (9).
TABLE 1.
Mitochondrial respiration rates of lung homogenates
| Substrate | State | WT | HT | KO |
|---|---|---|---|---|
| Pyruvate + malate (10 mM each) | Endogenous | 2.28 ± 0.73 | 1.53 ± 0.47 | 1.95 ± 0.52 |
| Substrates | 2.43 ± 0.42 | 1.87 ± 0.58 | 2.16 ± 0.34 | |
| ADP | 10.85 ± 1.51 | 9.07 ± 2.48 | 11.23 ± 0.74 | |
| Oligomycin | 1.50 ± 0.33 | 1.28 ± 0.40 | 1.60 ± 0.18 | |
| RCR | 7.62 ± 1.87 | 7.37 ± 1.48 | 7.14 ± 1.08 | |
| Succinate (10 mM) | Endogenous | 2.70 ± 0.74 | 2.18 ± 0.60 | 2.34 ± 0.52 |
| Substrates | 6.85 ± 0.94 | 5.29 ± 1.05* | 6.10 ± 0.45 | |
| ADP | 24.37 ± 2.81 | 18.79 ± 3.63* | 22.20 ± 2.03 | |
| Oligomycin | 3.79 ± 0.75 | 2.81 ± 0.30* | 3.23 ± 0.24# | |
| RCR | 6.57 ± 0.93 | 6.65 ± 0.74 | 6.87 ± 0.30 | |
| Glycerol 3-phosphate (10 mM) | Endogenous | 2.44 ± 0.65 | 2.14 ± 0.28 | 2.24 ± 0.32 |
| Substrates | 2.81 ± 0.54 | 2.45 ± 0.55 | 2.65 ± 0.59 | |
| ADP | 8.35 ± 0.92 | 6.93 ± 1.56 | 7.41 ± 1.14 | |
| Oligomycin | 2.45 ± 0.57 | 2.13 ± 0.56 | 2.14 ± 0.48 | |
| RCR | 3.53 ± 0.56 | 3.29 ± 0.20 | 3.53 ± 0.34 |
Data are presented as means ± se (n=4). WT, wild type; HT, heterozygote; KO, knockout. Respiration rate is defined as picomoles oxygen consumed per second per milligram wet tissue. Measurements were performed with a final concentration of 3.5 mg protein/ml.
P ≤ 0.05 vs. WT;
P ≤ 0.05 vs. HT.
Lung function is altered in COX4i2-knockout mice
Since COX4i2 is expressed across all cell types in the lung but shows higher expression levels in smooth muscle, we focused on a basic characterization of arterial and airway function. Arterial and airway constriction require ATP, so we expected decreased PAP and airway resistance in the COX4i2-knockout mice. Under basal conditions, PAP in isolated perfused lungs from knockout and wild-type mice was found not to be significantly different, giving mean values of 8.4 ± 0.2 and 8.6 ± 0.3 mmHg, respectively (n=9).
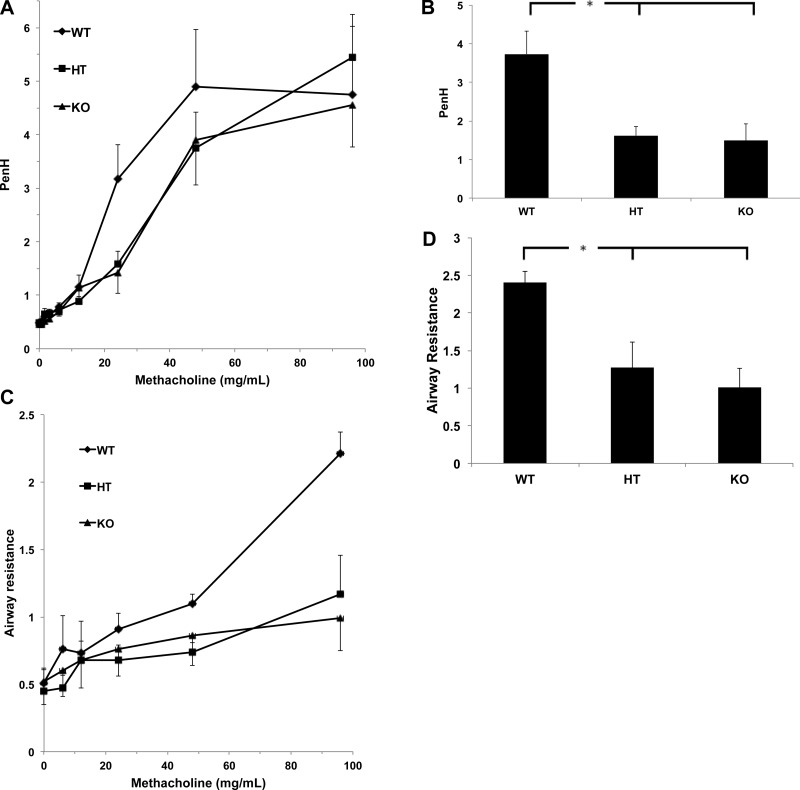
We next analyzed in vivo airway responses to bronchoconstrictor challenge via inhalation exposures to increasing concentrations of methacholine aerosols using a noninvasive method. Unrestrained and unanesthetized mice with an average age of 24 wk were placed in a barometric plethysmograph, and changes in box pressure were recorded as a measure of changes in breathing pattern (26). Penh measurements based on plethysmography to determine the delayed pause in expiration resulting from methacholine-induced airway constriction have been used as an empirical surrogate measure of changes in airway resistance (29). Airway Penh responses to methacholine were analyzed with mice exposed to increasing methacholine aerosol concentrations that range from 0 to 100 mg/ml for 1 min with 5 min postexposure recording of Penh. Although Penh values plateaued at the higher levels of methacholine challenge (Fig. 4A), knockout and heterozygote mice showed significantly (60 and 57%, respectively) decreased Penh responses at the lower methacholine levels, suggesting reduced airway reactivity in the lungs of animals lacking COX4i2 (Fig. 4B).
Figure 4.
Lung function. A) Enhanced pause (Penh) was determined in unrestrained and unanesthetized mice that were placed in a barometric plethysmograph and exposed to increasing amounts of methacholine aerosol. A bias airflow of 2 L/min was applied to prevent buildup of CO2. COX4i2-knockout (KO) and heterozygous (HT) mice show reduced Penh values at intermediate methacholine concentrations compared to wild-type (WT) mice, which plateau at high methacholine concentrations (n=7–11). B) Polynomial regression analysis (y = a + bx + cx2 …) of data shown in panel C, solved for x = 25 mg methacholine, shows significantly reduced Penh responses for KO and HT in comparison to WT mice. *P < 0.02. C) Invasive lung function was determined in anesthetized (urethane, 1.5 g/kg body wt) mice using a tracheal and esophageal cannula. Airway resistance was determined as the ratio of transpulmonary pressure (ΔP) to flow from pressure changes across box pneumotach (ΔF) at points of equal volume at 70% of the breathing cycle. At methacholine concentrations >48 mg/ml, differences in airway resistance between the KO and HT compared with WT mice became significant in that the KO and HT show reduced airway resistance (n=4). D) Polynomial regression analysis (as in panel B) of data shown in panel C, solved for x = 100 mg methacholine, shows significantly reduced airway resistance for the KO and HT in comparison to the WT mice. *P < 0.02.
Since Penh is not dependent on direct measurements of changes in airway caliber, we next analyzed airway reactivity using an invasive approach. Spontaneously breathing (via a tracheal cannula) anesthetized mice from the same cohort were subjected to inhalation exposures to increasing concentrations of methacholine aerosol. The animals were placed in a whole body plethysmograph to analyze airway flow with measurements of transpulmonary pressure achieved using an esophageal cannula. Heterozygote and knockout mice again showed 47 and 58% reduced airway reactivity to methacholine, as illustrated by the greater resistance to airflow at a methacholine aerosol concentration of 100 mg/ml when compared with wild-type mice (Fig. 4C, D). These results confirm that mice lacking the COX4i2 isoform have hyporeactive airways.
Comprehensive phenotypic characterization of the COX4i2-knockout mice at the GMC
In addition to the studies above, the knockout mice underwent a broad phenotypic screening protocol at the GMC. Experimental results are shown in Supplemental Data for the following screens: dysmorphology, eye, and metabolism (Supplemental Fig. S1), neurology, and clinical chemistry (Supplemental Fig. S2), and hematology immunology (Supplemental Fig. S3). A detailed description of the GMC workflow has been published (36, 37). As part of this characterization, which was restricted to wild-type and knockout mice and did not include heterozygotes, we here present results of selected screens.
COX4i2-knockout mice present with lung pathology
A pathology screen was conducted on skin, heart, muscle, lung, cerebrum, cerebellum, thymus, spleen, cervical lymph nodes, trachea, thyroid and parathyroid glands, adrenal glands, esophagus, stomach, intestine, liver, pancreas, kidneys, reproductive organs, and urinary bladder. Of those, only lung tissue showed pathological alterations in the animals not challenged by allergen (Table 2). At the age of 18 wk, approximately half of the knockout animals presented with lung inflammation (Fig. 5A). The cytological analysis revealed a cell-type range from predominantly eosinophilic to a lymphocytic, macrophage, and plasma cell-rich composition (Fig. 5B–F). Nearly all of those animals presenting with inflammation also exhibited Charcot-Leyden crystals, hexagonal colorless crystals within the granulomas or the bronchi (Fig. 5B, D). In contrast, none of the wild-type animals had Charcot-Leyden crystals at 18 wk, despite the fact that 2 wild-type males presented with a mild pneumonia (Table 2). At the age of 40 wk, all female knockout animals analyzed showed a mild to moderate chronic inflammation of the lung tissue, predominantly with plasma cells and macrophages, as well as mild architectural changes of the lung, such as emphysema due to fibrosis. All of those animals had Charcot-Leyden crystals to various degrees. Similar histopathological changes were observed in the majority of the old mutant males. Among the old wild-type animals, few presented with mild pneumonia, and one male and female showed the formation of crystals (Table 2 and Fig. 5B–D).
TABLE 2.
Increased inflammation and crystal formation in the lungs of COX4i2-knockout mice
| Group | Crystals | Inflammation | Animals analyzed |
|---|---|---|---|
| Age 18 wk | |||
| WT females | 0 | 0 | 7 |
| KO females | 3 | 4 | 8 |
| WT males | 0 | 2 | 8 |
| KO males | 5 | 5 | 8 |
| Age 40 wk | |||
| WT females | 1? | 1 | 5 |
| KO females | 5 | 5 | 5 |
| WT males | 1 | 2 | 6 |
| KO males | 4 | 5 | 6 |
Columns show number of animals presenting with crystals and inflammation, respectively. WT, wild type; KO, knockout.
Figure 5.
Lung pathology of 18-wk-old (A) and 40-wk-old (B–F) COX4i2-knockout mice. Lungs were fixed in 4% buffered formalin and embedded in paraffin for histological examination. Sections (4 μm thick) were cut and stained with hematoxylin and eosin. Slides were analyzed using an Axioplan microscope (Zeiss, Oberkochen, Germany) and pictures were taken using the virtual imaging system Dotslide2.0 (Olympus, Tokyo, Japan). A) Crystalline structure (arrow) in the bronchus of an 18-wk-old mutant mouse. Right bronchus shows a mild increase of mucus secretion. B) Accumulation of crystals (circled area) surrounded by macrophages in a bronchus. Some alveolar macrophages are also seen in bronchi that are free of crystals (asterisk). The lung tissue shows an unspecific inflammation (arrow). C) Granulomas (arrows) in the lung. D) Higher magnification of the granuloma in panel C. The center contains crystals (a) that are surrounded by lymphocytes (b) and macrophages (c). E) Inflamed lung tissue with thickened arteries (asterisks). F) Higher magnification of the infiltrate in panel E, showing giant cells (circled area). It should be noted that pulmonary arteries that appear thickened in the images presented here are not thickened in the normal, i.e., noninflamed, areas of the lung (not shown).
To obtain a quantitative assessment of possible differences in baseline inflammatory status, lungs from both male and female wild-type, heterozygote, and knockout mice with an average age of 33 wk were subjected to collagenase dispersion, and the resulting inflammatory cells were differentially counted for nonspecific esterase-positive cell recoveries, representing macrophage and monocyte populations, and lymphocytes, neutrophils, and eosinophils (Table 3). Cells recovered by collagenase dispersion represent inflammatory cells from parenchymal airway and interstitial and marginated inflammatory cell pools (26). Less than 2.0% of recovered cells were unidentified but presumably represent noninflammatory cells that survived destruction of the extracellular matrix. Since no significant differences were observed between cell recoveries from male and female mice, the data were combined (Table 3). Although the total number of inflammatory cells recovered was enhanced in both heterozygote and knockout mice when compared with wild-type mice, considerable variability occurred, with nonparametric statistical analysis failing to demonstrate significance. However, the percentage distribution among the different cell populations demonstrated that although the combined populations of monocytes and macrophages (nonspecific esterase-positive cells) were similar among the 3 groups, the percentage of lymphocytes and eosinophils recovered increased significantly in the heterozygote and knockout mice (Table 3). Interestingly, tissue-recoverable neutrophils were relatively diminished in these two mutant groups when compared with wild-type mice. These results, generated at Wayne State University, are consistent with the enhanced baseline inflammation observed in the lungs of mice used by the GMC (Table 2). It should be noted that in a separate series of analyses, inflammatory cell recoveries from the same 24-wk-old mice used for assessing airway reactivity (Fig. 4) did not show any major differences in lung tissue inflammatory cell recoveries among the three genotypes (data not shown). These data suggest that inflammation cannot account for the observed strain differences in response to methacholine (Fig. 4) and indicate that major pathological strain differences do not occur until after 24 wk (Fig. 5).
TABLE 3.
Immune cell composition from collagenase dispersions of lung tissue
| Genotype | Total cells | NSE+ (%) | Lymphocytes (%) | Neutrophils (%) | Eosinophils (%) |
|---|---|---|---|---|---|
| WT | 196 ± 13 | 26.7 ± 3.6 | 18.1 ± 2.0 | 52.3 ± 3.8 | 2.2 ± 0.7 |
| HT | 242 ± 29 | 29.9 ± 2.6 | 34.6 ± 3.2* | 27.9 ± 5.5* | 6.2 ± 1.6* |
| KO | 280 ± 71 | 33.8 ± 2.8 | 35.9 ± 2.0* | 22.7 ± 3.8* | 7.5 ± 1.3* |
Results represent means ± se × 105 for 6 mice/genotype. NSE+, nonspecific esterase-positive cells (representing the combined monocyte and macrophage cell populations); WT, wild type; HT, heterozygote; KO, knockout.
P < 0.05 vs. WT.
In the ovalbumin-challenged mice, the hematoxylin and eosin (H&E)-stained lung sections revealed mild to moderate eosinophilic pneumonia, granulomas, and multinucleated giant cells (so-called foreign body giant cells) in all challenged animals, without obvious differences between mutant and wild-type animals (not shown). The granulomas formed around the bronchi and blood vessels, therefore originating from the bronchi-associated lymphatic tissue. The vessel-associated granulomas presented the tendency to compress the vessels and to invade the vessel wall as a form of a vasculitis (not shown). The eosinophilic pneumonia within the challenged group is a normal reaction of immune-competent animals treated with proteins of foreign origin. Mainly characterized in humans, except for Löffler Syndrome, eosinophilic pneumonia is caused either by asthma due to an allergic reaction or by parasitic infection (39). Consistent with the above observation of no genotype differences in response to ovalbumin challenge and allergen-induced enhancement in airway lymphocyte and eosinophil numbers, bronchoalveolar lavage (BAL) recoveries of inflammatory cells from all three ovalbumin-challenged sensitized mouse groups demonstrated similar patterns of inflammatory cell recovery. BAL recoveries from wild-type mice were 23, 16, 2, and 79% for alveolar macrophages, T lymphocytes, neutrophils, and eosinophils, respectively. Comparable results from these unchallenged wild-type mice demonstrated the alveolar macrophage to be the major recoverable cell, representing a mean value of 89% of total bronchoalveolar lavageable cells, with lymphocyte, neutrophil, and eosinophil recoveries giving values of 1, 3, and 8%, respectively. Unlike whole-lung measurements of baseline inflammatory cells (Table 3), increased percentage recoveries of lymphocytes and eosinophils were found not to be statistically significant.
COX4i2-knockout mice display distinct sex-dependent features
Phenotypically, lean mass of COX4i2-knockout female mice was reduced (12% reduction compared to wild type), as opposed to their male counterparts, which had a reduction of ∼2% (Fig. 6A), suggesting a possible COX4i2-dependent sex-based imbalance in energy regulation in muscle. Consistent with this possibility, muscle force of female knockout mice was decreased, as evidenced by a reduction in their 4-paw grip strength. Grip strength was ∼14% lower in the female knockout mice compared to female wild-type mice, as opposed to only a nonsignificant 6% reduction in males (Fig. 6B).
Figure 6.
Sex-based differences in the COX4i2-knockout mice. A) Total body lean mass measured for age-matched wild-type (WT) and knockout (KO) mice. Compared to WT females, KO females had significantly lower lean masses, whereas no such effect was seen for the males (P=0.04, Wilcoxon rank sum test). B) Muscle strength, measured as grip force using all four paws, reveals reduction for female KO in comparison to female WT animals, but no such effect was seen for the males (P=0.01, Wilcoxon rank sum test). C) Intraperitonial glucose tolerance test on WT and KO mice. Glucose was injected intraperitoneally after a period of food withdrawal, and absolute blood glucose levels were measured at 0, 15, 30, 60, and 120 min postinjection. Female KO mice had lower glucose levels compared to WT females at all time points tested (differences at 15 and 30 min were statistically significant; P=0.003, Wilcoxon rank sum test). D) Localization of the putative estrogen-responsive element (ERE) in the COX4i2 promoter relative to the start ATG (arrow) located in exon II. Also depicted is the corresponding sequence in the mouse promoter and the consensus ERE.
Glucose is an important cellular energy source. To test whether COX4i2-knockout mice showed sex-specific differences in glucose handling, an intraperitoneal glucose tolerance test was performed. On the whole, knockout females but not males tolerated glucose challenge better than controls. Female knockout mice had lower blood glucose levels than the wild-type controls at all time points tested, whereas no genotype-related differences were observed in males (Fig. 6C). Kinetically, the glucose levels in female knockout mice remained lower than in the wild-type counterparts until 2 h after glucose administration. The difference in blood glucose levels among the female knockout mice compared to female wild-type mice was statistically significant at 15 and 30 min (Fig. 6C, compare open and closed squares).
A second sex-related difference was observed in a preliminary immunological profile. Both COX4i2-knockout and wild-type mice showed an increase in IgE levels in females but not in males (Table 4). Although female mice are known to have higher basal IgE levels than males, the absence of COX4i2 in females resulted in a trend toward even higher IgE levels. IgE is the immunoglobulin known to play a pivotal role in the immune response to allergens, and although no statistical significance was reached, it can be hypothesized that COX4i2 could be involved in the regulation of the immune response to allergens. A difference was also observed in the mean oxygen consumption rate. Although COX4i2 is mainly expressed in the lung and trachea, a trend toward a reduction in the overall rate of oxygen consumption was observed in the knockout females (8% decrease relative to wild type), whereas the knockout males displayed no such change. If oxygen consumption is adjusted for lean mass, female knockout mice appear to be comparable to wild-type females.
TABLE 4.
Sex-based differences in whole-body oxygen consumption and IgE
| Parameter | Male |
Female |
||
|---|---|---|---|---|
| WT | KO | WT | KO | |
| Vo2 (ml/h) | 113.36 ± 11.49 | 112.04 ± 5.01 | 101.42 ± 8.59 | 93.55 ± 4.99 |
| IgE (ng/ml) | 57 ± 110 | 46 ± 64 | 819 ± 578 | 1099 ± 838 |
Whole-body oxygen consumption (Vo2) was reduced in knockout (KO) females compared to wild-type (WT) females, whereas males did not show such a pattern. IgE levels also suggested a COX4i2-knockout sex-dependent effect, with a trend to increasing levels in the KO females but not males compared to WT mice.
Some of the observed sex-specific differences may be explained by the presence of a putative estrogen-responsive element (ERE) in the COX4i2 promoter (Fig. 6D). This notion is supported by reporter-gene experiments using COX4i2 promoter constructs. Cotransfection with an estrogen receptor α (ERα) expression plasmid revealed significant induction of reporter-gene activity only in COX4i2 promoter constructs containing the ERE (Supplemental Fig. S4B).
DISCUSSION
COX subunit 4 operates as an allosteric energy sensor by binding allosteric inhibitor ATP or activator ADP, adjusting COX activity to cellular energy demand, and it was postulated as a pivotal regulator of COX activity in higher organisms in contrast to bacteria that lack this subunit (40).
In mammals, the COX4i1/COX4i2 isoform pair arose ∼320 million years ago, which represents the earliest gene duplication event compared to other COX subunit isoform pairs (16). During this ancient period, oxygen concentrations fluctuated from ∼13% atmospheric O2 in the Devonian period ∼350 million years ago to 35% near the Carboniferous-Paleozoic transition ∼280 million years ago (41). COX4i2 expression is induced strongly after birth when the lung is exposed to atmospheric O2 (16), and transcription of COX4i2 but not COX4i1 is regulated by the oxygen concentration (42, 43).
The presence of a tissue-specific isoform of this regulatory subunit in lung suggests an energetic tissue-specific adaptation. We show that knockout of COX4i2 results in ∼50% reduction in COX activity, both in the presence of allosteric activator ADP and inhibitor ATP (Fig. 3). Consistent results were obtained when COX was isolated from cow lung and liver, showing that isolated COX4i2-containing lung COX is ∼2-fold more active compared to the liver isozyme (Fig. 1).
COX is the only complex in the mammalian OxPhos system with known tissue-specific subunit isoforms. Apparently, COX activity can be linked to mitochondrial capacity found in various organs (44): For the better-studied COX isozymes from liver and heart (45) and lung as presented here, COX activity increases in the sequence heart type < liver type < lung type, which inversely correlates with the mitochondrial capacity of the corresponding tissues. This observation suggests an adaptation of COX activity based on mitochondrial mass per tissue volume and equips lung tissue with an enzyme with a higher basal turnover compared to the liver and heart isozymes. This model is also consistent with the observation that bacterial enzymes, which lack homologs of the 10 nuclear-encoded COX subunits, are more active compared to mammalian COX. Therefore, in higher organisms COX activity is modulated depending on isoform composition and other regulatory mechanisms including allosteric regulation and reversible phosphorylations.
Loss of COX4i2 results in decreased COX activity and, as expected, in decreased ATP levels in the lung (Fig. 3). We reported previously that treatment of liver tissue with the oldest antiasthma drug, theophylline, a phosphodiesterase inhibitor, leads to Tyr304 phosphorylation of COX catalytic subunit I and enzyme inhibition, and we observed a similar effect of decreased COX activity and decreased ATP levels in lung tissue after theophylline treatment, applying concentrations used in therapy (18): theophylline treatment → COX phosphorylation → COX activity ↓ → ATP ↓ → airway constriction ↓ → asthma relief. Because energy is required for airway constriction, reduction of cellular energy levels would lead to smooth muscle relaxation and asthma relief. Indeed, COX4i2-knockouts showed decreased airway hyperresponsiveness after challenge with methacholine (Fig. 4), supporting our model: absence of COX4i2 → COX activity ↓ → ATP ↓ → airway constriction ↓ → reduced airway-responsiveness. Interestingly, airway responsiveness of the heterozygotes and knockouts are almost indistinguishable (Fig. 4B–E), rather than showing a graded response as a function of copy number. This finding suggests that having only a single copy of the higher activity isoform in bronchial smooth muscle represents a functional haploinsufficiency with respect to airway constriction.
While improvements have been made to known drug classes for asthma therapy, no novel treatments have been identified or developed for the past 3 decades (46). Surprisingly, one possible functional target for asthma therapy, the mitochondrial respiratory chain, has not yet been analyzed in detail. In the few asthma studies tangentially considering mitochondria, altered respiratory chain function was observed, e.g., mitochondrial dysfunction due to a mutation in the mitochondrial DNA (47), or lack of COX activity in a patient after treatment with long-acting β2-agonist formoterol (48), perhaps due to phosphorylation of COX. Furthermore, mitochondrial proliferation was required for the bronchial smooth muscle thickening during airway remodeling in asthma (49).
Asthma is typified by airway inflammation and hyperreactivity, mucus overproduction, and airway remodeling. Key second messengers calcium and cAMP are involved in these processes and are currently exploited for treatment. If asthma pathology can be linked to mitochondria through increased calcium release, e.g., induced by adenosine, a mediator of inflammation during asthma (50), mitochondria would be a functional target for asthma therapy. Calcium is the strongest activator of mitochondrial function (51), likely through dephosphorylation of proteins including COX (9). In contrast, the most common asthma medications, β-agonists and phosphodiesterase inhibitors acting on the cAMP pathway, may counteract calcium signaling and alter energy production through phosphorylation and inhibition of COX and perhaps other components of the OxPhos system.
Absence of COX4i2 results in lung pathology that worsens over time (Fig. 5), suggesting that its expression is essential for preserving a healthy lung. Since maintenance of cellular function is an energy-costly process, reduced energy levels due to loss of COX4i2 may be insufficient to sustain key functions, such as mucus production and ciliary escalator action for fighting airborne pathogens and removal of airborne dust particles. In addition, it may lessen wound repair processes of the airway epithelium, which are triggered by cellular ATP release (52).
The deposition of inert material in the form of crystals (Fig. 5) is a common mechanism in the pathophysiology of tissues that increases with age. Absence of COX4i2 increases Charcot-Leyden crystal formation, which is a characteristic finding in patients with eosinophilic pneumonia, especially in those with a longstanding disease. These crystals are thought to consist of breakdown products of eosinophils (53, 54). It is possible that lack of COX4i2 in lung tissue triggers a higher turnover of the eosinophilic cells accompanied by increased cell death, therefore leading to increased accumulation of breakdown products.
COX4i2-knockout mice displayed interesting sex-specific features. Female knockout mice possessed a reduced lean mass and showed a reduction in muscle force compared to wild-type females, suggesting a possible sex-dependent imbalance in energy regulation. In addition, intraperitoneal glucose tolerance tests revealed no genotype related difference in glucose levels in male knockouts compared to male controls, but a significant decrease of blood glucose levels was seen in the female knockout mice (Fig. 6C). In addition, the immunological screen showed an increase in the basal IgE levels in female mice, in agreement with previous studies emphasizing higher IgE levels in females compared to males (55, 56). IgE has been the immunoglobulin traditionally linked to an allergic response in conditions such as asthma. A female predominance exists in the susceptibility of young adults to allergic airway inflammation (57). In an allergic response, neutrophils are the first cells that are recruited to the antigenic sites (58), and patients with asthma have been shown to express high-affinity IgE receptors (59). IgE prolongs the survival of neutrophils in asthma by retaining Bax in the cytoplasm and decreasing Smac release from mitochondria (60). IgE, after binding its receptor, also primes other immune cells such as basophils and mast cells to release inflammatory mediators that further aggravate the symptoms of asthma. Female sex hormones estrogen and progesterone inhibit neutrophil apoptosis by inhibiting Cytc release from the mitochondria (61). In contrast, testosterone reduces the amount of superoxide generated by neutrophils thereby attenuating their function (62). This pattern might at least in part explain reduced susceptibility of males to allergic inflammation since testosterone and estrogen have opposing roles on neutrophil response. IgE levels in female COX4i2-knockouts are further augmented giving rise to the possibility of more tissue damage in an allergic response compared to the males.
A preliminary assessment of a possible hormonal regulation of the lung isoform gene, which might explain some of the aforementioned sex-specific differences, revealed a putative ERE in the proximal promoter (Fig. 6D). Scanning of the 3-kb DNA sequence 5′ of the transcription start site of the human COX4i2 gene revealed a sequence that was similar to an ERE. This sequence encompasses the −1547 to −1535 region from the start codon, which is located in exon 2 (5′-TGACCtcaGGTGA-3′). The identified putative ERE in the COX4i2 promoter is not identical to the classical consensus sequence (5′-GGTCAnnnTGACC-3′). However, the majority of functional EREs contain imperfect EREs (63), and a recent study has shown that approximately half of the DNA sequences to which ERα binds are not consensus sequences (64). Indeed, reporter-gene analysis using COX4i2 constructs containing the ERE showed increased reporter-gene activity when cotransfected with estrogen receptor 1α isoform, whereas a shorter construct lacking the element is not induced (Supplemental Fig. S4B). Furthermore, there is evidence that COX4i2 can be induced in brain astrocytes under stress conditions and that this effect shows sex-specific differences (65), and earlier work revealed that estrogen up-regulates COX activity in the brain (66), which may be explained with an induction of COX4i2. Taken together, estrogen, the predominant sex hormone in females, may modulate COX4i2 expression; however, more work is necessary to prove or disprove the functionality of the proposed ERE.
In the human COX4i2 gene, only one mutation has been reported to date, leading to a homozygous change of glutamic acid at codon 138 to lysine. Interestingly, this mutation causes congenital exocrine pancreatic insufficiency (67). However, lung function was never examined in the patients (Dr. Orly Elpeleg, Hadassah Hebrew University Medical Center, Jerusalem, Israel; personal communication; March 10, 2012). In the COX4i2-knockout mice, the pancreas histology was indistinguishable from the wild-type mice and showed no signs of dysfunction (Supplemental Fig. S4C). In addition, we performed quantitative real-time PCR experiments to determine COX4i2 expression levels in the pancreas of wild-type mice and detected only negligible (<1% compared to lung) amounts (Supplemental Fig. S4D). Our data suggest that, at least in mice, COX4i2 is not involved directly in pancreatic function. It remains to be seen whether COX4i2 is expressed in the pancreas of humans, or whether the observed pathology is indirectly caused by deficient functions of organs such as the lung, where the gene is highly expressed.
In summary, we here characterize the effect of the COX4i2 gene knockout in vivo for the first time. Our results highlight the importance of cellular energetics for lung function and provide a direct link between COX activity and airway reactivity, identifying COX as a possible functional target for future treatment of airway diseases.
Supplementary Material
Acknowledgments
The authors thank R. Seeliger, S. Bothur, R. Fischer, M. Grandl, E. Holupirek, M. Kugler, A. Langer, K. Laube, J. Müller, E. Samson, F. Schleicher, D. Schmidt, W. Schneider, A. E. Schwarz, W. Stettinger, L. Thumann, S. Wittich, and Anja Wohlbier, as well as the German Mouse Clinic (GMC; Neuherberg, Germany) animal caretaker team, M. Huber, B. Schön, H. Marr, A. Miedl, T. Reichelt, M. Gerstlauer, R. Huber, and H. Wenig, for expert technical help. The authors thank Christopher Sinkler (Wayne State University) for conducting quantitative real-time PCR experiments.
Research described in this article was supported by Philip Morris USA Inc. and Philip Morris International (M.H.), the Center for Molecular Medicine and Genetics, Wayne State University School of Medicine (M.H.), grant GM089900 from the U.S. National Institutes of Health (NIH; M.H.), and a grant supplement from the NIH (GM48517; L.I.G.). Work performed at the GMC was funded by a grant from the German Federal Ministry of Education and Research (BMBF) to the German Center for Diabetes Research (DZD eV) and to the GMC (NGFN-Plus grants 01GS0850, 01GS0851, 01GS0868, 01GS0869, and 01GS0854) as well as by an EU grant (EUMODIC, LSHG-2006-037188, GMC).
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
- ΔΨm
- mitochondrial membrane potential
- COX
- cytochrome c oxidase
- COX4i1
- COX subunit 4 isoform 1
- COX4i2
- COX subunit 4 isoform 2
- Cytc
- cytochrome c
- EGTA
- ethylene glycol-bis-(B-aminoethyl ether)-N,N,N′,N′–tetraacetic acid
- ERE
- estrogen-responsive element
- ETC
- electron transport chain
- OxPhos
- oxidative phosphorylation
- PAP
- pulmonary arterial pressure
REFERENCES
- 1. Tsukihara T., Aoyama H., Yamashita E., Tomizaki T., Yamaguchi H., Shinzawa-Itoh K., Nakashima R., Yaono R., Yoshikawa S. (1996) The whole structure of the 13-subunit oxidized cytochrome c oxidase at 2.8 Å. Science 272, 1136–1144 [DOI] [PubMed] [Google Scholar]
- 2. Villani G., Greco M., Papa S., Attardi G. (1998) Low reserve of cytochrome c oxidase capacity in vivo in the respiratory chain of a variety of human cell types. J. Biol. Chem. 273, 31829–31836 [DOI] [PubMed] [Google Scholar]
- 3. Acin-Perez R., Bayona-Bafaluy M. P., Bueno M., Machicado C., Fernandez-Silva P., Perez-Martos A., Montoya J., Lopez-Perez M. J., Sancho J., Enriquez J. A. (2003) An intragenic suppressor in the cytochrome c oxidase I gene of mouse mitochondrial DNA. Hum. Mol. Genet. 12, 329–339 [DOI] [PubMed] [Google Scholar]
- 4. Villani G., Attardi G. (1997) In vivo control of respiration by cytochrome c oxidase in wild-type and mitochondrial DNA mutation-carrying human cells. Proc. Natl. Acad. Sci. U. S. A. 94, 1166–1171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Piccoli C., Scrima R., Boffoli D., Capitanio N. (2006) Control by cytochrome c oxidase of the cellular oxidative phosphorylation system depends on the mitochondrial energy state. Biochem. J. 396, 573–583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dalmonte M. E., Forte E., Genova M. L., Giuffre A., Sarti P., Lenaz G. (2009) Control of respiration by cytochrome c oxidase in intact cells: role of the membrane potential. J. Biol. Chem. 284, 32331–32335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pacelli C., Latorre D., Cocco T., Capuano F., Kukat C., Seibel P., Villani G. (2010) Tight control of mitochondrial membrane potential by cytochrome c oxidase. Mitochondrion 11, 334–341 [DOI] [PubMed] [Google Scholar]
- 8. Hinkle P. C., Kumar M. A., Resetar A., Harris D. L. (1991) Mechanistic stoichiometry of mitochondrial oxidative phosphorylation. Biochemistry 30, 3576–3582 [DOI] [PubMed] [Google Scholar]
- 9. Hüttemann M., Lee I., Pecinova A., Pecina P., Przyklenk K., Doan J. W. (2008) Regulation of oxidative phosphorylation, the mitochondrial membrane potential, and their role in human disease. J. Bioenerg. Biomembr. 40, 445–456 [DOI] [PubMed] [Google Scholar]
- 10. Grossman L. I., Lomax M. I. (1997) Nuclear genes for cytochrome c oxidase. Biochim. Biophys. Acta 1352, 174–192 [DOI] [PubMed] [Google Scholar]
- 11. Hüttemann M., Jaradat S., Grossman L. I. (2003) Cytochrome c oxidase of mammals contains a testes-specific isoform of subunit VIb—the counterpart to testes-specific cytochrome c? Mol. Reprod. Dev. 66, 8–16 [DOI] [PubMed] [Google Scholar]
- 12. Hüttemann M., Schmidt T. R., Grossman L. I. (2003) A third isoform of cytochrome c oxidase subunit VIII is present in mammals. Gene 312, 95–102 [DOI] [PubMed] [Google Scholar]
- 13. Napiwotzki J., Shinzawa-Itoh K., Yoshikawa S., Kadenbach B. (1997) ATP and ADP bind to cytochrome c oxidase and regulate its activity. Biol. Chem. 378, 1013–1021 [DOI] [PubMed] [Google Scholar]
- 14. Arnold S., Kadenbach B. (1999) The intramitochondrial ATP/ADP-ratio controls cytochrome c oxidase activity allosterically. FEBS Lett. 443, 105–108 [DOI] [PubMed] [Google Scholar]
- 15. Acin-Perez R., Gatti D. L., Bai Y., Manfredi G. (2011) Protein phosphorylation and prevention of cytochrome oxidase inhibition by ATP: coupled mechanisms of energy metabolism regulation. Cell Metab. 13, 712–719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hüttemann M., Kadenbach B., Grossman L. I. (2001) Mammalian subunit IV isoforms of cytochrome c oxidase. Gene 267, 111–123 [DOI] [PubMed] [Google Scholar]
- 17. Lee I., Salomon A. R., Yu K., Samavati L., Pecina P., Pecinova A., Hüttemann M. (2009) Isolation of regulatory-competent, phosphorylated cytochrome c oxidase. Methods Enzymol. 457, 193–210 [DOI] [PubMed] [Google Scholar]
- 18. Lee I., Salomon A. R., Ficarro S., Mathes I., Lottspeich F., Grossman L. I., Hüttemann M. (2005) cAMP-dependent tyrosine phosphorylation of subunit I inhibits cytochrome c oxidase activity. J. Biol. Chem. 280, 6094–6100 [DOI] [PubMed] [Google Scholar]
- 19. Tybulewicz V. L., Crawford C. E., Jackson P. K., Bronson R. T., Mulligan R. C. (1991) Neonatal lethality and lymphopenia in mice with a homozygous disruption of the c-abl proto-oncogene. Cell 65, 1153–1163 [DOI] [PubMed] [Google Scholar]
- 20. Chomczynski P., Sacchi N. (1987) Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 162, 156–159 [DOI] [PubMed] [Google Scholar]
- 21. Bassett D. J., Elbon C. L., Reichenbaugh S. S. (1992) Respiratory activity of lung mitochondria isolated from oxygen-exposed rats. Am. J. Physiol. 263, L439–445 [DOI] [PubMed] [Google Scholar]
- 22. Fisher A. B., Scarpa A., LaNoue K. F., Bassett D., Williamson J. R. (1973) Respiration of rat lung mitochondria and the influence of Ca 2+ on substrate utilization. Biochemistry 12, 1438–1445 [DOI] [PubMed] [Google Scholar]
- 23. Lee I., Pecinova A., Pecina P., Neel B. G., Araki T., Kucherlapati R., Roberts A. E., Hüttemann M. (2010) A suggested role for mitochondria in Noonan syndrome. Biochim. Biophys. Acta 1802, 275–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Weissmann N., Akkayagil E., Quanz K., Schermuly R. T., Ghofrani H. A., Fink L., Hanze J., Rose F., Seeger W., Grimminger F. (2004) Basic features of hypoxic pulmonary vasoconstriction in mice. Respir. Physiol. Neurobiol. 139, 191–202 [DOI] [PubMed] [Google Scholar]
- 25. Weissmann N., Dietrich A., Fuchs B., Kalwa H., Ay M., Dumitrascu R., Olschewski A., Storch U., Mederos y Schnitzler M., Ghofrani H. A., Schermuly R. T., Pinkenburg O., Seeger W., Grimminger F., Gudermann T. (2006) Classical transient receptor potential channel 6 (TRPC6) is essential for hypoxic pulmonary vasoconstriction and alveolar gas exchange. Proc. Natl. Acad. Sci. U. S. A. 103, 19093–19098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bassett D., Hirata F., Gao X., Kannan R., Kerr J., Doyon-Reale N., Wilson S., Lieh-Lai M. (2010) Reversal of methylprednisolone effects in allergen-exposed female BALB/c mice. J. Toxicol. Environ. Health A. 73, 711–724 [DOI] [PubMed] [Google Scholar]
- 27. Birrell M. A., Battram C. H., Woodman P., McCluskie K., Belvisi M. G. (2003) Dissociation by steroids of eosinophilic inflammation from airway hyperresponsiveness in murine airways. Respir. Res. 4, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. DeLorme M. P., Moss O. R. (2002) Pulmonary function assessment by whole-body plethysmography in restrained versus unrestrained mice. J. Pharmacol. Toxicol. Methods 47, 1–10 [DOI] [PubMed] [Google Scholar]
- 29. Hamelmann E., Schwarze J., Takeda K., Oshiba A., Larsen G. L., Irvin C. G., Gelfand E. W. (1997) Noninvasive measurement of airway responsiveness in allergic mice using barometric plethysmography. Am. J. Respir. Crit. Care Med. 156, 766–775 [DOI] [PubMed] [Google Scholar]
- 30. Whitehead G. S., Walker J. K., Berman K. G., Foster W. M., Schwartz D. A. (2003) Allergen-induced airway disease is mouse strain dependent. Am. J. Physiol. Lung Cell. Mol. Physiol. 285, L32–L42 [DOI] [PubMed] [Google Scholar]
- 31. Hahn Y. S., Taube C., Jin N., Takeda K., Park J. W., Wands J. M., Aydintug M. K., Roark C. L., Lahn M., O'Brien R. L., Gelfand E. W., Born W. K. (2003) V gamma 4+ gamma delta T cells regulate airway hyperreactivity to methacholine in ovalbumin-sensitized and challenged mice. J. Immunol. 171, 3170–3178 [DOI] [PubMed] [Google Scholar]
- 32. DeLorme M. P., Yang H., Elbon-Copp C., Gao X., Barraclough-Mitchell H., Bassett D. J. (2002) Hyperresponsive airways correlate with lung tissue inflammatory cell changes in ozone-exposed rats. J. Toxicol. Environ. Health A 65, 1453–1470 [DOI] [PubMed] [Google Scholar]
- 33. Holt P. G., Degebrodt A., Venaille T., O'Leary C., Krska K., Flexman J., Farrell H., Shellam G., Young P., Penhale J., Robertson T., Papadimitriou J. M. (1985) Preparation of interstitial lung cells by enzymatic digestion of tissue slices: preliminary characterization by morphology and performance in functional assays. Immunology 54, 139–147 [PMC free article] [PubMed] [Google Scholar]
- 34. Schultheis A. H., Bassett D. J. (1994) Guinea pig lung inflammatory cell changes following acute ozone exposure. Lung 172, 169–181 [DOI] [PubMed] [Google Scholar]
- 35. Li C. Y., Lam K. W., Yam L. T. (1973) Esterases in human leukocytes. J. Histochem. Cytochem. 21, 1–12 [DOI] [PubMed] [Google Scholar]
- 36. Gailus-Durner V., Fuchs H., Becker L., Bolle I., Brielmeier M., Calzada-Wack J., Elvert R., Ehrhardt N., Dalke C., Franz T. J., Grundner-Culemann E., Hammelbacher S., Hölter S. M., Hölzlwimmer G., Horsch M., Javaheri A., Kalaydjiev S. V., Klempt M., Kling E., Kunder S., Lengger C., Lisse T., Mijalski T., Naton B., Pedersen V., Prehn C., Przemeck G., Rácz I., Reinhard C., Reitmeir P., Schneider I., Schrewe A., Steinkamp R., Zybill C., Adamski J., Beckers J., Behrendt H., Favor J., Graw J., Heldmaier G., Höfler H., Ivandic B., Katus H., Kirchhof P., Klingenspor M., Klopstock T., Lengeling A., Müller W., Ohl F., Ollert M., Quintanilla-Martinez L., Schmidt J., Schulz H., Wolf E., Wurst W., Zimmer A., Busch D. H., Hrabě de Angelis M. (2005) Introducing the German Mouse Clinic: open access platform for standardized phenotyping. Nat. Methods 2, 403–404 [DOI] [PubMed] [Google Scholar]
- 37. Fuchs H., Gailus-Durner V., Adler T., Aguilar-Pimentel J. A., Becker L., Calzada-Wack J., Da Silva-Buttkus P., Neff F., Gotz A., Hans W., Hölter S. M., Horsch M., Kastenmüller G., Kemter E., Lengger C., Maier H., Matloka M., Moller G., Naton B., Prehn C., Puk O., Rácz I., Rathkolb B., Römisch-Margl W., Rozman J., Wang-Sattler R., Schrewe A., Stöger C., Tost M., Adamski J., Aigner B., Beckers J., Behrendt H., Busch D. H., Esposito I., Graw J., Illig T., Ivandic B., Klingenspor M., Klopstock T., Kremmer E., Mempel M., Neschen S., Ollert M., Schulz H., Suhre K., Wolf E., Wurst W., Zimmer A., Hrabě de Angelis M. (2011) Mouse phenotyping. Methods 53, 120–135 [DOI] [PubMed] [Google Scholar]
- 38. Pecinova A., Drahota Z., Nuskova H., Pecina P., Houstek J. (2011) Evaluation of basic mitochondrial functions using rat tissue homogenates. Mitochondrion 11, 722–728 [DOI] [PubMed] [Google Scholar]
- 39. Rosai J., Ed. (2004) Rosai and Ackerman's Surgical Pathology, Edinburgh, Mosby [Google Scholar]
- 40. Ludwig B., Bender E., Arnold S., Hüttemann M., Lee I., Kadenbach B. (2001) Cytochrome c oxidase and the regulation of oxidative phosphorylation. Chembiochem 2, 392–403 [DOI] [PubMed] [Google Scholar]
- 41. Dudley R. (1998) Atmospheric oxygen, giant Paleozoic insects and the evolution of aerial locomotor performance. J. Exp. Biol. 201(Pt. 8), 1043–1050 [DOI] [PubMed] [Google Scholar]
- 42. Hüttemann M., Lee I., Liu J., Grossman L. I. (2007) Transcription of mammalian cytochrome c oxidase subunit IV-2 is controlled by a novel conserved oxygen responsive element. FEBS J. 274, 5737–5748 [DOI] [PubMed] [Google Scholar]
- 43. Fukuda R., Zhang H., Kim J. W., Shimoda L., Dang C. V., Semenza G. L. (2007) HIF-1 regulates cytochrome oxidase subunits to optimize efficiency of respiration in hypoxic cells. Cell 129, 111–122 [DOI] [PubMed] [Google Scholar]
- 44. Benard G., Faustin B., Passerieux E., Galinier A., Rocher C., Bellance N., Delage J. P., Casteilla L., Letellier T., Rossignol R. (2006) Physiological diversity of mitochondrial oxidative phosphorylation. Am. J. Physiol. Cell Physiol. 291, C1172–C1182 [DOI] [PubMed] [Google Scholar]
- 45. Vijayasarathy C., Biunno I., Lenka N., Yang M., Basu A., Hall I. P., Avadhani N. G. (1998) Variations in the subunit content and catalytic activity of the cytochrome c oxidase complex from different tissues and different cardiac compartments. Biochim. Biophys. Acta 1371, 71–82 [DOI] [PubMed] [Google Scholar]
- 46. Holgate S. T. (2005) Exacerbations: the asthma paradox. Am. J. Respir. Crit. Care Med. 172, 941–943 [DOI] [PubMed] [Google Scholar]
- 47. Shanske A. L., Shanske S., Silvestri G., Tanji K., Wertheim D., Lipper S. (1993) MELAS point mutation with unusual clinical presentation. Neuromuscul. Disord 3, 191–193 [DOI] [PubMed] [Google Scholar]
- 48. Kiernan M. C., Bullpitt P., Chan J. H. (2004) Mitochondrial dysfunction and rod-like lesions associated with administration of beta2 adrenoceptor agonist formoterol. Neuromuscul. Disord 14, 375–377 [DOI] [PubMed] [Google Scholar]
- 49. Trian T., Benard G., Begueret H., Rossignol R., Girodet P. O., Ghosh D., Ousova O., Vernejoux J. M., Marthan R., Tunon-de-Lara J. M., Berger P. (2007) Bronchial smooth muscle remodeling involves calcium-dependent enhanced mitochondrial biogenesis in asthma. J. Exp. Med. 204, 3173–3181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Yamano K., Inoue M., Masaki S., Saki M., Ichimura M., Satoh M. (2005) Human adenosine A(3) receptor leads to intracellular Ca(2+) mobilization but is insufficient to activate the signaling pathway via phosphoinositide 3-kinase gamma in mice. Biochem. Pharmacol. 70, 1487–1496 [DOI] [PubMed] [Google Scholar]
- 51. Robb-Gaspers L. D., Burnett P., Rutter G. A., Denton R. M., Rizzuto R., Thomas A. P. (1998) Integrating cytosolic calcium signals into mitochondrial metabolic responses. EMBO J. 17, 4987–5000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Wesley U. V., Bove P. F., Hristova M., McCarthy S., van der Vliet A. (2007) Airway epithelial cell migration and wound repair by ATP-mediated activation of dual oxidase 1. J. Biol. Chem. 282, 3213–3220 [DOI] [PubMed] [Google Scholar]
- 53. Weller P. F., Bach D. S., Austen K. F. (1984) Biochemical characterization of human eosinophil Charcot-Leyden crystal protein (lysophospholipase). J. Biol. Chem. 259, 15100–15105 [PubMed] [Google Scholar]
- 54. Ackerman S. J., Liu L., Kwatia M. A., Savage M. P., Leonidas D. D., Swaminathan G. J., Acharya K. R. (2002) Charcot-Leyden crystal protein (galectin-10) is not a dual function galectin with lysophospholipase activity but binds a lysophospholipase inhibitor in a novel structural fashion. J. Biol. Chem. 277, 14859–14868 [DOI] [PubMed] [Google Scholar]
- 55. Corteling R., Trifilieff A. (2004) Gender comparison in a murine model of allergen-driven airway inflammation and the response to budesonide treatment. BMC Pharmacol. 4, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Okuyama K., Wada K., Chihara J., Takayanagi M., Ohno I. (2008) Sex-related splenocyte function in a murine model of allergic asthma. Clin. Exp. Allergy 38, 1212–1219 [DOI] [PubMed] [Google Scholar]
- 57. Carey M. A., Card J. W., Voltz J. W., Arbes S. J., Jr., Germolec D. R., Korach K. S., Zeldin D. C. (2007) It's all about sex: gender, lung development and lung disease. Trends Endocrinol. Metab. 18, 308–313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Monteseirin J. (2009) Neutrophils and asthma. J. Investig. Allergol. Clin. Immunol. 19, 340–354 [PubMed] [Google Scholar]
- 59. Alphonse M. P., Saffar A. S., Shan L., HayGlass K. T., Simons F. E., Gounni A. S. (2008) Regulation of the high affinity IgE receptor (Fc epsilonRI) in human neutrophils: role of seasonal allergen exposure and Th-2 cytokines. PLoS One 3, e1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Saffar A. S., Alphonse M. P., Shan L., Hayglass K. T., Simons F. E., Gounni A. S. (2007) IgE modulates neutrophil survival in asthma: role of mitochondrial pathway. J. Immunol. 178, 2535–2541 [DOI] [PubMed] [Google Scholar]
- 61. Molloy E. J., O'Neill A. J., Grantham J. J., Sheridan-Pereira M., Fitzpatrick J. M., Webb D. W., Watson R. W. (2003) Sex-specific alterations in neutrophil apoptosis: the role of estradiol and progesterone. Blood 102, 2653–2659 [DOI] [PubMed] [Google Scholar]
- 62. Marin D. P., Bolin A. P., de Cassia Macedo dos Santos R., Curi R., Otton R. (2010) Testosterone suppresses oxidative stress in human neutrophils. Cell Biochem. Funct. 28, 394–402 [DOI] [PubMed] [Google Scholar]
- 63. Driscoll M. D., Sathya G., Muyan M., Klinge C. M., Hilf R., Bambara R. A. (1998) Sequence requirements for estrogen receptor binding to estrogen response elements. J. Biol. Chem. 273, 29321–29330 [DOI] [PubMed] [Google Scholar]
- 64. Mason C. E., Shu F. J., Wang C., Session R. M., Kallen R. G., Sidell N., Yu T., Liu M. H., Cheung E., Kallen C. B. (2010) Location analysis for the estrogen receptor-alpha reveals binding to diverse ERE sequences and widespread binding within repetitive DNA elements. Nucleic Acids Res. 38, 2355–2368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Roemgens A., Singh S., Beyer C., Arnold S. (2011) Inducers of chemical hypoxia act in a gender- and brain region-specific manner on primary astrocyte viability and cytochrome c oxidase. Neurotox. Res. 20, 1–14 [DOI] [PubMed] [Google Scholar]
- 66. Stirone C., Duckles S. P., Krause D. N., Procaccio V. (2005) Estrogen increases mitochondrial efficiency and reduces oxidative stress in cerebral blood vessels. Mol. Pharmacol. 68, 959–965 [DOI] [PubMed] [Google Scholar]
- 67. Shteyer E., Saada A., Shaag A., Al-Hijawi F. A., Kidess R., Revel-Vilk S., Elpeleg O. (2009) Exocrine pancreatic insufficiency, dyserythropoeitic anemia, and calvarial hyperostosis are caused by a mutation in the COX4I2 gene. Am. J. Hum. Genet. 84, 412–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.