Abstract
Female mice lacking group IVA phospholipase A2 (Pla2g4a−/−) have a smaller litter size, which is due, in part, to defective implantation. We examined PLA2G4A dependence of the processes of ovulation and fertilization. Following induction of ovulation by equine chorionic gonadotropin (eCG)/human CG (hCG) treatment and mating, ovulation and fertilization rates were reduced significantly in Pla2g4a−/− mice as compared to wild-type littermates. Human CG triggered robust ovarian prostaglandin (PG) E2 production in the preovulatory period that was significantly attenuated in Pla2g4a−/− mice. Human CG transiently enhanced ovarian expression of PLA2G4A and prostaglandin endoperoxide synthase 2 (PTGS2) in wild-type mice. This PTGS2 induction was decreased in Pla2g4a−/− mice and also in immature rats treated with the PLA2G4A inhibitor, archidonyl trifluoromethyl ketone. A close spatiotemporal association of PLA2G4A with PTGS2 was found in mouse and rat preovulatory follicles examined by immunohistochemistry. Less association was observed with 4 other forms of PLA2. Our data strongly suggest that PLA2G4A amplifies hCG induction of PTGS2 and colocalizes with the induced PTGS2, thus contributing to robust PG production required for optimal ovulation and fertilization in rodents.—Kurusu, S., Sapirstein, A., Bonventre, J. V. Group IVA phospholipase A2 optimizes ovulation and fertilization in rodents through induction of and metabolic coupling with prostaglandin endoperoxide synthase 2.
Keywords: PLA2 isoforms, prostaglandin E2, preovulatory follicle, female reproduction
Prostaglandins (PGs) are important mediators of several key processes in female fertility and reproduction (1–3). These lipid metabolites are synthesized through the concerted action of PG endoperoxide synthase (PTGS) and specific PG synthase enzymes on intracellular arachidonic acid (AA). Local concentrations of PGs are regulated by the availability of AA and cell-specific expression of these enzymes. The PGs signal by binding to a family of G-protein-coupled cell surface receptors (4) and also through intracellular binding of peroxisome proliferator-activated receptor (PPAR) in regulating fertility (5, 6) and other processes. Two PTGS isoforms exist: the constitutive type PTGS1 with “housekeeping ” functions and the inducible-type PTGS2 expressed in response to proinflammatory or mitogenic stimuli (3). Studies performed with mice deficient for PTGS1 or PTGS2 have demonstrated their roles in female reproduction. In rodents, PTGS1 is required for normal induction of luteolysis and parturition (7). In contrast, the surge of luteinizing hormone (LH) induces ovarian PTGS2 (8–12), and PTGS2-knockout mice have defective ovulation (13–15), embryonic implantation, and uterine decidualization (5, 14).
Intracellular AA concentrations are determined largely by the activity of phospholipase A2 (PLA2) enzymes. The PLA2s hydrolyze the release of free fatty acid from the sn-2 position of membrane glycerophospholipids, and the sn-2 position is enriched with AA (16). PLA2s constitute a large enzyme superfamily that is divided into 5 subfamilies, characterized by their structural homologies and Ca2+ requirements (16). The secreted PLA2s (sPLA2s; groups I, II, III, V, IX, X, XI, XII, XIII, and XIV) are small (14-kDa) proteins that require millimolar Ca2+ for catalytic activity. The prototype cytosolic PLA2 (cPLA2; group IV) is the cPLA2α or group IVA PLA2 (PLA2G4A) isoform, which is an 85-kDa cytosolic protein that requires submicromolar Ca2+ for translocation to membranes (17, 18). The Ca2+-independent PLA2s (iPLA2s; group VI) can be cytosolic or membrane bound and have no Ca2+ requirement for activity. The platelet-activating factor acetylhydrolases (PAF-AHs; groups VII and VIII) are Ca2+ independent and have 4 isoforms. The lysosomal PLA2 (group XV) is glycosylated and also has 1-O-acylceramide synthase activity. The mammalian PLA2s have highly diverse regulatory mechanisms, patterns of expression, and mechanisms of action (16). Because of this diversity, different PLA2s may coexist in given cell types and may exhibit activity in cooperative, independent, and/or redundant manners (16).
The PLA2G4A is a particularly important enzyme in the eicosanoid signaling pathway because of its unique molecular and biochemical characteristics. PLA2G4A has a substrate preference for glycerophospholipids, which have AA at the sn-2 position, and its activity is regulated synergistically by agonist-evoked signaling mechanisms, including physiological elevation of intracellular Ca2+, and phosphorylation by mitogen-activated protein kinases (16, 19). Mice lacking the Pla2g4a gene were created on the C57BL/6J background, and the females were found to have reproductive defects, including reduced litter size and delayed parturition that usually results in loss of pregnancy or stillbirth of pups (20–22). Pla2g4a gene deletion causes a nearly 60% reduction in litter size in mice of C57BL/6J strain (20, 21). Because the C57BL/6J strain lacks Pla2g2a (23, 24), PLA2G4A knockout was also examined in the Pla2g2a-intact CD1 strain, where reproductive defects were also manifest (25). Subsequent studies with Pla2g4a−/− mice found that the primary reproductive defect was in the embryo implantation process (25, 26). Although exogenous addition of PGE2 or a PGI2 analog partially rescued defective implantation and parturition, this treatment had no effect on the smaller litter size of Pla2g4a−/− mice (25, 26). These results indicate that PLA2G4A influences litter size at steps that precede implantation. Ovulation and fertilization rates determine the number of preimplanted embryos in polytocous species, and both processes have been shown to be potentially regulated by PTGS2 (1, 14, 15) and PGE2-EP2 (a subtype of PGE2 receptor) signaling (15, 27, 28) in mice and other species. Ovarian induction of PTGS2 is needed for this process, and PLA2G4A is required for both substrate generation and normal PTGS2 induction in both cellular and in vivo models (29–31). To better define the roles of PLA2G4A in reproduction, we studied the events that precede embryo implantation in Pla2g4a−/− mice and examined its ovarian expression/localization and its role in PTGS2 induction and periovulatory PGE2 synthesis.
MATERIALS AND METHODS
Animals
C57BL/6J mice used in the present study were generated previously by gene targeting to disrupt an exon of the Pla2g4a gene that resulted in creation of a null allele (21, 32). Heterozygotes were interbred to produce homozygous null and wild-type littermate mice. Mice were genotyped by PCR analysis of genomic DNA isolated from tail biopsies. In all experiments comparing wild-type (Pla2g4a+/+), heterozygous (Pla2g4a+/−), and homozygous null (Pla2g4a−/−) mice, littermates or age-matched mice derived from the same breeding colony were used. In other experiments, we used mice that have a congenital inactivation of Pla2g2a (23, 24) and rat strains that express the full complement of PLA2s activities (Wistar-Imamichi or Wistar; ref. 33). Gonadotropin-primed immature rats were used in experiments using a PLA2G4A inhibitor or immunohistochemistry. Animal handling and all procedures employed in this study were performed following the Guidelines of the Animal Care and Use Committees of Massachusetts General Hospital and Harvard Medical School and of Kitasato University School of Veterinary Medicine.
Ovulation and fertilization in gene engineered mice
To validate the spontaneous ovulation rate, cycling mice were sacrificed regardless of estrous stages. The ovaries and oviducts were removed, and oocytes in the oviduct ampulla were counted under a light microscope. Ovaries were weighed and processed for hematoxylin and eosin staining. In the superovulation model, adult female mice received a single dose of equine chorionic gonadotropin (eCG; 0.2 IU/g of body weight, intraperitoneally; Sigma Chemical Co., St. Louis, MO, USA) and 48 h later, were injected with human CG (hCG; 5 IU/mouse, intraperitoneally; Sigma). Female mice were housed overnight with a fertile male of various Pla2g4a genotypes (21, 32), and mating was confirmed by the presence of a vaginal plug the next morning [day 1 of pregnancy (PRG1)]. Between 9:00 and 11:00 AM on day 2 of pregnancy (PRG2) we sampled blood by cardiac puncture and sacrificed the mice. The ovulation rate (sum of single-cell oocytes and 2-cell-stage embryos in the oviduct) was determined, and for mice with vaginal plugs, the fertilization rate was determined as the number of embryos divided by the sum of oocytes and embryos. Plasma samples were stored at −20°C until assayed.
For ovarian PG and PTGS2 analyses, ovaries were harvested from naturally cycling mice [pre-eCG (cycling) group] and gonadotropin-treated mice [48 h after eCG treatment (hCG0h) and 6 h after hCG injection (hCG6h) groups]. Superovulated mice were examined at 40–42 h after hCG (PRG2). The samples were stored at −20°C for subsequent biochemical analyses. The ovaries were also collected at hCG6h for immunohistochemistry.
Ovulation in gonadotropin-primed immature rats
Superovulation was induced in 25- to 27-d-old rats (50–70 g body weight) by priming with eCG (10 IU/rat, intraperitoneally) followed 48 h later by hCG (10 IU/rat, intraperitoneally) (34, 35). In some experiments, a PLA2G4A preferential inhibitor, arachidonyl trifluoromethyl ketone [AACOCF3; Cayman Chemical Co., Ann Arbor, MI, USA; 1.0 mg in 50 μl 20% dimethyl sulfoxide (DMSO)] or equal volume of vehicle was administered in the left ovarian bursa immediately before hCG. In a separate group, vehicle was injected, and these and contralateral untreated ovaries served as control groups. In other experiments, a nonselective PTGS inhibitor, indomethacin (1.0 mg/rat, Sigma), or vehicle (DMSO, 0.2 ml) was subcutaneously administered at hCG3h. Ovaries and oviducts were collected for immunohistochemistry at hCG8h or for determination of ovulation rate at hCG24h.
Assay of PGE2 and progesterone
Ovarian PGE2 in Pla2g4a−/− mice was determined using a commercial enzyme immunoassay (EIA) kit (Cayman) as described previously (35). Briefly, the ovary was homogenized in 0.05 M Tris-HCl (pH 7.4) containing 0.9% NaCl, 0.01% Triton X-100, and 0.0057% thimerosal. After centrifugation at 10,000 g for 20 min at 4°C, the supernatant was assayed directly. PGE2 values were normalized to protein (Bio-Rad, Hercules, CA, USA). To examine whether superovulated Pla2g4a−/− mice might have defective luteinization and modified luteal function, blood plasma samples collected on PRG2 were assayed for progesterone. The steroid was extracted by diethyl ether and was assayed using an EIA kit (Cayman) according to the manufacturer's instruction.
Western blot analysis
Western blot analysis was performed as previously reported (30, 32). Whole ovarian tissue samples were transferred into lysis buffer (20 mM HEPES, pH7.4; 2 mM EGTA; 1 mM DTT; 1 mM NaVO4; 1% Triton X-100; 10% glycerol; 2 μM leupeptin; 400 μM PMSF; and 50 μM β-glycerophosphate), homogenized, and stored at −80°C until processing. Lysates were spun at 5000 rpm for 5 min. Proteins (15 μg) were loaded on each lane of an SDS-PAGE 10% gel, and proteins were transferred onto Immobilon-P membranes. The primary antibodies against PLA2G4A and PTGS2 were kindly donated by Dr. A. Cybulsky (McGill University, Montreal, QC, Canada) and purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA), respectively. Immunodetection followed standard protocol using horseradish peroxidase-conjugated secondary antibodies and enhanced chemiluminescence detection system (Perkin Elmer Life Sciences, Branchburg, NJ, USA).
Immunohistochemistry
Expression of 5 PLA2s (G1B, G2A, G5, G4A, and G6A) and localization relative to PTGS2 in preovulatory follicles were analyzed by immunohistochemistry, as reported previously (34, 35). Normal mouse ovaries sampled at hCG6h were fixed in Bouin's solution, dehydrated, and embedded in paraffin. Rat ovaries sampled at hCG0h and ovaries of control and experimental groups sampled at hCG8h were also processed. In each group, samples from 3 mice and 3–4 individual rats were collected and examined. Tissues were serially sectioned (5–6 μm in thickness), deparaffinized, and examined immunochemically. Adjacent sections were used to investigate the spatial association of PTGS2 and 5 PLA2 isoforms in preovulatory follicles of both species. The mirror-image technique was additionally applied to test the colocalization of immunoreactive PTGS2 and PLA2G4A in rat cumulus and granulosa cells. Endogenous peroxidase activity was quenched by pretreatment with 0.3% H2O2 in methanol for 30 min. Specific antibodies consisted of the following: anti-mouse PTGS2 (1:500; Cayman); anti-rat PLA2G1B (1:1000; a generous gift from Dr. K. Nomura, Shionogi Research Laboratories, Osaka, Japan); anti-rat PLA2G2A (1:1000; a generous gift from Dr. I. Kudo, Showa University, Tokyo, Japan); anti-mouse PLA2G5 (1:400; Cayman); anti-human PLA2G4A (1:500; a generous gift from Wyeth Co., Cambridge, MA, USA); and anti-PLA2G6A (1:400; Cayman). These primary antibodies were added to the sections at room temperature for 90 min or at 4°C overnight. The antigen/antibody complex was visualized as a brown color using the Vectastain Elite ABC staining kit (Vector Laboratories, Burlingame, CA, USA) and 3,3′-diaminobenzidine tetrahydrochrolide. Negative controls were performed with normal rabbit serum or nonspecific mouse IgG in place of specific primary antibodies or by antibody adsorption by incubation with recombinant human PLA2G4A protein (a gift of Wyeth Co.). Some slides were counterstained with hematoxylin (blue).
Murine graafian follicles >600 μm in the longest diameter seen in the tissue section were selected as putative ovulatory follicles. Rat graafian follicles >700 μm in the longest diameter were selected as well from 3–5 different ovaries (n=20–32 follicles) for each experimental group. In experiments to test the spatial association of multiple enzymes in rat ovaries, smaller antral follicles that were found to be negative for PTGS2 and very probably would not ovulate were also included in the analysis. The effects of inhibitors on rat follicular expression of immunoreactive PTGS2 were also examined for each of the experimental groups. The relative immunoreactivity was evaluated semiquantitatively using criteria for both intensity and numbers of positive cells in the granulosa and cumulus tissues and was scored on a scale of 0–3, grading from negative, faintly positive, moderately positive, to strongly positive.
Statistical analysis
Data are presented as means ± se. The means among different groups were analyzed by 1-way ANOVA, Student's t test, Tukey-Kramer's multiple comparison test, Mann-Whitney U test, or χ2 test as appropriate. A value of P < 0.05 was considered significant.
RESULTS
Ovulation and fertilization in Pla2g4a−/− mice
We performed gross examinations and measured tissue weight of cycling ovaries in Pla2g4a−/− mice and Pla2g4a+/+ mice. No differences were found in these measures (Table 1) or in a histological analysis between genotypes (data not shown). The rates of spontaneous ovulation in Pla2g4a−/− mice and Pla2g4a+/+ mice were determined. Oocytes were detected in about half the cycling mice irrespective of genotype. In mice that had ovulated, the number of oocytes was significantly reduced in Pla2g4a −/− mice (6.0±0.7; ranging from 3 to 9, n=9) as compared to Pla2g4a +/+ mice (8.0±0.6; ranging from 5 to 11, n=11) (Table 1).
TABLE 1.
Ovarian weight and ovulation and fertilization rates in untreated and eCG/hCG-treated mice
| Parameter | Genotype |
||
|---|---|---|---|
| +/+ | +/− | −/− | |
| Ovarian weight (mg) | |||
| Untreated | 6.0 ± 0.7 (6) | ND | 5.3 ± 1.1 (6) |
| eCG/hCG-treated | 15.9 ± 1.2 (7) | 15.3 ± 0.5 (9) | 12.4 ± 0.7 (7)* |
| Ovulation rate | |||
| Untreated | 8.0 ± 0.6 (11) | ND | 6.0 ± 0.7 (9)* |
| eCG/hCG-treated | 21.1 ± 2.6 (9) | 23.6 ± 3.8 (8) | 9.6 ± 3.1 (8)* |
| Fertilization rate (%) | |||
| eCG/hCG-treated | 76 (95/125) (6) | 77.9 (141/181) (8) | 56.7 (34/60) (5)* |
Ovary pairs were weighed in untreated and eCG/hCG-treated mice. Number of oocytes released per ovulation is expressed as the ovulation rate in untreated mice. For eCG/hCG-treated mice, ovulation rate is the sum of 2-cell embryos and 1-cell, unfertilized oocytes. Fertilization rates in eCG/hCG-treated mice were statistically analyzed by χ2 test. Numbers in parentheses indicate numbers of mice. ND, not determined.
P < 0.05 vs. other genotypes in same row.
Because of the difference observed in natural ovulation between the genotypes, we investigated the PLA2G4A defect further in a mouse superovulation model. Pla2g4a+/+, Pla2g4a+/−, and Pla2g4a−/− mice were treated with a bolus of eCG; ovarian tissue weight was measured 48 h later. This treatment produced a 265% increase in ovarian tissue weight in Pla2g4a+/+ mice compared to untreated mice of the same genotype. The tissue weight after eCG stimulation was comparable in Pla2g4a+/+ mice (15.9±1.2 mg, n=7) and Pla2g4a+/− mice (15.3±0.5 mg, n=9). While Pla2g4a−/− ovaries had a growth response (a 234% increase) to eCG, the absolute value was significantly less than those in other genotypes (P<0.05). The ovulation rate (the sum of oocytes and embryos) following the gonadotropin treatment was similar in Pla2g4a+/+ (21.1±2.6, n=9) and Pla2g4a+/− (23.6±3.8, n=8) mice. In contrast, the ovulation rate in Pla2g4a−/− mice was <50% of the other genotypes (9.6±3.1, n=8, P<0.05).
When we compared the number of recovered oocytes to embryos that were at the 2-cell stage, a marked reduction was found in the fertilization rate of Pla2g4a−/− mice (56.7%, 34/60, n=5) compared to Pla2g4a+/+ (76.6%, 95/124, n=6), and Pla2g4a+/− (75.4%, 141/187, n=8) mice (P<0.05, Pla2g4a−/− vs. other two genotypes). Pla2g4a−/− mice had only 33.7% of the numbers of preimplanted embryos that Pla2g4a+/+ mice had in the superovulation model when the rates of ovulation and fertilization were combined.
Gonadotropins-induced changes in ovarian PGE2 content in mice
Because Pla2g4a−/− mice had reduced ovulation and fertilization rates, we determined whether ovarian PGE2 content was altered by the absence of PLA2G4A. Prior to eCG treatment, PGE2 levels of ovaries from Pla2g4a+/+ (1.93±0.34 ng/mg protein, n=6) and Pla2g4a−/− (1.27±0.34 ng/mg protein, n=6) were not statistically different (Fig. 1). At hCG0h, PGE2 levels of Pla2g4a+/+, Pla2g4a+/−, and Pla2g4a−/− ovaries were similar. PGE2 levels of Pla2g4a+/+ and Pla2g4a−/− ovaries were also similar to their respective values before eCG treatment. PGE2 levels in the Pla2g4a+/+ and Pla2g4a+/− groups at hCG6h were 3.6 times higher than that of the same genotypes at hCG0h (P<0.01). The 6 h posttreatment PGE2 level in Pla2g4a−/− ovaries increased by 2.7-fold compared to baseline (P<0.01), but this PGE2 concentration was only 61.0% of the Pla2g4a+/+ ovaries (P<0.05). By the morning of PRG2, the PGE2 levels in luteinized ovaries in both Pla2g4a+/+ and Pla2g4a−/− groups returned to their basal, prehCG levels with no difference between groups.
Figure 1.
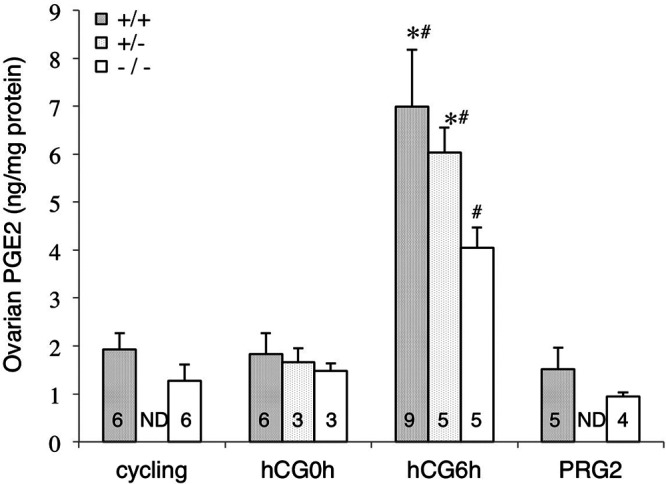
Alteration in mouse ovarian PGE2 levels during periovulatory period. Mouse ovarian PGE2 levels were determined at pre-eCG treatment (labeled as cycling), hCG0h, hCG6h, and in the morning of PRG2. Data are means ± se of the numbers indicated in each column, and statistically evaluated by ANOVA and Tukey-Kramer multiple comparison test. ND, not determined.*P < 0.05 vs. Pla2g4a−/− at the same time point; #P < 0.01 vs. same genotype at hCG0h and PRG2.
Mouse ovarian PLA2G4A and PTGS2 expression and effects of gonadotropin treatment
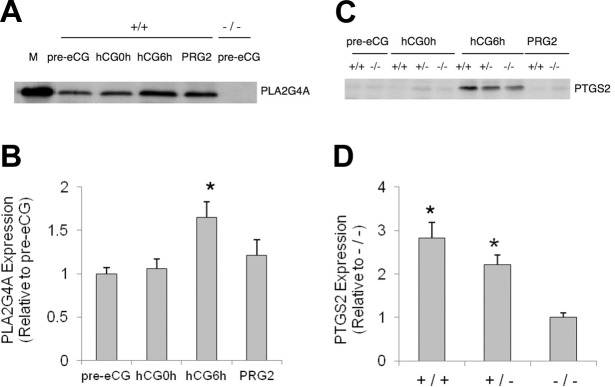
To further analyze the mechanism by which hCG-evoked PGE2 synthesis was reduced in Pla2g4a−/− ovaries, we examined the temporal changes in PLA2G4A and PTGS2 expression. We detected PLA2G4A protein in whole ovarian extract of cycling Pla2g4a+/+ mice but not in Pla2g4a−/− mice (Fig. 2A). The eCG treatment had little effect on this expression, but the subsequent hCG treatment increased PLA2G4A expression in Pla2g4a+/+ mice (∼1.6-fold, P<0.05) at hCG6h (Fig. 2A, B). On PRG2, the ovarian level of PLA2G4A protein was nearly at basal level (115% of pre-hCG level). Consistent with other rodent studies (2, 10, 11), PTGS2 protein in Pla2g4a+/+ ovaries was virtually absent before hCG treatment but was increased markedly in all genotypes at hCG6h and was absent by PRG2 (Fig. 2C). Notably, the PTGS2 protein level at hCG6h was significantly less in Pla2g4a−/− ovaries as compared to that in Pla2g4a+/+ ovaries (35.4%, P<0.05; Fig. 2D).
Figure 2.
Mouse ovarian expression of PLA2G4A and PTGS2 and regulation by gonadotropins. Ovarian expression of PLA2G4A and PTGS2 proteins and their regulation by gonadotropins were determined by Western blot analysis. A) PLA2G4A expression by the Pla2g4a+/+ ovary in unstimulated and stimulated conditions, with Pla2g4a−/− ovary as negative control. M, PLA2G4A standard protein. B) Pla2g4a+/+ ovarian expression of PLA2G4A, quantified as fold induction relative to that in unstimulated ovaries (means±se, n=3). *P < 0.05 vs. pre-eCG group. C) PTGS2 expression by the mouse ovary during the time course of gonadotropin treatment. D) Comparison in PTGS2 level at hCG6h among genotypes was made from three different samples (relative to that of Pla2g4a−/− ovary, means±se, n=3). *P < 0.05 vs. Pla2g4a−/− group at hCG6h.
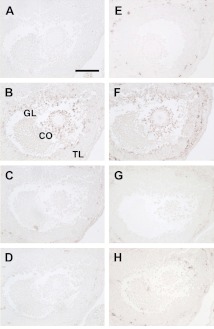
We performed immunohistochemistry on serial sections to determine whether spatial associations of PTGS2 expression existed with any of 5 PLA2s in mouse preovulatory follicles at hCG6h (Fig. 3). PTGS2 immunoreactivity localized to cumulus cells and mural granulosa cells adjacent to the antrum (Fig. 3B), consistent with earlier reports (10, 14, 35). Immunoreactivity of PLA2G1B was very modest (Fig. 3C). As expected in this C57BL/6J mouse (23, 24), PLA2G2A immunoreactivity was absent (Fig. 3D). Immunoreactivity of PLA2G5 was intense in the thecal layer but not in the granulosa layer (Fig. 3E), and that of PLA2G6A was slightly present only in the thecal layer (Fig. 3H). In contrast, PLA2G4A immunoreactivity was highly expressed in mural and cumulus granulosa cells and thecal layers (Fig. 3F) and was closely associated with that of PTGS2 (Fig. 3B). Nonspecific mouse IgG-challenged samples had no signal (Fig. 3A), and preabsorption of purified PLA2G4A antigen with the specific antibody abolished the reactions (Fig. 3G).
Figure 3.
Localization of immunoreactive PTGS2 and 5 forms of PLA2 in mouse preovulatory follicles. Serial sections (except for E stained for PLA2G5) of mouse ovaries at hCG6h were immunostained for PTGS2 and 5 PLA2s. A) Pan-negative control (nonspecific IgG). B) PTGS2 signals localize mainly to the cumulus oophrus (CO) and the granulosa layer (GL) but not the thecal layer (TL). C, D) Signals in cumulus and granulosa cells were very faintly positive for PLA2G1B (C) but negative for PLA2G2A (D). E) Signals for PLA2G5 were evident in the thecal layer. F) PLA2G4A signals are associated with PTGS2. G) PLA2G4A signal is eliminated by neutralization of the antibody. H) Signals for PLA2G6A were slightly positive in the thecal layer. Scale bar = 100 μm.
Effect of PLA2G4A inhibition on PTGS2 induction and ovulation in rats
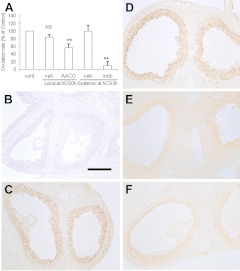
The effects of acute inhibition of PLA2G4A activity on PTGS2 induction and ovulation rate were examined in immature rats. In a gonadotropin treatment protocol similar to that in mice, the ovulation rate per rat ovary was highly variable (22.1±10.1, mean±sd) but was similar between the two ovaries of each individual. The ovulation rate was not affected by vehicle but was reduced markedly by indomethacin (P<0.01; Fig. 4A). The treatment with the PLA2G4A inhibitor AACOCF3 at hCG0h also reduced ovulation (P<0.01).
Figure 4.
Inhibition by AACOCF3 or indomethacin of PTGS2 induction and ovulation in rats. Effects of local AACOCF3 or systemic indomethacin on ovulation rate (A) and PTGS2 induction (B–F) were investigated in superovulated rats. See Materials and Methods for details. A) Both AACOCF3 (AACO) and indomethacin (indo) significantly reduced ovulation rate. In experiments with local treatment, data were transformed as percentage of oocyte numbers of each contralateral (cont) intact ovary (n=6). In experiments with systemic treatment, data were transformed as percentage of the mean of vehicle (veh)-treated rats (n=6 in vehicle-treated group, n=4 in indomethacin-treated group). Data are shown as means ± se. NS, not significant. **P < 0.01 vs. intact control; paired t test. B–F) PTGS2 immunoreactivity in intact animals at hCG0h (B) and hCG8h (C) and in vehicle (local; D)-, AACOCF3 (E)-, or indomethacin (F)-treated follicles at hCG8h. Each photo was taken with the same camera with identical lighting and exposure time. Semiquantitative comparison in staining intensity among treatment groups is summarized in Table 2. Scale bar = 200 μm.
We examined the effect of PLA2G4A on PTGS2 expression in the mural granulosa cells of mature follicles using immunohistochemistry. Treatment with vehicle did not affect PTGS2 expression in putative preovulatory follicles (Fig. 4D) compared to the untreated group (Fig. 4C). In contrast, treatment with AACOCF3 (Fig. 4E) and indomethacin (Fig. 4F) reduced PTGS2 immunoreactivity. Semiquantitative measurement of PTGS2 levels in large numbers of follicles is summarized in Table 2. Ovarian PTGS2 expression was attenuated significantly following pretreatment with AACOCF3 (P<0.05, vs. vehicle-treated group), while the effect of treatment with indomethacin was not statistically significant.
TABLE 2.
PTGS-2 immunoreactivity in rat preovulatory follicles
| Time | Group | Ovaries | Score |
P | |||
|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | ||||
| hCG0h | Untreated | 3 | 20 | 0 | 0 | 0 | |
| hCG8h | Vehicle | 4 | 5 | 3 | 5 | 19 | |
| hCG8h | AACOCF3 | 4 | 2 | 8 | 7 | 5 | <0.05 |
| hCG8h | Indomethacin | 5 | 6 | 4 | 3 | 7 | >0.05, <0.07 |
Preovulatory follicles that looked healthy and had an axis > 700 μm were examined (numbers of ovaries in each group are indicated). Staining intensity, as shown in Fig. 4, was evaluated semiquantitatively; scores, with a scale graded from 0 (negative), 1 (faintly positive), 2 (moderately positive) to 3 (strongly positive), were statistically compared between vehicle- and inhibitor (AACOCF3 or indomethacin)-treated groups by Mann-Whitney U test.
Association of PLA2s and PTGS2 expression in rat preovulatory follicles
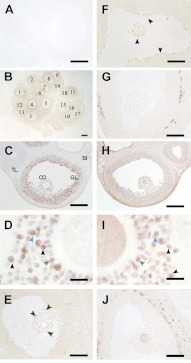
The possible association of PTGS2 and different PLA2s was further examined in rat ovarian follicles sampled at hCG8h. PTGS2 immunoreactivity was very intense in most of the antral and graafian follicles (Fig. 5B). PTGS2 was distributed in the cumulus oophrus (Fig. 5C, CO) and throughout the granulosa layer (Fig. 5C, GL) but not in oocytes, thecal layer (Fig. 5C, TL) or stromal tissue (Fig. 5C, St), as is consistent with previous reports (8–10, 11, 14). PTGS2 localized in many, but not all, cumulus oophrus and mural granulosa cells and very often on their nuclear envelopes (Fig. 5D, see black arrowheads).
Figure 5.
Localization of immunoreactive PTGS2 and 5 forms of PLA2 in rat preovulatory follicles. Normal rat ovaries at hCG8h were immunostained for PTGS2 and 5 PLA2s. A) Negative control (nonimmune serum). B, C) PTGS2 signals localization to most, but not all, mature follicles (B) and mainly to cumulus oophrus (CO) and throughout granulosa layers (GL) but not to oocyte, thecal layers (TL), and the stroma (St) (C). D) Abundant PTGS2 localization on nuclear membranes (black arrowheads). E, F) PLA2G1B (E) and PLA2G2A (F) localization to cumulus oophorus and granulosa cells lining the antral surface (arrowheads). G) PLA2G5 localization only to thecal layers of mature follicles. H, I) PLA2G4A signals in cumulus oophrus, granulosa layer, and oocytes and frequently on the hematoxylin-stained nucleus structures (I, black arrowheads). The mirror-image technique on the pair of adjacent sections (C and H, D and I) showed frequent colocalization of PTGS2 and PLA2G4A signals at the cellular level (blue and green arrowheads in each panel). J) PLA2G6A localization to thecal layers and oocytes of mature follicles. To investigate the association of PTGS2 and PLA2s in the identical follicles, serial sections were stained for PTGS2 and PLA2s. Immunoreactivities in a total of 47 follicles from 3 ovaries (see example in panel B) were semiquantitatively evaluated and summarized in Fig. 6. Scale bars = 400 μm (B); 200 μm (A, C, E, H); 100 μm (F, G, J); 20 μm (D, I).
Immunoreactivity of PLA2G1B was relatively weak but did localize in cumulus oophorus and granulosa cells lining the surface of the antrum (Fig. 5E). PLA2G2A immunoreactivity was evident in the rat follicles and restricted to cumulus oophrus and the innermost layer of granulosa tissues (Fig. 5F). The immunoreactivity of another sPLA2, PLA2G5, was intense in the thecal layer and almost absent in the granulosa layer and cumulus oophrus (Fig. 5G). Immunoreactive PLA2G4A was apparent in most cells of cumulus oophrus and mural granulosa layers and more ubiquitously in oocytes and interstitial tissues (Fig. 5H). Higher magnification revealed that PLA2G4A immunoreactivity frequently overlapped nuclear (hematoxylin) staining in cumulus and granulosa cells (Fig. 5I, see black arrowheads). Immunoreactivity of PLA2G6A was present in the thecal layer (Fig. 5J), similar to the mouse tissues (Fig. 3H). Similar to mouse preovulatory follicles (Fig. 3B, F), PTGS2 signals were most associated with that of PLA2G4A in rat tissues, as revealed in the pair of adjacent sections (Fig. 5C and H, D and I). Frequent colocalization of PTGS2 (Fig. 5D) and PLA2G4A (Fig. 5I) appeared in identical cells (See blue and green arrowheads in each panel).
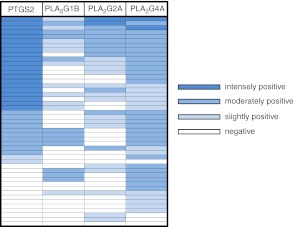
As 3 PLA2s among 5 isoforms tested were found to show similar tissue distribution to PTGS2 in mature follicles, we next determined the association of signal intensity of PTGS2 and the PLA2s semiquantitatively by immunostaining serial sections from preovulatory ovaries for each enzyme (n=47 antral and graafian follicles from 3 different ovaries; Figs. 5B and 6). PTGS2 expression was associated with follicular development, but none of the PLA2s appeared to have such a pattern of expression. PTGS2 was intensely positive in 44.7% of the follicles (21 of 47), moderately positive in 21.3% (10 of 47), slightly positive in 4.3% (2 of 47), and negative in the remaining 29.8% (Fig. 6). None of the follicles were intensely positive for PLA2G1B staining, but 22 follicles did have positive immunoreactivity. Among the 33 follicles positive for PTGS2, 15 follicles (45%) were negative, and the remaining 18 follicles (55%) were positive for PLA2G1B. Eleven of the 33 PTGS2-positive follicles were negative for PLA2G2A immunoreactivity, while the others varied from slightly to intensely positive. Immunoreactive PLA2G4A was present in most mature follicles (93.6%). Among the PTGS2-positive follicles, only one was negative for PLA2G4A. Of the follicles, 11 were positive for PLA2G4A but negative for PTGS2. The fact that PLA2G4A is almost always expressed in the mature follicles places it in a position to regulate expression of PTGS2 and supply AA for its metabolism.
Figure 6.
Semiquantitative analysis of expression of immunoreactive PTGS2 and 3 PLA2 isoforms in rat preovulatory follicles. Serial sections of rat ovaries at hCG8h were immunostained for PTGS2, PLA2G1B, PLA2G2A, and PLA2G4A. A total of 47 antral and graafian follicles in sections of 3 different ovarian samples (for example, Fig. 5B) were numbered, and the intensities of immunoreaction for each enzyme in each follicle were evaluated on a scale of 4 grades (negative, slightly positive, moderately positive, intensely positive) with different blue colors. One investigator scored the sections 2 or 3 times, and another investigator scored them independently. The readings resulted in a high level of agreement.
Progesterone secretion in Pla2g4a −/− mice
The plasma progesterone levels in Pla2g4a+/+ mice were 122.6 ± 23.7 ng/ml (n=7) on PRG2, whereas levels in Pla2g4a−/− mice were 78.7 ± 13.2 ng/ml (n=6) (P=0.139).
DISCUSSION
The LH surge-triggered induction of PTGS2 and the resulting increase in PGE2 production in preovulatory follicles are critical factors in oocyte maturation (12), ovulation (1, 14, 15, 27), and fertilization (14, 27, 28). We postulated that PLA2G4A was a primary candidate to regulate this signaling system and here examined ovarian causes for previously identified decrease in litter size of Pla2g4a−/− female mice (21, 22). We found that PLA2G4A contributes significantly to ovulation and fertilization, which maximizes the number of preimplanted embryos. Furthermore, our results show that PLA2G4A has a cellular and temporal association with follicular PTGS2 expression. This association creates an optimal environment for PGE2 synthesis for paracrine regulation of the ovulatory cascade and fertilization.
We observed a significant difference in the natural ovulation rate of Pla2g4a−/− mice in the C57BL/6J background. A previous report noted small reductions in the natural ovulation rate of Pla2g4a−/− mice that were insignificant in the C57BL/6J background but did reach significance in the PLA2G2A-expressing CD1 strain (25). The natural ovulation rate of the C57BL/6J background mice in the current study was slightly higher than in the previously published study (25), and this could be due to differences in housing or diet. Chemically induced superovulation demonstrated the significance of PLA2G4A on ovulation and localized the site of PLA2G4A action, at least in part, to ovarian factors that are responsive to gonadotropins. Two defects from Pla2g4a deficiency are possible, one is follicular maturation and the second is follicular rupture and oocyte release. A requirement for PLA2G4A in follicular maturation is supported by the decreased growth response to eCG of Pla2g4a−/− ovaries, which suggests a decreased number of LH/hCG-responsive mature follicles. The second model is more likely, because AACOCF3 treatment immediately before (this study) or 8 h (34) following hCG treatment decreased oocytes release in superovulated rodents.
We also found a significantly decreased fertilization rate in superovulated Pla2g4a−/− mice. This finding is consistent with a similar finding in natural ovulation (25) and is supported by morphological evidence of PLA2G4A localization in cumulus oophrus. The defects in natural ovulation and fertilization are modest, but taken together these defects can produce significant reductions in the putative number of fertilized, preimplanted embryos. Indeed, this reduction can largely account for the smaller litter size (∼60% decrease) of Pla2g4a−/− females (20, 21). This finding is supported by the fact that administration of exogenous PGE2 and PGI2 analogues to Pla2g4a−/− mice at the time of implantation partially restored embryo implantation but did not alter the decreased litter size (25, 26).
We ascribe defective ovulation and fertilization in the Pla2g4a−/− mouse to attenuated PTGS2-dependent PG synthesis. Western blot analysis showed a concomitant increase in PLA2G4A and PTGS2 levels in whole ovaries of adult mice following hCG stimulation. Because adult rodent ovaries contain several generations of corpora lutea, which have varying levels of PLA2G4A protein (34, 36), only a fraction of the protein in our Western blot analysis is derived specifically from periovulatory follicles. Following biochemical analyses of PGE2 and PLA2G4A/PTGS2 expression in whole ovaries, we investigated, using immunohistochemistry, the regional distribution and possible association of enzymes for PG production, focusing on graafian follicles. Previous work has shown that the rat ovary expresses PLA2G1B (37), PLA2G2A (37, 38), and PLA2G4A (34, 38) in an LH/hCG-dependent manner. The relationships of these PLA2s with PTGS2 expression have not been previously investigated, but here they are shown to associate predominantly in granulosa and cumulus oophrus tissues. Our current study also reveals the presence of immunoreactive PLA2G5 and PLA2G6A in rodent preovulatory follicles. Their distribution in the thecal layer, however, suggests association with PTGS1 but not with PTGS2 (8). The immunohistological results in both mice and rats indicate that PLA2G4A colocalizes with PTGS2 at the cellular and subcellular (nuclear) levels. This study is the first to reveal the frequent cellular association of PTGS2 expression with PLA2G4A in mammalian preovulatory follicles. We have further found that microsomal PGE2 synthase (PGES) type-1 (mPGES-1), but not cytosolic PGES or mPGES-2, was induced in rat cumulus oophrus tissues after ovulatory hCG stimulus (unpublished results). The mural granulosa and cumulus oophrus tissues in periovulatory follicles have different regulation of PTGS2 expression (2, 10), which may be related to their distinct roles in follicle wall degradation (ovulation) and cumulus expansion (fertilization) (2, 10, 11, 14, 27). Together with our data that the PGE2 response to hCG was attenuated in Pla2g4a−/− mice and that AACOCF3 treatment at hCG8h decreased PGE2 level and ovulation rate in rats (34) we hypothesized that PLA2G4A is also needed for optimal PTGS2 and PG responses to LH/hCG. Such a significant contribution of PLA2G4A to efficient coupling with PTGS2 and PG generation has been demonstrated in various cell lines (39, 40). In addition, PLA2G4A-dependent regulation of mammalian ovulation is highly conserved, since an ovulatory hCG stimulus induces both PLA2G4A and PTGS2 in the ovaries of cows (41) and monkeys (42). The latter species is an especially valuable model because it is most similar to humans. Information about PLA2G4A and PTGS2 in the early stage of human pregnancy is quite limited due to ethical and technical difficulties (43–45).
It is important to note that Pla2g4a deficiency does not cause total failure of ovulation or fertilization. This finding suggests that other forms of PLA2 or sources of AA can partially compensate for loss of PLA2G4A activity. Which PLA2 isoforms contribute to AA release and PG synthesis during these processes remains undefined. No other PLA2 has been reported to have such an effect on ovulation and fertilization and resultant litter size in mice (16, 24, 46–50). Follicular rupture is often considered analogous to an inflammatory response (2). The presence in rat preovulatory follicles of multiple PLA2s (G1B, G2A, G5, and G6A; Fig. 5) that are associated with inflammation (16) is consistent with their role in follicular rupture. This hypothesis is in agreement with previous findings that show PLA2G5 expression in monkey preovulatory follicles (42) and Pla2g1b mRNA expression in bovine preovulatory follicles (41). The fact that defects in ovulation and fertilization are more severe in Ptgs2−/− mice (14) than in Pla2g4a−/− mice is also consistent with the idea that multiple PLA2s can compensate for defective AA supply due to Pla2g4a deficiency. This implies that concerns about the effects on fertility of a clinical PLA2G4A inhibitor may be less than those of a traditional nonsteroidal antiinflammatory drug (51).
Previous findings on roles of PGs and their cell surface receptors in ovulation and fertilization (2, 14, 27, 28) led us to measure PGE2 levels in the periovulatory period. However, it is possible that other PGs and AA itself are also implicated in these processes. Our data demonstrating attenuated PTGS2 expression by PLA2G4A inhibition suggest that PLA2G4A enhances expression of PTGS2, which underlies the mechanism for LH/hCG-induced PG production in preovulatory follicles. This hypothesis is consistent with our previous findings that PTGS2 expression in Pla2g4a−/− mouse tissues after multiple stimuli was lower than in wild–type (29–31). Other in vitro evidence has shown that AA and several PGs can regulate PTGS2 expression through binding to PPARs (6, 52–54). Though colocalization of PLA2G4A and PTGS2 on nuclear membranes suggests its molecular effect, no direct biochemical evidence indicates that PLA2G4A contributes to direct the supply of AA to PTGS2 in preovulatory follicles. Similarly, the PLA2G4A-dependent biochemical metabolite and molecular mechanism for PTGS2 expression in ovarian cells have not been identified. Thus, future studies are needed to establish the PLA2G4A regulation of the AA cascade in preovulatory follicles.
Pregnancy is a unique mammalian process consisting of sequential events from follicular maturation to parturition and involving highly complicated interactions among pituitary, ovary, uterus, placenta, and embryo. In rodents, corpora lutea formed from ruptured follicles are the predominant source of progesterone governing pregnancy. We found no statistically significant changes, although there was a trend toward lower plasma progesterone levels in Pla2g4a−/− mice in the very early luteal phase (PRG2). Consistent with this finding, pregnant Pla2g4a−/− mice in the BALB/c strain also had a lower level of circulating progesterone (P=0.06, vs. Pla2g4a+/+) on PRG16 to PRG18 (data not shown). Moreover, it has been reported that the progesterone secretory response to hCG was inhibited by a specific PLA2G4A inhibitor, pyrrophenone, in rat luteal cells in vitro (55). Thus, the lack of PLA2G4A activity may reduce both the number and the intrinsic steroidogenic activity of corpora lutea, leading to reduced progesterone secretion. Less progesterone would lead to less successful induction of early pregnancy, a delay and reduction in implantation (25), and failure of luteolysis and subsequent parturition (20, 21). In particular, delayed parturition might result from progesterone-dependent and -independent effects of fewer embryos, as smaller litter size has been shown to delay parturition in rats (56, 57) and Pla2g4a−/− mice (26).
In summary, we believe that the inhibition or absence of ovarian PLA2G4A activity causes reductions in both ovulation and fertilization, which contribute to a significantly smaller litter size. PLA2G4A is molecularly and functionally in close association with the induction and activity of PTGS2 in mural granulosa and cumulus tissues of preovulatory follicles. Efficient metabolic coupling of these two enzymes is required for optimal ovarian prostanoid responses and related events. We propose that PLA2G4A-mediated enhancement of both the substrate precursor and its metabolizing enzyme creates a self-amplification mechanism for AA cascade that functions as an integral component of the ovulatory cascade and fertilization.
Acknowledgments
The authors greatly thank E. O'Leary and X.-M. Sun and other members of the J.V.B. laboratory for technical assistance and useful suggestions; H. Satoh and M. Jinno for experimental help; Dr. M. Kawaminami (Kitasato University, Towada, Japan) for supplying experimental materials; Drs. A. Cybulsky (McGill University, Montreal, QC, Canada), K. Nomura (Shionogi Research Laboratories, Osaka, Japan), and I. Kudo (Showa University, Tokyo, Japan) and Wyeth Co. (Cambridge, MA, USA) for antibodies; and M. Nakata for assistance with manuscript preparation.
The work with mice was supported by U.S. National Institute of Health grants DK39773 and DK38452 (to J.V.B.) and that with rats was by grants-in-aid for scientific research from the Japanese Society for the Promotion of Science and the Kieikai Research Foundation (to S.K.). S.K. received a fellowship for studies abroad from the Nakayama Foundation for Human Science.
The authors declare no conflicts of interest.
Footnotes
- AA
- arachidonic acid
- AACOCF3
- arachidonyl trifluoromethyl ketone
- DMSO
- dimethyl sulfoxide
- eCG
- equine chorionic gonadotropin
- EIA
- enzyme immunoassay
- hCG
- human chorionic gonadotropin
- LH
- luteinizing hormone
- PAF-AH
- platelet activating factor acetylhydrolase
- PG
- prostaglandin
- PGES
- prostaglandin E2 synthase
- PLA2
- phospholipase A2
- PPAR
- peroxisome proliferator-activated receptor
- PRG1
- day 1 of pregnancy
- PTGS
- prostaglandin endoperoxide synthase
- sPLA2
- secreted phospholipase A2
REFERENCES
- 1. Matsumoto H., Ma W., Smalley W., Trzaskos J., Breyer R. M., Dey S. K. (2001) Diversification of cyclooxygenase-2-derived prostaglandins in ovulation and implantation. Biol. Reprod. 64, 1557–1565 [DOI] [PubMed] [Google Scholar]
- 2. Espey L. L., Richards J. S. (2006) Ovulation. In The Physiology of Reproduction (Knobil E., Neill J. D., eds.) pp. 425–500, Raven Press, New York [Google Scholar]
- 3. Funk C. D., Song W-C, FitzGerald G. A. (2009) Prostaglandins and other lipid mediators in reproductive medicine. In Reproductive Endocrinology (Stauss J. F., III, Barbieri R. L. eds.) pp. 121–137, Saunders Elsevier, Philadelphia [Google Scholar]
- 4. Narumiya S., Sugimoto Y., Ushikubi F. (1999) Prostanoid receptors: structures, properties, and functions. Physiol. Rev. 79, 1193–1226 [DOI] [PubMed] [Google Scholar]
- 5. Lim H., Gupta R. A., Ma W., Paria B. C., Moller D. E., Morrow J. D., Dubois R. N., Trzaskos J. M., Dey S. K. (1999) Cylooxygenase-2-derived prostacyclin mediates embryo implantation in the mouse via PPARδ. Genes Dev. 131, 561–574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Halliwell R. J. A., Berry E. B. E., O'Carroll S. J., Michell M. D. (2004) Nuclear prostaglandin receptors: role in pregnancy and parturition? Prostaglandin Leukotr. Essent. Fatty Acids 70, 140–165 [DOI] [PubMed] [Google Scholar]
- 7. Langenbach R., Morham S. G., Tiano H. F., Loftin C. D., Ghanayem B. I., Chulada P. C., Mahler J. F., Lee C. A., Goulding E. H., Kluckman K. D., Kim H. S., Smithies O. (1995) Prostaglandin synthase 1 gene disruption in mice reduces arachidonic acid-induced inflammation and indomethacin-induced gastric ulceration. Cell 83, 483–492 [DOI] [PubMed] [Google Scholar]
- 8. Wong W. Y. L., Richards J. S. (1991) Evidence for antigenically distinct, mol wt variants of prostaglandin H synthase in the rat ovary. Mol. Endocrinol. 5, 1269–1279 [DOI] [PubMed] [Google Scholar]
- 9. Sirois J., Simmons D. L., Richards J. S. (1992) Hormonal regulation of messenger ribonucleic acid encoding a novel isoform of prostaglandin endoperoxide H synthase in rat preovulatory follicles. Induction in vivo and in vitro. J. Biol. Chem. 267, 11586–11592 [PubMed] [Google Scholar]
- 10. Joyce I. M., Pendola F. L., O'Brien M., Eppig J. J. (2001) Regulation of prostaglandin endoperoxide synthase 2 messenger ribonucleic acid in mouse granulose cells during ovulation. Endocrinology 142, 3187–3197 [DOI] [PubMed] [Google Scholar]
- 11. Sirois J., Sayasith K., Brown K. A., Stock A. E., Bouchard N., Dore M. (2004) Cyclooxygenase-2 and its role in ovulation: a 2004 account. Hum. Reprod. Update 10, 373–385 [DOI] [PubMed] [Google Scholar]
- 12. Takahashi T., Morrow J. D., Wang H., Dey S. K. (2006) Cyclooxygenase-2-derived prostaglandin E2 directs oocyte maturation by differentially influencing multiple signaling pathways. J. Biol. Chem. 281, 37117–37129 [DOI] [PubMed] [Google Scholar]
- 13. Morham S. G., Langenbach R., Loftin C. D., Tiano H. F., Vouloumanos N., Jennette J. C., Mahler J. F., Kluckman K. D., Ledford A., Lee C. A., Smithies O. (1995) Prostaglandin synthase 2 gene disruption causes severe renal pathology in the mouse. Cell 83, 473–482 [DOI] [PubMed] [Google Scholar]
- 14. Lim H., Paria B. C., Das S. K., Dinchuk J. E., Langenbach R., Trzaskos J. M., Dey S. K. (1997) Multiple female reproductive failures in cyclooxygenase-2 deficient mice. Cell 91, 197–208 [DOI] [PubMed] [Google Scholar]
- 15. Davis B. J., Lennard D. E., Lee C. A., Tiano H. F., Morham S. G., Wetsel W. C., Langenbach R. (1999) Anovulation in cyclooxygenase-2-deficient mice is restored by prostaglandin E2 and interleukin-1β. Endocrinology 140, 2685–2695 [DOI] [PubMed] [Google Scholar]
- 16. Murakami M., Taketomi Y., Miki Y., Sato H., Hirabayashi T., Yamamoto K. (2011) Recent progress in phospholipase A2 research: from cells to animals to humans. Prog. Lipid Res. 50, 152–192 [DOI] [PubMed] [Google Scholar]
- 17. Gronich J. H., Bonventre J. V., Nemenoff R. A. (1990) Purification of a high-molecular-mass form of phospholipase A2 from rat kidney activated at physiological calcium concentrations. Biochem. J. 271, 37–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Clark J. D., Lin L-L., Kriz R. W., Ramesha C. S., Sultzman L. A., Lin A. Y., Milona N., Knopf J. L. (1991) A novel arachidonic acid-selective cytosolic PLA2 contains a Ca2+-dependent translocation domain with homology to PKC and GAP. Cell 65, 1043–1051 [DOI] [PubMed] [Google Scholar]
- 19. Kita Y., Ohto T., Uozumi N., Shimizu T. (2006) Biochemical properties and pathophysiological roles of cytosolic phospholipase A2s. Biochim. Biophys. Acta 1761, 1317–1322 [DOI] [PubMed] [Google Scholar]
- 20. Uozumi N., Kume K., Nagase T., Nakatani N., Ishii S., Tashiro F., Komagata Y., Maki K., Ikuta K., Ouchi Y., Miyazaki J., Shimizu T. (1997) Role of cytosolic phospholipase A2 in allergic response and parturition. Nature 390, 618–622 [DOI] [PubMed] [Google Scholar]
- 21. Bonventre J. V., Huang Z., Reza Taheri M., O'Leary E., Li E., Moskowitz M. A., Sapirstein A. (1997) Reduced fertility and postischaemic brain injury in mice deficient in cytosolic phospholipase A2. Nature 390, 622–625 [DOI] [PubMed] [Google Scholar]
- 22. Sapirstein A., Bonventre J. V. (2000) Specific physiological roles of cytosolic phospholipase A2 as defined by gene knockouts. Biochim. Biophys. Acta 1488, 139–148 [DOI] [PubMed] [Google Scholar]
- 23. MacPhee M., Chepnik K. P., Liddell R. A., Nelson K. K., Siracusa L. D., Buchberg A. M. (1995) The secretory phospholipase A2 gene is a candidate for the Mom1 locus, a major modifier of ApcMin-induced intestinal neoplasia. Cell 81, 957–966 [DOI] [PubMed] [Google Scholar]
- 24. Kennedy B. P., Payette P., Mudgett J., Vadas P., Pruzanski W., Kwan M., Tang C., Rancourt D. E., Cromlish W. A. (1995) A natural disruption of the secretory group II phospholipase A2 gene in inbred mouse strain. J. Biol. Chem. 270, 22378–22385 [DOI] [PubMed] [Google Scholar]
- 25. Song H., Lim H., Paria B. C., Matsumoto H., Swift L. L., Morrow J., Bonventre J. V., Dey S. K. (2002) Cytosolic phospholiase A2α is crucial for “on-time ” embryo implantation that directs subsequent development. Development 129, 2879–2889 [DOI] [PubMed] [Google Scholar]
- 26. Brown N., Morrow J. D., Slaughter J. C., Paria B. C., Reese J. (2009) Restoration of on-time embryo implantation corrects the timing of parturition in cytosolic phospholipase A2 group IVA deficient mice. Biol. Reprod. 81, 1131–1138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hizaki H., Segi E., Sugimoto Y., Hirose M., Saji T., Ushikubi F., Matsuoka T., Noda Y., Tanaka T., Yoshida N., Narumiya S., Ichikawa A. (1999) Abortive expansion of the cumulus and impaired fertility in mice lacking the prostaglandin E receptor subtype EP2. Proc. Natl. Acad. Sci. U. S. A. 96, 10501–10506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tilley S. L., Audoly L. P., Hicks E. H., Kim H. S., Flannery P. J., Coffman T. M., Koller B. H. (1999) Reproductive failure and reduced blood pressure in mice lacking the EP2 prostaglandin E2 receptor. J. Clin. Invest. 103, 1539–1545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fujishima H., Sanchez Mejia R. O., Bingham C. O., 3rd, Lam B. K., Sapirstein A., Bonventre J. V., Austen K. F., Arm J. P. (1999) Cytosolic phospholipase A2 is essential for both the immediate and the delayed phases of eicosanoid generation in mouse bone marrow-derived mast cells. Proc. Natl. Acad. Sci. U. S. A. 96, 4803–4807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sapirstein A., Saito H., Texel S. J., Samad T. A., O'Leary E., Bonventre J. V. (2005) Cytosolic phospholipase A2α regulates induction of brain cyclooxygenase-2 in a mouse model of inflammation. Am. J. Physiol. 288, R1774–R1782 [DOI] [PubMed] [Google Scholar]
- 31. Kishimoto K., Li R. C., Zhang J., Klaus J. A., Kibler K. K., Doré S., Koehler R. C., Sapirstein A. (2010) Cytosolic phospholipase A2 alpha amplifies early cyclooxygenase-2 expression, oxidative stress and MAP kinase phosphorylation after cerebral ischemia in mice. J. Neuroinflammation 7, 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kurusu S., Sapirstein A., Sawada H., Kawaminami M., Bonventre J. V. (2011) Group IVA phospholiase A2 regulates testosterone biosynthesis by murine Leydig cells and is required for timely sexual maturation. Biochem. J. 439, 403–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ishiguro Y., Ochiai M., Sugimura T., Nagao M., Nakagama H. (1999) Strain differences of rats in the susceptibility to aberrant crypt foci formation by 2-amino-1-methyl-6-phenylimidazo-[4,5-b]pyridine: no implication of Apc and Pla2g2a genetic polymorphisms in differential susceptibility. Carcinogenesis 20, 1063–1068 [DOI] [PubMed] [Google Scholar]
- 34. Kurusu S., Iwao M., Kawaminami M., Hashimoto I. (1998) Involvement of cytosolic phospholipase A2 in the ovulatory process in gonadotropin-primed immature rats. Prostaglandins Leukotr. Essent. Fatty Acids 58, 405–411 [DOI] [PubMed] [Google Scholar]
- 35. Kurusu S., Jinno M., Ehara H., Yonezawa T., Kawaminami M. (2009) Inhibition of ovulation by a lipoxygenase inhibitor involves reduced cyclooxygenase-2 expression and prostaglandin E2 production in gonadotropin-primed immature rats. Reproduction 137, 59–66 [DOI] [PubMed] [Google Scholar]
- 36. Kurusu S., Sakaguchi S., Kawaminami M., Hashimoto I. (2001) Sustained activity of luteal cytosolic phospholipase A2 during luteolysis in pseudopregnant rats: its possible implication in tissue involution. Endocrine 14, 337–342 [DOI] [PubMed] [Google Scholar]
- 37. Nomura K., Fujita H., Arita H. (1994) Gene expression of pancreatic-type phospholipase A2 in rat ovaries: stimulatory action on progesterone release. Endocrinology 135, 603–609 [DOI] [PubMed] [Google Scholar]
- 38. Kol S., Ruutiainen-Altman K., Ben-Shlomo I., Payne D. W., Ando M., Adashi E. Y. (1997) The rat ovarian phospholipase A2 system: gene expression, cellular localization, activity characterization, and interleukin-1 dependence. Endocrinology 138, 322–331 [DOI] [PubMed] [Google Scholar]
- 39. Reddy S. T., Herschman H. R. (1995) Prostaglandin synthase 1 and prostaglandin synthase 2 are coupled to distinct phospholipases for the generation of prostaglandin D2 in activated mast cells. J. Biol. Chem. 272, 3231–3237 [DOI] [PubMed] [Google Scholar]
- 40. Morita I., Schindler M., Regier M. K., Otto J. C., Hori T., DeWitt D. L., Smith W. L. (1995) Different intracellular locations for prostaglandin endoperoxide H synthase 1 and 2. J. Biol. Chem. 270, 10902–10908 [DOI] [PubMed] [Google Scholar]
- 41. Diouf M. N., Sayasith K., Lefebvre R., Silversides D. W., Sirois J., Lussier J. G. (2006) Expression of phospholipase A2 group IVA (PLA2G4A) is upregulated by human chorionic gonadotropin in bovine granulosa cells of ovulatory follicles. Biol. Reprod. 74, 1096–1103 [DOI] [PubMed] [Google Scholar]
- 42. Duffy D. M., Seachord C. L., Dozier B. L. (2005) An ovulatory gonadotropin stimulus increases cytosolic phospholipase A2 expression and activity in granulosa cells of primate periovulatory follicles. J. Clin. Endocrinol. Metabol. 90, 5858–5865 [DOI] [PubMed] [Google Scholar]
- 43. Tokuyama O., Nakamura Y., Musoh A., Honda K., Ishiko O. (2003) Expression and distribution of cyclooxygenase-2 in human ovary during follicular development. Osaka City Med. J. 49, 39–47 [PubMed] [Google Scholar]
- 44. Stavreus-Evers A., Koraen L., Scott J. E., Zhang P., Westlund P. (2005) Distribution of cyclooxygenase-1, cyclooxygenase-2, and cytosolic phospholipase A2 in the luteal phase human endometrium and ovary. Fertil. Steril. 83, 156–162 [DOI] [PubMed] [Google Scholar]
- 45. Lappas M., Rice G. E. (2004) Phospholipase A2 isozymes in pregnancy and parturition. Prostaglandin Luekotr. Essent. Fatty Acids 70, 87–100 [DOI] [PubMed] [Google Scholar]
- 46. Richmond B. L., Boileau A. C., Zheng S., Huggins K. W., Granholm N. A., Tso P., Hui D. Y. (2001) Compensatory phospholipid digestion is required for cholesterol absorption in pancreatic phospholipase A2 deficient mice. Gastroenterology 120, 1193–1202 [DOI] [PubMed] [Google Scholar]
- 47. Bao S., Miller D. J., Ma Z., Wohltmann M., Eng G., Ramanadham S., Moley K., Turk J. (2004) Male mice that do not express group VIA phospholipase A2 produce spermatozoa with impaired motility and have greatly reduced fertility. J. Biol. Chem. 279, 38194–38200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Satake Y., Ziaz B. L., Balestrieri B., Lam B. K., Kanaoka Y., Grusby M. J., Arm J. P. (2004) Role of group V phospholipase A2 in zymosan-induced eicosanoid generation and vascular permeability revealed by targeted gene disruption. J. Biol. Chem. 279, 16488–16494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Henderson W. R., Jr., Chi E. Y., Bollinger J. G., Tien Y. T., Ye X., Castelli L., Rubtsov Y. P., Singer A. G., Chiang G. K., Nevalainen T., Rudensky A. Y., Gelb M. H. (2007) Importance of group X-secreted phospholipase A2 in allergen-induced airway inflammation and remodeling in a mouse asthma model. J. Exp. Med. 204, 865–877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Sato H., Taketomi Y., Isogai Y., Miki Y., Yamamoto K., Masuda S., Hosono T., Arata S., Ishikawa Y., Ishii T., Kobayashi T., Nakanishi H., Ikeda K., Taguchi R., Hara S., Kudo I., Murakami M. (2010) Group III secreted phospholipase A2 regulates epididymal sperm maturation and fertility in mice. J. Clin. Invest. 120, 1400–1414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Mendonca L. L. F., Khamashta M. A., Nelson-Piercy C., Hunt B. J., Hughes G. R. V. (2000) Non-steroidal anti-inflammatory drugs as a possible cause for reversible infertility. Rhuematology 39, 880–882 [DOI] [PubMed] [Google Scholar]
- 52. Inoue H., Tanabe T., Umesono K. (2000) Feedback control of cyclooxygenase-2 expression through PPARγ. J. Biol. Chem. 275, 28028–28032 [DOI] [PubMed] [Google Scholar]
- 53. Tsubouchi Y., Kawahito Y., Kohno M., Inoue K., Hla T., Sano H. (2001) Feedback control of the arachidonate cascade in rheumatoid synoviocytes by 15-deoxy-Delta(12,14)-prostaglandin J2. Biochem. Biophys. Res. Commun. 283, 750–755 [DOI] [PubMed] [Google Scholar]
- 54. Sheldrick E. L. R., Derecka K., Marshall E., Chin E. C., Hodges L., Wathes D. C., Abayasekara D. R. E., Flint A. P. F. (2007) Peroxisome-proliferator-activated receptors and the control of levels of prostaglandin-endoperoxide synthase 2 by arachidonic acid in the bovine uterus. Biochem. J. 406, 175–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kurusu S., Ohkawa M., Kawaminami M. (2007) Effects of arachidonate metabolism inhibitors on basal and human chorionic gonadotropin-stimulated progesterone secretion by rat corpus luteum cells in vitro. Prostaglandins Other Lipid Mediat. 83, 139–145 [DOI] [PubMed] [Google Scholar]
- 56. Sherwood O. D., Downing S. J., Rieber A. J., Fraley S. W., Bohrer R. E., Richardson B. C., Shanks R. D. (1985) Influence of litter size on antepartum serum relaxin and progesterone immunoactivity levels and on birth in the rat. Endocrinology 116, 2554–2562 [DOI] [PubMed] [Google Scholar]
- 57. Ochiai K., Kato H., Kelly P. A., Rothchild I. (1983) The importance of a luteolytic effect of the pituitary in understanding the placental control of the rat corpus luteum. Endocrinology 112, 1687–1695 [DOI] [PubMed] [Google Scholar]