Abstract
Extracellular ATP and ADP trigger inflammatory, vasodilatatory, and prothrombotic signaling events in the vasculature, and their turnover is governed by networks of membrane-associated enzymes. The contribution of soluble activities to intravascular nucleotide homeostasis remains controversial. By using thin-layer chromatographic assays, we revealed transphosphorylation of [γ-32P]ATP and AMP by human and murine sera, which was progressively inhibited by specific adenylate kinase (AK) inhibitor Ap5A. This phosphotransfer reaction was diminished markedly in serum from knockout mice lacking the major AK isoform, AK1, and in human serum immunodepleted of AK1. We also showed that ∼75% ADP in cell-free serum is metabolized via reversible AK1 reaction 2ADP ↔ ATP + AMP. The generated ATP and AMP are then metabolized through the coupled nucleotide pyrophosphatase/phosphodiesterase and 5′-nucleotidase/CD73 reactions, respectively. Constitutive presence of another nucleotide-converting enzyme, nucleoside triphosphate diphosphohydrolase-1 (NTPDase1, known as CD39), was ascertained by the relative deficiency of serum from CD39-null mice to dephosphorylate [3H]ADP and [γ-32P]ATP, and also by diminished [3H]ADP hydrolysis by human serum pretreated with NTPDase1 inhibitors, POM-1 and ARL-67156. In summary, we have identified hitherto unrecognized soluble forms of AK1 and NTPDase1/CD39 that contribute in the active cycling between the principal platelet-recruiting agent ADP and other circulating nucleotides.—Yegutkin, G. G., Wieringa, B., Robson, S. C., Jalkanen, S. Metabolism of circulating ADP in the bloodstream is mediated via integrated actions of soluble adenylate kinase-1 and NTPDase1/CD39 activities
Keywords: purinergic signaling, human and murine serum, intravascular nucleotide homeostasis, purinergic signaling
An important role for extracellular ATP and ADP as key signaling molecules has been established in several tissues, including the cardiovascular system, where they mediate immunomodulatory, vasoactive, and prothrombotic responses (1–4). Most models of purinergic signaling depend on functional interactions between distinct processes, including transient release of endogenous ATP and ADP; triggering of signaling events via a series of ligand-gated P2X and G-protein coupled P2Y receptors; ectoenzymatic nucleotide inactivation; and interaction of the resulting adenosine with own nucleoside-selective G-protein coupled receptors (1, 5). The duration and magnitude of purinergic signaling can be coordinated via opposite ectoenzymatic pathways, where sequential nucleotide breakdown to adenosine and further to inosine/hypoxanthine is counterbalanced by ATP resynthesis through backward phosphotransfer reactions (5).
Platelet activation and recruitment, followed by hemostatic plug formation, is generally initiated either via formation of thromboxane-A2 by cyclooxygenase or secretion of ADP from dense granules with subsequent activation of platelet ADP-selective P2Y1/P2Y12 receptors (6). In turn, vascular endothelium controls platelet reactivity and prevents thrombus formation via 3 pathways, including nitric oxide and prostaglandin-I2 synthesis and ADP scavenging by nucleoside triphosphate diphosphohydrolase-1 (NTPDase1, known as ecto-ATPDase, apyrase, CD39) (2, 7). Like other cell-surface members of NTPDase family, NTPDase1/CD39 represents an integral ∼70-kDa membrane glycoprotein with 2 transmembrane domains; it exists either in monomeric or homooligomeric states and can efficiently hydrolyze nucleoside tri- and diphosphates in the presence of Ca2+ and Mg2+ (5, 8–10). Sequence analysis of the proximal CD39 promoter in vascular endothelial cells revealed a binding site for hypoxia-inducible transcription factor Sp1 (11), and more recent studies demonstrated that Stat3 activation and TGF-β-mediated down-regulation of zinc finger protein growth factor independent-1 (Gfi-1) both transcriptionally regulate CD39 expression during differentiation of immunosuppressive Th17 cells (12). Data from mutant mice deficient in NTPDase1/CD39 (9, 13–15) or overexpressing human CD39 (16) and also from transgenic swine expressing human CD39 (17) further confirmed an important role for this ectoenzyme in the control of hemostasis, immune responses, and prothrombotic reactions. Engineered soluble forms of human NTPDase1/CD39 (18, 19) and other NTPDases (10), comprising only the extracellular domain, have also been expressed in mammalian cells, purified, and kinetically characterized. In addition, substantial ATP- and ADP-hydrolyzing activities may circulate in the blood serum or plasma, either as soluble forms (20–23) or incorporated into cell-derived microparticles (22, 24).
Adenylate kinase (AK), by virtue of its ability to catalyze the reaction ATP + AMP ↔ 2ADP, has been considered another key enzyme of nucleotide homeostasis (25). The enzyme isoforms are mainly localized in the cytosol (AK1), mitochondrial intermembrane space (AK2), mitochondrial matrix (AK3), and nucleus (AK6) of different tissues (25, 26). Studies to unravel the biological significance of AK have mainly focused on the role of AK1, the major enzyme isoform abundantly expressed in the heart, skeletal muscles, brain, and other tissues (26–29). Disruption of the network function by deletion of AK1 lowers the muscle energetic efficiency and reduces cell ability to maintain adenine nucleotide pools (27). AK1-mediated intracellular signaling coupled with AMP-responsive elements such as AMP-sensitive protein kinase, ATP-sensitive potassium channels (KATP) and AMP-sensitive metabolic enzymes, comprise a key metabolic sensing system regulating vital cellular processes (29). Plasma membrane-associated isoform AK1β has also been described (28, 30, 31), and the presence of AK activity on surfaces of vascular endothelial, lymphoid, and other cells is currently appreciated (5, 32, 33). Moreover, the ability of human and murine serum to transphosphorylate ATP and AMP into ADP suggests the presence of a soluble form of AK (21, 23).
The nature, origin, and exact mechanisms underlying the appearance of soluble purinergic enzymes in the blood and the physiological relevance of coupling these activities to intravascular nucleotide homeostasis remain largely unknown. By using thin-layer chromatograpy (TLC), in combination with competitive and immunodepleting approaches, we present here a dissection of the metabolism of radiolabeled nucleotides in the sera from human volunteers, as well as from wild-type and knockout mice lacking AK1 (27) or NTPDase1/CD39 (13) genes. The obtained results provide clear evidence that both AK1 and NTPDase1/CD39 are constitutively present in the human and murine blood as soluble enzymes and contribute, in conjunction with other purinergic enzymes, to the metabolism of ADP and other circulating nucleotides.
MATERIALS AND METHODS
Mice
Gene-targeted mice carrying a HygroBR replacement mutation in the exon-3-5 region of the AK1 gene were derived as described in detail elsewhere (27). Age- and sex-matched homozygous AK1−/− and wild-type control animals (both with 50–50% C56Bl/6×129/Ola mixed inbred background) were used throughout the experiments. We also studied wild-type C57Bl/6 mice (Taconic, Germantown, NY, USA) and CD39-null mice backcrossed with the same derivation of C57Bl/6 for >6 generations (13). All animals were raised in pathogen-free conditions, provided with food and water ad libitum, and used in the experiments at age 20 to 30 wk.
Tissue preparation
For murine serum preparation, mice were euthanized with carbon dioxide, and the blood was collected by heart puncture. For preparation of human serum and heparinized plasma, venous blood from healthy volunteers was collected into silicone- or lithium/heparin-containing Venosafe tubes (Terumo Europe N.V., Leuven, Belgium), respectively. Plasma samples were immediately centrifuged, while for serum preparation the blood was allowed to clot before centrifugation (10 min at 1000 g). Serum was additionally centrifuged for 10 min at 15,400 g, followed by freezing the resultant supernatants at −70°C. In some assays, serum was depleted of potential microparticles by passing it through Millex-GV 0.22 μm filters (Millipore, Core, Ireland). Murine tissue lysates were also prepared from freeze-clamped gastrocnemius and soleus muscles excised from the hind legs and from liver of AK1−/− mice and wild-type controls. The tissues were minced by scissors, incubated in phosphate buffered saline containing 0.5% Triton X-100 for 2 h with rocking at 4°C, clarified by centrifugation (20 min at 15,400 g), and stored at −70°C.
Immunodepletion of human serum of AK1 protein
Human serum was depleted of AK1 by using Protein A Sepharose CL-4B (GE Healthcare Bio-Science AB, Uppsala, Sweden), according to the manufacturer's instructions. Briefly, the swollen slurry was incubated for 2 h at room temperature in the appropriate binding buffer (Dulbecco's phosphate buffered saline supplemented with 1 mM MgCl2 and 1.2 mM CaCl2) containing either rabbit anti-AK1 antibody fused to the glutathione S-transferase protein (28) or normal rabbit serum (NRS) as a negative control. Human serum (100 μl) was then incubated under rotation for 2 h in the total volume of 250 μl medium containing binding buffer and ∼50 μl of AK1- or NRS-coupled Sepharose CL-4B beads. After centrifugation, supernatants containing the AK1- or mock-depleted sera were assayed for AK activity, as specified below. In addition, the sedimented slurry fractions were washed, resuspended in 150 μl binding buffer, and also used in the enzyme assays.
Analysis of purine-converting activities
Soluble purinergic activities were determined at 37°C in a final volume of 80 μl RPMI 1640 medium supplemented with 25 mM HEPES (pH 7.35) and containing 4 mM β-glycerophosphate and 4 μl murine serum in the following ways: for ADPase assays, serum was pretreated for 20 min with 80 μM diadenosine pentaphosphate (Ap5A; Sigma, St. Louis, MO, USA), followed by 60 min incubation with 100 μM [2,8-3H]ADP (Perkin Elmer, Boston, MA, USA); 5′-nucleotidase activity was determined after 60 min incubation of serum with 300 μM [2-3H]AMP (Quotient Bioresearch; GE Healthcare, Rushden, UK); AK and NDP kinase were assayed by incubating the serum with 400 μM [3H]AMP (for 45 min) or [3H]ADP (for 15 min) as respective phosphorus acceptors in the presence of 750 μM γ-phosphate-donating ATP; adenosine deaminase was measured by incubating the serum for 60 min with 300 μM [2-3H]adenosine (Amersham, Little Chalfont, UK). In addition, human AK was determined after 45 min incubation of serum or plasma (10 μl) with 400 μM [3H]AMP plus 750 μM ATP, while for ADPase assays, blood samples were incubated for 60 min with 50 μM [3H]ADP in the presence of 100 μM Ap5A. Catalytic reactions were terminated by applying aliquots of the mixture onto Alugram SIL G/UV254 sheets (Macherey-Nagel, Duren, Germany). 3H-labeled nucleotides and nucleosides were separated by TLC and then either quantified by scintillation β-counting (21) or exposed to Kodak BioMax MS films (Eastman Kodak, Rochester, NY, USA) for 2 wk at −70°C and developed by autoradiography. Autoradiographic analysis was also performed by incubating 4 μl murine serum for 30 min in 60 μl RPMI 1640 containing 4 mM β-glycerophosphate, 50 μM [γ-32P]ATP (Perkin Elmer) and various unlabeled nucleotides, followed by TLC separation of mixture aliquots on Polygram CEL-300 PEI sheets (Macherey-Nagel) with 0.75 M KH2PO4 (pH 3.5) as solvent. In some competitive assays, the samples were pretreated for 20 min with various concentrations of specific AK inhibitor Ap5A (25), as well as NTPDase1 inhibitors sodium polyoxotungstate-1 (POM-1; ref. 34) and ARL-67156 (35), and nonselective antagonist of P2 receptors pyridoxalphosphate-6-azophenyl-2′,4′-disulfonic acid (PPADS; ref. 1) (all from Tocris Bioscience, Bristol, UK), prior to addition of nucleotide substrates.
Statistical analysis
Data from competitive experiments were subjected to computer analysis by nonlinear least-squares curve fitting to determine IC50 values (GraphPad Prism 4.03; GraphPad, San Diego, CA, USA). The results are presented mainly as column bars (means±se) and statistical comparisons were made using Student's t test. The significance level was set at P < 0.05.
RESULTS
Absence of AK-mediated phosphotransfer in serum and skeletal muscle, but not in liver, from AK1−/− mice
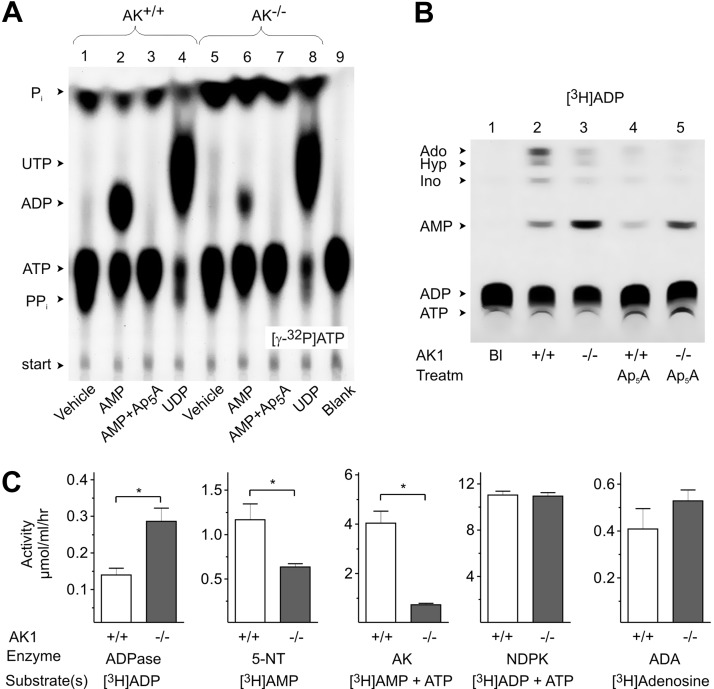
First, we took advantage of radio-TLC for characterizing the major soluble nucleotide-converting pathways in the sera from wild-type (i.e., AK1+/+) and AK1−/− mice. As shown in Fig. 1A, incubation of serum from AK+/+ mice with [γ-32P]ATP caused its breakdown into 32Pi and 32PPi (lane 1). Addition of AMP diminished the [γ-32P]ATP-hydrolyzing capability of AK+/+ serum and, at the same time, triggered formation of [β-32P]ADP (lane 2), which can be prevented by specific AK inhibitor Ap5A (Fig. 1A, lane 3). The relative deficiency of serum from AK1−/− mice to transphosphorylate [γ-32P]ATP and AMP into [β-32P]ADP (Fig. 1A, lane 6) indicates the predominant contribution of soluble AK1 isoform to the measured exchange activities. The employment of [γ-32P]ATP and UDP as another appropriate pair of γ-phosphate-donating and -accepting nucleotide substrates also revealed their transphosphorylation into [γ-32P]UTP and ADP. This reaction occurred at fairly similar rates both in AK+/+ (Fig. 2A; lane 4) and AK1−/− (Fig. 2A, lane 8) sera, remains insensitive to Ap5A treatment (data not shown) and most likely, is mediated by other soluble phosphorylating enzyme, NDP kinase.
Figure 1.
TLC analysis of purine-converting pathways in the serum from AK1−/− mice. A, B) Serum from AK1−/− and wild-type (AK+/+) mice was incubated with 50 μM [γ-32P]ATP in the absence (vehicle) or presence of 1 mM AMP and UDP (A), as well as with 100 μM [3H]ADP (B). Some samples were pretreated with 80 μM Ap5A prior to addition of tracer substrates. Blanks (Bl) show the radiochemical purity of tracer substrates in the absence of serum. Arrows indicate the positions of nucleotide standards, adenosine (Ado), hypoxanthine (Hyp), and inosine (Ino), as well as inorganic phosphate (Pi), and pyrophosphate (PPi). C) Sera from AK1−/− and AK+/+ mice were assayed for specific AK, ADPase, 5′-nucleotidase (5-NT), adenosine deaminase (ADA), and NDP kinase (NDPK) activities using saturated concentrations of indicated 3H-labeled and unlabeled substrates (means±se n=10–12). *P < 0.05 vs. wild-type controls.
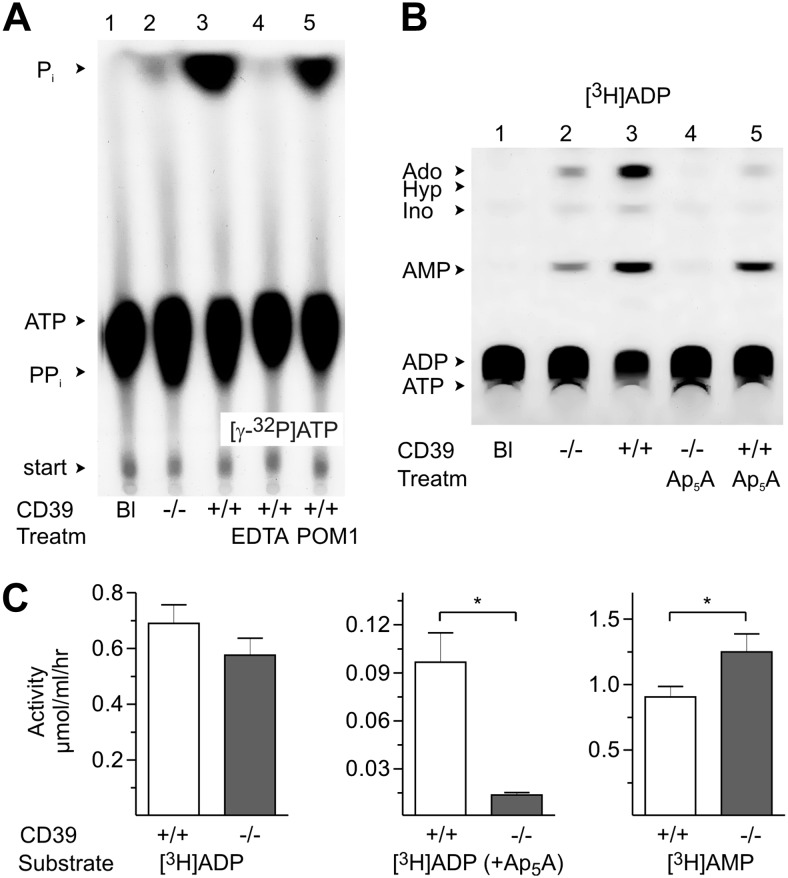
Figure 2.
TLC analysis of soluble nucleotidase activities in CD39−/− mice. A, B) Sera from CD39−/− and wild-type (CD39+/+) mice were incubated with 50 μM [γ-32P]ATP (A) or with 100 μM [3H]ADP (B). Some samples were pretreated with EDTA (2 mM), POM-1 (100 μM), or Ap5A (80 μM) prior to addition of tracer substrates. Blanks (Bl) show the radiochemical purities of [γ-32P]ATP and [3H]ADP. Arrows indicate the positions of nucleotide and nucleoside standards, inorganic phosphate (Pi) and pyrophosphate (PPi). C) Rate of [3H]ADP (100 μM) hydrolysis was measured in sera from CD39+/+ and CD39−/− mice in the absence (left panel) or presence (middle panel) of 80 μM Ap5A, while 5′-nucleotidase (5-NT) was assayed using 300 μM [3H]AMP as substrate (right panel). Data are presented as means ± se (n=12). *P < 0.05 vs. wild-type controls.
Additional autoradiographic analysis of the pattern of [γ-32P]ATP metabolism in tissue lysates revealed the absence of AK activity in skeletal muscles from AK1−/− mice, whereas livers from both AK+/+ and AK1−/− animals could efficiently transphosphorylate [γ-32P]ATP and AMP in a similar Ap5A-dependent manner (Supplemental Fig. S1). These data are consistent with the current view that AK1 is abundantly expressed in cytosol of skeletal muscles (26–28), while liver primarily contains another AK isozyme, AK2, which is located in mitochondria and intermembrane space (26). Furthermore, here for the first time, we provide evidence that AK1 represents the major soluble AK isoform accounting for AMP-ATP transphosphorylation in murine serum.
Circulating ADP is primarily metabolized via reverse soluble AK1 reaction in murine serum
Autoradiographic and quantitative TLC analyses of purine-converting activities were then performed in the serum from AK+/+ and AK−/− mice by using [3H]ADP and other 3H-labeled substrates. As shown in Fig. 1B, incubation of serum from wild-type mice with [3H]ADP as an initial substrate was accompanied by its hydrolysis into [3H]AMP and further to [3H]adenosine and other 3H-nucleosides (lane 2). This catalytic conversion was markedly inhibited in the presence of Ap5A (Fig. 1B, lane 4). Addition of [3H]ADP to the AK−/− serum triggered its breakdown into [3H]AMP as the major end-reaction product (Fig. 1B, lane 3), and this reaction was only slightly prevented by Ap5A (Fig. 1B, lane 5). In comparison with wild-type controls, AK1−/− mice are characterized by ∼80% lower rate of AK-mediated [3H]AMP/ATP transphosphorylation (Fig. 1C). Interestingly, serum from AK1−/− mice also displayed an enhanced rate of [3H]ADP dephosphorylation (determined in the presence of Ap5A) and diminished 5′-nucleotidase activity. Evaluation of the activities of other soluble enzymes, NDP kinase and adenosine deaminase, did not reveal significant differences between wild-type and AK1−/− animals (Fig. 1C).
Soluble NTPDase1/CD39 circulates in murine blood and contributes to the catabolism of intravascular ATP and ADP
For further elucidation of the nature of soluble nucleotide-hydrolyzing activities, the pattern of nucleotide metabolism was evaluated in CD39−/− mice. As can be seen in Fig. 2A, incubation of serum from the wild-type (CD39+/+) mice with [γ-32P]ATP caused efficient formation of 32Pi, and this reaction was completely abolished in the presence of cation-chelating agent EDTA and partially inhibited by NTPDase1 inhibitor POM-1. Strikingly, serum from CD39−/− mice displayed a markedly diminished [γ-32P]ATP-hydrolyzing capability, thus indicating the predominant contribution of NTPDase1/CD39 to the measured catalytic reaction. This conclusion was ascertained in studies with another preferred substrate for NTPDase1, [3H]ADP, which was dephosphorylated into [3H]AMP and other metabolites during incubation with serum from CD39+/+ (Fig. 2B; lane 5), but not from CD39−/− (lane 4), mice. Notably, clearcut distinctions between CD39+/+ and CD39−/− sera with dramatically reduced [3H]ADP hydrolysis in the latter case can be detected only under complete exclusion of overlapping AK1 activity with inhibitor Ap5A (Fig. 2B, C). Sera from CD39−/− mice also displayed elevated 5′-nucleotidase activity, when compared with wild-type controls (Fig. 2C). Notably, performance of enzyme assays at neutral pH in the presence of a large excess of β-glycerophosphate as an alternative phosphorylated substrate allowed us to exclude the potential contribution of nonspecific alkaline phosphatases to the measured activities.
Evidence for the presence of soluble AK1 and NTPDase1/CD39 in human blood and their contribution to the metabolism of ADP and other nucleotides
AK activities were then assayed in human and murine sera pretreated with the increasing concentrations of Ap5A. The rate of ATP-mediated [3H]AMP phosphorylation was progressively inhibited in the presence of Ap5A, and IC50 values for this inhibitory effect were ∼4–5 μM for both human and murine serum (Fig. 3A). Concurrent measurements of [3H]ADP metabolism by serum samples revealed that, despite similar pattern of concentration-dependent inhibition of reverse AK reaction by Ap5A, the residual ∼20–25% of [3H]ADP-converting activity remained insensitive even to high micromolar concentrations of this dinucleotide (Fig. 3B), and therefore may be considered as “true” soluble nucleotide hydrolase, most likely NTPDase1/CD39.
Figure 3.
Progressive inhibition of soluble AK activity in human and murine blood by Ap5A. Sera from human volunteers (n=5) and from C57Bl/6 wild-type mice (n=3) were preincubated for 20 min with increasing concentrations of Ap5A (0–120 μM). Direct and reverse AK activities were determined by the rates of [3H]AMP and ATP transphosphorylation (A) and [3H]ADP conversion into dephosphorylated 3H-metabolites (B), respectively. Results are plotted as the percentage of maximal activity measured in the absence of Ap5A (defined as 100%), and IC50 values were calculated from the obtained competitive curves using a nonlinear curve-fitting program (means±se).
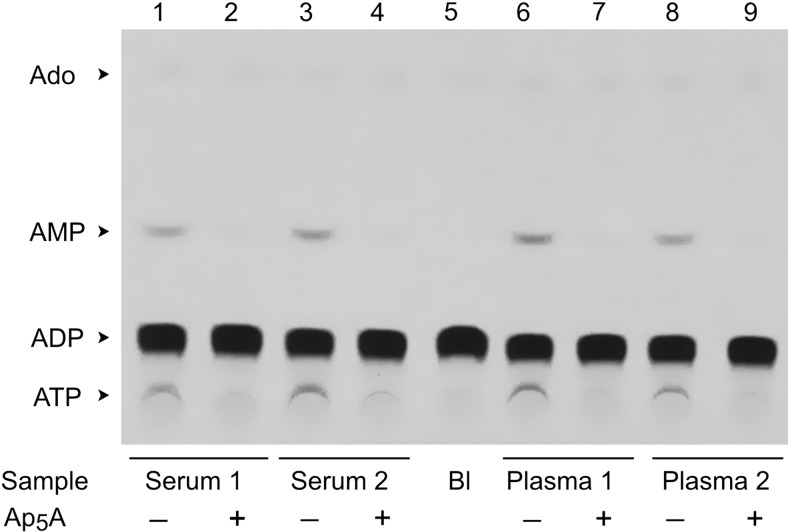
Additional autoradiographic imaging detected low but clearly visible capacity of the human serum to convert [3H]ADP into [3H]AMP and [3H]ATP (Fig. 4, lanes 1, 3), which can be prevented by Ap5A (Fig. 4, lanes 2, 4). Interestingly, similar pattern of Ap5A-dependent [3H]ADP phosphorylation was observed in studies with heparinized plasma (Fig. 4, lanes 6–9). Quantitative analysis also did not reveal any differences in specific AK (Fig. 5A) and NTPDase/ADPase (Fig. 5B) activities between serum and heparinized plasma samples prepared from the same subjects, as well as after supplementary serum filtration through the 0.22-μm devices. These data allow excluding potential release of membrane-bound enzymes from the formed blood elements in the course of serum preparation and provide further evidence for constitutive presence of soluble and “microparticle-free” purinergic activities in the human blood.
Figure 4.
Autoradiographic analysis of [3H]ADP metabolism in human blood. Serum and heparinized plasma were collected from venous blood of 2 volunteers. Blood samples (10 μl) were pretreated for 20 min in the absence or presence of 100 μM Ap5A prior to addition of 50 μM [3H]ADP. After 60 min incubation, aliquots of the reaction mixture were separated by TLC and developed by autoradiography. Blank (Bl) shows the radiochemical purity of [3H]ADP. Arrows indicate the positions of nucleotide standards and adenosine.
Figure 5.
Pattern of nucleotide metabolism in human blood. Purinergic activities were determined in human serum and heparinized plasma, as well as in serum filtrates passed through 0.22-μm filters (n=4). A) Left panel: Soluble AK was assayed by TLC using 400 μM [3H]AMP plus 750 μM ATP. Right panel: human serum was also depleted of AK1 with Protein A Sepharose CL-4B beads to which rabbit anti-AK1 antibodies (AK1) or normal rabbit serum (NRS; serving as irrelevant negative control) had been coupled. AK activity was assayed both in serum supernatants and resuspended bead pellets and expressed as micromoles of [3H]AMP phosphorylated by 1 ml of serum or beads per hour (means±se; n=3). B) Soluble NTPDase/ADPase was assayed by TLC as in A, using 50 μM [3H]ADP in the presence of 100 μM Ap5A. C) Serum was pretreated with 300 μM POM-1, ARL-67156, and PPADS prior to addition of [3H]ADP. Graph shows soluble [3H]ADP-hydrolyzing activity plotted as the percentage of control nucleotidase activity measured in the absence of inhibitors (means±se; n=6–8). *P < 0.05 vs. corresponding controls.
The presence of human soluble AK1 was ascertained in immunodepleting studies, by incubating serum samples with Protein A Sepharose CL-4B beads coupled to anti-AK1 antibody. Compared with mock-treated controls, AK1-depleted serum is characterized by ∼50–60% lower rate of [3H]AMP-ATP transphosphorylation with respective accumulation of AK activity in the resuspended pellet fraction (Fig. 5A). Competitive analysis of ADPase activity was then performed in the presence of known NTPDase1 inhibitors, POM-1 (4, 34) and ARL-67156 (35, 36), as well as P2 receptor antagonist PPADS, which may also inhibit membrane-bound ATPase/NTPDase (37, 38). Pretreatment of human serum samples with 300 μM POM-1 or ARL-67156, but not PPADS, diminished their [3H]ADP-hydrolyzing capacity by ∼50% (Fig. 5C). Together with the above data on POM-1-mediated inhibition of [γ-32P]ATP hydrolysis by murine serum (see Fig. 2A, lane 5), these data provide independent line of evidence that soluble NTPDase1/CD39 presents both in human and murine blood and contributes to the metabolism of intravascular nucleotides.
DISCUSSION
By using radio-TLC assay as the most sensitive and versatile approach for screening the purine-converting pathways, we have evaluated the pattern of nucleotide metabolism in sera from wild-type and knockout mice lacking AK1 or NTPDase1/CD39, as well as in serum and heparinized plasma from healthy human volunteers. A salient finding of this work is the demonstration that AK1 and NTPDase1/CD39 represent two key soluble ADP-converting enzymes in human and murine blood and, in conjunction with other soluble and membrane-bound enzymes, comprise an efficient network regulating nucleotide homeostasis in circulation.
The ability of human plasma to dephosphorylate ATP and other adenine nucleotides into adenosine and further to inosine/hypoxanthine was first demonstrated over half a century ago (39). Subsequent chromatographic and colorimetric assays allowed the identification and kinetic characterization of several purine-inactivating activities in the plasma and serum from humans and other species; i.e., nucleotide pyrophosphatases/ phosphodiesterases (NPPs; refs. 40, 41), ATPase and ADPase (20, 23, 42), 5′-nucleotidase (AMPase; refs. 20, 21, 43) and adenosine deaminase (5, 21). While the initial step of ATP breakdown in cell-free plasma or serum is mainly attributable to NPP (presumably, NPP1) activity, recent studies elicited the coexistence of another soluble nucleotidase which accounts for ∼10–15% of the total ATP-hydrolyzing pool in human serum and, in addition, may utilize ADP as another preferred substrate (23). Though, given the broad substrate specificity of the enzymes of NPP family which can hydrolyze pyrophosphate and phosphodiester bonds (40), potential contribution of certain NPPs to this “NTPDase-like” reaction cannot be excluded. The noted ability of serum from CD39+/+, but not from CD39−/−, mice to efficiently dephosphorylate [γ-32P]ATP and [3H]ADP, as well as marked inhibition of [3H]ADP hydrolysis by human serum in the presence of known NTPDase1 inhibitors POM-1 and ARL-67156, provide unambiguous evidence for constitutive presence of soluble NTPDase1/CD39 in the human and murine bloodstream.
Another novelty of this study is that soluble AK1 was identified as another key player in the metabolism of circulating ADP. The existence of AK activity in human plasma has long been proposed (42, 44). Subsequent studies confirmed the ability of human and murine serum to convert [γ-32P]ATP and AMP into [β-32P]ADP in an Ap5A-dependent manner (21, 23). Strikingly, drastic diminishment of this reaction in the sera from AK1−/− mice (see Fig. 1), as well as in AK1-depleted human serum (Fig. 5) indicates the predominant contribution of AK1 isoform to the measured phosphoryl transfers. AK is known to be a highly flexible protein containing ATP- and AMP-binding domains that substantially change on concurrent binding of both substrates (25). Therefore, the relevance of AK1-mediated transphosphorylation of ATP and AMP is presumably restricted by acute settings of inflammation or traumatic shock, where concurrent release of intravascular nucleotides would be sufficient for additive conformational changes of soluble AK. On the other hand, progressive inhibition of [3H]ADP metabolism by bisubstrate analog Ap5A provides evidence that up to 75% of ADP in cell-free serum is converted via reverse AK1 reaction: 2ADP ↔ ATP + AMP. The generated ATP and AMP are then metabolized through the coupled soluble NPP and 5′-nucleotidase reactions, respectively. This work additionally pointed to the existence of a network of soluble enzymes coordinately maintaining nucleotide homeostasis, where AK1- and CD39-mediated reactions play a complementary and functionally synergistic role. Indeed, the impaired ability of serum from AK1−/− mice to transphosphorylate ADP was compensated by up-regulated soluble ADPase/NTPDase activity (see Fig. 1).
Additional studies are required to elucidate the origin of soluble enzymes and mechanisms of their appearance into blood. Interestingly, a certain portion of cellular AK was shown to be released from the platelets and red blood cells (44), skeletal muscles (31), and epithelial cells (33), while the potential sources for secreted NTPDase1/CD39 might include nerve terminals (45), pancreatic acini and juice (46, 47), vascular endothelium (9, 48) and other cells. Alternatively, CD39 can also be incorporated into membrane-derived microvesicles where it maintains catalytic activity and impacts endothelial activation (24), platelet aggregation (22), and pancreatic secretion (46). However, absence of any difference in purine-converting activities between human serum and heparinized plasma, together with data on unchanged nucleotide metabolism after additional serum filtration (see Fig. 5) or ultracentrifugation for 60 min at 100,000 g (23), provide evidence for the constitutive presence of true soluble enzymes in circulation.
Assessment of the relative contribution of soluble vs. membrane-bound enzymes is another important issue. While endothelial NTPDase1/CD39, in concert with ecto-5′-nucleotidase/CD73, are thought to represent the major regulators of intravascular nucleotide homeostasis (5, 9, 49), soluble enzymes could comprise an important auxiliary effector system for tuned regulation of purinergic responses, especially within larger vessels where the capacity to metabolize nucleotides resides equally at the luminal surface of the vessel and in the flowing blood (20). Moreover, the balance between endothelial and soluble nucleotidases can be shifted under various pathological and inflammatory conditions, including hypoxia, ischemia, endothelial dysfunction, atherogenesis, and tissue injury (4, 7, 50). Specifically, previous studies revealed substantial hypoxia-induced induction of endothelial NTPDase1/CD39 expression in vitro (11, 49) as well as in acute myocardial ischemia (11) and hepatic injury (15) murine models, which is regulated by hypoxia-inducible transcription factor Sp1. However, chronic hypoxic exposure, oxidative stress, and pathological vascular remodeling may trigger the opposite effect of down-regulated NTPDase activity in the endothelial and other cells (4, 7, 51, 52).
It is pertinent to note that regulation of local concentrations of ADP by the recombinant human NTPDase1/CD39 inhibits platelet aggregation in vitro (2, 18) and diminishes thrombus formation and platelet reactivity at various experimental prothrombotic conditions, including murine models of cerebral stroke (14), hepatic injury (7, 15), and hypothermia (53); swine models of balloon arterial (54); and myocardial ischemia/reperfusion injuries (17), as well as acute and chronic models of transplant rejection (7). To date, only scattered evidence indicates factors controlling the expression and release of AK1. Nevertheless, given that cellular AK1, in conjunction with AMPK and other AMP-responsive components, is recognized as key regulator of the cellular energy state during hypoxia and other metabolic stress conditions (27, 29), it might be hypothesized that regulation of soluble AK1 activity might provide a network coordinating both energetic homeostasis and intravascular signal transduction pathways. Interestingly, transiently elevated circulating ATP/ADP levels and augmented platelet activity occurring during strenuous exercise in humans was shown to be accompanied by up-regulation of serum ADPase/NTPDase activity without any shifts in soluble AK and other purinergic enzymes (23).
In summary, our studies provide novel insights into the regulatory mechanisms of purine homeostasis in the fluid phase of the blood, as schematically outlined in Fig. 6. Consistent with previous findings (21, 23), the elements of inactivating cascade involve NPP, 5′-nucleotidase/CD73 and adenosine deaminase, while the counteracting ATP-regenerating pathway comprises AK and NDP kinase. Ultimately, the generated inosine can be converted into hypoxanthine by purine nucleoside phosphorylase and further via xanthine to uric acid through xanthine oxidoreductase reaction (55). The ability of soluble NPP to directly convert ATP into AMP and PPi allows bypassing the generation of ADP as the principal platelet-recruiting agent. Here we have shown for the first time that AK1 represents the major soluble AK isoform, which freely circulates in the blood and governs the majority of ADP homeostasis in cell-free serum. Furthermore, the identification of the hitherto unrecognized “natural antithrombotic enzyme” NTPDase1/CD39 in the serum, which shares substrate specificity with AK toward ADP (and also with NPP toward ATP), may provide an important auxiliary effector system for local scavenging of intravascular nucleotides. Identifying the precise mechanisms underlying the appearance of purinergic activities in the bloodstream at various pathophysiological states and their relevance within the larger framework of membrane-associated ectoenzymes may open up further research to assess the potential therapeutic and diagnostic applications of soluble purinergic enzymes in the vasculature.
Figure 6.
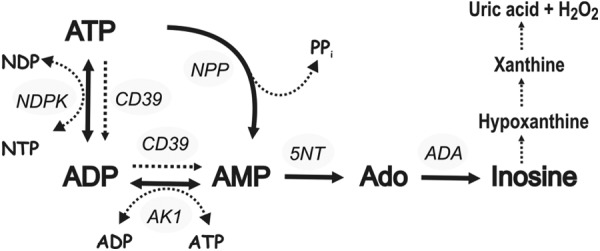
Major purine-converting pathways in cell-free serum. Scheme highlights the potential exchange activities of circulating adenine nucleotides and adenosine and additionally outlines further conversion of adenosine-derived inosine into hypoxanthine, xanthine, and uric acid. The following soluble purinergic activities have been identified: nucleotide pyrophosphatase/ phosphodiesterase (NPP), NTPDase1/CD39, 5′-nucleotidase/CD73 (5NT), adenylate kinase-1 (AK1), NDP kinase (NDPK), and adenosine deaminase (ADA).
Acknowledgments
This work was supported by grants from the Academy of Finland and the Sigrid Juselius Foundation (G.G.Y. and S.J.) and the U.S. National Institutes of Health (S.C.R.).
The authors are grateful to Frank Oerlemans for help in collecting the tissues from AK−/− and wild-type AK+/+ mice and to Drs. Keiichi Enjyoji and Guido Beldi (Harvard Medical School, Boston, MA, USA) for supplying plasma samples from CD39-null mice. The authors also thank Sari Mäki for technical assistance and Anne Sovikoski-Georgieva for secretarial help.
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
- AK
- adenylate kinase
- Ap5A
- diadenosine pentaphosphate
- NPP
- nucleotide pyrophosphatases/phosphodiesterases
- NTPDase
- nucleoside triphosphate diphosphohydrolase
- POM-1
- polyoxotungstate-1
- PPADS
- pyridoxalphosphate-6-azophenyl-2′,4′-disulfonic acid
- TLC
- thin-layer chromatography
REFERENCES
- 1. Ralevic V., Burnstock G. (1998) Receptors for purines and pyrimidines. Pharmacol. Rev. 50, 413–492 [PubMed] [Google Scholar]
- 2. Marcus A. J., Broekman M. J., Drosopoulos J. H., Islam N., Pinsky D. J., Sesti C., Levi R. (2003) Metabolic control of excessive extracellular nucleotide accumulation by CD39/ecto-nucleotidase-1: implications for ischemic vascular diseases. J. Pharmacol. Exp. Ther. 305, 9–16 [DOI] [PubMed] [Google Scholar]
- 3. Erlinge D., Burnstock G. (2008) P2 receptors in cardiovascular regulation and disease. Purinergic Signal. 4, 1–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mercier N., Kiviniemi T. O., Saraste A., Miiluniemi M., Silvola J., Jalkanen S., Yegutkin G. G. (2012) Impaired ATP-induced coronary blood flow and diminished aortic NTPDase activity precede lesion formation in apolipoprotein E-deficient mice. Am. J. Pathol. 180, 419–428 [DOI] [PubMed] [Google Scholar]
- 5. Yegutkin G. G. (2008) Nucleotide- and nucleoside-converting ectoenzymes: important modulators of purinergic signalling cascade. Biochim. Biophys. Acta 1783, 673–694 [DOI] [PubMed] [Google Scholar]
- 6. Gachet C. (2006) Regulation of platelet functions by P2 receptors. Ann. Rev. Pharm. Toxicol. 46, 277–300 [DOI] [PubMed] [Google Scholar]
- 7. Robson S. C., Wu Y., Sun X., Knosalla C., Dwyer K., Enjyoji K. (2005) Ectonucleotidases of CD39 family modulate vascular inflammation and thrombosis in transplantation. Semin. Thromb. Hemost. 31, 217–233 [DOI] [PubMed] [Google Scholar]
- 8. Kaczmarek E., Koziak K., Sevigny J., Siegel J. B., Anrather J., Beaudoin A. R., Bach F. H., Robson S. C. (1996) Identification and characterization of CD39/vascular ATP diphosphohydrolase. J. Biol. Chem. 271, 33116–33122 [DOI] [PubMed] [Google Scholar]
- 9. Robson S. C., Sevigny J., Zimmermann H. (2006) The E-NTPDase family of ectonucleotidases: Structure function relationship and pathophysiological significance. Purinergic Signal. 2, 409–430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Knowles A. F. (2011) The GDA1_CD39 superfamily: NTPDases with diverse functions. Purinergic Signal. 7, 21–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Eltzschig H. K., Kohler D., Eckle T., Kong T., Robson S. C., Colgan S. P. (2009) Central role of Sp1-regulated CD39 in hypoxia/ischemia protection. Blood 113, 224–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chalmin F., Mignot G., Bruchard M., Chevriaux A., Vegran F., Hichami A., Ladoire S., Derangere V., Vincent J., Masson D., Robson S. C., Eberl G., Pallandre J. R., Borg C., Ryffel B., Apetoh L., Rebe C., Ghiringhelli F. (2012) Stat3 and Gfi-1 transcription factors control Th17 cell immunosuppressive activity via the regulation of ectonucleotidase expression. Immunity 36, 362–373 [DOI] [PubMed] [Google Scholar]
- 13. Enjyoji K., Sevigny J., Lin Y., Frenette P. S., Christie P. D., Esch J. S., 2nd, Imai M., Edelberg J. M., Rayburn H., Lech M., Beeler D. L., Csizmadia E., Wagner D. D., Robson S. C., Rosenberg R. D. (1999) Targeted disruption of cd39/ATP diphosphohydrolase results in disordered hemostasis and thromboregulation. Nat. Med. 5, 1010–1017 [DOI] [PubMed] [Google Scholar]
- 14. Pinsky D. J., Broekman M. J., Peschon J. J., Stocking K. L., Fujita T., Ramasamy R., Connolly E. S., Jr., Huang J., Kiss S., Zhang Y., Choudhri T. F., McTaggart R. A., Liao H., Drosopoulos J. H., Price V. L., Marcus A. J., Maliszewski C. R. (2002) Elucidation of the thromboregulatory role of CD39/ectoapyrase in the ischemic brain. J. Clin. Invest. 109, 1031–1040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hart M. L., Gorzolla I. C., Schittenhelm J., Robson S. C., Eltzschig H. K. (2010) SP1-dependent induction of CD39 facilitates hepatic ischemic preconditioning. J. Immunol. 184, 4017–4024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dwyer K. M., Robson S. C., Nandurkar H. H., Campbell D. J., Gock H., Murray-Segal L. J., Fisicaro N., Mysore T. B., Kaczmarek E., Cowan P. J., d'Apice A. J. (2004) Thromboregulatory manifestations in human CD39 transgenic mice and the implications for thrombotic disease and transplantation. J. Clin. Invest. 113, 1440–1446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wheeler D. G., Joseph M. E., Mahamud S. D., Aurand W. L., Mohler P. J., Pompili V. J., Dwyer K. M., Nottle M. B., Harrison S. J., d'Apice A. J., Robson S. C., Cowan P. J., Gumina R. J. (2012) Transgenic swine: expression of human CD39 protects against myocardial injury Wheeler, CD39 reduces myocardial injury in swine. J. Mol. Cell. Cardiol. 52, 958–961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gayle R. B., 3rd, Maliszewski C. R., Gimpel S. D., Schoenborn M. A., Caspary R. G., Richards C., Brasel K., Price V., Drosopoulos J. H., Islam N., Alyonycheva T. N., Broekman M. J., Marcus A. J. (1998) Inhibition of platelet function by recombinant soluble ecto-ADPase/CD39. J. Clin. Invest. 101, 1851–1859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Schulte am Esch J., 2nd, Sevigny J., Kaczmarek E., Siegel J. B., Imai M., Koziak K., Beaudoin A. R., Robson S. C. (1999) Structural elements and limited proteolysis of CD39 influence ATP diphosphohydrolase activity. Biochemistry 38, 2248–2258 [DOI] [PubMed] [Google Scholar]
- 20. Coade S. B., Pearson J. D. (1989) Metabolism of adenine nucleotides in human blood. Circ. Res. 65, 531–537 [DOI] [PubMed] [Google Scholar]
- 21. Yegutkin G. G., Samburski S. S., Jalkanen S. (2003) Soluble purine-converting enzymes circulate in human blood and regulate extracellular ATP level via counteracting pyrophosphatase and phosphotransfer reactions. FASEB J. 17, 1328–1330 [DOI] [PubMed] [Google Scholar]
- 22. Cauwenberghs S., Feijge M. A., Hageman G., Hoylaerts M., Akkerman J. W., Curvers J., Heemskerk J. W. (2006) Plasma ectonucleotidases prevent desensitization of purinergic receptors in stored platelets: importance for platelet activity during thrombus formation. Transfusion 46, 1018–1028 [DOI] [PubMed] [Google Scholar]
- 23. Yegutkin G. G., Samburski S. S., Mortensen S. P., Jalkanen S., Gonzalez-Alonso J. (2007) Intravascular ADP and soluble nucleotidases contribute to acute prothrombotic state during vigorous exercise in humans. J. Physiol. 579, 553–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Banz Y., Beldi G., Wu Y., Atkinson B., Usheva A., Robson S. C. (2008) CD39 is incorporated into plasma microparticles where it maintains functional properties and impacts endothelial activation. Br. J. Haematol. 142, 627–637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yan H., Tsai M.-D. (1999) Nucleoside monophosphate kinases: structure, mechanism, and substrate specificity. Adv. Enzymol. Relat. Areas Mol. Biol. 73, 103–134 [DOI] [PubMed] [Google Scholar]
- 26. Noma T. (2005) Dynamics of nucleotide metabolism as a supporter of life phenomena. J. Med. Invest. 52, 127–136 [DOI] [PubMed] [Google Scholar]
- 27. Janssen E., Dzeja P. P., Oerlemans F., Simonetti A. W., Heerschap A., de Haan A., Rush P. S., Terjung R. R., Wieringa B., Terzic A. (2000) Adenylate kinase 1 gene deletion disrupts muscle energetic economy despite metabolic rearrangement. EMBO J. 19, 6371–6381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Janssen E., Kuiper J., Hodgson D., Zingman L. V., Alekseev A. E., Terzic A., Wieringa B. (2004) Two structurally distinct and spatially compartmentalized adenylate kinases are expressed from the AK1 gene in mouse brain. Mol. Cell. Biochem. 256–257, 59–72 [DOI] [PubMed] [Google Scholar]
- 29. Dzeja P. P., Chung S., Terzic A. (2007) Integration of adenylate kinase, glycolytic and glycogenolytic circuits in cellular energetics. In Molecular System Bioenergetics, Vol. 8 (Saks V., ed.) pp. 265–302, Wiley-VCH, Weinheim [Google Scholar]
- 30. Collavin L., Lazarevic D., Utrera R., Marzinotto S., Monte M., Schneider C. (1999) wt p53 dependent expression of a membrane-associated isoform of adenylate kinase. Oncogene 18, 5879–5888 [DOI] [PubMed] [Google Scholar]
- 31. Choo H. J., Kim B. W., Kwon O. B., Lee C. S., Choi J. S., Ko Y. G. (2008) Secretion of adenylate kinase 1 is required for extracellular ATP synthesis in C2C12 myotubes. Exp. Mol. Med. 40, 220–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yegutkin G. G., Henttinen T., Jalkanen S. (2001) Extracellular ATP formation on vascular endothelial cells is mediated by ecto-nucleotide kinase activities via phosphotransfer reactions. FASEB J. 15, 251–260 [DOI] [PubMed] [Google Scholar]
- 33. Donaldson S. H., Picher M., Boucher R. C. (2002) Secreted and cell-associated adenylate kinase and nucleoside diphosphokinase contribute to extracellular nucleotide metabolism on human airway surfaces. Am. J. Respir. Cell Mol. Biol. 26, 209–215 [DOI] [PubMed] [Google Scholar]
- 34. Muller C. E., Iqbal J., Baqi Y., Zimmermann H., Rollich A., Stephan H. (2006) Polyoxometalates–a new class of potent ecto-nucleoside triphosphate diphosphohydrolase (NTPDase) inhibitors. Bioorg. Med. Chem. Lett. 16, 5943–5947 [DOI] [PubMed] [Google Scholar]
- 35. Crack B. E., Pollard C. E., Beukers M. W., Roberts S. M., Hunt S. F., Ingall A. H., McKechnie K. C., AP I. J., Leff P. (1995) Pharmacological and biochemical analysis of FPL 67156, a novel, selective inhibitor of ecto-ATPase. Br. J. Pharmacol. 114, 475–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Levesque S. A., Lavoie E. G., Lecka J., Bigonnesse F., Sevigny J. (2007) Specificity of the ecto-ATPase inhibitor ARL 67156 on human and mouse ectonucleotidases. Br. J. Pharmacol. 152, 141–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chen B. C., Lee C. M., Lin W. W. (1996) Inhibition of ecto-ATPase by PPADS, suramin and reactive blue in endothelial cells, C6 glioma cells and RAW 264.7 macrophages. Br. J. Pharmacol. 119, 1628–1634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yegutkin G. G., Burnstock G. (2000) Inhibitory effects of some purinergic agents on ecto-ATPase activity and pattern of stepwise ATP hydrolysis in rat liver plasma membranes. Biochim. Biophys. Acta 1466, 234–244 [DOI] [PubMed] [Google Scholar]
- 39. Jorgensen S. (1956) Breakdown of adenine and hypoxanthine nucleotides and nucleosides in human plasma. Acta Pharmacol. Toxicol. 12, 294–302 [PubMed] [Google Scholar]
- 40. Goding J. W., Grobben B., Slegers H. (2003) Physiological and pathophysiological functions of the ecto-nucleotide pyrophosphatase/phosphodiesterase family. Biochim. Biophys. Acta 1638, 1–19 [DOI] [PubMed] [Google Scholar]
- 41. Park W., Masuda I., Cardenal-Escarcena A., Palmer D. W., McCarty D. J. (1996) Generation of inorganic pyrophosphate from extracellular adenosine triphosphate by human serum and plasma. J. Rheumatol. 23, 1233–1236 [PubMed] [Google Scholar]
- 42. Ireland D. M., Mills D. C. (1966) Detection and determination of adenosine diphosphate and related substances in plasma. Biochem. J. 99, 283–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sunderman F. W., Jr. (1990) The clinical biochemistry of 5′-nucleotidase. Ann. Clin. Lab. Sci. 20, 123–139 [PubMed] [Google Scholar]
- 44. Haslam R. J., Mills D. C. (1967) The adenylate kinase of human plasma, erythrocytes and platelets in relation to the degradation of adenosine diphosphate in plasma. Biochem. J. 103, 773–784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Todorov L. D., Mihaylova-Todorova S., Westfall T. D., Sneddon P., Kennedy C., Bjur R. A., Westfall D. P. (1997) Neuronal release of soluble nucleotidases and their role in neurotransmitter inactivation. Nature 387, 76–79 [DOI] [PubMed] [Google Scholar]
- 46. Sorensen C. E., Amstrup J., Rasmussen H. N., Ankorina-Stark I., Novak I. (2003) Rat pancreas secretes particulate ecto-nucleotidase CD39. J. Physiol. 551, 881–892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Yegutkin G. G., Samburski S. S., Jalkanen S., Novak I. (2006) ATP-consuming and ATP-generating enzymes secreted by pancreas. J. Biol. Chem. 281, 29441–29447 [DOI] [PubMed] [Google Scholar]
- 48. Yegutkin G., Bodin P., Burnstock G. (2000) Effect of shear stress on the release of soluble ecto-enzymes ATPase and 5′-nucleotidase along with endogenous ATP from vascular endothelial cells. Br. J. Pharmacol. 129, 921–926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Eltzschig H. K., Ibla J. C., Furuta G. T., Leonard M. O., Jacobson K. A., Enjyoji K., Robson S. C., Colgan S. P. (2003) Coordinated adenine nucleotide phosphohydrolysis and nucleoside signaling in posthypoxic endothelium: role of ectonucleotidases and adenosine A2B receptors. J. Exp. Med. 198, 783–796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Eltzschig H. K., Carmeliet P. (2011) Hypoxia and inflammation. N. Engl. J. Med. 364, 656–665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Robson S. C., Kaczmarek E., Siegel J. B., Candinas D., Koziak K., Millan M., Hancock W. W., Bach F. H. (1997) Loss of ATP diphosphohydrolase activity with endothelial cell activation. J. Exp. Med. 185, 153–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Yegutkin G. G., Helenius M., Kaczmarek E., Burns N., Jalkanen S., Stenmark K., Gerasimovskaya E. V. (2011) Chronic hypoxia impairs extracellular nucleotide metabolism and barrier function in pulmonary artery vasa vasorum endothelial cells. Angiogenesis 14, 503–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Straub A., Krajewski S., Hohmann J. D., Westein E., Jia F., Bassler N., Selan C., Kurz J., Wendel H. P., Dezfouli S., Yuan Y., Nandurkar H., Jackson S., Hickey M. J., Peter K. (2011) Evidence of platelet activation at medically used hypothermia and mechanistic data indicating ADP as a key mediator and therapeutic target. Arterioscler. Thromb. Vasc. Biol. 31, 1607–1616 [DOI] [PubMed] [Google Scholar]
- 54. Buergler J. M., Maliszewski C. R., Broekman M. J., Kaluza G. L., Schulz D. G., Marcus A. J., Raizner A. E., Kleiman N. S., Ali N. M. (2005) Effects of SolCD39, a novel inhibitor of platelet aggregation, on platelet deposition and aggregation after PTCA in a porcine model. J. Thromb. Thrombolysis. 19, 115–122 [DOI] [PubMed] [Google Scholar]
- 55. Berry C. E., Hare J. M. (2004) Xanthine oxidoreductase and cardiovascular disease: molecular mechanisms and pathophysiological implications. J. Physiol. 555, 589–606 [DOI] [PMC free article] [PubMed] [Google Scholar]