Abstract
Biomechanics plays a pivotal role in articular cartilage development, pathophysiology, and regeneration. During embryogenesis and cartilage maturation, mechanical stimuli promote chondrogenesis and limb formation. Mechanical loading, which has been characterized using computer modeling and in vivo studies, is crucial for maintaining the phenotype of cartilage. However, excessive or insufficient loading has deleterious effects and promotes the onset of cartilage degeneration. Informed by the prominent role of biomechanics, mechanical stimuli have been harnessed to enhance redifferentiation of chondrocytes and chondroinduction of other cell types, thus providing new chondrocyte cell sources. Biomechanical stimuli, such as hydrostatic pressure or compression, have been used to enhance the functional properties of neocartilage. By identifying pathways involved in mechanical stimulation, chemical equivalents that mimic mechanical signaling are beginning to offer exciting new methods for improving neocartilage. Harnessing biomechanics to improve differentiation, maintenance, and regeneration is emerging as pivotal toward producing functional neocartilage that could eventually be used to treat cartilage degeneration.—Responte, D. J., Lee, J. K., Hu, J. C., Athanasiou, K. A. Biomechanics-driven chondrogenesis: from embryo to adult.
Keywords: cartilage, tissue engineering, chondrocyte
Cartilage lines the articulating surfaces of long bones, functioning in the mechanically demanding environment of the joint space. The extracellular matrix of cartilage primarily includes collagen and proteoglycans. Cartilage is relatively acellular, with chondrocytes only comprising 1–5% of the tissue by volume (1). Although chondrocytes represent a small fraction of articular cartilage, they are crucial because they synthesize the matrix that imparts mechanical integrity to the tissue.
The significance of biomechanical stimuli has been well established for cartilage. Joint loading results in direct compression of chondrocytes inside a relatively impermeable matrix. Following tissue loading, hydrostatic pressure initially develops in the interstitial fluid, which is followed by fluid flow-induced shear. However, in time scales > 10 μs, the solid matrix begins to bear the applied load, resulting in deformation. Consequently, the cells residing in the matrix experience hydrostatic pressure, shear, compression, and, to a lesser extent, tension. This mechanical stimulation produces a signaling cascade, resulting in increased gene expression (2), matrix protein production (3), and intracellular ion influx (4). Understanding how mechanics influences chondrocytes is crucial because the resulting signaling cascades can alter matrix production and ultimately influence how well the tissue can function in the rigorous joint environment.
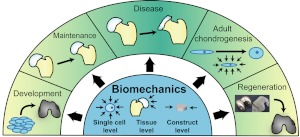
As summarized in Fig. 1, biomechanics plays a significant role throughout life. Biomechanics contributes to development during embryogenesis and cartilage formation, and it maintains the chondrocyte phenotype in adult tissues. Alternatively, abnormal loading can promote the onset of cartilage degeneration. Informed by these biomechanics studies, various mechanical stimuli have been employed to enhance stem cell differentiation and the development of more robust regeneration strategies. This perspective article highlights the importance of biomechanics in chondrogenesis from the early stages of neocartilage formation to pathophysiology of adult tissue. In particular, it describes the role of biomechanics in cartilage development, maintenance, disease, adult cell chondrogenesis, and cartilage regeneration.
Figure 1.
Multilevel role of biomechanics in chondrogenesis. Biomechanics plays a key role in cartilage development by promoting differentiation of stem cells and limb formation. After cartilage forms, normal loading is crucial for maintaining cartilage phenotype and preventing pathogenesis. Biomechanical stimuli can also be applied to enhance cartilage regeneration.
BIOMECHANICS IN EMBRYOGENESIS AND FETAL DEVELOPMENT
Mechanical stimuli during embryonic and fetal development are crucial for cartilage differentiation and limb morphogenesis. The mechanical microenvironment acts as a potent regulator of stem cell fate and contributes to chondrogenesis in the early embryo. Biomechanics also plays a key role in skeletogenesis, which begins with a primary cartilaginous matrix that later calcifies and forms bone via a process known as endochondral ossification (5). Immobilization experiments in vivo and computer models of chondrogenesis indicate the central role of mechanical stimuli in proper fetal cartilage development (6–14).
Stem cell response to changing mechanical microenvironment
Much of embryogenesis relies on resident stem cell differentiation into the needed cell types. Likewise, chondrogenesis involves the condensation of precartilaginous progenitor cells to form tightly packed cellular aggregates followed by differentiation into early chondrocytes (5). The contributions of mechanics to this process are currently unknown, as most studies focus on chemical pathways leading to embryonic cartilage formation. Some groups postulate that mechanical forces contribute to de novo chondrogenesis from early stem cells (15, 16); however, this hypothesis is difficult to test and current studies use in vitro culture platforms or computer simulations. The probable role of mechanics during development has prompted the use of biomechanics to characterize single stem cell populations, control differentiation, and further understand development.
At the single cell level, differentiating stem cells exhibit altered mechanical properties. For example, mouse stem cells demonstrate increased stiffness after just 6 d of chemical differentiation, with up to 3-fold higher stiffness values compared to undifferentiated cells (17). Similarly, the mechanical properties of single cells can be employed to evaluate differentiation states of embryonic (18) and marrow-derived (19) stem cells. Because of the relationship between mechanical properties and differentiation, biomechanics could provide a novel method of stem cell phenotyping and validation of isolation techniques.
The control of stem cell fate via cell-exerted forces (by modulating substrate stiffness) and exogenous mechanical stimulation is increasingly being explored as a differentiation strategy. By changing the substrate stiffness, the resultant cell-exerted forces can be altered to control stem cell lineage commitment. Growing embryonic stem cells on 2-dimensional substrates of various stiffnesses modulates differentiation gene expression (20) and growth rate kinetics (20) and can differentiate stem cells into all 3 germ layers, recapitulative of their native environments (21). Alternatively, applying compressive loading in 3 dimensions enhances chondrogenesis of progenitor cells, generating up to 3-fold increases in matrix protein synthesis (15). As evidenced, the ability of both cell-exerted and exogenous forces to chondrodifferentiate stem cells suggests that biomechanics is a key stimulus during cartilage development.
Though the mechanisms underlying precartilaginous condensation require elucidation, the differential adhesion hypothesis (DAH) offers insight into the segregation of cellular subpopulations (22). Spatiotemporal changes in progenitor cell adhesion molecule expression cause similar cells to transiently associate during chondrogenesis (23). However, cell-cell adhesion strength correlates linearly with cellular surface tension, irrespective of a homogeneous or heterogeneous interaction, suggesting surface tension as the primary driver of differential adhesion (24). Therefore, precartilaginous condensation may be the result of mesenchymal progenitor cells exhibiting similar surface tensions rather than similar biomarkers. Furthermore, disruption of surface tension inhibits differential adhesion (25). These findings indicate that cellular biomechanics is essential to the DAH and initiation of chondrogenesis. Gravity, the most basic of mechanical forces, also contributes to chondrogenesis: the absence of gravitational force reduces precartilaginous condensations in mesenchymal limb bud cells (26). Although these hypotheses for precartilaginous condensation remain untested at the early stages of chondrogenesis, the DAH and gravitational hypothesis allow extrapolation from mesenchymal stem cell studies to in vivo biomechanics-based embryonic development.
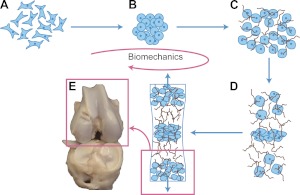
Studying chondrogenesis in embryonic stem cells can inform the mechanisms of in vivo cartilage development. As depicted in Fig. 2, in vivo chondrogenesis occurs in 5 stages, each subject to various biomechanical forces (27): 1) mesenchymal progenitor cell migration to limb buds and progenitor cell condensation, 2) chondrocyte differentiation and scaffold formation, 3) continued extracellular matrix synthesis, 4) chondrocyte maturation, and 5) chondrocyte hypertrophy and tissue maturation. While the biomechanical influence in the suggested progression is studied extensively in vitro, in vivo validation of the role of biomechanics requires further experimentation.
Figure 2.
Biomechanics-driven development of cartilage from embryo to fetal stages and beyond. A, B) Progenitor cells migrate from the early mesoderm to sites of skeletogenesis (A), where they undergo precartilaginous condensations (B). C) Chondrocyte progenitors secrete cartilage-specific matrix and decrease expression of cell-cell interaction proteins. D) Proliferation continues at the subchondral growth front, while endochondral ossification occurs throughout the juvenile stages to transform cartilage into bone. E) Ends of long bones remain capped with a layer of articular cartilage throughout adulthood.
In summary, stem cell chondrogenesis not only provides a platform for which to study embryonic cartilage tissue formation, it can also be used to study mechanical forces as differentiation stimuli. The significance of biomechanics in stem cell chondrogenesis has been demonstrated for single-cell mechanics, cell-exerted forces, and condensation. Elucidating how specific forces can be employed to promote differentiation into a chondrocyte lineage could advance de novo cartilage formation efforts and inform how biomechanics can influence chondrogenesis in vivo.
Mechanical stimulation promotes cartilage formation in animal models
Studies of vertebrate limb development reveal that mechanical stimulation promotes chondrogenesis: compression of embryonic limb bud mesenchymal cells triggers chondrogenic marker expression—most notably, SOX9, a master gene responsible for activating many of the cartilage genes expressed in terminally differentiated cells (15, 16). These results indicate mechanical stimulation as a genetic regulator of chondrogenesis in progenitor cells.
Because of the difficulties associated with experimentally controlling biomechanics during embryogenesis, embryos with impaired limb mobility are used to determine the effects of mechanics on limb development (6, 7). Strategies, such as chemically paralyzing limbs (28) or using animals with skeletal muscle defects, create abnormal joint-loading conditions to subsequently disrupt skeletogenesis. For instance, early chemical immobilization of chick limbs results in abnormal limb shape and complete failure to form elements of the synovial joint (7), while later immobilization decreases cartilage matrix production and mechanical properties (9). In addition, muscle removal hinders articular cartilage development (8). These studies demonstrate that joint mechanics are vital at various stages of cartilage formation and development.
Models corroborate animal studies
While joint immobilization provides insight into in vivo cartilage development, the difficulties associated with this procedure have led to modeling of joint development (10), which can help elucidate the stages of chondrogenesis during limb development. Ultimately, comparing models to in vivo studies demonstrates the accuracy of these simulations.
The modeling approaches to simulate chondrogenesis under mechanical stimulation vary widely, incorporating stimuli based on muscle contraction (7), hydrostatic pressure (11), and shear (11). Intermittent and cyclic hydrostatic pressure and strain both help regulate matrix protein synthesis to affect macromolecular organization of collagen fibers, which, in turn, leads to changes in the mechanical properties of the tissue (11). When applied to a mesenchymal tissue model, a simulation can estimate the mechanical properties of different cartilage types. These results reveal a successful cartilage differentiation algorithm based on mechanical forces recapitulative of the native chondrogenic environment.
Alternatively, models predict that intermittent hydrostatic pressure inhibits degeneration and ossification of cartilage, while intermittent strain or shear stresses accelerate ossification and degeneration (13). Balance of such mechanical forces dictates the progression of what is known as the ossification front, or the line at which cartilage begins to calcify. These forces, thus, generate cartilage of suitable thickness during development. These models can predict the extent of cartilage formation based on chondrocyte proliferation and hypertrophy in response to biomechanical forces. Ultimately, algorithms can estimate an anatomically correct long bone shape based on cartilage growth and ossification (14), thus validating the simulation.
Despite having models of in vivo chondrogenesis and their contributions to understanding cartilage development, the role of biomechanics still requires validation via animal studies. In addition, models focusing on the interplay between solute transport and mechanical loading (29) will need to be refined to help distinguish the effects of biotransport and biomechanical stimuli. Animal and computer models must build on each other to provide insight into the effects of mechanical stimulation during in vivo chondrogenesis.
Tissue mechanics during maturation
In addition to exploring how mechanics drives chondrogenesis, it is important to investigate how these stimuli promote cartilage maturation from fetus to newborn. As skeletal tissues mature, mechanical forces help determine their intrinsic mechanical properties via matrix enhancement and organization.
Studies have examined tissue mechanics at different maturation stages (30, 31). For example, human fetal articular cartilage at 20 to 36 wk old exhibits an age-dependent increase in cartilage tissue compressive stiffness by a factor of 2.5 and in collagen content by 3-fold; proteoglycan decreases by 18% (30). Similarly, comparing the mechanical properties of fetal and newborn bovine tissue reveals a correlation between tissue strength and specimen age (31). These studies illustrate how biomechanical properties progress with age.
Mechanical stimulation also plays a vital role in generating regional variation in cartilage. Although fetal cartilage does not demonstrate regional variation, newborn and adult cartilage exhibits stiffer tissues in regions bearing the greatest static or dynamic loads (32). Similarly, the compressive and tensile properties of bovine fetal, newborn, and adult tissues exhibit age-dependent and regional variation (33). These results suggest that joint loading is a potent regulator of regional chondrogenesis.
As described above, during cartilage maturation, the tissue exhibits increases in mechanical properties and concomitant development of regional variation. While the underlying mechanisms of biomechanics-based chondrogenesis remain unclear, it appears that regional variation stems from joint loading. Beginning at the embryo stage, biomechanics undoubtedly influences chondrogenesis, as demonstrated by inhibiting embryo movement in utero. By understanding the potential role of biomechanics from a developmental biology standpoint, mechanical stimuli may be rationally designed to differentiate stem cells.
MECHANICAL STIMULATION PROMOTES MAINTENANCE OF CARTILAGE HOMEOSTASIS
In addition to playing a vital role during development, biomechanics is integral for maintaining cartilage homeostasis. In particular, cartilage homeostasis depends on the maintenance of chondrocyte phenotype and production of cartilage matrix molecules without the expression of inflammatory cytokines or catabolic factors. The effects of different forces on cartilage maintenance are assessed using models of load distribution (34–37), in vivo imaging techniques (38–40), and in vitro experiments on cartilage explants (41–45). The physiological magnitude of stresses present in articular cartilage range from 3 to 10 MPa (46, 47), typically at a frequency of 1 Hz (48). The role of forces, such as hydrostatic pressure and compression to maintain cartilage phenotype in vivo, has been confirmed experimentally in vitro using biomechanical stimuli to promote cartilage maintenance.
Assessing native cartilage mechanical forces
Modeling studies are employed to identify forces that maintain tissue properties. Using the biphasic theory of cartilage (i.e., cartilage is composed of a solid and liquid phase that each contribute mechanical properties to the tissue; ref. 49) and applying appropriate assumptions in a finite element model, investigators have found that simulations, including compression of 1 MPa and hydrostatic pressure (HP), ranging from 2.8 to 10 MPa, show that mechanical forces exhibit depth dependence and can influence cellular phenotype: forces preserving the upper layers of cartilage are inherently different from those sustaining the lower layers (34). Simulations are also used to model the collagen network at strains up to 4% (35), and to model the pericellular matrix (36) at 10% strain, to illustrate how the matrix microenvironment is responsible for transducing tissue-level mechanical force to cells. Models also delineate how static compression (0.1 MPa) promotes matrix protein synthesis (37). A major shortcoming of these models lies in their simplification of a complex tissue. For example, models that follow the biphasic theory separate the solid and fluid phases of cartilage and may not include the solid-fluid interaction. Despite these limitations, modeling represents a useful approach for understanding force transduction at the cellular and tissue levels.
In vivo imaging techniques can noninvasively elucidate in vivo biomechanical forces during joint movement. For example, 2-photon laser microscopy and magnetic resonance imaging are used to reveal chondrocyte deformation at the single-cell level in response to muscle-induced mechanical loading (38), and at the tissue level during physiological loading (39). Imaging of articulating surfaces indicates that proper contact kinematics—that is, the spatial relationship of cartilage contact points within a joint during motion—is vital to preventing cartilage degeneration (40). In general, imaging methods noninvasively examine in vivo cartilage biomechanics, which could have important implications for studying cartilage disease.
Mechanical stimulation promotes cartilage homeostasis
Both in silico and in vivo work demonstrate that biomechanics plays a key role in cartilage maintenance. To verify the role of mechanics, the effects of stimuli, such as HP (41, 43, 50) and compression (37, 44, 45, 51–53), have been examined in vitro. HP does not result in deformation of incompressible media, so it is not expected to deform cells. Direct compression results in deformation of matrix and cells, which will also create fluid flow that is not observed with HP. These stimuli, which are described mechanistically in the section on chemically induced stimulation, have been studied with respect to cartilage homeostasis.
At the cellular level, applying HP to chondrocytes at 0.25–10 MPa (1 Hz, 4 h) up-regulates the expression of genes for cartilage matrix proteins (50). HP, thus, promotes and maintains appropriate expression levels of cartilage genes, which results in the synthesis of matrix proteins. Excessive HP stimulation (50 MPa, 12 h) of cells in damaged matrices leads to further degeneration of the surrounding matrix, indicating that HP can be detrimental in abnormal microenvironments (43). These results suggest that HP, especially in the range of 0.5-10 MPa, could act in vivo to increase the synthesis of cartilage extracellular matrix components.
Compression also promotes cartilage homeostasis. For example, cyclic compression has been shown to increase both collagen and proteoglycan content (54). Cartilage response to these effects is depth-dependent (45). In addition, studies demonstrate that passaging cells modulates their response to dynamic compression (5% strain, 0.1 Hz), suggesting a differentiation-dependent response to mechanical loading (53). Other factors, such as compressive load duration (37) and magnitude, are shown to alter the cellular response to stimulation, indicating that different compression regimens have differential effects on cartilage homeostasis.
Various biomechanical stimuli, including HP and compression, influence cartilage homeostasis. Modeling and imaging have been used to identify native mechanical forces, which have also been shown to promote in vitro homeostasis. As discussed below, when these forces are outside the range of physiological magnitudes, durations, or frequencies, they result in disuse or overuse regimens with detrimental effects.
ABNORMAL LOADING CAN PROMOTE DISEASE
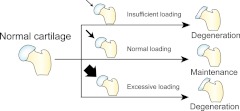
Although mechanical loading can promote cartilage maintenance, insufficient or excessive mechanical stimulation, resulting in disuse or overuse, respectively, can induce degeneration (Fig. 3). These degenerative changes parallel osteoarthritis (OA), highlighting how abnormal biomechanical stimuli may initiate cartilage disease.
Figure 3.
Role of biomechanics in cartilage pathophysiology. Physiological loading promotes the maintenance of cartilage phenotype. Abnormal loading, whether insufficient or excessive, can induce disease progression and eventually lead to degenerative conditions like osteoarthritis.
Excessive loading leads to cartilage degeneration
Excessive cartilage loading can promote the onset of cartilage degradation and OA. Cartilage trauma produces various degenerative effects, including matrix degradation and decreased mechanical properties (55). Cases in which the meniscus is removed, increasing loading on the tibia, also result in articular cartilage degeneration (56). Similarly, transecting the anterior cruciate ligament produces hypertrophic cartilage repair and increases bone volume (57), which is characteristic of OA. In contrast to the dramatic cases above, even slight changes in load distribution during daily activities, such as walking, may initiate early osteoarthritic effects in healthy knee joints (58). Collectively, these studies illustrate how elevated loading leads to osteoarthritic responses.
Cartilage disuse promotes degeneration
A lack of loading can also produce deleterious effects, resulting in changes that parallel the development of OA. Immobilizing joints externally or surgically allows observation of how reduced loading influences cartilage properties. In general, cartilage disuse for several weeks results in a more hydrated tissue (59, 60), with decreased proteoglycan content (59–62). Disuse also produces gross changes, such as thinner cartilage (61, 62). These effects parallel the swelling that occurs during OA (63), suggesting that a lack of joint motion could produce effects similar to those of OA. In addition, these changes in cartilage morphology and biochemical content do not return to control values after discontinuing the immobilization (62). The detrimental effects of disuse via immobilization have prompted the design of clinical modalities, e.g., continuous passive motion (64), to introduce biomechanics soon after joint surgeries.
HARNESSING BIOMECHANICS TO PROMOTE CHONDROGENESIS OF ADULT CELLS
In addition to promoting chondrogenesis during development, various efforts have focused on harnessing biomechanics to promote the chondrogenesis of adult cells (Fig. 4). These efforts aim to combat the detrimental effects of monolayer culture and expand cell sources that can be used for in vitro cartilage formation. In particular, biomechanical stimulation proves to be an exciting new strategy for effecting adult stem cell migration and differentiation, chondroinduction, and redifferentiation of chondrocytes.
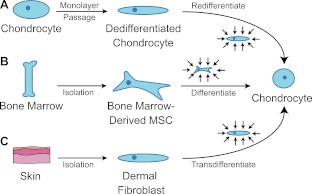
Figure 4.
Harnessing biomechanics to drive adult cell chondrogenesis. A) Following monolayer culture, chondrocytes rapidly dedifferentiate. Biomechanical stimuli, such as HP, can promote redifferentiation. B) Under mechanical stimulation, mesenchymal stem cells migrate and chondrodifferentiate. C) Mechanical stimulation can be used to induce transdifferentiation into chondrocytes.
Mechanical loading influences adult stem cell migration and chondrodifferentiation
To a limited degree, adult stem cells also exhibit an ability to respond in vivo to heal cartilage defects. In addition to chemical factors that promote adult stem cell migration, the mechanical microenvironment influences stem cell recruitment. A rabbit study demonstrates the effects of mechanics by grafting periosteal tissue—a source of progenitor cells—in an environment subject to mechanical loading (65). The loaded environment stimulates stem cell migration and subsequent in vivo chondrogenesis of stem cells present in the periosteum, demonstrating how biomechanics can initiate adult cell chondrodifferentiation. Similarly, simulations of joint motion show that loading and fluid flow can prompt stem cell release to heal cartilage defects (66). Mechanical loading [10% strain, 1 Hz (67) or HP between 3–10 MPa (68)] of cultured mesenchymal stem cells can also promote chondrodifferentiation. These studies illustrate how biomechanics can alter the behavior of adult stem cells and thus influence in vivo cartilage repair.
Effects of mechanical stimulation on redifferentiation
Chondrocyte dedifferentiation in monolayer limits the potential for cell expansion and large-scale experiments. Chondrocytes exhibit signs of phenotype loss (reduced gene and protein expression of molecules, such as collagen II and aggrecan), even after the first passage (69). Expansion also alters cellular mechanical properties (70) and changes the chondrocyte response to mechanical stimulation (71).
Biomechanical stimulation can be employed to enhance redifferentiation, particularly by using HP. Applying static 0.3 MPa HP for 6 h/d during redifferentiation increases synthesis of cartilage-specific matrix molecules up to 65% (72). Similarly, applying HP (5 MPa, 0.5 Hz) to dedifferentiated chondrocytes in pellet culture up-regulates chondrogenic gene expression and matrix production up to 5-fold (73). Certain regimens of HP have also been shown to decrease collagen II production (74), illustrating that more work needs to be done to understand how HP can be used as a redifferentiation agent.
Compression has also been used to redifferentiate chondrocytes. Applying compression (10% strain, 0.1 Hz) to passaged chondrocytes seeded on a collagen II scaffold has been shown to increase collagen and proteoglycan production (75). Another study has demonstrated that compression (5% strain, 0.1 Hz) increases glycosaminoglycan (GAG) production of expanded chondrocytes but does not alter collagen II expression (53). The beneficial responses to compression and HP indicate that mechanical stimuli can be effective redifferentiation agents.
Biomechanics-driven chondroinduction
Biomechanical stimuli are potent chondroinductive agents for various cell types. For example, applying HP (5 MPa, 1 Hz) to murine embryonic fibroblasts results in 2-fold increases in collagen synthesis and GAG production (76). Similarly, HP (5 MPa, 1 Hz) increases chondrogenic gene expression in neonatal human dermal fibroblasts (77). Mechanical forces have also been postulated to induce chondrogenic gene and protein expression in smooth muscle cells following atherosclerotic calcification (78). These studies illustrate that biomechanics can drive the chondroinduction of various cell types and thus can act as a differentiation agent.
Although several studies have shown promising results for using HP to promote chondroinduction, the effects of other forms of mechanical stimulation need to be investigated. Applying biomechanical stimuli on differentiated cells for chondroinduction could eventually provide new cell sources to supplement stem cell efforts. Additional refinement of mechanical stimuli for adult cell chondrogenesis will increase the availability of cell sources for applications like tissue engineering.
BIOMECHANICS PROMOTES TISSUE ENGINEERING AND REGENERATION
Biomechanical stimuli have been widely employed to enhance tissue-level chondrogenesis and subsequently promote de novo cartilage formation. Several methods of mechanical stimulation, including HP, compression, and even shear have been used to improve the biomechanical properties of neocartilage, which could enable it to function in vivo. In addition, mechanotransduction research is used to rationally select chemical agents that can reproduce the effects of direct mechanical stimulation (Fig. 5).
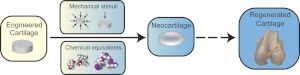
Figure 5.
Direct biomechanical stimulation and mechanical equivalents. Biomechanical stimuli, such as compression and HP, can be applied to enhance neotissue formation, improving the biochemical and biomechanical properties of neocartilage. Analogously, application of chemical equivalents, including ionophores and hyperosmotic media, mimic mechanotransduction and produce effects similar to those of direct stimulation. Mechanical equivalents offer opportunities for rationally designing stimulation regimens based on known biomechanics pathways.
Direct mechanical stimulation
HP has been applied to various cartilage engineering systems to improve neocartilage properties. Differences in the response to HP may be attributed to the variety of magnitudes and regimens utilized (79). Applying 3.44 MPa HP to chondrocyte-seeded polyglycolic acid meshes increases GAG production 10-fold (80). HP at 10 MPa increases collagen production but reduces GAG content in self-assembled cartilage constructs (3). Finally, HP above physiological levels at 50 MPa produces harmful effects when applied to HCS-2/8 cells (a chondrosarcoma cell line often used to model cartilage), resulting in decreased matrix production and increased expression of inflammatory cytokines (81). Differences in HP effects can also be attributed to different chondrocyte culture techniques: e.g., the anabolic response to dynamic HP is evident for chondrocytes in pellet culture but not for cells cultured in alginate (82). The widespread use of HP to successfully enhance neotissue suggests that, with the appropriate identification of stimulation regimens, HP is a potent stimulus for in vitro cartilage formation.
Direct compression is also used to modulate matrix composition and influence neocartilage mechanical properties. Early work with cartilage explants demonstrates that compression can act as a catabolic (83) or anabolic (84) stimulus. In general, dynamic compression is more beneficial than static regimens, which tend to produce catabolic effects (75). More recent efforts, many focusing on tissue-engineering applications, demonstrate that compression can be used to improve matrix production and subsequently improve the functional properties of neocartilage. For example, compression at 1 Hz increases matrix deposition and consequently increases the equilibrium aggregate modulus 6-fold (85). The beneficial role of dynamic compression and the deleterious effects of static compression highlight the importance of selecting an appropriate compression regimen.
Although most biomechanical stimulation work focuses on HP and compression, shear forces are also investigated. Treating cartilage explants with direct dynamic shear (0.01–1 Hz) increases both collagen and proteoglycan synthesis (86). Similar results are observed for neocartilage exposed to shear, which show 40% more collagen, 35% more proteoglycans, and 3- and 6-fold increases in compressive strength and stiffness, respectively, over static controls (87). Fluid flow-induced shear increases the expression of proinflammatory cytokines (88), suggesting that direct shear application is more beneficial. There is comparatively little work on the effects of shear alone, since its application is often coupled with either compression or fluid flow.
Chemically induced mechanical stimulation
Various studies have elucidated pathways underlying the responses to mechanical stimuli, particularly regarding how these stimuli modulate ion transport. For example, cellular deformation increases intracellular concentrations of Ca2+ and Na+ by enhancing Na+/H+ exchanger activity and stimulating stretch-activated ion channels (89, 90). The influx of Ca2+ leads to the production of intermediate signaling molecules, such as inositol triphosphate and diacylglycerol, which activate kinase cascades that are crucial for cartilage homeostasis (91). Applying agents like histamine, which increase intracellular Ca2+ levels, has also been shown to modulate signaling intermediates like cyclic AMP (92). These findings have spurred recent investigations of agents, including ionophores and hyperosmotic media, that recapitulate mechanotransduction pathways.
Exogenous chemicals can be applied to modulate intracellular ion levels, which are known to vary following compression (4), shear (93), and HP (90, 94). To recapitulate these signaling events, one study uses ouabain (a Na+/K+-ATPase inhibitor) and ionomycin (a Ca2+ ionophore) or a combination of the two to increase the tensile modulus of cartilage constructs by 40–95% (95). Electromagnetic fields, which can also affect ion channels, are also applied to increase matrix synthesis (96). These studies suggest that it is possible to apply exogenous agents to modulate ion influx, which mimics biomechanical stimulation, and subsequently enhance functional tissue-level properties.
Applying agents that increase osmolarity mimics the hyperosmotic environment that is created when compressive loading forces fluid out of cartilage. Hyperosmolarity modulates intracellular ion levels (97), suggesting that it could be employed like ion channel modulators to alter tissue properties. Studies with native cartilage demonstrate that hyperosmolarity up-regulates key cartilage genes, such as SOX9 and aggrecan (98). Although applying hyperosmolarity to neocartilage has not been studied extensively, expanding chondrocytes in hypertonic medium enhances construct mechanical properties (99). This promising result indicates that hyperosmotic environments need to be investigated further in the context of cartilage regeneration.
FUTURE DIRECTIONS
Elucidating the role of biomechanics improves the study of cartilage development, pathophysiology, and regeneration. Despite exciting recent advances, several areas need further examination to more fully understand biomechanics and develop biomechanics-driven strategies for improving cartilage differentiation and regeneration.
To refine a biomechanics-driven approach, it will be necessary to develop a better understanding of how mechanics drives chondrogenesis. The load-bearing environment of cartilage, which influences chondrocyte differentiation and biomechanics, should, therefore, be a central part of cell efforts focusing on chondrogenesis. A more thorough understanding of how mechanics influences embryogenesis could improve stem cell biology, chondroinduction, and redifferentiation methods. Although there is strong evidence for biomechanics influencing cartilage development, further in vivo work is needed to clarify the role of mechanics in chondrogenesis.
As knowledge of biomechanical stimuli and their downstream pathways becomes more developed, it will be possible to rationally apply chemical agents that mimic mechanotransduction. These exogenous agents are considerably easier to administer than direct mechanical stimulation and will make it simpler to apply regimens involving multiple stimuli. Because the native joint environment presents a complex combination of mechanical and biochemical stimuli, it will be advantageous to combine agents such as growth factors with mechanical equivalents. This multistimulus approach will enable researchers to leverage biomechanics knowledge to improve cartilage regeneration.
An appreciation of the influence of biomechanics on development, maintenance, disease, adult cell chondrogenesis, and regeneration, plays a crucial role in disease prevention and therapy development. Future therapies, probably those based on tissue engineering efforts, will be solidly based on biomechanics due to its multifaceted role in driving chondrodifferentiation and synthesis of new tissue. The outcome of future therapies is also biomechanical as de novo cartilage has to be able to withstand the strenuous environment of the diarthrodial joint. Biomechanics research has driven recent developments in musculoskeletal medicine and will continue to be at the frontiers of cartilage biology and regeneration.
Acknowledgments
The authors acknowledge funding support from U.S. National Institutes of Health grants R01 AR053286, R01 AR047839, R01 DE019666, and R01 DE015038, and an Arthritis Foundation Innovative Research grant.
Footnotes
- GAG
- glycosaminoglycan
- HP
- hydrostatic pressure
- OA
- osteoarthritis
- DAH
- differential adhesion hypothesis
REFERENCES
- 1. Hu J. C., Athanasiou K. A. (2003) Structure and function of articular cartilage. In Handbook of Histology Methods for Bone and Cartilage (An Y. H., Martin K. L., eds.), Humana Press, Totowa, NJ, USA [Google Scholar]
- 2. Smith R. L., Lin J., Trindade M. C., Shida J., Kajiyama G., Vu T., Hoffman A. R., van der Meulen M. C., Goodman S. B., Schurman D. J., Carter D. R. (2000) Time-dependent effects of intermittent hydrostatic pressure on articular chondrocyte type II collagen and aggrecan mRNA expression. J. Rehabil. Res. Dev. 37, 153–161 [PubMed] [Google Scholar]
- 3. Hu J. C., Athanasiou K. A. (2006) The effects of intermittent hydrostatic pressure on self-assembled articular cartilage constructs. Tissue Eng. 12, 1337–1344 [DOI] [PubMed] [Google Scholar]
- 4. D'Andrea P., Calabrese A., Capozzi I., Grandolfo M., Tonon R., Vittur F. (2000) Intercellular Ca2+ waves in mechanically stimulated articular chondrocytes. Biorheology 37, 75–83 [PubMed] [Google Scholar]
- 5. Lefebvre V., Bhattaram P. (2010) Vertebrate skeletogenesis. In: Current Topics in Developmental Biology, Vol. 90 (Peter K., ed.) pp. 291–317, Academic Press, San Diego, CA, USA: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nowlan N. C., Sharpe J., Roddy K. A., Prendergast P. J., Murphy P. (2010) Mechanobiology of embryonic skeletal development: insights from animal models. Birth Defects Res. C Embryo Today 90, 203–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Roddy K. A., Prendergast P. J., Murphy P. (2011) Mechanical influences on morphogenesis of the knee joint revealed through morphological, molecular and computational analysis of immobilised embryos. PLoS ONE 6, e17526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Solem R. C., Eames B. F., Tokita M., Schneider R. A. (2011) Mesenchymal and mechanical mechanisms of secondary cartilage induction. Dev. Biol. 356, 28–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mikic B., Isenstein A. L., Chhabra A. (2004) Mechanical modulation of cartilage structure and function during embryogenesis in the chick. Annals Biomed. Eng. 32, 18–25 [DOI] [PubMed] [Google Scholar]
- 10. Nowlan N. C., Murphy P., Prendergast P. J. (2007) Mechanobiology of embryonic limb development. Ann. N. Y. Acad. Sci. 1101, 389–411 [DOI] [PubMed] [Google Scholar]
- 11. Loboa E. G., Wren T. A. L., Beaupré G. S., Carter D. R. (2003) Mechanobiology of soft skeletal tissue differentiation—a computational approach of a fiber-reinforced poroelastic model based on homogeneous and isotropic simplifications. Biomech. Model. Mechanobiol. 2, 83–96 [DOI] [PubMed] [Google Scholar]
- 12. Carter D. R., Orr T. E., Fyhrie D. P., Schurman D. J. (1987) Influences of mechanical stress on prenatal and postnatal skeletal development. Clin. Orthop. Rel. Res. 219, 237–250 [PubMed] [Google Scholar]
- 13. Carter D. R., Wong M. (1988) Mechanical stresses and endochondral ossification in the chondroepiphysis. J. Orthop. Res. 6, 148–154 [DOI] [PubMed] [Google Scholar]
- 14. Carter D. R., Wong M. (1988) The role of mechanical loading histories in the development of diarthrodial joints. J. Orthop. Res. 6, 804–816 [DOI] [PubMed] [Google Scholar]
- 15. Takahashi I., Nuckolls G. H., Takahashi K., Tanaka O., Semba I., Dashmer R., Shum L., Slavkin H. C. (1998) Compressive force promotes Sox9, type II collagen and aggrecan and inhibits IL-1 beta expression resulting in chondrogenesis in mouse embryonic limb bud mesenchymal cells. J. Cell Sci. 111, 2067–2076 [DOI] [PubMed] [Google Scholar]
- 16. Elder S. H., Kimura J. H., Soslowsky L. J., Lavagnino M., Goldstein S. A. (2000) Effect of compressive loading on chondrocyte differentiation in agarose cultures of chick limb-bud cells. J. Orthop. Res. 18, 78–86 [DOI] [PubMed] [Google Scholar]
- 17. Pillarisetti A., Desai J. P., Ladjal H., Schiffmacher A., Ferreira A., Keefer C. L. (2011) Mechanical phenotyping of mouse embryonic stem cells: increase in stiffness with differentiation. Cell. Reprogram. 13, 371–380 [DOI] [PubMed] [Google Scholar]
- 18. Ofek G., Willard V. P., Koay E. J., Hu J. C., Lin P., Athanasiou K. A. (2009) Mechanical characterization of differentiated human embryonic stem cells. J. Biomech. Eng. 131, 061011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chiang H., Hsieh C.-H., Lin Y.-H., Lin S., Tsai-Wu J.-J., Jiang C.-C. (2011) Differences between chondrocytes and bone marrow-derived chondrogenic cells. Tissue Eng. A 17, 2919–2929 [DOI] [PubMed] [Google Scholar]
- 20. Evans N. D., Minelli C., Gentleman E., LaPointe V., Patankar S. N., Kallivretaki M., Chen X., Roberts C. J., Stevens M. M. (2009) Substrate stiffness affects early differentiation events in embryonic stem cells. Eur. Cell. Mater. 18, 1–14 [DOI] [PubMed] [Google Scholar]
- 21. Zoldan J., Karagiannis E. D., Lee C. Y., Anderson D. G., Langer R., Levenberg S. (2011) The influence of scaffold elasticity on germ layer specification of human embryonic stem cells. Biomaterials 32, 9612–9621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Steinberg M. S. (1970) Does differential adhesion govern self-assembly processes in histogenesis? Equilibrium configurations and the emergence of a hierarchy among populations of embryonic cells. J. Exp. Zool. 173, 395–433 [DOI] [PubMed] [Google Scholar]
- 23. Wada N. (2011) Spatiotemporal changes in cell adhesiveness during vertebrate limb morphogenesis. Dev. Dyn. 240, 969–978 [DOI] [PubMed] [Google Scholar]
- 24. Foty R. A., Steinberg M. S. (2005) The differential adhesion hypothesis: a direct evaluation. Dev. Biol. 278, 255–263 [DOI] [PubMed] [Google Scholar]
- 25. Krieg M., Arboleda-Estudillo Y., Puech P. H., Kafer J., Graner F., Muller D. J., Heisenberg C. P. (2008) Tensile forces govern germ-layer organization in zebrafish. Nat. Cell Biol. 10, 429–436 [DOI] [PubMed] [Google Scholar]
- 26. Crawford-Young S. J. (2006) Effects of microgravity on cell cytoskeleton and embryogenesis. Int. J. Dev. Biol. 50, 183–191 [DOI] [PubMed] [Google Scholar]
- 27. Yamashita A., Nishikawa S., Rancourt D. E. (2010) Identification of five developmental processes during chondrogenic differentiation of embryonic stem cells. PLoS One 5, e10998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hall B. K., Herring S. W. (1990) Paralysis and growth of the musculoskeletal system in the embryonic chick. J. Morphol. 206, 45–56 [DOI] [PubMed] [Google Scholar]
- 29. Yao H., Gu W. Y. (2007) Three-dimensional inhomogeneous triphasic finite-element analysis of physical signals and solute transport in human intervertebral disc under axial compression. J. Biomech. 40, 2071–2077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mahmoodian R., Leasure J., Philip P., Pleshko N., Capaldi F., Siegler S. (2011) Changes in mechanics and composition of human talar cartilage anlagen during fetal development. Osteoarthritis Cartilage 19, 1199–1209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Klein T. J., Chaudhry M., Bae W. C., Sah R. L. (2007) Depth-dependent biomechanical and biochemical properties of fetal, newborn, and tissue-engineered articular cartilage. J. Biomech. 40, 182–190 [DOI] [PubMed] [Google Scholar]
- 32. Brommer H., Brama P. A. J., Laasanen M. S., Helminen H. J., van Weeren P. R., Jurvelin J. S. (2005) Functional adaptation of articular cartilage from birth to maturity under the influence of loading: a biomechanical analysis. Equine Vet. J. 37, 148–154 [DOI] [PubMed] [Google Scholar]
- 33. Williamson A. K., Chen A. C., Masuda K., Thonar E. J., Sah R. L. (2003) Tensile mechanical properties of bovine articular cartilage: variations with growth and relationships to collagen network components. J. Orthop. Res. 21, 872–880 [DOI] [PubMed] [Google Scholar]
- 34. Carter D. R., Wong M. (2003) Modelling cartilage mechanobiology. Philos. Trans. R. Soc. London B Biol. Sci. 358, 1461–1471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Korhonen R., Julkunen P., Rieppo J., Lappalainen R., Konttinen Y., Jurvelin J. (2006) Collagen network of articular cartilage modulates fluid flow and mechanical stresses in chondrocyte. Biomech. Model. Mechanobiol. 5, 150–159 [DOI] [PubMed] [Google Scholar]
- 36. Kim E., Guilak F., Haider M. A. (2010) An axisymmetric boundary element model for determination of articular cartilage pericellular matrix properties in situ via inverse analysis of chondron deformation. J. Biomech. Eng. 132, 031011–031013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bachrach N. M., Valhmu W. B., Stazzone E., Ratcliffe A., Lai W. M., Mow V. C. (1995) Changes in proteoglycan synthesis of chondrocytes in articular cartilage are associated with the time-dependent changes in their mechanical environment. J. Biomech. 28, 1561–1569 [DOI] [PubMed] [Google Scholar]
- 38. Abusara Z., Seerattan R., Leumann A., Thompson R., Herzog W. (2011) A novel method for determining articular cartilage chondrocyte mechanics in vivo. J. Biomech. 44, 930–934 [DOI] [PubMed] [Google Scholar]
- 39. Eckstein F., Reiser M., Englmeier K.-H., Putz R. (2001) In vivo morphometry and functional analysis of human articular cartilage with quantitative magnetic resonance imaging—from image to data, from data to theory. Anat. Embryol. 203, 147–173 [DOI] [PubMed] [Google Scholar]
- 40. Li G., DeFrate L. E., Park S. E., Gill T. J., Rubash H. E. (2005) In vivo articular cartilage contact kinematics of the knee. Am. J. Sports Med. 33, 102–107 [DOI] [PubMed] [Google Scholar]
- 41. Juang Y.-M., Lee C.-Y., Hsu W.-Y., Lin C.-T., Lai C.-C., Tsai F.-J. (2010) Proteomic analysis of chondrocytes exposed to pressure. Biomed. Chromatogr. 24, 1273–1282 [DOI] [PubMed] [Google Scholar]
- 42. Knight M. M., Toyoda T., Lee D. A., Bader D. L. (2006) Mechanical compression and hydrostatic pressure induce reversible changes in actin cytoskeletal organisation in chondrocytes in agarose. J. Biomech. 39, 1547–1551 [DOI] [PubMed] [Google Scholar]
- 43. Kunitomo T., Takahashi K., Arai Y., Sakao K., Honjo K., Saito M., Inoue A., Tonomura H., Morihara T., Mazda O., Imanishi J., Kubo T. (2009) Influence of extracellular matrix on the expression of inflammatory cytokines, proteases, and apoptosis-related genes induced by hydrostatic pressure in three-dimensionally cultured chondrocytes. J. Orthop. Sci. 14, 776–783 [DOI] [PubMed] [Google Scholar]
- 44. Park S., Hung C. T., Ateshian G. A. (2004) Mechanical response of bovine articular cartilage under dynamic unconfined compression loading at physiological stress levels. Osteoarthritis Cartilage 12, 65–73 [DOI] [PubMed] [Google Scholar]
- 45. Chen A. C., Bae W. C., Schinagl R. M., Sah R. L. (2001) Depth- and strain-dependent mechanical and electromechanical properties of full-thickness bovine articular cartilage in confined compression. J. Biomech. 34, 1–12 [DOI] [PubMed] [Google Scholar]
- 46. Hodge W. A., Carlson K. L., Fijan R. S., Burgess R. G., Riley P. O., Harris W. H., Mann R. W. (1989) Contact pressures from an instrumented hip endoprosthesis. J. Bone Joint Surg. Am. 71, 1378–1386 [PubMed] [Google Scholar]
- 47. Afoke N. Y., Byers P. D., Hutton W. C. (1987) Contact pressures in the human hip joint. J. Bone Joint Surg. Br. 69, 536–541 [DOI] [PubMed] [Google Scholar]
- 48. Waters R. L., Lunsford B. R., Perry J., Byrd R. (1988) Energy-speed relationship of walking: standard tables. J. Orthop. Res. 6, 215–222 [DOI] [PubMed] [Google Scholar]
- 49. Mow V. C., Kuei S. C., Lai W. M., Armstrong C. G. (1980) Biphasic creep and stress relaxation of articular cartilage in compression: Theory and experiments. J. Biomech. Eng. 102, 73–84 [DOI] [PubMed] [Google Scholar]
- 50. Toyoda T., Seedhom B. B., Yao J. Q., Kirkham J., Brookes S., Bonass W. A. (2003) Hydrostatic pressure modulates proteoglycan metabolism in chondrocytes seeded in agarose. Arthritis Rheumatism 48, 2865–2872 [DOI] [PubMed] [Google Scholar]
- 51. Guilak F., Meyer B. C., Ratcliffe A., Mow V. C. (1994) The effects of matrix compression on proteoglycan metabolism in articular cartilage explants. Osteoarthritis Cartilage 2, 91–101 [DOI] [PubMed] [Google Scholar]
- 52. Schinagl R. M., Gurskis D., Chen A. C., Sah R. L. (1997) Depth-dependent confined compression modulus of full-thickness bovine articular cartilage. J. Orthop. Res. 15, 499–506 [DOI] [PubMed] [Google Scholar]
- 53. Démarteau O., Wendt D., Braccini A., Jakob M., Schäfer D., Heberer M., Martin I. (2003) Dynamic compression of cartilage constructs engineered from expanded human articular chondrocytes. Biochem. Biophys. Res. Commun. 310, 580–588 [DOI] [PubMed] [Google Scholar]
- 54. Waldman S. D., Couto D. C., Grynpas M. D., Pilliar R. M., Kandel R. A. (2006) A single application of cyclic loading can accelerate matrix deposition and enhance the properties of tissue-engineered cartilage. Osteoarthritis Cartilage 14, 323–330 [DOI] [PubMed] [Google Scholar]
- 55. Natoli R. M., Scott C. C., Athanasiou K. A. (2008) Temporal effects of impact on articular cartilage cell death, gene expression, matrix biochemistry, and biomechanics. Ann. Biomed. Eng. 36, 780–792 [DOI] [PubMed] [Google Scholar]
- 56. LeRoux M. A., Arokoski J., Vail T. P., Guilak F., Hyttinen M. M., Kiviranta I., Setton L. A. (2000) Simultaneous changes in the mechanical properties, quantitative collagen organization, and proteoglycan concentration of articular cartilage following canine meniscectomy. J. Orthop. Res. 18, 383–392 [DOI] [PubMed] [Google Scholar]
- 57. Brandt K. D., Myers S. L., Burr D., Albrecht M. (1991) Osteoarthritic changes in canine articular cartilage, subchondral bone, and synovium fifty-four months after transection of the anterior cruciate ligament. Arthritis Rheum. 34, 1560–1570 [DOI] [PubMed] [Google Scholar]
- 58. Andriacchi T. P., Mundermann A. (2006) The role of ambulatory mechanics in the initiation and progression of knee osteoarthritis. Curr. Opin. Rheumatol. 18, 514–518 [DOI] [PubMed] [Google Scholar]
- 59. Setton L. A., Mow V. C., Muller F. J., Pita J. C., Howell D. S. (1997) Mechanical behavior and biochemical composition of canine knee cartilage following periods of joint disuse and disuse with remobilization. Osteoarthritis Cartilage 5, 1–16 [DOI] [PubMed] [Google Scholar]
- 60. Behrens F., Kraft E. L., Oegema T. R., Jr. (1989) Biochemical changes in articular cartilage after joint immobilization by casting or external fixation. J. Orthop. Res. 7, 335–343 [DOI] [PubMed] [Google Scholar]
- 61. Palmoski M. J., Colyer R. A., Brandt K. D. (1980) Joint motion in the absence of normal loading does not maintain normal articular cartilage. Arthritis Rheum. 23, 325–334 [DOI] [PubMed] [Google Scholar]
- 62. Palmoski M. J., Brandt K. D. (1981) Running inhibits the reversal of atrophic changes in canine knee cartilage after removal of a leg cast. Arthritis Rheum. 24, 1329–1337 [DOI] [PubMed] [Google Scholar]
- 63. Venn M., Maroudas A. (1977) Chemical composition and swelling of normal and osteoarthrotic femoral head cartilage. I. Chemical composition. Ann. Rheum. Dis. 36, 121–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Salter R. B., Simmonds D. F., Malcolm B. W., Rumble E. J., MacMichael D., Clements N. D. (1980) The biological effect of continuous passive motion on the healing of full-thickness defects in articular cartilage. An experimental investigation in the rabbit. J. Bone Joint Surg. Am. 62, 1232–1251 [PubMed] [Google Scholar]
- 65. Moukoko D., Pourquier D., Pithioux M., Chabrand P. (2010) Influence of cyclic bending loading on in vivo skeletal tissue regeneration from periosteal origin. Orthop. Traumatol. Surg. Res. 96, 833–839 [DOI] [PubMed] [Google Scholar]
- 66. Kelly D. J., Prendergast P. J. (2005) Mechano-regulation of stem cell differentiation and tissue regeneration in osteochondral defects. J. Biomech. 38, 1413–1422 [DOI] [PubMed] [Google Scholar]
- 67. Huang C. Y. C., Hagar K. L., Frost L. E., Sun Y., Cheung H. S. (2004) Effects of cyclic compressive loading on chondrogenesis of rabbit bone-marrow-derived mesenchymal stem cells. Stem Cells 22, 313–323 [DOI] [PubMed] [Google Scholar]
- 68. Angele P., Yoo J. U., Smith C., Mansour J., Jepsen K. J., Nerlich M., Johnstone B. (2003) Cyclic hydrostatic pressure enhances the chondrogenic phenotype of human mesenchymal progenitor cells differentiated in vitro. J. Orthop. Res. 21, 451–457 [DOI] [PubMed] [Google Scholar]
- 69. Darling E. M., Athanasiou K. A. (2005) Rapid phenotypic changes in passaged articular chondrocyte subpopulations. J. Orthop. Res. 23, 425–432 [DOI] [PubMed] [Google Scholar]
- 70. Darling E., Pritchett P., Evans B., Superfine R., Zauscher S., Guilak F. (2009) Mechanical properties and gene expression of chondrocytes on micropatterned substrates following dedifferentiation in monolayer. Cell. Mol. Bioeng. 2, 395–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Das R. H., Jahr H., Verhaar J. A., van der Linden J. C., van Osch G. J., Weinans H. (2008) In vitro expansion affects the response of chondrocytes to mechanical stimulation. Osteoarthritis Cartilage 16, 385–391 [DOI] [PubMed] [Google Scholar]
- 72. Heyland J., Wiegandt K., Goepfert C., Nagel-Heyer S., Ilinich E., Schumacher U., Portner R. (2006) Redifferentiation of chondrocytes and cartilage formation under intermittent hydrostatic pressure. Biotechnol. Lett. 28, 1641–1648 [DOI] [PubMed] [Google Scholar]
- 73. Kawanishi M., Oura A., Furukawa K., Fukubayashi T., Nakamura K., Tateishi T., Ushida T. (2007) Redifferentiation of dedifferentiated bovine articular chondrocytes enhanced by cyclic hydrostatic pressure under a gas-controlled system. Tissue Eng. 13, 957–964 [DOI] [PubMed] [Google Scholar]
- 74. Domm C., Fay J., Schunke M., Kurz B. (2000) [Redifferentiation of dedifferentiated joint cartilage cells in alginate culture. Effect of intermittent hydrostatic pressure and low oxygen partial pressure]. Orthopade 29, 91–99 [DOI] [PubMed] [Google Scholar]
- 75. Lee C. R., Grodzinsky A. J., Spector M. (2003) Biosynthetic response of passaged chondrocytes in a type II collagen scaffold to mechanical compression. J. Biomed. Mater. Res. A 64, 560–569 [DOI] [PubMed] [Google Scholar]
- 76. Elder S. H., Fulzele K. S., McCulley W. R. (2005) Cyclic hydrostatic compression stimulates chondroinduction of C3H/10T1/2 cells. Biomech. Model. Mechanobiol. 3, 141–146 [DOI] [PubMed] [Google Scholar]
- 77. Singh M., Pierpoint M., Mikos A. G., Kasper F. K. (2011) Chondrogenic differentiation of neonatal human dermal fibroblasts encapsulated in alginate beads with hydrostatic compression under hypoxic conditions in the presence of bone morphogenetic protein-2. J. Biomed. Mater. Res. A 98, 412–424 [DOI] [PubMed] [Google Scholar]
- 78. Doehring L. C., Heeger C., Aherrahrou Z., Kaczmarek P. M., Erdmann J., Schunkert H., Ehlers E. M. (2010) Myeloid CD34+CD13+ precursor cells transdifferentiate into chondrocyte-like cells in atherosclerotic intimal calcification. Am. J. Pathol. 177, 473–480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Elder B. D., Athanasiou K. A. (2009) Hydrostatic pressure in articular cartilage tissue engineering: from chondrocytes to tissue regeneration. Tiss. Eng. B Rev. 15, 43–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Carver S. E., Heath C. A. (1999) Increasing extracellular matrix production in regenerating cartilage with intermittent physiological pressure. Biotechnol. Bioeng. 62, 166–174 [PubMed] [Google Scholar]
- 81. Takahashi K., Kubo T., Arai Y., Kitajima I., Takigawa M., Imanishi J., Hirasawa Y. (1998) Hydrostatic pressure induces expression of interleukin 6 and tumour necrosis factor alpha mRNAs in a chondrocyte-like cell line. Ann. Rheum. Dis. 57, 231–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Elder S. H., Sanders S. W., McCulley W. R., Marr M. L., Shim J. W., Hasty K. A. (2006) Chondrocyte response to cyclic hydrostatic pressure in alginate versus pellet culture. J. Orthop. Res. 24, 740–747 [DOI] [PubMed] [Google Scholar]
- 83. Burton-Wurster N., Vernier-Singer M., Farquhar T., Lust G. (1993) Effect of compressive loading and unloading on the synthesis of total protein, proteoglycan, and fibronectin by canine cartilage explants. J. Orthop. Res. 11, 717–729 [DOI] [PubMed] [Google Scholar]
- 84. Sah R. L., Kim Y. J., Doong J. Y., Grodzinsky A. J., Plaas A. H., Sandy J. D. (1989) Biosynthetic response of cartilage explants to dynamic compression. J. Orthop. Res. 7, 619–636 [DOI] [PubMed] [Google Scholar]
- 85. Mauck R. L., Soltz M. A., Wang C. C., Wong D. D., Chao P. H., Valhmu W. B., Hung C. T., Ateshian G. A. (2000) Functional tissue engineering of articular cartilage through dynamic loading of chondrocyte-seeded agarose gels. J. Biomech. Eng. 122, 252–260 [DOI] [PubMed] [Google Scholar]
- 86. Jin M., Frank E. H., Quinn T. M., Hunziker E. B., Grodzinsky A. J. (2001) Tissue shear deformation stimulates proteoglycan and protein biosynthesis in bovine cartilage explants. Arch. Biochem. Biophys. 395, 41–48 [DOI] [PubMed] [Google Scholar]
- 87. Waldman S. D., Spiteri C. G., Grynpas M. D., Pilliar R. M., Kandel R. A. (2003) Long-term intermittent shear deformation improves the quality of cartilaginous tissue formed in vitro. J. Orthop. Res. 21, 590–596 [DOI] [PubMed] [Google Scholar]
- 88. Smith R. L., Donlon B. S., Gupta M. K., Mohtai M., Das P., Carter D. R., Cooke J., Gibbons G., Hutchinson N., Schurman D. J. (1995) Effects of fluid-induced shear on articular chondrocyte morphology and metabolism in vitro. J. Orthop. Res. 13, 824–831 [DOI] [PubMed] [Google Scholar]
- 89. Browning J. A., Walker R. E., Hall A. C., Wilkins R. J. (1999) Modulation of Na+ × H+ exchange by hydrostatic pressure in isolated bovine articular chondrocytes. Acta Physiol. Scand. 166, 39–45 [DOI] [PubMed] [Google Scholar]
- 90. Mizuno S. (2005) A novel method for assessing effects of hydrostatic fluid pressure on intracellular calcium: a study with bovine articular chondrocytes. Am. J. Physiol. Cell Physiol. 288, C329–C337 [DOI] [PubMed] [Google Scholar]
- 91. Shakibaei M., Schulze-Tanzil G., de Souza P., John T., Rahmanzadeh M., Rahmanzadeh R., Merker H. J. (2001) Inhibition of mitogen-activated protein kinase kinase induces apoptosis of human chondrocytes. J. Biol. Chem. 276, 13289–13294 [DOI] [PubMed] [Google Scholar]
- 92. Taylor D. J., Yoffe J. R., Brown D. M., Woolley D. E. (1985) Histamine H2 receptors on chondrocytes derived from human, canine and bovine articular cartilage. Biochem. J. 225, 315–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Edlich M., Yellowley C. E., Jacobs C. R., Donahue H. J. (2001) Oscillating fluid flow regulates cytosolic calcium concentration in bovine articular chondrocytes. J. Biomech. 34, 59–65 [DOI] [PubMed] [Google Scholar]
- 94. Browning J. A., Saunders K., Urban J. P., Wilkins R. J. (2004) The influence and interactions of hydrostatic and osmotic pressures on the intracellular milieu of chondrocytes. Biorheology 41, 299–308 [PubMed] [Google Scholar]
- 95. Natoli R. M., Skaalure S., Bijlani S., Chen K. X., Hu J., Athanasiou K. A. Intracellular Na+ and Ca2+ modulation increases the tensile properties of developing engineered articular cartilage. Arthritis Rheum 62, 1097–1107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Ongaro A., Pellati A., Masieri F. F., Caruso A., Setti S., Cadossi R., Biscione R., Massari L., Fini M., De Mattei M. (2011) Chondroprotective effects of pulsed electromagnetic fields on human cartilage explants. Bioelectromagnetics 32, 543–551 [DOI] [PubMed] [Google Scholar]
- 97. Yellowley C. E., Hancox J. C., Donahue H. J. (2002) Effects of cell swelling on intracellular calcium and membrane currents in bovine articular chondrocytes. J. Cell. Biochem. 86, 290–301 [DOI] [PubMed] [Google Scholar]
- 98. Tew S. R., Peffers M. J., McKay T. R., Lowe E. T., Khan W. S., Hardingham T. E., Clegg P. D. (2009) Hyperosmolarity regulates SOX9 mRNA posttranscriptionally in human articular chondrocytes. Am. J. Physiol. Cell Physiol. 297, C898–906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Oswald E. S., Ahmed H. S., Kramer S. P., Bulinski J. C., Ateshian G. A., Hung C. T. (2011) Effects of hypertonic (NaCl) two-dimensional and three-dimensional culture conditions on the properties of cartilage tissue engineered from an expanded mature bovine chondrocyte source. Tissue Eng. C Methods 17, 1041–1049 [DOI] [PMC free article] [PubMed] [Google Scholar]