Abstract
Background:
The objectives of this cross-sectional study were to assess the prevalence and severity of sleep apnea in patients with multiple sclerosis (MS) referred for overnight polysomnography (PSG) and to explore the radiographic and clinical features that might signal risk for undiagnosed sleep apnea.
Methods:
Apnea-hypopnea (AHI) and central apnea indices (CAI) from laboratory-based PSG among 48 patients with MS were compared with those of group A, 84 sleep laboratory−referred patients without MS matched for age, gender, and body mass index; and group B, a separate group of 48 randomly selected, referred patients.
Results:
Mean AHI was higher among patients with MS than among control groups A or B (2-way analysis of variance and multiple linear regression, p = 0.0011 and 0.0118, respectively). Median and mean CAI were also increased among patients with MS in comparison to control groups (Wilcoxon signed rank and multiple linear regression, p = 0.0064 and 0.0027, respectively). Among MS patients with available data, those with evidence of brainstem involvement, compared with groups A and B, showed particularly robust differences in AHI (p = 0.0060 and 0.0016) and CAI (p = 0.0215 and <0.0001). In contrast, MS patients without brainstem involvement, compared with groups A and B, showed diminished differences in AHI, and CAI did not significantly differ among groups.
Conclusions:
These data suggest a predisposition for obstructive sleep apnea and accompanying central apneas among patients with MS, particularly among those with brainstem involvement.
Obstructive sleep apnea (OSA) and central sleep apnea (CSA), 2 common forms of sleep-disordered breathing (SDB), are treatable risk factors for cardiovascular disease, motor vehicle accidents, fatigue, and decreased quality of life.1–5 Patients with multiple sclerosis (MS) commonly experience fatigue and impaired quality of life,6 but the extent to which sleep apnea contributes to these symptoms remains unclear. Although some contend that the prevalence and severity of SDB are higher in persons with MS,7,8 previous studies have generated conflicting results.9 Moreover, as fatigue is often viewed as a cardinal symptom of MS, providers may be less likely to order sleep studies in these patients. Opportunities to diagnose and treat sleep apnea may therefore be underrecognized. The purpose of this study was to assess the prevalence and severity of sleep apnea in patients with MS referred for nocturnal sleep studies and to explore radiographic and clinical features that might facilitate recognition of occult obstructive or CSA in MS.
METHODS
Standard protocol approvals, registrations, and patient consents.
This retrospective data analysis was approved by the University of Michigan (U-M) Institutional Review Board.
Subjects/data collection.
Patients with MS.
Demographic, clinical, and polysomnographic (PSG) data were assembled from medical records of 48 subjects, 18 years or older, who had an established diagnosis of MS and had completed clinical overnight PSG between March 1999 and June 2010. Subjects with relevant PSG data were identified from the U-M Sleep Disorders Center database and from U-M MS clinic lists. For subjects identified from MS clinic lists, patient registration numbers were used to search electronic charts for PSG reports using EMERSE, the Electronic Medical Record Search Engine. EMERSE is a web-based tool used to search for patient-specific information from the U-M Clinical Data Repository.
Controls.
Demographic, clinical, and PSG data from the U-M Sleep Disorders Center database were assembled for 2 separate control groups. These groups were selected from more than 8,000 adult patients referred for diagnostic sleep studies. For group A, 2 controls were matched to each patient with MS for paired analyses, based on age (±5 years), gender, and body mass index (BMI) (±2 kg/m2). Matched controls were also selected based on date of study, before or after January 1, 2008, to control for minor changes in PSG scoring criteria that took place after January 1, 2008.10 Another sample of 48 controls (group B) was selected using a random numbers table for unpaired analyses that were not constrained a priori by matching criteria.
PSG.
Full laboratory-based PSG and scoring followed existing standards before 200811 and then slightly different, newly published standards from that point forward.10 The main relevant change concerned use of nasal pressure to identify hypopneas and rules used to score them. Our laboratory had already been using thoracic or abdominal excursion changes, in addition to thermocouple airflow changes (when any of these were followed by awakenings, arousals, or ≥4% oxygen desaturations), to identify hypopneas in a sensitive manner before the American Academy of Sleep Medicine 2007 standards were published.10 However, because the 2 approaches are not identical, we were careful to include in the MS and matched control group A the same proportions of patients studied before and after the change in scoring rules.
The apnea-hypopnea index (AHI) was calculated as the number of obstructive apneas, central apneas, or hypopneas per hour of sleep. The presence of OSA was defined by an AHI of at least 5 episodes per hour of sleep.13 Hypopneas are difficult to ascribe to obstructive or central etiologies, but they often make the major contribution to the AHI. In addition, central apneas were included in the AHI as generally practiced in sleep laboratories, in part because some degree of CSA is a common feature in OSA. For our study, the separate presence of CSA or a significant component of CSA was defined by a central apnea index (CAI) of at least 5 episodes per hour of sleep, in the absence of severe OSA.13,14 For individuals with severe OSA (AHI ≥30 episodes per hour of sleep), we identified concomitant CSA by a CAI of 15 or more central apneas per hour of sleep.
Data collection.
The following variables were extracted from the sleep database: gender, PSG date, age, BMI, PSG diagnosis, AHI (rate of apneas and hypopneas per hour of sleep), and CAI. Data regarding total sleep time spent in the supine position and the periodic leg movement index were also collected. Subjects who did not have BMI data recorded in either the sleep database or medical records (n = 6) were excluded from paired analyses but included in unpaired analyses. Individuals with concomitant diseases that could increase the risk of SDB, including severe cardiopulmonary disease, neurologic diseases other than MS, or patients with coexistent non-MS brainstem neuropathology were excluded. For subjects with MS, additional variables recorded included MS subtype (relapsing-remitting vs progressive), disease duration (years), use of disease modifying therapy (DMT) (defined as glatiramer acetate or interferon β use at the time of PSG), estimates of disability (defined as an Expanded Disability Status Scale [EDSS] score of ≥6.0), physical examination findings, and MRI results.
For subgroup analyses, MS patients with available MRI scans or physical examinations were segregated by the presence or absence of brainstem involvement, as suggested by the following: documented evidence of dysarthria or dysphagia; physical examination findings of lower cranial nerve dysfunction (diminished gag reflex, palatal asymmetry); or the presence of T2-weighted or gadolinium-enhancing midbrain, pontine, or medullary MRI lesions. Faculty neuroradiologist−generated MRI reports from medical records were used to determine the presence of brainstem lesions. When reports did not specifically address the brainstem in the interpretation, the MRI studies were personally reviewed by an investigator (T.J.B., a fellowship-trained MS specialist) to assess the presence of T2-weighted signal changes or gadolinium-enhancing lesions. These reviews were performed before any comparison to PSG data.
Statistical methods.
Statistical tests were performed using SAS version 9.2. Tests were 2-sided with the level of statistical significance set at 0.05.
Paired (matched) analyses.
Outcome variables AHI and CAI were compared between the subjects with MS and matched controls (group A). Comparisons were also made between MS subjects with brainstem involvement and their matched controls and between MS subjects without brainstem involvement and their matched controls. The AHI comparisons were made using 2-way analysis of variance (ANOVA). Because of right skewness in AHI distribution, values were log-transformed [ln (x + 1)] before 2-way ANOVA was performed. Due to persistent nonnormality in the CAI distribution despite log transformation, CAI comparisons were done using Wilcoxon rank sum tests. Differences in SDB prevalence were analyzed with χ2 tests.
Unmatched analyses.
Multiple linear regression models were used to identify significant predictors of AHI and CAI. To ensure residual normalization, CAI values were log-transformed before analysis. Raw AHI residuals were sufficiently normal for the regression models. Potential confounders taken into account in the regression model included BMI, age, and gender.
MS-specific unpaired analyses.
We also conducted exploratory, post hoc multiple linear regression analyses among MS subjects only, to assess whether MS subtype, duration, or treatment with DMT predicted AHI or log CAI, with adjustment for the presence of brainstem lesions, age, gender, and BMI. Because our sample size precluded use of a valid 7-variable model, we used the R2 selection method to select the 5 predictor variables that resulted in the highest R2 value for each model.
RESULTS
Baseline data.
Baseline characteristics for patients with MS, matched (group A) controls, and randomly selected (group B) controls are shown in table 1. Forty-eight subjects with MS with complete PSG data were identified. Mean disease duration was 13.1 years. No significant differences between subjects with MS and matched (group A) controls emerged for BMI, gender, or age. For those with available data, 1 of 28 (3.6%) patients with relapsing-remitting MS and 14 of 19 (73.7%) patients with progressive MS had an EDSS score ≥6.0. Twenty-one of 29 (72.4%) patients with relapsing-remitting MS and 12 of 19 (63.2%) patients with progressive MS were receiving DMT. Among MS subjects, 41 of 48 had MRI data available for unpaired analyses (24 with brainstem involvement and 17 without brainstem involvement). Among 42 MS subjects with available BMI data for paired analyses, 36 had MRI data available for matched subgroup analyses (20 with brainstem involvement and 16 without brainstem involvement).
Table 1.
Baseline characteristics of patients with MS, matched controls (group A), and randomly selected controls (group B)a
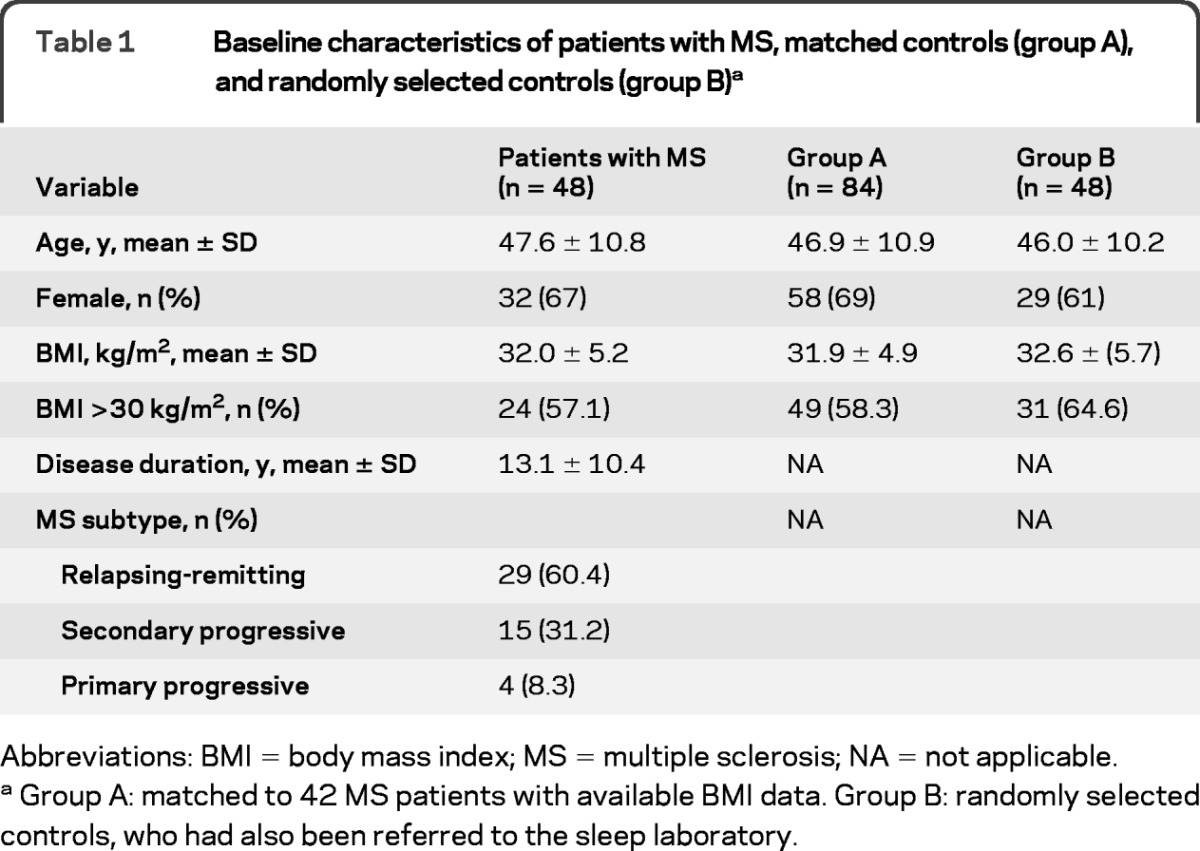
Abbreviations: BMI = body mass index; MS = multiple sclerosis; NA = not applicable.
Group A: matched to 42 MS patients with available BMI data. Group B: randomly selected controls, who had also been referred to the sleep laboratory.
Paired (matched) analyses.
Differences in mean AHI are summarized in table 2. Compared with 84 matched controls, the 42 subjects with MS on average had a higher AHI (log-transformed, p = 0.0011). Among subjects with MS, 27 had OSA, 2 had CSA, and 3 met the diagnostic criteria for both. Total sleep time spent supine during sleep did not differ among patients with MS and matched controls (165.2 and 197.8 minutes, respectively; 2-way ANOVA, p = 0.1988).
Table 2.
AHI, log-transformed AHI, and mean and median CAI are shown for MS patients, MS patients with brainstem involvement, MS patients without brainstem involvement, and their matched controls
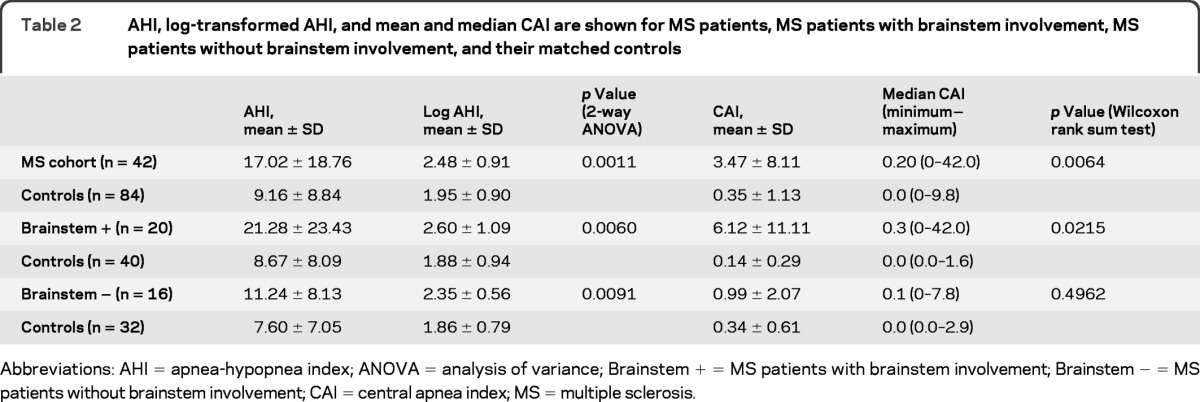
Abbreviations: AHI = apnea-hypopnea index; ANOVA = analysis of variance; Brainstem + = MS patients with brainstem involvement; Brainstem − = MS patients without brainstem involvement; CAI = central apnea index; MS = multiple sclerosis.
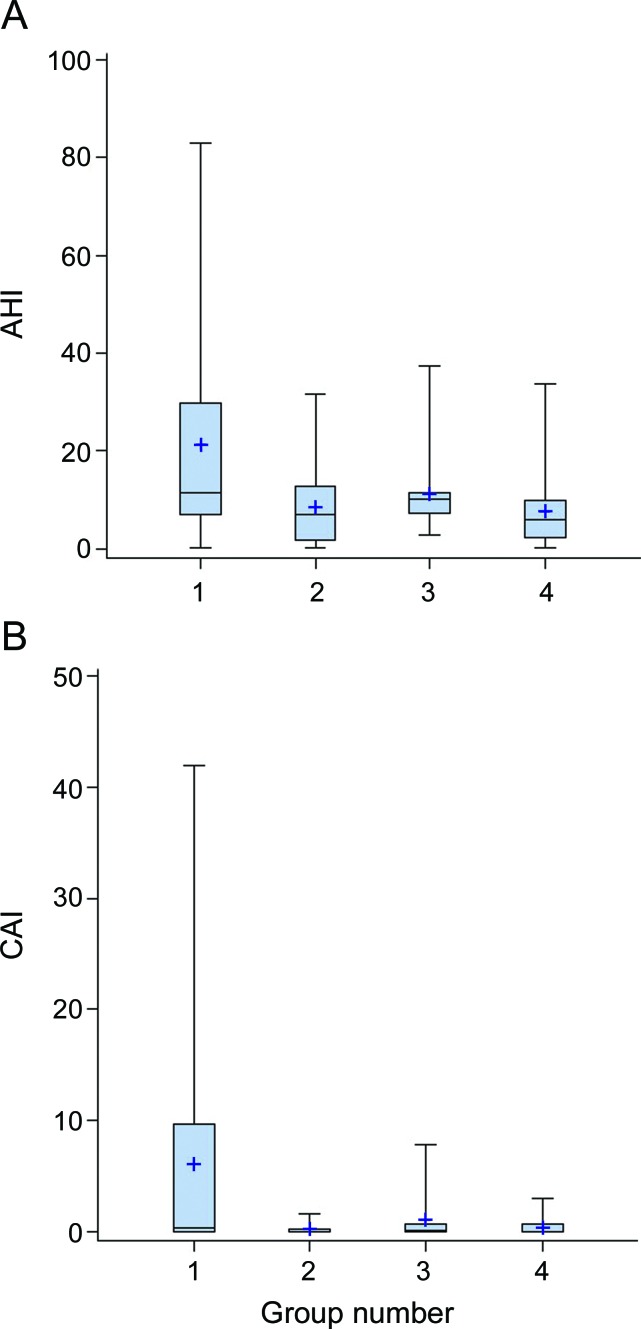
Mean AHI among the 20 MS subjects with brainstem involvement was larger (in absolute value) than that of the entire MS cohort and remained higher than the mean AHI for their 40 matched controls (p = 0.0060) (table 2, figure). In contrast, absolute AHI differences between the 16 MS subjects without brainstem involvement and their 32 matched controls was less impressive.
Figure. Boxplots of AHI and CAI for patients with MS, stratified by the presence or absence of brainstem involvement and their matched controls.
(A) AHI for 20 patients with MS with brainstem involvement vs 40 matched controls (groups 1 and 2), and 16 patients with MS without brainstem involvement vs 32 matched controls (groups 3 and 4). (B) CAI for 20 patients with MS with brainstem involvement vs 40 matched controls (groups 1 and 2) and 16 patients with MS without brainstem involvement vs 32 matched controls (groups 3 and 4). AHI = apnea-hypopnea index; CAI = central apnea index; MS = multiple sclerosis.
Median CAI also differed between patients with MS and controls (p = 0.0064) (table 2). This difference increased when the analysis was focused on patients with brainstem involvement (p = 0.0215) (table 2, figure). In contrast, there was no difference between MS patients without brainstem involvement and their respective controls (p = 0.4962).
Unmatched analyses.
Table 3 shows differences between patients with MS and randomly selected controls (group B) in mean AHI and log-transformed mean CAI values. Consistent with the matched analyses, these unmatched analyses showed differences in AHI and log CAI between the patients with MS and controls, both before and after adjustment for BMI, age, and gender (p = 0.0118 and 0.0027, respectively). These differences increased in magnitude when the analysis focused on patients with brainstem involvement and controls and diminished when the analysis was constrained to patients without brainstem involvement and controls.
Table 3.
Multiple linear regression results for 3 separate regression models in which all MS patients, MS patients with brainstem involvement, or MS patients without brainstem involvement, compared to group B controls, were modeled as predictors of AHI and log-transformed CAIa
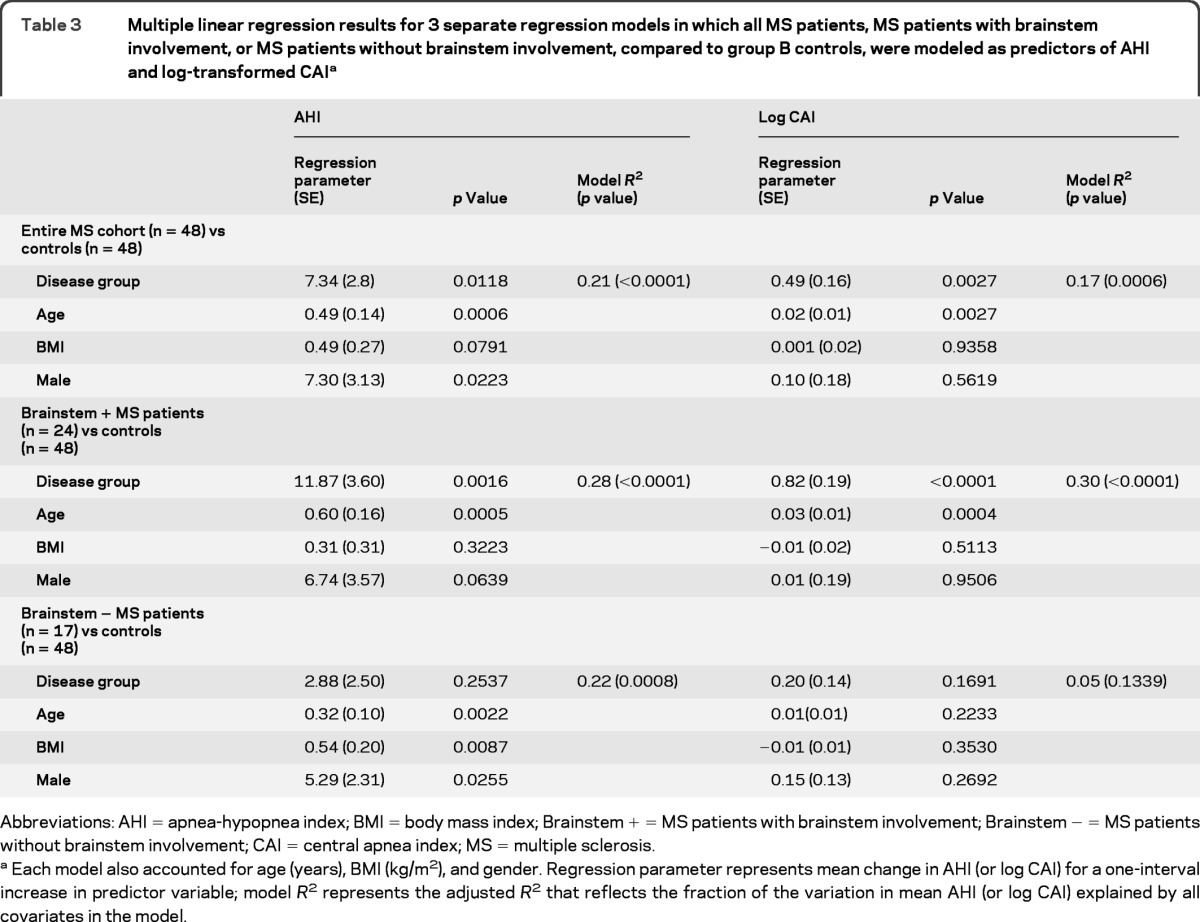
Abbreviations: AHI = apnea-hypopnea index; BMI = body mass index; Brainstem + = MS patients with brainstem involvement; Brainstem − = MS patients without brainstem involvement; CAI = central apnea index; MS = multiple sclerosis.
Each model also accounted for age (years), BMI (kg/m2), and gender. Regression parameter represents mean change in AHI (or log CAI) for a one-interval increase in predictor variable; model R2 represents the adjusted R2 that reflects the fraction of the variation in mean AHI (or log CAI) explained by all covariates in the model.
MS-specific unpaired analyses.
The presence of brainstem lesions, DMT use, disease subtype, age, and gender were the best predictors of AHI (table 4). Variables that best predicted log CAI included the presence of brainstem lesions, DMT use, disease subtype, disease duration, and BMI.
Table 4.
Results for separate MS cohort regression analysis modeled using the R2 selection methoda
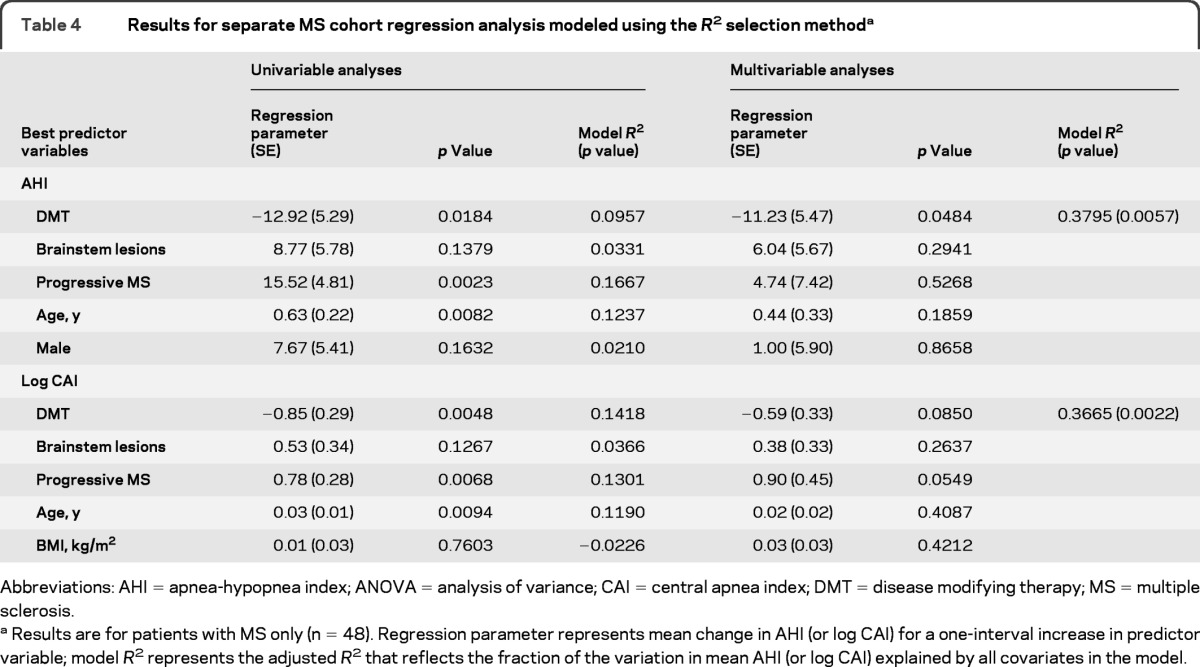
Abbreviations: AHI = apnea-hypopnea index; ANOVA = analysis of variance; CAI = central apnea index; DMT = disease modifying therapy; MS = multiple sclerosis.
Results are for patients with MS only (n = 48). Regression parameter represents mean change in AHI (or log CAI) for a one-interval increase in predictor variable; model R2represents the adjusted R2 that reflects the fraction of the variation in mean AHI (or log CAI) explained by all covariates in the model.
There was no correlation between BMI and apnea indices AHI and CAI (Pearson ρ = −0.0316 and 0.0224, p = 0.8423 and 0.8882, respectively). The periodic leg movement index did not differ among subjects with or without brainstem involvement (6.8 and 16.0, respectively, 2-sample t test, p = 0.1974).
DISCUSSION
In this carefully controlled study, patients with MS referred for clinical sleep studies had more severe OSA, as well as more severe CSA, than referred individuals without MS. Furthermore, MS patients with clinical or radiographic evidence of brainstem involvement showed a mean AHI of 21, nearly double the result (11) for patients without brainstem involvement. Mean and median CAI values were also substantially higher among MS patients with brainstem involvement than among those without brainstem involvement. In exploratory analyses, DMT use was associated with lower apnea scores, and a trend suggested a relationship between progressive subtypes of MS and sleep apnea. Although a causal relationship cannot be proven from cross-sectional data, our findings provide strong evidence that regional brainstem dysfunction due to MS plaque formation might contribute to both obstructive and central sleep apnea severity. Furthermore, apnea severity may be influenced by disease subtype and DMT use. These results could have important implications for the care of patients with MS, among whom fatigue is one of the most common and debilitating symptoms.15 The findings also may shed light on neuroanatomic and pathophysiologic mechanisms that can produce OSA and CSA.
OSA is characterized by upper airway obstruction during sleep, despite efforts to resume normal respiration.16 Upper airway patency, normally maintained by afferent sensory input to cranial nuclei and efferent output to the upper airway, is altered in OSA.17–19 Cases of both OSA and CSA in structural and ischemic brainstem syndromes are well documented,20–27 yet few studies have addressed the association between MS-related brainstem pathology and OSA. Our study is novel in that it explores MS clinical features that may increase OSA risk, while controlling for other MS-unrelated clinical features known to influence this risk. Although some data suggest an increased prevalence of SDB in patients with MS,8 other studies have produced conflicting results. Two cases of OSA were identified in a recent study of 28 subjects with MS.9 Although these 2 patients, compared with the remainder, showed no difference in MRI lesion location, the small sample of subjects with OSA may have precluded detection of clinically meaningful differences. In another study of 25 patients with MS and 25 age- and sex-matched controls,28 3 patients with MS had an AHI of >5, and 2 of these had predominantly central apnea. This study was one of the first to address the effect of infratentorial lesions on nocturnal respiration, but the report did not specify for these 3 patients the specific infratentorial locations involved.
In contrast to OSA, CSA results from recurrent complete or partial absence of respiratory effort. CSA can be caused by impaired respiratory control at the level of the medullary reticular formation. Although in the general population the prevalence of CSA is lower than that of OSA, patients with disorders such as MS that affect the brainstem may be at increased risk for CSA or apnea-related sudden death.29
It is noteworthy that 2 additional MS variables, DMT use and disease subtype, emerged as predictors of apnea severity. DMT use in particular eclipsed the individual effects of other modeled variables known to influence AHI. This finding may be explained in part by multicollinearity, because nearly all predictor variables were associated with each other to some extent in bivariate analyses. Furthermore, the exclusion of controls in these models reduced the sample size, decreasing our power to detect significant associations. Nonetheless, considering the strong predictive power of our model as a whole, our data suggest that the remaining variables still influence AHI but are perhaps overshadowed by the effects of DMT use.
Definitive conclusions on the relationship between DMT use and OSA cannot be drawn from exploratory analyses that were not the focus of our original hypotheses; however, these findings raise interesting questions about the pathogenesis of OSA. Inflammatory cytokines tumor necrosis factor-α and interleukin-6 are expressed at higher levels in individuals with OSA,30 and treatment with agents that influence these cytokine levels may improve apnea severity.31 Our data allow speculation that systemic or local inflammation not only reflects OSA but could contribute to it.
Among potential study limitations, observed differences in sleep apnea severity between patients with MS and controls could conceivably reflect referral practices. Because of the high prevalence of MS-related fatigue, physicians who see patients with MS may have a tendency to refer only the most obviously symptomatic patients for sleep evaluations. Although we cannot exclude this possibility, it is unlikely to explain the association between brainstem involvement and elevated AHI and CAI.
In addition, because this study was retrospective, some potential variables that could influence apnea severity were not available in a format that could be useful in the analyses. These include information on whole-brain quantitative MRI lesion burden, spinal cord involvement, respiratory function, and medications (such as antispasmodic drugs or narcotics) that are often used by patients with MS. The possibility exists that the association between CAI and disease subtype in our exploratory analyses may in part reflect overall MRI lesion burden, because patients with progressive subtypes of MS are more likely to have widespread parenchymal damage that may affect both brainstem and nonbrainstem pathways that control nocturnal respiration. Patients with progressive MS may also require more antispasmodic drugs and pain medications. However, medication use would not be expected to differ substantially between MS patients with and without brainstem findings.
Patients with OSA often have concomitant central apneas. The reason is not well understood but may involve alterations in hypocapnic sensitivity thresholds32 that can be reversed by continuous positive airway pressure.33 Conceivably, the increase in CAI among patients with MS may be explained in part by concomitant OSA. However, given the disproportionate elevation in CAI among patients with brainstem involvement, specific effects of brainstem lesions seem more likely.
Finally, the available number of MS patients with evidence of brainstem involvement forced their consideration within one category and did not allow for further substratification to distinguish between midbrain, pontine, or medullary locations that could differentially influence sleep apnea risk. This may also explain in part the large AHI and CAI standard deviations within the brainstem lesion group.
Despite these limitations, the robust differences between subjects with MS and controls indicate that MS and, in particular, MS-related brainstem pathology, could increase vulnerability to OSA and central sleep apnea. This vulnerability may be further influenced by other MS-specific variables including MS subtype and use of DMT. The data suggest that complaints of fatigue from patients with MS may frequently merit consideration of sleep apnea as a potential cause. Patients with sleep apnea complain about fatigue, lack of energy, or tiredness more often than sleepiness.3 This observation is particularly relevant to patients with MS who already experience, presumably because of their neuroimmunologic disorder, disproportionate disability and fatigue. Moreover, fatigue and related complaints, in addition to sleepiness, tend to improve when sleep apnea is treated.34
Results of this study indicate that patients with MS referred for PSG, compared with those without MS, may be at risk for more severe OSA and CSA and that patients with evidence of brainstem involvement may be particularly susceptible. Our findings underscore the importance of screening MS patients for sleep apnea and considering referral for PSG. The latter may be particularly useful for patients with signs of lower cranial nerve dysfunction, infratentorial lesions, and perhaps those with progressive MS or lack of DMT.
ACKNOWLEDGMENT
The authors thank Judy Fetterolf, RPSGT, REEGT, for her assistance in retrieval of information from the Sleep Disorders Center database and Brenda Gillepsie, PhD, for her statistical consultative assistance.
GLOSSARY
- AHI
apnea-hypopnea index
- ANOVA
analysis of variance
- BMI
body mass index
- CAI
central apnea index
- CSA
central sleep apnea
- DMT
disease modifying therapy
- EDSS
Expanded Disability Status Scale
- MS
multiple sclerosis
- OSA
obstructive sleep apnea
- PSG
polysomnography
- SDB
sleep-disordered breathing
- U-M
University of Michigan
AUTHOR CONTRIBUTIONS
Tiffany J. Braley: study concept and design, acquisition of data, statistical analysis, drafting/revising the manuscript for content. Benjamin M. Segal: interpretation of data, drafting/revising the manuscript for content. Ronald D. Chervin: study concept and design, analysis and interpretation of data, drafting/revising the manuscript for content.
DISCLOSURE
T. Braley is site principal investigator for several industry-funded studies at the University of Michigan (sponsors include Genzyme-Sanofi, Biogen-Idec, AB Science, EMD-Serono, and Genentech-Roche) but receives no direct compensation for this work. She received support from the National Multiple Sclerosis Society for a 3-year clinical research fellowship. She has previously received honoraria for speaking engagements for Teva, Pfizer, Serono, and Biogen-Idec Pharmaceuticals and has attended consultant's meetings for Biogen-Idec and Acorda Pharmaceuticals (none within the last 2 years). She has also served as a multiple sclerosis subject matter expert (SME) for Blue Cross/Blue Shield of Michigan. B. Segal has conducted research supported by the NIH, National Multiple Sclerosis Society, Veteran's Administration, Dana Foundation, Serono, Inc., and Centrocor, Inc. He serves on the Scientific Advisory Board for the National Multiple Sclerosis Society and has held consulting positions for Teva, Centrocor, Serono, Biogen-Idec, and Innate Therapeutics Limited. R. Chervin has conducted research funded by the NIH and the Fox Foundation; was involved with unrestricted educational gifts to the University of Michigan from Philips Respironics and Fisher Paykel; holds a professorship endowed in part by contributions from Philips Respironics and Sepracor; serves on boards of directors for the American Academy of Sleep Medicine, American Sleep Medicine Foundation, American Board of Sleep Medicine, and International Pediatric Sleep Association; is a member of advisory boards for the non-profit Sweet Dreamzzz and the NHLBI; was a member of the Scientific Advisory Board for Pavad Medical, Inc.; has consulted for Proctor & Gamble; serves as a section editor for UpToDate, Inc.; and is named in University of Michigan patents or patent applications for innovations designed to improve assessment and treatment of obstructive sleep apnea. Go to Neurology.org for full disclosures.
REFERENCES
- 1. Teran-Santos J, Jimenez-Gomez A, Cordero-Guevara J. The association between sleep apnea and the risk of traffic accidents. Cooperative Group Burgos-Santander N Engl J Med 1999;340:847–851 [DOI] [PubMed] [Google Scholar]
- 2. Yaggi HK, Concato J, Kernan WN, Lichtman JH, Brass LM, Mohsenin V. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med 2005;353:2034–2041 [DOI] [PubMed] [Google Scholar]
- 3. Chervin RD. Sleepiness, fatigue, tiredness, and lack of energy in obstructive sleep apnea. Chest 2000;118:372–379 [DOI] [PubMed] [Google Scholar]
- 4. Jenkinson C, Stradling J, Petersen S. Comparison of three measures of quality of life outcome in the evaluation of continuous positive airways pressure therapy for sleep apnoea. J Sleep Res 1997;6:199–204 [DOI] [PubMed] [Google Scholar]
- 5. Yang EH, Hla KM, McHorney CA, Havighurst T, Badr MS, Weber S. Sleep apnea and quality of life. Sleep 2000;23:535–541 [PubMed] [Google Scholar]
- 6. Janardhan V, Bakshi R. Quality of life in patients with multiple sclerosis: the impact of fatigue and depression. J Neurol Sci 2002;205:51–58 [DOI] [PubMed] [Google Scholar]
- 7. Veauthier C, Radbruch H, Gaede G, et al. Fatigue in multiple sclerosis is closely related to sleep disorders: a polysomnographic cross-sectional study. Mult Scler 2011;17:613–622 [DOI] [PubMed] [Google Scholar]
- 8. Ajayi OF, Chang-McDowell T-Y, Culpepper WJ, 2nd, Royal W, Bever C. High prevalence of sleep disorders in veterans with multiple sclerosis. Neurology 2008;50;141 [Google Scholar]
- 9. Tachibana N, Howard RS, Hirsch NP, Miller DH, Moseley IF, Fish D. Sleep problems in multiple sclerosis. Eur Neurol 1994;34:320–323 [DOI] [PubMed] [Google Scholar]
- 10. Iber C, Ancoli-Israel S, Chesson AL, Jr, American Academy of Sleep Medicine The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. Westchester, IL: American Academy of Sleep Medicine; 2007 [Google Scholar]
- 11. Rechtshaffen A, Kales A. A Manual of Standardized Terminology, Techniques and Scoring System for Sleep Stages of Human Subjects. Los Angeles: Brain Information Service/ Brain Research Institute, UCLA; 1968 [Google Scholar]
- 12. Deleted in proof.
- 13. Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The report of an American Academy of Sleep Medicine Task Force. Sleep 1999;22:667–689 [PubMed] [Google Scholar]
- 14. White DP. Central sleep apnea. Med Clin North Am 1985;69:1205–1219 [DOI] [PubMed] [Google Scholar]
- 15. Krupp LB, Alvarez LA, LaRocca NG, Scheinberg LC. Fatigue in multiple sclerosis. Arch Neurol 1988;45:435–437 [DOI] [PubMed] [Google Scholar]
- 16. Guilleminault C, Tilkian A, Dement WC. The sleep apnea syndromes. Annu Rev Med 1976;27:465–484 [DOI] [PubMed] [Google Scholar]
- 17. Friberg D. Heavy snorer's disease: a progressive local neuropathy. Acta Otolaryngol 1999;119:925–933 [DOI] [PubMed] [Google Scholar]
- 18. Hwang JC, St John WM, Bartlett D., Jr. Receptors responding to changes in upper airway pressure. Respir Physiol 1984;55:355–366 [DOI] [PubMed] [Google Scholar]
- 19. Fogel RB, Malhotra A, Pillar G, et al. Genioglossal activation in patients with obstructive sleep apnea versus control subjects: mechanisms of muscle control. Am J Respir Crit Care Med 2001;164:2025–2030 [DOI] [PubMed] [Google Scholar]
- 20. Levitt P, Cohn MA. Sleep apnea and the Chiari I malformation: case report. Neurosurgery 1988;23:508–510 [DOI] [PubMed] [Google Scholar]
- 21. Lam B, Ryan CF. Arnold-Chiari malformation presenting as sleep apnea syndrome. Sleep Med 2000;1:139–144 [DOI] [PubMed] [Google Scholar]
- 22. Pasterkamp H, Cardoso ER, Booth FA. Obstructive sleep apnea leading to increased intracranial pressure in a patient with hydrocephalus and syringomyelia. Chest 1989;95:1064–1067 [DOI] [PubMed] [Google Scholar]
- 23. Rabec C, Laurent G, Baudouin N, et al. Central sleep apnoea in Arnold-Chiari malformation: evidence of pathophysiological heterogeneity. Eur Respir J 1998;12:1482–1485 [DOI] [PubMed] [Google Scholar]
- 24. Ely EW, McCall WV, Haponik EF. Multifactorial obstructive sleep apnea in a patient with Chiari malformation. J Neurol Sci 1994;126:232–236 [DOI] [PubMed] [Google Scholar]
- 25. Doherty MJ, Spence DP, Young C, Calverley PM. Obstructive sleep apnoea with Arnold-Chiari malformation. Thorax 1995;50:690–691; discussion 696−697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chaudhary BA, Elguindi AS, King DW. Obstructive sleep apnea after lateral medullary syndrome. South Med J 1982;75:65–67 [DOI] [PubMed] [Google Scholar]
- 27. Askenasy JJ, Goldhammer I. Sleep apnea as a feature of bulbar stroke. Stroke 1988;19:637–639 [DOI] [PubMed] [Google Scholar]
- 28. Ferini-Strambi L, Filippi M, Martinelli V, et al. Nocturnal sleep study in multiple sclerosis: correlations with clinical and brain magnetic resonance imaging findings. J Neurol Sci 1994;125:194–197 [DOI] [PubMed] [Google Scholar]
- 29. Auer RN, Rowlands CG, Perry SF, Remmers JE. Multiple sclerosis with medullary plaques and fatal sleep apnea (Ondine's curse). Clin Neuropathol 1996;15:101–105 [PubMed] [Google Scholar]
- 30. Alberti A, Sarchielli P, Gallinella E, Floridi A, Mazzotta G, Gallai V. Plasma cytokine levels in patients with obstructive sleep apnea syndrome: a preliminary study. J Sleep Res 2003;12:305–311 [DOI] [PubMed] [Google Scholar]
- 31. Vgontzas AN, Zoumakis E, Lin HM, Bixler EO, Trakada G, Chrousos GP. Marked decrease in sleepiness in patients with sleep apnea by etanercept, a tumor necrosis factor-α antagonist. J Clin Endocrinol Metab 2004;89:4409–4413 [DOI] [PubMed] [Google Scholar]
- 32. Salloum A, Rowley JA, Mateika JH, Chowdhuri S, Omran Q, Badr MS. Increased propensity for central apnea in patients with obstructive sleep apnea: effect of nasal continuous positive airway pressure. Am J Respir Crit Care Med 2010;181:189–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dernaika T, Tawk M, Nazir S, Younis W, Kinasewitz GT. The significance and outcome of continuous positive airway pressure-related central sleep apnea during split-night sleep studies. Chest 2007;132:81–87 [DOI] [PubMed] [Google Scholar]
- 34. Chottinaiwattarakul W, O'Brien L, Chervin R. Fatigue, tiredness and lack of energy improve with treatment for OSA. J Clin Sleep Med 2009;5:222–227 [PMC free article] [PubMed] [Google Scholar]