Abstract
Objective:
It has been hypothesized that individuals without dementia with Alzheimer disease (AD) neuropathology may be in the preclinical stages of dementia and could be experiencing subtle cognitive decline. The purpose of this study was to compare longitudinal cognitive performance in oldest-old individuals without dementia with and without AD neuropathology.
Methods:
The study included 58 individuals without dementia from The 90+ Autopsy Study, a population-based study of aging and dementia in individuals aged 90 and older. Participants had neurologic and neuropsychological testing every 6 months with an average of 3 years of follow-up. We compared the trajectory of cognitive performance on the Modified Mini-Mental State Examination (3MS) and the California Verbal Learning Test II (CVLT) by level of AD neuropathology. Based on Consortium to Establish a Registry for Alzheimer's Disease plaque staging, individuals were categorized as having low (none or sparse) or high (moderate or frequent) plaques. Based on Braak and Braak staging, participants were classified as having low (stages I–III) or high (IV–VI) tangles.
Results:
No significant differences were found in 3MS or CVLT cognitive performance over time based on plaque or tangle staging. Both high and low pathology groups showed modest improvements on the 3MS and CVLT consistent with learning effects.
Conclusions:
AD neuropathology at autopsy is not associated with the trajectory of cognitive performance in the 3 years before death in oldest-old without dementia. Despite the presence of AD neuropathology at death, oldest-old without dementia display learning effects on cognitive tests. Further research is necessary to understand factors other than AD neuropathology that may affect cognition in the oldest-old.
Studies of Alzheimer disease (AD) neuropathology and cognition1–4 suggest poorer cognitive performance and more rapid cognitive decline in individuals with high levels of amyloid plaques and neurofibrillary tangles. It has been hypothesized that individuals without dementia with AD neuropathology may be in the preclinical stages of disease and experiencing subtle cognitive decline. The percentage of individuals without dementia with AD neuropathology increases with age.5–7 Findings from The 90+ Study have shown that approximately 50% of individuals without clinical dementia have high levels of AD neuropathology.8 For this investigation, we examined declines in cognitive performance and AD neuropathology among oldest-old without dementia.
Longitudinal cognitive performance in late adulthood has primarily been described with linear trends and modeled with rates of cognitive decline in individuals with and without dementia. Linear trends may not be appropriate for describing cognitive trajectories due to learning and other effects. The main objective of the current study was to compare cognitive performance over time in oldest-old individuals without dementia with varying degrees of AD neuropathology (plaques or tangles), using models that do not assume linear cognitive trajectories.
METHODS
Study population (subjects).
The 90+ Autopsy Study is an ancillary study of The 90+ Study, a population-based investigation of aging and dementia in individuals aged 90 years and older. As of April 2011, we obtained brains from 137 individuals participating in The 90+ Autopsy Study. Of these participants, 58 individuals were without dementia at death, and had completed cognitive testing at 1 or more visits.
Assessments.
All participants received a neurologic examination and neuropsychological testing every 6 months. Medical histories and medication usage were gathered at each visit. Medical records, including brain scans, were requested from the participant's physicians. Interviews with informants were also used to determine the participant's cognitive and functional status.
Cognitive testing.
A neuropsychological test battery was administered to participants to assess various cognitive domains including global cognition and memory. In the present study, global cognition (Modified Mini-Mental State Examination [3MS])9 and memory (10 minute California Verbal Learning Test–II Long Delay [CVLT])10 were used for the primary cognitive outcomes.
For this study, individual test scores were converted to z scores for comparison across cognitive measures. z Scores for each cognitive measure at each visit were computed using the same baseline test scores (mean and SD) among all participants without dementia in the entire cohort (3MS: mean = 89.23, SD = 8.11 and CVLT: mean = 5.08, SD = 2.67).
Standard protocol approvals, registrations, and patient consents.
All procedures were approved by the Institutional Review Board at the University of California, Irvine, and all participants gave written informed consent.
Determination of cognitive status.
After a participant died, all available information including participants' longitudinal evaluations, medical records, and informant information were discussed during a multidisciplinary diagnostic consensus conference. Participants were assigned a cognitive diagnosis of normal, cognitive impairment with no dementia (CIND), or dementia. Dementia diagnosis was established using DSM-IV criteria. CIND diagnosis was assigned to individuals with cognitive or functional loss that did not meet criteria for dementia. Investigators were blinded to the participants' pathologic evaluation when assigning cognitive diagnoses.
Neuropathologic measures and diagnosis.
Postmortem brain tissue procurement and preparation was done according to procedures from the Alzheimer's Disease Research Center (ADRC) protocols. Neuritic plaque staging (none, sparse, moderate, or frequent) was determined based on Consortium to Establish a Registry for Alzheimer's Disease (CERAD) criteria.11 Neurofibrillary tangle staging (I–VI) was based on Braak and Braak criteria.12 For this study, individuals were categorized into 2 groups: low plaques (none or sparse) or high plaques (moderate or frequent). Similarly, individuals were classified as having low tangles (I–III) or high tangles (IV–VI). Pathologic evaluations were performed blinded to clinical diagnoses.
Statistical analysis.
To assess the effect of AD neuropathology on cognitive performance over time we fitted 2 random effects models. Random effects models were used because these models can account for correlations that may exist across multiple measurements in the same individual across time.13 Model 1 included the intercept as a random effect, thus allowing the trajectory of cognitive performance to vary across individuals. Age at baseline visit was included as a fixed effect and modeled as a continuous variable. Dummy variables for each follow-up visit were created (Zi = 1 if visit i, 0 else) and included as fixed effects. The dummy visit variables represent the change in cognitive performance at each visit compared to the baseline visit. As most individuals did not have more than 7 visits, all visits 7 and higher were collapsed into the dummy variable for visit 7. Because change in cognitive performance is allowed to vary from visit to visit, model 1 does not impose a linear trajectory and allows for potential learning effects.
Model 2 included all variables from model 1 plus variables related to AD neuropathology. AD neuropathologies (plaques or tangles) were included as fixed effects and were coded as binary variables (0 = low, 1 = high). Model 2 also included interaction terms between each dummy visit variable and the AD neuropathology variable, which were modeled as fixed effects. The interaction terms represent the difference between low and high pathology groups in the change in cognitive performance from each person's follow-up visit compared to the baseline visit.
As the 2 models described were nested within one another, we compared the fit of the 2 models with a likelihood ratio test (LRT). This test allowed us to determine if the fit of the model that included AD neuropathology was significantly different from the model that did not include AD neuropathology.
The outcome variable in all of these models was the z score for the cognitive measure (3MS or CVLT). Analyses were done separately for each cognitive measure (3MS or CVLT) and for each AD neuropathology (plaques or tangles). Sex, education, and APOE were not significant in our initial analyses and were excluded from further analyses. All analyses were completed in SAS, version 9.2 (SAS Institute, Cary, NC). The SAS PROC MIXED procedure and maximum likelihood estimation option (METHOD = ML) were used to fit all models.
RESULTS
Descriptive characteristics for the 58 participants in this study are summarized in table 1. Participants were mostly women (67%) and had a mean age of 95 years at the first visit. On average, participants completed 6 visits over 3 years and the mean age at death was 98 years. Of all participants, 52% had a clinical diagnosis of normal cognition and 48% had a clinical diagnosis of CIND. In regards to AD neuropathology, 48% of the participants had high plaque neuropathology, 40% had high tangle neuropathology, and 29% had high levels of both neuropathologies. At the first visit, the mean 3MS score was 90 and the mean CVLT score was 6. Overall, autopsy participants did not differ from nonautopsy participants on baseline age, education, cognitive diagnosis, sex, or baseline cognitive performance.8
Table 1.
Descriptive characteristics of deceased participants without dementia in The 90+ Autopsy Study (n = 58)
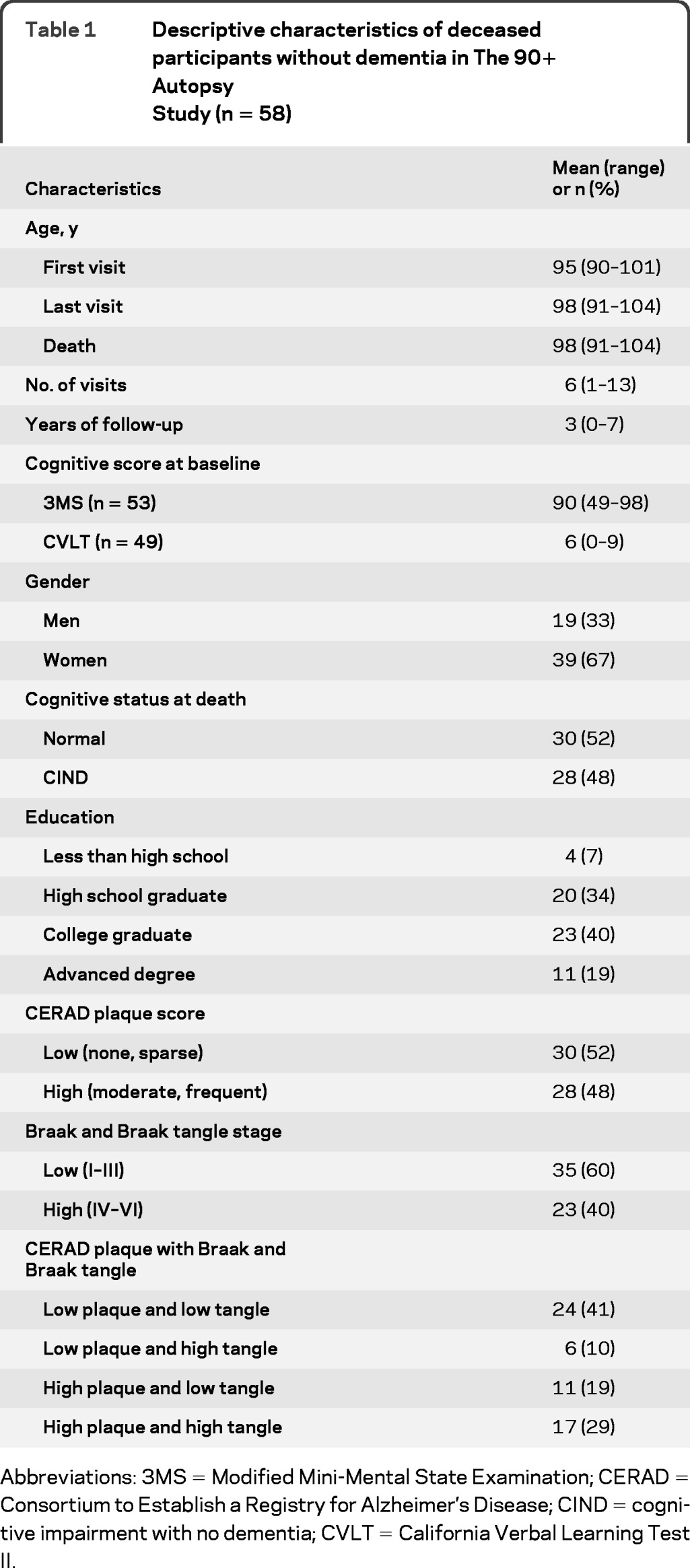
Abbreviations: 3MS = Modified Mini-Mental State Examination; CERAD = Consortium to Establish a Registry for Alzheimer's Disease; CIND = cognitive impairment with no dementia; CVLT = California Verbal Learning Test II.
There were no differences in trajectory of cognitive performance by AD neuropathology. Individuals with low and high levels of plaques or low and high levels of tangles had similar performance trajectories on the 3MS and CVLT. Regardless of the level of AD pathology, all individuals showed evidence of modest learning effects across visits. The parameter estimates, standard errors, and p values from the fully adjusted model with AD neuropathology (model 2) are reported in table 2.
Table 2.
Results from random effects model 2: pathology and cognitive performance among deceased 90+ autopsy participants without dementiaa

Abbreviations: 3MS = Modified Mini-Mental State Examination; CI = confidence interval; CVLT = California Verbal Learning Test II.
Each pathologic variable and cognitive test were analyzed in separate models.
Pathology is a dummy coded variable that refers to the level of plaques or tangles (0 = low, 1 = high).
Visit 2 to Visit 7+ refer to dummy coded visit variables (Visit i = 1 if ith visit, 0 else).
Visit 7+: Visits 7 and more were collapsed in dummy coded visit variable 7.
Plaque neuropathology.
There were no significant differences in cognitive performance on the 3MS or the CVLT based on plaque staging. Based on the t test for the parameter estimates from random effects model 2, individuals with high plaque pathology showed slightly worse cognitive test scores on the 3MS (β estimate = −0.17 units; p = 0.48) and CVLT (β estimate = −0.04 units; p = 0.88), although this difference was not significant. Similarly, the interactions between plaques and the dummy visit variables showed no differences across visits. Based on comparisons of model 1 to model 2, we found no significant differences in trajectory of cognitive performance on the 3MS (p = 0.55) or CVLT (p = 0.88) based on plaque staging (table 3). Thus, including plaque parameters in the statistical model did not explain better the observed trajectory of cognitive performance on the 3MS and CVLT.
Table 3.
Fit statistics for AD neuropathology and cognitive performancea
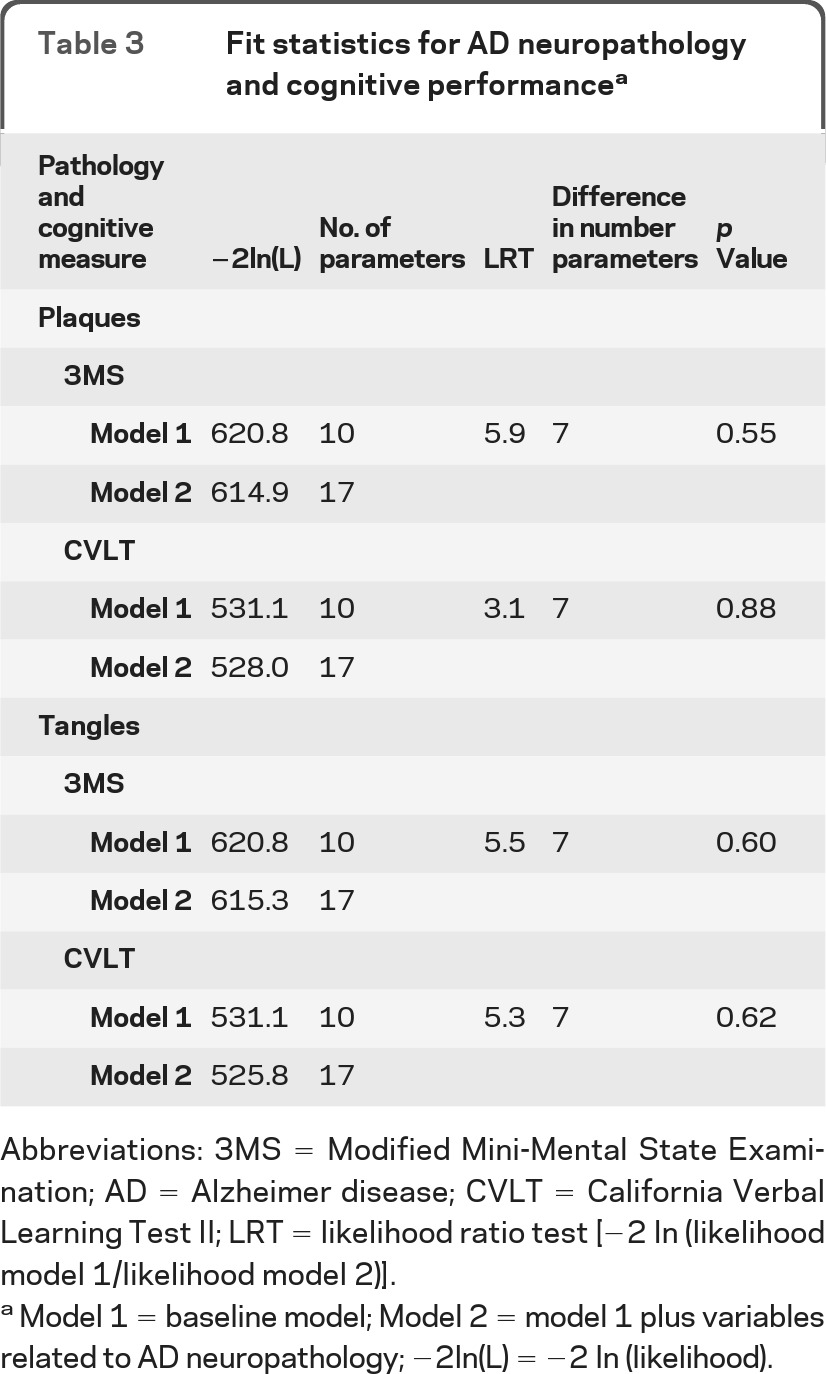
Abbreviations: 3MS = Modified Mini-Mental State Examination; AD = Alzheimer disease; CVLT = California Verbal Learning Test II; LRT = likelihood ratio test [−2 In (likelihood model 1/likelihood model 2)].
Model 1 = baseline model; Model 2 = model 1 plus variables related to AD neuropathology; −2ln(L) = −2 In (likelihood).
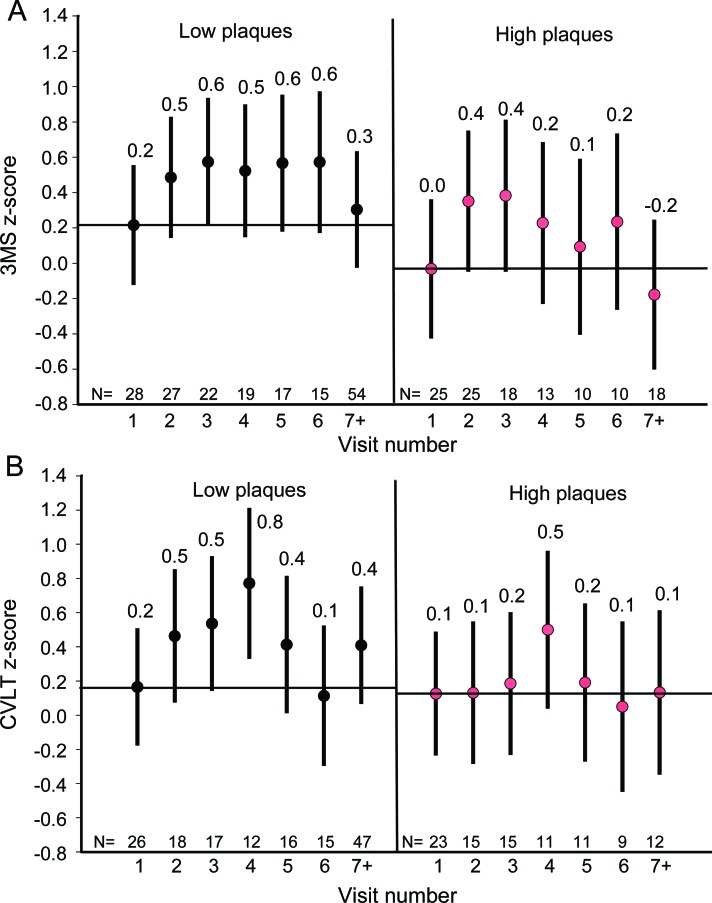
In general, participants showed improvement on the 3MS and the CVLT on subsequent visits compared to visit 1. For the 3MS, these improvements were either statistically significant or trended toward significance for all visits except visits 7 and higher (visit 7+). Although individuals with higher plaque pathology appeared to have somewhat smaller learning effects, as evidenced by the negative visit × plaque interaction terms, these effects were nonsignificant for the 3MS and CVLT. Based on the parameter estimates from model 2 we calculated the predicted cognitive z scores at each visit for a participant who was 94 years old at baseline (average baseline age) with and without pathology. See figure 1 for the described differences in overall trajectory of cognitive performance by plaque pathology and the observed learning effects.
Figure 1. Predicted cognitive scores at each visit by plaque group.
The predicted cognitive score at each visit for the Modified Mini-Mental State Examination (3MS) (A) and the California Verbal Learning Test II (CVLT) (B) was based on the parameter estimates derived from model 2 for an individual who was 94 years of age at baseline.
Tangle neuropathology.
There was no difference in 3MS or CVLT cognitive performance by tangle staging. Individuals with high tangle pathology showed slightly worse cognitive test performance on the 3MS (β estimate = −0.28 units; p = 0.27) and CVLT (β estimate = −0.37 units; p = 0.15), although these results were not significant. Similarly, the interactions between tangles and the dummy visit variables were not significant, suggestive of no differences in cognitive performance across visits based on tangle staging. Based on comparisons of model 1 to model 2, we found no significant differences in trajectory of cognitive performance on the 3MS (p = 0.60) or CVLT (p = 0.62) based on tangle staging (table 3). Thus, inclusion of the tangle parameters in the statistical model did not explain better the observed trajectory of cognitive performance on the 3MS or CVLT.
Similar to plaque pathology, both tangle neuropathology groups demonstrated learning effects, but these were not significant. See figure 2 for the described differences in overall trajectory of cognitive performance by tangle pathology level and the observed learning effects.
Figure 2. Predicted cognitive scores at each visit by tangle group.
The predicted cognitive score at each visit for the Modified Mini-Mental State Examination (3MS) (A) and the California Verbal Learning Test II (CVLT) (B) was based on the parameter estimates derived from model 2 for an individual who was 94 years of age at baseline.
We tested whether different categorizations of plaques and tangles would affect our results.
Results were similar when individuals with frequent CERAD plaques (12%) were compared to individuals with fewer plaques (3MS: p = 0.50 and CVLT: p = 0.19) or when individuals with Braak and Braak tangle stages V-VI (21%) were compared to individuals with lower stages (3MS: p = 0.13; CVLT: p = 0.85). We also repeated the statistical analyses by dropping the visits past visit 7 to see if the observed results were driven by cognitive test scores past visit 7. No significant differences in levels of cognitive performance were found for plaques (3MS: p = 0.54 and CVLT: p = 0.82) or tangles (3MS: p = 0.87 and CVLT: p = 0.41).
DISCUSSION
Individuals who died with high AD neuropathology had a similar trajectory of global cognition and memory performance before death when compared to individuals with low AD neuropathology. We found evidence of learning effects in these participants; however, these learning effects were greater in individuals with lower AD neuropathology.
The relationship between neuropathology and cognition may be different for the oldest-old compared to younger elderly. Alternatively, it is possible that our participants may have been in the preclinical stages of AD14,15 and cognitive decline may have become apparent if the participants had lived longer. Supporting this notion, individuals with high levels of plaques or high levels of tangles tended to have poorer cognitive test scores and smaller learning effects compared to participants with low level of plaques or low level of tangles, but these results were not significant.14,15 An earlier study suggests that AD risk can be identified up to 15 years prior to clinical diagnosis of AD based on tests of visual memory cognitive performance.15
Some investigations found no association between AD neuropathology and rates of global cognitive decline among elderly without dementia; however, these studies did find differences in memory performance.16,17 In one study, 134 individuals with no cognitive impairment or dementia before death were examined,17 and there was a significant relationship between AD neuropathology and declines in episodic memory suggesting that memory was more susceptible to changes in AD neuropathology. Similarly, another study examined cognitive performance in 48 clinically normal individuals with varying degrees of AD neuropathology at autopsy.16 Significant differences in rates of decline were only found on a memory test (cued selective reminding test). Declines in memory may serve as an earlier indication of preclinical dementia, although we were not able to detect this in our sample.
Many studies have found1–4 a relationship between AD neuropathology and rates of cognitive decline in older populations. Some of these studies, however, differ from our study in several important ways. First, some investigations1,4 have included individuals with dementia. Individuals already diagnosed with dementia could be more likely to have more rapid rates of cognitive decline and may be driving the association. Second, some of the studies2,3 examined cognitive performance at only one time point, precluding longitudinal assessment. Third, some studies were conducted in younger elderly individuals16 and the relationship between neuropathology and cognition may change with age. For example, in a recent study18 of younger elderly participants (mean baseline age: 79.8 years) who were followed 7.5 years on average, cognitively normal individuals with high levels of AD neuropathology showed significant declines on cognitive tests of praxis, verbal fluency, and delayed word list. Finally, methodology varied among different studies.
Typically, studies of cognitive decline in the elderly have been quantified with a linear rate of change or have used change point analyses to tease apart terminal declines from age-related changes.19–21 In contrast to these studies, we examined differences in trajectories of cognitive performance by using a model that incorporated potential learning effects at subsequent visits in relation to baseline cognitive performance. This approach enabled us to examine differences in trajectories of cognitive performance resulting from differences in level of AD pathology without imposing linear trajectories. Learning effects on the MMSE and the CVLT would not be as evident if we used a model that derived a rate of change from linear slopes.
We found evidence of learning effects on cognitive tests in individuals with low and high AD neuropathology. These findings suggest that after age 90, individuals without dementia have the ability to learn and retain content from these cognitive tests particularly when given at frequent intervals. Three recent studies that examined learning effects in the elderly found differing results.22–24 Two of these investigations show that learning can occur over time, even in persons with MCI.23,24 Interestingly, the study with the oldest-old22 did not suggest significant learning effects. Frequency of testing (annually vs biannually), differences in cognitive tests (i.e., CERAD Recognition vs CVLT), and the statistical methodology (regression slopes and mean change vs random effects model) are all factors that may have contributed to the differences in observed learning effects between that study and our study.
The current study in The 90+ Autopsy Study participants is unique in several ways. Participants were without dementia and aged 90 and older throughout the entire study (at entry and at death). These individuals were followed longitudinally and given comprehensive neuropsychological examinations and neurologic evaluations biannually. In addition, the sample of this study is larger than some of the previous studies, particularly for the oldest-old population. The frequency of cognitive testing, every 6 months, enabled us to look at cognitive performance across a large number of visits.
Limitations of this study deserve attention. First, our investigation is in the oldest-old, limiting our ability to comment on younger elderly. Furthermore, The 90+ Study participants are predominantly Caucasian, and well-educated, from the community of Laguna Woods, in Orange County, CA. These characteristics limit our ability to generalize our findings to other ethnic and racial groups. However, a recent report from the Census Bureau on the oldest-old suggests that characteristics of 90+ Study participants are actually similar to those of the oldest-old in the United States,25 particularly in regards to sex and ethnicity. Second, it is possible that we did not have enough power to find a true association that did exist. Third, we may have found differences by AD pathology if we had examined other cognitive tests. Only the CVLT and 3MS were examined because we thought we would be more likely to find cognitive declines in memory and global cognition with respect to greater AD pathology. In addition, these 2 cognitive tests provided us with the most complete data. Factors such as sensory deficits and fatigue make it difficult for participants to complete the entire test battery. For instance, 72% of participants in The 90+ Study have sensory deficits (i.e., vision loss) and at least 20% of participants report being fatigued.26 Fourth, we did not examine comorbid pathologies such as hippocampal sclerosis or cerebrovascular disease given the low prevalence of these pathologies in our participants without dementia. Hippocampal sclerosis is not found in the brains of our participants without dementia and less than 12% have infarcts at autopsy.8 Finally, we have recently shown in people without dementia that amyloid area shared a better relationship to cognition than CERAD plaque staging.27 This finding suggests that amyloid area, compared to CERAD plaque staging, may be a better measure of plaque neuropathology in the oldest-old.
The present study shows that in oldest-old without dementia, AD neuropathology is common, but not related to trajectories of cognitive performance over 3 years. These findings suggest cognition may not be affected by high levels of AD neuropathology in the oldest-old. It is possible that oldest-old individuals have the ability to withstand and tolerate the adverse effects associated with AD neuropathology. Alternatively, perhaps these individuals did not live long enough to develop the clinical effects of AD neuropathology. Health and lifestyle factors as well as other brain pathologies may be relevant for the expression of cognitive performance in the oldest-old. Further research must be conducted to better understand the relationship between cognitive performance and AD neuropathology in this extreme age group.
GLOSSARY
- 3MS
Modified Mini-Mental State Examination
- AD
Alzheimer disease
- ADRC
Alzheimer's Disease Research Center
- CERAD
Consortium to Establish a Registry for Alzheimer's Disease
- CIND
cognitive impairment with no dementia
- CVLT
California Verbal Learning Test II
- DSM-IV
Diagnostic and Statistical Manual of Mental Disorders, 4th edition
- LRT
likelihood ratio test
AUTHOR CONTRIBUTIONS
Statistical analyses: Archana B. Balasubramanian, PhD. Interpretation of data: Archana B. Balasubramanian, PhD, Claudia H. Kawas, MD, Carrie B. Peltz, PhD, Ron Brookmeyer, PhD, María M. Corrada, ScD. Drafting/revision of manuscript: Archana B. Balasubramanian, PhD, Claudia H. Kawas, MD, Carrie B. Peltz, PhD, Ron Brookmeyer, PhD, María M. Corrada, ScD.
DISCLOSURE
The authors report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1. Green MS, Kaye JA, Ball MJ. The Oregon Brain Aging Study: neuropathology accompanying healthy aging in the oldest old. Neurology 2000;54:105–113 [DOI] [PubMed] [Google Scholar]
- 2. Hulette CM, Welsh-Bohmer KA, Murray MG, Saunders AM, Mash DC, McIntyre LM. Neuropathological and neuropsychological changes in “normal” aging: evidence for preclinical Alzheimer disease in cognitively normal individuals. J Neuropathol Exp Neurol 1998;57:1168–1174 [DOI] [PubMed] [Google Scholar]
- 3. Price JL, McKeel DW, Jr, Buckles VD, et al. Neuropathology of nondemented aging: presumptive evidence for preclinical Alzheimer disease. Neurobiol Aging 2009;30:1026–1036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wilson RS, Leurgans SE, Boyle PA, Schneider JA, Bennett DA. Neurodegenerative basis of age-related cognitive decline. Neurology 2010;75:1070–1078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Crystal H, Dickson D, Fuld P, et al. Clinico-pathologic studies in dementia: nondemented subjects with pathologically confirmed Alzheimer's disease. Neurology 1988;38:1682–1687 [DOI] [PubMed] [Google Scholar]
- 6. Crystal HA, Dickson D, Davies P, Masur D, Grober E, Lipton RB. The relative frequency of “dementia of unknown etiology” increases with age and is nearly 50% in nonagenarians. Arch Neurol 2000;57:713–719 [DOI] [PubMed] [Google Scholar]
- 7. Savva GM, Wharton SB, Ince PG, Forster G, Matthews FE, Brayne C. Age, neuropathology, and dementia. N Engl J Med 2009;360:2302–2309 [DOI] [PubMed] [Google Scholar]
- 8. Corrada MM, Berlau DJ, Kawas CH. A population-based clinicopathological study in the oldest-old: The 90+ Study. Curr Alzheimer Res Epub 2012 Apr 2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Teng EL, Chui HC. The Modified Mini-Mental State (3MS) Examination. J Clin Psychiatry 1987;48:314–318 [PubMed] [Google Scholar]
- 10. Delis DC, Kramer JH, Kaplan E, Ober BA. California Verbal Learning Test (2nd ed.). San Antonio, TX: Psychological Corporation; 2000 [Google Scholar]
- 11. Mirra SS, Heyman A, McKeel D, et al. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD): part II: standardization of the neuropathologic assessment of Alzheimer's disease. Neurology 1991;41:479–486 [DOI] [PubMed] [Google Scholar]
- 12. Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol 1991;82:239–259 [DOI] [PubMed] [Google Scholar]
- 13. Diggle P, Liang KY, Zeger SL. Analysis of Longitudinal Data. New York: Oxford University Press; 1994 [Google Scholar]
- 14. Galvin JE, Powlishta KK, Wilkins K, et al. Predictors of preclinical Alzheimer disease and dementia: a clinicopathologic study. Arch Neurol 2005;62:758–765 [DOI] [PubMed] [Google Scholar]
- 15. Kawas CH, Corrada MM, Brookmeyer R, et al. Visual memory predicts Alzheimer's disease more than a decade before diagnosis. Neurology 2003;60:1089–1093 [DOI] [PubMed] [Google Scholar]
- 16. Driscoll I, Resnick SM, Troncoso JC, An Y, O'Brien R, Zonderman AB. Impact of Alzheimer's pathology on cognitive trajectories in nondemented elderly. Ann Neurol 2006;60:688–695 [DOI] [PubMed] [Google Scholar]
- 17. Bennett DA, Schneider JA, Arvanitakis Z, et al. Neuropathology of older persons without cognitive impairment from two community-based studies. Neurology 2006;66:1837–1844 [DOI] [PubMed] [Google Scholar]
- 18. Riley KP, Jicha GA, Davis D, et al. Prediction of preclinical Alzheimer's disease: longitudinal rates of change in cognition. J Alzheimers Dis 2011;25:707–717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sliwinski MJ, Stawski RS, Hall CB, Katz M, Verghese J, Lipton R. Distinguishing preterminal and terminal cognitive decline. Eur Psychologist 2006;11:172–181 [Google Scholar]
- 20. Thorvaldsson V, Hofer SM, Berg S, Skoog I, Sacuiu S, Johansson B. Onset of terminal decline in cognitive abilities in individuals without dementia. Neurology 2008;71:882–887 [DOI] [PubMed] [Google Scholar]
- 21. Wilson RS, Beck TL, Bienias JL, Bennett DA. Terminal cognitive decline: accelerated loss of cognition in the last years of life. Psychosom Med 2007;69:131–137 [DOI] [PubMed] [Google Scholar]
- 22. Hickman SE, Howieson DB, Dame A, Sexton G, Kaye J. Longitudinal analysis of the effects of the aging process on neuropsychological test performance in the healthy young-old and oldest-old. Dev Neuropsychol 2000;17:323–337 [DOI] [PubMed] [Google Scholar]
- 23. Dodge HH, Wang CN, Chang CC, Ganguli M. Terminal decline and practice effects in older adults without dementia: the MoVIES project. Neurology 2011;77:722–730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Duff K, Beglinger LJ, Moser DJ, Paulsen JS, Schultz SK, Arndt S. Predicting cognitive change in older adults: the relative contribution of practice effects. Arch Clin Neuropsychol 2010;25:81–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. He W, Muenchrath M. US Census Bureau, American Community Survey Reports, ACS-17, 90+ in the United States: 2006–2008: US Government Printing Office, Washington, DC, 2011. Available at: http://www.census.gov/prod/2011pubs/acs-17.pdf Accessed November 28, 2011 [Google Scholar]
- 26. Brumback-Peltz C, Balasubramanian AB, Corrada MM, Kawas CH. Diagnosing dementia in the oldest-old. Maturitas 2011;70:164–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Robinson JL, Geser F, Corrada MM, et al. Neocortical and hippocampal amyloid-beta and tau measures associate with dementia in the oldest-old. Brain 2011;134:3708–3715 [DOI] [PMC free article] [PubMed] [Google Scholar]