Abstract
Objective:
We developed the Recurrence and Recovery Questionnaire (RRQ) by converting the Pediatric Stroke Outcome Measure (PSOM) to a questionnaire for telephone interview and sought to validate the RRQ in a large cohort.
Method:
We analyzed parents' RRQ responses and same-day PSOM assessments for 232 children who had arterial ischemic stroke, cerebral sinovenous thrombosis, or presumed perinatal ischemic stroke. We assessed the agreement and consistency of the PSOM and RRQ, and we identified conditions that contributed to differences between the 2 measures. We tested selected factors as predictors of differences between the total PSOM and total RRQ (tPSOM and tRRQ) scores.
Results:
Median PSOM score was 1.5 and median RRQ score was 1.5. There was good agreement between tPSOM and tRRQ, and RRQ was a reliable estimator of PSOM at the total and component level. Preexisting neurologic deficits or chronic illnesses increased the difference between the tPSOM and tRRQ; the chronic illness effect was confirmed with univariate analysis.
Conclusions:
The RRQ can characterize poststroke function when a child cannot return for examination. While the RRQ is not identical to the PSOM, the 2 measures likely assess closely related aspects of recovery. The RRQ is particularly useful when assessing outcomes of large cohorts, and will be useful in performing long-term follow-up studies of pediatric stroke.
Pediatric stroke studies need measures to assess patient outcomes over time. Many outcome measures used for adult stroke1–3 are less useful with children. The adult tools assess functions that children typically do not perform, and do not account for the evolving functions that characterize child development. The Pediatric Stroke Outcome Measure (PSOM) has been used in multiple pediatric stroke studies,4–6 but can only be applied at a follow-up visit because a clinical examination is a key element of the PSOM. When the subject cannot return for a follow-up visit important outcome data are lost if one relies solely upon the PSOM. To date, no validated measure has been developed to assess poststroke function if a child cannot be present at a follow-up visit.
Pediatric stroke is unique since a child's caregiver can report a child's functional status and limitations. A measure that can take advantage of a caregiver's report would be particularly useful to capture data over extended follow-up times. We developed the Recovery and Recurrence Questionnaire (RRQ) to assess a child's poststroke function by telephone interview.7 The RRQ was based upon the 5 functional categories measured by the PSOM. In a small pilot study the RRQ results correlated with a validated quality of life measure7 but the RRQ has never been validated in a larger cohort.
This study assessed the RRQ as an estimator of the PSOM in a cohort of children by comparing the estimates of function provided by the RRQ with the PSOM measures generated during a neurologic examination.
METHODS
The International Pediatric Stroke Study (IPSS) is a multinational registry that has collected standardized data on subjects with pediatric ischemic stroke since January 2003. Subjects were included if they had arterial ischemic stroke, sinovenous thrombosis, or presumed perinatal arterial ischemic stroke, since these are the commonly recognized cerebral ischemia diagnoses in children.8,9 The current report examined cases that were submitted to the IPSS up to February 2010.
The PSOM (appendices e-1 and e-2 on the Neurology® Web site at www.neurology.org: measures 1 and 2) has been described previously4 and a validation study was recently performed.10 Briefly, the PSOM measures poststroke neurologic function in 5 domains: right sensorimotor, left sensorimotor, language comprehension, language production, and cognitive/behavioral function. Function is ranked on an ordinal 4-point scale—0 (no deficit), 0.5 (mild deficit), 1 (moderate deficit), 2 (severe deficit)—so that a total score ranges from 0 to 10, with 0 indicating normal function. The PSOM was developed so a trained examiner can perform the assessment during the course of a clinical visit.
The RRQ (appendix e-3: questionnaire) was developed by adapting the final summary of the PSOM for use by telephone interview. The RRQ retained the 5 subcomponent features of the PSOM and the ordinal 4-point severity rating scale. It adapted the terminology and descriptors for each subcomponent into language suitable for lay respondents including parents of children, or older children with stroke. For example, the RRQ asks whether the child has any problems with strength, coordination, or sensation as a result of the stroke and whether the right or left side is affected. The PSOM queries whether the child has a sensorimotor deficit affecting the right or left side on clinical examination. The language production, language comprehension, and cognition/behavior elements of the RRQ similarly parallel the PSOM queries of the clinical examination. The second part of the survey asked about stroke recurrence. Investigators in the IPSS network began using the RRQ in 2007 and 2008 so that after 2007 cases were reported to the IPSS that had both a PSOM and an RRQ.
The PSOM scores were recorded by the patient's neurologist during routine clinic visits. Most RRQ scores were performed by face-to-face interview by clinical staff or a research assistant on the day of the clinic visit. In a few cases the RRQ was performed by telephone interview by research staff. Most of the clinical or research staff who recorded the RRQs were blinded to the PSOM scores (75%), while a smaller number were aware of the PSOM score (14%) or in some cases the blinding status was not recorded (11%). Some subjects had multiple pairs of PSOM-RRQ assessments so we only analyzed the most recent PSOM-RRQ pair. The PSOM and RRQ scores were submitted by 14 centers from North America, South America, and Europe to the IPSS data center.
Patients were included if they presented with stroke symptoms between ages 30 days and 18 years and had a confirmed arterial ischemic stroke or sinovenous thrombosis on neuroimaging. Children who had presumed perinatal ischemic stroke did not have acute symptoms, but typically were identified because of hemiparesis, seizures, or developmental delay detected in early infancy.
Data analysis.
We used graphical analysis to test the agreement between the PSOM and RRQ total scores (tPSOM and tRRQ).11 We then assessed the correlation of the PSOM and RRQ total measures and their 5 components (right and left sensorimotor, language comprehension and production, cognition/behavior) with internal consistency, intraclass correlation, and ordinal association analysis.12 In preliminary examination of the tPSOM and tRRQ data, we noted that the difference tPSOM − tRRQ (tPSOM-tRRQ) was greater in subjects with preexisting conditions (chronic illness or neurologic abnormalities) so we used frequency distributions to evaluate the effect of prestroke chronic illnesses or neurologic abnormalities upon tPSOM-tRRQ. We used linear regression to determine how well tRRQ “predicted” tPSOM. Regression, analysis of variance, and nonparametric tests were used to examine the relationship between potential continuous (age at stroke, age at examination, time between stroke and examination) and categorical (gender, study site, and presence of selected illnesses) predictors upon tPSOM-tRRQ. We did not examine the effect of preexisting neurologic deficits in these analyses because of the number of cases with missing data. Because the PSOM and RRQ component scores were ordinal in nature we used the Mann-Whitney test to examine the effect of potential propensity factors.
All statistical analyses were performed using IBM SPSS 19.0 (www.SPSS.com, Somers, NY). This work was approved by the ethical review boards of the contributing centers.
RESULTS
As of February 1, 2010, there were 681 unique individuals who had a PSOM assessment. Of these, 232 had concurrent PSOMs and RRQs and comprise the subjects of this analysis. There were 130 male subjects (56%). Acute arterial ischemic stroke occurred in 195 cases (84%), 18 had cerebral sinovenous thrombosis (8%), and 21 had presumed perinatal ischemic stroke (9%). One child with arterial ischemic stroke had cerebral sinovenous thrombosis and one child with presumed perinatal ischemic stroke had a second arterial ischemic stroke. The median age at stroke onset was 2.7 years (range 0.0–17.8) and the median age at assessment was 5.1 years (range 0.1–19.4). Thirty-two (14%) had recurrent ischemic events, either TIA or stroke. There was no stroke recurrence in 197 (85%) and 3 (1%) had an unknown recurrence status. The median tPSOM score was 1.5 (interquartile range 1.0–3.0) and 80% of subjects had a tPSOM of 3.5 or less. The median tRRQ score was 1.5 (interquartile range 0.5–3.0) and 84% of subjects had a tRRQ of 3.5 or less (table e-1).
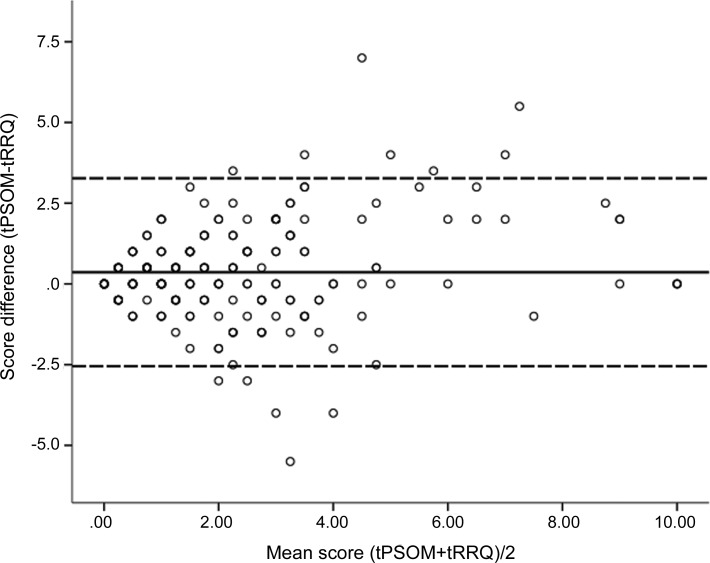
There was good agreement between the tPSOM and tRRQ scores, as demonstrated by graphical analysis (figure 1). To test the reliability of the RRQ as an estimator of the PSOM, we assessed internal consistency and intraclass correlation (ICC) for total scores and the 5 component scores (left and right sensorimotor, language production, language comprehension, and cognitive/behavior function). The internal consistency was excellent for tPSOM and tRRQ scores (table 1),3,13 good for the right and left sensorimotor function and language production components, and moderate for the language comprehension and cognition/behavior components. The ICC results for the tPSOM and tRRQ scores and for the component measures were similar. Ordinal association analysis showed that tRRQ and the 5 component scores were associated with the corresponding PSOM scores with the strength of association ranging from moderate for language comprehension (0.50) to strong for sensorimotor function (0.71).
Figure 1. A Bland-Altman plot demonstrates agreement between Recurrence and Recovery Questionnaire total score (tRRQ) and Pediatric Stroke Outcome Measure total score (tPSOM).
The mean of the tPSOM and RRQ scores is plotted on the X-axis while the difference between tPSOM and tRRQ (tPSOM-tRRQ) is plotted on the Y-axis. The overlying circles represent multiple subjects per data point. The solid line identifies the mean value. The dashed lines identify ± 2 SD. No. = 232.
Table 1.
Reliability of RRQ as an estimator of PSOM total and subcomponent scores
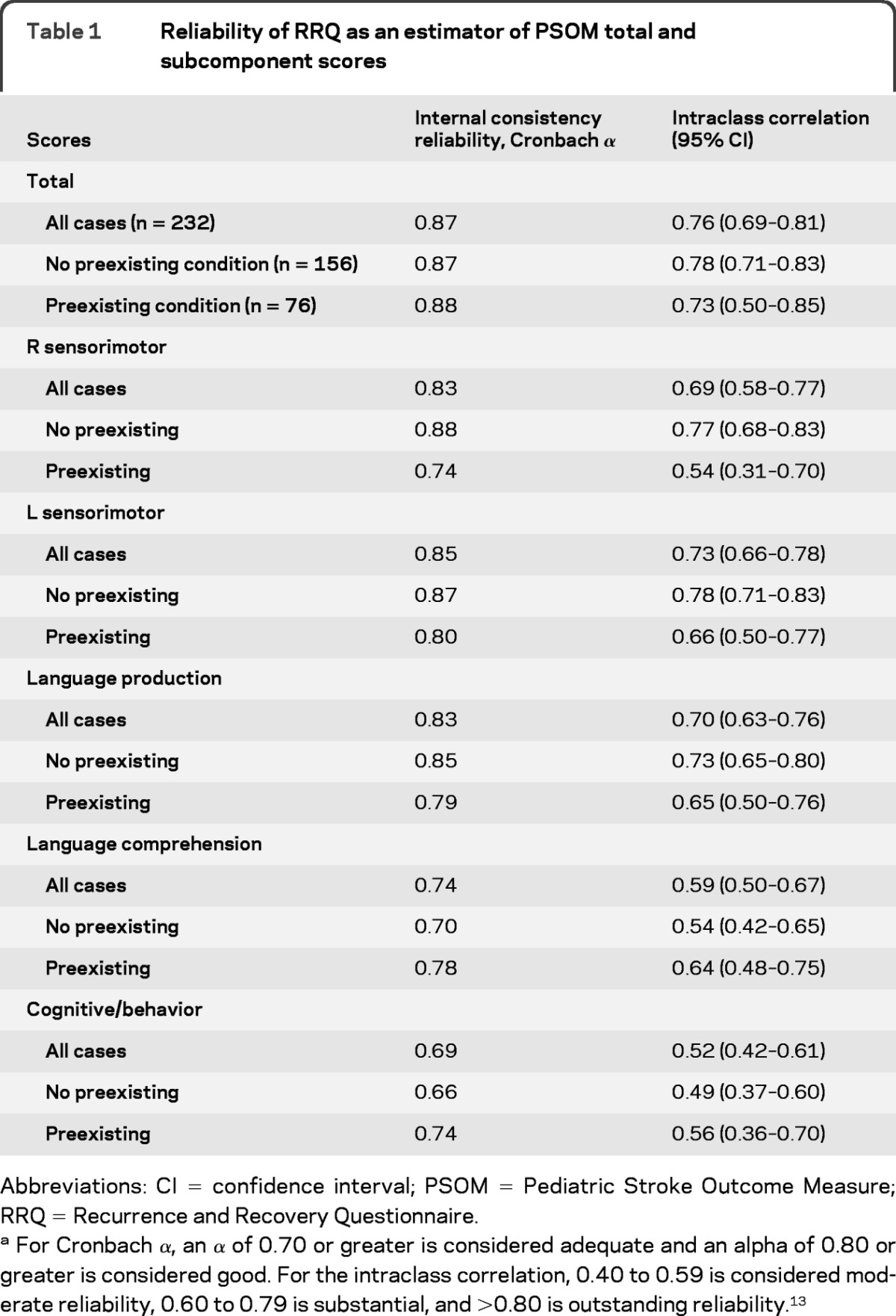
Abbreviations: CI = confidence interval; PSOM = Pediatric Stroke Outcome Measure; RRQ = Recurrence and Recovery Questionnaire.
For Cronbach α, an α of 0.70 or greater is considered adequate and an alpha of 0.80 or greater is considered good. For the intraclass correlation, 0.40 to 0.59 is considered moderate reliability, 0.60 to 0.79 is substantial, and >0.80 is outstanding reliability.13
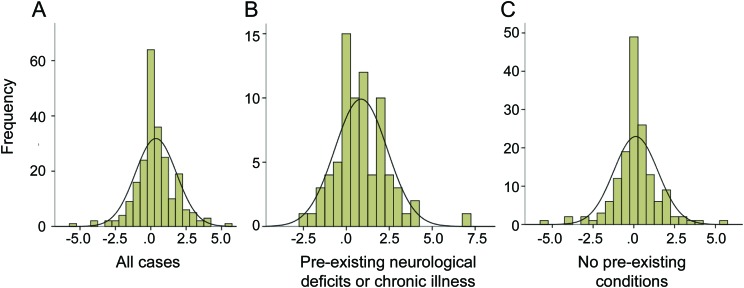
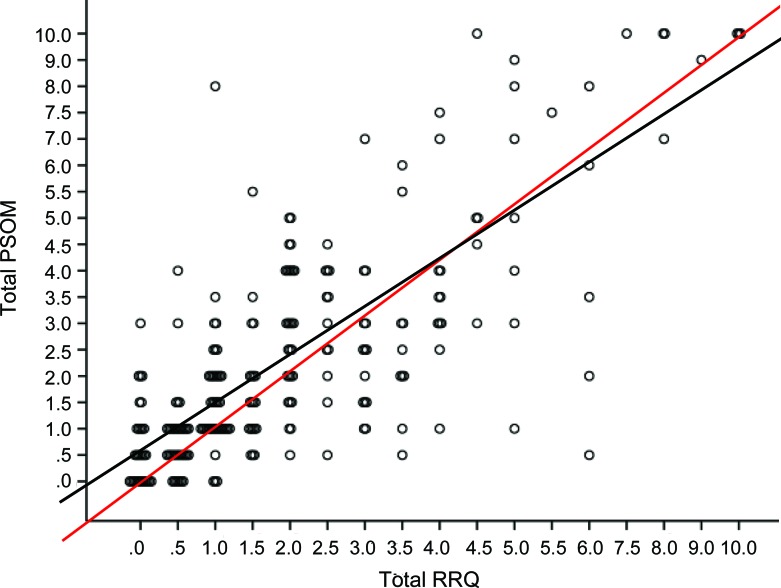
A frequency distribution plot of the difference between the tPSOM and tRRQ (tPSOM-tRRQ) (figure 2A) indicated that total PSOM scores were greater than total RRQ scores. This shift was greater in subjects who had preexisting neurologic deficits or chronic illnesses (figure 2B) and negligible in subjects who had no preexisting conditions (figure 2C). When we re-examined the reliability of RRQ as a predictor of PSOM component scores in the context of preexisting conditions we found a mixed effect (table 1). Cases with preexisting conditions had lower internal consistency for sensorimotor function and language production while cases without preexisting conditions had lower internal consistency for language production and cognitive/behavior function. The ICCs followed a similar pattern with lower ICCs for subjects with preexisting conditions in the sensorimotor and language production components, and lower ICCs for language comprehension and cognitive/behavior components in those subjects without preexisting conditions. tRRQ significantly predicted tPSOM (p < 0.001) in linear regression (figure 3).
Figure 2. Frequency plots for Pediatric Stroke Outcome Measure total score (tPSOM)–Recurrence and Recovery Questionnaire total score (tRRQ).
Note the different ranges on the respective Y axes. The smooth line represents the hypothetical normal distribution. (A) All cases. Mean 0.36, SD 1.453, n = 232. (B) Cases with preexisting neurologic deficits or chronic illness. Mean 0.86, SD 1.529, n = 76. (C) Cases without preexisting conditions. Mean 0.12, SD 1.355, n = 156.
Figure 3. Scatterplot and linear regression of Recurrence and Recovery Questionnaire total score (tRRQ) vs Pediatric Stroke Outcome Measure total score (tPSOM).
The overlying circles represent multiple subjects per data point. The dashed line is the line of identity. The solid line is the regression line fitting the data. tPSOM = (0.90 × tRRQ) + 0.55, R2 = 0.61, p < 0.001.
We found that a preexisting chronic illness predicted an increased difference in tPSOM-tRRQ (p < 0.001) (table 2). Other propensity factors, age at onset of stroke, age when stroke was recorded, age at examination, or time from stroke to examination did not predict significant tPSOM-tRRQ difference. At the level of the RRQ and PSOM subcomponent scores, prior chronic illness predicted an increased PSOM-RRQ difference for the right sensorimotor and cognitive-behavior subcomponents. Male gender, age at stroke, age when stroke was recorded, and age at examination predicted an increased PSOM-RRQ difference for the language comprehension subcomponent. When these 4 factors were analyzed with a general linear model, only male gender remained significant (p = 0.018).Other factors were not associated with subcomponent score differences. There were significant differences in tPSOM-tRRQ based upon study site largely because of significantly higher tPSOM scores from 2 sites. There were no site-related differences for tRRQ (table e-2). When the tPSOM and tRRQ were analyzed by blinding status, there were no significant differences.
Table 2.
Selected propensity and age/time factors predict greater differences between PSOM and RRQ total and subcomponent scores
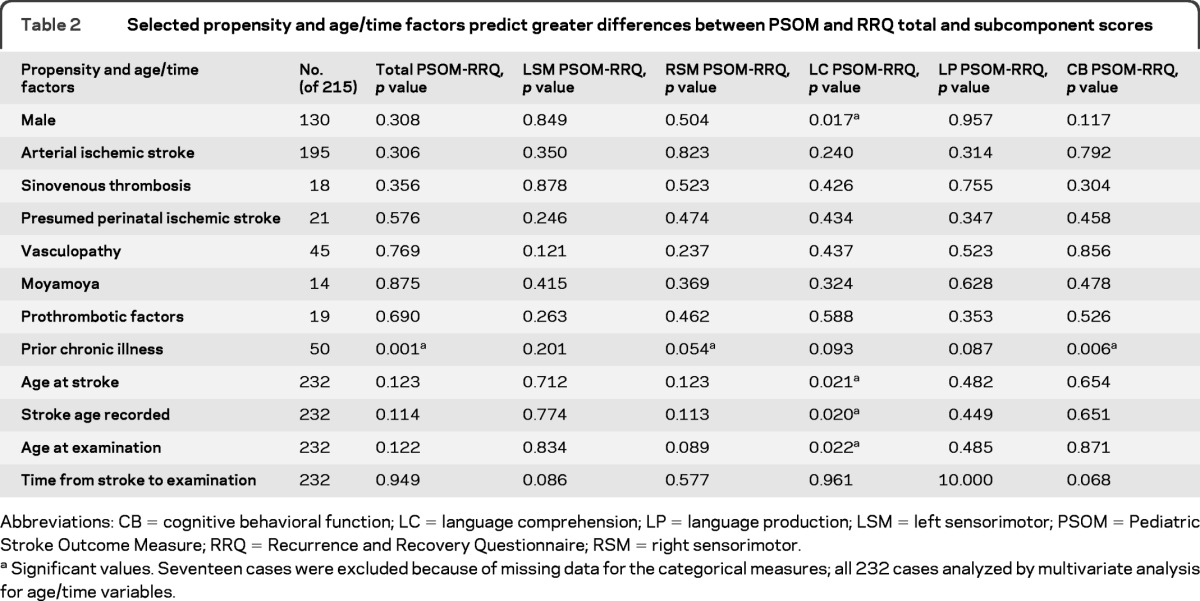
Abbreviations: CB = cognitive behavioral function; LC = language comprehension; LP = language production; LSM = left sensorimotor; PSOM = Pediatric Stroke Outcome Measure; RRQ = Recurrence and Recovery Questionnaire; RSM = right sensorimotor.
Significant values. Seventeen cases were excluded because of missing data for the categorical measures; all 232 cases analyzed by multivariate analysis for age/time variables.
DISCUSSION
Pediatric stroke patients often receive care at centers that may be far from their homes.14 The most widely used pediatric outcome measure, the PSOM, requires that the patient is physically present for an assessment. Another measure, the Pediatric Evaluation of Disability Inventory, has been used in stroke studies,15 but this also requires direct examination. When follow-up is difficult because of distance or if a child cannot be examined at a given time point, then valuable follow-up data may be lost. For this reason we developed the RRQ as an abbreviated version of the PSOM that could be performed via interview.
In a small pilot study7 we observed that the RRQ seemed to yield results consistent with a widely used quality of life measure. The current study has assessed the validity of the RRQ scores in a large cohort that had a concurrently performed PSOM. For the overall group there was good agreement between the tRRQ and tPSOM scores; indeed, the regression plot of tRRQ vs tPSOM nearly overlaid the line of identity. At the individual subject level the tRRQ and tPSOM scores varied, sometimes to a substantial degree, but 64% of PSOM scores were within 1 point of the RRQ scores. Since the RRQ was modified from the PSOM, the RRQ shares many of the face and content validity features of the PSOM.16 The PSOM is the current gold standard in pediatric stroke, so the regression results suggest that the RRQ has criterion and convergent validity compared with the PSOM. In terms of other clinimetric properties of the RRQ,1–3 there was good reliability between the total RRQ and PSOM, and the subcomponent right and left sensorimotor function and language production scores. Reliability for the language comprehension and cognition/behavior subcomponents was weaker, but still fell within the moderate range. We note that for language comprehension and cognition/behavior reliability was greater in subjects who had preexisting chronic illness or neurologic deficits than in subjects who were not affected; the reasons for this finding are not apparent. Two factors, preexisting chronic illness and gender, predicted increased differences between tPSOM and tRRQ or their subcomponent elements, but most factors had no effect.
In adults, telephone interviews have been used to assess poststroke outcomes and in some cases the telephone results compared favorably with face-to-face assessments (modified Rankin Scale after subarachnoid hemorrhage17 and Barthel Index after stroke18). The use of a patient proxy to report adult stroke outcome was performed using the Stroke Impact Scale (SIS).19 The SIS study found that patient proxies tended to report a greater degree of stroke severity than the patient's self-report, which raises the question whether a parent can reliably rate the severity of the child's deficit. The current study shows that parents can equally over- or under-rate the severity of the child's poststroke deficit (figure 2A). Since 64% of the PSOM scores were within 1 point of the RRQ scores (53% of PSOM scores were within one-half point), we conclude that at the cohort level, parental report can be used to estimate a child's poststroke function.
There are a number of limitations to this study. The RRQ scores were determined concurrently with the PSOM in patients who were clinically stable. We did not attempt to assess the RRQ in patients whose poststroke symptoms were evolving. In a retrospective study we were not able to test whether the RRQ was stable over time, i.e., whether an RRQ at time A accurately predicts an RRQ or PSOM at time A+B, and we were unable to assess test-retest validity or interobserver reliability.16 An obvious limitation is that while the RRQ correlates well with the PSOM for the group as a whole, at the individual subject level there can be substantial variability between the PSOM and RRQ. This individual variability raises the possibility that the RRQ and PSOM are measuring 2 different albeit closely related phenomena. The RRQ may be more a measure of parental impression of recovery while the PSOM is a measure of the neurologist's impression of recovery. This variation between the PSOM and parent reports of outcomes is similar to previous work with the PSOM. In an earlier study,4 the results of the PSOM moderately correlated with parental responses to 2 questions that were modified from the Euroqual measure. When there was disagreement between the PSOM and the 2 Euroqual responses, the parents equally over- or underestimated the subjects' outcomes in all but neonates with arterial ischemic stroke.
Caution should be applied to the interpretation of the RRQ. When there is a preexisting chronic illness the tRRQ tends to be smaller than the tPSOM at low scores. For all cases as the tRRQ increases to the high range of the scale, the tRRQ tends to overestimate the tPSOM. Multiple factors, such as age at onset or age at examination, did not significantly affect the tPSOM-tRRQ difference; however, we were unable to examine the effect of preexisting neurologic deficits in detail. The frequency distribution data suggest that neurologic deficits may contribute to the tRRQ underestimating the tPSOM.
Despite these limitations, the RRQ provides an important tool to assess a patient's poststroke function particularly when a subject cannot appear for a clinical assessment. While the RRQ does not supplant the PSOM, it can be used to estimate the PSOM. As such the RRQ tool can be useful in stroke outcome studies so that an investigator can capture clinical outcome data that might otherwise be lost. An ongoing project of the IPSS is to use the RRQ to capture long-term outcomes of the cohort. A future study should examine the interrater reliability and the test-retest validity of the RRQ obtained by different interviewers when compared with the PSOM.
Supplementary Material
ACKNOWLEDGMENT
The authors thank Elisa Wilson and Lauren Kielstra of the Hospital for Sick Children for technical assistance.
GLOSSARY
- ICC
intraclass correlation
- IPSS
International Pediatric Stroke Study
- PSOM
Pediatric Stroke Outcome Measure
- RRQ
Recurrence and Recovery Questionnaire
- SIS
Stroke Impact Scale
- tPSOM
Pediatric Stroke Outcome Measure total score
- tRRQ
Recurrence and Recovery Questionnaire total score
Footnotes
Supplemental data at www.neurology.org
Coinvestigators are listed on the Neurology® Web site at www.neurology.org.
AUTHOR CONTRIBUTIONS
Dr. Lo designed the Recurrence and Recovery Questionnaire, developed the study design, performed part of the statistical analysis, drafted the manuscript, and revised it. Dr. Ichord submitted subjects to the study, contributed to the conceptualization of the design, and participated in the revision of the manuscript. Dr. Dowling submitted subjects to the study and participated in the study design and in the revision of the manuscript. Dr. Rafay submitted subjects to the study and participated in the revision of the manuscript. Mr. Templeton coordinated the parent IPSS study, collated and cleaned the data, performed the initial statistical analysis, and participated in the revision of the manuscript. Ms. Halperin collected data on a subset of the subjects, performed a preliminary analysis of that subset, and participated in the revision of the manuscript. Dr. Smith submitted subjects to the study and participated in the revision of the manuscript. Dr. Licht submitted subjects to the study and participated in the revision of the manuscript. Dr. Moharir submitted subjects to the study and participated in the revision of the manuscript. Dr. Askalan submitted subjects to the study and participated in the revision of the manuscript. Dr. deVeber designed the parent IPSS study and obtained funding to maintain the study and to provide for the data collection and initial analysis. She participated in the study design and in the revision of the manuscript.
DISCLOSURE
W. Lo receives institutional support from the Research Institute of Nationwide Children's Hospital and has received support from NINDS. R. Ichord received support from NINDS. M. Dowling receives support from the Doris Duke Foundation. M. Rafay receives support from the Manitoba Institute of Child Health. J. Templeton, A. Halperin, and S. Smith report no disclosures. D. Licht is supported by grants R01 NS072338, R21 HD069390, The Dana Foundation, and The Wolfson Family Foundation for Neurological Research. M. Moharir and R. Askalan report no disclosures. G. deVeber receives support from NIH NS062820 and the Auxilium Foundation. Go to Neurology.org for full disclosures.
REFERENCES
- 1. Kasner SE. Clinical interpretation and use of stroke scales. Lancet Neurol 2006;5:603–612 [DOI] [PubMed] [Google Scholar]
- 2. Quinn TJ, Langhorne P, Stott DJ. Barthel index for stroke trials: development, properties, and application. Stroke 2011;42:1146–1151 [DOI] [PubMed] [Google Scholar]
- 3. Barak S, Duncan PW. Issues in selecting outcome measures to assess functional recovery after stroke. NeuroRx 2006;3:505–524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. deVeber GA, MacGregor D, Curtis R, Mayank S. Neurologic outcome in survivors of childhood arterial ischemic stroke and sinovenous thrombosis. J Child Neurol 2000;15:316–324 [DOI] [PubMed] [Google Scholar]
- 5. Ichord RN, Bastian R, Abraham L, et al. Interrater reliability of the Pediatric National Institutes of Health Stroke Scale (PedNIHSS) in a multicenter study. Stroke 2011;42:613–617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Beslow LA, Smith SE, Vossough A, et al. Hemorrhagic transformation of childhood arterial ischemic stroke. Stroke 2011;42:941–946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lo W, Zamel K, Ponnappa K, et al. The cost of pediatric stroke care and rehabilitation. Stroke 2008;39:161–165 [DOI] [PubMed] [Google Scholar]
- 8. Golomb MR, Fullerton HJ, Nowak-Gottl U, deVeber G. Male predominance in childhood ischemic stroke: findings from the international pediatric stroke study. Stroke 2009;40:52–57 [DOI] [PubMed] [Google Scholar]
- 9. Golomb MR, MacGregor DL, Domi T, et al. Presumed pre- or perinatal arterial ischemic stroke: risk factors and outcomes. Ann Neurol 2001;50:163–168 [DOI] [PubMed] [Google Scholar]
- 10. Kitchen L, Westmacott R, Friefeld S, et al. The Pediatric Stroke Outcome Measure: a validation and reliability study. Stroke 2012;43:1602–1608 [DOI] [PubMed] [Google Scholar]
- 11. Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986;1:307–310 [PubMed] [Google Scholar]
- 12. Garson GD. Ordinal association. Statnotes: Topics in Multivariate Analysis. Available at: http://faculty.chass.ncsu.edu/garson/PA765/reliab.htm#rater. Accessed August 16, 2011
- 13.Garson GD.Reliability analysis. Statnotes: Topics in Multivariate Analysis. [Accessed June 13, 2011]. Available at: http://faculty.chass.ncsu.edu/garson/PA765/reliab.htm#rater.
- 14. Perkins E, Stephens J, Xiang H, Lo W. The cost of pediatric stroke acute care in the United States. Stroke 2009;40:2820–2827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Galvin J, Hewish S, Rice J, Mackay MT. Functional outcome following paediatric stroke. Dev Neurorehabil 2011;14:67–71 [DOI] [PubMed] [Google Scholar]
- 16.Roach KE.Measurement of Health Outcomes: Reliability, Validity and Responsiveness. American Academy of Orthotists and Prosthetists. [Accessed July 5, 2011]. Available at: http://www.oandp.org/jpo/library/2006_01S_008.asp.
- 17. Janssen PM, Visser NA, Dorhout Mees SM, Klijn CJ, Algra A, Rinkel GJ. Comparison of telephone and face-to-face assessment of the modified Rankin Scale. Cerebrovasc Dis 2010;29:137–139 [DOI] [PubMed] [Google Scholar]
- 18. Korner-Bitensky N, Wood-Dauphinee S, Siemiatycki J, Shapiro S, Becker R. Health-related information postdischarge: telephone versus face-to-face interviewing. Arch Phys Med Rehabil 1994;75:1287–1296 [PubMed] [Google Scholar]
- 19. Duncan PW, Lai SM, Tyler D, Perera S, Reker DM, Studenski S. Evaluation of proxy responses to the Stroke Impact Scale. Stroke 2002;33:2593–2599 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.