Abstract
Objective
Patients with cervical spondylotic myelopathy (CSM) have the same clinical symptoms that vary according to the degree of spinal cord compression and the cross-sectional cord shape. We used a three-dimensional finite element method (3D-FEM) to analyze the stress distributions of the spinal cord with neck extension under three cross-sectional cord shapes.
Methods
Experimental condition for the 3D-FEM spinal cord, ligamentum flavum, and anterior compression shape (central, lateral, and diffuse types) was established. To simulate neck extension, the spinal cord was extended by 20° and the ligamentum flavum was shifted distally according to movement of the cephalad lamina.
Results
The stress distribution in the spinal cord increased due to invagination of the ligamentum flavum into the neck extension. The range of stress distribution observed for the diffuse type was wider than for the central and lateral types. In addition, the stress distribution in the spinal cord was increased by the pincer movement of the ligamentum flavum and by the anterior compression of the spinal cord. The range of stress distribution observed for the diffuse type under antero-posterior compression was also wider than for the central and lateral types.
Conclusion
This simulation model showed that the clinical symptoms of CSM due to compression of the diffuse type may be stronger than for the central and lateral types. Therefore, careful follow-up is recommended for anterior compression of the spinal cord of diffuse type.
Keywords: Spinal cord, Morphometry, Cervical spondylotic myelopathy, Finite element method, Ligamentum flavum, Pincer effect, Spinal cord, Anterior compression
Introduction
The number of patients with cervical spondylotic myelopathy (CSM) is increasing due to the aging population. The neurologic dysfunction may initially be mild and associated with minimal disability; however, progressive spinal cord compression can lead to severe neurologic deterioration major disability. CSM manifests itself following static or dynamic compression of the spinal cord. Static factors include developmental canal stenosis, bulging of the intervertebral disc posterior margin, and hypertrophy of the ligamentum flavum. Dynamic factors include invagination of the ligamentum flavum (buckling) and a pincer effect (anterior and posterior cord impingement)1,2 during neck extension. Of note, patients with CSM do not necessarily share the same symptoms. These differ according to the degree of spinal cord compression and the cross-sectional cord shape.3,4 The latter factor was the basis of a study on patients with myelopathy that classified cord morphology according to the deformity.
There have been several reports on the relationship between cross-sectional cord shapes and prognosis.3–7 The spinal cord shape observed by computed tomography myelography (CTM) or magnetic resonance imaging (MRI) was classified into three general morphological types (central, lateral, and diffuse) by Fujiwara et al.9
The prognosis for patients with CSM varies according to the morphometry of spinal cord compression. Image-based analyses have been reported; however, so far there are no reports on morphometry-based analysis of stress distribution. In this study, we used a three-dimensional finite element method (3D-FEM) to analyze the stress distribution in the spinal cord with neck extension and under three anterior compression shapes.
Methods
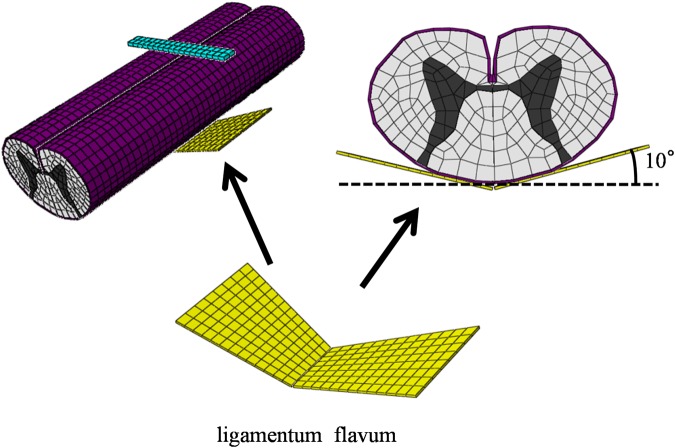
The ABAQUS 6.11 (Valley Street, Providence, RI, USA) finite element package was used for FEM simulation. The 3D-FEM spinal cord model used in this study consisted of the gray matter, white matter, and pia mater (Fig. 1). In order to simplify calculation in the model, the denticulate ligament, dura, and nerve root sheaths were not included. The pia mater was included as it has been demonstrated that the spinal cord with and without this component shows significantly different mechanical behavior.8 The spinal cord was assumed to be symmetrical about the mid-sagittal plane, such that only half the spinal cord required reconstruction and the whole model could be integrated by mirror image. The vertical length of the spinal cord for CTM measurement was two vertebral bodies (approximately 80 mm).
Figure 1.
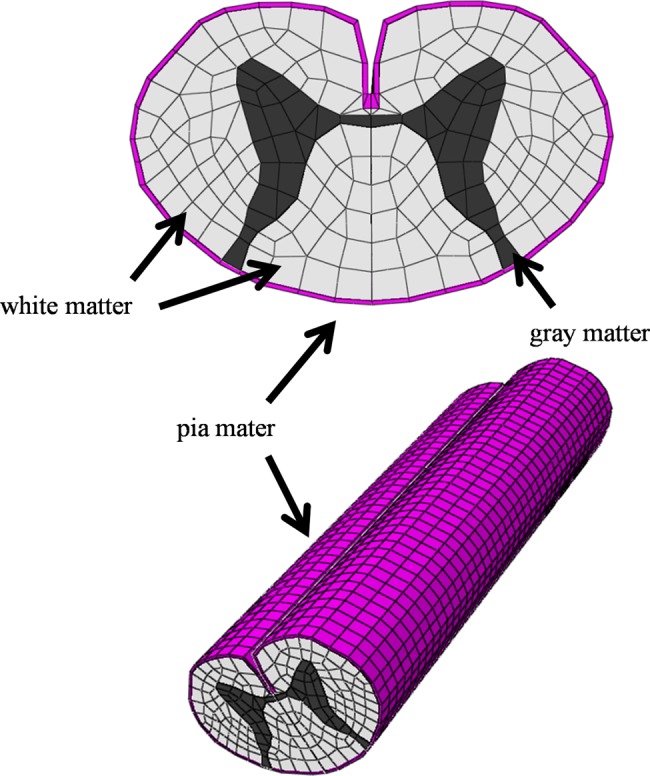
The three-dimensional finite element method model of the spinal cord consisted of the gray matter, white matter, and pia mater.
The ligamentum flavum model was established by measuring CTM and MRI. In order to simplify calculation in the model, the ligamentum flavum was a linear shape. The angle of the ligamentum flavum was set to open 10° outward from the posterior spinal cord center as deduced from the literature (Fig. 2).9
Figure 2.
The ligamentum flavum model was established using the rear of the spinal cord.
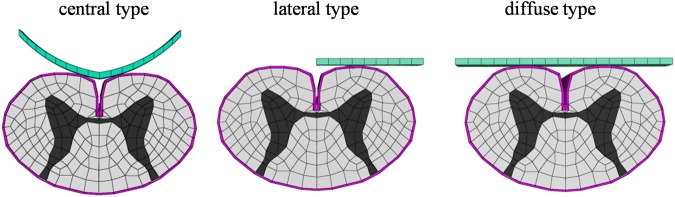
The shapes resulting from anterior compression on the spinal cord were classified as central, lateral, and diffuse type. A rigid flat plate was used as the compression factor on the anterior surface of the spinal cord for the lateral and diffuse types, while a semi-circular plate was used for the central type. The shape of the semi-circular plate for the central type was deduced from the literature (Fig. 3).9
Figure 3.
The shapes for anterior compression of the spinal cord were central type, lateral type, and diffuse type.
The model simulated CSM under three shapes of anterior compression of the spinal cord, as well as dynamic compression under neck extension of the cervical spinal cord. The rigid flat plate and the semi-circular plate were located at inferior margin of the posterior vertebral body. The ligamentum flavum was located at the anterior surface of the cephalad lamina and at the superior margin of the caudal lamina. The position of the three anterior compression plates and the ligamentum flavum was set according to MRI resulting from the literature.10
The spinal cord consists of three distinctive materials referred to as the white matter, gray matter, and pia mater. The mechanical properties (Young's modulus and Poisson's ratio) of the gray and white matter were determined using data obtained by the tensile stress strain curve and stress relaxation under various strain rates.11,12 The mechanical properties of the pia mater and the ligamentum flavum were obtained from the literature.13,14 The mechanical properties of the flat plate and semi-circular plate were stiff enough for the spinal cord to be pressed. On the basis of the assumption that no slippage occurs at the interfaces of the white matter, gray matter, and pia mater, these interfaces were glued together. There are no data on the friction coefficient between the ligamentum flavum and the spinal cord. The coefficient of friction between the ligamentum flavum and the spinal cord was frictionless at the contact interfaces and adjacent to the part in contact. Similarly, the coefficient of friction between the rigid flat plate and the spinal cord, or the semi-circular plate and the spinal cord, was frictionless at the contact interfaces and adjacent to the part in contact.
The spinal cord, the rigid flat plate, the semi-circular plate, and the ligamentum flavum model were symmetrically meshed with 20-node elements. With an FEM model for diffuse type of rigid flat plate of the spinal cord, the total number of isoperimetric 20-node elements was 10 104 and the total number of nodes was 57 433.
For the simulation of CSM under neck extension, nodes at the bottom of the spinal cord model were constrained in all directions and the extension angle of the superior margin of the spinal cord was 20°. The flat plate and the semi-circular plate were constrained in all directions. On the basis of assumption that the caudal vertebral body of the responsible lesion in patients with CSM was stable, the bottom of the ligamentum flavum was constrained in all directions. The superior margin of the ligamentum flavum shifted distally according to the movement of cephalad lamina. The angle of neck extension and the direction in which the ligamentum flavum moved were obtained from each angle of C3–C4 kinematic MRI published by Iwabuchi et al.15 (Fig. 4).
Figure 4.
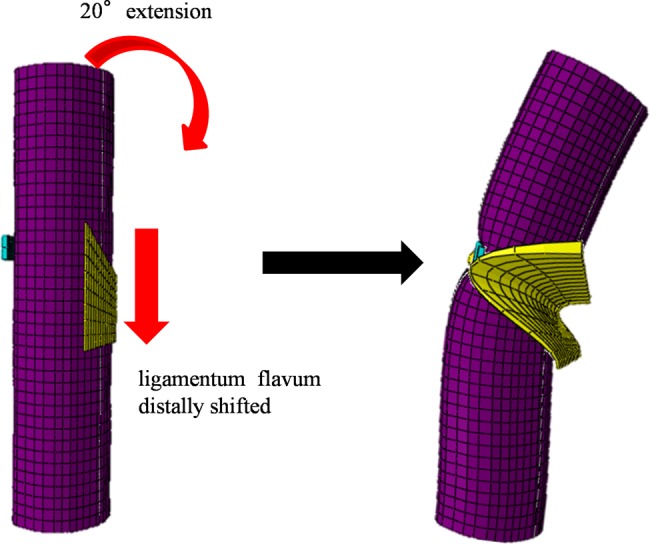
For the simulation of neck extension, an extension of 20° was applied to the spinal cord. The ligamentum flavum moved distally in accordance with movement of the cephalad lamina.
FEM analysis was conducted under each of the above conditions and in duplicate to test the reproducibility of results with this model.
Results
When the shape of the spinal cord resulting from anterior compression was the central type, stress distributions were observed in the posterior horn and in parts of the posterior funiculus, lateral funiculus, and anterior horn beneath the invagination by the ligamentum flavum. Under antero-posterior compression, high-stress distributions were observed in the gray matter, anterior funiculus, and part of the lateral funiculus (Fig. 5A).
Figure 5.
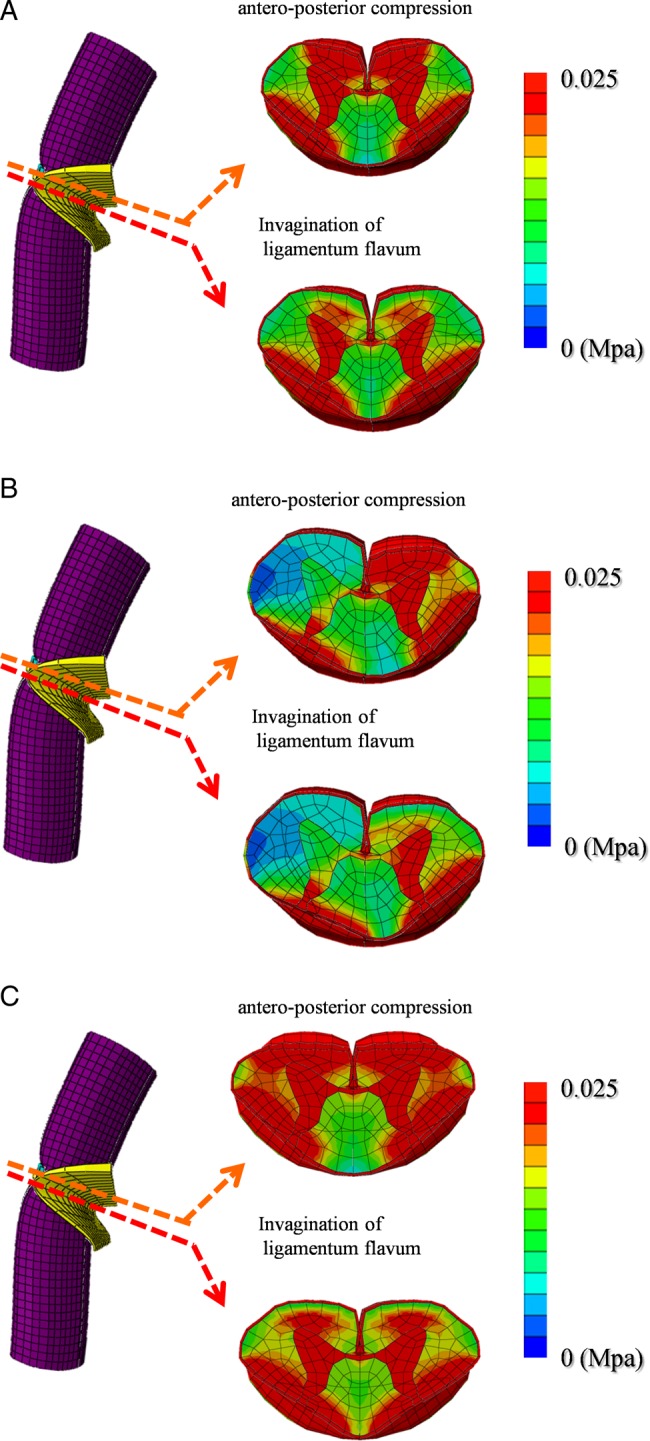
Stress distributions under invagination of the ligamentum flavum and under antero-posterior compression are shown for the central, lateral, and diffuse type shapes following anterior compression of the spinal cord (A–C).
When the shape of the spinal cord resulting from anterior compression was the lateral type, stress distributions were observed in the gray matter and lateral funiculus on the compression side, as well as parts of the posterior funiculus and posterior horn on the non-compression side beneath the invagination by the ligamentum flavum. Under antero-posterior compression, high stress distributions were observed in the gray matter, anterior funiculus, and lateral funiculus on the compression side (Fig. 5B).
When the shape of the spinal cord resulting from anterior compression was the diffuse type, stress distributions were observed in the gray matter, lateral funiculus, and posterior funiculus beneath the invagination by the ligamentum flavum. Under antero-posterior compression, high-stress distributions were observed in the gray matter, anterior funiculus, lateral funiculus, and part of the posterior funiculus (Fig. 5C).
Discussion
CSM is the most common type of spinal cord dysfunction in patients over the age of 55 years, but can sometimes be difficult to recognize. Although it is the most common reason for spinal cord dysfunction in the elderly individuals, CSM has no pathognomonic symptoms or physical signs. Because no single neurologic feature is unique to cervical myelopathy, diagnosis must be established by the affirmation of associated clinical signs and symptoms, in parallel with the exclusion of other clinical entities that may also show these signs.
CSM is a well-described clinical syndrome that can arise due to a combination of etiological factors. This clinical entity manifests itself following static or dynamic compression of the spinal cord, causing compression of the enclosed cord and leading to local tissue ischemia, injury, and neurological impairment. Static factors include developmental canal stenosis, bulging of the intervertebral disc posterior margin, and hypertrophy of the ligamentum flavum. With regard to dynamic factors, the spinal cord of patients with CSM is likely to be compressed during neck extension because of the protrusion of the ligamentum flavum and intervertebral discs into the spinal canal (pincer mechanism).2 Recent developments in imaging have enabled closer examination of the cervical spine through improved CT and MRI. Using multidetector-row CT, Machino et al.7 reported narrowing of the spinal cord cross-sectional area during neck extension in patients with CSM. In healthy individuals, Muhle et al.16,17 found that the subarachnoid space was smallest at extension compared with its size at mid-position and at flexion of the cervical spine. These workers also showed an increasing prevalence of functional cord impingement from the posterior aspect and from both the anterior and posterior aspects during neck extension in patients with advancing stages of degenerative disease of the cervical spine.
With regard to morphological changes in the spinal cord, the symptoms of CSM patients differ according to the degree of spinal cord compression and the cross-sectional cord shape. Yu et al.5 reported that the severity of symptoms correlated with the degree of spinal cord deformity. Kameyama et al.3 reported that a triangular-shaped spinal cord was associated with severe and irreversible pathological changes, whereas Shimomura and Washimi4 found this shape was associated with a more severe prognosis compared with oval-shaped or boomerang-shaped spinal cords. The spinal cord shape was classified into three general morphological types (central, lateral, and diffuse) by Fujiwara et al.9 These investigators reported that the prognosis of CSM patients with the diffuse-type shape was poorer than those with the central or lateral types.
Using this prior knowledge, we investigated whether the morphology of the spinal cord following anterior compression was associated with difference in stress distribution for the same dynamic motion. Our first goal was to develop a 3D-FEM spinal cord model that simulates the clinical situation, whereas our second goal was to analyze the clinical condition of patients. Similar to previous studies by Kato et al.,18–20 Xin-Feng Li et al.,21,22 and Nishida et al.,23 bovine spinal cord was used in the current analytical model as it was impossible to obtain fresh human spinal cord. Xin-Feng et al.21 noted that it was reasonable to use the mechanical properties of bovine spinal cord because the brain and spinal cord of cattle and humans show similar injury changes. For the purpose of this study, we therefore assumed that the mechanical properties of the spinal cord from these two species were similar.
Our study was limited to the investigation of stress distribution caused by compression. Other casual factors that can contribute to CSM include ischemia, congestion, and spinal cord stretch injury.24 Blood flow was not factored into this FEM analysis and only one movement (neck extension) was investigated for association with CSM. Long-term compression and apoptotic factors were not considered in the FEM analysis. Moreover, the FEM model used here was simplified in order to facilitate the calculations. Analysis errors were reduced by using a FEM mesh, by assuming the spinal cord was symmetric, by not including the denticulate ligament, dura, and nerve root sheaths, and by setting a close distance between the spinal cord and ligamentum flavum, spinal cord and anterior compression of the spinal cord.
In pathology-based studies, Ono et al.25 and Ogino et al.26 described how patients with severe myelopathy had spinal cord atrophy and also presented with extensive degeneration and infarction of the entire gray matter and white columns, except for the anterior funiculus. In this analysis, stress distributions in the anterior funiculus were also increased. However, as this increase is not associated with any clinical symptoms or with apoptosis, the current results merely provide an estimate for the possible range of increased stress distribution at this site. Nevertheless, the observed morphometric pattern was similar to a prior clinical report and hence our results may still be applicable to the clinical situation. More complex materials and structural characteristics of the spinal cord model should be included in future investigations.
In this study, stress distribution in the spinal cord was increased by the invagination of the ligamentum flavum during neck extension. However, the range of stress distributions for the three types of anterior compression of the spinal cord was not uniform (Figs. 5A–5C). The range of stress distribution observed for the diffuse type was wider than for the central and lateral types.
In addition, stress distribution in the spinal cord was increased by the pincer movement of the ligamentum flavum and by anterior compression of the spinal cord. However, in this situation the range of stress distribution for the three types of anterior compression of the spinal cord was not uniform. The range of stress distribution under antero-posterior compression was also wider for the diffuse type compared with the central and lateral types. Moreover, the compressed spinal cord shape with diffuse type was a triangular shape and was supposed to be a severe prognosis.
Conclusion
This simulation model showed that the stress distribution with the diffuse type of compression was wider than with the central and lateral types. The symptoms of CSM associated with diffuse type may therefore be stronger than for CSM with the central or lateral types. We recommend more careful follow-up of anterior compression of the spinal cord when the resultant shape is the diffuse type.
References
- 1.Taylor AR. Mechanism and treatment of spinal-cord disorders associated with cervical spondylosis. Lancet 1953;1(6763):717–20 [DOI] [PubMed] [Google Scholar]
- 2.Penning L. Some aspects of plain radiography of the cervical spine in chronic myelopathy. Neurology 1962;12:513–9 [DOI] [PubMed] [Google Scholar]
- 3.Kameyama T, Hashizume Y, Ando T, Takahashi A, Yanagi T, Mizuno J. Spinal cord morphology and pathology in ossification of the posterior longitudinal ligament. Brain 1995;118:263–78 [DOI] [PubMed] [Google Scholar]
- 4.Shimomura T, Washimi M. The natural course of cervical spondylotic myelopathy: follow-up of conservative treatment after admission. Rinsho-Seikeigeka 2004;39(4):439–44 (Japan) [Google Scholar]
- 5.Yu YL, Stevens JM, Kandall B, du Bourlay GH. Cord shape and measurements in cervical spondylotic myelopathy and radiculopathy. AJNR 1983;4(3):839–42 [PMC free article] [PubMed] [Google Scholar]
- 6.Hayashi H, Okada K, Hashimoto J, Tada K, Ueno R. Cervical spondylotic myelopathy in the aged patient. A radiographic evaluation of the aging changes in the cervical spine and etiologic factors of myelopathy. Spine 1988;13(6):618–25 [PubMed] [Google Scholar]
- 7.Machino M, Yukawa Y, Ito K, Nakashima H, Kato F. Dynamic changes in dural sac and spinal cord cross-sectional area in patients with cervical spondylotic myelopathy. Spine 2011;36(5):399–403 [DOI] [PubMed] [Google Scholar]
- 8.Cecilia P, Jon S, Richard M. The importance of Fluid-Structure Interaction in spinal trauma models. J Neurotrauma 2011;28(1):113–25 [DOI] [PubMed] [Google Scholar]
- 9.Fijiwara K, Yonenobu K, Hiroshima K, Fuji T, Ehara S, Yamashita K, et al. The evaluation of viability of the spinal cord by CT myelography. Rinsho Seikei Geka 1986;21:355–61 (Japan) [Google Scholar]
- 10.Tani T, Yamamoto H, Kimura J. Cervical spondylotic myelopathy in elderly people: a high incidence of conduction block at C3-4 or C4-5. J Neural Neurosurg Psychiatry 1999;66(4):456–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ichihara K, Taguchi T, Yoshinori S, Ituo S, Shunichi K, Shinya K. Gray matter of the bovine cervical spinal cord is mechanically more rigid and fragile than the white matter. J Neurotrauma 2001;18(3):361–7 [DOI] [PubMed] [Google Scholar]
- 12.Ichihara K, Taguchi T, Sakuramoto I, Kawano S, Kawai S. Mechanism of the spinal cord injury and the cervical spondylotic myelopathy: new approach based on the mechanical features of the spinal cord white and gray matter. J Neurosurg 2003;993 suppl.:278–85 [DOI] [PubMed] [Google Scholar]
- 13.Tunturi AR. Elasticity of the spinal cord, pia and denticulate ligament in the dog. J Neurosurg 1978;48(6):975–9 [DOI] [PubMed] [Google Scholar]
- 14.Ueno H, Taguchi T, Toyoda K, Ichihara K, Kawai S, Kawano K. Mechanical characteristic with the aging of the lumbar vertebrae ligamentum flavum. Seikeigeka to Saigaigeka 2002;51(4):367–9 (Japan) [Google Scholar]
- 15.Iwabuchi M, Kikuchi S, Yabuki S. Kinematic MRI and preoperative evaluation in cervical spondylotic myelopathy. Rinsyo Seikeigeka. 2005;40(1):13–7 (Japan) [Google Scholar]
- 16.Muhle C, Wiskirchen J, Weinert D, Falliner A, Wesner F, Brinkmann G, et al. Biomechanical aspects of the subarachnoid space and cervical cord in healthy indivisuals examined with kinematic magnetic resonance imaging. Spine 1998;23(5):556–67 [DOI] [PubMed] [Google Scholar]
- 17.Muhle C, Metzner J, Weinert D, Falliner A, Brinkmann G, Mehdorn MH, et al. Classification system based on kinematic MR imaging in cervical spomdylotic myelopathy. AJNR 1998;19(9):1763–71 [PMC free article] [PubMed] [Google Scholar]
- 18.Kato Y, Kataoka H, Ichihara K, Imajo Y, Kojima T, Kawano S, et al. Biomechanical study of cervical flexion myelopathy using a three-dimensional finite element method. J Neurosurg Spine 2008;8(5):436–41 [DOI] [PubMed] [Google Scholar]
- 19.Kato Y, Kanchiku T, Imajo Y, Ichihara K, Kawano S, Hamanaka D, et al. Flexion model simulating spinal cord injury without radiographic abnormality in patients with ossification of the longitudinal ligament: the influence of flexion speed on the cervical spine. J Spinal Cord Med 2009;32(5):555–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kato Y, Kanchiku T, Imajo Y, Kimura K, Ichihara K, Kawano S, et al. Biomechanical study of the effect of the degree of static compression of the spinal cord in ossification of the posterior longitudinal ligament. J Neurosurg Spine 2010;12(3):301–5 [DOI] [PubMed] [Google Scholar]
- 21.Xin-Feng Li, MSc T, Li-Yang Dai. Three-dimensional finite element model of the cervical spinal cord. Spine 2009;34(11):1140–7 [DOI] [PubMed] [Google Scholar]
- 22.Xin-Feng Li, MSc T, Li-Yang Dai. Acute central cord syndrome. Spine 2010;35(19):E955–E964 [DOI] [PubMed] [Google Scholar]
- 23.Nishida N, Kato Y, Imajo Y, Kawano S, Taguchi T. Biomechanical study of the spinal cord in thoracic ossification of the posterior longitudinal ligament. J Spinal Cord Med 2011;34(5):518–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Henderson FC, Geddes JF, Vaccaro AR, Woodard E, Berry KJ, Benzel EC, et al. Stretch-associated injury in cervical spondylotic myelopathy: new concept and review. Neurosurgery 2005;56(5):1101–13 [PubMed] [Google Scholar]
- 25.Ono K, Ota H, Tada K, Yamamoto T. Cervical myelopathy secondary to multiple spondylotic protrusions: a clinicopathologic study. Spine 1977;2(2):109–25 [Google Scholar]
- 26.Ogino H, Tada K, Okada K, Yonenobu K, Yamamoto T, Ono K, et al. Canal diameter, anteroposterior compression ration, and spondylotic myelopathy of the cervical spine. Spine 1983;8(1):1–15 [DOI] [PubMed] [Google Scholar]