Abstract
Context
Long extensive transverse myelitis (LETM) seldom develops in patients with breast cancer who are aquaporin-4 antibody (Aqp-4 Ab)-positive. Whether this association is coincidental is not well understood.
Findings
A 62-year-old woman presented with treatment-resistant LETM and Aqp-4 Ab. Two months later, a stage 3 invasive ductal carcinoma was detected in her right breast. Following tumor resection and chemotherapy, her neurologic symptoms and magnetic resonance imaging findings significantly improved and serum Aqp-4 Ab disappeared. The breast tumor samples of this patient and neurologically normal patients showed inflammatory infiltrates and Aqp-4 expressing cells.
Conclusion/Clinical Relevance
The temporal association between tumor treatment, amelioration of clinical findings, and seroreversion suggest that coexistence of cancer and LETM is not coincidental. Cancer screening should be considered at least in treatment-resistant LETM cases.
Keywords: Transverse myelitis, Myelopathy, Aquaporin-4, Breast cancer, Antibody, Paraneoplastic, Neuromyelitis optica
Introduction
Neuromyelitis optica and long extensive transverse myelitis (LETM) occasionally develop in patients with cancer in relation to aquaporin-4 antibody (Aqp-4 Ab).1,2 Whether this is a coincidental relationship is unclear, partially because the temporal associations between clinical symptoms, serological findings, tumor occurrence, and treatment response are not well known.
Case report
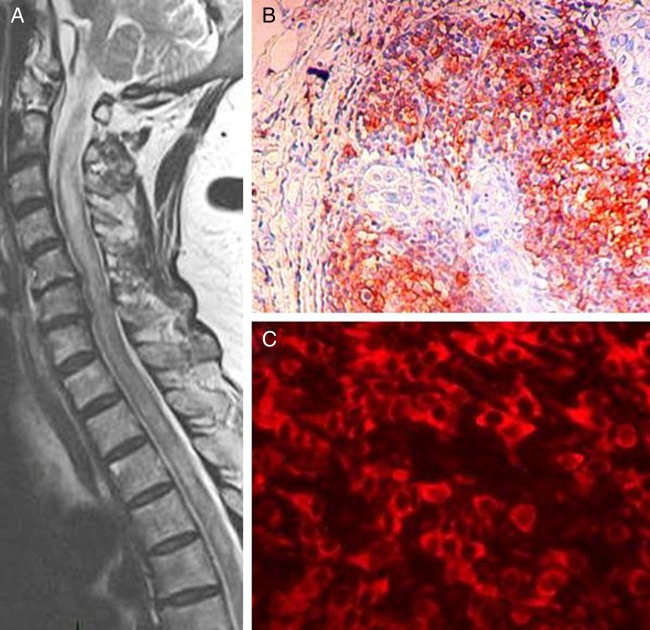
A 62-year-old woman with no history of cancer or prior neurological disease presented with severe fatigue and nausea-vomiting episodes. Gastrointestinal evaluation was normal. Within 1 month band-like left-sided chest tightness appeared, extending to the left armpit together with weakness and numbness in both legs and urinary retention. Neurological examination revealed flaccid tone and severe weakness (2/5 at Medical Research Council scale) in both legs and a sensory level at D4. The patient also showed decreased proprioception in both feet, was areflexic, and had extensor plantar responses. Cerebrospinal fluid (CSF) examination was acellular, with a protein level of 78 mg/dl, and a normal glucose level; CSF IgG index was slightly elevated (0.72), and oligoclonal bands were negative. While her brain magnetic resonance imaging (MRI) was unremarkable, the spinal MRI revealed a hyperintense T2 lesion extending from C2 to C6 (Fig. 1A), with patchy contrast enhancement. Routine blood tests and a comprehensive panel for autoimmune diseases (anti-nuclear, anti-double-stranded DNA, anti-neutrophil cytoplasmic, cardiolipin antibodies, and extractable nuclear antigen antibody (ENA) screening) were negative. Serum Aqp-4 Ab was detected with a cell-based assay using Aqp-4-transfected HEK-293 cells (Euroimmun, Lübeck, Germany). Her visual evoked potential examination was normal. The patient responded suboptimally to a 10-day treatment of 1 g/day intravenous methylprednisolone, which was followed by oral steroid and azathioprine with little improvement. Bilateral lower-extremity muscle strength increased to 3/5, and slight spasticity developed, so that she could stand and make a few steps with bilateral assistance.
Figure 1.
Sagittal T2-weighted MRI showing long extensive spinal cord lesion at C2–C6 (A) breast tumor section exhibiting CD20+ B cells (avidin–biotin–peroxidase technique with mild hematoxylin counterstaining) (B) aquaporin-4 expressing cells (C, red). B and C panels, original magnification ×100 and ×400, respectively.
Two months later, a non-tender mass was palpated in her right breast. Following mammography, whole-body computed tomography scan, and biopsy, a stage 3 invasive ductal carcinoma was diagnosed. She was seronegative for paraneoplastic antibodies (Hu, Ri, Yo, Ma2, CV2, and amphiphysin; Euroimmun) but was seropositive for Aqp-4 Ab. Shortly after radical mastectomy with axillary clearance and introduction of chemotherapy (5-fluorouracil, epirubicin, and cyclophosphamide), her symptoms started improving. Two months later, she had only mild left leg weakness (4/5), whereas the motor strength of her right leg was normal. She had no sensory symptoms, markedly improved vibration sense, and no sphincter disturbance. Brain and spinal MRIs were normal. At that time, in two different assays, her new sera failed to show Aqp-4 Ab, while her archived old sera were Aqp-4 Ab-positive.
Histology findings
Immunohistochemistry of paraffin-embedded tumor sections of our patient and three additional neurologically normal patients with invasive ductal breast carcinoma using rabbit anti-human CD3-, CD20-, and CD68-antibodies (all 1:200, Novocastra, UK)3 exhibited extensive perivascular and parenchymal infiltrates of all three lymphocyte subtypes (Fig. 1B). Moreover, tumor sections were incubated overnight at 4°C with rabbit anti-human Aqp-4 Ab (1:200, Santa Cruz, Santa Cruz, CA, USA) and appropriate secondary Alexa-Fluor 594-conjugated antibody (1:1000, Invitrogen, Grand Island, NY, USA). All four tumor samples displayed cells expressing Aqp-4 in the membranes (Fig. 1C), as described previously.4 Control sections incubated only with the secondary antibody did not yield any staining. Aqp-4 expression could not be verified with western blotting and reverse transcription-polymerase chain reaction due to the unavailability of frozen tumor samples.
Discussion/conclusion
This patient adds to the growing list of cases of possibly paraneoplastic LETM.1,5 Moreover, immunotherapy-resistance before tumor detection and amelioration of symptoms and seroreversion after tumor treatment suggest that coexistence of cancer and LETM is not coincidental. A similar chemotherapy-responsive LETM case with a history of breast cancer has been reported.5 This case and previously reported ones show that LETM might also precede and act as a harbinger of a forthcoming cancer.1 Cancer screening should therefore be considered at least in immunotherapy-resistant LETM cases. Presence of inflammatory cells and Aqp-4 expression at the patient's tumor tissue supports the notion that Aqp-4 Ab-positive LETM might develop by autoimmune mechanisms. However, Aqp-4 is also expressed in neurologically normal patients’ tumors, suggesting that additional factors are required for myelitis induction in patients with cancer.
References
- 1.Pittock SJ, Lennon VA. Aquaporin-4 autoantibodies in a paraneoplastic context. Arch Neurol 2008;65(5):629–32 [DOI] [PubMed] [Google Scholar]
- 2.De Santis G, Caniatti L, De Vito A, De Gennaro R, Granieri E, Tola MR. A possible paraneoplastic neuromyelitis optica associated with lung cancer. Neurol Sci 2009;30(5):397–400 [DOI] [PubMed] [Google Scholar]
- 3.Cattoretti G, Pileri S, Parravicini C, Becker MH, Poggi S, Bifulco C, et al. Antigen unmasking on formalin-fixed, paraffin-embedded tissue sections. J Pathol 1993;171(2):83–98 [DOI] [PubMed] [Google Scholar]
- 4.Chan KH, Kwan JS, Ho PW, Ho SL, Chui WH, Chu AC, et al. Aquaporin-4 water channel expression by thymoma of patients with and without myasthenia gravis. J Neuroimmunol 2010;227(1–2):178–84 [DOI] [PubMed] [Google Scholar]
- 5.Mueller S, Dubal DB, Josephson SA. A case of paraneoplastic myelopathy associated with the neuromyelitis optica antibody Nat Clin Pract Neurol 2008;4(5):284–8 [DOI] [PubMed] [Google Scholar]