Abstract
Background
This pilot feasibility study examined the role of genetics in laboratory-induced cocaine craving.
Methods
Thirty-Four African American, cocaine-dependent male subjects underwent a baseline assessment, cue-exposure session and genetic analysis. Subjects were classified as either cue-reactive or non-reactive.
Results
Among SNP markers in 13 candidate genes examined for association with cocaine cue-reactivity, two were statistically significant: GABRA2 (coding for GABA-A receptor alpha-2 subunit; rs11503014, nominal p = 0.001) and OPRM1 (coding for mu opioid receptor; rs2236256, nominal p = 0.03).
Conclusions
These pilot results suggest that cocaine craving shows variability among cocaine-dependent subjects, and that GABRA2 and OPRM1 polymorphisms have differential influences on cocaine cue-reactivity, warranting studies in future research.
Introduction
Because cocaine craving is thought to play a role in ongoing use, researchers have been interested in studying the neural architecture underlying laboratory induced craving.1–5 This research has not yet been extended to examine the genetics underlying laboratory-induced cocaine craving, which is unfortunate given that studies have identified genes that predict laboratory induced craving for opiates, alcohol and marijuana.6–10 Furthermore, researchers have examined individual differences in cocaine cue-reactivity with some subjects displaying high cue-reactivity, whereas others display minimal or no-reactivity to the cues and an underlying neurobiological link.8,11–16 Therefore, the current pilot study intended to fill this void by beginning to examine the feasibility of identifying which candidate gene polymorphisms correlate with cocaine cue-reactivity. This study examined 13 candidate genes thought to play a role in cocaine addiction or in cue-reactivity of abused drugs other than cocaine.
Methods
Thirty-four cocaine-dependent male patients were recruited from the Veterans Administration (VA) New Jersey Health Care System, and the study was approved by an Institutional Review Board. Sample selection was restricted to African-American individuals, given the better statistical power in a relatively homogeneous ethnic group. Selection criteria included: (1) meeting DSM-IV criteria for an Axis I diagnosis of cocaine dependence; (2) cocaine as a primary drug of choice; (3) male; (4) cocaine and alcohol-free at initial evaluation; and (5) English-speaking, required for completing the protocol. Exclusion criteria were: (1) meeting DSM-IV criteria for a substance dependence disorder other than cocaine, or other mental health conditions, including PTSD, schizophrenia, bipolar I disorder, or major depressive disorder; and (2) taking any psychoactive medication.
After signing informed consent, eligible subjects underwent a baseline assessment that included: a Structured Clinical Interview for DSM-IV Axis I Diagnosis (SCID),17 the Addiction Severity Index (ASI),18,19 and the Cocaine Craving Questionnaire Brief (CCQ).15,20 Subjects also completed the within-session Voris Cocaine Craving Scale (VCCS)21 which is a 4-item self-report 50-point within-session visual analogue measure that covers four dimensions of craving; craving intensity, mood, energy and feelings, with sub-scales range from 0 (indicating no desire) to 50 (high desire).
In the cue-exposure laboratory, subjects first completed a non-stimulating control procedure that includes the presentation of different shapes of varying colors. Subjects were subsequently exposed to both a neutral-non-substance use cue and an active cocaine cue presentations, while being monitored for changes in self report craving. During the neutral cue-session, subjects were shown a videotape of a person handling items from nature, including a shell, pinecone and rock. Following the videotape, the subjects were instructed to manually handle those items previously presented in video form. During the active cue phase, subjects were exposed to videotaped cues of people using and handling drugs. Following the videotape, the subjects were instructed to handle drug paraphernalia (e.g., pipes, stems, and vials). Subjects completed the VCCS before and after each cue-presentation. After the cue-exposure procedures, subjects underwent a debriefing session to minimize the impact of experimentally induced relapse. On a separate day, subjects provide a blood sample in the phlebotomy laboratory.
Blood and DNA samples were processed at the Rutgers University Cell and DNA Repository (RUCDR). DNA from venous blood sample was extracted from whole blood using an inorganic, salt-precipitation (i.e. phenol free) method. Typical yields for extracted DNA were approximately 30 micrograms DNA per ml blood, and genomic DNA samples were of high quality suitable for genotyping studies. For genotyping, three oligonucleotides were designed for each SNP assay, comprised of two PCR primers for amplification and a third tagged SNP primer (SBE primer) used in the primer extension phase of the assay. Each extension mix contained two fluorescently labeled terminators; there were a total of 6 extension types that allowed for the successful multiplexing of assays in this process. The 5' tag sequences on the SBE primer were matched to the tags immobilized on the SNPware array plate, to each SNP primer.
The 13 candidate genes were selected from several neurotransmission systems important for drug abuse in general, and cocaine dependence in particular.22–30 These included dopamine receptors, dopamine and serotonin transporters, tryptophan hydroxylase-2, Catechol-O-methyltransferase, pro-opiomelanocortin, mu-opioid receptor, and GABA-A receptor alpha subunit 2 (Supplementary Table 1). SNPs in these candidate genes were tested for association with cocaine cue-reactivity using a logistic model and four different genetic models: additive, dominant, recessive, and the 2 degrees of freedom genotype model. Because these models can be expected to give correlated results, we adjusted for multiple testing within each SNP by using permutation-based testing to determine the SNP-specific p-value. Reported p-values are “nominal” in the sense that after this permutation-based correction for multiple models by SNP we did not adjust for multiple testing across SNPs.
Cocaine cue-reactivity status was determined blind to the genotype data. To determine a classifier, we did not rely on any particular a priori set of variables or functions thereof but took an exploratory approach, looking for a metric that would show a spike, corresponding to no change from neutral to active cue, mixed with a wider distribution showing change from neutral to active cue. Multi-dimensional scaling,31 pairwise plots, density estimation, and two-way clustering were all used informally as dimension-reduction techniques to look for a parsimonious set of variables showing a spike and spread distribution. In light of the modest sample size of this pilot study, we sought a simple classifier and settled on using the change in the craving intensity subscale of the Voris Cocaine Craving Scale as a single-variable indicator of cocaine cue-reactivity. This subscale directly measures self-reported craving and is a continuous variable that ranges from 0–50. Subjects were dichotomized as non-reactive (NCR) or reactive (CR) based on whether there was a change on this subscale from after neutral cue to after active cue. Individuals who showed zero change were placed in the NCR group, and those scoring “delta>0,” reflecting a change from neutral to active cue, were placed in the reactive CR group.
Of the 34 subjects enrolled, one was excluded from the analysis because of poor quality DNA. Three additional subjects were removed from the analysis because they showed a reduction in craving from the neutral to the active cocaine cues and could not be classified as cue reactive or non-reactive.
Results
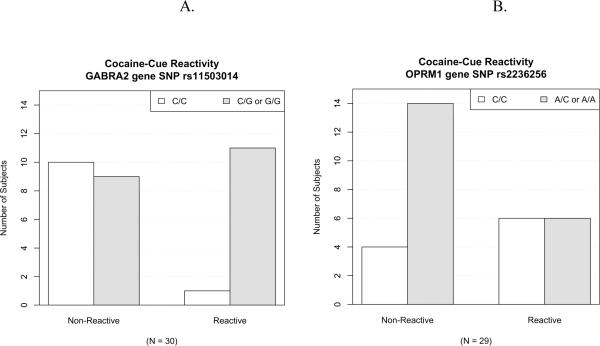
Following standard quality control procedures, 103 non-monomorphic SNPs were available for analysis (Supplementary Table 1). Patient demographics did not show any significant differences between the cue-reactive (N = 12) and non-reactive (N = 18) groups in age, education, employment, or current or lifetime cocaine use or treatments (P > 0.05). Of the SNPs examined for association with cocaine cue-reactivity, there was a statistically significant effect for a SNP in GABRA2 (coding for GABA-A receptor alpha-2 subunit; rs11503014, p = 0.001) and in OPRM1 (coding for mu opioid receptor; rs2236256, p = 0.03) (Table 1). To explore genotype-phenotype relation for cocaine cue-reactivity, the 30 subjects were separated into cue-reactive and non-reactive groups, and SNP allele distribution was examined. Interestingly, for cocaine cue-reactivity phenotype, the minor allele of the GABRA2 gene SNP rs11503014 was associated with high cocaine cue-reactivity; that is, of all 12 cue-reactive subjects, 11 of them have the G allele (Figure 1A, “Reactive”). In contrast, the major allele of the OPRM1 gene SNP rs2236256 was associated with a lack of cocaine cue-reactivity; that is, of all 18 non-reactive subjects, 14 of them lack the A allele (Figure 1B, “Non-Reactive”).
Table 1.
Cue-reactivity status by genotype for rs11503014 and rs2236256.
| GABRA2 gene rs11503014 SNP (p = .001) | |||
|---|---|---|---|
|
| |||
| Genotype | |||
| Status | C/C | C/G | G/G |
| Non-Reactive | 10 | 8 | 0 |
| Reactive | 1 | 10 | 1 |
| OPRM1 gene rs2236256 SNP (p = .025) | |||
|---|---|---|---|
|
| |||
| Genotype | |||
| Status | A/A | A/C | C/C |
| Non-Reactive* | 1 | 12 | 4 |
| Reactive | 4 | 2 | 6 |
One subject was a “no-call” at this locus.
Figure 1.
Genetic marker association with cocaine cue-reactivity. Distributions of SNP genotypes vs. the phenotypes of cocaine cue-reactivity are shown. (A) In GABRA2, the gene for the GABA-A receptor alpha-2 subunit, the G allele of the SNP rs11503014 was associated with high cocaine cue-reactivity, with 11 out of the 12 cue-reactive subjects (right-side “Reactive” pair) having this allele. (B) In OPRM1, the gene for the mu opioid receptor, the A allele of the SNP rs2236256 was associated with non-cocaine cue-reactivity, with 14 of the 18 non-reactive subjects (left-side “Non-Reactive” pair) lacking the A allele.
Discussion
In this study, we measured cocaine cue-reactivity, and dichotomized the subjects as either cue-reactive or non-reactive. The rationale for cue-reactivity dichotomization was two-fold. First of all, literature on cocaine cue-reactivity has shown a bimodal distribution, with some subjects displaying high cue-reactivity, whereas others displaying minimal or no-reactivity to the cues.8,11–16 Furthermore, our data support this pattern of bimodal distribution, with 18 of 30 subjects displaying a zero value for cue-reactivity, and only 12 of 30 subjects displaying a non-zero value. Thus, dichotomizing such a bimodal distribution appears to better describe our dataset.
Based on this approach of dichotomization, association with cocaine cue-reactivity was observed for two candidate gene SNPs, GABRA2 gene SNP rs11503014 and OPRM1 gene SNP rs2236256. This implicates the involvement of inhibitory neurobiological systems in cocaine cue-reactivity: GABRA2 is part of the GABA-A receptor complex, which functions as a chloride channel, and mediates inhibitory synapse throughout the central nervous system; OPRM1 is the gene for the mu opioid receptor, which also mediates synaptic inhibition. Thus, the association of both GABRA2 and OPRM1 suggest that, for the biological processes that underlie cue-reactivity variability in cocaine dependence, inhibitory neurobiological systems may play an important role. In examining the genotype-phenotype relation for cocaine cue-reactivity, we propose considering the G allele of rs11503014 in GABRA2 as a predictor of cue-reactivity and the A allele of rs2236256 in OPRM1 as a predictor of non-reactivity. It is noteworthy that rs11503014 in GABRA2 has been shown to be associated with heroin dependence,29 suggesting a potential involvement of this SNP with a functional effect in drug addiction. Previous literature provides little information regarding cocaine cue-reactivity as a biological phenotype for cocaine craving; our findings suggest that synaptic inhibitory systems may participate in drug reward, particularly an individual's intrinsic predisposition for drug craving.
While not directly examining cocaine cue-reactivity, other research findings support the role of GABRA2 and OPRM1 in cocaine addiction. GABA-A receptor alpha-2 subunit was involved in mediating the stimulant effects of cocaine in mice,32 with reduced level in rats in cocaine-induced behavioral sensitization.33 In GABRA2-deficient mice, behavioral sensitization by cocaine was absent, and the GABRA2 gene was associated with cocaine addiction.34 OPRM1 encodes the mu opioid receptor,35 and its level was increased by cocaine in several brain regions of the rat, including nucleus accumbens and caudate putamen.36–40 In addition, mu opioid receptor binding was increased in cocaine-dependent patients,41,42 well correlated with cocaine craving42 and relapse after abstinence.43 Further, mu opioid receptor agonist was shown to inhibit cocaine-induced motor activities,43 influence cocaine self-administration in rats,44 and enhance discriminative stimulus effects of cocaine in monkeys.45 In OPRM1-deficient mice, cocaine-induced locomotion and dopamine release were attenuated,46 with less robust cocaine-induced conditioned place preference47,48 and behavioral sensitization.48,49 Taken together, these studies indicate that both GABRA2 and OPRM1 are involved in several aspects of cocaine effects. The two SNPs identified in this study as related to cocaine cue-reactivity, rs11503014 in GABRA2 and rs2236256 in OPRM1, both reside in intron sequences of the respective genes; as such, they are unlikely to directly affect coding capacities of the genes. Further studies will need to be carried out to explore their potential impact on gene expression in human brain tissues,50 as a potential mechanism of the functional effect of these SNPs. It is noteworthy that other SNPs have been studied in these genes, and one SNP, rs11503014, was found to be associated with heroin dependence.29
Our initial focus was on the dopamine system genes, which showed relatively weak associations in this study (Supplementary Table 1), while two other genes well-documented in drug abuse and cocaine addiction showed more promise. The sample size is modest, however, thus limiting statistical power to detect potentially weaker association in dopamine and other genes. Furthermore, without counterbalancing the order of the cue-presentations, we cannot be sure that the observed effects were not related to the order of the cue-presentations, and will need to address this in future studies. These cautionary notes notwithstanding, this is the first study to examine the role of candidate genes in cocaine cue-reactivity. While clearly needing replication in a larger sample, our findings suggest a possible new research direction in cocaine cue-reactivity and genetics — implicating inhibitory synaptic neurotransmission (GABAergic and opioidergic) as a biological mechanism in modulating cocaine cue-reactivity.
Supplementary Material
Acknowledgments
This research was supported by grant R03 DA020434 from the National Institute on Drug Abuse, Rockville, MD (Dr. Smelson).
The authors wish to acknowledge the support by veterans who participated in this research. We also wish to thank the Bedford VA Medical Center.
Footnotes
Declaration of Interest The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- 1.Childress A, Mozley P, McElgin W, et al. Limbic activation during cue-induced cocaine craving. Am J Psychiatry. 1999;156(1):11. doi: 10.1176/ajp.156.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Garavan H, Pankiewicz J, Bloom A, et al. Cue-induced cocaine craving: neuroanatomical specificity for drug users and drug stimuli. Am J Psychiatry. 2000;157(11):1789–98. doi: 10.1176/appi.ajp.157.11.1789. [DOI] [PubMed] [Google Scholar]
- 3.Kosten TR, Scanley BE, Tucker KA, et al. Cue-induced brain activity changes and relapse in cocaine-dependent patients. Neuropsychopharmacology. 2006;31(3):644–50. doi: 10.1038/sj.npp.1300851. [DOI] [PubMed] [Google Scholar]
- 4.Maas LC, Lukas SE, Kaufman MJ, et al. Functional magnetic resonance imaging of human brain activation during cue-induced cocaine craving. Am J Psychiatry. 1998;155(1):124–126. doi: 10.1176/ajp.155.1.124. [DOI] [PubMed] [Google Scholar]
- 5.Wexler BE, Gottschalk CH, Fulbright RK, et al. Functional magnetic resonance imaging of cocaine craving. Am J Psychiatry. 2001;158(1):86–95. doi: 10.1176/appi.ajp.158.1.86. [DOI] [PubMed] [Google Scholar]
- 6.Franklin TR, Lohoff FW, Wang Z, et al. DAT genotype modulates brain and behavioral responses elicited by cigarette cues. Neuropsychopharmacology. 2009;34:717–728. doi: 10.1038/npp.2008.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li Y, Shao C, Zhang D, et al. The effect of dopamine D2, D5 receptor and transporter (SLC6A3) polymorphisms on the cue-elicited heroin craving in Chinese. Am J Med Genet. 2006;141B:269–273. doi: 10.1002/ajmg.b.30264. [DOI] [PubMed] [Google Scholar]
- 8.Shao C, Li Y, Jiang K, et al. Dopamine D4 receptor polymorphism modulates cue-elicited heroin craving in Chinese. Psychoparmacology (Berl) 2006;186(2):185–190. doi: 10.1007/s00213-006-0375-6. [DOI] [PubMed] [Google Scholar]
- 9.van den Wildenberg E, Wiers RW, Dessers J, et al. A functional polymorphism of the mu-opioid receptor gene (OPRM1) influences cue-induced craving for alcohol in male heavy drinkers. Alcohol Clin Exp Res. 2007;31(1):1–10. doi: 10.1111/j.1530-0277.2006.00258.x. [DOI] [PubMed] [Google Scholar]
- 10.Filbey FM, Schacht JP, Myers US, Chavez RS, Hutchison KE. Individual and additive effects of the CNR1 and FAAH genes on brain response to marijuana cues. Neuropsychopharmacology. 2010;35(4):967–75. doi: 10.1038/npp.2009.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Avants KS, Margolin A, Kosten T, Cooney NL. Differences between responders and non-responders to cocaine cues in a laboratory. Addict Behav. 1995;20:215–224. doi: 10.1016/0306-4603(94)00066-2. [DOI] [PubMed] [Google Scholar]
- 12.Childress A, Ehrman R, McLellan A, O'Brien C. Conditioned craving and arousal in cocaine addiction: a preliminary report. Problems of drug dependence. 1987:88–1564. [PubMed] [Google Scholar]
- 13.Smelson DA, Roy A, Roy M, Torshikovic D. Electroretinogram blue cone amplitude and cue-eliciting cocaine craving: A replication. Am J Drug Alcohol Abuse. 2001;27:391–397. doi: 10.1081/ada-100103716. [DOI] [PubMed] [Google Scholar]
- 14.Smelson DA, Williams J, Ziedonis DM, Losonczy M, Kaune M. The efficacy of olanzapine for decreasing cue-elicited craving in individuals with schizophrenia and cocaine dependence: A preliminary report. J Clin Psychopharmacol. 2006;26(1):9–12. doi: 10.1097/01.jcp.0000194624.07611.5e. [DOI] [PubMed] [Google Scholar]
- 15.Sussner BD, Smelson DA, Rodrigues S, et al. The validity and reliability of a brief measure of cocaine craving. Drug Alcohol Depend. 2006;83(3):233–7. doi: 10.1016/j.drugalcdep.2005.11.022. [DOI] [PubMed] [Google Scholar]
- 16.Suzuki T, Abe S, Yamaguchi M, et al. Effects of cocaine administration on receptor binding and subunits mRNA of GABA(A)-benzodiazepine receptor complexes. Synapse. 2000;38(2):198–215. doi: 10.1002/1098-2396(200011)38:2<198::AID-SYN11>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 17.First MB, Spitzer RL, Gibbon M, Williams J.b.W. Structured clinical interview for DSM-IV access I disorders, clinical version. Columbia University; New York: 1997. [Google Scholar]
- 18.Alterman AI, Droba M, McLellan AT. Response to day hospital treatment by patients with cocaine and alcohol dependence. Hosp Community Psychiatry. 1992;43(9):930–2. doi: 10.1176/ps.43.9.930. [DOI] [PubMed] [Google Scholar]
- 19.McLellan AT, Luborsky L, Woody GE, Obrien CP. Improved Diagnostic Evaluation Instrument for Substance Abuse Patients - Addiction Severity Index. J Nerv Ment Dis. 1980;168(1):26–33. doi: 10.1097/00005053-198001000-00006. [DOI] [PubMed] [Google Scholar]
- 20.Tiffany ST, Singleton E, Haertzen CA, Henningfield JE. The development of a cocaine craving questionnaire. Drug Alcohol Depend. 1993;34(1):19–28. doi: 10.1016/0376-8716(93)90042-o. [DOI] [PubMed] [Google Scholar]
- 21.Voris J, Elder I, Sebastian P. A simple test of cocaine craving and related responses. J Clin Psychol. 1991;47(2):320–3. doi: 10.1002/1097-4679(199103)47:2<320::aid-jclp2270470221>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 22.Mattay VS, Goldberg TE, Fera F, et al. Catechol O-methyltransferase val158-met genotype and individual variation in the brain response to amphetamine. Proc Natl Acad Sci US A. 2003;100(10):6186. doi: 10.1073/pnas.0931309100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vandenbergh DJ, Rodriguez LA, Miller IT, Uhl GR, Lachman HM. High-activity catechol-O-methyltransferase allele is more prevalent in polysubstance abusers. Am J. Med Genet. 1997;74(4):439–442. [PubMed] [Google Scholar]
- 24.Volkow ND, Fowler JS, Wang GJ, Goldstein RZ. Role of Dopamine, the Frontal Cortex and Memory Circuits in Drug Addiction: Insight from Imaging Studies. Neurobiol Learn Mem. 2002;78(3):610–624. doi: 10.1006/nlme.2002.4099. [DOI] [PubMed] [Google Scholar]
- 25.Wang T, Franke P, Neidt H, et al. Association study of the low-activity allele of catechol-O-methyltransferase and alcoholism using a family-based approach. Mol Psychiatry. 2001;6(1):109–111. doi: 10.1038/sj.mp.4000803. [DOI] [PubMed] [Google Scholar]
- 26.Lohoff FW, Weller AE, Bloch PJ, et al. Association between the catechol-O-methyltransferase Val158Met polymorphism and cocaine dependence. Neuropsychopharmacology. 2008;33(13):3078–3084. doi: 10.1038/npp.2008.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang H, Kranzler HR, Weiss RD, et al. Pro-opiomelanocortin gene variation related to alcohol or drug dependence: evidence and replications across family-and population-based studies. Biol Psychiatry. 2009;66(2):128–136. doi: 10.1016/j.biopsych.2008.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bloch PJ, Nall AH, Weller AE, et al. Association analysis between polymorphisms in the dopamine D3 receptor (DRD3) gene and cocaine dependence. Psychiatric genetics. 2009;19(5):275. doi: 10.1097/YPG.0b013e32832cec12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Enoch MA, Hodgkinson CA, Yuan Q, et al. The influence of GABRA2, childhood trauma, and their interaction on alcohol, heroin, and cocaine dependence. Biol. Psychiatry. 2010;67(1):20–27. doi: 10.1016/j.biopsych.2009.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Haile CN, Kosten TR, Kosten TA. Pharmacogenetic treatments for drug addiction: cocaine, amphetamine and methamphetamine. Am J Drug Alcohol Abuse. 2009;35(3):161–177. doi: 10.1080/00952990902825447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mardia KV, Kent JT, Bibby JM. Chapter 14, in Multivariate Analysis. Academic Press; London: 1979. [Google Scholar]
- 32.Morris HV, Dawson GR, Reynolds DS, et al. Alpha2-containing GABA(A) receptors are involved in mediating stimulant effects of cocaine. Pharmacol Biochem Behav. 2008;90(1):9–18. doi: 10.1016/j.pbb.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 33.Chen Q, Lee TH, Wetsel WC, et al. Reversal of cocaine sensitization-induced behavioral sensitization normalizes GAD67 and GABA(A) receptor alpha 2 subunit expression, and PKC zeta activity. Biochem Biophys Res Commun. 2007;356(3):733–738. doi: 10.1016/j.bbrc.2007.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dixon CI, Morris HV, Breen G, et al. Cocaine effects on mouse incentive-learning and human addiction are linked to alpha 2 subunit-containing GABA(A) receptors. Proc Natl Acad Sci US A. 2010;107(5):2289–2294. doi: 10.1073/pnas.0910117107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen Y, Mestek A, Liu J, Hurley JA, Yu L. Molecular cloning and functional expression of a mu-opioid receptor from rat brain. Mol Pharmacol. 1993;44(1):8–12. [PubMed] [Google Scholar]
- 36.Bailey A, Gianotti R, Ho A, Kreek MJ. Persistent upregulation of mu-opioid, but not adenosine, receptors in brains of long-term withdrawn escalating dose “binge” cocaine-treated rats. Synapse. 2005;57(3):160–6. doi: 10.1002/syn.20168. [DOI] [PubMed] [Google Scholar]
- 37.Unterwald EM, Horne-King J, Kreek MJ. Chronic cocaine alters brain mu opioid receptors. Brain Res. 1992;584(1–2):314–8. doi: 10.1016/0006-8993(92)90912-s. [DOI] [PubMed] [Google Scholar]
- 38.Unterwald EM, Rubenfeld JM, Kreek MJ. Repeated Cocaine Administration up-Regulates Kappa-Opioid and Mu-Opioid, but Not Delta-Opioid Receptors. Neuroreport. 1994;5(13):1613–1616. doi: 10.1097/00001756-199408150-00018. [DOI] [PubMed] [Google Scholar]
- 39.Yuferov V, Zhou Y, Spangler R, et al. Acute “binge” cocaine increases mu-opioid receptor mRNA levels in areas of the rat mesolimbic mesocortical dopamine system. Brain Res Bull. 1999;48(1):109–12. doi: 10.1016/s0361-9230(98)00155-5. [DOI] [PubMed] [Google Scholar]
- 40.Azaryan AV, Coughlin LJ, Buzas B, Clock BJ, Cox BM. Effect of chronic cocaine treatment on mu- and delta-opioid receptor mRNA levels in dopaminergically innervated brain regions. J Neurochem. 1996;66(2):443–448. doi: 10.1046/j.1471-4159.1996.66020443.x. [DOI] [PubMed] [Google Scholar]
- 41.Zubieta JK, Gorelick DA, Stauffer R, et al. Increased mu opioid receptor binding detected by PET in cocaine-dependent men is associated with cocaine craving. Nat Med. 1996;2(11):1225–1229. doi: 10.1038/nm1196-1225. [DOI] [PubMed] [Google Scholar]
- 42.Gorelick DA, Kim YK, Bencherif B, et al. Imaging brain mu-opioid receptors in abstinent cocaine users: Time course and relation to cocaine craving. Biol Psychiatry. 2005;57(12):1573–1582. doi: 10.1016/j.biopsych.2005.02.026. [DOI] [PubMed] [Google Scholar]
- 43.Ukai M, Mizutani M, Kameyama T. Opioid peptides selective for receptor types modulate cocaine-induced behavioral responses in mice. Nihon Shinkei Seishin Yakurigaku Zasshi. 1994;14(3):153–9. [PubMed] [Google Scholar]
- 44.Corrigall WA, Coen KM, Adamson KL, Chow BLC. Manipulations of mu-opioid and nicotinic cholinergic receptors in the pontine tegmental region alter cocaine self-administration in rats. Psychopharmacology. 1999;145(4):412–417. doi: 10.1007/s002130051075. [DOI] [PubMed] [Google Scholar]
- 45.Rowlett JK, Spealman RD. Opioid enhancement of the discriminative stimulus effects of cocaine: evidence for involvement of mu and delta opioid receptors. Psychopharmacology (Berl) 1998;140(2):217–24. doi: 10.1007/s002130050760. [DOI] [PubMed] [Google Scholar]
- 46.Chefer VI, Kieffer BL, Shippenberg TS. Contrasting effects of mu opioid receptor and delta opioid receptor deletion upon the behavioral and neurochemical effects of cocaine. Neuroscience. 2004;127(2):497–503. doi: 10.1016/j.neuroscience.2004.05.021. [DOI] [PubMed] [Google Scholar]
- 47.Becker A, Grecksch G, Kraus J, et al. Rewarding effects of ethanol and cocaine in mu opioid receptor-deficient mice. Naunyn Schmiedebergs Arch Pharmacol. 2002;365(4):296–302. doi: 10.1007/s00210-002-0533-2. [DOI] [PubMed] [Google Scholar]
- 48.Hall FS, Goeb M, Li XF, Sora L, Uhl GR. mu-opioid receptor knockout mice display reduced cocaine conditioned place preference but enhanced sensitization of cocaine-induced locomotion. Molecular Brain Research. 2004;121(1–2):123–130. doi: 10.1016/j.molbrainres.2003.10.024. [DOI] [PubMed] [Google Scholar]
- 49.Hummel M, Ansonoff MA, Pintar JE, Unterwald EM. Genetic and pharmacological manipulation of mu opioid receptors in mice reveals a differential effect on behavioral sensitization to cocaine. Neuroscience. 2004;125(1):211–220. doi: 10.1016/j.neuroscience.2004.01.025. [DOI] [PubMed] [Google Scholar]
- 50.Johnson AD, Zhang Y, Papp AC, et al. Polymorphisms affecting gene transcription and mRNA processing in pharmacogenetic candidate genes: detection through allelic expression imbalance in human target tissues. Pharmacogenet Genomics. 2008;18(9):781. doi: 10.1097/FPC.0b013e3283050107. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.