Abstract
In this issue of Science Translational Medicine, Wallin et al. have identified a subset of breast and ovarian cancer cell lines that show synergistic response to the combination of doxorubicin and GDC-0941, a class IA phosphatidylinositol 3-kinase (PI3K) inhibitor. Here, we discuss the potential implications of these data on the clinical development of PI3K pathway inhibitors as cancer therapeutics.
In this issue of Science Translational Medicine, Wallin et al. (1) explore the effects of the small molecule GDC-0941, a specific inhibitor of type 1A isoforms of phosphatidylinositol 3-kinase (PI3K), in combination with the chemotherapeutic agent doxorubicin in a collection of breast and ovarian cancer cell lines. This work is timely and important because it focuses on breast and ovarian cancers, two leading causes of cancer-related mortalities (2); it evaluates the mechanistic effects of a combination of anticancer agents—doxorubicin, a traditional cytotoxic drug, and GDC-0941, one of many PI3K pathway inhibitors entering the clinical arena; and it touches upon the importance of biomarker development, which promises to be one of the greatest clinical challenges in the era of personalized cancer medicine.
PI3K AND ITS PARTNERS
The PI3K signaling pathway (Fig. 1) regulates cell growth, proliferation, and differentiation. PI3K enzymes are activated by G protein–coupled receptors and receptor tyrosine kinases and transfer phosphate groups to the inositol ring of phosphatidylinositol to produce the signaling molecule phosphatidylinositol 3-phosphate (PIP3) (3). Alterations in the PI3K pathway are among the most common molecular aberrations found in tumors and are important in both the development and maintenance of cancer (4). There are many underlying mechanisms for PI3K pathway activation in cancer, including receptor tyrosine kinase activation or amplification; mutation, deletion, or silencing of negative regulators of PI3K; and activation or amplification of downstream kinase mediators (Fig. 1) (4). The PI3K pathway also interacts with the Ras–extracellular signal–related kinase (ERK) signaling cascade, which is also regulated by receptor tyrosine kinases. These two pathways have redundant functions, and because they share multiple negative-feedback loops, isolated inhibition of either pathway will often result in compensatory activation of the other (5).
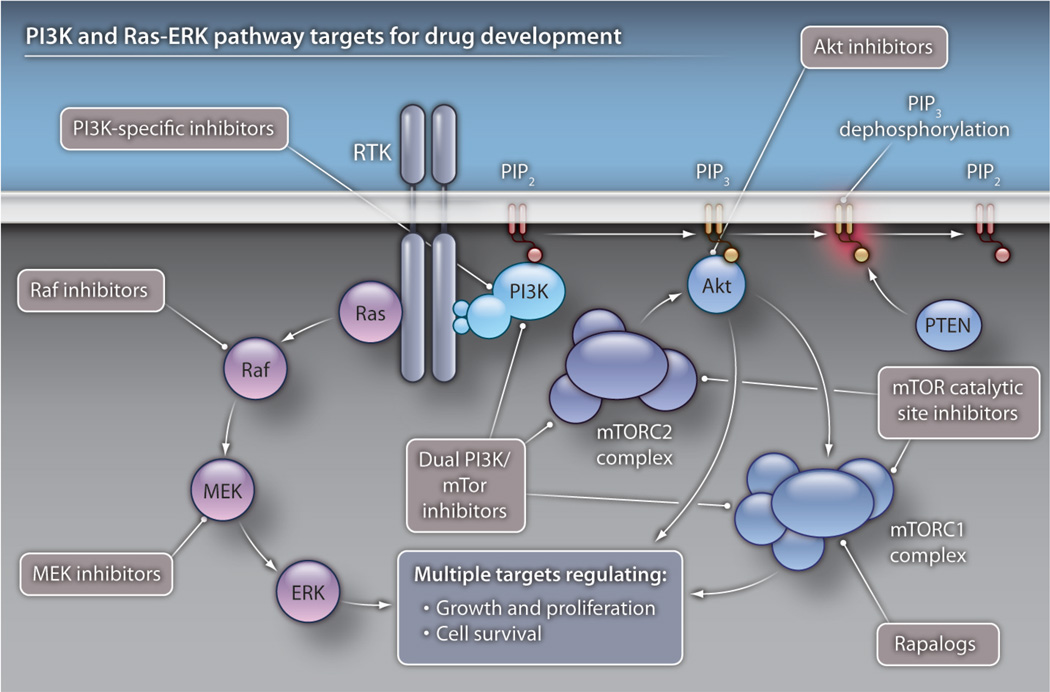
Fig. 1. The PI3K pathway: tons of targets.
In normal physiological situations, the PI3K pathway is activated by an extracellular stimulus that results in activation of a receptor tyrosine kinase (RTK). This activation causes recruitment of PI3K (which comprises the p85 regulatory subunit and the p110 catalytic subunit) to the intracellular component of the RTK. PI3K phosphorylates the lipid moiety phosphatidylinositol 2-phosphate (PIP2) to form phosphatidylinositol 3-phosphate (PIP3), which further recruits other downstream kinases, including Akt, to the membrane for phosphorylation, activation, and targeting of effector molecules that regulate energy uptake, growth, and proliferation. Negative regulators of this pathway include PTEN and inositol polyphosphate 4-phosphatase (INPP4), which are lipid phosphatases. The p85 subunit of PI3K also has important regulatory roles. Several classes of drug agents that target this pathway are in clinical development and include class IA–specific PI3K inhibitors, PI3K/mTOR dual inhibitors, Akt inhibitors, mTORC catalytic inhibitors, and rapamycin analogs (rapalogs). Inhibitors that are specific for selected PI3K isoforms are also in clinical development. The Ras-ERK pathway is also activated by RTKs. Both Raf and MEK inhibitors are in clinical development.
Breast and ovarian cancers are clear disease targets for PI3K inhibitor use. The most common modes of PI3K activation in breast cancer include amplification of the human epidermal growth factor receptor 2 gene (HER2), which encodes a receptor tyrosine kinase that interacts with PI3K (30%) (6); activating mutations in the PIK3CA gene, which encodes p110α, the catalytic subunit of PI3K (25 to 30%) (7–9); and loss of the phosphatase and tensin (PTEN) tumor suppressor gene, which encodes a protein phosphatase and negatively regulates PI3K signaling pathways (10%) (9, 10). Activation of estrogen receptor alpha (ERα) on breast cancer cells stimulates tumor growth, and ERα also has been shown to activate PI3K signaling (11). Genomic analyses reveal that high-grade ovarian cancers are characterized by chromosomal amplifications and deletions. Amplification of the PIK3CA gene is noted in 10 to 30% of ovarian cancers (8, 12). And the loss of chromosome locus 4q31.21, which carries the candidate tumor suppressor gene INPP4, may also be important in as many as 30% of ovarian cancers; similarly to PTEN, INPP4 encodes a phosphatase protein that inhibits the PI3K signaling pathway (13).
The small molecule GDC-0941 is an inhibitor of all class IA PI3Ks and is one of the several kinds of PI3K pathway inhibitors currently in clinical development. Others include PI3K/mammalian target of rapamycin (mTOR) dual inhibitors, which are catalytic inhibitors of both class IA PI3K and mTOR complexes (mTORC1 and mTORC2), downstream mediators of PI3K; inhibitors of Akt, a serine/threonine kinase and the main effector of PI3K; mTORC catalytic inhibitors, which target both the mTORC1 and mTORC2 complexes; and rapamycin analogs (rapalogs), which target only mTORC1. Inhibitors that are specific for selected PI3K isoforms are also in clinical development (14, 15). Although the rapalogs are approved by the Food and Drug Administration for the treatment of renal cell carcinoma, the remainder of these agents are in either phase I or II clinical trials. As such, clinical experience with them is limited. But data from these early trials suggest that these drugs are generally safe, and although phase I studies are not designed to address drug efficacy, a few clinical responses to these agents have been documented, including in patients with breast and ovarian cancers (15–17). Phase I and II clinical trials investigating combination drug regimens that include treatment with both traditional cytotoxic chemotherapeutic drugs (such as doxorubicin) and targeted therapeutics (such as GDC-0941) also are under way (18).
LESSONS LEARNED
Although the efficacy of PI3K inhibitors in patients has yet to be established, the clinical development of other molecularly targeted agents offers important considerations as these drugs move forward. First, the identification of specific oncogenic alterations within a given tumor can have clinical utility. Second, the development of reproducible, widely applicable biomarkers can be deceptively difficult. Third, even in molecularly characterized patient subpopulations, responses to single agents are often not durable because of the development of drug resistance. The history of HER2-targeted therapeutics exemplifies these points. Trastuzumab, a monoclonal antibody that binds to and inhibits the activity of HER2, has had substantial impact in the treatment of HER2-expressing breast cancers but is minimally efficacious in patients without HER2-driven cancers (19). Even given the clear clinical utility of targeting HER2, classification of HER2 expression in breast tumors remains imperfect. Although several professional societies have submitted guidelines for the test conditions and interpretation of HER2-overexpression assays, they remain susceptible to variable reporting (20). Clinically, trastuzumab has some efficacy used as a single agent (21) but is currently used primarily in combination therapy, which has resulted in more durable responses than is achieved with trastuzumab alone (22, 23).
MECHANISMS OF SYNERGY
Results presented by Wallin et al. (1) are pertinent to our understanding of the cellular effects of PI3K inhibition in combination with a cytotoxic chemotheraputic agent. Briefly, the study describes the effects of treatment of 32 cancer cell lines with a combination of GDC-0941 and doxorubicin. Twenty of these cell lines were from breast cancers, representing luminal and basal subtypes, and 12 were from ovarian cancers. When treated with doxorubicin, there was variable phosphorylation of Akt (a measurement that represents Akt activation) among the cell lines. As compared to those without increased Akt phosphorylation (pAkt), cell lines with greater than a 1.5-fold increase in pAkt were relatively resistant to treatment with single-agent doxorubicin but relatively sensitive to a synergistic effect of doxorubicin and GDC-0941. This suggests that in the setting of doxorubicin exposure, these cell lines are particularly dependent on PI3K signaling. Focusing on two cell lines, one sensitive (MCF-7) and one resistant (HDQ-P1) to the drug combination, the mechanism of these treatment effects was explored. When treated with doxorubicin alone, both the MCF-7 and HDQ-P1 cell lines underwent cell-cycle arrest, most likely in response to doxorubicin-induced DNA damage. However, with the addition of GDC-0941, the MCF-7 lines progressed through the cell cycle in spite of the DNA damage, triggered the DNA-damage stress response, and succumbed to cell death. The HDQ-P1 cell line remained in cell-cycle arrest and did not undergo cellular death. This suggests that the MCF-7 cell line (which has increased pAkt in the presence of doxorubicin), as compared with HDQ-P1 (which displayed no doxorubicin-induced change in pAkt), may be more reliant on PI3K signaling for its DNA damage or stress response. Consistent with this notion, the authors show that the doxorubicin-induced pAkt in MCF-7 cell lines relies in part on DNA-PK, a protein kinase implicated in the DNA damage response. Finally, xenograft studies in which the MCF-7 cell lines were transplanted into immunocompromised mice suggest the potential utility of this therapeutic combination in vivo. These experiments showed an enhanced effect of doxorubicin and GDC-0941 in tumor stasis compared with that achieved with either agent alone. Although still in early preclinical stages, the results presented by Wallin et al. (1) offer additional ideas for how PI3K inhibitors can be optimally developed for clinical use.
BETTER PREDICTIVE BIOMARKERS
Several preclinical studies suggest that the most promising patient subpopulation for response to PI3K pathway inhibitors as single agents is that comprising individuals with cancers that carry PI3K pathway–driving mutations, such as tumors with activating mutations in PIK3CA (15, 24, 25). Molecular data that include the mutation status of PIK3CA and the presence or absence of PTEN protein have been collected from the tumors of some of the patients enrolled in early clinical trials of PI3K pathway inhibitors (16, 17, 26–28). Clear association of these biomarkers with drug response has not yet been verified, but such associations are difficult to prove in Phase I trials, which enroll heterogeneous populations of patients, many of whom have progressed through several chemotherapy regimens and, by design, receive subtherapeutic doses of the agent under investigation. Moving forward, the collection of molecular data from patient tumors—including information on mutations, amplifications, and deletions in genes that encode components of the PI3K pathway—may help to identify patients who are more or less likely to respond to PI3K inhibitors as single agents.
Phase I safety trials of PI3K inhibitors in combination with other agents, either molecularly targeted or cytotoxic, are in early stages. Clinical biomarkers for combinations of molecularly targeted agents have been suggested by preclinical studies and cotranslational studies. For example, in a mouse model of a K-Ras–driven lung tumor, PI3K inhibition is not sufficient for tumor stasis. However, the addition of a mitogen-activated protein/extracellular signal–regulated kinase kinase (MEK) inhibitor results in tumor shrinkage (25). In addition, as demonstrated in pre- and posttreatment patient biopsies, the Ras-ERK pathway is activated in a subset of patients who had been taking a rapalog (5, 29). Taken together, these data support the hypothesis that patients who have tumors that display either primary or secondary activation of the Ras-ERK pathway may benefit from a combination of therapies that target both this and the PI3K signaling pathways.
Molecular markers that are able to predict tumor response to a combination of PI3K inhibition and a cytotoxic agent have not been extensively studied. Wallin et al. note that the molecular characteristics of cancer cell lines that were responsive to the synergistic effect of doxorubicin and GDC-0941, including PIK3CA mutation, PTEN protein loss, and mutations in the gene encoding the tumor supressor p53, were not significantly associated with the effects of the combined drug treatment. However, within the breast cancer cell line studies, the three cell lines that had PIK3CA mutations, including the MCF-7 line, were the most sensitive to the combination treatment. These observations suggest that investigating this association in a larger number of PIK3CA mutant breast cancer cell lines may be warranted.
The synergistic effect of doxorubicin and GDC-0941 was associated with another molecular marker, an elevation in pAkt primarily in the nuclear fractions of responsive cancer cell extracts, and so it was postulated that nuclear pAkt may serve as a clinical predictive biomarker. However, cancer cell lines—even in the context of a xenograft—do not always reflect the biology of an endogenous tumor. As such, the association between elevated pAkt and a synergistic response to PI3K inhibition and doxorubicin will need to be further corroborated in other experimental systems, such as genetic mouse models and then, perhaps, patient samples. In this context, it is important to note that measuring real-time pathway activation in a patient’s tumor is quite challenging.
Clinical diagnosis and treatment decisions rely primarily on the analysis of formalin-fixed, paraffin-embedded tumor samples, usually from a biopsy or surgery at the time of initial diagnosis. Although this method of sample preparation is optimal for maintaining the tissue architecture required for histological diagnosis, it is not optimal for immunohistochemical studies using antibodies directed against labile phosphorylated proteins, such as pAkt. Moreover, as we continue to search for biomarkers that predict response to treatment, it is quite possible that results obtained from the study of an original biopsy sample may not reflect the patient’s disease after undergoing DNA-damaging treatments such as radiation or chemotherapy. Cotranslational studies that employ pre- and posttreatment tumor biopsies will probably become more common in studies of molecularly targeted anticancer agents because this approach will allow for optimal sample preparation for protein studies and reflect the changing biology of a tumor, including the responses of signaling pathways to treatment. Recognizing that these procedures are invasive, the development of radiographic techniques to probe the molecular underpinning of tumors and methods for the isolation of tumor cells from blood are under way.
DRUG COMBOS IN THE CLINICS
The synergistic effect of doxorubicin and GDC-0941 in both the in vitro and in vivo studies in the work by Wallin et al. (1) suggests the clinical utility of this combination. What remains unclear, but certainly will be of both scientific and clinical interest, is whether or not these effects will be generalizable to combinations of other PI3K inhibitors and other chemotherapeutic agents. GDC-0941 is a class IA inhibitor. Will Akt, mTOR, or dual PI3K/mTOR inhibitors display the same synergistic effect with doxorubicin? Most chemotherapeutic drugs inflict DNA damage and cellular stress. Will other chemotherapeutic agents or even radiation therapy create similar responses in PI3K signaling? In the meantime, the clinical safety of such combinations will need to be evaluated before studies of efficacy.
In conclusion, PI3K pathway inhibitors are rapidly entering the clinical arena, and their role in the treatment of cancers is actively being designed. Broadly, this new study may suggest that, given the pleiotropic effects of PI3K signaling in cellular physiology, PI3K inhibition may have a variety of mechanisms for inducing tumor death. Moreover, there may be uses for PI3K inhibitors, both singly and in combination with other cytotoxic drugs, in a broader subset of patients than previously imagined. Although some lessons learned from other targeted anticancer agents suggest that the utility of identifying specific patient responders is great, other lessons stress that many of the practical challenges of targeted drug discovery and development remain.
Acknowledgments
Funding: The authors acknowledge the Stand Up To Cancer Foundation, the National Institutes of Health, and the Dana-Farber/Harvard Cancer Center for grant support to study the PI3K pathway in women’s cancers. A.P.M. also receives funding from the Dana-Farber/Harvard Cancer Center.
Footnotes
Competing interests: L.C.C. has consulted for companies that are generating PI3K pathway inhibitors, including Genentech, Novartis, GlaxoSmithKline, Bristol- Myers Squibb, and AstraZeneca. A.P.M. declares that she has no competing interests.
References and Notes
- 1.Wallin JJ, Guan J, Prior WW, Edgar KA, Kassees R, Sampath D, Belvin M, Friedman LS. Nuclear phosphoAkt increase predicts synergy of PI3K inhibition and doxorubicin in breast and ovarian cancer. Sci. Transl. Med. 2010;2:48ra66. doi: 10.1126/scitranslmed.3000630. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J. Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 3.Engelman JA, Luo J, Cantley LC. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat. Rev. Genet. 2006;7:606–619. doi: 10.1038/nrg1879. [DOI] [PubMed] [Google Scholar]
- 4.Yuan TL, Cantley LC. PI3K pathway alterations in cancer: Variations on a theme. Oncogene. 2008;27:5497–5510. doi: 10.1038/onc.2008.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carracedo A, Pandolfi PP. The PTEN-PI3K pathway: Of feedbacks and cross-talks. Oncogene. 2008;27:5527–5541. doi: 10.1038/onc.2008.247. [DOI] [PubMed] [Google Scholar]
- 6.Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: Correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 7.Samuels Y, Wang Z, Bardelli A, Silliman N, Ptak J, Szabo S, Yan H, Gazdar A, Powell SM, Riggins GJ, Willson JKV, Markowitz S, Kinzler KW, Vogelstein B, Velculescu VE. High frequency of mutations of the PIK3CA gene in human cancers. Science. 2004;304:554. doi: 10.1126/science.1096502. [DOI] [PubMed] [Google Scholar]
- 8.Campbell IG, Russell SE, Choong DY, Montgomery KG, Ciavarella ML, Hooi CS, Cristiano BE, Pearson RB, Phillips WA. Mutation of the PIK3CA gene in ovarian and breast cancer. Cancer Res. 2004;64:7678–7681. doi: 10.1158/0008-5472.CAN-04-2933. [DOI] [PubMed] [Google Scholar]
- 9.Forbes SA, Bhamra G, Bamford S, Dawson E, Kok C, Clements J, Menzies A, Teague JW, Futreal PA, Stratton MR. The catalogue of somatic mutations in cancer (COSMIC). Chapter 10, p. Unit 10.11. Curr. Protoc. Hum. Genet. 2008 doi: 10.1002/0471142905.hg1011s57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li J, Yen C, Liaw D, Podsypanina K, Bose S, Wang SI, Puc J, Miliaresis C, Rodgers L, McCombie R, Bigner SH, Giovanella BC, Ittmann M, Tycko B, Hibshoosh H, Wigler MH, Parsons R. PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science. 1997;275:1943–1947. doi: 10.1126/science.275.5308.1943. [DOI] [PubMed] [Google Scholar]
- 11.Sun M, Paciga JE, Feldman RI, Yuan Z, Coppola D, Lu YY, Shelley SA, Nicosia SV, Cheng JQ. Phosphatidylinositol- 3-OH kinase (PI3K)/AKT2, activated in breast cancer, regulates and is induced by estrogen receptor α (ERalpha) via interaction between ERalpha and PI3K. Cancer Res. 2001;61:5985–5991. [PubMed] [Google Scholar]
- 12.Nakayama K, Nakayama N, Kurman RJ, Cope L, Pohl G, Samuels Y, Velculescu VE, Wang TL, Shih I-M. Sequence mutations and amplification of PIK3CA and AKT2 genes in purified ovarian serous neoplasms. Cancer Biol. Ther. 2006;5:779–785. doi: 10.4161/cbt.5.7.2751. [DOI] [PubMed] [Google Scholar]
- 13.Gewinner C, Wang ZC, Richardson A, Teruya-Feldstein J, Etemadmoghadam D, Bowtell D, Barretina J, Lin WM, Rameh L, Salmena L, Pandolfi PP, Cantley LC. Evidence that inositol polyphosphate 4-phosphatase type II is a tumor suppressor that inhibits PI3K signaling. Cancer Cell. 2009;16:115–125. doi: 10.1016/j.ccr.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Engelman JA. Targeting PI3K signalling in cancer: Opportunities, challenges and limitations. Nat. Rev. Cancer. 2009;9:550–562. doi: 10.1038/nrc2664. [DOI] [PubMed] [Google Scholar]
- 15.Courtney KD, Corcoran RB, Engelman JA. The PI3K pathway as drug target in human cancer. J. Clin. Oncol. 2010;28:1075–1083. doi: 10.1200/JCO.2009.25.3641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Von Hoff DD, LoRusso P, Tibes R, Shapiro G, Weiss GJ, Ware JA, Fredrickson J, Mazina KE, Levy GG, Wagner AJ. A first-in-human phase I study to evaluate the pan-PI3K inhibitor GDC-0941 administered QD or BID in patients with advanced solid tumors. J. Clin. Oncol. 2010;28:15s:2541. [Google Scholar]
- 17.Baselga J, De Jonge MJ, Rodon J, Burris HA, III, Birle DC, De Buck SS, Demanse D, Ru QC, Goldbrunner M, Bendell JC. A first-in-human phase I study of BKM120, an oral pan-class I PI3K inhibitor, in patients (pts) with advanced solid tumors. J. Clin. Oncol. 2010;28:15s:3003. doi: 10.1200/JCO.2011.36.1360. [DOI] [PubMed] [Google Scholar]
- 18.See the U.S. National Institutes of Health clinical trials Web site, http://clinicaltrials.gov.
- 19.Mass RD, Press MF, Anderson S, Cobleigh MA, Vogel CL, Dybdal N, Leiberman G, Slamon DJ. Evaluation of clinical outcomes according to HER2 detection by fluorescence in situ hybridization in women with metastatic breast cancer treated with trastuzumab. Clin. Breast Cancer. 2005;6:240–246. doi: 10.3816/CBC.2005.n.026. [DOI] [PubMed] [Google Scholar]
- 20.Henry NL, Hayes DF. Uses and abuses of tumor markers in the diagnosis, monitoring, and treatment of primary and metastatic breast cancer. Oncologist. 2006;11:541–552. doi: 10.1634/theoncologist.11-6-541. [DOI] [PubMed] [Google Scholar]
- 21.Baselga J, Tripathy D, Mendelsohn J, Baughman S, Benz CC, Dantis L, Sklarin NT, Seidman AD, Hudis CA, Moore J, Rosen PP, Twaddell T, Henderson IC, Norton L. Phase II study of weekly intravenous recombinant humanized anti-p185HER2 monoclonal antibody in patients with HER2/neu-overexpressing metastatic breast cancer. J. Clin. Oncol. 1996;14:737–744. doi: 10.1200/JCO.1996.14.3.737. [DOI] [PubMed] [Google Scholar]
- 22.Pegram MD, Lipton A, Hayes DF, Weber BL, Baselga JM, Tripathy D, Baly D, Baughman SA, Twaddell T, Glaspy JA, Slamon DJ. Phase II study of receptorenhanced chemosensitivity using recombinant humanized anti-p185HER2/neu monoclonal antibody plus cisplatin in patients with HER2/neu-overexpressing metastatic breast cancer refractory to chemotherapy treatment. J. Clin. Oncol. 1998;16:2659–2671. doi: 10.1200/JCO.1998.16.8.2659. [DOI] [PubMed] [Google Scholar]
- 23.National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology, Breast Cancer V.2.2010. available at www.nccn.org/professionals/physician_gls/f_guidelines.asp.
- 24.She QB, Chandarlapaty S, Ye Q, Lobo J, Haskell KM, Leander KR, DeFeo-Jones D, Huber HE, Rosen N, Ouchi T. Breast tumor cells with PI3K mutation or HER2 amplification are selectively addicted to Akt signaling. PLoS ONE. 2008;3:e3065. doi: 10.1371/journal.pone.0003065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Engelman JA, Chen L, Tan X, Crosby K, Guimaraes AR, Upadhyay R, Maira M, McNamara K, Perera SA, Song Y, Chirieac LR, Kaur R, Lightbown A, Simendinger J, Li T, Padera RF, García-Echeverría C, Weissleder R, Mahmood U, Cantley LC, Wong KK. Effective use of PI3K and MEK inhibitors to treat mutant Kras G12D and PIK3CA H1047R murine lung cancers. Nat. Med. 2008;14:1351–1356. doi: 10.1038/nm.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Burris H, Rodon J, Sharma S, Herbst RS, Tabernero J. First-in-human phase I study of the oral PI3K inhibitor BEZ235 in patients with advanced solid tumors. J. Clin. Oncol. 2010;28:15s:3005. [Google Scholar]
- 27.Edelman G, Bedell C, Shapiro G, Pandya SS, Kwak EL, Scheffold C, Nguyen LT, Laird A, Baselga J, Rodon J. A phase I dose-escalation study of XL147 (SAR245408), a PI3K inhibitor administered orally to patients (pts)with advanced malignancies. J. Clin. Oncol. 2010;28:15s:3004. [Google Scholar]
- 28.Yap TA, Patnaik A, Fearen I, Olmos D, Papadopoulos K, Tunariu N, Sullivan D, Yan L, De Bono JS, Tolcher AW. First-in-class phase I trial of a selective Akt inhibitor, MK2206 (MK), evaluating alternate day (QOD) and once weekly (QW) doses in advanced cancer patients (pts) with evidence of target modulation and antitumor activity. J. Clin. Oncol. 2010;28:15s:3009. [Google Scholar]
- 29.Carracedo A, Ma L, Teruya-Feldstein J, Rojo F, Salmena L, Alimonti A, Egia A, Sasaki AT, Thomas G, Kozma SC, Papa A, Nardella C, Cantley LC, Baselga J, Pandolfi PP. Inhibition of mTORC1 leads to MAPK pathway activation through a PI3K-dependent feedback loop in human cancer. J. Clin. Invest. 2008;118:3065–3074. doi: 10.1172/JCI34739. [DOI] [PMC free article] [PubMed] [Google Scholar]