Abstract
Introduction
Platelet activation/aggregation plays a key role in the dysregulation of coagulation and the development of thrombotic microangiopathy in nonhuman primate recipients of pig xenografts. As a preliminary to the study of anti-platelet therapy in vitro and in vivo, the present study aimed to compare platelet aggregation in whole blood from humans, baboons, and cynomolgus monkeys.
Methods
Using ‘Chrono-log’ technology (two-sample four-channel Chrono-log Whole Blood Aggregometer), we studied aggregation of platelets in healthy humans (n=8), baboons (n=5), and monkeys (n=8). Whole blood (blood) samples were collected, and platelet aggregation was assessed using three different volumes of blood (1, 0.5, and 0.25 mL). Platelet activation was induced using collagen (at 3 and 5 µg/mL), ristocetin (at 0.5 and 1.0 mg/ml), adenosine diphosphate (ADP; at 10, 20, and 40 µM), or thrombin (at 1 and 5 IU/ml). Inhibition of agonist-induced platelet aggregation by heparin and low molecular weight heparin (LMWH) (at 1, 10, and 100 IU/mL) was evaluated.
Results
Mean platelet counts were 222.1, 263.2, and 276.1 (x103/ul) in humans, baboons, and monkeys, respectively. In all 3 species, platelet aggregation was induced by collagen, ristocetin, ADP, or thrombin in a dose-dependent manner. A blood volume of 0.5mL provided the most consistent results with all agonists in all 3 species. Dilution studies indicated that there was a significant positive correlation between platelet count and percent aggregation of platelets (p<0.05). Collagen (3 and 5µg/mL), ADP (10, 20 and 40 µM) and thrombin (1 and 5 IU/ml) induced significantly greater platelet aggregation in humans than in baboons. ADP (20 and 40 µM) and thrombin (1 and 5 IU/ml) induced significantly greater platelet aggregation in monkeys than in baboons. There was no species difference with ristocetin (0.5 or 1.0 mg/ml). In all species, thrombin (1 or 5 IU) induced greater platelet aggregation than any of the other reagents. Heparin at 1 IU/mL and LMWH at 10 IU/ml in all species almost completely abrogated thrombin-induced platelet aggregation. Heparin at 100 IU/mL effectively inhibited platelet aggregation induced by collagen, but only partially inhibited aggregation induced by ADP or ristocetin. LMWH only partially inhibited aggregation induced by collagen, ristocetin and ADP.
Conclusions
The ‘Chrono-log’ technology proved to be a reliable method of evaluating platelet activation and aggregation in vitro in primates. Species differences may play a role in platelet aggregation, with the monkey being more comparable to the human than the baboon, though overall trends were similar. In all species, thrombin induced greater platelet aggregation than other agonists. Even a concentration of heparin of 1 IU/ml, which is probably the maximal concentration that is clinically-applicable, prevented platelet aggregation induced by thrombin, but were less effective in preventing aggregation induced by collagen, ADP, or, particularly, ristocetin.
Keywords: Aggregation, Coagulation, Platelets, Primates, Xenotransplantation
INTRODUCTION
The use of porcine organs or cells as alternatives to those from deceased human donors is viewed by many as a potential solution to the increasing shortage of allografts (1). However, the transplantation of porcine tissue into humans or nonhuman primates elicits a severe and rapid rejection response, resulting in graft loss, even when intensive immunosuppressive therapy is administered. Although many advances have been made with respect to understanding the immunological basis of rejection of highly-disparate organs, there are several remaining barriers to the transplantation of porcine organs into humans or nonhuman primates, of which graft injury caused by thrombotic microangiopathy, with associated consumptive coagulopathy is proving particularly problematic (2, 3, 4). Incompatibilities between the human and porcine coagulation systems have been shown to play a role in the dysregulation of coagulation in xenograft recipients (5, 6, 7). Inhibition of platelet activation, recruitment, and sequestration might inhibit development of the thrombotic microangiopathy and/or consumptive coagulopathy (8). However, details of the mechanism of platelet activation in xenotransplantation remain uncertain.
Platelet aggregation in organ xenografts and in the circulation after xenotransplantation has been reported (9). In porcine hematopoietic progenitor cell xenotransplantation studies (10), Appel, et al. utilized standard aggregometry to assess the effects of the individual components of an immunosuppressive regimen on platelet aggregation in vitro.
As a preliminary study for the evaluation of anti-platelet therapy in vitro and in vivo, we assessed aggregation of human and nonhuman primate (baboons and monkeys) platelets using different agonists. Additionally, we evaluated the inhibitory effect of heparin and low molecular weight heparin (LMWH) on agonist-induced platelet aggregation.
MATERIALS AND METHODS
Blood preparation
Whole blood samples were obtained from healthy human volunteers (n=8), naive baboons (n=5; Papio anubis, average age, 3.1±0.4years, 10.4±3.7kg), naïve cynomolgus monkeys (n=8; 5.0±2.1years, 4.1±1.2kg). None of the blood donors was taking any anti-platelet therapy, e.g., aspirin, or nonsteroidal anti-inflammatory drugs. Blood was drawn through a butterfly cannula (21-gauge) into an EDTA plastic tube (for platelet count), and into a 3.2% trisodium citrate plastic tube (for platelet aggregation). Blood samples were tested for total blood count, prothrombin time, partial thromboplastin time, and fibrinogen at the University of Pittsburgh Medical Center Central Laboratory, Presbyterian Hospital, Pittsburgh, PA.
Platelet agonists and antagonists
Four standard platelet agonists/stimulators (agents that are known to initiate platelet aggregation) at various concentrations were added to the experimental samples in the platelet aggregometer. The agonists used were (i) collagen (3 and 5 ug/ml, #385, ristocetin (0.5 and 1.0 mg/ml, #396), adenosine diphosphate (ADP; 10, 20 and 40 µM, #384) (all from Chronolog, Havertown, PA), and thrombin (1 and 5 IU/ml, #T7009, Sigma-Aldrich, St. Louis, MO). Heparin was obtained from APP Pharmaceuticals, LLC, (#504011, Schaumburg, IL), and LMWH (Fragmin®, 2,500IU/0.2ml) from Eisai, Woodcliff Lake, NJ.
Platelet aggregation assay
Blood samples were analyzed at three different concentrations - (i) undiluted blood, (ii) 50% diluted blood, or (iii) 75% diluted blood. Blood was diluted with pre-warmed (37°C) normal saline (Baxter, Deerfield, IL). The assay was commenced within 30min after blood withdrawal, and completed within 3h.
Platelet aggregation was measured using a platelet aggregometer (two-sample, four-channel, model 592 Whole Blood Aggregometer, Chrono-log), according to the manufacturer’s instructions. Blood samples were placed in plastic cuvettes with electrodes and magnetic stir bars, and incubated at 37°C for 5min in incubation wells. By adding a small rotating magnet (disposable stir bar) to each sample, a shear stress was created to simulate intravascular flow conditions. Platelet agonists were then added to induce platelet aggregation, which increases resistance. These changes of impedance were automatically transformed into arbitrary aggregation units (ohms), which were calculated as percentages, and then presented as aggregation curves by an integrated computer system. Changes of electrical impedance were recorded for 6min, and the maximum platelet aggregation induced was determined.
Inhibition of platelet aggregation with heparin and LMWH
Inhibition of agonist-induced platelet aggregation by heparin and LMWH was investigated by blood aggregometry using three different concentrations (1, 10, and 100 IU/ml) of heparin (of which only a concentration of 1 IU/ml is clinically-relevant) or LMWH. Blood samples were incubated with either heparin or LMWH for 30min at 37°C. The effect of heparin was assessed in humans (n=5), baboons (n=5), and in monkeys (n=3). The effect of LMWH was assessed in humans (n=3), baboons (n=3), and monkeys (n=3).
Statistical analysis
Statistical analysis was performed using social sciences software GraphPad Prism 5.0 (GraphPad Software, San Diego, CA). Association analysis was evaluated using Spearman’s rank-order correlation. Comparison among groups was assessed by nonparametric statistical analysis of variance by Kruskal-Wallis’ test followed by Dunn’s multiple comparison. Values of p<0.05 were considered statistically significant.
RESULTS
Induction of platelet aggregation in blood
We evaluated platelet aggregation in 8 healthy humans, 5 baboons, and 8 monkeys. Hematological and coagulation parameters were comparable in the three species (Table 1). No platelet aggregation was observed in the absence of any agonist (Figure 1). Within 6min of adding an agonist to the blood sample, platelet aggregation was observed (Figure 1). The percentage of platelet aggregation was dependent on the extent of blood dilution (undiluted, 50% diluted, or 75% diluted), with a tendency towards more platelet aggregation with greater dilution (Figures 2–5). Fifty percent (50%) diluted blood provided the most consistent platelet aggregation in all three species, and platelet aggregation was dependent on the dose of the agonist used (Figures 2–5).
Table 1.
Hematology and coagulation data in healthy humans, baboons and cynomolgus monkeys
| Parameter (unit) | Human | Baboon | Monkey | P value |
|---|---|---|---|---|
| White blood cell count (×103/µl) | 7.71±0.95 | 8.20±1.72 | 9.93±1.66 | ns |
| Red blood cell count (×106/µl) | 5.01±0.24 (a) | 4.89±0.16 (b) | 5.75±0.29 | (a) (p<0.05) (b) (p<0.01) |
| Hematocrit (%) | 40±1.7 | 38±1.3 | 38±1.6 | ns |
| Platelet count (×103/µl) | 222±49.3 | 263±29.1 | 276±25.7 | ns |
| Prothrombin time (s) | 13.2±0.61 | 13.4±0.46 | 12.7±0.40 | ns |
| Activated partial thromboplastin time (s) | 28.5±2.10 | 29.7±2.18 | 26.7±1.61 | ns |
| Fibrinogen (mg/dl) | 275±26.6 (c) | 202±10.5 | 234±37.0 | (c) (p<0.01) |
Human vs Monkey,
Baboon vs Monkey,
Human vs Baboon
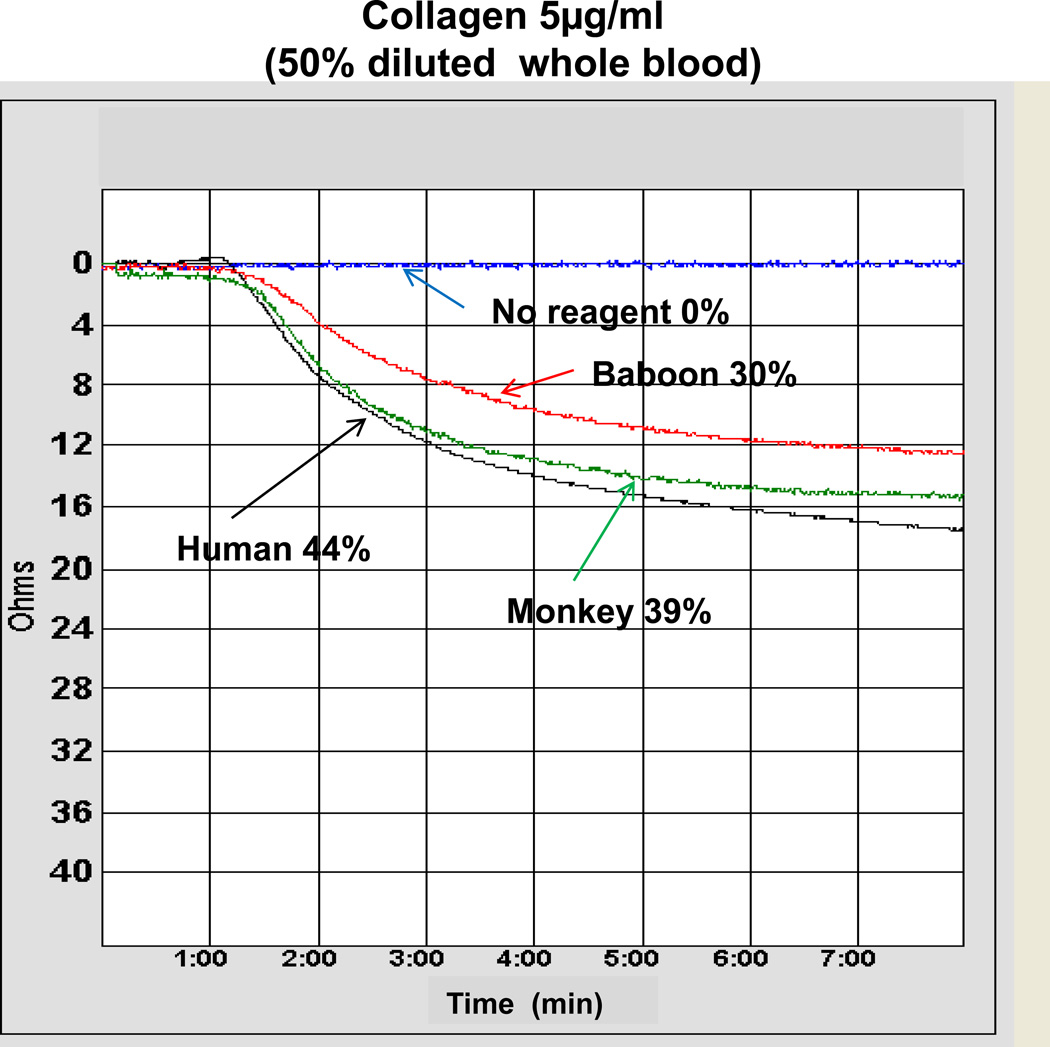
Figure 1. Platelet aggregation representative curve induced with and without collagen (at 5ug/ml).
Platelet aggregation curves from a representative experiment. The platelet aggregation that developed in 50% diluted whole blood, with (i) no agonist, or (ii) collagen (5ug/ml) in humans, baboons, and monkeys. In the absence of any agonist, no platelet aggregation was seen. Percentages of aggregation were 44% (human), 30% (baboon), and 39% (monkey).
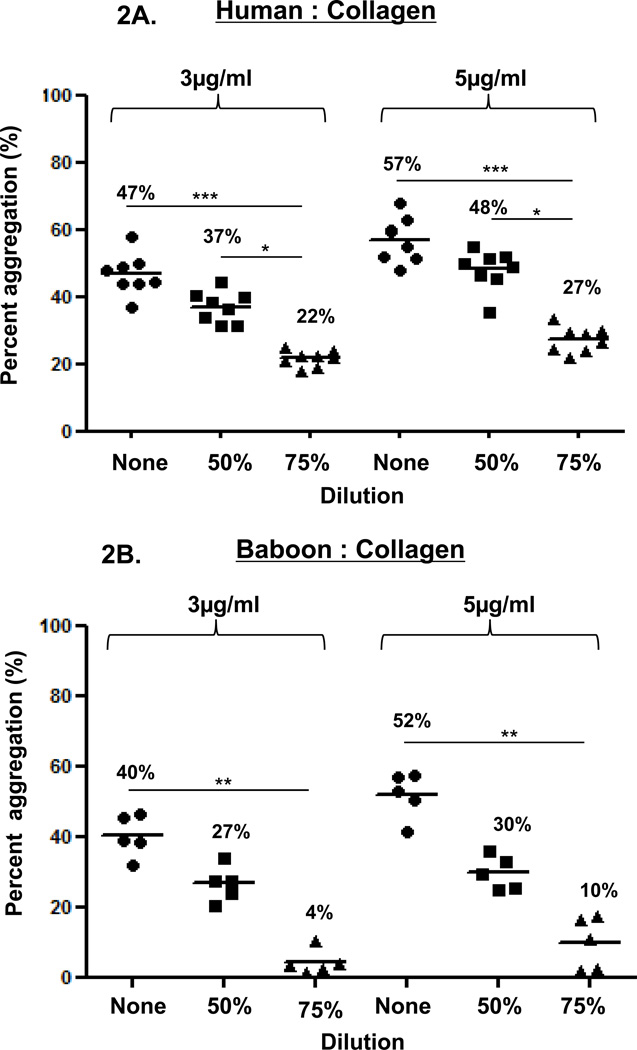
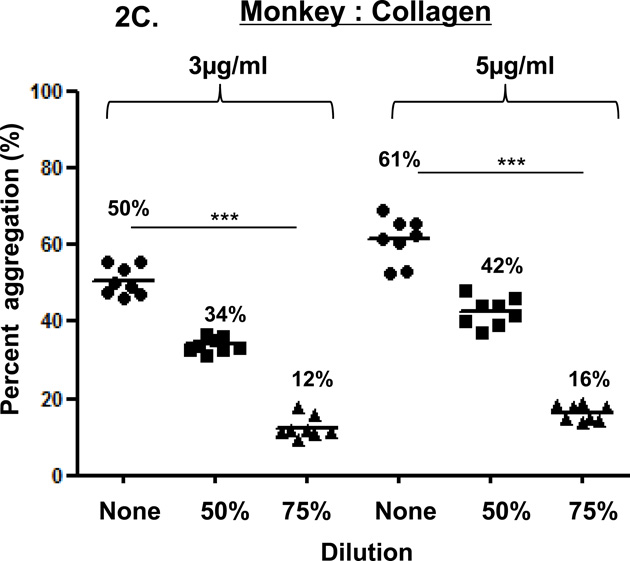
Figure 2. Collagen-induced platelet aggregation.
In humans (A), baboons (B), and monkeys (C), platelet aggregation with collagen at 3 and 5 mg/ml was dose- and dilution-dependent. Collagen induced significantly greater aggregation in undiluted than in 75% diluted blood in all three species. Collagen induced significantly greater aggregation in 50% diluted blood than in 75% diluted blood only in humans. Values of mean percentage (%) of aggregation are indicated in the figure. (*p<0.05, **p<0.01, ***p<0.001).
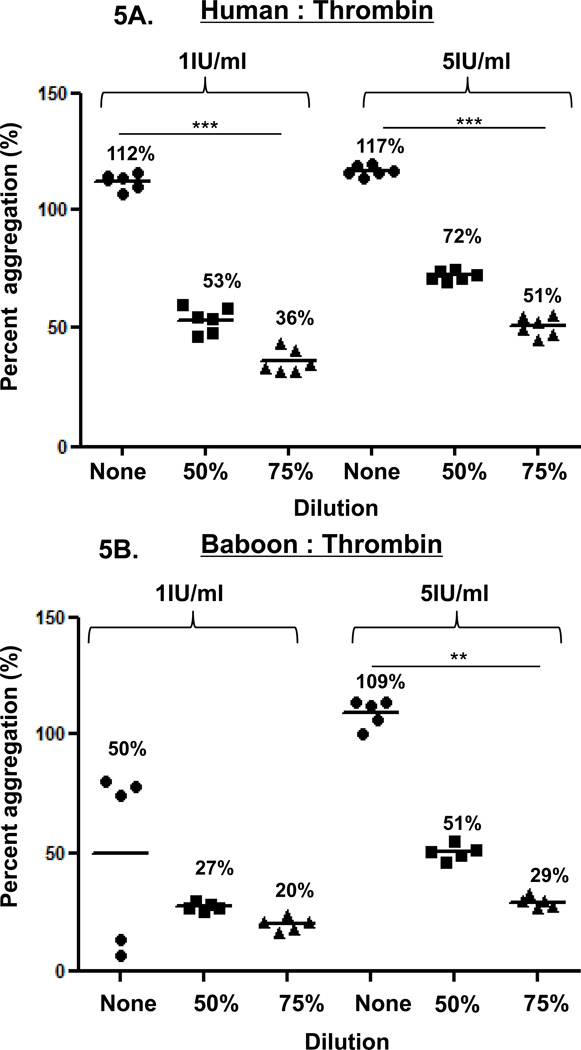
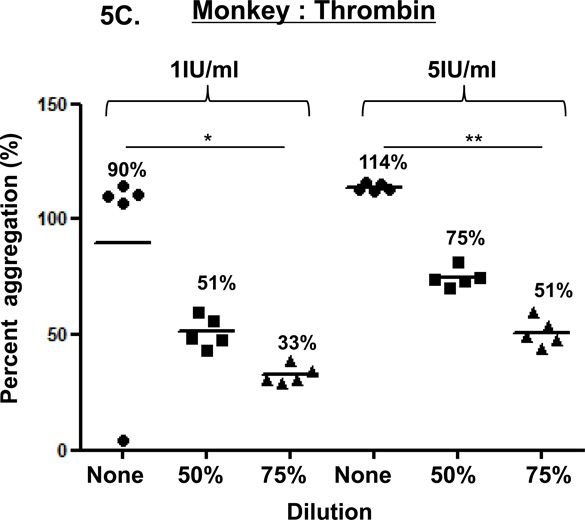
Figure 5. Thrombin-induced platelet aggregation.
In humans (A), baboons (B), and monkeys (C), platelet aggregation with thrombin (at 1 and 5 IU/ml) was dose-and dilution-dependent. Thrombin induced significantly greater aggregation in undiluted blood than in 75% diluted blood in all three species, except in baboons at 1IU/ml. Values of mean percentage (%) of aggregation are indicated in the figure. (*p<0.05, **p<0.01, ***p<0.001).
Collagen-induced platelet aggregation
In the three species, collagen (3 or 5 µg/mL) induced dose-dependent platelet aggregation in a comparable manner (Figure 2). Aggregation was consistently greater using undiluted blood than diluted blood. In humans, aggregation in undiluted and 50% diluted blood was significantly greater than in 75% diluted blood (p<0.001and p<0.05, respectively). In baboons and monkeys, aggregation in undiluted blood was significantly greater than in 75% diluted blood (p<0.01 and p<0.001, respectively).
Ristocetin-induced platelet aggregation
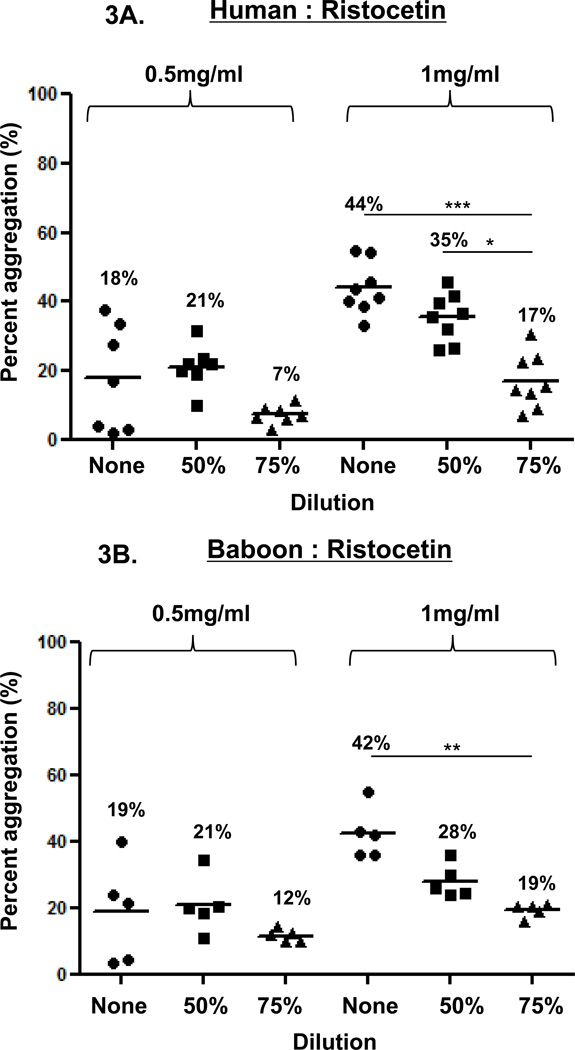
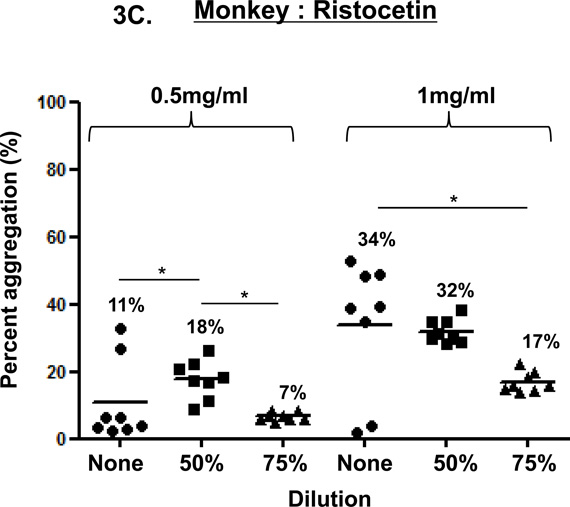
In the three species, ristocetin-induced platelet aggregation (at 0.5 and 1 mg/ml), was dose-dependent (Figure 3). Using 0.5mg/ml, aggregation in undiluted blood was variable in all three species, while using 1mg/ml, aggregation showed variability in undiluted blood in monkeys. In 50% diluted blood, 0.5mg/ml induced consistently greater aggregation than in 75% diluted blood, but the difference was significant only in monkeys (p<0.05), and 1mg/ml induced consistently greater aggregation than in 75% diluted blood, but this difference was significant only in humans (p<0.05).
Figure 3. Ristocetin-induced platelet aggregation.
In humans (A), baboons (B), and monkeys (C), platelet aggregation with ristocetin at 1mg/ml was dose-and dilution-dependent. Ristocetin (at 1mg/ml) induced significantly greater aggregation in undiluted blood than in 75% diluted blood in all three species. Ristocetin induced significantly greater aggregation in 50% diluted blood than in 75% diluted blood only in humans. In all three species, ristocetin at 0.5mg/ml induced dilution-dependent aggregation, but significant differences were only observed in monkeys. Values of mean percentage (%) of aggregation are indicated in the figure. (*p<0.05, **p<0.01, ***p<0.001).
ADP-induced platelet aggregation
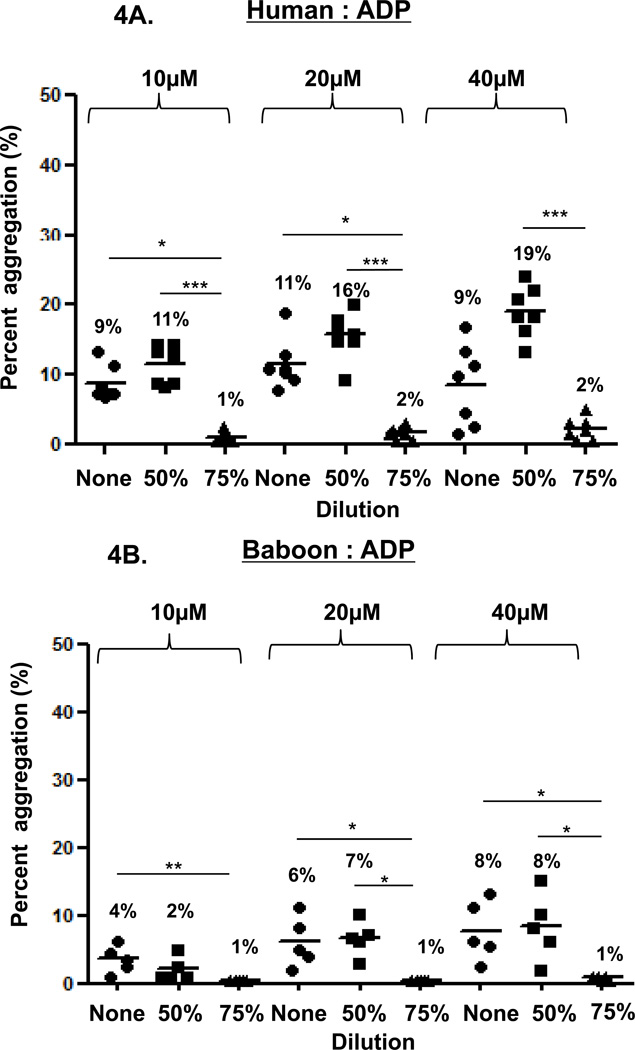
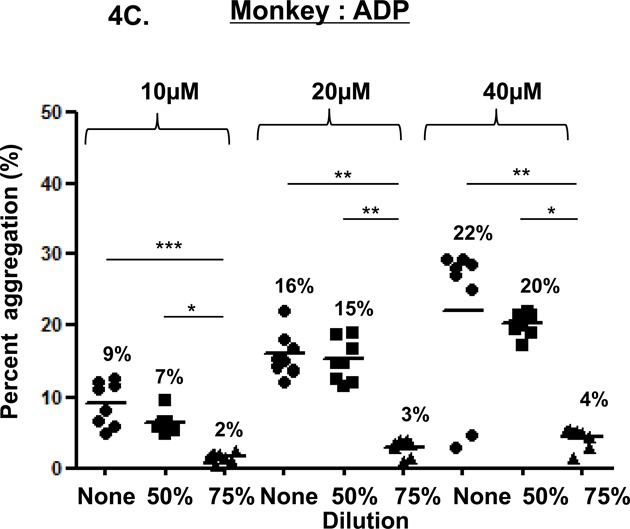
In the three species, ADP-induced platelet aggregation (at 10, 20, and 40µM) was dose-dependent (Figure 4). In undiluted blood, aggregation was not consistently greater than in 50% or 75% diluted blood. Except in baboons (at 10µM ADP), aggregation in 50% diluted blood was significantly greater than in 75% diluted blood (humans p<0.001, monkeys p<0.05).
Figure 4. ADP-induced platelet aggregation.
In humans (A), baboons (B), and monkeys (C), platelet aggregation with ADP (at 10, 20 and 40 µM) was dose-and dilution-dependent. ADP induced significantly greater aggregation in undiluted blood than in 75% diluted blood in all three species, except at 40µM in humans. ADP induced significantly greater aggregation in 50% diluted blood than in 75% diluted blood in all three species, except at 10µM in baboons. Values of mean percentage (%) of aggregation are indicated in the figure. (*p<0.05, **p<0.01, ***p<0.001).
Thrombin-induced platelet aggregation
In the three species, thrombin-induced platelet aggregation (at 1 and 5 IU/mL) was dose-dependent (Figure 5). In undiluted blood, aggregation was consistently greater than in 50% or 75% diluted blood in humans, but not in baboons or monkeys. In 50% diluted blood, aggregation was greater than in 75% diluted blood in the three species, but this increase was not significant.
Correlation between platelet aggregation and platelet count
In 50% diluted blood, there was a significant positive correlation between platelet count and percent agonist-induced platelet aggregation for each agonist in the three species.
Comparison of platelet aggregation between humans, baboons, and monkeys
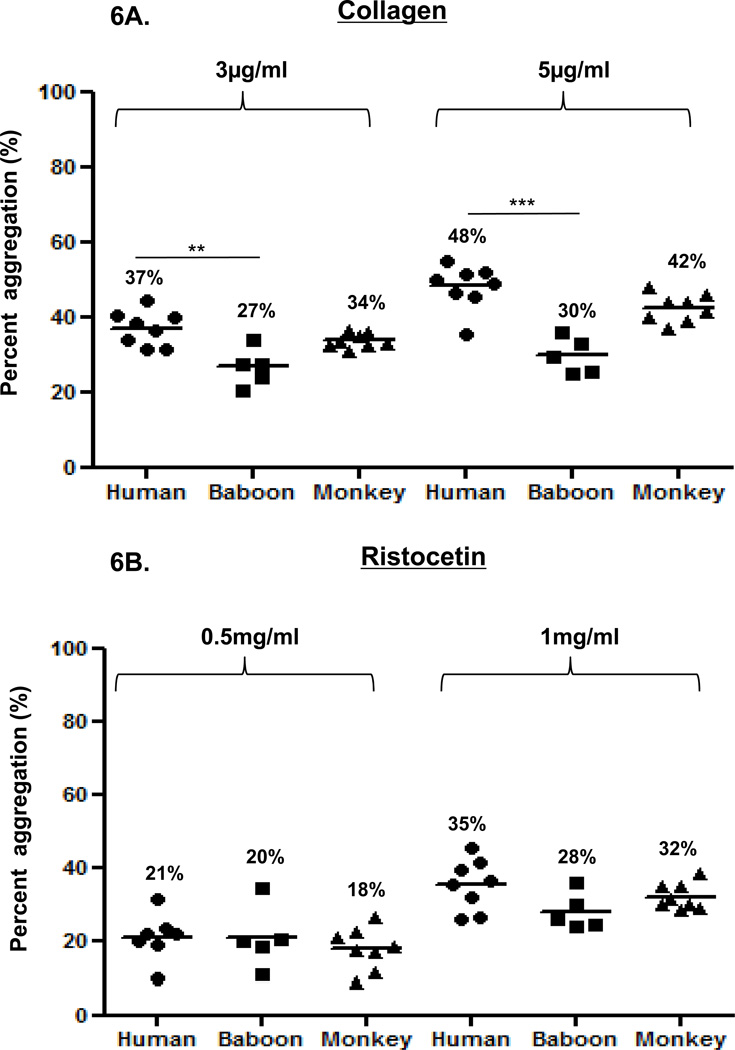
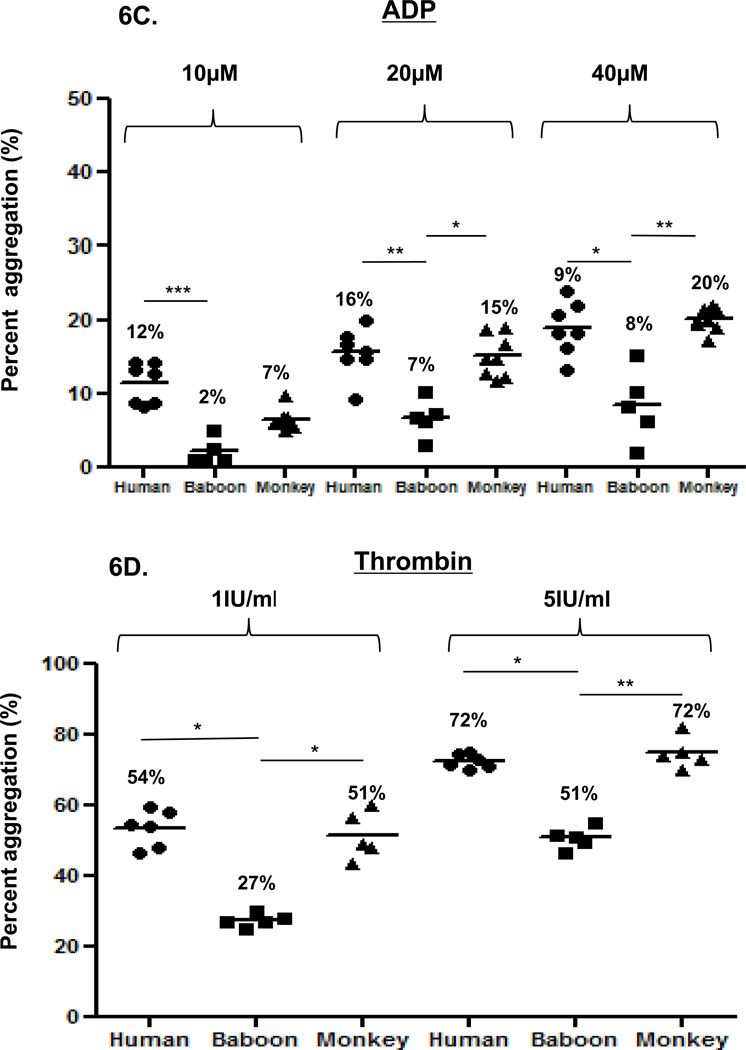
Agonist-induced platelet aggregation in 50% diluted blood provided the most consistent results. Accordingly, we compared agonist-induced platelet aggregation in 50% diluted blood among the three species. Collagen induced greater platelet aggregation in humans than in baboons, but aggregation was not significantly different from that in monkeys (Figure 6A). Ristocetin induced comparable and insignificantly different platelet aggregation in the three species (Figure 6B). ADP induced significantly greater platelet aggregation in humans than in baboons (Figure 6C). At 10µM, aggregation was greater in humans than monkeys, and at 20 and 40µM aggregation in monkeys was significantly greater than in baboons. Thrombin induced significantly greater aggregation in humans and monkeys than in baboons, but was comparable between human and monkeys (Figure 6D).
Figure 6. Comparison of platelet aggregation between humans, baboons and monkeys.
Platelet aggregation in 50% diluted blood was compared between humans, baboons, and monkeys. Collagen at 3 and 5 µg/mL (A) induced significantly greater platelet aggregation in humans than in baboons, and comparable aggregation in monkeys. With ristocetin at 0.5 or 1 mg/ml (B), no significant differences were found between species. Platelet activation with ADP at 20 and 40 µM (C) induced significantly greater platelet aggregation in humans and monkeys than in baboons. Platelet activation with thrombin at 1 and 5 IU/ml (D) induced significantly greater platelet aggregation in humans and monkeys than in baboons. Values of mean percentage (%) of aggregation are indicated in the figure. (*p<0.05, **p<0.01, ***p<0.001).
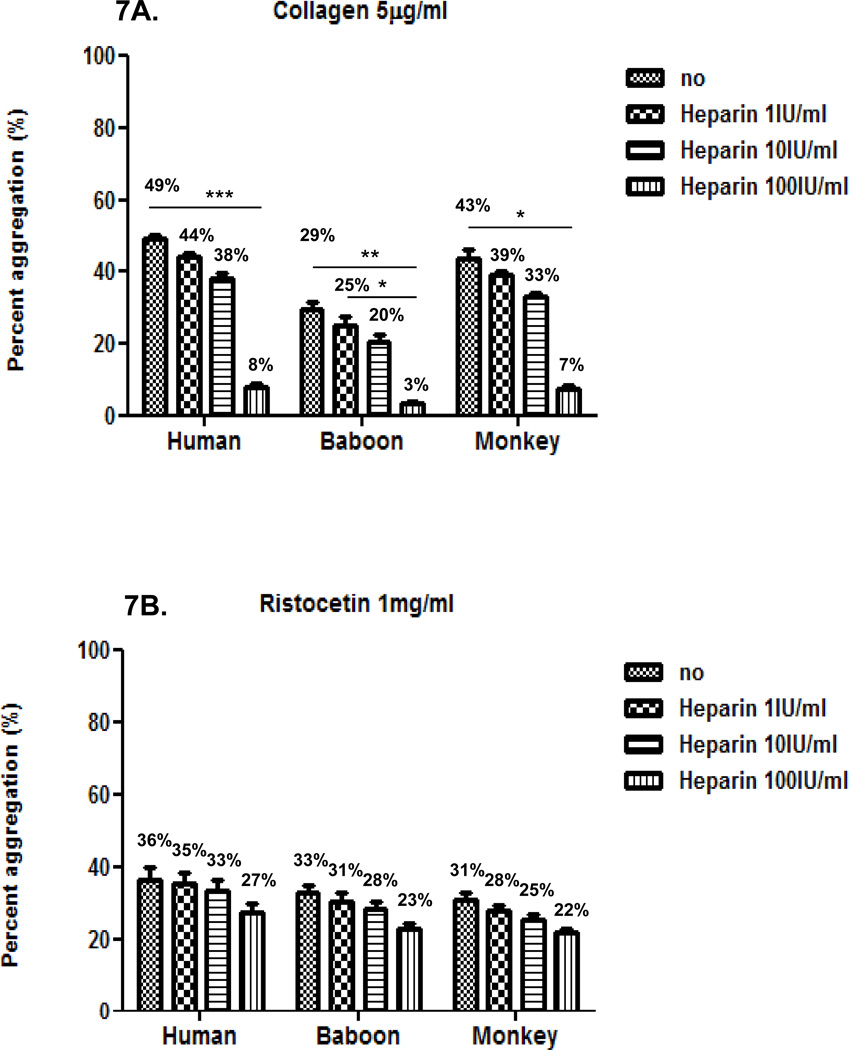
Inhibition of agonist-induced platelet aggregation by heparin
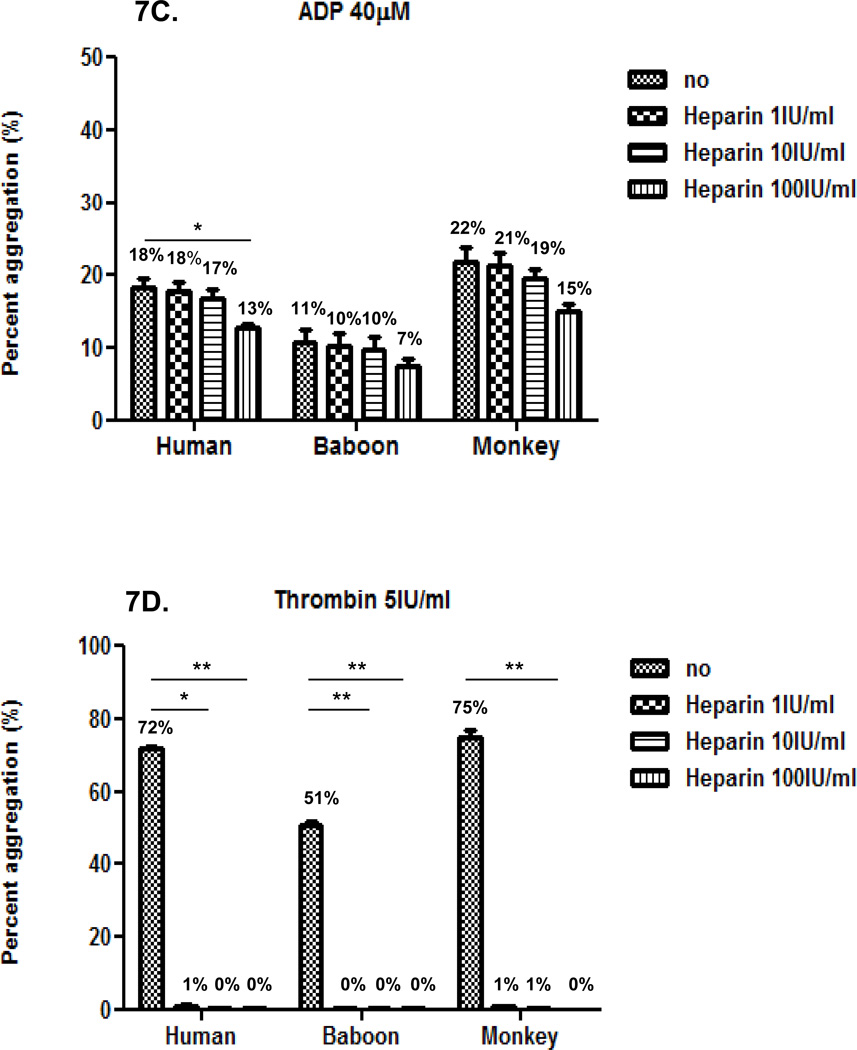
Next, we evaluated the effect of heparin at three different concentrations (1, 10 and 100IU/ml) on platelet aggregation induced by each agonist in the three species using 50% diluted blood. In the three species, heparin inhibition of agonist-induced platelet aggregation was comparable and dose-dependent (Figure 7). Heparin was relatively efficient at reducing collagen-induced aggregation, but inefficient at reducing ristocetin- or ADP-induced aggregation (Figures 7A, B, and C). Heparin was very efficient at inhibiting thrombin-induced aggregation (Figure 7D). Mean percentage inhibition of thrombin-induced aggregation was almost 100% at all concentrations.
Figure 7. Effect of heparin on agonist-induced platelet aggregation.
In the three species, heparin at 100 IU/ml effectively inhibited collagen-induced platelet aggregation (A), but had a very weak effect on inhibiting aggregation induced by ristocetin (B) and ADP (C). Heparin at 1, 10 and 100 IU/ml equally and effectively inhibited thrombin-induced platelet aggregation (D). Values of mean percentage (%) of aggregation are indicated in the figure. (*p<0.05, **p<0.01, ***p<0.001).
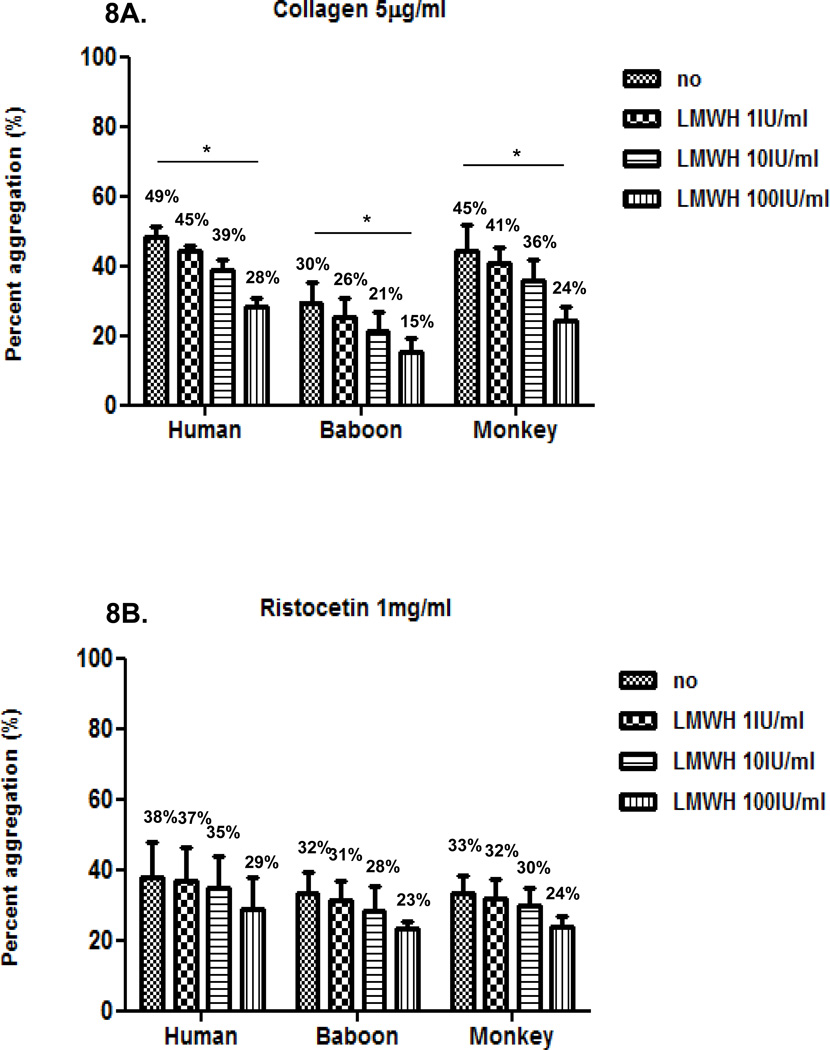
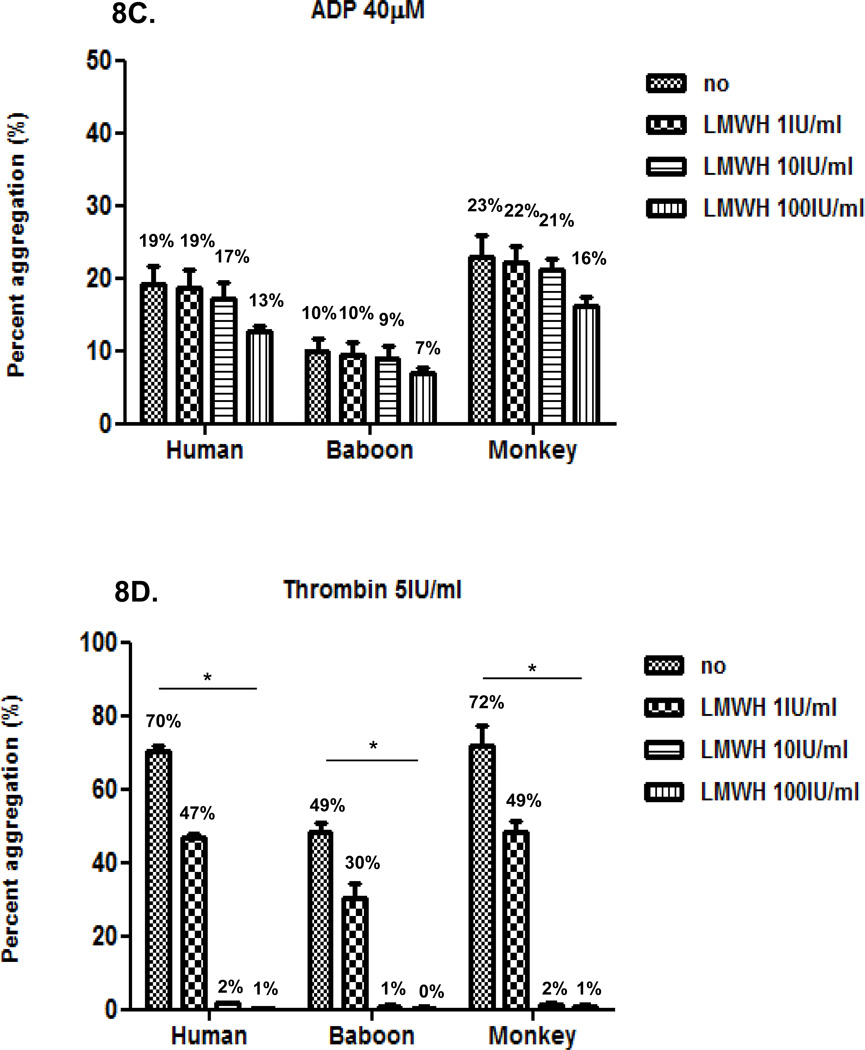
Inhibition of agonist-induced platelet aggregation by LMWH in humans and baboons
We then compared the effect of LMWH at three different concentrations (1, 10 and 100IU/ml) on platelet aggregation induced by each agonist in the three species, using 50% diluted blood. LMWH inhibition of agonist-induced aggregation was dose-dependent (Figure 8). LMWH was less effective than heparin in inhibiting collagen-induced and thrombin-induced aggregation, though at the highest concentration (100 IU/ml) it effectively prevented thrombin-induced aggregation. Inhibition of ristocetin and ADP was limited, but comparable to heparin.
Figure 8. Effect of LMWH on agonist-induced platelet aggregation In all three species.
LMWH at 100 IU/ml moderately inhibited collagen-induced platelet aggregation (A), but had relatively little effect on reducing aggregation induced by ristocetin (B) or ADP (C). LMWH at 10 and 100 IU/ml equally and effectively inhibited thrombin-induced platelet aggregation (D). Values of mean percentage (%) of aggregation are indicated in the figure. (*p<0.05).
DISCUSSION
Substantial advances in xenotransplantation, particularly through the availability of genetically-engineered pigs, have allowed hyperacute rejection to be overcome and the onset of acute vascular rejection to be delayed or inhibited. However, even if rejection is prevented, an imbalance in clotting homeostasis, tending towards hypercoagulation (11, 12), has been a major barrier to long-term survival in pig-to-primate organ xenotransplantation. When a pig organ (e.g., heart, kidney) is transplanted into a nonhuman primate, microvascular thrombosis develops (4, 13, 14). Although the exact mechanisms inducing coagulation after xenotransplantation are not fully understood, activation of donor vascular endothelium and/or recipient platelets plays an important role (15).
Prevention of recipient platelet activation may be crucial for successful pig-to-primate xenotransplantation. After pig-to-baboon kidney xenotransplantation model, activated platelets express tissue factor at an earlier stage (1–2 days) than do peripheral blood mononuclear cells (PBMC) (4 days) (9). In liver xenografts, profound thrombocytopenia associated with aggregation of platelets with white blood cell subtypes was observed within one hour after pig liver reperfusion (16). Recipient platelets may bind directly to pig endothelial cells and subsequently become activated (17). Human platelets upregulate tissue factor expression by direct contact with porcine aortic endothelial cells in the absence of human serum or antibodies (15). Porcine, but not human, von Willebrand Factor (vWF) binds and activates human platelets in the absence of shear stress (18).
Platelet aggregation develops in two stages. The primary phase occurs in the form of a change in shape and reversible aggregation, followed by a secondary phase of secretion of platelet mediators and irreversible aggregation. Platelets carry specific receptors for known agonists (19–21) (Table 2).
Table 2.
Platelet agonists and receptors
| Agonist | Receptor |
|---|---|
| Collagen | GpIa/IIa, GpIV, GpVI |
| Ristocetin (vWF) | GPIb, GPIb/V/IX |
| ADP | P2Y1, P2Y12 |
| Thrombin | PAR1, PAR2 |
In the present study, we found a positive correlation between the platelet count and platelet aggregation, as reported by others (22, 23). At the blood volumes used, platelet aggregation using 50% diluted blood generated the most consistent results, while undiluted blood was less consistent. This inconsistency might be related to technical and environmental factors (24–26).
As nonhuman primates are frequently used in xenotransplantation studies as surrogates for humans, it is important to establish baseline biological values in these species. In the present study, platelet aggregation in humans and monkeys was greater than in baboons, at least with regard to collagen, ADP, and thrombin. Significantly lower levels of fibrinogen were observed in baboons than in humans, though not in monkeys (Table 1), as reported by others (27), which may help explain the reduced aggregation seen in baboons. It is reported that higher levels of fibrinogen are associated with increased aggregation (28, 29).
Red and white blood cells are known to modulate platelet function by providing either physical scaffolding or chemicals (e.g., ADP) that have a direct impact on platelet function (30). An increased hematocrit has been associated with a reduction in in vitro aggregation, possibly as a result of reduced levels of ADP (31, 32), but this could be a result of technical problems (see above). In the present study, we found no significant differences in white blood cell count, hematocrit, platelet count, prothrombin time, and partial thromboplastin time between the three species, although the red blood cell count in monkeys was significantly higher than in humans and baboons (Table 1). While lower fibrinogen levels were observed in monkeys than in humans, higher red blood cell counts, FVIII, and platelet counts were observed in monkeys, and no substantial difference in hematocrit (33).
In xenotransplantation experiments, heparin has often been administered at doses higher than that used clinically (100–200IU/kg; q6–8h), with resulting relatively high concentrations of heparin in the blood. Heparin leads to efficient inhibition of thrombin-induced platelet aggregation (34) by increasing the affinity of antithrombin III to thrombin to form thrombin-antithrombin complexes. Heparin can also inhibit the binding of xenogeneic (bovine) vWF to human platelets, where increased inhibition of binding was observed with an increased molecular weight of heparin (35). While heparin can bind a number of platelet membrane proteins, including the GPIb receptor (36), heparin binding to platelets is not completely prevented by monoclonal antibodies directed against platelet receptors (GPIa/IIa, GPIb, GPIIb/IIIa, and GPIV) (37). In the current study, we found that heparin at a (maximal) clinically-applicable concentration (1 IU/mL) was very effective in inhibiting thrombin-induced platelet aggregation, but not collagen-, ADP-, or ristocetin- induced platelet aggregation. As thrombin activation is a key step in the dysregulation of coagulation in xenograft recipients, this might explain the importance of anticoagulation using continuous heparin infusion in our own studies and others (38).
Heparin and LMWH have similar inhibitory effects on platelet aggregation (38, 39), with similar anti-thrombin (FIIa) activity, but with a greater anti-FXa activity by LMWH (40, 41). Weaker LMWH effect on aggregation has been reported (42). In our study, in comparison to heparin, inhibition of thrombin-induced platelet aggregation by LMWH was also efficient, though not at a concentration of 1 IU/mL (and therefore probably not clinically useful). Because, in contrast to heparin, LMWH can be administered subcutaneously, these data suggest a possible role for it in reducing dysregulation of coagulation in xenograft recipients.
Although most studies have used the Chrono-log method for evaluation of platelet hypofunction or dysfunction, the method is also useful for assessment of hyperactivity of platelets (29, 43). Methodology using blood has several advantages over the use of platelet-rich plasma for the assessment of hyperactive platelets (44, 45). For example, studies in blood (i) allow evaluation of platelets in a more physiologic milieu (30); (ii) have a greater sensitivity than the optical platelet-rich plasma method (45); (iii) avoid the need for centrifugation (iv) allow a faster technique; and (v) are more suitable for a routine laboratory setting. However, there are some limitations of the whole blood aggregation assay - (i) platelet aggregation studies must be performed within 3 hours after blood collection (46); (ii) platelet activation can be caused by improper sample collection; and (iii) there are limitations to the interpretation of results in thrombocytopenic samples (22, 31).
Platelet aggregometry (using the optical method) has been used previously in studies of xenotransplantation (47). In vitro, porcine, but not baboon, PBMC directly induced aggregation of baboon platelets in a dose-dependent manner in the absence of any agonist (47). Xenotransplantation of mobilized porcine PBMC in baboons was followed by immediate severe thrombotic microangiopathy (in lungs, heart, and kidneys), associated with platelet aggregation and thrombocytopenia (14, 48). Benatuil et al. documented that pig PBMC induced human platelet aggregation to a similar extent to collagen (49).
At present, it is not absolutely clear which factors influence the hypercoagulable state that develops in a primate after the transplantation of a pig organ. There may therefore be a role for platelet aggregometry assays in the management of primates with pig organ grafts, not only as part of the coagulation profile, but also to assess the efficacy of anti-thrombotic therapy.
ACKNOWLEGMENTS
Work on xenotransplantation in the Thomas E. Starzl Transplantation Institute of the University of Pittsburgh is supported in part by NIH grants #U19 AI090959-01, #U01 AI068642, and # R21 A1074844, and by Sponsored Research Agreements between the University of Pittsburgh and Revivicor, Blacksburg, VA. Burcin Ekser, MD, is a recipient of a NIH NIAID T32 AI 074490 Training Grant. The baboons were provided by the Oklahoma University Health Sciences Center, Division of Animal Resources, which is supported in part by NIH P40 sponsored grant RR012317-09.
Abbreviations
- ADP
adenosine diphosphate
- LMWH
low molecular weight-heparin
- PBMC
peripheral blood mononuclear cell
- vWF
von Willebrand factor
Footnotes
CONFLICT OF INTEREST
None of the authors has a conflict of interest.
REFERENCES
- 1.Ekser B, Ezzelarab M, Hara H, et al. Clinical xenotransplantation: the next medical revolution? 2011 Oct 20; doi: 10.1016/S0140-6736(11)61091-X. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 2.Cowan PJ, Aminian A, Barlow H, et al. Renal xenografts from triple-transgenic pigs are not hyperacutely rejected but cause coagulopathy in nonimmunosuppressed baboons. Transplantation. 2000;69:2504–2515. doi: 10.1097/00007890-200006270-00008. [DOI] [PubMed] [Google Scholar]
- 3.Shimizu A, Yamada K, Yamamoto S, et al. Thrombotic microangiopathic glomerulopathy in human decay accelerating factor-transgenic swine-to-baboon kidney xenografts. J Am Soc Nephrol. 2005;16:2732–2745. doi: 10.1681/ASN.2004121148. [DOI] [PubMed] [Google Scholar]
- 4.Kuwaki K, Tseng YL, Dor FJ, et al. Heart transplantation in baboons using alpha1, 3-galactosyltransferase gene-knockout pigs as donors:initial experience. Nat Med. 2005;11:29–31. doi: 10.1038/nm1171. [DOI] [PubMed] [Google Scholar]
- 5.Mazzucato M, De Marco L, Pradella P, et al. Porcine von Willebrand factor binding to human platelet GPIb induces transmembrane calcium influx. Thromb Haemost. 1996;75:655–660. [PubMed] [Google Scholar]
- 6.Kopp CW, Siegel JB, Hancock WW, et al. Effect of porcine endothelial tissue factor pathway inhibitor on human coagulation factors. Transplantation. 1997;63:749–758. doi: 10.1097/00007890-199703150-00023. [DOI] [PubMed] [Google Scholar]
- 7.Siegel JB, Grey ST, Lesnikoski BA, et al. Xenogeneic endothelial cells activate human prothrombin. Transplantation. 1997;64:888–896. doi: 10.1097/00007890-199709270-00017. [DOI] [PubMed] [Google Scholar]
- 8.Robson SC, Schulte am Esch J, 2nd, Bach FH. Factors in xenograft rejection. Ann N Y Acad Sci. 1999;875:261–276. doi: 10.1111/j.1749-6632.1999.tb08509.x. [DOI] [PubMed] [Google Scholar]
- 9.Lin CC, Ezzelarab M, Shapiro R, et al. Recipient tissue factor expression is associated with consumptive coagulopathy in pig-to-primate kidney xenotransplantation. Am J Transplant. 2010;10:1556–1568. doi: 10.1111/j.1600-6143.2010.03147.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Appel JZ, 3rd, Alwayn IP, Buhler L, et al. Modulation of platelet aggregation in baboons:implications for mixed chimerism in xenotransplantation. I. The roles of individual components of a transplantation conditioning regimen and of pig peripheral blood progenitor cells. Transplantation. 2001;72:1299–1305. doi: 10.1097/00007890-200110150-00020. [DOI] [PubMed] [Google Scholar]
- 11.Cozzi E, Simioni P, Boldrin M, et al. Alterations in the coagulation profile in renal pig-to-monkey xenotransplantation. Am J Transplant. 2004;4:335–345. doi: 10.1046/j.1600-6143.2003.00349.x. [DOI] [PubMed] [Google Scholar]
- 12.Lin CC, Cooper DK, Dorling A. Coagulation dysregulation as a barrier to xenotransplantation in the primate. Transpl Immunol. 2009;21:75–80. doi: 10.1016/j.trim.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ierino FL, Kozlowaski T, Siegel JB, et al. Disseminated intravascular coagulation in association with the delayed rejection of pig-to-baboon renal xenografts. Transplantation. 1998;66:1439–1450. doi: 10.1097/00007890-199812150-00006. [DOI] [PubMed] [Google Scholar]
- 14.Buhler L, Basker M, Alwayn IPJ, et al. Coagulation and thrombotic disorders associated with pig organ and hematopoietic cell transplantation in nonhuman primates. Transplantation. 2000;70:1323–1331. doi: 10.1097/00007890-200011150-00010. [DOI] [PubMed] [Google Scholar]
- 15.Lin CC, Chen D, McVey JH, Cooper DKC, Dorling A. Expression of tissue factor and initiation of clotting by human platelets and monocytes after incubation with porcine endothelial cells. Transplantation. 2008;86:702–709. doi: 10.1097/TP.0b013e31818410a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ezzelarab M, Ekser B, Gridelli B, et al. Thrombocytopenia after pig to baboon liver xenotransplantation:where do platelets go? Xenotransplantation. 2011;18:320–327. doi: 10.1111/j.1399-3089.2011.00679.x. [DOI] [PubMed] [Google Scholar]
- 17.Bombeli T, Schwartz BR, Harlan JM. Endothelial cells undergoing apoptosis become proadhesive for nonactivated platelets. Blood. 1999;93:3831–3838. [PubMed] [Google Scholar]
- 18.Schulte Am Esch J, II, Robson SC, Knoefel WT, Hosch SB, Rogiers X. O-linked glycosylation and functional incompatibility of porcine von Willebrand factor for human platelet GPIb receptors. Xenotransplantation. 2005;12:30–37. doi: 10.1111/j.1399-3089.2004.00187.x. [DOI] [PubMed] [Google Scholar]
- 19.Rand ML, Leung R, Packham MA. Platelet function assays. Transfus Apher Sci. 2003;28:307–317. doi: 10.1016/S1473-0502(03)00050-8. [DOI] [PubMed] [Google Scholar]
- 20.Marcus AJ, Safier LB. Thromboregulation:multicellular modulation of platelet reactivity in hemostasis and thrombosis. FASEB J. 1993;7:516–522. doi: 10.1096/fasebj.7.6.8472890. [DOI] [PubMed] [Google Scholar]
- 21.Body SC. Platelet activation and interactions with the microvasculature. J Cardiovasc Pharmacol. 1996;27:S13–S25. doi: 10.1097/00005344-199600001-00006. [DOI] [PubMed] [Google Scholar]
- 22.Sweeney JD, Labuzetta JW, Fitzpatrick JE. The effect of the platelet count on the aggregation response and adenosine triphosphate release in an impedance lumi-aggregometer. Am J Clin Pathol. 1988;89:655–659. doi: 10.1093/ajcp/89.5.655. [DOI] [PubMed] [Google Scholar]
- 23.Seyfert UT, Haubelt H, Vogt A, Hellstern P. Platelets. Variables influencing Multiplate(TM) whole blood impedance platelet aggregometry and turbidimetric platelet aggregation in healthy individuals. Platelets. 2007;18:199–206. doi: 10.1080/09537100600944277. [DOI] [PubMed] [Google Scholar]
- 24.Ingerman-Wojenski C, Smith JB, Silver MJ. Evaluation of electrical aggregometry: comparison with optical aggregometry, secretion of ATP, and accumulation of radiolabeled palatelets. J Lab Clin Med. 1983;101:44–52. [PubMed] [Google Scholar]
- 25.George JN, Shattil SJ. The clinical importance of acquired abnormalities of platelet function. N Engl J Med. 1991;324:27–39. doi: 10.1056/NEJM199101033240106. [DOI] [PubMed] [Google Scholar]
- 26.Thiagarajan P, Wu KK. In vitro assays for evaluating platelet function. In: Grisele P, Page CP, Fuster V, Vermylen J, editors. Platelets in Thrombotic and Non-Thrombotic Disorders: Pathophysiology, Pharmacology and Therapeutics. Cambridge, UK: Cambridge University Press; 2002. pp. 459–470. [Google Scholar]
- 27.Schuurman HJ, Smith HT, Cozzi E. Reference values for clinical chemistry and clinical hematology parameters in baboons. Xenotransplantation. 2004;11:511–516. doi: 10.1111/j.1399-3089.2004.00171.x. [DOI] [PubMed] [Google Scholar]
- 28.Feng D, Lindpaintner K, Larson MG, et al. Platelet glycoprotein IIIa Pl(a) polymorphism, fibrinogen, and platelet aggregability: The Framingham Heart Study. Circulation. 2001;104:140–144. doi: 10.1161/01.cir.104.2.140. [DOI] [PubMed] [Google Scholar]
- 29.Yee DL, Sun CW, Bergeron AL, Dong JF, Bray PF. Aggregometry detects platelet hyperreactivity in healthy individuals. Blood. 2005;106:2723–2729. doi: 10.1182/blood-2005-03-1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bouchard BA, Tracy PB. Platelets, leukocytes, and coagulation. Curr Opin Hematol. 2001;8:263–269. doi: 10.1097/00062752-200109000-00001. [DOI] [PubMed] [Google Scholar]
- 31.Mackie IJ, Jones R, Machin SJ. Platelet impedance aggregation in whole blood and its inhibition by aniplatelet drugs. J Clin Pathol. 1984;37:874–878. doi: 10.1136/jcp.37.8.874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Muller MR, Salat A, Pulaki S, et al. Influence of hematocrit and platelet count on impedance and reactivity of whole blood for electrical aggregometry. J Pharmacol Toxicol Methods. 1995;34:17–22. doi: 10.1016/1056-8719(94)00075-f. [DOI] [PubMed] [Google Scholar]
- 33.Spiezia L, Bertini D, Boldrin M, et al. Reference values for thromboelastometry (ROTEM®) in cynomolgus monkeys (Macaca fascicularis) Thromb Res. 2010;126:e294–e297. doi: 10.1016/j.thromres.2010.07.016. [DOI] [PubMed] [Google Scholar]
- 34.Alwayn IPJ, Appel JZ, Goepfert C, et al. Inhibition of platelet aggregation in baboons:therapeutic implications for xenotransplantation. Xenotransplantation. 2000;7:247–257. doi: 10.1034/j.1399-3089.2000.00965.x. [DOI] [PubMed] [Google Scholar]
- 35.Sobel M, McNeill PM, Carlson PL, Kermode JC, Adelman B, Conroy R, Marques D. Heparin inhibition of von Willebrand factor-dependent platelet function in vitro and in vivo. J Clin Invest. 1991;87:1787–1793. doi: 10.1172/JCI115198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Adelman B, Sobel M, Fujimura Y, Ruggeri ZM, Zimmerman TS. Heparin-associated thrombocytopenia:observations on the mechanism of platelet aggregation. J Lab Clin Med. 1989;113:204–210. [PubMed] [Google Scholar]
- 37.Mcdonald K, Chao ES. Heparin binding to resting and activated platelets. Blood. 1989;74:238–243. [PubMed] [Google Scholar]
- 38.Chen J, Karlberg KE, Sylven C, et al. Heparin and low molecular weight heparin but not hirudin stimulate platelet aggregation in whole blood from acetylsalicylic acid treated healthy volunteers. Thromb Res. 1991;63:319–329. doi: 10.1016/0049-3848(91)90135-j. [DOI] [PubMed] [Google Scholar]
- 39.Ljungberg B, Beving H, Egberg N, et al. Immediate effects of heparin and LMW heparin on some platelet and endothelial derived factors. Thromb Res. 1988;51:209–217. doi: 10.1016/0049-3848(88)90064-3. [DOI] [PubMed] [Google Scholar]
- 40.Hirsh J, Levin MN. Low molecular weight heparin. Blood. 1992;79:1–17. [PubMed] [Google Scholar]
- 41.Ravn HB, Bregengaard C, Vissinger H, et al. Effect of low-molecular-weight heparin (Tinzaprin) and unfractionated heparin on platelet aggregation. Clin Appl Thromb Hemost. 1996;2:209–213. [Google Scholar]
- 42.Brace LD, Fareed J. Heparin-induced platelet aggregation:Dose/response relationships for a low molecular weight heparin derivative (PK 10169) and its subfractions. Thromb Res. 1986;42:769–782. doi: 10.1016/0049-3848(86)90113-1. [DOI] [PubMed] [Google Scholar]
- 43.Manoharan A, Gemmell R, Hartwell T. Use of whole blood platelet lumi-aggregometry to optimize anti-platelet therapy in patients with chronic myeloproliferative disorders. Am J Hematol. 2006;81:676–683. doi: 10.1002/ajh.20698. [DOI] [PubMed] [Google Scholar]
- 44.Balduini CL, Bertolino G, Noris P, Piletta GC. Platelet aggregation in platelet-rich plasma and whole blood in 120 patients with myeloproliferative disorders. Am J Clin Pathol. 1991;95:82–86. doi: 10.1093/ajcp/95.1.82. [DOI] [PubMed] [Google Scholar]
- 45.Dyszkiewicz-Korpanty AM, Frenkel EP, Sarode R. Approach to the assessment of platelet function:comparison between optical-based platelet-rich plasma and impedance-based wholeblood platelet aggregation methods. Clin Appl Thromb Hemost. 2005;11:25–35. doi: 10.1177/107602960501100103. [DOI] [PubMed] [Google Scholar]
- 46.Sweeney JD, Hoernig LA, Michnik A, Fitzpatrick JE. Whole blood aggregometry. Influence of sample collection and delay in study performance on test results. Am J Clin Pathol. 1989;92:676–679. doi: 10.1093/ajcp/92.5.676. [DOI] [PubMed] [Google Scholar]
- 47.Alwayn IPJ, Buhler L, Appel JZ, et al. Mechanisms of thrombotic microangiopathy following xenogeneic hematopoietic progenitor cell transplantation. Transplantation. 2001;71:1601–1609. doi: 10.1097/00007890-200106150-00020. [DOI] [PubMed] [Google Scholar]
- 48.Buhler L, Goepfert C, Kitamura H, et al. Porcine hematopoietic cell transplantation in nonhuman primates is complicated by thrombotic microangiopathy. Bone Marrow Transplant. 2001;27:1227–1236. doi: 10.1038/sj.bmt.1703067. [DOI] [PubMed] [Google Scholar]
- 49.Benatuil L, Fernandez AZ, Romano E. Aggregation of human platelets in plasma by porcine blood cells in vitro is probably mediated by thrombin generation. Xenotransplantation. 2003;10:454–459. doi: 10.1034/j.1399-3089.2003.00061.x. [DOI] [PubMed] [Google Scholar]