Abstract
Background:
Inhibitors of the mammalian target of rapamycin (mTOR) might become a novel tool to treat advanced prostate cancer. However, chronic drug exposure may trigger resistance, limiting the utility of mTOR inhibitors.
Methods:
Metastatic potential of PC3 prostate cancer cells, susceptible (PC3par) or resistant (PC3res) to the mTOR-inhibitor RAD001 was investigated. Adhesion to vascular endothelium or immobilised collagen, fibronectin and laminin was quantified. Motility, migration and invasion were explored by modified Boyden chamber assay. Integrin α and β subtypes were analysed by flow cytometry, western blotting and real-time PCR. Integrin-related signalling, EGFr, Akt, p70S6kinase and ERK1/2 activation were determined.
Results:
Adhesion was reduced, whereas motility, migration and invasion were enhanced in PC3res. The α2 and β1 integrin subtypes were dramatically elevated, integrins α1 and α6 were lowered, whereas α5 was nearly lost in PC3res. Activation of the Akt signalling pathway was strongly upregulated in these cells. Treating PC3par cells with RAD001 reduced motility, migration and invasion and deactivated Akt signalling. Blocking studies revealed that α2 and β1 integrins significantly trigger the motile behaviour of the tumour cells.
Conclusion:
Chronic RAD001 treatment caused resistance development characterised by distinct modification of the integrin-expression profile, driving prostate cancer cells towards high motility.
Keywords: prostate cancer, RAD001 resistance, integrins, Akt, migration
Prostate cancer, once metastasised, is difficult to treat. Surgical castration or hormonal manipulation provides initial success. However, patients progressively become hormone-resistant. During the last years, specific target proteins have been identified, which are involved in neoplastic development and tumour progression. In vitro investigation points to a close relationship between hormone resistance and activation of the mammalian target of rapamycin (mTOR) pathway (Wu et al, 2010). Analysis of tumour specimens has documented the association between mTOR variations and prostate cancer risk (Campa et al, 2011). Indeed, most patients with prostate cancer have at least one activated component of the mTOR signalling pathway (Kremer et al, 2006; Dai et al, 2009).
Hence, inactivating mTOR could become an attractive option to treat advanced prostate cancer. Among the number of mTOR inhibitors that have have been developed, the rapamycin analogues temsirolimus and RAD001 (everolimus) are the most prevalent in clinical use. Both have received US Food and Drug Administration approval, which however, is restricted to the treatment of advanced renal cell carcinoma. The relevance of temsirolimus and RAD001 in treating prostate cancer is still unclear. Although preclinical studies show mTOR inhibitors reverting prostatic neoplasia and reducing cell growth and proliferation (Morgan et al, 2009), the clinical experience of mTOR inhibition in men with castrate-resistant prostate cancer has been disappointing. Only a few patients have benefited from an mTOR inhibition-based regimen, and disease progression inevitably occured during treatment (Amato et al, 2008; Armstrong et al, 2010). It has, therefore, been argued that chronic drug exposure triggers the development of resistance, ultimately limiting the utility of mTOR inhibitors (Amato et al, 2008). Knowledge about the precise mechanism of resistance, however, is limited. Based on a RAD001-resistant prostate cancer cell line, we recently reported that drug non-responsiveness is characterised by an increased level of cdk1 and cyclin B, which counteracts growth-blocking effects of this drug (Tsaur et al, 2011). These studies have now been extended to explore the consequences of RAD001 resistance on the metastatic behaviour of prostate tumour cells. Additionally, the activity of RAD001-target proteins, as well as the expression pattern of α and β integrin adhesion receptors in resistant and non-resistant tumour cells, was analysed.
Materials and methods
Cell culture
The human prostate tumour cell line PC3 was obtained from DSMZ (Braunschweig, Germany). Tumour cells were grown and subcultured in RPMI 1640 (Gibco/Invitrogen, Karlsruhe, Germany) containing 10% fetal calf serum (FCS), 2% HEPES buffer (1 ℳ, pH 7.4), 2% glutamine and 1% penicillin/streptomycin. The RAD001-resistant subline was developed by 12 months of exposure to RAD001, starting at 1 nℳ and increasing stepwise to 1 μℳ. The control cells were designated PC3par, the resistant variant was termed PC3res.
Human endothelial cells (HUVECs) were isolated from human umbilical veins and harvested by enzymatic treatment with dispase (Gibco/Invitrogen). Human endothelial cells were grown in Medium 199 (M199; Biozol, Munich, Germany), supplemented with 10% FCS, 10% pooled human serum, 20 μg ml−1 endothelial cell growth factor (Boehringer, Mannheim, Germany), 0.1% heparin, 100 ng ml−1 gentamycin and 20 mℳ HEPES buffer (pH 7.4). Subcultures from passages 2–6 were selected for experimental use.
RAD001
RAD001 (provided by Novartis Pharma AG, Basel, Switzerland) was dissolved in DMSO as 10 mℳ stock solution and stored in aliquots at −20 °C. Prior to the experiments, RAD001 was diluted in cell culture medium. To analyse the influence of RAD001 on chemotactic movement, migration and invasion of PC3par versus PC3res cells, cell culture medium of PC3res cells containing 1 μℳ RAD001 was replaced by RAD001-free medium to avoid unspecific effects. A medium change was also carried out in the PC3par cell culture system. After 3 days, 5 nℳ RAD001 was added to both PC3par versus PC3res cells (controls were treated with fresh medium without RAD001) and chemotactic movement, migration and invasion were analysed.
To exclude toxic effects of the compound, cell viability was determined by trypan blue (Gibco/Invitrogen). For apoptosis detection, the expression of annexin V/propidium iodide (PI) was evaluated using the Annexin V-FITC Apoptosis Detection kit (BD Pharmingen, Heidelberg, Germany). Tumour cells were washed twice with PBS and then incubated with 5 μl of Annexin V-FITC and 5 μl of PI in the dark for 15 min at room temperature. Cells were analysed on a FACScalibur (BD Biosciences, Heidelberg, Germany). The percentage of apoptotic cells (early and late) in each quadrant was calculated using CellQuest software (BD Biosciences).
Tumour cell adhesion
To analyse tumour cell adhesion, HUVECs were transferred to six-well multiplates (Falcon Primaria; BD Biosciences) in complete HUVEC medium. When confluency was reached, PC3par or PC3res cells were detached from the culture flasks by accutase treatment (PAA Laboratories, Cölbe, Germany) and 0.5 × 106 cells were then added to the HUVEC monolayer for 30, 60 or 120 min. Subsequently, non-adherent tumour cells were washed off using warmed (37 °C) Medium 199. The remaining cells were fixed with 1% glutaraldehyde. Adherent tumour cells were counted in five different fields of a defined size (5 × 0.25 mm2) using a phase contrast microscope and the mean cellular adhesion rate was calculated.
Attachment to extracellular matrix components
Six-well plates were coated with collagen G (extracted from calfskin, consisting of 90% collagen type I and 10% collagen type III; seromed; diluted to 400 μg ml−1 in PBS), laminin (derived from the Engelbreth–Holm–Swarm mouse tumour; diluted to 50 μg ml−1 in PBS; BD Biosciences) or fibronectin (derived from human plasma; diluted to 50 μg ml−1 in PBS; BD Biosciences) overnight. Unspecific cell binding was evaluated by culture plates treated with poly-𝒟-lysine (Nunc, Wiesbaden, Germany). Plastic dishes were served as the background control. Plates were washed with 1% bovine serum albumin (BSA) in PBS to block nonspecific cell adhesion. Thereafter, 0.5 × 106 tumour cells were added to each well for 60 min. Subsequently, non-adherent tumour cells were washed off, the remaining adherent cells were fixed with 1% glutaraldehyde and counted microscopically. The mean cellular adhesion rate, defined by adherent cellscoated well−adherent cellsbackground, was calculated from five different observation fields.
Measurement of tumour cell motility (chemotaxis), migration and invasion
Serum-induced chemotactic movement was examined using six-well Transwell chambers (Greiner, Frickenhausen, Germany) with 8-μm pores. A total of 0.5 × 106 PC3par versus PC3res cells per ml were placed in the upper chamber in serum-free medium. To evaluate cell migration, Transwell chambers were precoated with collagen (400 μg ml−1). Cell invasion was explored by coating the Transwell chambers with collagen (400 μg ml−1), which were then overlaid with HUVEC. The lower chamber contained 10% serum. After 20 h incubation, the upper surface of the Transwell membrane was gently wiped with a cotton swab to remove non-migrating cells. Cells, which had moved to the lower surface of the membrane, were stained using hematoxylin and counted microscopically. The mean chemotaxis, migration or invasion rate was calculated from five different observation fields.
Integrin surface expression
PC3par versus PC3res cells were washed in blocking solution (PBS, 0.5% BSA) and then incubated for 60 min at 4 °C with phycoerythrin (PE)-conjugated monoclonal antibodies directed against the following integrin subtypes: anti-α1 (IgG1; clone SR84, dilution 1 : 1000), anti-α2 (IgG2a; clone 12F1-H6, dilution 1 : 250), anti-α3 (IgG1; clone C3II.1, dilution 1 : 1000), anti-α4 (IgG1; clone 9F10, dilution 1 : 200), anti-α5 (IgG1; clone IIA1, dilution 1 : 5000), anti-α6 (IgG2a; clone GoH3, dilution 1 : 200), anti-β1 (IgG1; clone MAR4, dilution 1 : 2500), anti-β3 (IgG1; clone VI-PL2, dilution 1 : 2500) or anti-β4 (IgG2a; clone 439–9B, dilution 1 : 250; all: BD Biosciences). Integrin expression of tumour cells was then measured using a FACscan (BD Biosciences; FL-2H (log) channel histogram analysis; 1 × 104 cells per scan) and expressed as mean fluorescence units. A mouse IgG1-PE (MOPC-21) or IgG2a-PE (G155–178; all: BD Biosciences) was used as an isotype control.
Western blot analysis
To explore the integrin protein level after 24 h drug incubation, tumour cell lysates were applied to a 7% polyacrylamide gel and electrophoresed for 90 min at 100 V. The protein was then transferred to nitrocellulose membranes. After blocking with non-fat dry milk for 1 h, the membranes were incubated overnight with the monoclonal antibodies listed above. Additionally, integrin-related signalling was explored by anti-integrin-linked kinase (ILK; clone 3, dilution 1 : 1000), anti-focal adhesion kinase (FAK; clone 77, dilution 1 : 1000) and anti-phospho-specific FAK (pY397; clone 18, dilution 1 : 1000) antibodies (all: BD Biosciences). HRP-conjugated goat-anti-mouse IgG (Upstate Biotechnology, Lake Placid, NY, USA; dilution 1 : 5.000) served as the secondary antibody. The membranes were briefly incubated with ECL detection reagent (ECL, Amersham/GE Healthcare, München, Germany) to visualise the proteins and then analysed by the Fusion FX7 system (Peqlab, Erlangen, Germany). β-actin (1 : 1.000; Sigma, Taufenkirchen, Germany) served as the internal control.
Real-time (RT)–qPCR
RT–qPCR was also done in triplicate. cDNA synthesis was performed using 3 μg of total RNA per sample according to the manufacturer’s protocol by AffinityScript QPCR cDNA Synthesis Kit (Stratagene, Amsterdam, The Netherlands). Quantitative gene-expression analysis by RT–PCR was performed by the Mx3005p (Stratagene) using SYBR-Green SuperArray (SABioscience Corporation, Valencia, CA, USA) and SuperArray primer sets: GAPDH (NM_002046.3, Hs.592355), integrin α1 (ITGA1, NM_181501, Hs.644352), integrin α2 (ITGA2, NM_002203, Hs.482077), integrin α3 (ITGA3, NM_002204, Hs.265829), integrin α4 (ITGA4, NM_000885, Hs.694732), integrin α5 (ITGA5, NM_002205, Hs.505654), integrin α6 (ITGA6, NM_000210, Hs.133397), integrin β1 (ITGB1, NM_002211, Hs.643813), integrin β3 (ITGB3, NM_000212, Hs.218040) and integrin β4 (ITGB4, NM_000213, Hs.632226; all: SABioscience Corporation). Calculation of the relative expression of each gene was done by the ΔΔCt method in the analysis programme of SABioscience Corporation. The housekeeping gene GAPDH was used for normalisation.
Cell signalling
Cell signalling was explored by using the following monoclonal antibodies: Akt (IgG1, clone 55, dilution 1 : 500), phospho Akt (pAkt; IgG1, clone 104A282, dilution 1 : 500), EGFr (IgG1, clone 13/EGFR, dilution 1 : 500), phospho EGFr (pEGFr; IgG1, clone 74, dilution 1 : 1000), ERK1 (IgG1, clone MK12, dilution 1 : 5000), ERK2 (IgG2b, clone 33, dilution 1 : 5000), phospho ERK1/2 (pERK; IgG1, clone 20A, dilution 1 : 1000; all: BD Biosciences), p70S6k (IgG, clone 49D7, dilution 1 : 1000) and phospho p70S6k (pp70S6k; IgG, clone 108D2, dilution 1 : 1000; all: New England Biolabs, Frankfurt, Germany).
Blocking studies
PC3par and PC3res cells were preincubated for 60 min with function-blocking anti-integrin β1 (clone 6S6), anti-integrin α2 (clone P1E6) or anti-integrin α5 (clone P1D6; all: Millipore, Schwalbach, Germany) monoclonal antibodies (20 μg ml−1). Controls remained untreated. Cells were then subjected to the chemotaxis and migration assay as indicated above. Adhesion to immobilised collagen was evaluated additionally. An anti-Akt function-blocking antibody was used to analyse the influence of Akt on PC3par and PC3res cell chemotaxis (Akt inhibitor VIII, 20 μg ml−1; Chemdea, Ridgewood, NJ, USA).
Statistics
All experiments were performed three to six times. Statistical significance was investigated by the Wilcoxon Mann–Whitney U-test. Differences were considered statistically significant at a P-value <0.05.
Results
Adhesion characteristics
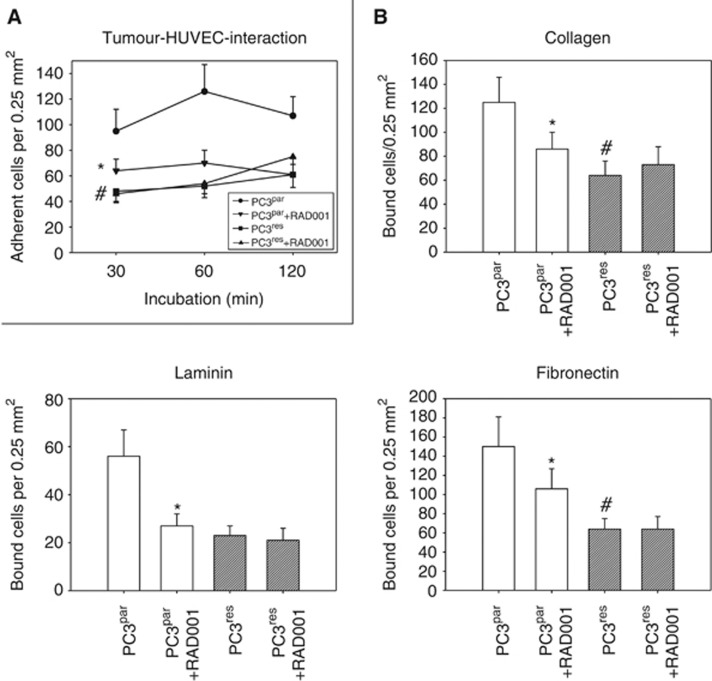
Dynamic evaluation of tumour cell–endothelial cell interaction revealed that more PC3par cells adhered to HUVEC over time than did PC3res cells (Figure 1A). Addition of 5 nℳ RAD001 significantly reduced the adhesion capacity of PC3par but not of PC3res cells. A similar phenomenon was seen in the extracellular matrix binding assay. More PC3par cells bound to immobilised collagen, laminin or fibronectin than PC3res cells (Figure 1B), and application of 5 nℳ RAD001 resulted in a diminished attachment rate of PC3par but not of PC3res cells.
Figure 1.
Cell–cell and cell–matrix interaction of PC3par versus PC3res cells. (A) Adhesion to HUVEC. PC3par and PC3res cells were treated with fresh medium (without RAD001) for 3 days and then added to HUVEC monolayers for 30, 60 or 120 min. (B) Adhesion to extracellular matrix proteins. PC3par and PC3res cells were treated with fresh medium (without RAD001) for 3 days and then added to immobilised collagen, laminin or fibronectin for 60 min. The adhesion rate of PC3par versus PC3res cells was compared in both experimental settings. Adhesion rate of PC3par and PC3res cells was also compared with the number of PC3par and PC3res cells treated with fresh medium for 3 days and subsequently with 5 nℳ RAD001. Mean values were calculated from five counts. Mean adhesion (A) or binding capacity (B) is depicted as adherent cells per 0.25 mm2. One representative of six experiments is shown. *indicates significant difference between the PC3 subline not treated with 5 nℳ RAD001 and the PC3 subline treated with 5 nℳ RAD001. #indicates significant difference between PC3par and PC3res cells.
Tumour motility, migration and invasion
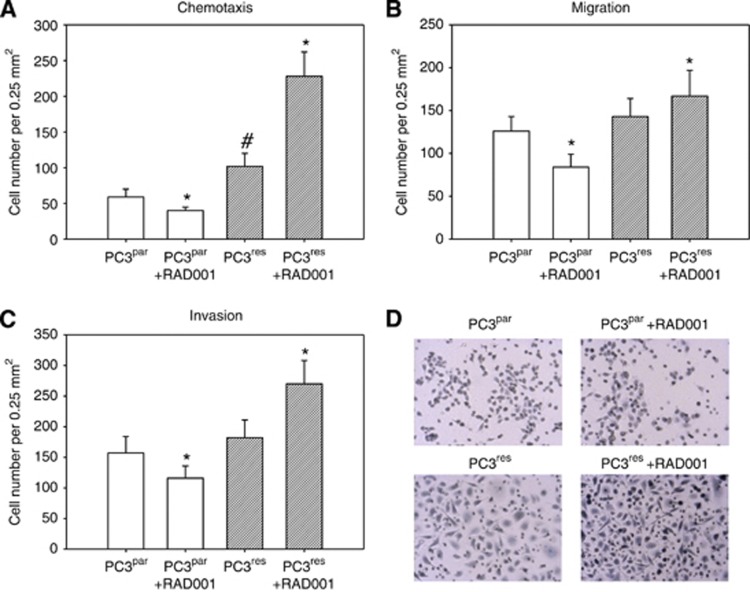
Chemotactic movement was significantly elevated in PC3res, compared with PC3par cells (Figure 2A). Furthermore, application of 5 nℳ RAD001 blocked chemotaxis of PC3par but strongly increased the motile activity of PC3res cells. PC3res cells also tended to cross collagen (Figure 2B) or HUVEC (Figure 2C) at a higher rate than PC3par cells did. The addition of 5 nℳ RAD001 decreased migration and invasion of PC3par cells. In contrast, migration and invasion of PC3res cells were upregulated in the presence of 5 nℳ RAD001. Figure 2D shows chemotaxis of penetrated PC3par cells, PC3par cells treated with RAD001, PC3res cells and PC3res cells treated with RAD001.
Figure 2.
RAD001 resistance alters PC3 chemotaxis (A, D), migration (B) and invasion (C) as assessed in a Transwell chamber assay. PC3par and PC3res cells were used, as well as PC3par and PC3res cells, additionally treated with 5 nℳ RAD001, as indicated in Materials and Methods. To evaluate chemotaxis, tumour cells were seeded in the upper chamber in serum-free medium, and 10% FCS, as the chemoattractant, was placed in the lower well. To evaluate cell migration, Transwell chambers were precoated with collagen. Invasion was analysed by adding the tumour cells to the upper chamber, which was coated with collagen and overlaid with HUVEC. Cells, which moved to the lower surface of the membrane, were stained using hematoxylin and counted. (D) Representative chemotaxis assays. Mean values were calculated from five counts and depicted as cell number per 0.25 mm2. One representative of six experiments is shown. *indicates significant difference between the PC3 subline not treated with 5 nℳ RAD001 and the PC3 subline treated with 5 nℳ RAD001. #indicates significant difference between PC3par and PC3res cells.
Integrins are modified in PC3res cells
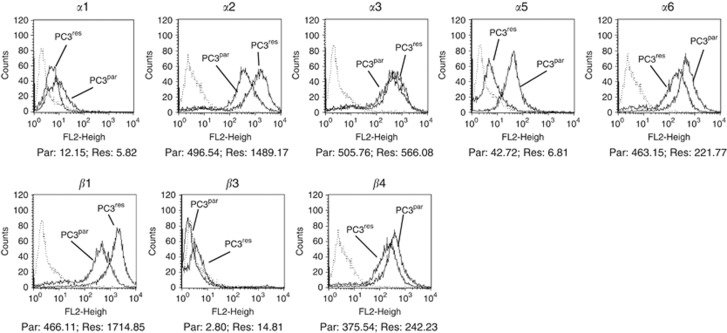
Surface levels of integrin α and β adhesion receptors were analysed next. The integrin subtypes α2, α3, α6, β1 and β4 were strongly expressed, α1 and α5 were moderately expressed and β3 was not expressed on PC3par cells (Figure 3). The α4 integrin subtype was not detected by flow cytometry, either on PC3par or PC3res cells (data not shown). PC3res cells were characterised by distinct differences in the integrin-expression pattern, compared with the controls. The α2 and β1 subtypes were dramatically elevated. Integrins α1 and α6 were lowered, whereas α5 was nearly lost on the PC3res cell membrane. The β3 subtype appeared on PC3res cells. Only slight differences were seen with respect to the α3 and β4 integrins. In a further experimental setting, PC3res and PC3par cells were treated short-term with 5 nℳ RAD001. Integrin α2 and β1 expression (most altered under chronic RAD001 treatment) was then evaluated. Exposing PC3par cells to RAD001 led to an upregulation of α2 (+69.7±14.8%) and β1 (+39.7±7.6%), compared with untreated PC3par cells. Short-term treatment of PC3res cells with RAD001 also evoked an upregulation of α2 (+14.2±4.9%) and β1 (+17.3±4.0%), although to a lesser extent than in PC3par cells.
Figure 3.
FACS analysis of integrin α and β subtype expression on PC3par versus PC3res cells. Cells were washed in blocking solution and then stained with specific monoclonal antibodies as listed in Materials and Methods. To evaluate background staining of PE-conjugated antibodies, goat anti-mouse IgG1-PE or IgG2a-PE was used (dotted lines). Fluorescence was analysed using a FACScan flow cytometer. Mean fluorescence values are given below the histograms. One from three independent experiments.
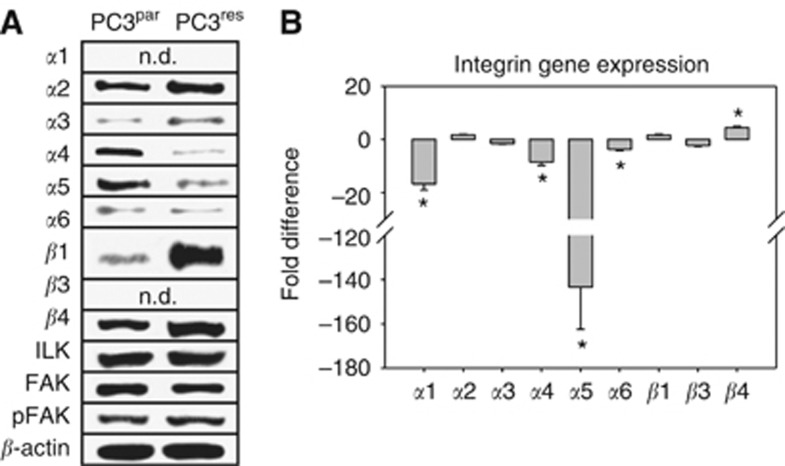
Western blotting demonstrated slight elevation of α2, α3 and β4 integrins in PC3res cells, compared with the control cell line. Notably, the β1 protein content was found to be drastically upregulated in cells resistant to RAD001 (Figure 4A). In contrast, the α4 integrin protein, which was detectable in the PC3par cytoplasm, as well as the α5 integrin, was suppressed in PC3res cells. Protein bands specific for the α1 and β3 integrin were not seen in the cell cultures. FAK, pFAK and ILK analysis showed similar protein amounts in drug-resistant compared with drug-sensitive tumour cells.
Figure 4.
Modification of intracellular integrin protein level. (A) Lysates of PC3par or PC3res cells were subjected to SDS–PAGE and blotted on the membrane incubated with respective monoclonal antibodies. β-actin served as the internal control. The figure shows one representative from three separate experiments. (B) is related to the integrin gene-expression pattern. Primer sets used for evaluation are listed in materials and methods. Calculation of the relative expression of each gene was done by the ΔΔCt method in the analysis programme of SABioscience Corporation. The housekeeping gene GAPDH was used for normalisation. Values are given as fold difference to PC3par cells. *indicates significant difference.
Additionally, integrin-coding genes were evaluated. The most distinct differences became evident on α5 integrin mRNA, which was expressed in PC3res at a very low level, compared with the PC3par cells (Figure 4B). There was also a significant reduction of α1, α4 and α6 integrins, accompanied by an enhancement of β4 integrin mRNA in the resistant compared with non-resistant cells.
Cell signalling is altered in PC3res cells
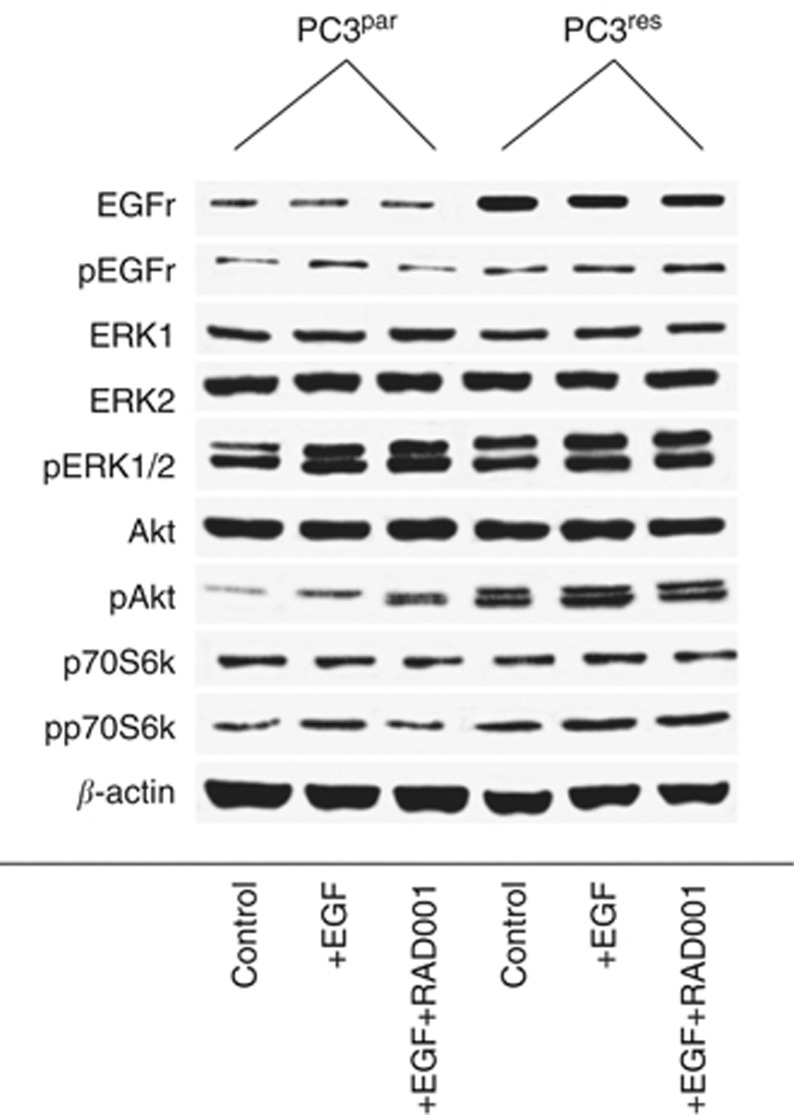
The consequence of resistance development for intracellular signalling was subsequently investigated. EGFr was expressed to a higher extent in PC3res compared with PC3par cells. This was also true with respect to EGFr activation (pEGFr; PC3res>PC3par). Stimulation with EGF further elevated pEGFr in both cell types. RAD001 reverted this process in PC3par but not in PC3res cells (Figure 5). p70S6k displayed a similar characteristic, whose activation in PC3res exceeded that in PC3par cells. RAD001 deactivated p70S6k in PC3par, whereas pp70S6k expression remained high in PC3res cells. Akt was visualised as a faint band in PC3par cells, whereas two distinct protein bands were detected in PC3res cells. EGF elevated pAkt in both PC3par and PC3res cells. Addition of RAD001 further enhanced pAkt in PC3par but slightly diminished pAkt in PC3res cells. Phosphorylation of ERK was enhanced in PC3res compared with PC3par cells. EGF additionally enhanced pERK in both cell lines. However, RAD001 did not alter the activation status of this protein.
Figure 5.
Western blot analysis of cell signalling proteins, listed in methods. PC3par or PC3res cells remained untreated (control). They were kept for 2 h in serum-free cell culture medium and subsequently stimulated for 30 min with EGF (100 ng ml−1; +EGF) or they were stimulated with EGF and additionally treated with 5 nℳ RAD001 (+EGF+RAD001). Cell lysates were then subjected to SDS–PAGE and blotted on the membrane incubated with the respective monoclonal antibodies. β-actin served as the internal control. The figure shows one representative from three separate experiments.
Blocking studies
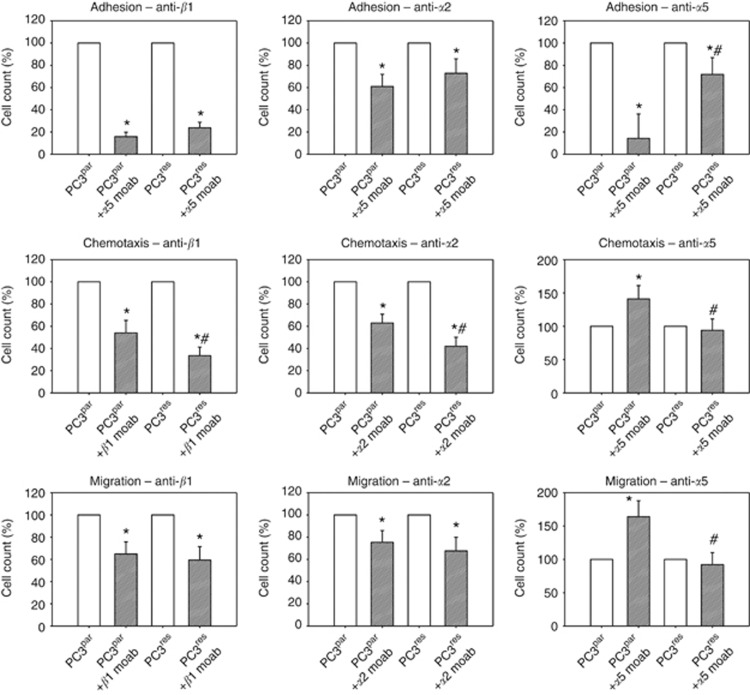
To investigate the functionality of β1 and α2 integrins, which were strongly elevated in PC3res, compared with PC3par cells, blocking studies were carried out. Figure 6 reveals that β1 and α2 integrins are significantly involved in adhesion, chemotactic movement and migration of both PC3res and PC3par cells. Chemotaxis was significantly more intensely diminished in PC3res compared with PC3par cells. We also investigated the relevance of the α5 integrin loss, which was evident in PC3res cells. Blocking α5 in PC3par cells led to a significant downregulation of adhesion and upregulation of chemotaxis and migration. However, blocking α5 in PC3res caused no effect on the motile behaviour of this cell type. To explore whether activation of Akt, evident in the drug-resistant cells, is also involved in invasion and metastasis, PC3par and PC3res cells were treated with an Akt inhibitor and chemotaxis was investigated. Interestingly, Akt blockade strongly diminished chemotaxis of PC3par (−43.9±10.2%) but not of PC3res cells.
Figure 6.
Influence of integrin α2, α5 or β1 blockade on tumour cell adhesion, chemotaxis or migration. PC3par or PC3res cells were preincubated for 60 min with function-blocking anti-integrin β1, anti-integrin α2 or anti-integrin α5 monoclonal antibodies. Controls remained untreated. Cells were then subjected to the adhesion, chemotaxis and migration assay as indicated in Materials and Methods. Values are shown as percentage difference to the 100% control. *indicates significant difference between the PC3 control subline and the PC3 subline treated with the function-blocking antibody. #indicates significant difference between PC3par and PC3res cells whose integrin subtype was blocked.
Discussion
Despite encouraging preclinical and clinical results of mTOR inhibitors, resistance has emerged as a problem. Because metastasis is a critical step in tumour dissemination and progression, the consequences of RAD001 resistance in prostate cancer adhesion and invasion was investigated in the present study. The PC3res cells were defined by an IC50 value for RAD001, which was 70-fold higher than that for PC3par cells (Tsaur et al, 2011). Evidence is presented here that drug non-responsiveness is coupled to downregulation of tumour adhesion to endothelial cells and extracellular matrix proteins, accompanied by increased chemotactic activity. Tumour-cell amoeboid motility is necessary for metastasis (Yilmaz and Christofori, 2010; van Zijl et al, 2011). Hence, the differences seen between PC3res and PC3par cells indicate that long-term exposure to RAD001 alters intracellular mechanisms, which are closely involved in controlling metastatic spread. The differences in the motile behaviour of PC3res and PC3par cells became particularly evident in the chemotaxis assay, which only evaluates cell movement. Differences were not clearly seen in the migration and invasion assay, probably because these assays include cell movement as well as the interaction of the tumour cells with collagen or HUVEC, respectively. The interaction with HUVECs was downregulated in PC3res compared with PC3par cells. Consequently, the total count of PC3res and PC3par cells might be equalised in the migration and invasion assay. Most importantly, treating PC3res cells with a therapeutically relevant RAD001 dosage dramatically increased their motile capability as shown in the chemotaxis, migration and invasion assays. Chronic drug treatment, therefore, may drive the tumour cell to acquire a more invasive phenotype, and continuing RAD001 application may further accelerate the metastatic dissemination.
Evaluation of the mechanism responsible for the elevated motile behaviour of PC3res cells points to a modified integrin-expression pattern. Particularly, the α2 and β1 subtypes were upregulated, whereas the α5 subtype was absent in the drug-resistant cells. The role of α2 in prostate cancer metastasis is not yet clear. Neal et al (2011) have reported that α2 expression inversely correlates with prostate cancer cell migration into collagen, whereas the opposite was seen by Van Slambrouck et al (2009). Based on our own blocking studies, increased α2 seems more likely connected with elevated motile behaviour, because functional blocking of the integrin α2 subunit distinctly inhibited both chemotactic movement and migration through a collagen matrix. The blocking effect was significantly stronger in the resistant sublines than in the parental cells. This is important. Obviously, metastatic spreading of RAD001-resistant prostate cancer is accelerated by two strategies: (1) by upregulating the α2 expression level (quantitative regulation) and (2) by strengthening the relevance of α2 in controlling invasion (qualitative regulation). In fact, cell migration has been demonstrated to depend on the number of α2 integrin receptors expressed on the cell surface (Li et al, 2011), as well as on qualitative parameters, such as activation of intracellular signalling cascades and/or receptor cross-talk (Ning et al, 2005; Sawhney et al, 2006). We assume that the conversion of prostate cancer cells from a drug-sensitive to a drug-insensitive state is accompanied by an elevated α2 level, α2–cytoskeleton interaction and cytoskeleton-related signalling, finally enforcing actin turnover and remodelling.
The same mode of action may be attributed to β1 as to α2 integrin receptors, because β1 blockade leads to a distinct downregulation of chemotaxis and migration (PC3res>PC3par). However, the role of the β1 receptor seems to be complex. Blocking β1 also reduced tumour cell adhesion properties. Because β1 was strongly increased in the PC3res variant, an enhanced attachment rate of these cells, compared with the controls, could be expected, but was not the case. PC3res–HUVEC and PC3res–matrix interaction were even lowered. Consequently, integrin β1 may not serve as a pure mechanistic binding element. Live cell imaging of fibroblast spreading has demonstrated that β1 undergoes an affinity switch, which allows disassembly of adhesion structures and dynamic crawling (Millon-Frémillon et al, 2008). In line with this, alteration of β1-actin cross-linking has been reported to weaken adhesion and increase migratory activity of cancer cells (Mouneimne and Brugge, 2007). Chronic treatment with RAD001 possibly induces a functional switch in PC3 cells. In fact, a greater dependency of tumour migration on β1 was seen in the resistant compared with the non-resistant cell line. With this in mind, β1 (as well as α2) integrin elevation in PC3par induced by short-term RAD001 application may strengthen adhesive forces and thereby prevent motile spreading, whereas the same effect may cause enhanced chemotactic activity of PC3res cells. In fact, treating PC3par cells with RAD001 led to a significant reduction of tumour cell chemotaxis, migration and invasion, whereas short-term treatment of PC3res cells with RAD001 evoked the opposite effect. The interpretation of the integrin data obtained after short-term RAD001 treatment is speculative. However, the same integrin has recently been shown to control cell spreading and retraction by switching the direction of integrin outside-in signalling (Flevaris et al, 2007). Deshmukh et al (2011) have provided a complex paradigm where integrin function depends on the secondary structure pattern and overall folding of the integrin cytoplasmic tail, shifting the integrin influence to different signalling proteins and the intracellular pathways. Therefore, it seems plausible that resistance development of PC3 cells may be accompanied by two different processes: (A) quantitative alterations of the integrin-expression level and (B) structural changes of the integrin molecules, leading to a switch of the intracellular pathway direction following short-term RAD001 treatment.
Apart from being involved in metastasis, β1 integrins are required for Akt phosphorylation and contribute to cell survival and growth (Riaz et al, 2012). Downregulation of β1 in prostate cancer cells inhibited Akt activation and retarded tumour proliferation (Niewiarowska et al, 2009; Goel et al, 2010). Meanwhile, β1 is considered to be a key component in regulating the conversion from a dormant state to active proliferation and metastasis (Barkan and Chambers, 2011). Our data point to a strong activation of Akt (along with EGFr and pERK) in PC3res cells. We cannot definitively declare that the massive accumulation of β1 in PC3res cells activates growth-related signals, because we did not analyse β1–Akt cross communication. However, Akt activation points towards a speed up of the cell-cycle machinery. It is of particular interest that β1 has been shown to contribute to chemoresistance in head and neck (Eke et al, 2012), pancreatic (Danilov et al, 2011), breast (Huang et al, 2011), lung (Ju et al, 2010) and ovarian cancer (Chen et al, 2010a), and targeting β1 integrins has provided benefit in overcoming drug non-responsiveness (Mori et al, 2008; Park et al, 2008; Huang et al, 2011). Whatever the precise mechanism of β1 in PC3res is, it represents a significant prognostic and therapeutic marker molecule. From a clinical viewpoint, patients should be carefully controlled when a β1 increase becomes overt. Ongoing studies should explore whether β1 increases during chronic RAD001 treatment and whether this increase correlates with resistance development in cancer patients.
An interesting phenomenon is seen with respect to the α5 integrin. Blocking α5 led to decreased adhesion and increased chemotaxis and migration of PC3par cells. It has recently been postulated that α5 may be crucial for cell detachment and subsequent metastasis of prostate cancer (Neal et al, 2011), which is in line with our results. However, this relationship does not seem transferable to the PC3res cells, whose adhesion properties were only slightly, and motile behaviour not at all, modified following α5 blockade. Another mode of action must be assumed here. Experiments with breast (Wang et al, 2011), melanoma (Landreville et al, 2011) or colon cancer cells (De Wever et al, 2011) have shown that α5 subunit functions as a tumour-growth suppressor. Indeed, a link between the α5 integrin and cell-cycle controlling proteins exists, because overexpression of α5 triggers downregulation of CDK2, thereby inhibiting cellular entry into the S phase (Wang et al, 2011). Vice versa, loss of α5 as seen in the PC3res cells may trigger enhanced CDK2 expression, resulting in elevated mitotic activity. This is speculative. However, a recent publication points to the accumulation of CDK1 and CDK2 in RAD001-resistant prostate cancer cells (Tsaur et al, 2011), which supports our hypothesis that the reduction of α5 evoked by long-term RAD001 exposure may cause an increase in tumour growth.
With respect to intracellular signalling, the most striking differences were seen in the activation level of Akt, which was strongly enhanced in PC3res compared with PC3par cells. Much data point to the relevance of this protein in resistance development. Upregulation of phosphorylated Akt has been shown to correlate to docetaxel resistance and progression to castration-resistant prostate cancer after androgen ablation (Kosaka et al, 2011). Evidence has also been provided that the Akt pathway has an important role in TRAIL resistance in cancer cells (Xu et al, 2010). It is not clear how long-term inhibition of mTOR triggers Akt activation. mTOR consists of two complexes, mTORC1, which is located downstream of Akt and is sensitive to mTOR inhibitors, and mTORC2, which is upstream of Akt and is resistant to mTOR inhibitors (Ma and Blenis, 2009). Long-term application of RAD001 may, therefore, induce feedback activation of Akt via mTORC2 signalling.
Concerning metastatic progression, activation of the Akt pathway has been shown to correlate with the chemotactic motility of prostate cancer cells in vitro (Jeong et al, 2012) and prostate tumour progression to metastasis in the transgenic adenocarcinoma mouse prostate mouse model (Sakamoto et al, 2010). Indeed, Akt blockade strongly diminished chemotaxis of PC3par cells, which corroborates both reports. Surprisingly, chemotactic activity of PC3res cells was not diminished following Akt blockade, perhaps indicating uncoupling of the integrin–Akt axis during resistance development. Similarly, Chen et al (2010b), recently observed an uncoupling of the Akt-connected pathways in drug-resistant breast cancer cells. This finding could be clinically relevant because therapeutic suppression of Akt may no longer prevent metastatic progression once tumour cells have acquired resistance. Whether the action of Akt in PC3res cells is exclusively focused on increasing the tumour mass (e.g., by speeding up tumour cell proliferation and blocking apoptosis) is not yet clear.
This study demonstrates that RAD001 resistance drives prostate cancer cells to become highly motile. The process is accompanied by significant alterations of the integrin-expression profile, particularly α2, α5 and α1, and by reactivating Akt. Further studies should be directed towards answering whether α5 integrin undergoes a functional switch from adhesion/migration to proliferation under chronic RAD001 treatment and whether Akt is connected to integrins during resistance development.
Acknowledgments
We would like to thank Karen Nelson for critically reading the manuscript. This work was supported by the ‘Alfons und Getrud Kassel-Stiftung’.
Footnotes
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
The authors declare no conflict of interest.
References
- Amato RJ, Jac J, Mohammad T, Saxena S (2008) Pilot study of rapamycin in patients with hormone-refractory prostate cancer. Clin Genitourin Cancer 6: 97–102 [DOI] [PubMed] [Google Scholar]
- Armstrong AJ, Netto GJ, Rudek MA, Halabi S, Wood DP, Creel PA, Mundy K, Davis SL, Wang T, Albadine R, Schultz L, Partin AW, Jimeno A, Fedor H, Febbo PG, George DJ, Gurganus R, De Marzo AM, Carducci MA (2010) A pharmacodynamic study of rapamycin in men with intermediate- to high-risk localized prostate cancer. Clin Cancer Res 16: 3057–3066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkan D, Chambers AF (2011) β1-integrin: a potential therapeutic target in the battle against cancer recurrence. Clin Cancer Res 17: 7219–7223 [DOI] [PubMed] [Google Scholar]
- Campa D, Hüsing A, Stein A, Dostal L, Boeing H, Pischon T, Tjønneland A, Roswall N, Overvad K, Østergaard JN, Rodríguez L, Sala N, Sánchez MJ, Larrañaga N, Huerta JM, Barricarte A, Khaw KT, Wareham N, Travis RC, Allen NE, Lagiou P, Trichopoulou A, Trichopoulos D, Palli D, Sieri S, Tumino R, Sacerdote C, van Kranen H, Bueno-de-Mesquita HB, Hallmans G, Johansson M, Romieu I, Jenab M, Cox DG, Siddiq A, Riboli E, Canzian F, Kaaks R (2011) Genetic variability of the mTOR pathway and prostate cancer risk in the European Prospective Investigation on Cancer (EPIC). PLoS One 6: e16914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Gomes AR, Monteiro LJ, Wong SY, Wu LH, Ng TT, Karadedou CT, Millour J, Ip YC, Cheung YN, Sunters A, Chan KY, Lam EW, Khoo US (2010b) Constitutively nuclear FOXO3a localization predicts poor survival and promotes Akt phosphorylation in breast cancer. PLoS One 5: e12293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YX, Wang Y, Fu CC, Diao F, Song LN, Li ZB, Yang R, Lu J (2010a) Dexamethasone enhances cell resistance to chemotherapy by increasing adhesion to extracellular matrix in human ovarian cancer cells. Endocr Relat Cancer 17: 39–50 [DOI] [PubMed] [Google Scholar]
- Dai B, Kong YY, Ye DW, Ma CG, Zhou X, Yao XD (2009) Activation of the mammalian target of rapamycin signalling pathway in prostate cancer and its association with patient clinicopathological characteristics. BJU Int 104: 1009–1016 [DOI] [PubMed] [Google Scholar]
- Danilov AV, Neupane D, Nagaraja AS, Feofanova EV, Humphries LA, DiRenzo J, Korc M (2011) DeltaNp63alpha-mediated induction of epidermal growth factor receptor promotes pancreatic cancer cell growth and chemoresistance. PLoS One 6: e26815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Wever O, Sobczak-Thépot J, Vercoutter-Edouart AS, Michalski JC, Ouelaa-Benslama R, Stupack DG, Bracke M, Wang JY, Gespach C, Emami S (2011) Priming and potentiation of DNA damage response by fibronectin in human colon cancer cells and tumor-derived myofibroblasts. Int J Oncol 39: 393–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshmukh L, Meller N, Alder N, Byzova T, Vinogradova O (2011) Tyrosine phosphorylation as a conformational switch: a case study of integrin β3 cytoplasmic tail. J Biol Chem 286: 40943–40953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eke I, Deuse Y, Hehlgans S, Gurtner K, Krause M, Baumann M, Shevchenko A, Sandfort V, Cordes N (2012) β1 integrin/FAK/cortactin signaling is essential for human head and neck cancer resistance to radiotherapy. J Clin Invest 122: 1529–1540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flevaris P, Stojanovic A, Gong H, Chishti A, Welch E, Du X (2007) A molecular switch that controls cell spreading and retraction. J Cell Biol 179: 553–565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goel HL, Underwood JM, Nickerson JA, Hsieh CC, Languino LR (2010) Beta1 integrins mediate cell proliferation in three-dimensional cultures by regulating expression of the sonic hedgehog effector protein, GLI1. J Cell Physiol 224: 210–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C, Park CC, Hilsenbeck SG, Ward R, Rimawi MF, Wang YC, Shou J, Bissell MJ, Osborne CK, Schiff R (2011) β1 integrin mediates an alternative survival pathway in breast cancer cells resistant to lapatinib. Breast Cancer Res 13: R84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong JW, Jin CY, Park C, Han MH, Kim GY, Moon SK, Kim CG, Jeong YK, Kim WJ, Lee JD, Choi YH (2012) Inhibition of migration and invasion of LNCaP human prostate carcinoma cells by cordycepin through inactivation of Akt. Int J Oncol 40: 1697–1704 [DOI] [PubMed] [Google Scholar]
- Ju L, Zhou C, Li W, Yan L (2010) Integrin beta1 over-expression associates with resistance to tyrosine kinase inhibitor gefitinib in non-small cell lung cancer. J Cell Biochem 111: 1565–1574 [DOI] [PubMed] [Google Scholar]
- Kosaka T, Miyajima A, Shirotake S, Suzuki E, Kikuchi E, Oya M (2011) Long-term androgen ablation and docetaxel up-regulate phosphorylated Akt in castration resistant prostate cancer. J Urol 185: 2376–2381 [DOI] [PubMed] [Google Scholar]
- Kremer CL, Klein RR, Mendelson J, Browne W, Samadzedeh LK, Vanpatten K, Highstrom L, Pestano GA, Nagle RB (2006) Expression of mTOR signaling pathway markers in prostate cancer progression. Prostate 66: 1203–1212 [DOI] [PubMed] [Google Scholar]
- Landreville S, Vigneault F, Bergeron MA, Leclerc S, Gaudreault M, Morcos M, Mouriaux F, Salesse C, Guérin SL (2011) Suppression of α5 gene expression is closely related to the tumorigenic properties of uveal melanoma cell lines. Pigment Cell Melanoma Res 24: 643–655 [DOI] [PubMed] [Google Scholar]
- Li HY, Liao CY, Lee KH, Chang HC, Chen YJ, Chao KC, Chang SP, Cheng HY, Chang CM, Chang YL, Hung SC, Sung YJ, Chiou SH (2011) Collagen IV significantly enhances migration and transplantation of embryonic stem cells: involvement of α2β1 integrin-mediated actin remodeling. Cell Transplant 20: 893–907 [DOI] [PubMed] [Google Scholar]
- Ma XM, Blenis J (2009) Molecular mechanisms of mTOR-mediated translational control. Nat Rev Mol Cell Biol 10: 307–318 [DOI] [PubMed] [Google Scholar]
- Millon-Frémillon A, Bouvard D, Grichine A, Manet-Dupé S, Block MR, Albiges-Rizo C (2008) Cell adaptive response to extracellular matrix density is controlled by ICAP-1-dependent beta1-integrin affinity. J Cell Biol 180: 427–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan TM, Koreckij TD, Corey E (2009) Targeted therapy for advanced prostate cancer: inhibition of the PI3K/Akt/mTOR pathway. Curr Cancer Drug Targets 9: 237–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori R, Ishiguro H, Kuwabara Y, Kimura M, Mitsui A, Tomoda K, Mori Y, Ogawa R, Katada T, Harata K, Fujii Y (2008) Targeting beta1 integrin restores sensitivity to docetaxel of esophageal squamous cell carcinoma. Oncol Rep 20: 1345–1351 [PubMed] [Google Scholar]
- Mouneimne G, Brugge JS (2007) Tensins: a new switch in cell migration. Dev Cell 13: 317–319 [DOI] [PubMed] [Google Scholar]
- Neal CL, Mckeithen D, Odero-Marah VA (2011) Snail negatively regulates cell adhesion to extracellular matrix and integrin expression via the MAPK pathway in prostate cancer cells. Cell Adh Migr 5: 249–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niewiarowska J, Sacewicz I, Wiktorska M, Wysocki T, Stasikowska O, Wagrowska-Danilewicz M, Cierniewski CS (2009) DNAzymes to mouse beta1 integrin mRNA in vivo: targeting the tumor vasculature and retarding cancer growth. Cancer Gene Ther 16: 713–722 [DOI] [PubMed] [Google Scholar]
- Ning Y, Zeineldin R, Liu Y, Rosenberg M, Stack MS, Hudson LG (2005) Down-regulation of integrin alpha2 surface expression by mutant epidermal growth factor receptor (EGFRvIII) induces aberrant cell spreading and focal adhesion formation. Cancer Res 65: 9280–9286 [DOI] [PubMed] [Google Scholar]
- Park CC, Zhang HJ, Yao ES, Park CJ, Bissell MJ (2008) Beta1 integrin inhibition dramatically enhances radiotherapy efficacy in human breast cancer xenografts. Cancer Res 68: 4398–4405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riaz A, Zeller KS, Johansson S (2012) Receptor-specific mechanisms regulate phosphorylation of AKT at Ser473: role of rictor in β1 integrin-mediated cell survival. PLoS One 7: e32081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto S, McCann RO, Dhir R, Kyprianou N (2010) Talin1 promotes tumor invasion and metastasis via focal adhesion signaling and anoikis resistance. Cancer Res 70: 1885–1895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawhney RS, Cookson MM, Omar Y, Hauser J, Brattain MG (2006) Integrin alpha2-mediated ERK and calpain activation play a critical role in cell adhesion and motility via focal adhesion kinase signaling: identification of a novel signaling pathway. J Biol Chem 281: 8497–8510 [DOI] [PubMed] [Google Scholar]
- Tsaur I, Makarević J, Hudak L, Juengel E, Kurosch M, Wiesner C, Bartsch G, Harder S, Haferkamp A, Blaheta RA (2011) The cdk1-cyclin B complex is involved in everolimus triggered resistance in the PC3 prostate cancer cell line. Cancer Lett 313: 84–90 [DOI] [PubMed] [Google Scholar]
- Van Slambrouck S, Jenkins AR, Romero AE, Steelant WF (2009) Reorganization of the integrin alpha2 subunit controls cell adhesion and cancer cell invasion in prostate cancer. Int J Oncol 34: 1717–1726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Zijl F, Krupitza G, Mikulits W (2011) Initial steps of metastasis: cell invasion and endothelial transmigration. Mutat Res 728: 23–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Shenouda S, Baranwal S, Rathinam R, Jain P, Bao L, Hazari S, Dash S, Alahari SK (2011) Integrin subunits alpha5 and alpha6 regulate cell cycle by modulating the chk1 and Rb/E2F pathways to affect breast cancer metastasis. Mol Cancer 13(10): 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Chhipa RR, Cheng J, Zhang H, Mohler JL, Ip C (2010) Androgen receptor-mTOR crosstalk is regulated by testosterone availability: implication for prostate cancer cell survival. Anticancer Res 30: 3895–3901 [PMC free article] [PubMed] [Google Scholar]
- Xu J, Zhou JY, Wei WZ, Wu GS (2010) Activation of the Akt survival pathway contributes to TRAIL resistance in cancer cells. PLoS One 5: e10226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yilmaz M, Christofori G (2010) Mechanisms of motility in metastasizing cells. Mol Cancer Res 8: 629–642 [DOI] [PubMed] [Google Scholar]