Abstract
Background:
Bowel cancer is a serious health burden and its early diagnosis improves survival. The Bowel Cancer Screening Programme (BCSP) in England screens with the Faecal Occult Blood test (FOBt), followed by colonoscopy for individuals with a positive test result. Socioeconomic inequalities have been demonstrated for FOBt uptake, but it is not known whether they persist at the next stage of the screening pathway. The aim of this study was to assess the association between colonoscopy uptake and area socioeconomic deprivation, controlling for individual age and sex, and area ethnic diversity, population density, poor self-assessed health, and region.
Methods:
Logistic regression analysis of colonoscopy uptake using BCSP data for England between 2006 and 2009 for 24 180 adults aged between 60 and 69 years.
Results:
Overall colonoscopy uptake was 88.4%. Statistically significant variation in uptake is found between quintiles of area deprivation (ranging from 86.4 to 89.5%), as well as age and sex groups (87.9–89.1%), quintiles of poor self-assessed health (87.5–89.5%), non-white ethnicity (84.6–90.6%) and population density (87.9–89.3%), and geographical regions (86.4–90%).
Conclusion:
Colonoscopy uptake is high. The variation in uptake by socioeconomic deprivation is small, as is variation by subgroups of age and sex, poor self-assessed health, ethnic diversity, population density, and region.
Keywords: colonoscopy, colorectal cancer, screening, socioeconomic status, deprivation
Colorectal cancer (CRC) accounts for 8% of cancer deaths worldwide, making it the fourth leading cause of cancer death (Ferlay et al, 2010). In the United Kingdom there are ∼38 000 CRC cases and 16 000 CRC deaths each year (Cancer Research UK, 2010). Survival is strongly related to stage of disease, with up to 90% survival if the disease is diagnosed at an early stage (Smith et al, 2001). Combined results from four randomised controlled trials show that annual or biennial screening for CRC using the Faecal Occult Blood test (FOBt), with further investigation by colonoscopy or other diagnostic procedure following a positive result, reduces CRC mortality by 16% (Hewitson et al, 2008). After adjusting for screening participation, screening is associated with a 25% relative risk reduction in CRC mortality (NHS Bowel Cancer Screening Programme, 2011).
Colorectal cancer screening in England is organised by the NHS Bowel Cancer Screening Programme (BCSP; NHS Bowel Cancer Screening Programme, 2011), which began in 2006, offering biennial FOBt to all adults aged 60–69 years. Screening is coordinated by five regional screening hubs. Eligible individuals within each hub are sent a FOBt kit with instructions on sample collection and return, followed by a reminder if the kit is not returned within 4 weeks. Participants with a positive FOBt result are given an appointment at a local specialist nurse clinic where the significance of a positive FOBt is explained and the merits of further investigations, usually by colonoscopy, are assessed and discussed. Individuals who are not contraindicated are offered a colonoscopy at the local screening centre.
There is good evidence that among those tested CRC screening is both effective at reducing CRC mortality (Hewitson et al, 2008) and cost-effective (Tappenden et al, 2004). However, uptake of FOBt was only 56.8% among 478 250 invitations sent out in the first round of the UK CRC Screening Pilot from 2000 to 2003 (UK Colorectal Cancer Screening Pilot Group, 2004), 52.1% among 127 746 invitations sent out in the second round of the Pilot from 2003 to 2005 (Weller et al, 2007), and 53.6% in the first 2.6 million invitations sent out in the NHS BCSP between 2006 and 2009 (von Wagner et al, 2011). FOBt uptake has also been shown to vary significantly by area deprivation and ethnic diversity (UK Colorectal Cancer Screening Pilot Group, 2004; Weller et al, 2007; von Wagner et al, 2009; von Wagner et al, 2011). For example, von Wagner et al (2011) found a gradient in FOBt uptake across quintiles of deprivation, ranging from 35% in the most deprived quintile to 61% in the least deprived. These inequalities occur against a background of widening socioeconomic inequalities in CRC survival: the deprivation gap in 5-year survival between rich and poor became significantly wider among patients diagnosed in England and Wales in 1996–1999, reaching 6% (for men) and 7% (women) for colon cancer, and 9% (men) and 8% (women) for rectal cancer (Coleman et al, 2004). Reducing these inequalities depends, at least in part, on reducing inequalities in uptake at each stage of the BCSP screening pathway, which first involves identifying the stages in the pathway where inequalities occur, so that appropriate interventions for increasing uptake can be designed and implemented.
Although a socioeconomic gradient in FOBt uptake has been established (UK Colorectal Cancer Screening Pilot Group, 2004; Weller et al, 2007; von Wagner et al, 2009; von Wagner et al, 2011), little is known about variation in uptake of colonoscopy following a positive FOBt result (Steele et al, 2010a). The first round of the UK CRC screening pilot showed that 81.5% participants who had a positive FOBt test received a colonoscopy (UK Colorectal Cancer Screening Pilot Group, 2004). In the second round of the pilot, 91.7% of 1171 participants who had a positive FOBt test attended the follow-up specialist nurse clinic and 82.8% had a colonoscopy (Weller et al, 2007). Deprivation was negatively associated with colonoscopy uptake in a pilot study in North East Scotland between 2000 and 2006; the effect was greater in men than in women and did not persist across the whole period (Steele et al, 2010a). Colonoscopy uptake was no different between South Asian and non-South Asian participants during the first two rounds of the UK CRC screening pilot (Szczepura et al, 2008).
The aim of this study was to assess the association between colonoscopy uptake and socioeconomic status, measured by area socioeconomic deprivation. We used a large, national dataset for England from the national screening programme to investigate whether or not uptake was associated with area deprivation, controlling for individual age and sex, and area poor self-assessed health, ethnic diversity and region (all of which have been associated with FOBt uptake). We also assessed the role of population density, as a measure of rurality, which has been associated with lower use of primary and secondary health care services, with rural populations having poorer access than others (Watt et al, 1994).
Materials and methods
Data and variables
Our main source of data was the NHS BCSP. We extracted data on individuals who completed an FOBt test between October 2006 and January 2009, and received a positive result. Our outcome measure was uptake of colonoscopy, defined as undergoing the colonoscopy procedure. We excluded those who had a positive FOBt less than 60 days before the data were extracted. The mean time interval between notification of a positive FOBt result and colonoscopy was 29 days. From the extracted data we excluded the small number of individuals who self-referred, were outside the 60–69 year age range, or for whom data on postcode of residence were not available. We also excluded individuals who attended the specialist nurse clinic following a positive FOBt result and were judged to be unsuitable for colonoscopy due to significant cardiovascular or respiratory morbidity, too frail to undergo standard laxative preparation, taking warfarin, or history of incomplete colonoscopy (NHS BCSP, 2010). In these groups, imaging, for example, by computer tomographic colonography, may be indicated (NHS BCSP, 2010).
For each individual, data were recorded on age, sex, and postcode of residence. We used the National Statistics Postcode Directory to link each postcode to a corresponding lower layer super output area (LSOA). There are 32 482 LSOAs in England, each with a minimum population of 1000 residents and 400 households, and with a national mean of 1500 residents. LSOAs were designed to include postcodes of similar social backgrounds based on housing tenure and dwelling type, and to be as geographically compact as possible. We used this linkage to merge LSOA-level data from the Neighbourhood Statistics website to individual BCSP participants (Neighbourhood Statistics website, 2011). We used LSOA data on deprivation, ethnic diversity, population density, and self-rated health. Deprivation was assessed using the Index of Multiple Deprivation (IMD) 2007 (Noble et al, 2008), which combines seven domains (income, employment, health deprivation, and disability, education training and skills, barriers to housing and services, crime, and living environment) based on 38 indicators, into a single deprivation score for each LSOA, with higher IMD scores representing greater deprivation. LSOAs are the smallest geographical units for which IMD scores are available. Ethnic diversity was measured by the percentage of the LSOA population who are non white. This was derived from 2001 Census data, and based on the percentage of residents who described themselves as being from ethnic groups other than ‘white British’, ‘white Irish’, and ‘white other’. Rurality was measured by population density, calculated as the number of people resident in each LSOA (based on 2001 Census data) divided by the size of the LSOA in hectares. We controlled for poor health, which was measured using self-assessed health status data from the 2001 Census, in which individuals were asked to categorise their health in the previous 12 months as ‘good’, ‘fairly good’, or ‘poor’. Our measure was the percentage of the LSOA population reporting poor health. We also included age group (<65 years, ⩾65 years) and sex interactions.
To test whether the results depended on the functional form of the variables of interest, area deprivation, poor self-assessed health, ethnic diversity, and rurality were measured as categorical variables and also as continuous variables. For the former we constructed quintiles of each measure based on national distributions (see Supplementary Online Material). We also included indicators for geographical region (BCSP hub) to investigate regional variation in uptake.
Analysis
We tested for differences in colonoscopy uptake by quintiles of area deprivation, individual age and sex, and quintiles of poor self-assessed health, non-white ethnicity, and population density using χ2 tests. We used logistic regression to regress colonoscopy uptake (1=had colonoscopy, 0=otherwise) against these variables. We ran unadjusted and adjusted models, the former including each variable individually, the latter including all variables jointly, both with and without region indicators.
We also ran models with continuous versions of these variables; we controlled for age and sex in every model and included statistically significant terms for area deprivation, non-white ethnicity, population density, and poor self-assessed health. In these models we experimented with including combinations of linear, quadratic, and cubic terms for each variable and used the specification that best fitted the data in terms of the statistical significance of the individual terms and the explanatory power of the models.
We tested the joint significance of the variables (all odds ratios=1, all coefficients=0) using Wald tests. In our adjusted analyses using quintiles, we calculated predictive margins, that is, adjusted probabilities of colonoscopy uptake in each quintile, fixing the other variables at their sample mean values. We calculated average marginal effects for each quintile as the difference in the predictive margins between quintiles to give the difference in the adjusted probability of uptake between each quintile. We drew unadjusted plots of the raw data using locally weighted scatterplot smoothing. In our adjusted analyses, using continuous variables, we plotted the predicted probability of colonoscopy uptake against each variable, fixing the other variables at their sample mean values. In every model, we adjusted for clustering at the regional (BCSP hub) level to account for the possibility that observations were not independent within regions.
Results
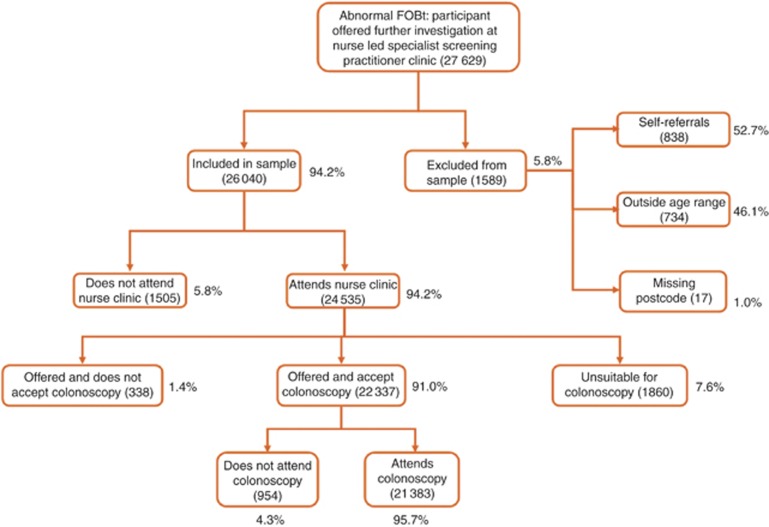
The total number of positive FOBt results in the BCSP between October 2006 and January 2009 was 27 629. Of these, 3449 individuals (12.5%) were excluded from the sample, either because they were self-referrals and therefore more likely to attend subsequent investigations all else equal (n=838), or because they were outside the 60–69 year age range (n=734), or because data on postcode of residence were not available (n=17), or because they attended the nurse clinic and were found to be unsuitable for colonoscopy for the reasons stated (n=1860). Hence, the final sample was 24 180 individuals, of whom 21 383 (88.4%) received a colonoscopy and 2797 (11.6%) did not (Figure 1). Among the 2797 who did not have a colonoscopy, 1505 (53.8%) did not attend the nurse clinic, 338 (12.1%) attended and were offered a colonoscopy but declined it, and 954 (34.1%) attended the clinic and were offered and accepted a colonoscopy appointment but did not attend.
Figure 1.
Colonoscopy uptake among individuals with a positive FOBt result. Number of individuals during the period October 2006 to January 2009 is shown in brackets. Abbreviation: FOBt=Faecal Occult Blood test.
There were statistically significant differences in mean colonoscopy uptake by quintiles of area deprivation in both unadjusted (P<0.001; Table 1) and adjusted models controlling for age and sex, poor self-assessed health, non-white ethnicity and population density, both with and without controls for region (P<0.001; Table 2). On the basis of the predictive margins not adjusting for region, between quintiles of area-level deprivation, uptake varied between 86.4 and 89.5% (Table 2); adjusting for region the range was 86.3–89.5% in both models there was little variation between quintiles. Statistically significant variation in uptake (all P<0.05) was also found using continuous variables rather than quintiles (Supplementary Online Material). These findings were borne out by unadjusted plots of the raw data using locally weighted scatterplot smoothing (Supplementary Online Material) and adjusted plots of colonoscopy uptake by continuous measures of area deprivation (Figure 2A). The plots show that colonoscopy uptake declines slightly at higher levels of area deprivation, but the extent of the decline is small.
Table 1. Unadjusted analyses of variables associated with colonoscopy uptake among individuals with a positive FOBt result (24 180 observations in every model).
| Sample (%) | Mean uptake (%) | OR (95% CI) | P -value | |
|---|---|---|---|---|
| Deprivation (IMD score) | ||||
| 1(most deprived) | 19.7 | 85.2 | 1.000 | — |
| 2 | 19.1 | 88.4 | 1.333*** (1.238–1.435) | <0.001 |
| 3 | 21.3 | 89.8 | 1.538*** (1.369–1.729) | <0.001 |
| 4 | 20.9 | 89.5 | 1.482*** (1.320–1.664) | <0.001 |
| 5 (least deprived) | 19.1 | 89.1 | 1.429*** (1.281–1.595) | <0.001 |
| Age (years) and sex | ||||
| <65* Female | 17.0 | 89.1 | 1.000 | — |
| <65* Male | 26.1 | 88.7 | 0.887 (0.861–1.090) | 0.595 |
| ⩾65* Female | 22.5 | 87.8 | 0.928*** (0.831–0.946) | <0.001 |
| ⩾65* Male | 34.4 | 88.3 | 0.969 (0.820–1.051) | 0.239 |
| % SAH: poor | ||||
| 1 (highest % SAH: poor) | 23.6 | 87.3 | 1.000 | — |
| 2 | 21.3 | 88.6 | 1.136** (1.003–1.287) | 0.045 |
| 3 | 20.6 | 89.9 | 1.293** (1.057–1.582) | 0.012 |
| 4 | 19.2 | 88.6 | 1.137 (0.974–1.327) | 0.104 |
| 5 (lowest % SAH: poor) | 15.4 | 87.8 | 1.049 (0.820–1.344) | 0.702 |
| % Non white | ||||
| 1 (highest % non white) | 20.0 | 84.1 | 1.000 | — |
| 2 | 15.0 | 87.3 | 1.301*** (1.226–1.380) | <0.001 |
| 3 | 17.3 | 89.1 | 1.553*** (1.272–1.896) | <0.001 |
| 4 | 21.4 | 89.8 | 1.666*** (1.451–1.914) | <0.001 |
| 5 (lowest % non white) | 26.4 | 90.9 | 1.887*** (1.647–2.161) | <0.001 |
| Population density | ||||
| 1 (lowest population density) | 21.7 | 90.3 | 1.000 | — |
| 2 | 20.1 | 88.8 | 0.857** (0.759–0.967) | 0.012 |
| 3 | 20.5 | 89.4 | 0.905 (0.777–1.054) | 0.199 |
| 4 | 19.5 | 87.6 | 0.759*** (0.640–0.899) | 0.001 |
| 5 (highest population density) | 18.2 | 85.7 | 0.647*** (0.516–0.811) | <0.001 |
| Region | ||||
| London hub | 16.0 | 83.7 | 1.000 | — |
| Eastern hub | 20.4 | 90.1 | 1.762*** (1.553–1.999) | <0.001 |
| North East hub | 16.0 | 90.4 | 1.833*** (1.599–2.103) | <0.001 |
| North West hub | 31.7 | 88.6 | 1.511*** (1.353–1.688) | <0.001 |
| Southern hub | 15.9 | 88.8 | 1.535*** (1.346–1.751) | <0.001 |
| Tests of joint significance (P-value) | ||||
| Deprivation (IMD score) | <0.001*** | <0.001*** | ||
| Age and sex | 0.241 | <0.001*** | ||
| % SAH: poor | 0.001*** | <0.001*** | ||
| % Non white | <0.001*** | <0.001*** | ||
| Population density | <0.001*** | <0.001*** | ||
| Region | <0.001*** | <0.001*** | ||
Abbreviations: FOBt=Faecal Occult Blood test; IMD=Index of Multiple Deprivation; OR=odds ratio; SAH=self-assessed health.
*P<0·1; **P<0·05; ***P<0·01. Associations expressed as OR.
Table 2. Adjusted analyses of variables associated with colonoscopy uptake among individuals with a positive FOBt result.
|
Adjusting for region
|
Not adjusting for region
|
|||||||
|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | P -value | PM | AME | OR (95% CI) | P -value | PM | AME | |
| Deprivation (IMD score) | ||||||||
| 1 (most deprived) | 1.000 | — | 0.863 | — | 1.000 | — | 0.864 | — |
| 2 | 1.262*** (1.112–1.433) | <0.001 | 0.888 | 0.025 | 1.246*** (1.100–1.412) | 0.001 | 0.888 | 0.024 |
| 3 | 1.358*** (1.092–1.690) | 0.006 | 0.895 | 0.032 | 1.346*** (1.088–1.665) | 0.006 | 0.895 | 0.031 |
| 4 | 1.251* (0.978–1.601) | 0.075 | 0.887 | 0.024 | 1.246* (0.973–1.595) | 0.082 | 0.888 | 0.024 |
| 5 (least deprived) | 1.271** (1.054–1.532) | 0.012 | 0.889 | 0.026 | 1.263** (1.052–1.517) | 0.012 | 0.889 | 0.025 |
| Age and sex | ||||||||
| Age <65 years * Female | 1.000 | — | 0.892 | — | 1.000 | — | 0.891 | — |
| Age <65 years * Male | 0.958 (0.849–1.081) | 0.484 | 0.887 | −0.004 | 0.960 (0.852–1.080) | 0.496 | 0.887 | −0.004 |
| Age ⩾65 years * Female | 0.881*** (0.831–0.934) | <0.001 | 0.879 | −0.013 | 0.881*** (0.829–0.936) | <0.001 | 0.879 | −0.013 |
| Age ⩾65 years * Male | 0.906 (0.795–1.031) | 0.135 | 0.882 | −0.010 | 0.910 (0.800–1.034) | 0.148 | 0.882 | −0.009 |
| % SAH: poor | ||||||||
| 1 (highest % SAH: poor) | 1.000 | — | 0.876 | — | 1.000 | — | 0.881 | — |
| 2 | 1.096 (0.977–1.231) | 0.119 | 0.886 | 0.010 | 1.061 (0.946–1.191) | 0.309 | 0.887 | 0.006 |
| 3 | 1.221*** (1.112–1.340) | <0.001 | 0.896 | 0.020 | 1.162*** (1.042–1.295) | 0.007 | 0.895 | 0.015 |
| 4 | 1.081 (0.898–1.302) | 0.411 | 0.884 | 0.008 | 1.014 (0.823–1.250) | 0.893 | 0.882 | 0.001 |
| 5 (lowest % SAH: poor) | 1.027 (0.878–1.202) | 0.737 | 0.879 | 0.003 | 0.948 (0.789–1.139) | 0.569 | 0.875 | −0.006 |
| % Non white | ||||||||
| 1 (highest % non white) | 1.000 | — | 0.858 | — | 1.000 | — | 0.846 | — |
| 2 | 1.145** (1.017–1.289) | 0.025 | 0.874 | 0.016 | 1.271*** (1.153–1.402) | <0.001 | 0.874 | 0.029 |
| 3 | 1.314** (1.024 to 1.685) | 0.032 | 0.888 | 0.030 | 1.496*** (1.162 to 1.927) | 0.002 | 0.891 | 0.046 |
| 4 | 1.404*** (1.192 to 1.654) | <0.001 | 0.894 | 0.036 | 1.588*** (1.334 to 1.890) | <0.001 | 0.897 | 0.051 |
| 5 (lowest % non white) | 1.548*** (1.227 to 1.953) | <0.001 | 0.903 | 0.045 | 1.767*** (1.416 to 2.206) | <0.001 | 0.906 | 0.061 |
| Population density | ||||||||
| 1 (lowest population density) | 1.000 | — | 0.882 | — | 1.000 | — | 0.885 | — |
| 2 | 0.986 (0.924–1.052) | 0.674 | 0.881 | −0.001 | 0.975 (0.918–1.035) | 0.405 | 0.882 | −0.003 |
| 3 | 1.104 (0.957–1.273) | 0.174 | 0.892 | 0.010 | 1.084 (0.950–1.238) | 0.230 | 0.893 | 0.008 |
| 4 | 0.973 (0.845–1.121) | 0.708 | 0.879 | −0.003 | 0.950 (0.838–1.077) | 0.423 | 0.879 | −0.005 |
| 5 (highest population density) | 1.056 (0.921–1.211) | 0.433 | 0.887 | 0.006 | 0.985 (0.872–1.112) | 0.804 | 0.883 | −0.002 |
| Region | ||||||||
| London hub | 1.000 | — | 0.864 | — | ||||
| Eastern hub | 1.318*** (1.137–1.528) | <0.001 | 0.893 | 0.029 | ||||
| North East hub | 1.413*** (1.186–1.683) | <0.001 | 0.900 | 0.036 | ||||
| North West hub | 1.245*** (1.072–1.445) | 0.004 | 0.888 | 0.024 | ||||
| Southern hub | 1.105 (0.953–1.281) | 0.187 | 0.875 | 0.011 | ||||
| Observations | 24 180 | 24 180 | ||||||
| C statistic | 0.5820 | 0.5782 | ||||||
| Tests of joint significance (P-value) | ||||||||
| Deprivation (IMD score) | <0.001*** | <0.001*** | ||||||
| Age and sex | <0.001*** | <0.001*** | ||||||
| % SAH: poor | <0.001*** | <0.001*** | ||||||
| % Non white | <0.001*** | <0.001*** | ||||||
| Population density | <0.001*** | <0.001*** | ||||||
| Region | <0.001*** | |||||||
Abbreviations: AME=average marginal effect (the difference in the adjusted probability of colonoscopy uptake between each group or quintile); CI=confidence interval; FOBt=Faecal Occult Blood test; IMD=Index of Multiple Deprivation; OR=odds ratio; PM=predictive margin (the adjusted probability of colonoscopy uptake in each group or quintile, fixing the other variables at their sample mean values); SAH=self-assessed health.
*P<0.1; **P<0.05; ***P<0.01.
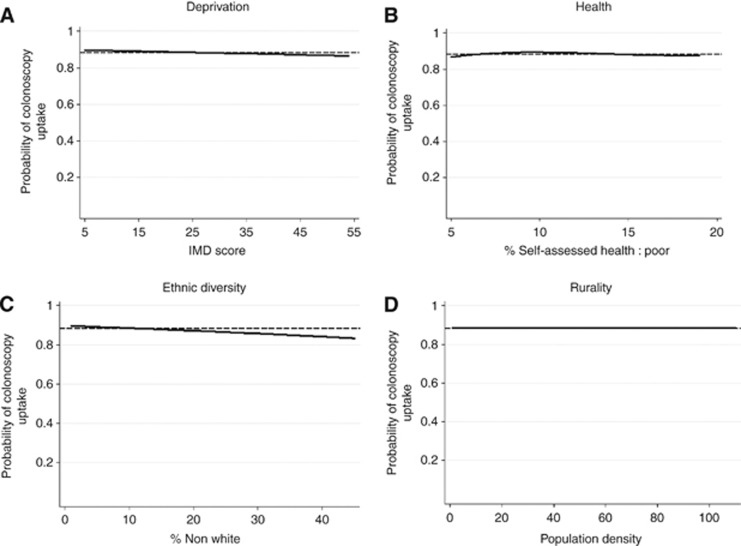
Figure 2.
Adjusted plots of colonoscopy uptake by deprivation (A), health (B), ethnic diversity (C) and rurality (D). The solid lines show the relationship between the variables and the probability of colonoscopy uptake based on the coefficients in Model (2) in Supplementary Table A3 in the Supplementary Online Material. Predicted values are computed by fixing the other variables at their sample mean values. The range of the x-axis is limited to the 5th to the 95th percentile of the sample values of each variable. The dashed lines show the mean uptake rate across the sample (88.4%).
In the unadjusted analyses (Table 1) we also find that colonoscopy uptake varies significantly by area-level poor health, non-white ethnicity and population density, and region (all P<0.05), but not by individual age and sex (P=0.241). Results in the adjusted analyses were similar (Table 2) with some small differences compared with the unadjusted models (all P<0.05, including for age and sex). The magnitude of the variation in colonoscopy uptake by quintile/region was small. On the basis of the predictive margins not adjusting for region, variation in uptake by age and sex groups was 87.9–89.1% (Table 2). Between quintiles of area-level self-assessed health, non-white ethnicity and population density, uptake varied in the ranges 87.5–89.5%, 84.6–90.6% and 87.9–89.3%, respectively. Variation by region was 86.4–90.0%, with uptake lowest in the London hub and highest in the North East hub. Statistically significant variation in uptake (all P<0.05) was also found using continuous variables rather than quintiles (Supplementary Online Material). The unadjusted plots of the raw data using locally weighted scatterplot smoothing (Supplementary Online Material) and adjusted plots of colonoscopy uptake by continuous measures show that colonoscopy uptake declines slightly at higher levels of non white, but the extent of the decline is small, and there is little evidence of any variation with respect to self-assessed health and rurality (Figures 2B, C and D).
We reran our models using more disaggregated ethnicity measures based on the percentage of residents in each LSOA in each of 10 non-white groups, and using an alternative rurality measure based on the Rural Definition produced by the Department of Environment, Food and Rural Affairs, which categorises areas according to their morphology and context (DEFRA, 2005; Supplementary Online Material). The relationship between colonoscopy uptake and area deprivation was qualitatively the same as in the original results, showing small but statistically significant variations (all P<0.05) in uptake by area deprivation. We found statistically significant variation in uptake by type of non-white ethnic group, and that uptake varied significantly by the rural classification of the area, but the extent of the variation was small (Supplementary Online Material).
We repeated the analysis using two different measures of colonoscopy uptake: undergoing a colonoscopy procedure but not excluding those who are contraindicated for colonoscopy (uptake across the whole sample was 82.1%), and attendance at the specialist nurse clinic (94.2%). The results were qualitatively the same in that for both alternatives variations in uptake by area deprivation, individual age and sex, and area poor health, ethnic diversity, rurality and region were statistically significant but small (Supplementary Online Material).
Discussion
Colonoscopy uptake was high (88.4%) among 24 180 participants in the NHS BCSP between October 2006 and January 2009 with a positive FOBt result. Statistically significant associations between uptake and area deprivation were observed, but the extent of the variation was small. These findings were found using unadjusted models and models adjusting for individual age and sex, and area self-assessed health, ethnic diversity, rurality and region. We also found that the variation in colonoscopy uptake by these covariates was small.
This is the first national study of socioeconomic variation in colonoscopy uptake following a positive FOBt. Our dataset was large (24 180 observations), recent (October 2006 to January 2009) and covered the whole of England. A strength of our analysis is that the size of the dataset has enabled us to investigate the association between colonoscopy uptake and deprivation, plus a number of variables simultaneously in a multivariate framework.
A potential weakness is that we have assessed the association between individual colonoscopy uptake and area variables. This was necessary because individual-level data, other than for colonoscopy uptake, age and sex, were not available. Given that we are primarily interested in socioeconomic variation, measured by IMD scores, our area variables are measured at the LSOA level, which is the lowest geographical unit at which IMD scores are available. Our method assumes that individuals living in the same area share similar characteristics. In defence of this approach, the area units were LSOAs, which are small both in terms of population size and geographical area, and are designed to be homogenous with respect to socioeconomic circumstances.
Data for 2003–2005 from the second round of the English site of the UK CRC Screening Pilot (Weller et al, 2007) showed that of 1171 individuals with a positive FOBt result, 1074 (91.7%) attended the follow-up specialist nurse clinic, 1001 of these (93.2%) were referred for colonoscopy and 970 (82.8% of all those with a positive FOBt result) attended the colonoscopy. Analogous figures using our data were 94.2% (24 535/26 040), 91.0% (22 337/24 535) and 82.1% (21 383/26 040), respectively. Studies in Denmark (Jørgensen et al, 2002), France (Faivre et al, 2004), Italy (Parente et al, 2009), and The Netherlands (Hol et al, 2010) have all reported similar uptake rates for colonoscopy after a positive FOBt result. In our main analysis we report a higher uptake rate (88.4%) because we excluded patients for whom colonoscopy was contraindicated.
One of the few studies to examine variation in colonoscopy uptake by population subgroups showed a diminishing SES gradient over time. Among 7388 individuals with a positive FOBT result who were invited for colonoscopy in a demonstration pilot in North East Scotland between 2000 and 2006, acceptance of colonoscopy was associated with area-level deprivation among men in 2000–2002 data (87.0% in the least deprived quintile to 77.8% in the most deprived quintile), and also in 2003–2004 data (93.6–85.1%), but not in 2005–2006 data (82.5–86.7%). Among women, acceptance of colonoscopy was associated with deprivation in 2003–2004 only (90.3–84.2; Steele et al, 2010a).
Although there were significant ethnic differences in FOBt uptake, colonoscopy uptake was no different between South Asian and non-South Asian individuals in England between 2000 and 2005 (Szczepur et al, 2008). In our study, although the extent of the variation in colonoscopy uptake between population subgroups was small, it varied most by quintiles of area-level ethnic diversity and by region. The London region (hub) had the lowest uptake (86.4%) and was also the most ethnically diverse (mean % non white 32.2% compared with mean 4.4% for all other hubs combined). Studies of screening uptake for breast cancer have also reported lower uptake in London compared with the rest of England (Eilbert et al, 2009; Renshaw et al, 2010). This may be due to the ethnic diversity in London (Eilbert et al, 2009; Renshaw et al, 2010), with cultural differences, and language and literacy influencing use of health care services among ethnic minority groups (Szczepura, 2005). Our results are consistent with these findings – in multivariate analyses we find that removing the regional indicators increases the impact of ethnic diversity on uptake – but we also note that the negative London effect persists even after controlling for ethnic diversity and deprivation, as well as other factors.
Approximately 10% of people with a positive FOBt result will have CRC (NHS Cancer Screening Programmes, 2006). Research and service innovations are therefore needed to improve uptake among the 11.6% who did not undergo colonoscopy. For example, over half of those who did not undergo colonoscopy did not attend the nurse clinic. Replacing the routine face-to-face nurse consultation with the choice of telephone interview or face-to-face consultation with the same nurse has been shown to reduce colonoscopy non-attendance rates from 14.9% to 0.8% (P<0.001; Rodger and Steele, 2008). In a recent Italian study, it was hypothesised that the high colonoscopy uptake rate among those with a positive FOBt (⩾92%) was achieved due to the use of a fast-track system carried out by a specialist nurse to contact FOBt-positive patients and arrange the colonoscopy appointment (Parente et al, 2011). This study also emphasised that the introduction of population-based screening for CRC can produce a change in attitudes towards colonoscopy among GPs and the general population over a relatively short period of time (two full rounds of screening), suggesting that awareness about CRC screening can change attitudes towards colonoscopy and increase uptake.
Our analysis shows that once people have made their decision to participate in FOBt screening, they are then highly likely to access and use appropriate follow-up health care. However, inequalities in use of the first step of the bowel cancer screening process need to be addressed, and future policy and research should focus on this issue, for example, using repeated invitations (Steele et al, 2010b). If strategies to increase FOBt uptake are successful, it remains to be seen whether the colonoscopy uptake rates shown by our data are maintained, given that these were achieved among respondents who agreed to FOBt using the current screening pathway and are therefore likely to have a higher propensity to participate than those who require more intensive recruitment efforts. Hence, our analysis ought to be repeated as and when FOBt uptake increases, and strategies to increase colonoscopy uptake ought to be introduced if current rates are not maintained.
Acknowledgments
This research was undertaken at UCLH/UCL that received a proportion of funding from the Department of Health’s NIHR Biomedical Research Centres funding scheme. EK is funded by a Medical Research Council Capacity Building Studentship in Health Economics.
Footnotes
Supplementary Information accompanies the paper on British Journal of Cancer website (http://www.nature.com/bjc)
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
The authors declare no conflict of interest.
Supplementary Material
References
- Cancer Research UK (2010) CancerStats key facts: large bowel cancer (colorectal cancer). Cancer Research UK: London [Google Scholar]
- Coleman M, Rachet B, Woods L, Miltry E, Riga M, Cooper N, Quinn MJ, Brenner H, Estève J (2004) Trends and socioeconomic inequalities in cancer survival in England and Wales up to 2001. Br J Cancer 90: 1367–1373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Department of Environment, Food and Rural Affairs (DEFRA) (2005) DEFRA Classification of Local Authority Districts and Unitary Authorities in England: an Introductory Guide. DEFRA: London [Google Scholar]
- Eilbert KW, Carrol K, Peach J, Khatoon S, Basnett I, McCulloch N (2009) Approaches to improving breast cancer screening uptake: evidence and experience from Tower Hamlets. Br J Cancer 101: S64–S67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faivre J, Dancourt V, Lejeune C, Tazi MA, Lamour J, Gerard D, Dassonville F, Bonithon-Kopp C (2004) Reduction in colorectal cancer mortality by faecal occult blood screening in a French controlled study. Gastroenterology 126: 1674–1680 [DOI] [PubMed] [Google Scholar]
- Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM (2010) Estimates of worldwide burden of cancer in 2008: GLOBOCAN. Int J Cancer 127: 2893–2917 [DOI] [PubMed] [Google Scholar]
- Hewitson P, Glasziou P, Watson E, Towler B, Irwig L (2008) Cochrane systematic review of colorectal cancer screening using the Fecal Occult Blood test (hemoccult): an update. Am J Gastroenterology 103: 1541–1549 [DOI] [PubMed] [Google Scholar]
- Hol L, van Leerdam M, van Ballegooijen M, van Vuuren AJ, van Dekken H, Reijerink JC, van der Togt AC, Habbema JD, Kuipers EJ (2010) Screening for colorectal cancer: randomised trial comparing guaiac-based and immunochemical faecal occult blood testing and flexible sigmoidoscopy. Gut 59: 62–68 [DOI] [PubMed] [Google Scholar]
- Jørgensen O, Kronborg O, Fenger C (2002) A randomised study of screening for colorectal cancer using faecal occult blood testing: results after 13 years and seven biennial screening rounds. Gut 50: 29–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neighbourhood Statistics website http://www.neighbourhood.statistics.gov.uk/dissemination/ (last accessed on 13 June 2011)
- NHS BCSP (2010) Guidelines for the use of imaging in the NHS Bowel Cancer Screening Programme NHS BCSP publication no. 5, September 2010 NHS Cancer Screening Programmes: Sheffield [Google Scholar]
- NHS Bowel Cancer Screening Programme http://www.cancerscreening.nhs.uk/bowel/ (last accessed on 13 June 2011)
- NHS Cancer Screening Programmes (2006) Bowel cancer Screening: The Colonoscopy Investigation (278490/Bowel Cancer Colonoscopy). Department of Health: London [Google Scholar]
- Noble M, McLennan D, Wilkinson K, Whitworth A, Barnes H (2008) The English Indices of Deprivation 2007. Communities and Local Government: London [Google Scholar]
- Parente F, Marino B, Ardizzio A, Ucci G, Ilardo A, Limonta F, Villani P, Morettie R, Zucchi A, Cremaschini M, Pirola M (2011) Impact of a population-based colorectal cancer screening program on local health services demand in Italy: a 7-year survey in a Northern province. Am J Gastroenterol 106: 1986–1993 [DOI] [PubMed] [Google Scholar]
- Parente F, Marino B, De Vecchi N, Morreti R, Ucci G, Tricomi P, Armellino A, Redaelli L, Bargiggia S, Cristofori E, Masala E, Tortorella F, Gattinoni A, Odinolfi F, Pirola ME Lecco Colorectal Cancer Screening Group (2009) Faecal occult blood test-based screening programme with high compliance for colonoscopy has a strong clinical impact on colorectal cancer. B J Surg 96: 533–540 [DOI] [PubMed] [Google Scholar]
- Renshaw C, Jack R, Dixon S, Møller H, Davies EA (2010) Estimating attendance for breast cancer screening in ethnic groups in London. BMC Public Health 10: 157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodger J, Steele RJC (2008) Telephone assessment increases uptake of colonoscopy in a FOBT colorectal cancer screening programme. J Med Screen 15: 105–107 [DOI] [PubMed] [Google Scholar]
- Smith RA, von Eschenbach AC, Wender R, Levin B, Byers T, Rothenberger D, Brooks D, Creasman W, Choen C, Runowicz C, Saslow D, Cokkinides V, Eyre H (2001) American Cancer Society guidelines for the early detection of cancer: update of early detection guidelines for prostate, colorectal, and endometrial cancers. Also: update 2001-testing for early lung cancer detection. CA Cancer J Clin 51: 38–75 [DOI] [PubMed] [Google Scholar]
- Steele R, Kostourou I, McClements P, Watling C, Libby G, Weller D, Brewster DH, Black R, Carey FA, Fraser C (2010a) Effect of gender, age and deprivation on key performance indicators of FOBT-based colorectal cancer screening. J Med Screen 17: 68–74 [DOI] [PubMed] [Google Scholar]
- Steele RJC, Kostourou I, McClements P, Watling C, Libby G, Weller G, Brewster DH, Black R, Carey FA, Fraser C (2010b) Effect of repeated invitations on uptake of colorectal cancer screening using faecal occult blood testing: analysis of prevalence and incidence screening. BMJ 341: c5531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szczepura A (2005) Access to health care for ethnic minority populations. Postgrad Med J 81: 141–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szczepura A, Price C, Gumber A (2008) Breast and bowel cancer screening uptake patterns over 15 years for UK south Asian ethnic minority populations, corrected for differences in socio-demographic characteristics. BMC Public Health 8: 346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tappenden P, Eggington S, Nixon R, Chilcott J, Sakai H, Karnon J (2004) Colorectal Cancer Screening Options Appraisal: Cost-Effectiveness, Cost-Utility and Resource Impact of Alternative Screening Options for Colorectal Cancer. Report to the English Bowel Cancer Screening Working Group September 2004. School of Health and Related Research: Sheffield [Google Scholar]
- UK Colorectal Cancer Screening Pilot Group (2004) Results of the first round of a demonstration pilot of screening for colorectal cancer in the United Kingdom. BMJ 329: 133–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Wagner C, Baio G, Raine R, Snowball J, Morris S, Atkin W, Obichere A, Jandley G, Logan RF, Rainbow S, Smith S, Halloran S, Wardle J (2011) Inequalities in participation in an organized national colorectal cancer screening programme: results from the first 2.6 million invitations in England. Int J Epidemiol 40: 712–718 [DOI] [PubMed] [Google Scholar]
- von Wagner C, Good A, Wright D, Rachet B, Obichere A, Bloom S, Wardle J (2009) Inequalities in colorectal cancer screening participation in the first round of the national screening programme in England. Br J Cancer 101: S55–S59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt I, Franks A, Sheldon T (1994) Health and health care of rural populations in the UK: is it better or worse? J Epidemiol Community Health 48: 16–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weller D, Coleman D, Robertson R, Butler P, Melia J, Campbell C, Parker R, Patnick J, Moss S (2007) The UK colorectal cancer screening pilot: results of the second round of screening in England. Br J Cancer 97: 1601–1605 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.