Abstract
Background:
We performed a cross-sectional study in Indonesia to evaluate the performance of a single-visit approach of cervical cancer screening, using visual inspection with acetic acid (VIA), histology and cryotherapy in low-resource settings.
Methods:
Women having limited access to health-care facilities were screened by trained doctors using VIA. If the test was positive, biopsies were taken and when eligible, women were directly treated with cryotherapy. Follow-up was performed with VIA and cytology after 6 months. When cervical cancer was suspected or diagnosed, women were referred. The positivity rate, positive predictive value (PPV) and approximate specificity of the VIA test were calculated. The detection rate for cervical lesions was given.
Results:
Screening results were completed in 22 040 women, of whom 92.7% had never been screened. Visual inspection with acetic acid was positive in 4.4%. The PPV of VIA to detect CIN I or greater and CIN II or greater was 58.7% and 29.7%, respectively. The approximate specificity was 98.1%, and the detection rate for CIN I or greater was 2.6%.
Conclusion:
The single-visit approach cervical cancer screening performed well, showing See and Treat is a promising way to reduce cervical cancer in Indonesia.
Keywords: single-visit approach, visual inspection with acetic acid (VIA), cryotherapy, Indonesia
Cervical cancer is the second most common cancer among women in developing countries, where more than 85% of the 530 000 new cervical cancer cases occur worldwide (Jemal et al, 2011). Cervical cancer rates in Indonesia are high, but because there is no population-based cancer registration, reports on the incidence of cervical cancer and efficacy of opportunistic screening procedures are limited. The governmental hospitals report cervical cancer to be up to 28% among all female cancer cases, representing 75% of all gynaecological cancers that are mostly diagnosed in advanced stages (Tjindarbumi and Mangunkusumo, 2002; Aziz, 2009).
In developed countries, introduction of organised Pap smear screening led to tremendous drops in cervical cancer rates, as it allows detection and treatment of precancerous lesions and early stage cervical cancer (Hakama et al, 1985; WHO Meeting, 1986; Laara et al, 1987; Anderson et al, 1988; Sankaranarayanan et al, 2001). Unfortunately, the health-care infrastructure in developing countries often prohibits successful implementation of organised conventional cytological screening due to lack of financial support, professional human resources and laboratory services (Vizcaino et al, 2000; Sankaranarayanan et al, 2004).
For many years, research has been done to develop alternative screening and treatment approaches for low-resource settings. Single-visit approaches, where visual inspection with acetic acid (VIA) and cryotherapy are applied in the same visit, are feasible, highly efficient and cost effective for these settings (University of Zimbabwe/JHPIEGO Cervical Cancer Project, 1999; Denny et al, 2000; Mandelblatt et al, 2002; Gaffikin et al, 2003a; Gaffikin et al, 2003b; Sankaranarayanan et al, 2004). Promising successes in decreasing cervical cancer incidence rates could be achieved using this method, reducing the lifetime risk of cervical cancer by 25% when 35-year-old women are screened only once in their life (Goldie et al, 2005).
This research describes a single-visit approach cervical cancer screening programme using VIA and cryotherapy in three areas in Indonesia, the first of its kind to be systematically performed and described in this setting. The main objective was to study the performance of this approach, targeting high-risk women aged 20–60 years, living in low-resource circumstances with limited access to health-care facilities. Information on prevalence of premalignant cervical cancer lesions and cervical cancer was collected and the performance of VIA was compared with histology, the reference diagnosis. When the VIA test was positive and women were eligible, they were directly treated with cryotherapy and registered for follow-up with VIA and cytology after 6 months. As follow-up was scheduled after therapy, the cure rate of cryotherapy could be measured.
The approval for this study was given by the institutional review boards of the three collaborating University Hospitals Cipto Mungunkusumo in Jakarta, Hasan Sadikin in Bandung, Sanglah Bali and the regional hospital in Tasikmalaya.
Materials and Methods
Study design
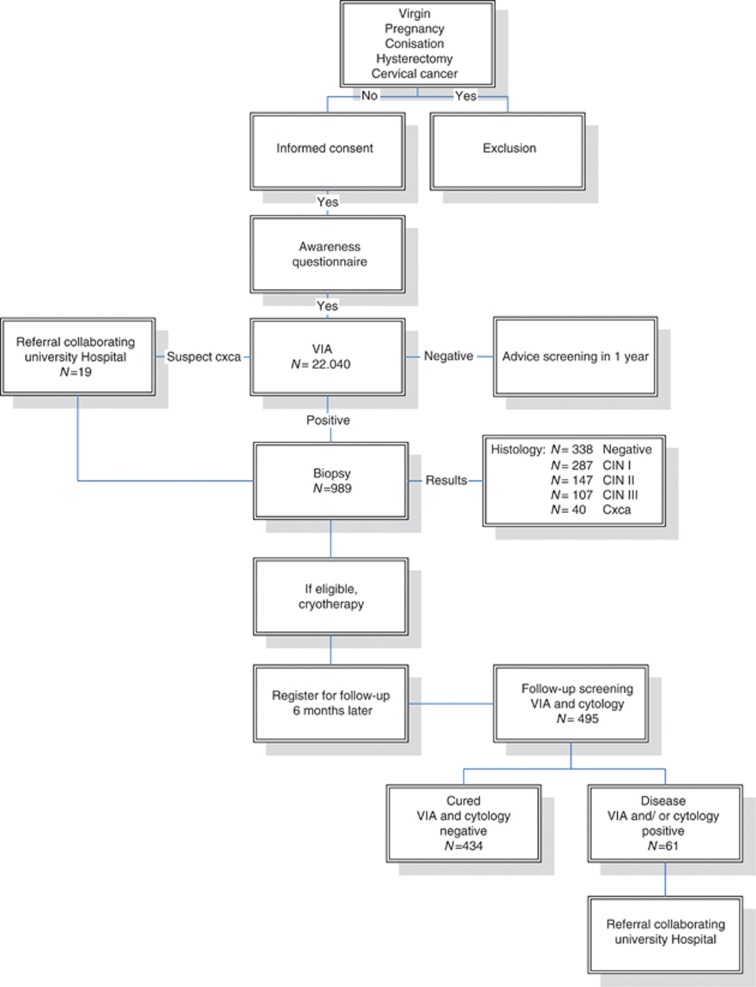
This community-based, single-visit approach, cervical cancer prevention programme was performed from October 2004 to June 2006. The programme was organised in collaboration with the University of Indonesia and the Cipto Mangunkusumo Hospital in Jakarta, the Padjadjaran University and the Hasan Sadikin Hospital in Bandung, Siliwangi University and General Hospital in Tasikmalaya, Udayana University and the Sanglah Hospital in Denpasar in Indonesia, and the Leiden University Medical Center in the Netherlands. This project aimed to screen women living in low-resource areas with limited access to health-care facilities. Screening was free of charge. Three mobile clinics with teams consisting of two doctors, two nurses, two public health nurses and a driver visited low-resource areas in Jakarta and Tasikmalaya (Java) and in rural areas on the island of Bali. All the members of the mobile clinic teams were trained in a 1-week central training course. In addition, the general practitioners (Jakarta and Tasikmalaya) and residents in gynaecology (Bali) were specially trained in VIA and cryotherapy and performed the screening using VIA. If the test was positive, biopsies were taken and women were directly treated with cryotherapy and registered for follow-up after 6 months. When cervical cancer was suspected or diagnosed, women were referred to one of the three collaborating regional University Hospitals for further evaluation and treatment. All costs for treatment, transportation and costs for an accompanying relative were covered by the Female Cancer Foundation. Figure 1 shows a flowchart of the study design.
Figure 1.
Flowchart of the study design.
Next to the screening through VIA, we performed conventional cytological screening in the same setting. However, in this paper, we focus on the VIA screening procedure; therefore, the cytological procedure and results will not be discussed.
Participants
The target group was women aged 20–60 years of whom we tried to reach 80% in each region. Women were excluded if they were virgin, pregnant, did not have an intact cervix or had a history of cervical cancer. To reach women for enrolment, collaboration was set up with the ‘Pembinaan Kesejahteraan Keluarga’ (PKK), the national Indonesian family welfare organisation that has access to the smallest villages. In the selected areas, the members of this local PKK encouraged women to participate in the project by visiting them in their homes, informing them about risk factors, prevention, early detection and treatment of cervical cancer. Printed information designed in collaboration with the Indonesian cancer foundation, ‘Yayasan Kanker Indonesia’ (YKI), was given to the women.
On scheduled days, the mobile clinic team arrived and set up a screening site in existing public facilities, mostly in small health facilities. Public health nurses counselled the women individually. After describing and explaining the procedure of screening and possible treatment, informed consent was obtained. Women were interviewed about cervical cancer risk factors, reproductive health and sociodemographic issues.
Test methods
With an adequate light source, the cervix was inspected visually. With a cotton swap, a 5% diluted acetic acid solution was applied to the cervix for 1 min. After application, acetowhite lesions in the transformation zone, close to the squamocolumnar junction, were considered positive and eligible for treatment (Sankaranarayanan, 2011). Cervicitis, nabothian cysts and polyps were considered negative. Biopsy was taken of all VIA-positive lesions and polyps were removed; both specimens were sent to the Department of Pathology of the collaborating University Hospital for diagnosis.
When the VIA result was negative, subsequent screening was advised at 1 year, according to the Indonesian guidelines. When the screening test was positive, women were counselled for immediate cryotherapy.
Cryotherapy was considered if the lesion was not suspected for cervical cancer, did not extend over more than 75% of the cervix, into the cervical canal or vaginal wall, and could be covered fully by the cryotherapy probe. Cryotherapy was provided in cycles of 3 min of freeze, 3 min of thaw and 3 min of freeze with an Erbe cryogun (Tuebingen, Germany) using a cone-shaped probe and CO2 gas (Jakarta and Tasikmalaya) or N2O (Bali). After cryotherapy, women received non-narcotic analgesics and one oral dose of antibiotics, according to the Indonesian guidelines. They were informed about side effects and instructed not to have sexual intercourse for 4 weeks and to return to the health centre in case of severe abdominal pain, fever (>38 °C) and purulent or bloody discharge.
In case of cervicitis, antibiotics were administered. Among women with a suspected diagnosis of cervical cancer based on clinical evaluation, biopsy was taken and they were referred to the collaborating University Hospitals for further diagnosis, staging and treatment. All histologic specimens were processed and diagnosed by the pathologists in the collaborating University Hospitals.
Follow-up 6 months after cryotherapy was scheduled at the initial screening. Women were informed about the date for their return appointment and received a follow-up card with the information. The mobile clinic came down to the village at the set date, if a patient did not show up at the follow-up appointment, she was contacted by phone or by the PKK.
The follow-up screening consisted of cytological and repeat VIA screening. The Pap smears were taken on the site and were diagnosed at the collaborating hospitals. In the follow-up, the cytopathologists reading the slides were aware of the VIA test results.
Patients were considered cured when both cytological screening and their VIA test were negative on follow-up. Cure rate was calculated by dividing the number of women who were negative on cytological and VIA follow-up screening by the number of women screened in the follow-up.
Treatment failure was diagnosed if at least one test was positive; these patients were referred to the collaborating University Hospitals for colposcopy.
During this study, site visits to guard the screening process and VIA quality were performed regularly in all three regions.
Calculations and data management
The positive predictive value (PPV) of the VIA test was calculated by dividing the histology-proven cases by the VIA test positive cases. The detection rate of CIN I, II, III and cervical cancer was calculated by dividing the number of screened women with these lesions by the total number of screened women. The specificity of the VIA test could not be given as there were no biopsies taken of the women who were negative on VIA screening but an approximate specificity of the VIA test was calculated following the example described by Sankaranarayanan et al (1998) by dividing the number of women with negative screening tests by the total number screened subjects minus the number of true-positive cases detected by the test.
Data management was performed using Microsoft Access 2003 and SPSS software (SPSS, version 17, SPSS Inc., Chicago, IL, USA).
Results
Demographics
Test results were completed in 22 040 women, all screened using VIA. Of these, 7480 (33.9%) were from Jakarta, 8007 (36.3%) from Tasikmalaya and 6553 (29.7%) from Bali. Mean age was 37.5 years (range 12–70) and 64.4% were age 30–49. Overall, 92.7% of women had never been screened before and one-third lived beneath the WHO poverty border of 1 US dollar a day. Table 1 reports participant characteristics.
Table 1. Characteristics of the participants.
| Overall | Jakarta | Tasik | Bali | |
|---|---|---|---|---|
| Number | n=22 040 | n=7480 | n=8007 | n=6553 |
| Characteristic |
Numbers (%)
|
|||
| Age | ||||
| <19 | 151 (0.7) | 47 (0.6) | 44 (0.5) | 60 (0.9) |
| 20–29 | 4652 (21.1) | 1545 (20.7) | 1535 (19.2) | 1572 (24.0) |
| 30–39 | 8169 (37.1) | 2642 (35.3) | 3133 (39.1) | 2394 (36.5) |
| 40–49 | 6006 (27.3) | 2151 (28.8) | 2346 (29.3) | 1509 (23.0) |
| 50–59 | 2607 (11.8) | 968 (12.9) | 808 (10.1) | 831 (12.7) |
| >60 | 351 (1.6) | 125 (1.7) | 136 (1.7) | 90 (1.4) |
| Missing | 104 (0.5) | 2 (0.0) | 5 (0.1) | 97 (1.5) |
| Ever screened before | ||||
| No | 20449 (92.8) | 6359 (85.0) | 7868 (98.3) | 6222 (94.9) |
| Yes | 1490 (6.8) | 1101 (14.7) | 124 (1.5) | 265 (4.0) |
| Missing | 101 (0.4) | 20 (0.3) | 15 (0.2) | 66 (1.0) |
| Education | ||||
| Illiterate | 2096 (9.5) | 317 (4.2) | 13 (0.2) | 1766 (26.9) |
| Primary school | 9293 (42.2) | 2773 (37.1) | 3771 (47.1) | 2749 (42.0) |
| High school | 8709 (39.5) | 3747 (50.1) | 3183 (39.8) | 1779 (27.1) |
| >High school | 1907 (8.7) | 635 (8.5) | 1036 (12.9) | 236 (3.6) |
| Missing | 35 (0.1) | 8 (0.1) | 4 (0.0) | 23 (0.4) |
| Income per day | ||||
| <1 US $ per day | 7367 (33.4) | 1016 (13.6) | 3504 (43.8) | 2847 (43.4) |
| 1–3 US $ per day | 7972 (36.2) | 3557 (47.6) | 1981 (24.7) | 2434 (37.1) |
| >3 US $ per day | 6092 (27.6) | 2667 (35.7) | 2306 (28.8) | 1119 (17.1) |
| Missing | 609 (2.8) | 240 (3.2) | 216 (2.7) | 153 (2.3) |
| Marital status | ||||
| Not married yet | 15 (0.1) | 0 (0.0) | 5 (0.1) | 10 (0.2) |
| Married | 21313 (96.7) | 7090 (94.8) | 7704 (96.2) | 6519 (99.5) |
| Separated | 134 (0.6) | 67 (0.9) | 67 (0.8) | 0 (0.0) |
| Widowed | 545 (2.5) | 315 (4.2) | 225 (2.8) | 5 (0.1) |
| Missing | 33 (0.1) | 8 (0.1) | 6 (0.1) | 19 (0.2) |
| Number of children | ||||
| 0 | 880 (4.0) | 360 (4.8) | 318 (4.0) | 202 (3.1) |
| 1/2 | 9513 (43.2) | 2766 (37.0) | 3139 (39.2) | 3608 (55.1) |
| 3/4 | 7606 (34.5) | 2555 (34.2) | 3028 (37.8) | 2023 (30.9) |
| 5/6 | 2754 (12.5) | 1143 (15.3) | 1095 (13.7) | 516 (7.9) |
| >7 | 1211 (5.5) | 649 (8.7) | 394 (4.9) | 168 (2.6) |
| Missing | 76 (0.3) | 7 (0.1) | 33 (0.4) | 36 (0.5) |
| Mean (s.d.) | ||||
| Menarche | 13.9 (1.5) | 13.7 (1.7) | 14.1 (1.5) | 13.9 (1.3) |
| Age at marriage | 19.9 (3.7) | 20.0 (4.1) | 19.4 (3.6) | 20.7 (3.2) |
| Age at first child | 21.1 (4.1) | 21.2 (4.5) | 21.1 (3.8) | 20.9 (4.0) |
| Sexual partners | 1.1 (0.4) | 1.2 (0.5) | 1.2 (0.5) | 1.0 (0.2) |
Screening
Of the 22 040 total women, 970 (4.4%) were VIA positive and 19 (0.1%) were suspected to have cervical cancer. The VIA positivity rate varied within the geographic regions from 2.3% in Jakarta to 3.9% in Bali and 6.8% in Tasikmalaya. A descending trend was seen when VIA positivity rate was calculated over time; when the total amount of women was divided in four even parts, the VIA positivity rate decreased from 5.3% within the first 25% of the screened women, to 4.8% in the second 25%, to 4.6% in the third 25%, finally, to a positivity rate of 3.3% within the last 25% of the screened women.
On histologic analysis, 338 (34.2%) had a normal histology, 287 (1.3%) had a diagnosis of CIN I, 147 (0.7%) CIN II, 105 (0.5%) CIN III and 42 (0.2%) adenocarcinoma in situ, adenocarcinoma, or squamous cell carcinoma. In 7% of the biopsies (70/989), there was no diagnosis as the quality of the biopsies was too poor for histological classification. The overall detection rates for lesions ⩾CIN I and ⩾CIN II were 2.6% and 1.3%, respectively.
Most positive results were in the 30–39-year-old age group, followed by the 40–49-year-old age group. Together, these groups account for 70.7% of VIA-positive cases and 73.0% of histologic-positive cases. For details on age distribution and positive test results and (pre)malignant detection rate, see Table 2.
Table 2. VIA positivity and (pre)malignancy detection rate.
| VIA+ | CIN I | CIN II | CIN III | Cxca | ||
|---|---|---|---|---|---|---|
| No. screened | Number (%) | Number (detection rate in %) | ||||
| Overall | 22 040 | 989 (4.5) | 287 (1.3) | 147 (0.7) | 107 (0.5) | 40 (0.2) |
| <19 | 151 | 3 (2.0) | 1 (0.7) | 0 (0.0) | 1 (0.7) | 0 (0.0) |
| 20–29 | 4652 | 174 (3.7) | 46 (1.0) | 25 (0.5) | 26 (0.6) | 1 (0.02) |
| 30–39 | 8169 | 412 (5.0) | 129 (1.6) | 63 (0.8) | 40 (0.5) | 8 (0.1) |
| 40–49 | 6006 | 282 (4.7) | 86 (1.4) | 46 (0.8) | 27 (0.4) | 14 (0.2) |
| 50–59 | 2607 | 84 (3.2) | 21 (0.8) | 8 (0.3) | 9 (0.3) | 6 (0.2) |
| >60 | 351 | 22 (6.3) | 1 (0.3) | 4 (1.1) | 3 (0.9) | 10 (2.8) |
| Age unknown | 104 | 12 (11.5) | 3 (2.9) | 1 (1.0) | 1 (1.0) | 1 (1.0) |
VIA-positive patients including suspect for malignancy. CIN III including 2 cases of adenocarcinoma in situ, 1 in the 20–29 and 1 in the 40–49 age group and including 18 cases diagnosed as carcinoma in situ. Cxca; cervical carcinoma, including micro- and macroinvasive squamous cell carcinoma and adenocarcinoma.
The overall PPV of VIA for a histological diagnosis of CIN I or greater was 581/989 × 100%=58.7%, and for a histological diagnosis of CIN II or greater was 294/989 × 100%=29.7%.
The approximate specificity was 21051/(22040−581) × 100%=98.1%.
Cervical malignancy was based on the histological specimen. The 40 cases of cervical cancer included 38 squamous cell carcinomas and 2 adenocarcinomas. All the 40 cases were referred to the collaborating University Hospitals.
Treatment
Based on positive VIA, 918 women were to receive cryotherapy. Most of these women received treatment directly, some after consultation with their husband or family.
Although during and directly after the cryotherapy, women had some abdominal cramps, dizziness, flushing and sometimes fainting, no major side effects such as severe pelvic cramps, severe bleeding, anaphylactic reactions or pelvic inflammatory disease were reported.
Follow-up
Follow-up screening consisted of cytologic and repeat VIA screening 6 months after cryotherapy. Of the 989 women that were VIA positive, 71 women were excluded from follow-up as they were referred; 19 women were clinically suspect of cervical cancer, 25 women were diagnosed cervical cancer on histology, 18 women were diagnosed with carcinoma in situ, 2 with adenocarcinoma in situ and 7 women were referred based on other reasons than suspect cervical cancer.
The follow-up screening results are shown in Table 3. Overall, 495 of the 918 scheduled women (53.9%) received follow-up screening. Of these women, 92.0% was diagnosed ‘cured’, based on negative cytological and negative VIA screening. The cure rate decreased when the severity of the initial cervical lesion increased.
Table 3. Follow-up on VIA-positive women.
| VIA+ | Number FU | FU VIA− | FU VIA+ | Missing | FU Cyt− | FU Cyt+ | Missing | Cyt and VIA neg | Cure rate (%) | |
|---|---|---|---|---|---|---|---|---|---|---|
| Overall | 918 | 495 | 471 | 20 | 4 | 450 | 43 | 2 | 434 | 92.0 |
| Histology neg | 336 | 147 | 141 | 6 | 0 | 143 | 3 | 1 | 139 | |
| CIN I | 285 | 177 | 171 | 5 | 1 | 165 | 12 | 0 | 160 | 93.6 |
| CIN II | 147 | 93 | 88 | 3 | 2 | 78 | 15 | 0 | 75 | 85.2 |
| CIN III | 81 | 56 | 50 | 5 | 1 | 43 | 12 | 1 | 40 | 80.0 |
| No result | 69 | 22 | 21 | 1 | 0 | 21 | 1 | 0 | 20 | 95.2 |
VIA+ are the women who received cryotherapy based on VIA-positive results. Number FU are the women that actually underwent follow-up screening. Missing results are given for VIA and cytology.
Overall, in 43 women, the cytological results in follow-up screening were positive, these consisted from 3 × ASCUS, 39 LSIL and 1 × HSIL on Bethesda classification.
Women with positive VIA and/or an abnormal result in cytology at follow-up screening were referred to the collaborating University Hospitals for further investigations.
Discussion
In three essentially different areas in Indonesia, the metropolitan Jakarta, the city and rural areas of Tasikmalaya, and the city and rural areas on the island of Bali, we studied the performance of the concept of single-visit approach cervical cancer screening using VIA, histology and cryotherapy.
Collaboration with existing health-care services, the Indonesian family welfare movement (PKK) and the Indonesian cancer foundation (YKI), proved to be efficient in setting up an infrastructure to reach women in the villages and to mobilise them for participating in the screening programme. The targeted population was reached, 92.7% of participants had never been screened before, 64.4% were in the high-risk age group of 30–49 years, and one-third lived below the WHO poverty border. All women attending the programme were informed about cervical cancer and prevention and underwent the screening procedures. Of the women with abnormal results scheduled for cryotherapy, most of these women directly received treatment, some after consultation with their husband or family. In conclusion, after 4 months of preparation, the screening programme and the precedent awareness programme could be adapted and implemented in the local system.
Our study gives insight in the use of VIA in the field conditions and its routine performance in health services in Indonesia. In this unscreened population, the overall prevalence of cervical cancer found was high, being 40 cervical cancer cases in 22 040 women. The overall VIA positivity rate, 4.4%, seemed rather low. During this study, we concentrated on reaching the target group and on the quality of the VIA screening. Regarding the characteristics of the studied population and the findings on the site visits, we do think these are representative. Although we expected to find higher positivity rates, our rates are comparable to population-based VIA screening programs in high incidence areas as Tanzania (VIA positivity 3.8%), Bangladesh (4.8%) and Angola (6.6%) (Ngoma et al, 2010; Nessa et al, 2010; Muwonge et al, 2010).
The high rate of VIA positivity in Tasikmalaya compared with Jakarta and Bali could be explained by epidemiological differences. The percentage of women that had never been screened was higher in Tasikmalaya. Moreover, promiscuity seems to be more common in Tasikmalaya as there is more labour migration than in Jakarta and Bali.
The highest prevalence of positive VIA were found in the high-risk group of women aged 30–49, accounting for 70.7% of positive cases. The overall PPV of VIA for histological diagnosis of CIN I or greater and CIN II or greater was 58.7% and 29.3%, respectively. The resultant overtreatment rates are 41.3% and 70.7%, respectively. We think these overtreatment rates are acceptable as the morbidity associated with cryotherapy is low and the overall benefit of treatment in reducing the risk of cervical cancer in high incidence areas is significant (Sankaranarayanan et al, 2007). There were no major side effects reported, although we might not have been fully informed as follow-up rates are limited. However, these findings are consistent with earlier performed studies on the safety, acceptability and feasibility of cryotherapy, where side effects were found to be rare (Alliance for Cervical Cancer Prevention, 2003; Gaffikin et al, 2003a).
The set up of our study is limited in further objectifying the accuracy of VIA other than the PPV, as it lacks the necessary data to calculate sensitivity, specificity and negative predictive value. We calculated the approximate specificity (Sankaranarayanan et al, 1998), which was found to be 98.1%. A review on VIA test by Gaffikin et al (2003a) reported a specificity range of 64–98% with a weighted mean of 83%. The fact that our approximate specificity is high can be explained by the low VIA positivity rate in our study (Sankaranarayanan et al, 2003).
The cure rate of the lesions 6 months after cryotherapy based on negative VIA and negative cytological follow-up screening in this study was 92.0%. As these cure rates are not based on histological diagnosis, they give an indication of cure in this study rather than being an absolute number. It must be taken into account that only 47.4% of the initial population that received cryotherapy came for follow-up screening, which may have led to a bias in these numbers.
Several conclusions can be drawn from our data. First, collaboration with existing health services in Indonesia, the Indonesian family welfare movement and the Indonesian cancer foundation, succeeded in reaching high-risk women having limited access to health-care facilities and living in low-resource settings. Further targeting coverage to reach high-risk women must be taken into account when new cost-effective screening programme policies are designed and expanded to other regions in Indonesia. Second, the use of VIA and cryotherapy performed well in this single-visit approach, and the acceptance of the therapy was high.
The cervical screening programme continued in all three areas. First, the programme was granted by the ‘Goede doelen van de Nederlandse Postcode Loterij’ through the Female Cancer Foundation. Granted by the Dutch Ministry of Foreign Affairs, the programme expanded from the original three regions described in this project to another five regions in Indonesia in North Sumatra, South Kalimantan and North Sulawesi. Now the Indonesian Ministry of Health and the Indonesian cancer Association ‘Yayasan Kanker Indonesia’ took over in cooperation with the faculties of Medicine in the collaborating regions.
This cross-sectional study showed that the See and Treat single-visit approach is a promising way to reduce cervical cancer in Indonesia.
Acknowledgments
This study was funded by the Female Cancer Foundation, Leiden University Medical Center, the Netherlands (www.femalecancerfoundation.org).
Footnotes
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
References
- Alliance for Cervical Cancer PreventionACCP. Effectiveness, safety, and acceptability of cryotherapy. A systematic literature review. Cervical cancer prevention issues in depth, no 10 (2003)
- Anderson GH, Boyes DA, Benedet JL, Le Riche JC, Matisic JP, Suen KC, Worth AJ, Millner A, Bennett OM (1988) Organisation and results of the cervical cytology screening programme in British Columbia, 1955–85. Br Med J (Clin Res Ed) 296(6627): 975–978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aziz MF (2009) Gynecological cancer in Indonesia. J Gynecol Oncol 20(1): 8–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denny L, Kuhn L, Pollack A, Wainwright H, Wright TC (2000) Evaluation of alternative methods of cervical cancer screening for resource-poor settings. Cancer 89(4): 826–833 [DOI] [PubMed] [Google Scholar]
- Gaffikin L, Blumenthal PD, Emerson M, Limpaphayom K (2003a) Safety, acceptability, and feasibility of a single-visit approach to cervical-cancer prevention in rural Thailand: a demonstration project. Lancet 361(9360): 814–820 [DOI] [PubMed] [Google Scholar]
- Gaffikin L, Lauterbach M, Blumenthal PD (2003b) Performance of visual inspection with acetic acid for cervical cancer screening: a qualitative summary of evidence to date. Obstet Gynecol Surv 58(8): 543–550 [DOI] [PubMed] [Google Scholar]
- Goldie SJ, Gaffikin L, Goldhaber-Fiebert JD, Gordillo-Tobar A, Levin C, Mahe C, Wright TC (2005) Cost-effectiveness of cervical-cancer screening in five developing countries. N Engl J Med 353(20): 2158–2168 [DOI] [PubMed] [Google Scholar]
- Hakama M, Chamberlain J, Day NE, Miller AB, Prorok PC (1985) Evaluation of screening programmes for gynaecological cancer. Br J Cancer 52(4): 669–673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D (2011) Global cancer statistics. CA Cancer J Clin 61(2): 69–90 [DOI] [PubMed] [Google Scholar]
- Laara E, Day NE, Hakama M (1987) Trends in mortality from cervical cancer in the Nordic countries: association with organised screening programmes. Lancet 1(8544): 1247–1249 [DOI] [PubMed] [Google Scholar]
- Mandelblatt JS, Lawrence WF, Gaffikin L, Limpahayom KK, Lumbiganon P, Warakamin S, King J, Yi B, Ringers P, Blumenthal PD (2002) Costs and benefits of different strategies to screen for cervical cancer in less-developed countries. J Natl Cancer Inst 94(19): 1469–1483 [DOI] [PubMed] [Google Scholar]
- Muwonge R, Manuel MG, Filipe AP, Dumas JB, Frank MR, Sankaranarayanan R (2010) Visual screening for early detection of cervical neoplasia in Angola. Int J Gynaecol Obstet 111(1): 68–72 [DOI] [PubMed] [Google Scholar]
- Nessa A, Hussain MA, Rahman JN, Rashid MH, Muwonge R, Sankaranarayanan R (2010) Screening for cervical neoplasia in Bangladesh using visual inspection with acetic acid. Int J Gynaecol Obstet 111(2): 115–118 [DOI] [PubMed] [Google Scholar]
- Ngoma T, Muwonge R, Mwaiselage J, Kawegere J, Bukori P, Sankaranarayanan R (2010) Evaluation of cervical visual inspection screening in Dar es Salaam, Tanzania. Int J Gynaecol Obstet 109(2): 100–104 [DOI] [PubMed] [Google Scholar]
- Sankaranarayanan R (2011) A Practical Manual on Visual Screening for Cervical Neoplasia. [2003]. IARC Press: Lyon [Google Scholar]
- Sankaranarayanan R, Budukh AM, Rajkumar R (2001) Effective screening programmes for cervical cancer in low- and middle-income developing countries. Bull World Health Organ 79(10): 954–962 [PMC free article] [PubMed] [Google Scholar]
- Sankaranarayanan R, Esmy PO, Rajkumar R, Muwonge R, Swaminathan R, Shanthakumari S, Fayette JM, Cherian J (2007) Effect of visual screening on cervical cancer incidence and mortality in Tamil Nadu, India: a cluster-randomised trial. Lancet 370(9585): 398–406 [DOI] [PubMed] [Google Scholar]
- Sankaranarayanan R, Rajkumar R, Theresa R, Esmy PO, Mahe C, Bagyalakshmi KR, Thara S, Frappart L, Lucas E, Muwonge R, Shanthakumari S, Jeevan D, Subbarao TM, Parkin DM, Cherian J (2004) Initial results from a randomized trial of cervical visual screening in rural south India. Int J Cancer 109(3): 461–467 [DOI] [PubMed] [Google Scholar]
- Sankaranarayanan R, Wesley R, Somanathan T, Dhakad N, Shyamalakumary B, Amma NS, Parkin DM, Nair MK (1998) Visual inspection of the uterine cervix after the application of acetic acid in the detection of cervical carcinoma and its precursors. Cancer 83(10): 2150–2156 [PubMed] [Google Scholar]
- Sankaranarayanan R, Wesley R, Thara S, Dhakad N, Chandralekha B, Sebastian P, Chithrathara K, Parkin DM, Nair MK (2003) Test characteristics of visual inspection with 4% acetic acid (VIA) and Lugol's iodine (VILI) in cervical cancer screening in Kerala, India. Int J Cancer 106(3): 404–408 [DOI] [PubMed] [Google Scholar]
- Tjindarbumi D, Mangunkusumo R (2002) Cancer in Indonesia, present and future. Jpn J Clin Oncol 32(Suppl): S17–S21 [DOI] [PubMed] [Google Scholar]
- University of Zimbabwe/JHPIEGO Cervical Cancer Project (1999) Visual inspection with acetic acid for cervical-cancer screening: test qualities in a primary-care setting. Lancet 353(9156): 869–873 [PubMed] [Google Scholar]
- Vizcaino AP, Moreno V, Bosch FX, Munoz N, Barros-Dios XM, Borras J, Parkin DM (2000) International trends in incidence of cervical cancer: II. Squamous-cell carcinoma. Int J Cancer 86(3): 429–435 [DOI] [PubMed] [Google Scholar]
- WHO Meeting (1986) Control of cancer of the cervix uteri. A WHO meeting. Bull World Health Organ 64(4): 607–618 [PMC free article] [PubMed] [Google Scholar]