Abstract
Transforming growth factor beta (TGFβ) is a multipotent cytokine that is sequestered in the extracellular matrix (ECM) through interactions with a number of ECM proteins. The ECM serves to concentrate latent TGFβ at sites of intended function, to influence the bioavailability and/or function of TGFβ activators, and perhaps to regulate the intrinsic performance of cell surface effectors of TGFβ signal propagation. The downstream consequences of TGFβ signaling cascades in turn provide feedback modulation of the ECM. This review covers recent examples of how genetic mutations in constituents of the ECM or TGFβ signaling cascade result in altered ECM homeostasis, cellular performance and ultimately disease, with an emphasis on emerging therapeutic strategies that seek to capitalize on this refined mechanistic understanding.
Keywords: Transforming growth factor beta, extracellular matrix, marfan syndrome, stiff skin syndrome, fibrillin, integrin
The role of ECM in TGFβ localization, sequestration and activation
The transforming growth factor beta (TGFβ) superfamily consists of a number of cytokines that regulate diverse cellular functions, including proliferation, differentiation, and synthesis of a wide array of gene products. TGFβ exists in at least three isoforms (TGFβ1-3), which are highly conserved. They are all synthesized within cells as large protein precursors. Each has an N-terminal signal peptide that is required to permit secretion from the cell, a pro-region (latency associated peptide, LAP), and a C-terminal portion that is cleaved from the pro-region to become the mature TGFβ molecule. Following cleavage within the cell, the mature TGFβ molecule remains non-covalently bound to the LAP as part of the Small Latent Complex (SLC). The SLC remains in the cell until it is bound by one of four latent TGFβ-binding proteins (LTBPs 1-4), forming the Large Latent Complex (LLC).
Following secretion from the cell, the LLC initially interacts via LTBPs with fibronectin fibrils and heparin sulfate proteoglycans at the cell membrane, and then subsequently relocates to fibrillin-rich microfibrils in the extracellular matrix (ECM). [1, 2] LTBPs and fibrillins belong to the same gene family. Both have two types of repeating cysteine-rich domains, epidermal growth factor (EGF)-like repeats, and the so-called eight-cysteine (8-cys) or transforming growth factor protein-binding (TB) domains. Fibrillins-1, -2 and -3 are large glycoproteins that are the major components of microfibrils. Fibrillin-1 is the most prevalent postnatal form, and is found in several elastic and nonelastic tissue types, including the skin, vasculature, bones, lungs and eye. Incorporation of the LLC into microfibrils involves interaction between LTBPs 1 and 4 and the N-terminus of fibrillin-1. [3] Activation of TGFβ occurs when free ligand is released from the microfibril-bound LLC. Various modes of activation have been proposed, including protease-mediated cleavage and integrin-mediated release, the latter of which appears to result from either mechanical force transduction or metalloprotease-mediated release. [4] Following its release from the ECM, TGFβ binds to its type II cell surface receptor (TβRII), which recruits and phosphorylates the type I receptor (TβRI). TβRI then recruits and phosphorylates a receptor regulated SMAD (R-SMAD) protein, namely SMAD2 and/or SMAD3. These R-SMADs then bind to the common SMAD (co-SMAD) SMAD4 to form a heterodimeric complex. This complex enters the cell nucleus where it acts as a transcription factor for various TGFβ-dependent genes, such as connective tissue growth factor (CTGF), plasminogen activator inhibitor-1 (PAI-1) and multiple collagens.
Mutations in FBN1 influence TGFβ bioavailability
Marfan syndrome (MFS) is an autosomal dominant systemic connective tissue disorder that is primarily associated with manifestations in the cardiovascular, skeletal and ocular systems. More specifically, it includes a strong predisposition for aortic root aneurysm, with consequent vessel tear (dissection) or rupture, long bone overgrowth and ocular lens dislocation. Other manifestations include mitral valve prolapse, spine curvature (both scoliosis and kyphosis), chest wall deformity, spontaneous pneumothorax, dilation of the dural sac (dural ectasia), near-sightedness (myopia) and skin stretch marks.
MFS is caused by mutations in the FBN1 gene, which encodes fibrillin-1. It was long thought that fibrillin-1 containing microfibrils were required for elastic fiber formation (elastogenesis) within the ECM. This led to the belief that individuals with MFS are born with an obligate predisposition for tissue structural weakness and ultimately failure. This was thought to explain why in MFS the aorta dilates at blood pressures that would not typically have any deleterious consequence. This boded poorly for the development of novel therapeutic strategies for the disease. However, it was evident that certain manifestations of MFS, such as bone overgrowth, mitral valve elongation and thickening, and skeletal muscle hypoplasia, were difficult to reconcile with a pathogenic model that singularly invoked compromised tissue structural integrity; they were more suggestive of altered cellular performance. The degree of homology between the fibrillins and LTBPs prompted the hypothesis that perhaps abnormal or insufficient fibrillin-1 containing microfibrils in the tissues of MFS patients might lead to altered TGFβ activation. Whether this was true, and whether TGFβ was driving disease pathogenesis in MFS, remained to be determined.
It was subsequently shown that mice heterozygous for a missense mutation in fibrillin-1 (Fbn1C1039G/+; hereafter referred to as MFS mice) show excess TGFβ signaling, as evidenced by increased Smad2/3 activation in the aortic root, lung, skeletal muscle and mitral valve. [5,6,7,8] To prove that increased TGFβ signaling was a driver rather than simply a marker or consequence of disease progression, MFS lung, skeletal muscle and mitral valve phenotypes in the mice, in association with blunted Smad2/3 activation.
The angiotensin II (ANG-II) type 1 receptor (AT1R) blocker (ARB) losartan is a clinically-available blood pressure lowering agent that had been shown to attenuate TGFβ signaling in certain disease states, such as chronic renal failure, by reducing the expression of TGFβ ligands, [9] receptors, [10,11] and activators. [12] Given its ability to inhibit TGFβ signaling in other disease states, as well as the fact that lowering blood pressure is a desirable effect in MFS, a trial of losartan was performed in MFS mice. Losartan treatment led to a significant rescue of aortic root aneurysm progression, aortic wall thickness and aortic wall architecture in MFS mice, compared to treatment with either placebo or a hemodynamically-equivalent dose of the β-blocker propranolol. [5] A similar phenotypic rescue with losartan was also observed in the lungs and skeletal muscle of MFS mice, tissues in which blood pressure changes would have no inferred relevance. Notably, rescue in all tissues correlated with a reduction in Smad2/3 activation, suggesting that losartan was achieving its therapeutic effect through antagonism of TGFβ signaling. [5]
There is preliminary evidence to suggest that these findings hold promise for patients with MFS. The effect of ARBs was assessed in a small cohort of pediatric MFS patients who had severe aortic root enlargement despite previous alternative medical therapy. This study found that ARBs significantly slowed the rate of aortic root and sinotubular junction dilatation, whereas the distal ascending aorta (which does not normally become dilated in MFS) remained unaffected. [13] In light of this growing body of evidence, several multi-center prospective clinical trials to assess the therapeutic potential of ARBs in MFS are underway. [14] It also appears that circulating TGFβ blood levels may represent a novel biomarker for aneurysm progression and response to therapy in MFS. [15] Circulating total Tgfb1 concentration is elevated in older untreated MFS mice compared to wild type (WT) littermates. Furthermore, losartan treatment of MFS mice leads to lower total Tgfb1 concentration compared with age-matched placebo-treated littermates, to levels indistinguishable from age-matched WT mice. In humans, circulating total TGFβ1 concentration is elevated in patients with MFS compared to controls, while MFS patients treated with losartan or β-blocker showed significantly lower circulating TGFβ1 concentration compared with untreated patients.
Intracellular TGFβ-dependent signaling cascades driving aneurysmal disease in MFS
Following aberrant matrix-dependent activation of TGFβ and subsequent interaction with its cell surface receptor, it remained to be determined what the critical TGFβ-dependent intracellular mediators were driving disease pathogenesis in MFS. Until recently, much of the focus in both MFS and other TGFβ-dependent disease states had been placed on so-called canonical (i.e. SMAD-dependent) pathways. However it was becoming increasingly recognized that TGFβ can also stimulate other (noncanonical) cascades, including the phosphatidylinositol-3-OH kinase (PI3K)/AKT, Rho-associated protein kinase (ROCK), and mitogen-activated protein kinase (MAPK) cascades, the latter of which includes extracellular signal-regulated kinase (ERK), Jun N-terminal kinase (JNK) and p38. [16] While Smad2/3 activation had been shown to correlate with both disease progression and therapeutic rescue by TGFβ NAb and losartan in MFS mice, a cause-and-effect relationship had yet to be determined.
Western blot analysis indicated that both Smad2 and Erk1/2 are activated in the proximal ascending aortas of MFS mice, and both are inhibited by TGFβ NAb and losartan. [17] This increase in Smad2 and Erk1/2 activation occurs selectively in portions of the aorta affected in MFS mice, since no such activation was seen in the descending thoracic aorta. [18] Furthermore, selective inhibition of Erk1/2 activation using the orally-bioavailable MEK1 inhibitor RDEA119 led to complete abrogation of abnormal aortic root growth in MFS mice. Erk1/2 activation, but not Smad2 activation, was reduced in RDEA119-treated MFS animals, confirming the specificity of the drug and illustrating that Smad2 activation in MFS mice is not Erk-dependent. Furthermore, RDEA119 had no effect on aortic root growth in WT mice, indicating that it selectively targets MFS-associated pathological aortic growth, rather than simply inducing aortic hypoplasia. This suggests that the noncanonical TGFβ-dependent Erk1/2 cascade plays a critical role in aortic disease progression in MFS mice.
There is considerable evidence in the literature linking the TGFβ and angiotensin signaling pathways. It is well recognized that ANG-II can mediate progression of aortic aneurysm, but the relative contributions of the AT1R and the angiotensin-II type 2 receptor (AT2R) remained unknown in MFS. Given losartan’s therapeutic efficacy via inhibition of the AT1R, this receptor appeared likely to play a role in disease pathogenesis in MFS. The contribution of the AT2R to aortic aneurysm progression in MFS remained controversial. On the one hand, AT2R signaling has been shown to oppose AT1R-mediated enhancement of TGFβ signaling in some cell types and tissues, [19] which would suggest it should provide protection. In contrast, AT2R signaling can also induce vascular smooth muscle cell (VSMC) apoptosis, theoretically contributing to aortic wall damage. [20] This had direct clinical relevance, as it left open to question the relative therapeutic merits of selective AT1R blockade with ARBs versus limiting signaling through both the AT1R and the AT2R using angiotensin-converting enzyme inhibitors (ACEi) that prevent production of ANG-II.
Genetic elimination of At2r was shown to accelerate aortic growth and rupture in MFS mice, indicating that AT2R signaling provides protection within this context. [21] Furthermore, losartan’s efficacy is reduced in At2r-deficient MFS mice, indicating that ARBs achieve their effect, at least in part, through selective AT1R blockade, which could stimulate signaling through the AT2R. To directly assess the efficacy of ARBs versus ACEi, a head-to-head trial of hemodynamically-equivalent doses of the ARB losartan and the ACEi enalapril was performed in MFS mice. Enalapril was less effective than losartan in reducing aortic root growth. Both agents attenuated canonical TGFβ (i.e. Smad2) signaling in the aortas of MFS mice, but losartan uniquely inhibited Erk1/2 activation. Although concordant effects of prior therapies on canonical and noncanonical TGFβ signaling cascades made it impossible to dissect out their relative contributions, the differential effects of losartan and enalapril suggest that noncanonical TGFβ signaling (specifically Erk1/2), is the predominant driver of aneurysm progression in MFS mice.
There is evidence in patients with MFS that ACEi therapy may be beneficial. Enalapril treatment was shown in a small cohort of MFS patients to be favorable over β-blocker therapy, [22] although no biochemical analysis was performed and no comparison to ARBs was made. Another study suggested that treatment with the ACEi perindopril is beneficial in patients with MFS, [23] although the analysis period was relatively brief (six months), ACEi therapy was not prescribed in isolation (but rather was used in combination with β-blockers), and no comparison to ARBs was made. However, the study did show that combined ACEi/β-blocker therapy led to a significant reduction in circulating latent and active TGFβ, as well as total matrix metalloproteinase (MMP)-2 and -3 levels. Hence it remains unclear what the relative therapeutic merits of ARBs versus ACEi are in patients with MFS, an issue that is further clouded by the fact that some ACEi such as perindopril may have a number of pleitropic effects.
It is also possible that other noncanonical TGFβ signaling cascades contribute to aortic disease in MFS. On the one hand, no evidence for increased Jnk, p38 or RhoA activation has been observed in the aortas of Fbn1C1039G/+MFS mice. [17] Furthermore, an in-vivo trial of fasudil, a well-established inhibitor of the RhoA/Rock pathway, failed to attenuate aortic root growth in these animals. However, Smad4 haploinsufficiency was found to exacerbate aortic disease and cause premature lethality in MFS mice, in association with enhanced Jnk activation, while a Jnk antagonist ameliorated aortic growth in MFS mice that either lacked or retained full Smad4 expression. This suggests that manipulation of Smad-dependent TGFβ signaling cascades (e.g. Smad4 haploinsufficiency) can directly or indirectly activate noncanonical signaling cascades (e.g. Jnk), and that Jnk inhibition may be another potential therapeutic strategy for MFS. This observation is consistent with prior work showing that Jnk1 inhibition can ameliorate abdominal aortic aneurysm (AAA) induced by the periaortic application of calcium chloride. [24]
There is some evidence that the p38 MAPK cascade may be increased in fibrillin-1 null states. [25] In-vitro elimination of fibrillin-1 deposition was shown to result in activation of Smad2, p38 and its upstream activator TGFβ-activated kinase 1 (Tak1). Furthermore, Western blot analysis of the aortic wall in fibrillin-1 null (Fbn1MgN/MgN) mice shows increased Smad2 and p38 activation relative to WT animals, although it remains to be seen whether selective p38 inhibition influences aortic disease progression in these animals. Furthermore, it is yet to be determined why in the aortas of mice haploinsufficient for a missense mutation in FBN1 (i.e. Fbn1C1039G/+), selective Erk1/2 and Smad2 activation occurs, with Smad2 activation being Erk1/2-independent; yet in the aortas of mice null for fibrillin-1 (i.e. Fbn1MgN/MgN), selective p38 and Smad2 activation occurs, with Smad2 activation perhaps being partially p38-dependent. Whether these differences relate to the animal models used, the timing of the analyses or some other aspect of TGFβ signaling is not yet known.
Aortic aneurysm also occurs in human conditions known to modify RAS signaling, a major upstream activator of ERK1/2. These include gain-of-function mutations in the PTPN11 gene (encoding the protein tyrosine phosphatase SHP2) which causes Noonan syndrome, and loss-of-function mutations in the NF1 gene (encoding a RAS GTPase-activating protein) which causes neurofibromatosis Type 1. In mouse models of Noonan syndrome, multiple manifestations of the disease, including cardiac, craniofacial and heart valve deformities have been shown to be Erk-dependent [reviewed in 26]. While the vast majority of individuals with MFS show aortic aneurysm, this phenotype is of low penetrance in Noonan syndrome or NF Type 1. One explanation for this may be that PTPN11 or NF1 are not highly expressed, or are of minor significance to ERK1/2 signaling, in the human aorta. Another possibility is that ERK1/2 activation alone is not sufficient for aneurysm to develop; while there is overlap in the intracellular cascades activated in MFS and Noonan syndrome mice (Erk1/2), other pathways appear to be MFS-specific (Smad2/3, p38 and possibly Jnk). Furthermore, in mouse models Erk1/2 activation appears to be TGFβ-dependent in MFS but may be TGFβ-independent in Noonan syndrome. Whether it is the nature or number of intracellular signaling pathways involved, the manner by which they are activated, their degree of activation (which itself may be magnitude- or threshold-based), the relative balance of different signaling pathways (e.g. Smad and Erk1/2 cascades), or some other variable, is yet to be determined.
Activation of TGFβ-dependent signaling cascades causes feedback modulation of the ECM
There are a number of downstream targets of TGFβ signaling, which can provide feedback modulation of the ECM, either directly through the deposition of gene products or indirectly through their influence on existing ECM components. TGFβ-dependent increases in CTGF, PAI-1 and collagen expression are well documented in the aorta and other tissues in patients with MFS. Another group of ECM proteins that have received much attention in MFS are MMPs. Both Mmp2 and Mmp9 have been shown to be upregulated in the aorta of MFS mice. [27,28] Furthermore, doxycycline, a nonspecific MMP inhibitor, has been shown to attenuate elastic fiber degeneration and improve vasomotor function in the aorta of MFS mice. This was in association with augmented tissue inhibitors of Mmp (Timp2/9) to Mmp2/9 ratio and suppression of TGFβ in the aortas of these mice. [27] Doxycycline has also been shown to reduce the rate of aortic dissection and improve survival in a more severe mouse model of MFS (Fbn1MgR/MgR), in association with reduced Mmp2 and 9 expression. [28] Given that losartan has also been shown to reduce Mmp2 and Mmp9 expression and activation in the aortas of MFS mice, [29] a trial assessing the therapeutic potential of combined doxycycline and losartan treatment was performed. In comparison to the two agents used in isolation, combination therapy led to a greater rescue of aortic diameter and aortic wall architecture. [30]
There is substantial evidence linking TGFβ, ERK1/2 and MMP activity. ERK1/2 has been shown to increase MMP9 expression in cultured aortic smooth muscle cells, while selective inhibition of ERK1/2 activation reduces this expression. [31] MMP2 and MMP9 have been shown to cleave latent TGFβ, with the protease-sensitive hinge region of the LAP complex being an obvious potential target for their activity. Furthermore, both MMP2 and MMP9, as well as other MMPs, have been shown to mediate ANG-II dependent transactivation of the epidermal growth factor receptor (EGFR) and consequent ERK1/2 activation in vascular cells. [32] This suggests that a feed-forward loop could develop in MFS, whereby increased TGFβ-dependent ERK1/2 activation drives MMP2/9 expression and/or activation; MMP2/9 could in turn augment TGFβ activation and/or ANG-II mediated EGFR-transactivation, leading to enhanced or prolonged canonical or noncanonical TGFβ signaling. Given the permissive role of ANG-II in this process, it raises the possibility that the efficacy of ARBs in MFS may at least in part derive from their ability to intervene in this potentially detrimental feed-forward loop.
Erk1/2 activation has also been shown to mediate Ang-II infusion driven AAA formation in apolipoprotein E-knockout mice through the modulation of Mmp2/9. Furthermore, selective inhibition of Erk1/2 activation using CI1040, or treatment with the HMGCoA reductase inhibitor simvastatin, led to amelioration of AAA progression in this disease model, in association with reduced Erk1/2 and Mmp2/9 activation. [33] In light of this, the potential therapeutic efficacy of HMGCoA reductase inhibitors was assessed in MFS mice. Compared to placebo-treated MFS animals, simvastatin-treated mice showed a significant reduction in performed, it remains to be determined whether the effects of simvastatin therapy in MFS mice are TGFβ] Since no biochemical analysis was -, Erk1/2- and/or Mmp2/9-related. An integrated model of our current understanding of the molecular mechanisms driving disease pathogenesis in MFS is illustrated in Figure 1.
Figure 1.
Integrated signaling cascades in Marfan syndrome and related diseases. Canonical (green) and noncanonical (blue) TGFβ signaling cascades, as well as intracellular proteins not currently implicated in downstream TGFβ signaling (red), are illustrated according. Disorders caused by a mutated gene product are shown in brackets next to the protein. Drugs shown in green have been tested in Marfan mice and/or patients. Drugs shown in red are untested but may have hypothetical benefit, based on our current understanding of disease pathogenesis. MFS: Marfan syndrome; SSS: Stiff skin syndrome; WMS: Weill-Marchesani syndrome; ELS: Ectopia lentis syndrome; GD: Geophysic dysplasia; AC: Acromelic dysplasia; HHT T1: Hereditary hemorrhagic telangiectasia Type 1; HHT T2: Hereditary hemorrhagic telangiectasia Type 2; ATS: Arterial tortuosity syndrome; PVNH: Periventricular nodular heterotopia; LDS: Loeys Dietz syndrome; iFTAA: Isolated familial thoracic aortic aneurysm; FSS: Ferguson Smith syndrome. NAb: neutralizing antibody; ARB: Angiotensin II Type 1 receptor blocker (e.g. losartan); ACEi: Angiotensin-converting enzyme inhibitor (e.g. enalapril).
Mutations in FBN1 cause TGFβ-dependent phenotypes distinct from MFS
As well as causing MFS, mutations in FBN1 can cause a number of other disorders, including stiff skin syndrome (SSS). SSS is a rare autosomal dominant fibrillinopathy characterized by thickened fibrotic skin throughout the entire body that is present at birth, as well as secondary joint contractures. Patients are also shorter than normal, with a mean adult male and female height of <15th and <5th percentile, respectively. Other occasional features include diffuse entrapment neuropathy (nerve injury and dysfunction because of local compression), focal lipodystrophy, muscle weakness, and cutaneous nodules, predominantly on the distal interphalangeal joints. Approximately 40 cases have been documented; one report identified 4 families with dominant SSS caused by heterozygous missense mutations in exon 38 of FBN1 that either create or destroy cysteine residues within the N-terminal portion of the fourth 8-cysteine domain (TB4). Interestingly, one patient who presented with both EL and SSS, which is more suggestive of an overlap phenotype between SSS and MFS, was found to possess a de novo mutation downstream in exon 39 of FBN1. [35] Relative to control skin, SSS skin has a widened zone of dense collagen, an excess of both fibrillin-1 and elastin throughout the dermis, excessive and disorganized microfibrillar aggregates, and patchy elastin distribution. Evidence for aberrant TGFβ signaling has been seen in dermal biopsies from SSS patients, including nuclear accumulation of phosphorylated SMAD2 and increased expression of collagen and CTGF. [35]
The phenotypic diversity of TGFβ-associated fibrillinopathies suggests that the effect of microfibrils on TGFβ regulation is complex. Data derived from mouse models of MFS have shown that microfibrils can limit TGFβ activation via binding to the LLC. In contrast, evidence from other mouse models suggests that microfibrils can enhance TGFβ signaling in certain contexts, by concentrating the cytokine at sites of intended function. Mutations in FBN2 cause autosomal dominant congenital contractural arachnodactyly (CCA or Beals syndrome), which is characterized by kyphoscoliosis, osteopenia, joint contracture and arachnodactyly. CCA mouse models deficient in fibrillin-2 do not show contractures, but instead show syndactyly. Genetic studies showed this to be a result of decreased local concentration of, and activity by, the TGFβ superfamily member bone morphogenetic protein 7 (Bmp-7) in the developing autopod. [36] Extrapolating from these data, it has been posited that microfibrils may help concentrate the LLC in a tissue-specific manner, thereby localizing TGFβ and its consequent signaling to specific sites of action. In this way, the level of TGFβ signaling in a given tissue may, at least in part, be determined by both positive and negative regulation by microfibrils. This could explain why the increased TGFβ signaling that occurs in both MFS and SSS can generate distinct phenotypes. In MFS, microfibrillar deficiency may drop below a critical threshold, beyond which a decreased concentration of the LLC in affected tissues (such as the aortic wall) may be offset by a dramatic increase in TGFβ activation. In contrast, increased microfibril accumulation in the skin of SSS patients may lead to excess LLC recruitment to the skin; this could lead to an inappropriately high concentration of latent TGFβ within the skin, which over time may result in chronically increased TGFβ signaling, fibrosis and ultimately stiffening of the dermis. [37]
Cell surface integrins mediate cell-ECM interactions and influence TGFβ signaling
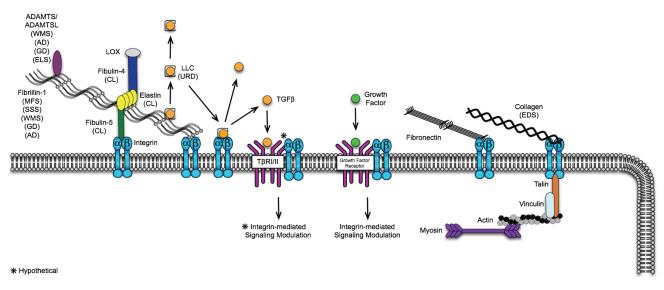
The highly focal repertoire of FBN1 mutations that cause SSS points to another possible role for microfibrils in TGFβ regulation. Mutations that cause MFS are found throughout the FBN1 gene. By contrast, FBN1 mutations causing SSS cluster in the TB4 domain. TB4 is the only domain that harbors an RGD motif, through which fibrillin-1 binds cell-surface receptors known as integrins. This implies that impaired integrin-ECM interaction may contribute to SSS pathogenesis. For example, if fibrillin-1 is a critical informant of ECM status for cells, and it signals this information via integrins, then perturbation of this mechanism may initiate a cascade of compensatory cellular events, leading to excess ECM production. In keeping with this hypothesis, cells expressing integrins αvβ3 and α5β1 plated on recombinant fibrillin-1 fragments bearing SSS mutations show a dramatically reduced ability to support integrin-mediated events, including cell attachment and spreading. More recent evidence suggests that SSS dermal cells respond to aberrant integrin-ECM interaction with compensatory changes in integrin expression and localization, altered responses to cytokines such as TGFβ, and increased ECM production, culminating in skin fibrosis (Dietz, unpublished; Figure 2).
Figure 2.
Extracellular matrix proteins influencing TGFβ and other pathways in disease. Disorders caused by a mutated gene product are shown in brackets next to the protein. Cells sense stiffened matrix through integrin-mediated bridging of ECM proteins (e.g. fibrillins, fibronectin, and collagens) to the cytoskeleletal proteins such as actin and myosin. Integrins can also modulate intracellular signaling via interactions with growth factor receptors (e.g. VEGFR, PDGFR, and IGFR). Diseases: MFS: Marfan syndrome; SSS: Stiff skin syndrome; WMS: Weill-Marchesani syndrome; ELS: Ectopia lentis syndrome; GD: Geophysic dysplasia; AC: Acromelic dysplasia; CL: Cutis Laxa, EDS: Ehlers Danlos Syndrome. *Hypothetical.
Studies of SSS-related fibrotic diseases also implicate altered integrin-ECM interaction in pathogenic TGFβ regulation. For example, altered integrin signaling has been implicated in the most commonly recognized form of acquired skin fibrosis, namely systemic sclerosis (SSc). SSc is characterized by excess ECM deposition in the skin, with additional features including autoantibody production, peripheral vasoconstriction, and fibrosis of other organs such as the kidney, heart and lungs. The excess ECM deposition in the SSc dermis is predominately composed of collagen, but also includes fibronectin and excess microfibril accumulation. [38,39] Evidence for increased TGFβ signaling in SSc includes increased activation of SMAD2 in the SSc dermis and in vitro dermal fibroblast culture, as well as increased expression of TGFβ-responsive genes in skin biopsies from a subset of SSc patients with severe skin fibrosis. [40] SSc fibroblasts have been shown to display atypical integrin presentation in vitro, [41,42] including the upregulation of integrin αvβ3, while administration of αvβ3-neutralizing antibody can inhibit αvβ3-dependent TGFβ activation and TGFβ-dependent collagen production. [43] This and other work has directly implicated integrin-mediated TGFβ activation in SSc and other fibrotic disorders. [44,45]
The cells responsible for increased TGFβ activation and excess ECM production in SSc and other fibrotic diseases are believed to be myofibroblasts. These arise from fibroblasts or other cell types such as epithelial or endothelial cells, which acquire smooth muscle characteristics, including contractile stress fibers and expression of alpha-smooth muscle actin (αSMA), through TGFβ-mediated mesenchymal transition. [46,47] When myofibroblasts contract, specific integrins at their cell surface mechanically pull the LAP, thus releasing and activating TGFβ from the LLC. Active TGFβ then drives ECM deposition, which in turn promotes myofibroblast contraction via integrins bound to the stiffened ECM. In addition, TGFβ activation, ECM remodeling and decreased ECM compliance all promote the differentiation of new myofibroblasts. [48] Hence in this context, stiffened ECM may serve to perpetuate myofibroblast contraction and excessive TGFβ signaling.
Integrins have also been shown to modulate intracellular signaling responses to a number of cytokines, including CCN2 [49], VEGF [50-51], IGF [52], PDGF [53] and TGFβ [49]. Proposed mechanisms by which altered ligand-integrin interactions affect these pathways include: 1) transcriptional or post-transcriptional induction of integrin and/or growth factor expression; 2) enhanced trafficking of integrins to the cell surface or recruitment to active focal adhesions; 3) altered selection of or affinity for integrin-ligand binding; and 4) altered levels of active integrins at the cell surface. [54,55] For example, loss of β1-integrin in cultured renal tubular cells has been shown to promote αvβ3 integrin recruitment to focal adhesions, its subsequent transactivation and resultant TGFβ-induced collagen expression. Critical steps in this process include αvβ3-dependent activation of both Rac1 and ERK. [56] There is also evidence that αvβ3 integrin directly interacts with TβRII, [57] or with other secreted factors such as CCN2 that associate with the TGFβ receptor. In light of these data, it seems feasible that altered integrin signaling may drive, or at least contribute to, some or all of the aforementioned TGFβ-dependent phenotypes, such as MFS-associated aortic disease, although this remains to be demonstrated.
There is evidence that ECM proteins other than fibrillin-1 may also serve as informants to cells of ECM status via their interaction with integrins (Figure 2). [58] Although the specific mechanism is unknown, the ECM protein fibronectin has also been implicated in fibrosis. For example, selective genetic deletion of fibronectin in the liver of mice results in increased collagen production and an enhanced fibrotic response to dimethylnitrosamine. [59] Furthermore, fibronectin splice variant extra-domain-A (ED-A) is thought to upregulate integrin-mediated signaling and myofibroblast differentiation, and has been implicated in fibrosis of the liver, [60] lungs [61] and retina. [62]
Mutations in cell surface TGFβ receptors have phenotypic overlap with MFS
Mutations in TGFβRI and TGFβRII, which encode the TGFβ receptor subunits TβRI (ALK5) and TβRII respectively, result in disease states that have clear phenotypic overlap with MFS, including Loeys-Dietz syndrome (LDS) and isolated familial thoracic aortic aneurysm (iFTAA).
Similar to MFS, patients with LDS typically show aortic aneurysm, mitral valve prolapse, skeletal changes (long fingers, chest wall deformity), dural ectasia and skin stretch marks. By contrast, they do not typically possess the lens dislocation seen in MFS, and they can show many unique features such as highly penetrant arterial tortuosity, widely spaced eyes (hypertelorism), cleft palate or bifid uvula, cervical spine malformation or instability, osteoporosis and club foot deformity. [63] Most importantly, the vascular disease observed in LDS is more diffuse and aggressive than is typically seen in MFS; patients have a strong predisposition for aneurysm and dissection throughout the arterial tree, with aortic rupture typically occurring at younger ages and at smaller aneurysm dimensions than in MFS. [64] Nearly all LDS patients harbor heterozygous missense substitutions in the kinase domain of TGFβRI or TGFβRII. Recombinant expression of mutant receptor subunits in cells naïve for the corresponding subunit failed to support TGFβ signaling, leading to the suggestion that disease manifestations result from decreased TGFβ signaling. [65] This seemed surprising, as it suggests that the concordant phenotypic manifestations between MFS and LDS are caused by directly opposing TGFβ signaling states, namely high TGFβ signaling in the former and low TGFβ signaling in the latter.
However, there is evidence to suggest that haploinsufficiency is not the mechanism by which the LDS phenotype arises. Firstly, haploinsufficiency for TβRI is already known to cause Ferguson-Smith syndrome, a condition characterized by multiple self-resolving squamous epitheliomas, but no phenotypic manifestations of LDS. LDS patients have no evidence of any such lesions. Furthermore, there have been no deletion or early nonsense mutations identified in LDS patients thus far, either of which would have favored haploinsufficiency as the causal mechanism. Instead, mutant receptors causing LDS are synthesized, transported to the cell surface and able to interact with TGFβ ligand. What happens after that remains less clear. Co-transfection studies in human cells using equimolar concentrations of normal and mutant receptors, as well as analysis of heterozygous patient cell lines have shown signaling activity of WT receptor subunits to be normal, excluding a conventional dominant-negative mechanism of action.
Interestingly, vascular tissue obtained at surgery or autopsy from LDS patients has consistently shown an apparent paradoxical increase in canonical TGFβ signaling, as evidenced by increased SMAD2 activation, and increased CTGF and collagen expression. The mechanisms underlying this paradoxical effect are yet to be resolved, but may include altered receptor trafficking, impaired autoregulation of TGFβ signaling, and/or aberrant cell autonomous signaling [reviewed in 66]. Another explanation may be that TGFβ-mediated activation of the canonical and noncanonical cascades relies on separate portions of the TβRI/RII complex. Distinct receptor kinase activities have been shown to drive the phosphorylation of SMAD proteins and the ShcA adaptor protein (an upstream mediator of ERK1/2 activation). [67] Alternatively, canonical and noncanonical TGFβ-dependent pathways may be activated through different processes. For example, while TGFβ-dependent SMAD activation occurs via receptor kinase mediated-phosphorylation (which may be affected in LDS), there is evidence that TGFβ-dependent activation of some MAPKs occurs via ubiquitylation of the TRAF6 ubiquitin ligase. [68]
Mutations in TGFβRII (and occasionally TGFβRI) have also been shown to cause iFTAA. [69] Analogous to the mutations causing LDS, most of the mutations causing iFTAA identified to date have been in close approximation in the intracellular domains of the receptors, including the serine/theonine domain that is involved in intracellular signal transduction. It seems notable that all mutations associated with iFTAA have also been observed in patients with classic LDS, suggesting that phenotypic variability may relate more to modifier effects than to discrete phenotype-genotype correlations.
Downstream mediators of the canonical TGFβ cascade have recently been shown to cause phenotypes with considerable overlap to LDS. Mutations in the SMAD3 gene have been associated with aneurysms, dissections and tortuosity throughout the arterial tree, in association with craniofacial (hypertelorism, cleft palate, bifid uvula), skeletal and cutaneous anomalies, as well as early-onset osteoarthritis. [70] Analogous to the aforementioned findings with TGFβRI and TGFβRII mutations in LDS, the authors found that mutations in SMAD3 lead to a paradoxical increase in aortic expression of several key players in the TGFβ pathway, including TGFβ1 and SMAD3 itself, increased output of TGFβ-dependent gene products such as CTGF and collagen, and direct evidence of increased TGFβ signaling (i.e. SMAD2 activation). Mutations in SMAD3 have since been described in five families possessing iFTAA, one of which also possessed intracranial and abdominal aortic aneurysms, but none of which displayed osteoarthritis. [71] No analysis was made of TGFβ signaling in the tissues of these patients. Recently a nonsense mutation in SMAD4 was identified in an individual whose family history was positive for aortopathy, in association with mitral valve disease and juvenile polyposis syndrome, [72] although again no analysis of TGFβ signaling status was performed. As with mutations in TGFβRI and TGFβRII, the basis for paradoxical enhancement of TGFβ signaling remains to be elucidated.
Mutations in other ECM components result in altered TGFβ signaling
The fibulins are a family of proteins thought to function as intermolecular bridges within the ECM. Both fibulin-4 and fibulin-5 bind to fibrillin-1 and elastin, and play a role in elastic fiber assembly and function (Figure 2). While fibulin-5 appears to promote elastin fiber assembly through the recruitment of tropoelastin to microfibrils, fibulin-4 leads to the recruitment of lysyl oxidase (LOX), which crosslinks elastin molecules. [73] In terms of disease states, deficiency of fibulin-4 causes autosomal recessive cutis laxa in association with arterial tortuosity and aortic aneurysm in both humans and mice, [74,75] while fibulin-5 deficiency causes cutis laxa with arterial tortuosity but not aneurysm. Evidence for increased canonical TGFβ signaling has been found in both humans and mice deficient in fibulin-4. Increased SMAD2 activation and CTGF expression were observed in the aortas and lungs of patients with fibulin-4 mutations, [76] while analysis of aortic tissue from mice heterozygous or homozygous for a fibulin-4 hypomorphic allele revealed a graded increase in Smad2 activation and nuclear translocation, Ctgf expression and collagen deposition compared to WT animals. [77] As with MFS mice, it appears that noncanonical TGFβ signaling cascades, specifically the Erk1/2 pathway, are also activated in the aortas of mice that are either globally- or smooth muscle cell selectively-deficient in fibulin-4. An increase in Mmp9 expression has also been observed in fibulin-4 deficient mice, although the potential therapeutic benefit of doxycycline and/or other MMP inhibitors are yet to be determined in this mouse model.
Given the apparent upregulation of both TGFβ signaling and Ang-II in tissues of fibulin-4 deficient mice, the effect of ARB therapy was assessed in these animals. [78] Prenatal losartan treatment prevented elastic fiber fragmentation in the aortic media of newborn homozygous targeted fibulin-4 deficient mice, while β-blocker therapy showed no beneficial effect on this parameter. By contrast, postnatal losartan treatment reduced hemodynamic stress and improved the lifespan of the animals, but did not ameliorate aortic architecture and failed to show a reduction in Smad2 activation. This suggests that in the context of fibulin-4 associated aneurysmal disease, ARBs can prevent aortic medial degeneration, but do not reverse pre-existing disease. The exact mechanism by which fibulin-4 deficiency leads to increased TGFβ activation remains to be determined. Failure of abnormal or absent fibulin-4 to recruit LOX, which can directly inactivate TGFβ ligand, [79] is one possible mechanism, although this is yet to be shown in-vivo. Why ARBs show less postnatal benefit in fibulin-4 deficient mice than in MFS mice is unknown, although this could conceivably relate to the differing roles of fibulin-4 and fibrillin-1 in elastic fiber formation and maintenance. Finally, why fibulin-4 deficient mice display upregulated BMP signaling (in the form of increased Smad1/5/8 activation) yet MFS mice do not, also remains unanswered.
LTBPs 1, 3 and perhaps 4 are ECM components that appear to be involved in the secretion and targeting of TGFβ to sites at which it is stored and/or activated. They may also exert effects independent of those associated with TGFβ, for example as structural proteins. Human LTBPs 1, 2 and 4 also contain an Arg-Gly-Asp (RGD) sequence that potentially promotes interaction with cell membrane-bound integrins. Mutations in LTBP4 have been associated with cutis laxa in association with severe pulmonary, gastrointestinal and urinary abnormalities (Urban-Rifkin-Davis syndrome). [80] Similarly, mice lacking Ltbp4 develop severe pulmonary emphysema, cardiomyopathy and colorectal cancer. [81] These highly tissue-specific abnormalities are associated with a lack of Ltbp4 deposition in the ECM, profound defects in elastic fiber structure and increased TGFβ activity in cultured fibroblasts. Mutations in LTBP2 have been shown to cause primary congenital glaucoma (PCG), [82] as well as an abnormally small ocular lens (microspherophakia), while mutations in LTBP3 have been associated with an absence of many permanent teeth and an apparent increase in bone density in the spine and skull base. [83] To date, no human diseases have been associated with mutations in LTBP1, although mice null for the long form of the gene (Ltbp1L-/-) die shortly after birth from persistent truncus arteriosus (PTA) and interrupted aortic arch (IAA), in association with aberrant cardiac neural crest (CNC) development and decreased TGFβ signaling. Ltbp1L-/- mice also show hypoplastic endocardial cushions in early heart valve development, followed by hyperplastic valves in late valvulogenesis, which appear to be the result of altered epithelial-to-mesenchymal transition (EMT), a well-known TGFβ-mediated process. [84]
Another group of ECM-associated proteins with clinical importance are members of the ADAMTS (a disintegrin-like and metalloprotease (reprolysin-type) with thrombospondin type 1 motif) family. The ADAMTS superfamily includes 19 zinc metalloproteases and 7 ADAMTS-like proteins that lack catalytic activity. While many functions of family members have yet to be identified, reports indicate involvement in maturation of procollagen and von Willebrand factor, as well as in ECM turn over, including proteolytic processing of proteoglycans and other ECM proteins. There is strong evidence that they are also involved in TGFβ sequestration and activation. They appear to exert their effect on TGFβ either directly via their interactions with LTBP1 (ADAMTSL2), or indirectly by regulating microfibril formation through their interaction with fibrillin-1 (ADAMTS10, ADAMTSL2, 4, 6) and/or enhancing microfibril biogenesis (ADAMTS10, ADAMTSL4, 6). [85,86]
Recessive forms of geophysic dysplasia (GD) are caused by mutations in ADAMTSL2. GD patients have short stature, facial dysmorphism, small hands and feet, thick skin, and progressive contractures of the limb joints, which can often result in an irregular gait. Importantly, these patients also suffer from progressive cardiac valvular disease and tracheal stenosis, resulting in frequent pulmonary infections and a high mortality rate. Dermal fibroblasts from GD patients with ADAMTSL2 mutations exhibit increased TGFβ secretion and SMAD2 activation. [87] Interestingly, dominant forms of GD and of the related disease acromelic dysplasia (AD) are caused by mutations affecting the TGFβ-binding protein-like domain 5 (TB5) of fibrillin-1, suggesting that ADAMTSL2 may directly interact with fibrillin-1 in-vivo. Microfibrillar network disorganization and enhanced TGFβ signaling have also been reported in fibroblasts from patients with dominant forms of both GD and AD. [88]
Analogous to the GD findings, mutations in genes encoding ADAMTS proteins can cause recessive Weill-Marchesani syndrome (WMS; ADAMTS10) [89] and recessive WMS-like syndrome (ADAMTS17), [90] while mutations in exons 9 and 41 of FBN1 can cause dominant WMS. [91] Similarly, recessive forms of ectopia lentis syndrome (ELS) are caused by mutations in ADAMTSL4 and ADAMTS17, [92] while mutations in FBN1 have been shown to cause dominant ELS. [93] It remains to be determined whether WMS or ELS are associated with aberrant TGFβ signaling.
Mutations in a number of other ECM components have been shown to cause aortic, skeletal, skin and/or ocular phenotypes that share some degree of phenotypic overlap with MFS and related conditions. Mutations in the gene encoding elastin (ELN) cause cutis laxa with low penetrance ascending aortic aneurysm and dissection. Fibroblast cultures of patients with cutis laxa and aortic aneurysm caused by ELN mutations have recently been found to show impaired deposition of tropoelastin onto microfibril-containing fibers, and enhanced tropoelastin coacervation and globule formation, leading to lower amounts of mature, insoluble elastin. They also displayed upregulated SMAD2 activation, implicating increased TGFβ signaling in disease pathogenesis. [94]
Mutations in genes that encode collagen or influence its metabolism can cause osteogenesis imperfecta (COL1A1, COL1A2), Ehlers-Danlos syndrome (COL3A1, PLOD1), X-linked Allport syndrome (COL4A5), hereditary angiopathy, nephropathy, muscle cramps and aneurysm (COL4A1), and bone fragility with contractures, deafness and arterial rupture (PLOD3), all of which can be associated with aortic aneurysm [reviewed in 66]. However, the aortic involvement in these diseases is typically of low penetrance, and is highly variable in both nature (i.e. progressive aneurysm without dissection, versus sudden dissection with minimal dilatation) and distribution (i.e. aortic root, ascending aorta, abdominal aorta or medium-sized arteries). Furthermore, the degree to which TGFβ signaling plays a role in these phenotypes, if any, remains largely unknown.
Mutations in other cell membrane proteins result in altered TGFβ signaling
Mutations in other cell surface proteins also appear to affect TGFβ signaling and result in various phenotypes. Individuals with autosomal recessive arterial tortuosity syndrome (ATS) show diffuse and severe arterial tortuosity, as well as vascular stenoses, segmental vascular hypoplasia and arterial aneurysms, in association with disruption of elastic fibers in the arterial wall. This disorder is caused by loss-of-function mutations in the SLC2A10 gene, which encodes the glucose transporter type 10 membrane protein (GLUT10). [95] Interestingly, vascular tissue from ATS patients displays upregulated TGFβ signaling, as evidenced by increased SMAD2 activation and CTGF expression. The exact mechanism by which this enhanced TGFβ signaling occurs in ATS remains unclear, although it may relate to impaired expression of the proteoglycan decorin (an antagonist of TGFβ superfamily signaling), which has been shown in fibroblasts cultured from ATS patients. [95]
Mutations in other membrane proteins that can lead to altered TGFβ signaling also cause aneurysmal phenotypes. Mutations in the genes encoding both endoglin (ENG) and activin receptor like kinase 1 (ALK1), which cause hereditary hemorrhagic telangiectasia (HHT) Type 1 and Type 2, respectively, occasionally present with aortic and medium-sized arterial aneurysms. [96,97] Endoglin (also known as CD105) is expressed on the surface of endothelial and hematopoietic cells and binds TGFβ1 and TGFβ3, in combination with the TβRI/TβRII complex. Its expression increases during angiogenesis, wound healing, and inflammation, all of which are associated with increased TGFβ signaling; hence it may serve to facilitate or enhance TGFβ-mediated downstream signaling events. ALK1 is a type I TGFβ receptor that appears to mediate TGFβ1 signaling in endothelial cells. More specifically, it binds to TGFβ1 and TβRII and inhibits TGFβ1-dependent transcriptional activity of TβRI. This suggests that the degree of binding of TGFβ1 to either ALK1 or TβRI may influence downstream signaling readout. Interestingly, mice homozygous for nonsense mutations in either Eng or Alk1 show remarkable phenotypic overlap to Tgfbr1 and Tgfbr2 knockout mice. [98,99] It remains to be determined how ENG and ALK1 mutations cause aneurysmal disease, and what effect they have on the modulation of the different TGFβ-dependent intracellular signaling cascades.
Mutations in the cellular contractile apparatus result in altered TGFβ signaling
In addition to ECM-mediated alterations in the contractile apparatus of cells (e.g. during the transformation from fibroblasts to myofibroblasts), recent work identifying genes causing iFTAA has implicated perturbation of the vascular contractile apparatus in aneurysm pathogenesis. Heterozygous mutations in MYH11, which encodes smooth muscle myosin heavy chain 11 (MYH11), can cause iFTAA in association with patent ductus arteriosus (PDA) and rarely bicuspid aortic valve (BAV). [100,101] Heterozygous mutations in ACTA2, which encodes αSMA, have been found to cause ascending aortic aneurysm and dissection, that can be found in association with descending aortic aneurysm and dissection, PDA and BAV. [102] Some patients show a lattice-like purplish discoloration of the skin, caused by dilatation of medium sized veins (livedo reticularis), as well as pigmented cysts of the iris (iris flocculi). As the number of documented mutations in these two genes has increased, it has become apparent that the resulting phenotypes are highly variable, with manifestations occurring at all ages and in tissues outside of the cardiovascular system. [103,104]
Most recently, heterozygous mutations in MYLK1, the kinase that controls SMC contractile function, have been identified in 2 families who demonstrated acute aortic dissection with little to no enlargement of the aorta. [105] The nature of the mutations identified in MYH11 and ACTA2 indicate that they impair protein assembly and act in a dominant negative manner. By contrast, the mutations in MYLK1 identified so far result in impaired calmodulin binding or kinase activity. In combination with the observation of aortic medial degeneration in mice possessing VSMC-specific knockdown of Mylk, these data implicate loss of function as the mechanistic basis of MYLK1 mutations.
While initial studies of VSMCs isolated from patients with MYH11 mutations showed upregulation of proliferation, insulin-like growth factor 1 (IGF-1) signaling, and components of the ANG-II signaling cascade, they failed to find evidence of increased TGFβ signaling. [101] More recent work using aortic tissue from patients harboring MYH11 mutations has found evidence of high VSMC expression of the calcium-binding protein S100A12, a protein which has been associated with high TGFβ expression and signaling in mice. [106] Furthermore, increased SMAD activation and CTGF expression has implicated high TGFβ signaling in aortic tissue from patients with missense ACTA2 mutations. [107]
The mechanism by which mutations in genes encoding or influencing the intracellular contractile apparatus cause aberrant activation of extracellularly-sequestered TGFβ remains to be determined. Binding and contractility of intracellular cytoskeletal components (such as actin and myosin) via membrane-embedded integrins is required to permit dimerization of extracellular fibronectin and formation of a stable insoluble fibrillar matrix. [108] This stable matrix is essential for the formation of fibrillin-1 microfibrils, [109] as well as incorporation of the LLC into microfibrils. [110,111] One hypothesis posits that mutations in genes that affect the structure or function of intracellular cytoskeletal proteins may prevent correct fibronectin assembly, leading to defective fibrillin-1 incorporation into microfibrils and reduced sequestration of latent TGFβ. This may leave it more prone to activation, resulting in enhanced TGFβ signaling. [107] Alternatively, TGFβ signaling can also be influenced by the cytoskeleton through alterations in the trafficking and/or activity of TGFβ receptors and intracellular signaling intermediates [reviewed in 112].
Finally, the large actin-binding protein filamin-A, encoded by the FLNA gene on the X-chromosome, is another cytoskeletal (but non-contractile) intracellular protein that has been implicated in aortic aneurysm disease and shows connection to TGFβ signaling. A small subset of women with the neurological disease periventricular nodular heterotopia (PVNH), in which filamin-A expression or function is altered, show a predisposition for aneurysm, usually in association with other systemic connective tissue findings. [113] Mutations in FLNA have also been implicated in myxomatous valve disease, a phenotype commonly seen in MFS and LDS. Filamin-A can function as a positive regulator of TGFβ signaling through modulation of RhoA and SMAD trafficking, as well as contributing to the negative regulation of both ERK1/2 and MMP signaling via a RAS-GRF1 dependent mechanism. [114,115]
Controversy surrounding the role of TGFβ signaling in cardiovascular disease states
While there is considerable evidence linking high TGFβ signaling to aneurysmal disease states, conflicting data exists. For example, juvenile Emilin1-knockout mice display a diffusely small vasculature and hypertension, despite high TGFβ signaling. [116] However, given the apparently paradoxical signaling that occurs with TGFβRI/II, SMAD3 and SMAD4 mutations, the precise vascular distribution and developmental timing of high TGFβ signaling in these animals may need closer scrutiny. Other work has shown that VSMC-specific elimination of TGFβ signaling in the ascending aorta of fetal mice results in PTA, impaired elastogenesis and vessel widening, [117] while VSMC-specific elimination of Tgfbr2 results in impaired elastogenesis in the descending aorta. [118]
Non-genetic models of vascular disease have also raised questions about the exact role that TGFβ signaling plays in aneurysmal disease. It is well known that ANG-II signaling through the AT1R can enhance TGFβ signaling, by inducing the expression of TGFβ ligands, receptors and activators. ANG-II can also activate SMAD and MAPK signaling cascades in VSMCs independently of TGFβ. [119] TGFβ NAb treatment appears to induce Ang-II infusion-mediated aneurysms in normocholesterolemic C57BL/6 mice, a mouse strain that does not typically incur prominent aneurysmal disease from Ang-II infusion. [120] The lesions were inflammatory in nature, and the incidence of aneurysm and dissection was ameliorated by monocyte depletion, suggesting that TGFβ may be protective specifically within the setting of acute and intense inflammation.
By contrast, increased adventitial interleukin-6 (IL-6) expression has been observed at sites of dissection in human aneurysms. Increased expression of IL-6, monocyte chemoattractant protein-1 (MCP-1) and its receptor (CCR2) have also been shown in models of Ang-II-induced aneurysm, with CCR2 signaling contributing to IL-6 expression. Interestingly, within the setting of Ang-II infusion, CCR2-positive macrophages accumulate in the adventitia, specifically at sites of aneurysm and most prominently at sites of dissection. [121] Furthermore, mice lacking Ccr2 show reduced macrophage accumulation, decreased IL-6 and Mcp-1 expression, and protection from early dissection in response to Ang-II infusion (although they still undergo dissection after chronic infusion). TGFβ has a number of anti-inflammatory properties, and is known to induce the expression of both IL-6 and MCP-1 in many cell types, including fibroblasts and VSMCs. Furthermore, TGFβ can positively regulate monocyte recruitment and macrophage differentiation, [122] while JNK1 signaling suppresses LOX, which normally negatively regulates MCP-1 expression. [123] Administration of TGFβ NAb has even been shown to provide significant protection from Ang-II induced inflammatory aneurysms after targeted silencing of Cxcl10, a known chemoattractant for monocytes and macrophages. [124] Therefore it remains unclear exactly what role TGFβ plays in Ang-II mediated aneurysm development and how this relates to human disease.
This complexity concerning the role of TGFβ in aneurysmal disease is closely mirrored by that observed in another cardiovascular disease state, namely cardiomyopathy [reviewed in 125]. Again, the data elucidating the exact role that TGFβ plays in this disease state are conflicting. On the one hand, spontaneous cardiac hypertrophy and interstitial fibrosis develop in Tfgb1-overexpressing mice. [126] Furthermore, mice haploinsufficient for Tgfb1 are protected from age-related cardiac fibrosis and diastolic dysfunction, [127] while losartan treatment ameliorates genetically-inherited fibrosis and cardiac failure in dystrophin-deficient mdx mice. [128] TGFβ antagonism can also attenuate inflammatory-associated cardiac fibrosis caused by cardiac-specific tumor necrosis factor alpha (TNFα) overexpression. [129] Similarly, TGFβ NAb treatment prevents collagen accumulation and attenuates diastolic dysfunction without affecting cardiac hypertrophy in pressure overload-induced cardiomyopathy. [130] Finally, treatment with the ARB telmisartan has been shown to lead to a significant improvement in cardiac hypertrophy, fibrosis and left ventricular function in rats after experimental autoimmune myocarditis (EAM)-induced dilated cardiomyopathy, in association with reduced TGFβ1, Mmp2 and 9, collagen I and III, IL-6, IL-1 and Mcp1 expression. [131]
However, there are conflicting results. For example, inhibition of TGFβ signaling using mice overexpressing an inducible dominant-negative form of Tgfbr2 show reduced collagen deposition, increased left ventricular dilation and systolic dysfunction in pressure-overload induced cardiomyopathy. [132] Hence it may be the magnitude or timing of TGFβ activation that is key to determining whether it plays a protective or deleterious role in disease progression. For example, basal TGFβ activity may be necessary to preserve cardiac structure and to protect the myocardium from uncontrolled ECM degradation that could lead to cardiac dilation; on the other hand excessive TGFβ activation may be deleterious due to the promotion of collagen deposition, increased myocardial stiffness and ultimately diastolic dysfunction.
In summary, much progress has been made in our understanding of TGFβ-related vasculopathies (summarized in Table 1). This has led to the development of a number of potential novel therapeutic strategies, several of which are clinically available and are now entering human trials. Notwithstanding this, many questions remain unresolved, highlighting the need to carefully consider context when developing pathogenic and therapeutic hypotheses, and to utilize model systems that adequately recapitulate the physiologic complexity of the human system.
Table 1.
| Gene (Exons) |
Protein (Specific Domains) |
Clinical Syndrome | Implicated Pathogenic Events |
References |
|---|---|---|---|---|
| Extracellular Proteins | ||||
| FBN1 | Fibrillin-1 | Marfan syndrome | SMAD2/3, SMAD4, ERK1/2, JNK1/2, p38, AT1R, AT2R, PAI-1, CTGF, MMP2/9 |
5-8, 13, 15, 17, 18, 21, 23, 25, 27-30 |
| Ectopia lentis syndrome (dominant) | Unknown | 91, 93 | ||
| FBN1 (9, 41) | Weill-Marchesani syndrome (dominant) | |||
| FBN1 (38) | Fibrillin-1 (TB4 domain) |
Stiff skin syndrome | SMAD2, CTGF, LTBP4, αSMA, integrins αvβ3 and α5β1 |
35, unpublished |
|
FBN1 (41, 42) |
Fibrillin-1 (TB5 domain) |
Geophysic dysplasia (dominant) | SMAD2, total TGFβ | 88 |
| Acromelic dysplasia (dominant) | ||||
| FBN2 | Fibrillin-2 | Congenital contractural arachnotactyly | BMP7 | 36 |
| EFEMP2 | Fibulin-4 | Cutis laxa (recessive) with arterial tortuosity and aortic aneurysm |
SMAD2, SMAD1/5/8, ERK1/2, CTGF, MMP9, ANG-II |
76-78 |
| FBLN5 | Fibulin-5 | Cutis laxa (recessive) with arterial tortuosity but no aneurysm |
ERK1/2 & p38, TSP1 ,VEGF and MMPs in cancer models |
133, 134, 135 |
| Age-related macular degeneration 3 | VEGF, CXCR4 and TGFβ1 | 136 | ||
| ELN | Elastin | Cutis laxa, low penetrance aortic aneurysm | SMAD2 | 94 |
| LTBP2 | Latent TGFβ binding protein 2 | Primary congenital glaucoma | ECM structural integrity | 82 |
| Microspherophakia | ||||
| LTBP3 | Latent TGFβ binding protein 3 | Altered permanent teeth and bone density | TGFβ bioavailability | 80, 81, 83 |
| LTBP4 | Latent TGFβ binding protein 4 | Urban-Rifkin-Davis syndrome | ||
| ADAMTS10 | ADAMTS protein 10 | Weill-Marchesani syndrome (recessive) | Microfibril biogenesis |
85, 86, 89, 90, 92 |
| ADAMTS17 | ADAMTS protein 17 | Weill-Marchesani-like syndrome (recessive) | ||
| Ectopia lentis syndrome (recessive) | ||||
| ADAMTSL4 | ADAMTS-like protein 4 | |||
| ADAMTSL2 | ADAMTS-like protein 2 | Geophysic dysplasia (recessive) | TGFβ, SMAD2 | 87 |
| Transmembrane Proteins | ||||
| TGFβR1 | TGF-β receptor type 1 | Loeys Dietz syndrome | Canonical (SMAD2, CTGF) and noncanonical TGFβ signaling |
63, 64 |
| Isolated familial thoracic aortic aneurysm | ||||
| TGFβR2 | TGF-β receptor type 2 | Loeys Dietz syndrome | ||
| Isolated familial thoracic aortic aneurysm | ||||
| ENG | Endoglin | Hereditary hemorrhagic telangiectasia Type 1 | TGFβ superfamily | 96-99 |
| ACVRL1 | Activin receptor-like kinase I | Hereditary hemorrhagic telangiectasia Type 2 | ||
| SLC2A10 | Glucose transporter type 10 | Arterial tortuosity syndrome | SMAD2, CTGF | 95 |
| Intracellular Proteins | ||||
| SMAD3 | SMAD3 | Loeys Dietz syndrome with osteoarthritis | TGFβ1, SMAD2, CTGF and noncanonical TGFβ signaling |
70 |
| Isolated familial thoracic aortic aneurysm | ||||
| SMAD4 | SMAD4 | Loeys Dietz syndrome with mitral valve disease and juvenile polyposis syndrome |
Canonical TGFβ signaling | 72 |
| ACTA2 | α-smooth muscle actin | Isolated familial thoracic aortic aneurysm with descending aortic aneurysm, patent ductus arteriosus, bicuspid aortic valve |
SMAD2, CTGF | 102, 107 |
| MYH11 | Smooth muscle myosin | Isolated familial thoracic aortic aneurysm with patent ductus arteriosus, bicuspid aortic valve |
IGF1, ANG-II, S100A12, SMAD2, CTGF |
100, 101, 106 |
| MYLK1 | Myosin light chain kinase | Aortic dissection without prior aneurysm | Unknown | 105 |
| FLNA | Filamin-A | Periventricular nodular heterotopia, ascending aortic aneurysm and valvular dystrophy |
RhoA, SMAD, ERK1/2, MMP | 113-115 |
| PTPN11 | Protein-tyrosine phosphatase 2C | Noonan and LEOPARD syndromes | RAS-RAF-MEK-ERK | 26 (Review) |
| NF1 | Neurofibromin-1 | Neurofibromatosis Type 1 | RAS-RAF-MEK-ERK | 137, 138, 139 |
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Dallas SL, Sivakumar P, Jones CJP, Chen Q, Peters DM, Mosher DF, Humphries MJ, Kielty CM. Fibronectin regulates latent transforming growth factor-β (TGFβ) by controlling matrix assembly of latent TGFβ binding protein-1. J. Biol. Chem. 2005;280:18871–80. doi: 10.1074/jbc.M410762200. [DOI] [PubMed] [Google Scholar]
- [2].Chen Q, Sivakumar P, Barley C, Peters DM, Gomes RR, Farach-Carson MC, Dallas SL. Potential role for heparan sulfate proteoglycans in regulation of transforming growth factor-β (TGF-β) by modulating assembly of latent TGF-β-binding protein-1. J. Biol. Chem. 2007;282:26418–30. doi: 10.1074/jbc.M703341200. [DOI] [PubMed] [Google Scholar]
- [3].Isogai Z, Ono RN, Ushiro S, Keene DR, Chen Y, Mazzieri R, Charbonneau NL, Reinhardt DP, Rifkin DB, Sakai LY. Latent transforming growth factor beta-binding protein 1 interacts with fibrillin and is a microfibril-associated protein. J. Biol. Chem. 2003;24:2750–7. doi: 10.1074/jbc.M209256200. [DOI] [PubMed] [Google Scholar]
- [4].Wipff P-J, Hinz B. Integrins and the activation of latent transforming growth factor β1-An intimate relationship. Eur. J. Cell Biol. 2008;87:601–15. doi: 10.1016/j.ejcb.2008.01.012. [DOI] [PubMed] [Google Scholar]
- [5].Habashi JP, Judge DP, Holm TM, Cohn RD, Loeys BL, Cooper TK, Myers L, Klein EC, Liu G, Calvi C, Podowski M, Neptune ER, Halushka MK, Bedja D, Gabrielson K, Rifkin DB, Carta L, Ramirez F, Huso DL, Dietz HC. Losartan, an AT1 antagonist, prevents aortic aneurysm in a mouse model of Marfan syndrome. Science. 2005;312:117–21. doi: 10.1126/science.1124287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Neptune ER, Frischmeyer PA, Arking DE, Myers L, Bunton TE, Gayraud B, Ramirez F, Sakai LY, Dietz HC. Dysregulation of TGF-beta activation contributes to pathogenesis in Marfan syndrome. Nat. Genet. 2003;33(3):407–11. doi: 10.1038/ng1116. [DOI] [PubMed] [Google Scholar]
- [7].Cohn RD, van Erp C, Habashi JP, Soleimani AA, Klein EC, Lisi MT, Gamradt M, ap Rhys CM, Holm TM, Loeys BL, Ramirez F, Judge DP, Ward CW, Dietz HC. Angiotensin II type 1 receptor blockade attenuates TGF-beta-induced failure of muscle regeneration in multiple myopathic states. Nat. Med. 2007;13:204–10. doi: 10.1038/nm1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Ng CM, Cheng A, Myers LA, Martinez-Murillo F, Jie C, Bedja D, Gabrielson KL, Hausladen JM, Mecham RP, Judge DP, Dietz HC. TGF-beta-dependent pathogenesis of mitral valve prolapse in a mouse model of Marfan syndrome. J. Clin. Invest. 2004;114:1586–92. doi: 10.1172/JCI22715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Everett AD, Tufro-McReddie A, Fisher A, Gomez RA. Angiotensin receptor regulates cardiac hypertrophy and transforming growth factor-beta 1 expression. Hypertension. 1994;23:587–92. doi: 10.1161/01.hyp.23.5.587. [DOI] [PubMed] [Google Scholar]
- [10].Fukuda N, Hu WY, Kubo A, Kishioka H, Satoh C, Soma M, Izumi Y, Kanmatsuse K. Angiotensin II upregulates transforming growth factor-beta type I receptor on rat vascular smooth muscle cells. Am. J. Hypertens. 2000;13:191–8. doi: 10.1016/s0895-7061(99)00152-1. [DOI] [PubMed] [Google Scholar]
- [11].Wolf G, Ziyadeh FN, Stahl RA. Angiotensin II stimulates expression of transforming growth factor beta receptor type II in cultured mouse proximal tubular cells. J. Mol. Med. 1999;77:556–64. doi: 10.1007/s001099900028. [DOI] [PubMed] [Google Scholar]
- [12].Naito T, Masaki T, Nikolic-Paterson DJ, Tanji C, Yorioka N, Kohno N. Angiotensin II induces thrombospondin-1 production in human mesangial cells via p38 MAPK and JNK: a mechanism for activation of latent TGF-beta1. Am. J. Physiol. Renal. Physiol. 2004;286(2):F278–87. doi: 10.1152/ajprenal.00139.2003. [DOI] [PubMed] [Google Scholar]
- [13].Brooke BS, Habashi JP, Judge DP, Patel N, Loeys B, Dietz HC. Angiotensin II blockade and aortic-root dilation in Marfan’s syndrome. N. Engl. J. Med. 2008;358:2787–95. doi: 10.1056/NEJMoa0706585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Lacro RV, Dietz HC, Wruck LM, Bradley TJ, Colan SD, Devereux RB, Klein GL, Li JS, Minich LL, Paridon SM, Pearson GD, Printz BF, Pyeritz RE, Radojewski E, Roman MJ, Saul JP, Stylianou MP, Mahony L. Pediatric Heart Network Investigators: Rationale and design of a randomized clinical trial of beta-blocker therapy (atenolol) versus angiotensin II receptor blocker therapy (losartan) in individuals with Marfan syndrome. Am. Heart J. 2007;154:624–31. doi: 10.1016/j.ahj.2007.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Matt P, Schoenhoff F, Habashi J, Holm T, Van Erp C, Loch D, Carlson OD, Griswold BF, Fu Q, De Backer J, Loeys B, Huso DL, McDonnell NB, Van Eyk JE, Dietz HC, GenTAC Consortium Circulating transforming growth factor-beta in Marfan syndrome. Circulation. 2009;120:526–32. doi: 10.1161/CIRCULATIONAHA.108.841981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature. 2003;425:577–84. doi: 10.1038/nature02006. [DOI] [PubMed] [Google Scholar]
- [17].Holm TM, Habashi JP, Doyle JJ, Bedja D, Chen Y, van Erp C, Lindsay ME, Kim D, Schoenhoff F, Cohn RD, Loeys BL, Thomas CJ, Patnaik S, Marugan JJ, Judge DP, Dietz HC. Noncanonical TGFβ signaling contributes to aortic aneurysm progression in Marfan syndrome mice. Science. 2011;332:358–61. doi: 10.1126/science.1192149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Haskett D, Doyle JJ, Gard C, Chen H, Ball C, Estabrook MA, Encinas AC, Dietz HC, Utzinger U, Vande Geest JP, Azhar M. Altered tissue behavior of a non-aneurysmal descending thoracic aorta in the mouse model of Marfan syndrome. Cell Tissue Res. 2012;347(1):267–77. doi: 10.1007/s00441-011-1270-y. [DOI] [PubMed] [Google Scholar]
- [19].Jones ES, Black MJ, Widdop RE. Angiotensin AT2 receptor contributes to cardiovascular remodelling of aged rats during chronic AT1 receptor blockade. J. Mol. Cell Cardiol. 2004;37:1023–30. doi: 10.1016/j.yjmcc.2004.08.004. [DOI] [PubMed] [Google Scholar]
- [20].Nagashima H, Sakomura Y, Aoka Y, Uto K, Kameyama K.i., Ogawa M, Aomi S, Koyanagi H, Ishizuka N, Naruse M, Kawana M, Kasanuki H. Angiotensin II type 2 receptor mediates vascular smooth muscle cell apoptosis in cystic medial degeneration associated with Marfan’s syndrome. Circulation. 2001;104:I282–87. doi: 10.1161/hc37t1.094856. [DOI] [PubMed] [Google Scholar]
- [21].Habashi JP, Doyle JJ, Holm TM, Aziz H, Schoenhoff F, Bedja D, Chen Y, Modiri AN, Judge DP, Dietz HC. Angiotensin II type 2 receptor signaling attenuates aortic aneurysm in mice through ERK antagonism. Science. 2011;332:361–65. doi: 10.1126/science.1192152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Yetman AT, Bornemeier RA, McCrindle BW. Usefulness of enalapril versus propranolol or atenolol for prevention of aortic dilation in patients with the Marfan syndrome. Am. J. Cardiol. 2005;95:1125–7. doi: 10.1016/j.amjcard.2005.01.032. [DOI] [PubMed] [Google Scholar]
- [23].Ahimastos AA, Aggarwal A, D’Orsa KM, Formosa MF, White AJ, Savarirayan R, Dart AM, Kingwell BA. Effect of perindopril on large artery stiffness and aortic root diameter in patients with Marfan syndrome: a randomized controlled trial. JAMA. 2007;298(13):1539–47. doi: 10.1001/jama.298.13.1539. [DOI] [PubMed] [Google Scholar]
- [24].Yoshimura K, Aoki H, Ikeda Y, Fujii K, Akiyama N, Furutani A, Hoshii Y, Tanaka N, Ricci R, Ishihara T, Esato K, Hamano K, Matsuzaki M. Regression of abdominal aortic aneurysm by inhibition of c-Jun N-terminal kinase. Nat. Med. 2005;11(12):1330–8. doi: 10.1038/nm1335. [DOI] [PubMed] [Google Scholar]
- [25].Carta L, Smaldone S, Zilberberg L, Loch D, Dietz HC, Rifkin DB, Ramirez F. p38 MAPK is an early determinant of promiscuous Smad2/3 signaling in the aortas of fibrillin-1 (Fbn1)-null mice. J. Biol. Chem. 2009;284(9):5630–6. doi: 10.1074/jbc.M806962200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Moga MA, Nakamura T, Robbins J. Genetic approaches for changing the heart and dissecting complex syndromes. J. Mol. Cell Cardiol. 2008;45(2):148–55. doi: 10.1016/j.yjmcc.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Chung AW, Yang HH, Radomski MW, van Breemen C. Long-term doxycycline is more effective than atenolol to prevent thoracic aortic aneurysm in marfan syndrome through the inhibition of matrix metalloproteinase-2 and -9. Circ. Res. 2008;102(8):e73–85. doi: 10.1161/CIRCRESAHA.108.174367. [DOI] [PubMed] [Google Scholar]
- [28].Xiong W, Knispel RA, Dietz HC, Ramirez F, Baxter BT. Doxycycline delays aneurysm rupture in a mouse model of Marfan syndrome. J. Vasc. Surg. 2008;47(1):166–72. doi: 10.1016/j.jvs.2007.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Yang HH, Kim JM, Chum E, van Breemen C, Chung AW. Long-term effects of losartan on structure and function of the thoracic aorta in a mouse model of Marfan syndrome. Br. J. Pharmacol. 2009;158(6):1503–12. doi: 10.1111/j.1476-5381.2009.00443.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Yang HH, Kim JM, Chum E, van Breemen C, Chung AW. Effectiveness of combination of losartan potassium and doxycycline versus single-drug treatments in the secondary prevention of thoracic aortic aneurysm in Marfan syndrome. J. Thorac. Cardiovasc. Surg. 2010;140(2):305–12. doi: 10.1016/j.jtcvs.2009.10.039. [DOI] [PubMed] [Google Scholar]
- [31].Nagata T, Kai H, Shibata R, Koga M, Yoshimura A, Imaizumi T. Oncostatin M, an interleukin-6 family cytokine, upregulates matrix metalloproteinase-9 through the mitogen-activated protein kinase kinase-extracellular signal-regulated kinase pathway in cultured smooth muscle cells. Arterioscler. Thromb. Vasc. Biol. 2003;23(4):588–93. doi: 10.1161/01.ATV.0000060891.31516.24. [DOI] [PubMed] [Google Scholar]
- [32].Lucchesi PA, Sabri A, Belmadani S, Matrougui K. Involvement of metalloproteinases 2/9 in epidermal growth factor receptor transactivation in pressure-induced myogenic tone in mouse mesenteric resistance arteries. Circulation. 2004;110(23):3587–93. doi: 10.1161/01.CIR.0000148780.36121.47. [DOI] [PubMed] [Google Scholar]
- [33].Zhang Y, Naggar JC, Welzig CM, Beasley D, Moulton KS, Park HJ, Galper JB. Simvastatin inhibits angiotensin II-induced abdominal aortic aneurysm formation in apolipoprotein E-knockout mice: possible role of ERK. Arterioscler. Thromb. Vasc. Biol. 2009;29(11):1764–71. doi: 10.1161/ATVBAHA.109.192609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].McLoughlin D, McGuinness J, Byrne J, Terzo E, Huuskonen V, McAllister H, Black A, Kearney S, Kay E, Hill AD, Dietz HC, Redmond JM. Pravastatin reduces Marfan aortic dilation. Circulation. 2011;124(11):S168–73. doi: 10.1161/CIRCULATIONAHA.110.012187. [DOI] [PubMed] [Google Scholar]
- [35].Loeys BL, Gerber EE, Riegert-Johnson D, Iqbal S, Whiteman P, McConnell V, Chillakuri CR, Macaya D, Coucke PJ, De Paepe A, Judge DP, Wigley F, Davis EC, Mardon HJ, Handford P, Keene DR, Sakai LY, Dietz HC. Mutations in fibrillin-1 cause congenital scleroderma: stiff skin syndrome. Sci. Transl. Med. 2010;2(23):23ra20. doi: 10.1126/scitranslmed.3000488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Putnam EA, Zhang H, Ramirez F, Milewicz DM. Fibrillin-2 (FBN2) mutations result in the Marfan-like disorder, congenital contractural arachnodactyly. Nat. Genet. 1995;11(4):456–8. doi: 10.1038/ng1295-456. [DOI] [PubMed] [Google Scholar]
- [37].Gerber EE, Dietz HC. Chapter 22: Fibrosis: Insights from Stiff Skin Syndrome in Scleroderma. In: Wigley FM, Varga J, Denton CP, editors. From Pathogenesis to Comprehensive Management. Springer Press; 2012. [Google Scholar]
- [38].Wipff J, Avouac J, Le Charpentier M, Varret M, Houtteman A, Ruiz B, Vacher-Lavenu MC, Kahan A, Boileau C, Allanore Y. Dermal tissue and cellular expression of fibrillin-1 in diffuse cutaneous systemic sclerosis. Rheumatology (Oxford) 2010;49(4):657–61. doi: 10.1093/rheumatology/kep433. [DOI] [PubMed] [Google Scholar]
- [39].Davis EC, Blattel SA, Mecham RP. Remodeling of elastic fiber components in scleroderma skin. Connect. Tissue Res. 1999;40(2):113–21. doi: 10.3109/03008209909029107. [DOI] [PubMed] [Google Scholar]
- [40].Varga J, Pasche B. Transforming growth factor beta as a therapeutic target in systemic sclerosis. Nat. Rev. Rheumatol. 2009;5(4):200–6. doi: 10.1038/nrrheum.2009.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Asano Y, Ihn H, Yamane K, Jinnin M, Mimura Y, Tamaki K. Involvement of alphavbeta5 integrin-mediated activation of latent transforming growth factor beta1 in autocrine transforming growth factor beta signaling in systemic sclerosis fibroblasts. Arthritis Rheum. 2005;52(9):2897–905. doi: 10.1002/art.21246. [DOI] [PubMed] [Google Scholar]
- [42].Iannone F, Matucci-Cerinic M, Falappone PC, Guiducci S, Cinelli M, Distler O, Lapadula G. Distinct expression of adhesion molecules on skin fibroblasts from patients with diffuse and limited systemic sclerosis. A pilot study. J. Rheumatol. 2005;32(10):1893–8. [PubMed] [Google Scholar]
- [43].Asano Y, Ihn H, Yamane K, Jinnin M, Mimura Y, Tamaki K. Increased expression of integrin alpha(v)beta3 contributes to the establishment of autocrine TGF-beta signaling in scleroderma fibroblasts. J. Immunol. 2005;175(11):7708–18. doi: 10.4049/jimmunol.175.11.7708. [DOI] [PubMed] [Google Scholar]
- [44].Nishimura S. Activation, Integrin-Mediated Transforming Growth Factor-β, a Potential Therapeutic Target in Fibrogenic Disorders. Am. J. Pathol. 2009;175(4):1362–70. doi: 10.2353/ajpath.2009.090393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Margadant C, Sonnenberg A. Integrin-TGF-beta crosstalk in fibrosis, cancer and wound healing. EMBO Rep. 2010;11(2):97–105. doi: 10.1038/embor.2009.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Hinz B. The myofibroblast: paradigm for a mechanically active cell. J. Biomech. 2010;43(1):146–55. doi: 10.1016/j.jbiomech.2009.09.020. [DOI] [PubMed] [Google Scholar]
- [47].Rajkumar VS, Howell K, Csiszar K, Denton CP, Black CM, Abraham DJ. Shared expression of phenotypic markers in systemic sclerosis indicates a convergence of pericytes and fibroblasts to a myofibroblast lineage in fibrosis. Arthritis Res. Ther. 2005;7(5):R1113–23. doi: 10.1186/ar1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat. Rev. Mol. Biol. 2002;3(5):349–63. doi: 10.1038/nrm809. [DOI] [PubMed] [Google Scholar]
- [49].Nakerakanti SS, Bujor AM, Trojanowska M. CCN2 is required for the TGF-β induced activation of Smad1-Erk1/2 signaling network. PLoS ONE. 2011;6:e21911. doi: 10.1371/journal.pone.0021911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Masson-Gadais B, Houle F, Laferriere J, Huot J. Integrin αvβ3, requirement for VEGFR2-mediated activation of SAPK2/p38 and for Hsp90-dependent phosphorylation of focal adhesion kinase in endothelial cells activated by VEGF. Cell Stress Chaperones. 2003;8:37–52. doi: 10.1379/1466-1268(2003)8<37:ivrfva>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].De S, Razorenova O, McCabe NP, O’Toole T, Qin J, Byzova TV. VEGF-integrin interplay controls tumor growth and vascularization. Proc. Natl. Acad. Sci. USA. 2005;102(21):7589–94. doi: 10.1073/pnas.0502935102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Maile LA, Busby WH, Sitko K, Capps BE, Sergent T, Badley-Clarke J, Clemmons DR. Insulin-like growth factor-I signaling in smooth muscle cells is regulated by ligand binding to the sequence of the β3-subunit of αvβ3. Mol. Endocrinol. 2006;20:405–13. doi: 10.1210/me.2005-0241. [DOI] [PubMed] [Google Scholar]
- [53].Ishigaki T, Imanaka-Yoshida K, Shimojo N, Matsushima S, Taki W, Yoshida T. Tenascin-C enhances crosstalk signaling of integrin αvβ3/PDGFR-β complex by SRC recruitment promoting PDGF-induced proliferation and migration in smooth muscle cells. J. Cell Physiol. 2011;226(10):2617–24. doi: 10.1002/jcp.22614. [DOI] [PubMed] [Google Scholar]
- [54].Calderwood DA, Tai V, Di Paolo G, De Camilli P, Ginsberg MH. Competition for talin results in trans-dominant inhibition of integrin activation. J. Biol. Chem. 2004;279(28):28889–95. doi: 10.1074/jbc.M402161200. [DOI] [PubMed] [Google Scholar]
- [55].Gonzalez AM, Claiborne J, Jones JC. Integrin cross-talk in endothelial cells is regulated by protein kinase A and protein phosphatase 1. J. Biol. Chem. 2008;283(46):31849–60. doi: 10.1074/jbc.M801345200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Hayashida T, Jones JC, Lee CK, Schnaper HW. Loss of beta1-integrin enhances TGF-beta1-induced collagen expression in epithelial cells via increased alphavbeta3-integrin and Rac1 activity. J. Biol. Chem. 2010;285(40):30741–51. doi: 10.1074/jbc.M110.105700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Scaffidi AK, Petrovic N, Moodley YP, Fogel-Petrovic M, Kroeger KM, Seeber RM, Eidne KA, Thompson PJ, Knight DA. Alpha(v)beta(3) integrin interacts with the transforming growth factor beta (TGFbeta) type II receptor to potentiate the proliferative effects of TGFbeta1 in living human lung fibroblasts. J. Biol. Chem. 2004;279:37726–33. doi: 10.1074/jbc.M403010200. [DOI] [PubMed] [Google Scholar]
- [58].Streuli CH, Akhtar N. Signal co-operation between integrins and other receptor systems. Biochem. J. 2009;418(3):491–506. doi: 10.1042/BJ20081948. [DOI] [PubMed] [Google Scholar]
- [59].Kawelke N, Vasel M, Sens C, Au A, Dooley S, Nakchbandi IA. Fibronectin protects from excessive liver fibrosis by modulating the availability of and responsiveness of stellate cells to active TGF-β. PLoS One. 2011;6(11):e28181. doi: 10.1371/journal.pone.0028181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Hackl NJ, Bersch C, Feick P, Antoni C, Franke A, Singer MV, Nakchbandi IA. Circulating fibronectin isoforms predict the degree of fibrosis in chronic hepatitis C. Scand. J. Gastroenterol. 2010;45(3):349–56. doi: 10.3109/00365520903490606. [DOI] [PubMed] [Google Scholar]
- [61].Muro AF, Moretti FA, Moore BB, Yan M, Atrasz RG, Wilke CA, Flaherty KR, Martinez FJ, Tsui JL, Sheppard D, Baralle FE, Toews GB, White ES. An essential role for fibronectin extra type III domain A in pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 2008;177(6):638–45. doi: 10.1164/rccm.200708-1291OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Khankan R, Oliver N, He S, Ryan SJ, Hinton DR. Regulation of fibronectin-EDA through CTGF domain-specific interactions with TGFβ2 and its receptor TGFβRII. Invest. Ophthalmol. Vis. Sci. 2011;52(8):5068–78. doi: 10.1167/iovs.11-7191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Loeys BL, Chen J, Neptune ER, Judge DP, Podowski M, Holm T, Meyers J, Leitch CC, Katsanis N, Sharifi N, Xu FL, Myers LA, Spevak PJ, Cameron DE, De Backer J, Hellemans J, Chen Y, Davis EC, Webb CL, Kress W, Coucke P, Rifkin DB, De Paepe AM, Dietz HC. A syndrome of altered cardiovascular, craniofacial, neurocognitive and skeletal development caused by mutations in TGFBR1 or TGFBR2. Nat. Genet. 2005;37(3):275–81. doi: 10.1038/ng1511. [DOI] [PubMed] [Google Scholar]
- [64].Loeys BL, Schwarze U, Holm T, Callewaert BL, Thomas GH, Pannu H, De Backer JF, Oswald GL, Symoens S, Manouvrier S, Roberts AE, Faravelli F, Greco MA, Pyeritz RE, Milewicz DM, Coucke PJ, Cameron DE, Braverman AC, Byers PH, De Paepe AM, Dietz HC. Aneurysm syndromes caused by mutations in the TGF-beta receptor. N. Engl. J. Med. 2006;355(8):788–98. doi: 10.1056/NEJMoa055695. [DOI] [PubMed] [Google Scholar]
- [65].Mizuguchi T, Collod-Beroud G, Akiyama T, Abifadel M, Harada N, Morisaki T, Allard D, Varret M, Claustres M, Morisaki H, Ihara M, Kinoshita A, Yoshiura K, Junien C, Kajii T, Jondeau G, Ohta T, Kishino T, Furukawa Y, Nakamura Y, Niikawa N, Boileau C, Matsumoto N. Heterozygous TGFBR2 mutations in Marfan syndrome. Nat. Genet. 2004;36(8):855–60. doi: 10.1038/ng1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Lindsay ME, Dietz HC. Lessons on the pathogenesis of aneurysm from heritable conditions. Nature. 2011;473(7347):308–16. doi: 10.1038/nature10145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Lee MK, Pardoux C, Hall MC, Lee PS, Warburton D, Qing J, Smith SM, Derynck R. TGF-beta activates Erk MAP kinase signalling through direct phosphorylation of ShcA. EMBO J. 2007;26(17):3957–67. doi: 10.1038/sj.emboj.7601818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Yamashita M, Fatyol K, Jin C, Wang X, Liu Z, Zhang YE. TRAF6 mediates Smad-independent activation of JNK and p38 by TGF-beta. Mol. Cell. 2008;31(6):918–24. doi: 10.1016/j.molcel.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Pannu H, Fadulu VT, Chang J, Lafont A, Hasham SN, Sparks E, Giampietro PF, Zaleski C, Estrera AL, Safi HJ, Shete S, Willing MC, Raman CS, Milewicz DM. Mutations in transforming growth factor-beta receptor type II cause familial thoracic aortic aneurysms and dissections. Circulation. 2005;112(4):513–20. doi: 10.1161/CIRCULATIONAHA.105.537340. [DOI] [PubMed] [Google Scholar]
- [70].van de Laar IM, Oldenburg RA, Pals G, Roos-Hesselink JW, de Graaf BM, Verhagen JM, Hoedemaekers YM, Willemsen R, Severijnen LA, Venselaar H, Vriend G, Pattynama PM, Collée M, Majoor-Krakauer D, Poldermans D, Frohn-Mulder IM, Micha D, Timmermans J, Hilhorst-Hofstee Y, Bierma-Zeinstra SM, Willems PJ, Kros JM, Oei EH, Oostra BA, Wessels MW, Bertoli-Avella AM. Mutations in SMAD3 cause a syndromic form of aortic aneurysms and dissections with early-onset osteoarthritis. Nat. Genet. 2011;43:121–26. doi: 10.1038/ng.744. [DOI] [PubMed] [Google Scholar]
- [71].Regalado ES, Guo DC, Villamizar C, Avidan N, Gilchrist D, McGillivray B, Clarke L, Bernier F, Santos-Cortez RL, Leal SM, Bertoli-Avella AM, Shendure J, Rieder MJ, Nickerson AD, Milewicz DM. Exome Sequencing Identifies SMAD3 Mutations as a Cause of Familial Thoracic Aortic Aneurysm and Dissection With Intracranial and Other Arterial Aneurysms. Circ. Res. 2011;109(6):680–6. doi: 10.1161/CIRCRESAHA.111.248161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Andrabi S, Bekheirnia MR, Robbins-Furman P, Lewis RA, Prior TW, Potocki L. SMAD4 mutation segregating in a family with juvenile polyposis, aortopathy, and mitral valve dysfunction. Am. J. Med. Genet. A. 2011;155A(5):1165–9. doi: 10.1002/ajmg.a.33968. [DOI] [PubMed] [Google Scholar]
- [73].Horiguchi M, Inoue T, Ohbayashi T, Hirai M, Noda K, Marmorstein LY, Yabe D, Takagi K, Akama TO, Kita T, Kimura T, Nakamura T. Fibulin-4 conducts proper elastogenesis via interaction with cross-linking enzyme lysyl oxidase. Proc. Natl. Acad. Sci. USA. 2009;106(45):19029–34. doi: 10.1073/pnas.0908268106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Dasouki M, Markova D, Garola R, Sasaki T, Charbonneau NL, Sakai LY, Chu ML. Compound heterozygous mutations in fibulin-4 causing neonatal lethal pulmonary artery occlusion, aortic aneurysm, arachnodactyly, and mild cutis laxa. Am. J. Med. Genet. A. 2007;143A(22):2635–41. doi: 10.1002/ajmg.a.31980. [DOI] [PubMed] [Google Scholar]
- [75].Huang J, Davis EC, Chapman SL, Budatha M, Marmorstein LY, Word RA, Yanagisawa H. Fibulin-4 deficiency results in ascending aortic aneurysms: a potential link between abnormal smooth muscle cell phenotype and aneurysm progression. Circ. Res. 2009;106(3):583–92. doi: 10.1161/CIRCRESAHA.109.207852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Renard M, Holm T, Veith R, Callewaert BL, Adès LC, Baspinar O, Pickart A, Dasouki M, Hoyer J, Rauch A, Trapane P, Earing MG, Coucke PJ, Sakai LY, Dietz HC, De Paepe AM, Loeys BL. Altered TGFbeta signaling and cardiovascular manifestations in patients with autosomal recessive cutis laxa type I caused by fibulin-4 deficiency. Eur. J. Hum. Genet. 2010;18(8):895–901. doi: 10.1038/ejhg.2010.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Hanada K, Vermeij M, Garinis GA, de Waard MC, Kunen MG, Myers L, Maas A, Duncker DJ, Meijers C, Dietz HC, Kanaar R, Essers J. Perturbations of vascular homeostasis and aortic valve abnormalities in fibulin-4 deficient mice. Circ. Res. 2007;100(5):738–46. doi: 10.1161/01.RES.0000260181.19449.95. [DOI] [PubMed] [Google Scholar]
- [78].Moltzer E, te Riet L, Swagemakers SM, van Heijningen PM, Vermeij M, van Veghel R, Bouhuizen AM, van Esch JH, Lankhorst S, Ramnath NW, de Waard MC, Duncker DJ, van der Spek PJ, Rouwet EV, Danser AH, Essers J. Impaired vascular contractility and aortic wall degeneration in fibulin-4 deficient mice: effect of angiotensin II type 1 (AT1) receptor blockade. PLoS One. 2011;6(8):e23411. doi: 10.1371/journal.pone.0023411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Atsawasuwan P, Mochida Y, Katafuchi M, Kaku M, Fong KS, Csiszar K, Yamauchi M. Lysyl oxidase binds transforming growth factor-beta and regulates its signaling via amine oxidase activity. J. Biol. Chem. 2008;283(49):34229–40. doi: 10.1074/jbc.M803142200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Urban Z, Hucthagowder V, Schürmann N, Todorovic V, Zilberberg L, Choi J, Sens C, Brown CW, Clark RD, Holland KE, Marble M, Sakai LY, Dabovic B, Rifkin DB, Davis EC. Mutations in LTBP4 cause a syndrome of impaired pulmonary, gastrointestinal, genitourinary, musculoskeletal, and dermal development. Am. J. Hum. Genet. 2009;85(5):593–605. doi: 10.1016/j.ajhg.2009.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Sterner-Kock A, Thorey IS, Koli K, Wempe F, Otte J, Bangsow T, Kuhlmeier K, Kirchner T, Jin S, Keski-Oja J, von Melchner H. Disruption of the gene encoding the latent transforming growth factor-beta binding protein 4 (LTBP-4) causes abnormal lung development, cardiomyopathy, and colorectal cancer. Genes Dev. 2002;16:2264–73. doi: 10.1101/gad.229102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Ali M, McKibbin M, Booth A, Parry DA, Jain P, Riazuddin SA, Hejtmancik JF, Khan SN, Firasat S, Shires M, Gilmour DF, Towns K, Murphy AL, Azmanov D, Tournev I, Cherninkova S, Jafri H, Raashid Y, Toomes C, Craig J, Mackey DA, Kalaydjieva L, Riazuddin S, Inglehearn CF. Null mutations in LTBP2 cause primary congenital glaucoma. Am. J. Hum. Genet. 2009;84:664–71. doi: 10.1016/j.ajhg.2009.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Noor A, Windpassinger C, Vitcu I, Orlic M, Rafiq MA, Khalid M, Malik MN, Ayub M, Alman B, Vincent JB. Oligodontia is caused by mutation in LTBP3, the gene encoding latent TGF-beta binding protein 3. Am. J. Hum. Genet. 2009;84:519–23. doi: 10.1016/j.ajhg.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Todorovic V, Finnegan E, Freyer L, Zilberberg L, Ota M, Rifkin DB. Long form of latent TGF-β binding protein 1 (Ltbp1L) regulates cardiac valve development. Dev. Dyn. 2011;240:176–87. doi: 10.1002/dvdy.22521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Apte S. A Disintegrin-like and Metalloprotease (Reprolysin-type) with Thrombospondin Type 1 Motif (ADAMTS) Superfamily: Functions and Mechanisms. JBC. 2009;284:31493–31497. doi: 10.1074/jbc.R109.052340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Hubmacher D, Apte S. Genetic and functional linkage between ADAMTS superfamily proteins and fibrillin-1: a novel mechanism influencing microfibril assembly and function. Cell. Mol. Life Sci. 2011;68:3137–3148. doi: 10.1007/s00018-011-0780-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Le Goff C, Morice-Picard F, Dagoneau N, Wang LW, Perrot C, Crow YJ, Bauer F, Flori E, Prost-Squarcioni C, Krakow D, Ge G, Greenspan DS, Bonnet D, Le Merrer M, Munnich A, Apte SS, Cormier-Daire V. ADAMTSL2 mutations in geleophysic dysplasia demonstrate a role for ADAMTS-like proteins in TGF-beta bioavailability regulation. Nat. Genet. 2008;40:1119–23. doi: 10.1038/ng.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Le Goff C, Mahaut C, Wang LW, Allali S, Abhyankar A, Jensen S, Zylberberg L, Collod-Beroud G, Bonnet D, Alanay Y, Brady AF, Cordier MP, Devriendt K, Genevieve D, Kiper PÖ, Kitoh H, Krakow D, Lynch SA, Le Merrer M, Mégarbane A, Mortier G, Odent S, Polak M, Rohrbach M, Sillence D, Stolte-Dijkstra I, Superti-Furga A, Rimoin DL, Topouchian V, Unger S, Zabel B, Bole-Feysot C, Nitschke P, Handford P, Casanova JL, Boileau C, Apte SS, Munnich A, Cormier-Daire V. Mutations in the TGFβ binding-protein-like domain 5 of FBN1 are responsible for acromicric and geleophysic dysplasias. Am. J. Hum. Genet. 2011;89(1):7–14. doi: 10.1016/j.ajhg.2011.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Dagoneau N, Benoist-Lasselin C, Huber C, Faivre L, Mégarbané A, Alswaid A, Dollfus H, Alembik Y, Munnich A, Legeai-Mallet L, Cormier-Daire V. ADAMTS10 mutations in autosomal recessive Weill-Marchesani syndrome. Am. J. Hum. Genet. 2004;75(5):801–6. doi: 10.1086/425231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Morales J, Al-Sharif L, Khalil DS, Shinwari JM, Bavi P, Al-Mahrouqi RA, Al-Rajhi A, Alkuraya FS, Meyer BF, Al Tassan N. Homozygous mutations in ADAMTS10 and ADAMTS17 cause lenticular myopia, ectopia lentis, glaucoma, spherophakia, and short stature. Am. J. Hum. Genet. 2009;85(5):558–68. doi: 10.1016/j.ajhg.2009.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Faivre L, Gorlin RJ, Wirtz MK, Godfrey M, Dagoneau N, Samples JR, Le Merrer M, Collod-Beroud G, Boileau C, Munnich A, Cormier-Daire V. In-frame fibrillin-1 gene deletion in autosomal dominant Weill-Marchesani syndrome. J. Med. Genet. 2003;40:34–6. doi: 10.1136/jmg.40.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Ahram D, Sato TS, Kohilan A, Tayeh M, Chen S, Leal S, Al-Salem M, El-Shanti H. A homozygous mutation in ADAMTSL4 causes autosomal-recessive isolated ectopia lentis. Am. J. Hum. Genet. 2009;84:274–8. doi: 10.1016/j.ajhg.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Ades LC, Holman KJ, Brett MS, Edwards MJ, Bennetts B. Ectopia lentis phenotypes and the FBN1 gene. Am. J. Med. Genet. 2004;126A:284–9. doi: 10.1002/ajmg.a.20605. [DOI] [PubMed] [Google Scholar]
- [94].Callewaert B, Renard M, Hucthagowder V, Albrecht B, Hausser I, Blair E, Dias C, Albino A, Wachi H, Sato F, Mecham RP, Loeys B, Coucke PJ, De Paepe A, Urban Z. New insights into the pathogenesis of autosomal-dominant cutis laxa with report of five ELN mutations. Hum. Mutat. 2011;32(4):445–55. doi: 10.1002/humu.21462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Coucke PJ, Willaert A, Wessels MW, Callewaert B, Zoppi N, De Backer J, Fox JE, Mancini GM, Kambouris M, Gardella R, Facchetti F, Willems PJ, Forsyth R, Dietz HC, Barlati S, Colombi M, Loeys B, De Paepe A. Mutations in the facilitative glucose transporter GLUT10 alter angiogenesis and cause arterial tortuosity syndrome. Nat. Genet. 2006;38(4):452–7. doi: 10.1038/ng1764. [DOI] [PubMed] [Google Scholar]
- [96].Hsi DH, Ryan GF, Hellems SO, Cheeran DC, Sheils LA. Large aneurysms of the ascending aorta and major coronary arteries in a patient with hereditary hemorrhagic telangiectasia. Mayo Clin. Proc. 2003;78(6):774–6. doi: 10.4065/78.6.774. [DOI] [PubMed] [Google Scholar]
- [97].Andersen ND, Dubose J, Shah A, Lee T, Wechsler SB, Hughes GC. Thoracic endografting in a patient with hereditary hemorrhagic telangiectasia presenting with a descending thoracic aneurysm. J. Vasc. Surg. 2010;51(2):468–470. doi: 10.1016/j.jvs.2009.08.058. [DOI] [PubMed] [Google Scholar]
- [98].Arthur HM, Ure J, Smith AJ, Renforth G, Wilson DI, Torsney E, Charlton R, Parums DV, Jowett T, Marchuk DA, Burn J, Diamond AG. Endoglin, an ancillary TGFbeta receptor, is required for extraembryonic angiogenesis and plays a key role in heart development. Dev. Biol. 2000;217(1):42–53. doi: 10.1006/dbio.1999.9534. [DOI] [PubMed] [Google Scholar]
- [99].Oh SP, Seki T, Goss KA, Imamura T, Yi Y, Donahoe PK, Li L, Miyazono K, ten Dijke P, Kim S, Li E. Activin receptor-like kinase 1 modulates transforming growth factor-beta 1 signaling in the regulation of angiogenesis. Proc. Natl. Acad. Sci. USA. 2000;97(6):2626–31. doi: 10.1073/pnas.97.6.2626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Zhu L, Vranckx R, Khau Van Kien P, Lalande A, Boisset N, Mathieu F, Wegman M, Glancy L, Gasc JM, Brunotte F, Bruneval P, Wolf JE, Michel JB, Jeunemaitre X. Mutations in myosin heavy chain 11 cause a syndrome associating thoracic aortic aneurysm/aortic dissection and patent ductus arteriosus. Nat. Genet. 2006;38(3):343–9. doi: 10.1038/ng1721. [DOI] [PubMed] [Google Scholar]
- [101].Pannu H, Tran-Fadulu V, Papke CL, Scherer S, Liu Y, Presley C, Guo D, Estrera AL, Safi HJ, Brasier AR, Vick GW, Marian AJ, Raman CS, Buja LM, Milewicz DM. MYH11 mutations result in a distinct vascular pathology driven by insulin-like growth factor 1 and angiotensin II. Hum. Mol. Genet. 2007;16(20):2453–62. doi: 10.1093/hmg/ddm201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Guo DC, Pannu H, Tran-Fadulu V, Papke CL, Yu RK, Avidan N, Bourgeois S, Estrera AL, Safi HJ, Sparks E, Amor D, Ades L, McConnell V, Willoughby CE, Abuelo D, Willing M, Lewis RA, Kim DH, Scherer S, Tung PP, Ahn C, Buja LM, Raman CS, Shete SS, Milewicz DM. Mutations in smooth muscle alpha-actin (ACTA2) lead to thoracic aortic aneurysms and dissections. Nat. Genet. 2007;39(12):1488–93. doi: 10.1038/ng.2007.6. [DOI] [PubMed] [Google Scholar]
- [103].Milewicz DM, Østergaard JR, Ala-Kokko LM, Khan N, Grange DK, Mendoza-Londono R, Bradley TJ, Olney AH, Adès L, Maher JF, Guo D, Buja LM, Kim D, Hyland JC, Regalado ES. De novo ACTA2 mutation causes a novel syndrome of multisystemic smooth muscle dysfunction. Am. J. Med. Genet. A. 2010;152A(10):2437–43. doi: 10.1002/ajmg.a.33657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Roder C, Peters V, Kasuya H, Nishizawa T, Wakita S, Berg D, Schulte C, Khan N, Tatagiba M, Krischek B. Analysis of ACTA2 in European Moyamoya disease patients. Eur. J. Paediatr. Neurol. 2011;15(2):117–22. doi: 10.1016/j.ejpn.2010.09.002. [DOI] [PubMed] [Google Scholar]
- [105].Wang L, Guo DC, Cao J, Gong L, Kamm KE, Regalado E, Li L, Shete S, He WQ, Zhu MS, Offermanns S, Gilchrist D, Elefteriades J, Stull JT, Milewicz DM. Mutations in myosin light chain kinase cause familial aortic dissections. Am. J. Hum. Genet. 2010;87(5):701–7. doi: 10.1016/j.ajhg.2010.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Bowman M. Hofmann, Wilk J, Heydemann A, Kim G, Rehman J, Lodato JA, Raman J, McNally EM. S100A12 mediates aortic wall remodeling and aortic aneurysm. Circ. Res. 2010;106(1):145–54. doi: 10.1161/CIRCRESAHA.109.209486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Renard M, Callewaert B, Baetens M, Campens L, Macdermot K, Fryns JP, Bonduelle M, Dietz HC, Gaspar IM, Cavaco D, Stattin EL, Schrander-Stumpel C, Coucke P, Loeys B, De Paepe A, De Backer J. Novel MYH11 and ACTA2 mutations reveal a role for enhanced TGFβ signaling in FTAAD. Int. J. Cardiol. 2011 doi: 10.1016/j.ijcard.2011.08.079. [ePub ahead of Print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Singh P, Carraher C, Schwarzbauer JE. Assembly of fibronectin extracellular matrix. Annu. Rev. Cell Dev. Biol. 2010;26:397–419. doi: 10.1146/annurev-cellbio-100109-104020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Sabatier L, Chen D, Fagotto-Kaufmann C, Hubmacher D, McKee MD, Annis DS, Mosher DF, Reinhardt DP. Fibrillin assembly requires fibronectin. Mol. Biol. Cell. 2009;20(3):846–58. doi: 10.1091/mbc.E08-08-0830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Dallas SL, Sivakumar P, Jones CJ, Chen Q, Peters DM, Mosher DF, Humphries MJ, Kielty CM. Fibronectin regulates latent transforming growth factor-beta (TGF beta) by controlling matrix assembly of latent TGF beta-binding protein-1. J. Biol. Chem. 2005;280(19):18871–80. doi: 10.1074/jbc.M410762200. [DOI] [PubMed] [Google Scholar]
- [111].Chen Q, Sivakumar P, Barley C, Peters DM, Gomes RR, Farach-Carson MC, Dallas SL. Potential role for heparan sulfate proteoglycans in regulation of transforming growth factor-beta (TGF-beta) by modulating assembly of latent TGF-beta-binding protein-1. J. Biol. Chem. 2007;282(36):26418–30. doi: 10.1074/jbc.M703341200. [DOI] [PubMed] [Google Scholar]
- [112].Moustakas A, Heldin CH. The regulation of TGFbeta signal transduction. Development. 2009;136(22):3699–3714. doi: 10.1242/dev.030338. [DOI] [PubMed] [Google Scholar]
- [113].Sheen VL, Jansen A, Chen MH, Parrini E, Morgan T, Ravenscroft R, Ganesh V, Underwood T, Wiley J, Leventer R, Vaid RR, Ruiz DE, Hutchins GM, Menasha J, Willner J, Geng Y, Gripp KW, Nicholson L, Berry-Kravis E, Bodell A, Apse K, Hill RS, Dubeau F, Andermann F, Barkovich J, Andermann E, Shugart YY, Thomas P, Viri M, Veggiotti P, Robertson S, Guerrini R, Walsh CA. Filamin A mutations cause periventricular heterotopia with Ehlers-Danlos syndrome. Neurology. 2005;64(2):254–62. doi: 10.1212/01.WNL.0000149512.79621.DF. [DOI] [PubMed] [Google Scholar]
- [114].Zhu TN, He HJ, Kole S, D’Souza T, Agarwal R, Morin PJ, Bernier M. Filamin A-mediated down-regulation of the exchange factor Ras-GRF1 correlates with decreased matrix metalloproteinase-9 expression in human melanoma cells. J. Biol. Chem. 2007;282(20):14816–26. doi: 10.1074/jbc.M611430200. [DOI] [PubMed] [Google Scholar]
- [115].Sasaki A, Masuda Y, Ohta Y, Ikeda K, Watanabe K. Filamin associates with Smads and regulates transforming growth factor-beta signaling. J. Biol. Chem. 2001;276(21):17871–7. doi: 10.1074/jbc.M008422200. [DOI] [PubMed] [Google Scholar]
- [116].Zacchigna L, Vecchione C, Notte A, Cordenonsi M, Dupont S, Maretto S, Cifelli G, Ferrari A, Maffei A, Fabbro C, Braghetta P, Marino G, Selvetella G, Aretini A, Colonnese C, Bettarini U, Russo G, Soligo S, Adorno M, Bonaldo P, Volpin D, Piccolo S, Lembo G, Bressan GM. Emilin1 links TGF-beta maturation to blood pressure homeostasis. Cell. 2006;124(5):929–42. doi: 10.1016/j.cell.2005.12.035. [DOI] [PubMed] [Google Scholar]
- [117].Choudhary B, Zhou J, Li P, Thomas S, Kaartinen V, Sucov HM. Absence of TGFbeta signaling in embryonic vascular smooth muscle leads to reduced lysyl oxidase expression, impaired elastogenesis, and aneurysm. Genesis. 2009;47(2):115–21. doi: 10.1002/dvg.20466. [DOI] [PubMed] [Google Scholar]
- [118].Langlois D, Hneino M, Bouazza L, Parlakian A, Sasaki T, Bricca G, Li JY. Conditional inactivation of TGF-β type II receptor in smooth muscle cells and epicardium causes lethal aortic and cardiac defects. Transgenic Res. 2010;19(6):1069–82. doi: 10.1007/s11248-010-9379-4. [DOI] [PubMed] [Google Scholar]
- [119].Rodríguez-Vita J, Sánchez-López E, Esteban V, Rupérez M, Egido J, Ruiz-Ortega M. Angiotensin II activates the Smad pathway in vascular smooth muscle cells by a transforming growth factor-beta-independent mechanism. Circulation. 2005;111(19):2509–17. doi: 10.1161/01.CIR.0000165133.84978.E2. [DOI] [PubMed] [Google Scholar]
- [120].Wang Y, Ait-Oufella H, Herbin O, Bonnin P, Ramkhelawon B, Taleb S, Huang J, Offenstadt G, Combadière C, Rénia L, Johnson JL, Tharaux PL, Tedgui A, Mallat Z. TGF-beta activity protects against inflammatory aortic aneurysm progression and complications in angiotensin II-infused mice. J. Clin. Invest. 2010;120(2):422–32. doi: 10.1172/JCI38136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Tieu BC, Lee C, Sun H, Lejeune W, Recinos A, 3rd, Ju X, Spratt H, Guo DC, Milewicz D, Tilton RG, Brasier AR. An adventitial IL-6/MCP1 amplification loop accelerates macrophage-mediated vascular inflammation leading to aortic dissection in mice. J. Clin. Invest. 2009;119(12):3637–51. doi: 10.1172/JCI38308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Dietz HC. TGF-beta in the pathogenesis and prevention of disease: a matter of aneurysmic proportions. J. Clin. Invest. 2010;120(2):403–7. doi: 10.1172/JCI42014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Onoda M, Yoshimura K, Aoki H, Ikeda Y, Morikage N, Furutani A, Matsuzaki M, Hamano K. Lysyl oxidase resolves inflammation by reducing monocyte chemoattractant protein-1 in abdominal aortic aneurysm. Atherosclerosis. 2010;208(2):366–9. doi: 10.1016/j.atherosclerosis.2009.07.036. [DOI] [PubMed] [Google Scholar]
- [124].King VL, Lin AY, Kristo F, Anderson TJ, Ahluwalia N, Hardy GJ, Owens AP, 3rd, Howatt DA, Shen D, Tager AM, Luster AD, Daugherty A, Gerszten RE. Interferon-gamma and the interferon-inducible chemokine CXCL10 protect against aneurysm formation and rupture. Circulation. 2009;119(3):426–35. doi: 10.1161/CIRCULATIONAHA.108.785949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Dobaczewski M, Chen W, Frangogiannis NG. Transforming growth factor (TGF)-β signaling in cardiac remodeling. J. Mol. Cell Cardiol. 2011;51(4):600–6. doi: 10.1016/j.yjmcc.2010.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Rosenkranz S, Flesch M, Amann K, Haeuseler C, Kilter H, Seeland U, Schlüter KD, Böhm M. Alterations of beta-adrenergic signaling and cardiac hypertrophy in transgenic mice overexpressing TGF-beta(1) Am. J. Physiol. Heart Circ. Physiol. 2002;283(3):H1253–62. doi: 10.1152/ajpheart.00578.2001. [DOI] [PubMed] [Google Scholar]
- [127].Brooks WW, Conrad CH. Myocardial fibrosis in transforming growth factor beta 1 heterozygous mice. J. Mol. Cell Cardiol. 2000;32(2):187–95. doi: 10.1006/jmcc.1999.1065. [DOI] [PubMed] [Google Scholar]
- [128].Spurney CF, Sali A, Guerron AD, Iantorno M, Yu Q, Gordish-Dressman H, Rayavarapu S, van der Meulen J, Hoffman EP, Nagaraju K. Losartan decreases cardiac muscle fibrosis and improves cardiac function in dystrophin-deficient mdx mice. J. Cardiovasc. Pharmacol. Ther. 2011;16(1):87–95. doi: 10.1177/1074248410381757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Sakata Y, Chancey AL, Divakaran VG, Sekiguchi K, Sivasubramanian N, Mann DL. Transforming growth factor-beta receptor antagonism attenuates myocardial fibrosis in mice with cardiac-restricted overexpression of tumor necrosis factor. Basic Res. Cardiol. 2008;103(1):60–8. doi: 10.1007/s00395-007-0689-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Kuwahara F, Kai H, Tokuda K, Kai M, Takeshita A, Egashira K, Imaizumi T. Transforming growth factor-beta function blocking prevents myocardial fibrosis and diastolic dysfunction in pressure-overloaded rats. Circulation. 2002;106(1):130–5. doi: 10.1161/01.cir.0000020689.12472.e0. [DOI] [PubMed] [Google Scholar]
- [131].Sukumaran V, Watanabe K, Veeraveedu PT, Thandavarayan RA, Gurusamy N, Ma M, Yamaguchi K, Suzuki K, Kodama M, Aizawa Y. Telmisartan, an angiotensin-II receptor blocker ameliorates cardiac remodeling in rats with dilated cardiomyopathy. Hypertens. Res. 2010;33(7):695–702. doi: 10.1038/hr.2010.67. [DOI] [PubMed] [Google Scholar]
- [132].Lucas JA, Zhang Y, Li P, Gong K, Miller AP, Hassan E, Hage F, Xing D, Wells B, Oparil S, Chen YF. Inhibition of transforming growth factor-beta signaling induces left ventricular dilation and dysfunction in the pressure-overloaded heart. Am. J. Physiol. Heart Circ. Physiol. 2010;298(2):H424–32. doi: 10.1152/ajpheart.00529.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional References
- [133].Schiemann WP, Blobe GC, Kalume DE, Pandey A, Lodish HF. Context-specific effects of fibulin-5 (DANCE/EVEC) on cell proliferation, motility, and invasion. Fibulin-5 is induced by transforming growth factor-beta and affects protein kinase cascades. J. Biol. Chem. 2002;277:27367–77. doi: 10.1074/jbc.M200148200. [DOI] [PubMed] [Google Scholar]
- [134].Albig AR, Schiemann WP. Fibulin-5 antagonizes vascular endothelial growth factor (VEGF) signaling and angiogenic sprouting by endothelial cells. DNA Cell Biol. 2004;23(6):367–79. doi: 10.1089/104454904323145254. [DOI] [PubMed] [Google Scholar]
- [135].Lee YH, Albig AR, Regner M, Schiemann BJ, Schiemann WP. Fibulin-5 initiates epithelial-mesenchymal transition (EMT) and enhances EMT induced by TGF-beta in mammary epithelial cells via a MMP-dependent mechanism. Carcinogenesis. 2008;29(12):2243–51. doi: 10.1093/carcin/bgn199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Li F, Xu H, Zeng Y, Yin ZQ. Overexpression of Fibulin-5 in Retinal Pigment Epithelial Cells Inhibits Cell Proliferation and Migration and Downregulates VEGF, CXCR4, and TGFB1 Expression in Cocultured Choroidal Endothelial Cells. Curr. Eye Res. 2012 doi: 10.3109/02713683.2012.665561. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- [137].Lasater EA, Bessler WK, Mead LE, Horn WE, Clapp DW, Conway SJ, Ingram DA, Li F. Nf1+/− mice have increased neointima formation via hyperactivation of a Gleevec sensitive molecular pathway. Hum. Mol. Genet. 2008;17:2336–44. doi: 10.1093/hmg/ddn134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Cui Y, Costa RM, Murphy GG, Elgersma Y, Zhu Y, Gutmann DH, Parada LF, Mody I, Silva AJ. Neurofibromin regulation of ERK signaling modulates GABA release and learning. Cell. 2008;135:549–60. doi: 10.1016/j.cell.2008.09.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Wang W, Nyman JS, Ono K, Stevenson DA, Yang X, Elefteriou F. Mice lacking Nf1 in osteochondroprogenitor cells display skeletal dysplasia similar to patients with neurofibromatosis type I. Hum. Mol. Genet. 2011;20:3910–24. doi: 10.1093/hmg/ddr310. [DOI] [PMC free article] [PubMed] [Google Scholar]