In this issue Arderiu and colleagues (1) investigate the role of tissue factor (TF) in angiogenesis using both in vitro and in vivo models. They find that TF expression in endothelial cells (ECs) stimulates the expression of chemokine ligand 2 (CCL2). This facilitates the recruitment of vascular smooth muscle cells (VSMCs) and the stabilization of EC-VSMC networks.
Judah Folkman, a pioneer in angiogenesis research, was intrigued by the connection between blood coagulation and blood vessel development and proposed that the two processes were intimately connected (2, 3). A summary of the key observations connecting TF and angiogenesis is shown in Figure 1. In 1994, Zhang and colleagues (4) were the first to show that TF expression by Meth-A sarcoma cells regulates their angiogenic activity in vivo. A High level of TF expression was associated with enhanced expression of the pro-angiogenic factor vascular endothelial cell growth factor (VEGF). Later, Yu and colleagues (5) demonstrated that antisense silencing of TF expression in a human colorectal cancer cell line reduced the growth of tumor cells in mice. A study with human MDA-MB-231 breast cancer cells revealed that the TF/FVIIa complex regulated the expression of interleukin 8 (IL-8), another angiogenic factor, via activation of protease-activated receptor 2 (PAR2) (6). Interestingly, the transcriptional program induced by activation of PAR2 was similar to that induced by activation of the thrombin receptor, PAR1, and included many angiogenic factors and chemokines (7). A summary of the proposed coagulation protease-PAR pathways that lead to the expression of angiogenic factors by tumors cells is shown in Figure 1A. One area of controversy is whether TF is expressed by ECs within tumors. One study (8) reported TF expression by ECs in invasive breast cancer but not by ECs of benign tumors. However, Luther and colleagues (9) did not observe TF expression by tumor ECs. Indeed, TF expression by ECs would be expected to induce clotting which would reduce rather than increase tumor growth. Host TF does appear to have a subtle contribution to angiogenesis in some tumors. For instance, B16F1 melanoma tumors grown in low TF mice had smaller vessels than tumors grown in mice with higher levels of TF (10). The host cell type that contributes to this phenomenon is unknown but could be macrophages, VSMCs or even ECs. The role of TF in tumor angiogenesis is summarized in two recent reviews (11, 12).
Figure 1. Roles of TF in angiogenesis.
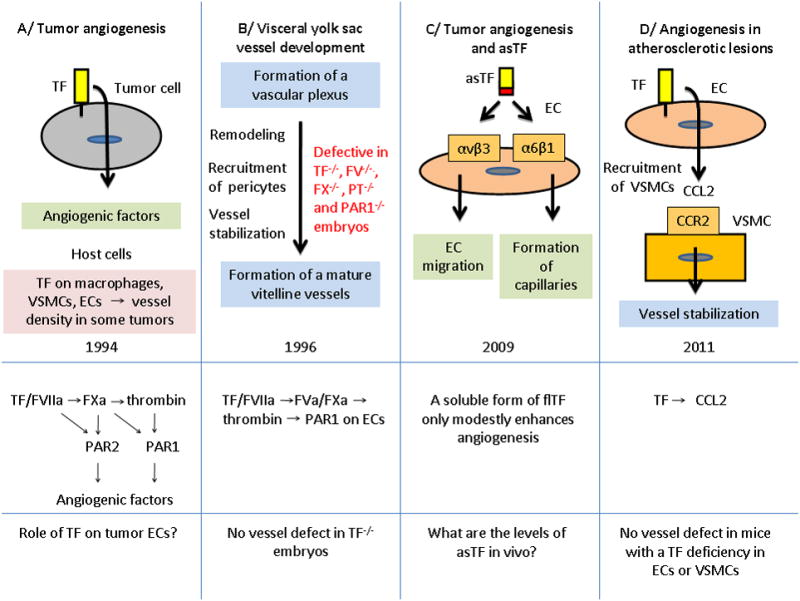
The figure shows a time line for studies on TF and angiogenesis. The different pathways that are thought to contribute to angiogenesis are shown below. PT, prothrombin.
The next major discovery was that embryos lacking TF died at mid-gestation (13-15) (Figure 1B). One study concluded that yolk sac vessels of TF−/− embryos were more fragile due to a deficit in mesenchymal cells/pericyte accumulation (13). TF was found to be expressed by the visceral endoderm within the yolk sac (13). Intriguingly, the defect in the formation of the yolk sac vasculature observed in TF−/− embryos was remarkably similar to the defect observed in PAR1−/− embryos (16). PAR1 is not expressed on platelets in mice, which suggested that the defect in TF−/− embryos maybe due to a reduction in TF-dependent thrombin generation and subsequent activation of PAR1 (13). Interestingly, PAR1 expression on ECs was able to rescue the defect in PAR1−/− embryos (17). The defect in the remodeling of the vascular plexus of this extra-embryonic tissue of TF−/− embryos has led some investigators to propose that TF is “essential” for angiogenesis in general. However, no blood vessel defects have been reported within the TF−/− embryos themselves or in the few TF−/− mice that have survived to wean (Mackman, unpublished data). Why does the TF-thrombin-PAR1 pathway contribute to the development of the yolk sac vasculature and not other vascular beds? One possibility is that a role of this pathway in physiological angiogenesis is only revealed in rapidly forming vasculature, such as the yolk sac (18, 19). TF may also play a role in the maintenance of vascular integrity in the placenta (20).
Other studies have suggested a role for the cytoplasmic domain of TF and an alternatively spliced version of TF (asTF) in angiogenesis. AsTF lacks the C-terminal region that includes the transmembrane and cytoplasmic domains (21). For instance, mice lacking the TF cytoplasmic domain have been reported to exhibit enhanced PAR2-dependent retinal angiogenesis (22). However, no defect in retinal angiogenesis was observed in mice that express very low levels of TF (Erlich and Mackman, unpublished data). In addition, there are no reported angiogenic defects in PAR2−/− mice. Therefore, at present, the role of the TF cytoplasmic domain in angiogenesis is uncertain. Interestingly, over-expression of asTF in a human pancreatic cell line increased the growth and microvascular density of tumors in mice (23). A further study found that that asTF enhanced angiogenesis ex vivo in a manner that was independent of either FVII or PAR2 (24). asTF was found to interact with the integrin αvβ3 to enhance EC migration and with α6β1 to increase the formation of capillaries in vitro (24) (Figure 1C). AsTF was also found to enhance angiogenesis in Matrigel plugs in mice (24). These studies suggest that asTF may play a role in angiogenesis, although it remains unclear how much asTF is expressed in vivo.
Arderiu and colleagues (1) modulated TF expression in human ECs and VSMCs and analyzed the ability of these cells to form capillary-like networks on 3 dimensional (3D) basement membrane (Matrigel) surfaces. Interestingly, TF mRNA was transiently expressed in the ECs. We have observed a similar transient expression of TF mRNA from ECs forming tubes within a 3D collagen matrix (Mackman and Davis, unpublished data). Gene silencing of TF was associated with a reduction in the formation of networks formed by ECs (1). Similar results were observed by silencing TF in VSMCs. Next, the authors examined networks formed by co-culture of ECs with VSMCs in vitro. A reduction of TF expression in either cell type inhibited network formation. Interestingly, TF expression has been shown to increase cell survival (25). Although the authors did not find any differences in apoptosis in ECs or VSMCs containing TF siRNA, a role of TF in cell survival in these experiments cannot be excluded. Lastly, a reduction in TF expression was associated with reduced “angiogenesis” in Matrigel plugs implanted into mice. However, it should be noted that many cell types, including tumors cells and VSMCs, align to form networks in matrigel and therefore one must be cautious in interpreting these structures as capillaries without demonstrating a lumen in the structures formed in vitro or the presence of blood in the structures formed in vivo (26). Thus, one of the complexities of the Matrigel system in vitro is that there is primarily cord-like cell alignment and minimal tube morphogenesis, and therefore, the data presented with respect to mural cell recruitment needs to be interpreted with caution since pericytes and VSMCs are known to recruit to EC tubes (rather than cords) in vivo (27). Other systems where pericyte recruitment to EC-lined tubes in 3D matrices in vitro has been investigated in more detail would represent better experimental approaches that could be used to confirm the findings presented in this study and to further assess the role of TF in these events (28,29). For example, previous work has demonstrated a role for EC-derived platelet-derived growth factor (PDGF)-BB and heparin-binding epidermal growth factor (HB-EGF) in pericyte recruitment to EC-lined tubes in 3D collagen matrices (29).
What pathways are regulated by TF in ECs? Arderiu and colleagues (1) used an angiogenesis targeted microarray to compare transcripts in ECs containing either control or TF siRNA. Due to the previously reported association between TF and VEGF, one would have expected that TF silencing would reduce VEGF expression (4). However, VEGF expression was not reduced in the TF silenced cells. Instead, they found that expression of CCL2 (also known as monocyte chemotactic protein-1 [MCP-1]) was reduced in cells treated with TF siRNA. Further studies indicated that CCL2 acts as a chemoattractant for VSMCs by binding to the CCR2 receptor. Although silencing TF in VSMCs reduced network formation the TF-dependent pathway that function in VSMCs was not elucidated. Finally, Arderiu and colleagues (1) present data that ECs within atherosclerotic lesions express both TF and CCL2, although the resolution is low making it difficult to definitively conclude that ECs are the source of these proteins.
What regulates TF expression in ECs grown in 3D culture and how does it enhance CCL2 expression? At present, there is no information on how TF gene expression is regulated. Similarly, it is not known how TF expression increases the CCL2 expression. The fact that these changes are observed in cell culture suggests that this maybe a FVIIa and PAR2-independent pathway involving integrin αvβ3. Finally, it should be noted that mice with a TF deficiency in either ECs or VSMCs (30,32) have no apparent defects in angiogenesis, which again indicates that TF is not “essential” for angiogenesis. Further studies are necessary to determine the role of full length TF, asTF and other coagulation proteins in different forms of angiogenesis.
References
- 1.Arderiu G, Pena E, Aledo R, Juan-Babot O, Badimon L. Tissue Factor Regulates Microvessel Formation and Stabilization by Induction of CCL2 Expression. Arterioscler Thromb Vasc Biol. 2011 doi: 10.1161/ATVBAHA.111.233536. in press. [DOI] [PubMed] [Google Scholar]
- 2.Folkman J. Tumor angiogenesis and tissue factor. Nat Med. 1996;2:167–168. doi: 10.1038/nm0296-167. [DOI] [PubMed] [Google Scholar]
- 3.Folkman J. Angiogenesis and proteins of the hemostatic system. J Thromb Haemost. 2003;1:1681–1682. doi: 10.1046/j.1538-7836.2003.00344.x. [DOI] [PubMed] [Google Scholar]
- 4.Zhang Y, Deng Y, Luther T, Muller M, Ziegler R, Waldherr R, Ster DM, Nawroth PP. Tissue factor controls the balance of angiogenic and antiangiogenic properties of tumor cells in mice. J Clin Invest. 1994;94:1320–1327. doi: 10.1172/JCI117451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yu JL, May L, Lhotak V, Shahrzad S, Shirasawa S, Weitz JI, Coomber BL, Mackman N, Rak JW. Oncogenic events regulate tissue factor expression in colorectal cancer cells: implications for tumor progression and angiogenesis. Blood. 2005;105:1734–1741. doi: 10.1182/blood-2004-05-2042. [DOI] [PubMed] [Google Scholar]
- 6.Hjortoe GM, Petersen LC, Albrektsen T, Sorensen BB, Norby PL, Mandal SK, Pendurthi UR, Rao LV. Tissue factor-factor VIIa-specific up-regulation of IL-8 expression in MDA-MB-231 cells is mediated by PAR-2 and results in increased cell migration. Blood. 2004;103:3029–3037. doi: 10.1182/blood-2003-10-3417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Albrektsen T, Sorensen BB, Hjorto GM, Fleckner J, Rao LV, Petersen LC. Transcriptional program induced by factor VIIa-tissue factor, PAR1 and PAR2 in MDA-MB-231 cells. J Thromb Haemost. 2007;5:1588–1597. doi: 10.1111/j.1538-7836.2007.02603.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Contrino J, Hair G, Kreutzer DL, Rickles FR. In situ detection of tissue factor in vascular endothelial cells: correlation with the malignant phenotype of human breast disease. Nat Med. 1996;2:209–215. doi: 10.1038/nm0296-209. [DOI] [PubMed] [Google Scholar]
- 9.Luther T, Flossel C, Albrecht S, Kotzsch M, Muller M. Tissue factor expression in normal and abnormal mammary gland. Nat Med. 1996;2:491–492. doi: 10.1038/nm0596-491a. [DOI] [PubMed] [Google Scholar]
- 10.Yu J, May L, Milsom C, Anderson GM, Weitz JI, Luyendyk JP, Broze G, Mackman N, Rak J. Contribution of host-derived tissue factor to tumor neovascularization. Arterioscler Thromb Vasc Biol. 2008;28:1975–1981. doi: 10.1161/ATVBAHA.108.175083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shoji M, Abe K, Nawroth PP, Rickles FR. Molecular mechanisms linking thrombosis and angiogenesis in cancer. Trends Cardiovasc Med. 1997;7:52–59. doi: 10.1016/S1050-1738(96)00142-9. [DOI] [PubMed] [Google Scholar]
- 12.Ruf W, Yokota N, Schaffner F. Tissue factor in cancer progression and angiogenesis. Thromb Res. 2010;125(2):S36–38. doi: 10.1016/S0049-3848(10)70010-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carmeliet P, Mackman N, Moons L, Luther T, Gressens P, Van Vlaenderen I, Demunck H, Kasper M, Breier G, Evrard P, et al. Role of tissue factor in embryonic blood vessel development. Nature. 1996;383:73–75. doi: 10.1038/383073a0. [DOI] [PubMed] [Google Scholar]
- 14.Toomey JR, Kratzer KE, Lasky NM, Stanton JJ, Broze GJ., Jr Targeted disruption of the murine tissue factor gene results in embryonic lethality. Blood. 1996;88:1583–1587. [PubMed] [Google Scholar]
- 15.Bugge TH, Xiao Q, Kombrinck KW, Flick MJ, Holmback K, Danton MJ, Colbert MC, Witte DP, Fujikawa K, Davie EW, et al. Fatal embryonic bleeding events in mice lacking tissue factor, the cell-associated initiator of blood coagulation. Proc Natl Acad Sci U S A. 1996;93:6258–6263. doi: 10.1073/pnas.93.13.6258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Connolly AJ, Ishihara H, Kahn ML, Farese RV, Jr, Coughlin SR. Role of the thrombin receptor in development and evidence for a second receptor. Nature. 1996;381:516–519. doi: 10.1038/381516a0. [DOI] [PubMed] [Google Scholar]
- 17.Griffin CT, Srinivasan Y, Zheng YW, Huang W, Coughlin SR. A role for thrombin receptor signaling in endothelial cells during embryonic development. Science. 2001;293:1666–1670. doi: 10.1126/science.1061259. [DOI] [PubMed] [Google Scholar]
- 18.Carmeliet P, Moons L, Dewerchin M, Mackman N, Luther T, Breier G, Ploplis V, Muller M, Nagy A, Plow E, et al. Insights in vessel development and vascular disorders using targeted inactivation and transfer of vascular endothelial growth factor, the tissue factor receptor, and the plasminogen system. Ann N Y Acad Sci. 1997;811:191–206. doi: 10.1111/j.1749-6632.1997.tb52002.x. [DOI] [PubMed] [Google Scholar]
- 19.Mackman N. Role of tissue factor in hemostasis, thrombosis, and vascular development. Arterioscler Thromb Vasc Biol. 2004;24:1015–1022. doi: 10.1161/01.ATV.0000130465.23430.74. [DOI] [PubMed] [Google Scholar]
- 20.Erlich J, Parry GC, Fearns C, Muller M, Carmeliet P, Luther T, Mackman N. Tissue factor is required for unterine hemostasis and maintenance of the placental labyrinth during gestation. Proc Natl Acad Sci USA. 1999;96:8138–8143. doi: 10.1073/pnas.96.14.8138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bogdanov VY, Balasubramanian V, Hathcock J, Vele O, Lieb M, Nemerson Y. Alternatively spliced human tissue factor: a circulating, soluble, thrombogenic protein. Nat Med. 2003;9:458–462. doi: 10.1038/nm841. [DOI] [PubMed] [Google Scholar]
- 22.Belting M, Dorrell MI, Sandgren S, Aguilar E, Ahamed J, Dorfleutner A, Carmeliet P, Mueller BM, Friedlander M, Ruf W. Regulation of angiogenesis by tissue factor cytoplasmic domain signaling. Nat Med. 2004;10:502–509. doi: 10.1038/nm1037. [DOI] [PubMed] [Google Scholar]
- 23.Hobbs JE, Zakarija A, Cundiff DL, Doll JA, Hymen E, Cornwell M, Crawford SE, Liu N, Signaevsky M, Soff GA. Alternatively spliced human tissue factor promotes tumor growth and angiogenesis in a pancreatic cancer tumor model. Thromb Res. 2007;120(2):S13–21. doi: 10.1016/S0049-3848(07)70126-3. [DOI] [PubMed] [Google Scholar]
- 24.van den Berg YW, van den Hengel LG, Myers HR, Ayachi O, Jordanova E, Ruf W, Spek CA, Reitsma PH, Bogdanov VY, Versteeg HH. Alternatively spliced tissue factor induces angiogenesis through integrin ligation. Proc Natl Acad Sci U S A. 2009;106:19497–19502. doi: 10.1073/pnas.0905325106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Versteeg HH, Spek CA, Slofstra SH, Diks SH, Richel DJ, Peppelenbosch MP. FVIIa:TF induces cell survival via G12/G13-dependent Jak/STAT activation and BclXL production. Circ Res. 2004;94:1032–1040. doi: 10.1161/01.RES.0000125625.18597.AD. [DOI] [PubMed] [Google Scholar]
- 26.Vernon RB, Angello JC, Iruela-Arispe ML, Lane TF, Sage EH. Reorganization of basement membrane matrices by cellular traction promotes the formation of cellular networks in vitro. Lab Invest. 1992;66:536–547. [PubMed] [Google Scholar]
- 27.Stratman AN, Malotte KM, Mahan RD, Davis MJ, Davis GE. Pericyte recruitment during vasculogenic tube assembly stimulates endothelial basement membrane matrix formation. Blood. 2009;114:5091–5101. doi: 10.1182/blood-2009-05-222364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stratman AN, Schwindt AE, Malotte KM, Davis GE. Endothelial-derived PDGF-BB and HB-EGF coordinately regulate pericyte recruitment during vasculogenic tube assembly and stabilization. Blood. 2010;116:4720–4730. doi: 10.1182/blood-2010-05-286872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Armulik A, Genove G, Betsholtz C. Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Dev Cell. 2011;21:193–215. doi: 10.1016/j.devcel.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 30.Pawlinski R, Wang JG, Owens AP, 3rd, Williams J, Antoniak S, Tencati M, Luther T, Rowley JW, Low EN, Weyrich AS, et al. Hematopoietic and nonhematopoietic cell tissue factor activates the coagulation cascade in endotoxemic mice. Blood. 2010;116:806–814. doi: 10.1182/blood-2009-12-259267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang L, Miller C, Swarthout RF, Rao M, Mackman N, Taubman MB. Vascular smooth muscle-derived tissue factor is critical for arterial thrombosis after ferric chloride-induced injury. Blood. 2009;113:705–713. doi: 10.1182/blood-2007-05-090944. [DOI] [PMC free article] [PubMed] [Google Scholar]


