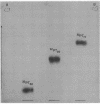
Abstract
By using anhydrous triethylamine-pyridine to selectively remove the cyanoethyl group from the fully protected oligonucleotide, a substantial improvement has been achieved in yields and the rates of condensation by the modified triester approach from the 5' leads to 3' end. The unreacted oligonucleotide containing the 5'-hydroxy group was removed by treatment with bis (triazolyl)-p-chlorophenyl phosphate after each condensation in situ. These modifications, as exemplified by the synthesis of fully protected T12, T18, T24 and T38 in 80%, 77%, 70% and 50% yields respectively, should allow the ready synthesis of polynucleotides of even longer chain lengths by purely chemical methods.
Full text
PDF




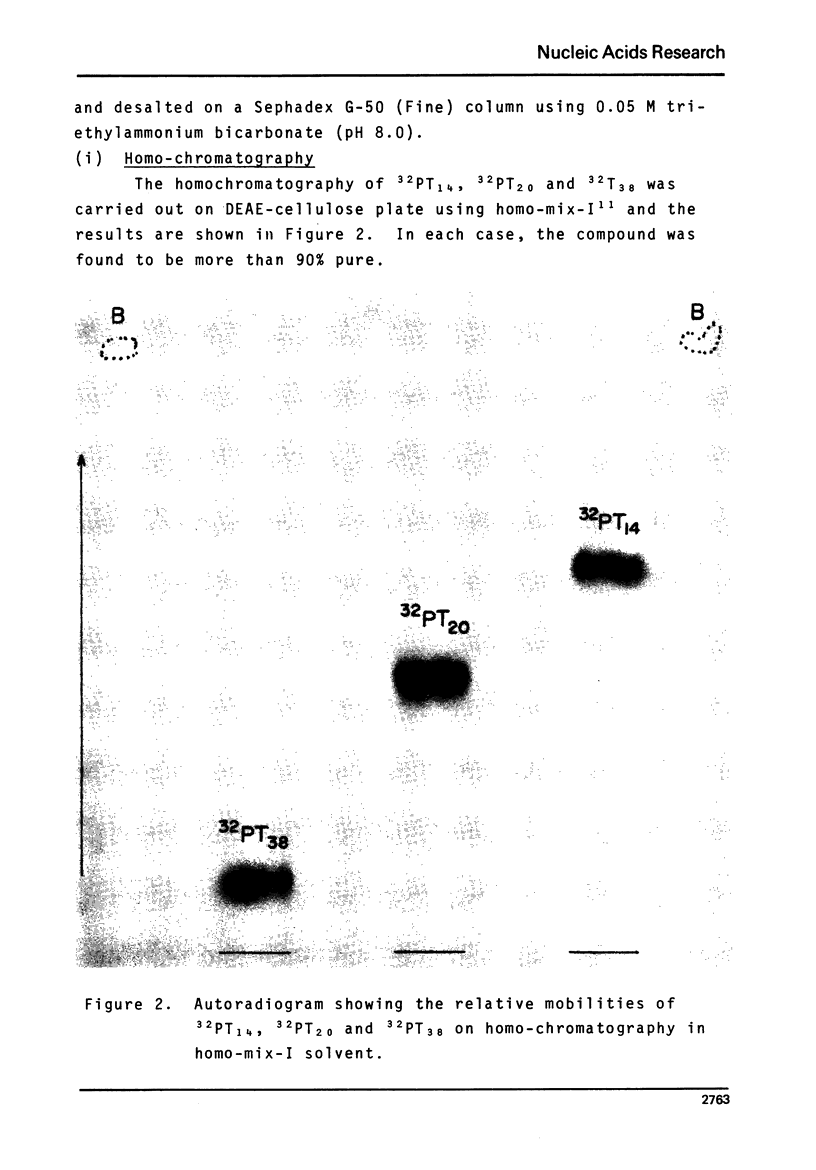
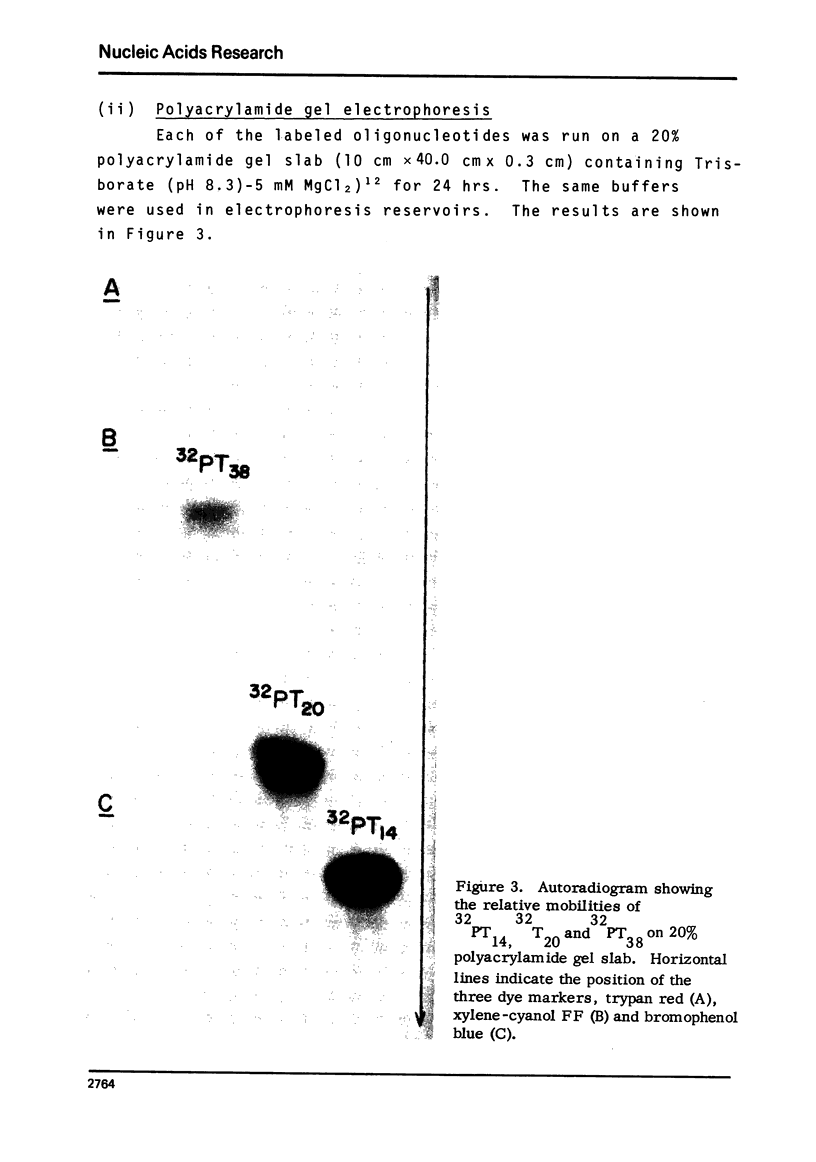

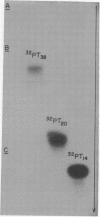
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adamiak R. W., Barciszewska M. Z., Biala E., Grzéskowiak K., Kierzek R., Kraszewski A., Markiewicz W. T., Wiewiórowski M. Nucleoside-3'-phosphotriesters as key intermediates for the oligoribonucleotide synthesis. III. An improved preparation of nucleoside 3'-phosphotriesters, their 1H NMR characterization and new conditions for removal of 2-cyanoethyl group. Nucleic Acids Res. 1976 Dec;3(12):3397–3408. doi: 10.1093/nar/3.12.3397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arentzen R., Reese C. B. The phosphotriester approach to oligonucleotide synthesis: Preparation of oligo- and poly-thymidylic acids. J Chem Soc Perkin 1. 1977;4:445–460. doi: 10.1039/p19770000445. [DOI] [PubMed] [Google Scholar]
- Brownlee G. G., Sanger F. Chromatography of 32P-labelled oligonucleotides on thin layers of DEAE-cellulose. Eur J Biochem. 1969 Dec;11(2):395–399. doi: 10.1111/j.1432-1033.1969.tb00786.x. [DOI] [PubMed] [Google Scholar]
- Catlin J. C., Cramer F. Deoxy oligonucleotide synthesis via the triester method. J Org Chem. 1973 Jan 26;38(2):245–250. doi: 10.1021/jo00942a011. [DOI] [PubMed] [Google Scholar]
- Eckstein F., Rizk I. Synthese von Oligodesoxynucleotiden über Phosphorsäure-triester. Chem Ber. 1969;102(7):2362–2377. doi: 10.1002/cber.19691020724. [DOI] [PubMed] [Google Scholar]
- Itakura K., Katagiri N., Bahl C. P., Wightman R. H., Narang S. A. Improved triester approach for the synthesis of pentadecathymidylic acid. J Am Chem Soc. 1975 Dec 10;97(25):7327–7332. doi: 10.1021/ja00858a020. [DOI] [PubMed] [Google Scholar]
- Itakura K., Katagiri N., Bahl C. P., Wightman R. H., Narang S. A. Improved triester approach for the synthesis of pentadecathymidylic acid. J Am Chem Soc. 1975 Dec 10;97(25):7327–7332. doi: 10.1021/ja00858a020. [DOI] [PubMed] [Google Scholar]
- Jay E., Bambara R., Padmanabhan R., Wu R. DNA sequence analysis: a general, simple and rapid method for sequencing large oligodeoxyribonucleotide fragments by mapping. Nucleic Acids Res. 1974 Mar;1(3):331–353. doi: 10.1093/nar/1.3.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katagiri N., Itakura K., Narang S. A. The use of arylsulfonyltriazoles for the synthesis of oligonucleotides by the triester approach. J Am Chem Soc. 1975 Dec 10;97(25):7332–7337. doi: 10.1021/ja00858a021. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., van deSande H. Chain length determination of small double- and single-stranded DNA molecules by polyacrylamide gel electrophoresis. Biochemistry. 1975 Aug 26;14(17):3787–3794. doi: 10.1021/bi00688a010. [DOI] [PubMed] [Google Scholar]
- Marians K. J., Wu R., Stawinski J., Hozumi T., Narang S. A. Cloned synthetic lac operator DNA is biologically active. Nature. 1976 Oct 28;263(5580):744–748. doi: 10.1038/263744a0. [DOI] [PubMed] [Google Scholar]
- Stawinski J., Hozumi T., Narang S. A., Bahl C. P., Wu R. Arylsulfonyltetrazoles, new coupling reagents and further improvements in the triester method for the synthesis of deoxyribooligonucleotides. Nucleic Acids Res. 1977 Feb;4(2):353–371. doi: 10.1093/nar/4.2.353. [DOI] [PMC free article] [PubMed] [Google Scholar]